ABSTRACT
Maternal health status is vital for the development of the offspring of humans, including physiological health and psychological functions. The complex and diverse microbial ecosystem residing within humans contributes critically to these intergenerational impacts. Perinatal factors, including maternal nutrition, antibiotic use and maternal stress, alter the maternal gut microbiota during pregnancy, which can be transmitted to the offspring. In addition, gestational age at birth and mode of delivery are indicated frequently to modulate the acquisition and development of gut microbiota in early life. The early-life gut microbiota engages in a range of host biological processes, particularly immunity, cognitive neurodevelopment and metabolism. The perturbed early-life gut microbiota increases the risk for disease in early and later life, highlighting the importance of understanding relationships of perinatal factors with early-life microbial composition and functions. In this review, we present an overview of the crucial perinatal factors and summarise updated knowledge of early-life microbiota, as well as how the perinatal factors shape gut microbiota in short and long terms. We further discuss the clinical consequences of perturbations of early-life gut microbiota and potential therapeutic interventions with probiotics/live biotherapeutics.
Keywords: prenatal and postnatal factors, gut microbiome, transmission, early life, infant, diseases
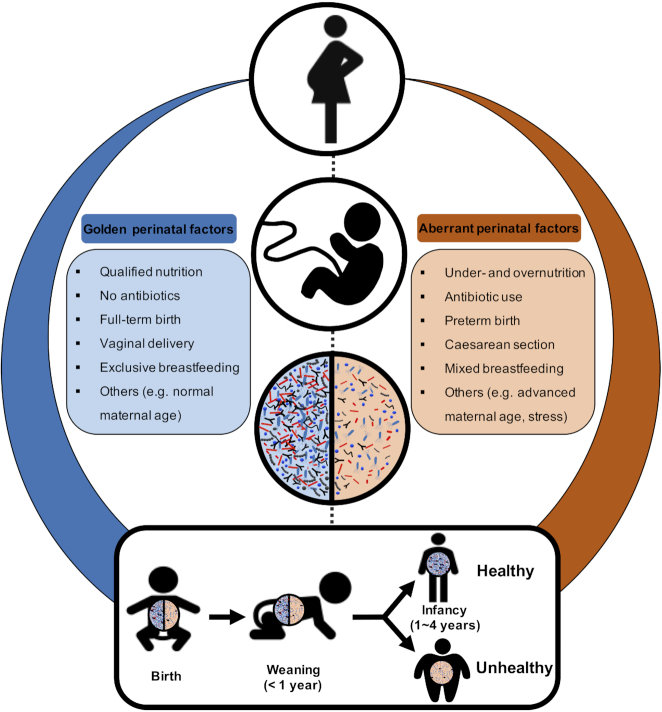
Perinatal factors impact the health of offspring through modulating the gut microbiota in mothers and infants, which leaves a lasting impression beyond infancy until childhood and adulthood.
INTRODUCTION
The first years of life are characterised by rapid growth and development of the infant, including physical growth, development of the immune system, and motor, cognitive and behavioural skills. In parallel, the acquisition, selection and colonisation of microbiota residing in the gastrointestinal tract as well as interactions with the host take place until homeostasis is achieved. Increasing evidence points to a mutualistic relationship between early-life gut microbiota and host health in the short and long terms. Although the mechanism and/or causality remain unclear, the accepted dogmatic belief indicates that the disrupted microbiota in early or later life can leave a lasting and potential footprint on health, with a subsequent risk of disease through an altered immune system (Gensollen et al. 2016). In comparison to adults, the assembly of the microbiota in early life is susceptible to influences from various factors (Tamburini et al. 2016), and a longer length of recovery time from the exposed factors is required (Yassour et al. 2016).
During pregnancy, the mother supports foetal development by transferring molecular nutrients and microbial molecules (e.g. short-chain fatty acids and bile acids) to the foetus through the umbilical cord (Macpherson, de Agüero and Ganal-Vonarburg 2017; Ganal-Vonarburg, Hornef and Macpherson 2020). It has been realised that any abnormal changes related to the mother during pregnancy and lactation are linked to the growth and health of the foetus and infant after birth. An important underlying contributor is the gut microbiota of both mother and infant. Numerous factors (collectively named ‘perinatal factors’) have been documented to influence the maternal and infant gut microbiota during pregnancy and lactation, mainly including maternal diet, antibiotic use, mode of delivery, breastfeeding and maternal stress (Table 1). These factors can shape the initial microbiota in infants by altering the composition and diversity of maternal microbiota per se that is transmitted to the infant (e.g. maternal gestational diet), or disrupting the vertical mother-to-infant microbial transmission (e.g. mode of delivery), thus predisposing the infants to colonisation by aberrant microbiota, such as opportunistic pathogens (Shao et al. 2019). Importantly, these perturbations in the early-life microbiota can persist beyond infancy until childhood and adulthood.
Table 1.
Summary of selected studies linking perinatal factors dictating early microbiota development and longer term maturation in humans.
| Perinatal factors | Cohort characteristics | Time/age at evaluation | Outcomes | Study |
|---|---|---|---|---|
| Maternal nutrition | 81 US mother–infant dyads | Mother: third trimester; infants: 1–2 days and 4–6 weeks | Association of maternal high-fat diet with the gut microbiota of infants at birth and 4–6 weeks of age | Chu et al. (2016a) |
| 145 US mother–infant dyads | Mother: 24–28 gestational weeks; infants: 6 weeks | Association of maternal diet with the gut microbiota of infants at 6 weeks of age dependent on mode of delivery | Lundgren et al. (2018) | |
| Antibiotic use | 40 Spanish mother–infant dyads [22 no antibiotics, 18 IAP (intrapartum antibiotic prophylaxis)] | Infants: 2, 10, 30 and 90 days | An altered establishment pattern of gut microbiota in IAP infants within the first weeks of life, and a delay in the increase of faecal acetate level in IAP infants | Nogacka et al. (2017) |
| 20 Italian mother–infant dyads (10 no antibiotics, 10 IAP) | Infants: 6–7 days | Infants from IAP group with decreased richness and diversity of gut microbiota, with a lower abundance of Actinobacteria and Bacteroidetes and higher Proteobacteria and Enterobacteriaceae family | Aloisio et al. (2016) | |
| 63 Finnish mother–infant dyads (32 no antibiotics, 31 IAP) | Infants: 1 day; 6 months | Influences of IAP on gut microbiota in infants lasting until 6 months after birth | Tapiainen et al. (2019) | |
| 16 Finnish mother–infant dyads (8 no antibiotics, 8 IAP) | Infants: 1 and 6 months | IAP affecting the antibiotic resistance genes and mobile genetic element composition until 6 months of life | Pärnänen et al. (2018) | |
| 36 Australian mother–infant dyads (13 no antibiotics, 23 intrapartum antibiotic use) | Infants: 3 days | The infant oral microbiota mainly from maternal oral microbiota; and antibiotic treatment at delivery shaping the initial oral microbiome in neonates | Gomez-Arango et al. (2017) | |
| Preterm birth | 84 US preterm infants | Infants: 6–158 days | Preterm infants harbouring 10% bacterial species of full-term infants; Enterococcus faecalis, Enterobacter cloacae, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae and Klebsiella oxytoca present in 99.8% samples with high abundance | Gibson et al. (2016) |
| 58 US preterm infants | Infants: 1 day to 11 weeks | Preterm infants mainly harbouring Bacilli, Gammaproteobacteria and Clostridia, and each of the three classes representing the preponderance at different period of life, with increasing proportions of Clostridia at the cost of Bacilli | La Rosa et al. (2014) | |
| 39 Irish preterm infants | Infants: 1–36 weeks | Gut microbiota in gut and metabolite in urine of infants changed in a gestational age-dependent manner | Hill et al. (2017) | |
| 23 Irish preterm infants | Infants: 1, 2 and 4 years | Impact of gestational age at birth on gut microbiota up to 4 years of age | Fouhy et al. (2019) | |
| 45 US preterm infants | Infants: 1–2 days | Preterm-associated bacteria expressing a series of proinflammatory cytokines into vaginal fluid, which was proposed to induce the preterm birth | Fettweis et al. (2019) | |
| Caesarean section (C-section) | 10 Venezuelan infants (4 vaginally, 6 C-section) | Infants: 1 day | C-section infants harbouring microbiota similar to those on maternal skin surface; vaginally born infants resembling their own mother's vaginal microbiota | Dominguez-Bello et al. (2010) |
| 75 US infants (53 vaginally, 22 C-section) | Infants: 1 day; 4–6 weeks | Mode of delivery clustering microbiota of the oral cavity, nares and skin but not the meconium at birth; no influence at 6 weeks for any body site | Chu et al. (2017) | |
| 43 US infants (24 vaginally, 19 C-section) | Infants: 1 day; 1–24 months | C-section-born infants having greater diversity, richness and evenness of gut microbiota in meconium, but declining during the first month and displaying lower diversity and richness up to 2 years of age compared with vaginally born infants | Bokulich et al. (2016) | |
| 596 UK infants (314 vaginally, 282 C-section) | Infants: 4, 7 and 21 days; 4–12 months | C-section as the main factor shaping the gut microbiota in early life increasing the opportunistic pathogen colonisation | Shao et al. (2019) | |
| 16 Luxembourg infants (7 vaginally, 9 C-section) | Infants: 1, 3 and 5 days | C-section disrupting the mother-to-infant microbial transmission and changing the associated functions | Wampach et al. (2018) | |
| Breastfeeding | 98 Swedish infants | Infants: <1, 4 and 12 months | Exclusively breastfeeding delaying the maturation of microbiota in infants | Bäckhed et al. (2015) |
| 903 infants (Germany, Sweden, Finland) | Infants: 3–46 months | Breastfeeding dominating the microbial development as measured from 3 to 14 months of life | Stewart et al. (2018) | |
| Maternal stress | 56 Dutch mother–infant dyads (28 low and 28 high prenatal stress of mothers) | Infants: <110 days | Infants of mothers with high cumulative stress during pregnancy harbouring a higher microbial diversity with enriched Proteobacteria and less lactic acid bacteria and Actinobacteria; the altered microbial colonisation pattern increasing the risk of gastrointestinal symptoms and allergic reactions in infants | Zijlmans et al. (2015) |
Here, we review the existing findings regarding the influence of perinatal factors on the health of infants to provide a comprehensive overview, which thus far is still lacking. Then, we describe the updated knowledge of the development and maturation of the gut microbiota in early life, including bacteria, viruses and fungi, and pay attention to impacts of perinatal factors on the gut microbiota in early life in short and long terms. Finally, we depict associations between early-life gut microbiota and disease, and discuss the potential therapeutic interventions with probiotics.
PERINATAL FACTORS DURING PREGNANCY, BIRTH AND POSTPARTUM
During pregnancy, simultaneous changes in hormone secretion, immunity and metabolism in the female take place until delivery and beyond, the process of which interplays with placental development and foetus growth. Perinatal factors mainly include maternal nutrition, antibiotic use, maternal stress and maternal age during pregnancy, as well as gestational age, mode of delivery and breastfeeding, which can interfere with the course of pregnancy and the health outcomes of offspring, in addition to the gut microbiota in early life of the offspring (as discussed below), likely leaving a lasting impression on the risk of disease in later life.
Maternal nutrition
The macro- and micronutrients during pregnancy provide the essential nutrition for growth of the foetus. Under- and overnutrition as well as diet composition and quality can influence the in utero nutrient levels that are associated with the placental and foetal immune development, thereby leading to adverse pregnancy outcomes. For example, in the human, the profile and intake (e.g. proteins, starch and fatty acids) of maternal macronutrients during pregnancy have been shown to be related to foetal body composition and fat distribution (Blumfield et al. 2012). Maternal dietary pattern during pregnancy dominated by intake of poultry, nuts, cheese, fruits, whole grains, added sugars and solid fats was associated with greater gestational weight gain but not newborn fat mass or adiposity. The other dietary pattern with an intake of eggs, starchy vegetables and non-whole grains led to a higher maternal fasting glucose and greater newborn adiposity (Starling et al. 2017). In addition, the influence of maternal diet during pregnancy on neonatal health could leave a lasting impression on the body composition of offspring (Chen et al. 2016; Crume et al. 2016). For example, a higher maternal energy intake during pregnancy mainly from carbohydrates and fat led to an increase in neonatal fat mass and risk of adiposity within 3 days after birth (Crume et al. 2016). Insufficient micronutrients during pregnancy, for example vitamin D, could reduce the whole-body and lumbar-spine bone-mineral content in children 9 years old (Javaid et al. 2006) and might increase the risk of wheeze symptoms in children 12 years old (Devereux et al. 2007).
Antibiotic use
Although prescription of a course of antibiotics to a pregnant woman is a contentious issue, the average number of medications (e.g. antibiotics) that pregnant women took increased from 2.5 in 1976 to 4.2 in 2008 at any period of pregnancy, and >90% of pregnant women took at least one medication in 2008 (Mitchell et al. 2011). Although the benefits of antibiotics during and after pregnancy cannot be denied, one concern about antibiotic intervention in pregnant and lactating women is the substances that can be transferred to the foetus via the placenta and umbilical vein by simple diffusion and blood flow. The concentration of antibiotics (e.g. ampicillin, cephalothin and clindamycin) in the umbilical blood reaches a peak within 1 h after maternal serum peak during pregnancy (Chow and Jewesson 1985). Due to the limited activity of foetal hepatic drug-metabolising enzymes compared with adults, the non-metabolised drug thus accumulates in the foetal tissues (Morgan 1997). During lactation, the transfer of maternal antibiotics to the newborn still occurs via breastfeeding as the maternal antibiotics can circulate into breast milk in multiple ways, including simple diffusion, active transport and pinocytosis, depending on the type of antibiotics (e.g. ampicillin, penicillin and cephalosporins) and circulation conditions (Reali et al. 2005).
Furthermore, exposure to antibiotics during pregnancy for either mother or foetus or newborn has been associated with an increased risk of multiple diseases, which may be attributed to increased expression of antibiotic resistance genes and altered gut microbiota (Mueller et al. 2015; Yassour et al. 2016; Neuman et al. 2018). For example, at 7 years of age, children whose mothers were exposed to antibiotics during the second or third trimester exhibited 84% higher risk of obesity, compared with children of non-exposed mothers (Mueller et al. 2015). Other studies suggest that antibiotic exposure during pregnancy may increase the risk of inflammatory bowel disease among 827 239 Swedish children (Örtqvist et al. 2019) and wheeze/asthma in childhood, as suggested by a cohort of 411 Danish children (Stensballe et al. 2013) and a meta-analysis (Zhao et al. 2015).
Gestational age
The global incidence of preterm birth (gestational age <37 weeks) is estimated to be up to 15 million each year, which is still rising and accounts for 5–18% of all births across 184 countries, with significant disparities in the frequency of preterm birth among countries [Purisch and Gyamfi-Bannerman 2017; World Health Organization (WHO) 2019]. Two-thirds of preterm births take place after the spontaneous onset of labour (Romero, Dey and Fisher 2014), which is responsible for over 50% of perinatal mortalities and morbidity below the age of 5 years (Goldenberg et al. 2008). However, the underlying mechanism for spontaneous preterm birth remains enigmatic. Of note, numerous causes, including infection or inflammation, environmental exposure, immune status, maternal and foetal genome, uteroplacental ischaemia or haemorrhage, maternal anxiety and stress, a decline in progesterone action and changes in microbiota, have all been considered as potential causes with an induction of spontaneous preterm delivery (Goldenberg et al. 2008; Romero, Dey and Fisher 2014). Among these determinants, infection and inflammatory responses seem to be the significant risk factors for preterm birth as elevated production of proinflammatory cytokines is associated with uterine activation and preterm birth (Cappelletti et al. 2016). Mother's genetics may be a grounded factor that influences the gestation period and the risk of preterm birth (Bezold et al. 2013). A genome-wide association study involving a total of 8 643 women found 6 genomic loci that were associated with gestational duration and 3 of these loci were involved with preterm birth. The functions of these genes were involved in uterine development, maternal nutrition and vascular control (Zhang et al. 2017). The advances of next-generation sequencing and metagenomic analysis have uncovered the potential association between preterm birth and specific microbiota in pregnancy (as discussed below) (Vinturache et al. 2016; Fettweis et al. 2019).
Mode of delivery
Over the years, the occurrence of Caesarean section (C-section) delivery has been unprecedentedly and steadily increasing to 18.6% of all births in 150 countries (Betrán et al. 2016) although the threshold of 10–15% was recommended by the WHO in 1985 (WHO 1985). Regionally, the highest proportion of 40.5% was in Latin America and the Caribbean region, followed by 32.3% in North America, 31.1% in Oceania, 25% in Europe, 19.2% in Asia and 7.3% in Africa (Betrán et al. 2016). Many efforts, including clinical and non-clinical interventions, have been made in order to reduce unnecessary C-section births, as there is an increasing body of evidence showing the association of short- and long-term risks from C-section with the health of the woman, baby and subsequent pregnancies. Mothers who gave birth via C-section had a decreased risk of urinary incontinence and pelvic organ prolapse, but an increased risk of placenta previa, placenta accrete and placental abruption, as well as miscarriage and stillbirth for subsequent pregnancy (Keag, Norman and Stock 2018). Children delivered by C-section had increased risk of asthma up to the age of 12 years and obesity up to 5 years of age (Keag, Norman and Stock 2018), which was consistent with other studies (Li, Zhou and Liu 2013; Kuhle, Tong and Woolcott 2015; Peters et al. 2018). Increased risks of subsequent subfertility, pelvic adhesion and small bowel obstruction have also been observed in mothers who gave birth via C-section (Gurol-Urganci et al. 2013; Abenhaim et al. 2018; Sandall et al. 2018). In addition, C-section could increase risk of overweight and obesity in childhood compared with infants delivered vaginally (Li, Zhou and Liu 2013).
Breastfeeding
Breast milk contains all the essential macro- [e.g. caseins, whey, fatty acids, human milk oligosaccharides (HMOs) and lactose] and micronutrients (e.g. Immunoglobulin A, Immunoglobulin G, Immunoglobulin M, calcium and vitamin A) (Andreas, Kampmann and Le-Doare 2015; Ahern et al. 2019). Based on the nutritional composition, breast milk is commonly classified into colostrum (first milk after birth), transitional milk and mature milk, which also differ in microbiota composition (Gomez-Gallego et al. 2016). Colostrum contains high concentrations of whey protein and low concentrations of both lactose and fat compared with mature milk. The nutritional composition of breast milk is dynamic (Andreas, Kampmann and Le-Doare 2015). In general, the content of protein in breast milk gradually decreases with a gradual increase in the concentration of lipid, and lactose production is highest in the fourth to seventh month (Andreas, Kampmann and Le-Doare 2015). The HMOs are an important part of the carbohydrate fraction of human milk, mainly consisting of 2′-fucosyllactose (20–30% of all HMOs) (Ahern et al. 2019).
During lactation, exclusive breastfeeding can meet all of the nutritional requirements for the infant's growth. In addition, the bioactive molecules (e.g. HMOs) and microbiota in breast milk help to guide the development and maturation of the infant immune system, promote the colonisation of beneficial microbiota and protect from invasive pathogenic bacteria (Gomez-Gallego et al. 2016). In comparison with the infant fed with formula, breastfed infants have lower incidences of various infectious morbidity and diseases, such as necrotising enterocolitis (NEC), respiratory tract infection and decreased risk of childhood obesity and diabetes (Salone, Vann and Dee Stuebe 2009; 2013).
Other perinatal factors
Chronic psychological distress of mothers (e.g. depression, anxiety and perceived stress) during pregnancy may increase the risk of adverse birth outcomes, including preterm birth (Coussons-Read et al. 2012), low birth weight (Lewis, Austin and Galbally 2016) and adverse child neurodevelopment (Sawyer et al. 2019). The intergenerational transmission of depression is mainly influenced by genetic inheritance, maternal antidepressant use during pregnancy and childhood maltreatment (Sawyer et al. 2019), as well as gut microbiota (Wang et al. 2020). In addition, maternal smoking during pregnancy is a potential cause of a range of behavioural problems and disorders in the offspring as the foetus is particularly vulnerable to numerous tobacco components compared with adults (Talati et al. 2016).
Due to changes in socioeconomic circumstances and lifestyle in recent years, the proportion of women giving birth after 35 years of age, considered advanced maternal age, has been rising considerably. Both mothers of early age (typically <17 years) at first childbirth and those of advanced age have been realised to be the most vulnerable to infant mortality and poor health outcomes for children and mothers, including high risk of foetal growth restriction, placental abruption, preterm birth, low birth weight, stillbirth, C-section, neonatal intensive care unit (NICU) admission and postnatal morbidity and mortality in children, as well as gestational hypertension and diabetes mellitus for advanced age mothers-to-be (Finlay, Özaltin and Canning 2011; Gibbs et al. 2012; Lean et al. 2017; Londero et al. 2019).
FEATURES OF THE INFANT GUT MICROBIOTA
Composition and development of the bacterial population in the infant gut
The infant gut microbiota contains members from the three domains of life, Archaea, Bacteria and Eukarya, as well as viruses. The majority of studies of the infant gut microbiota have utilised 16S ribosomal ribonucleic acid (rRNA) amplicon sequencing to profile the bacterial composition, which are limited by factors such as primer design and sample processing, leading to the underrepresentation of some of the key members of this population (Claesson et al. 2010; Hill et al. 2016). More recent studies have replaced 16S with whole-genome shotgun sequencing, which is more cost prohibitive but allows for higher taxonomic and functional analyses.
It has been long believed that bacterial colonisation of the infant gut begins at birth but recent studies have questioned if it actually begins in utero (as discussed below). Although the infant gut microbiota tends to have lower diversity and as much as six times fewer operational taxonomic units (OTUs) than adults, it is also more dynamic with rapid development over the first 6 months of life (Avershina et al. 2016; Yassour et al. 2016; Hill et al. 2017). The microbiota in infants is mostly represented by four phyla, namely Actinobacteria (genus Bifidobacterium), Proteobacteria (genus Enterobacteriaceae—unclassified), Firmicutes (e.g. genera of Streptococcus and Enterococcus) and Bacteroidetes (genus Bacteroides), which normally, with the exception of the Firmicutes, are represented by no more than one genus as indicated in the bracket (Dogra et al. 2015).
Immediately after birth, facultative and aerotolerant microorganisms such as Proteobacteria (genus Enterobacteriaceae—unclassified) and Firmicutes (genus Streptococcus) dominate, resulting in the depletion of oxygen in the gut and subsequent colonisation by strict anaerobes (Del Chierico et al. 2015; Dogra et al. 2015). However, studies vary on the exact timing of when such strict anaerobes become dominant. In a recent study, Shao et al. (2019) found that by the first 7 days, the microbiota of vaginally born infants was dominated by strict and facultative anaerobes, namely the genera Bifidobacterium, Escherichia, Bacteroides and Parabacteroides. Likewise, Hill et al. (2017) found that the Bifidobacterium genus was dominant 1 week after birth in vaginally born infants, while Jost et al. (2012) found that bifidobacteria were significantly more abundant between days 4 and 6. However, other studies have found that facultative aerobes such as Enterobacteriaceae could be dominant for up to 1 or 3 months before being supplanted by Bifidobacterium and Bacteroides (Dogra et al. 2015; Bokulich et al. 2016; Yassour et al. 2016). However, the majority of studies are in agreement that the Actinobacterium phylum (genus Bifidobacterium) becomes dominant between 3 and 6 months of age (Yatsunenko et al. 2012; Jakobsson et al. 2014; Dogra et al. 2015). The early bifidobacterial population at 1 month mainly consists of Bifidobacterium longumsubsp.longum, Bifidobacterium breve, Bifidobacterium bifidum, B. longumsubsp.infantis, Bifidobacterium adolescentis and Bifidobacterium pseudocatenulatum species (Duranti et al. 2017).
A key point in the development of the infant gut microbiota is weaning and the introduction of solid foods. The introduction of solid foods between 6 and 24 months led to a decrease in Bifidobacterium and Clostridium, and an increase in Bacteroides, Faecalibacterium and Clostridiales revealed by the longitudinal study of 43 US infants from birth to 2 years (Bokulich et al. 2016) and 39 Finnish infants from 2 months to 3 years (Yassour et al. 2016). On the contrary, a Danish longitudinal study found a significant increase of species within the Bacteroidetes (genus Bacteroides) phylum in line with weaning (Bergström et al. 2014). This study also noted that while the relative abundance of the Bifidobacterium genus decreased with weaning, certain species namely B. adolescentis and Bifidobacterium catenulatum actually increased in relative abundance (Bergström et al. 2014). This is likely due to the decrease of HMO-utilising bifidobacteria (e.g. B. bifidum) and an increase in species that are adapted to the utilisation of plant-derived fibres (Egan and van Sinderen 2018). The cessation of breastfeeding leads to an increase in butyrate-producing bacteria, such as Clostridium leptum, Clostridium coccoides and Lachnospiraceae, possibly associated with energy harvest to new food sources (Bergström et al. 2014).
By 2 to 3 years of age, the infant microbiota almost fully resembles the adult-like microbiota (Yatsunenko et al. 2012). Alpha diversity increases with age, in particular between 1, 2 and 4 years (Fouhy et al. 2019). At 2 years of age, the number of OTUs is almost two-thirds that of adults (Avershina et al. 2016), while the composition is also altered, with higher numbers of class Clostridia and Bacteroides and a decrease in the relative abundance of bifidobacteria (Bergström et al. 2014; Avershina et al. 2016). Interestingly, it was noted that the 10 most prevalent OTUs in the infant gut up to 1 year could not be identified in the mother, but at 2 years the most prevalent OTUs were found at the same level in the mother's microbiota (Avershina et al. 2016). In another study, two notable bacteria in the adult gut, namely Akkermansia muciniphila and Faecalibacterium prausnitzii, were detectable at 2 year and 1 year, respectively, having been absent in the first 2 months of life (Yassour et al. 2016). However, it has to be mentioned that the absence of microbes may be attributed to the limitation of current approaches, even with shotgun metagenomic sequencing. By 4 years of age, the bacterial profile is dominated by Ruminococcaceae, Dialister, Faecalibacterium, Bacteroides and Christensenellaceae (Fouhy et al. 2019).
The concept of enterotypes in the adult gut microbiota was introduced in 2011, when meta-analysis of faecal metagenome studies revealed that individuals could be grouped into three clusters or ‘enterotypes’ based on the relative abundances of Bacteroides, Prevotella and Ruminococcus in their microbiota (Arumugam et al. 2011). Bergström et al. (2014) studied the Bacteroides/Prevotella ratio in infants aged between 9 and 36 months and found that such enterotypes were established during this time. Interestingly, other correlations between certain families and genera have also been observed. Notably, there have been incidences of an inverse correlation between the levels of Bacteroides and Bifidobacterium (Jost et al. 2012; Yassour et al. 2016), perhaps due to competition for HMOs. Likewise, B. breve has been associated with delayed colonisation of certain Clostridia OTUs (Avershina et al. 2016). Bacteroides have also been negatively correlated with Clostridium (Nakayama et al. 2011). At a species level, B. longum was shown to co-exist with Lactobacillus and Enterococcus at 9 months, but this had ceased by 18 and 36 months (Bergström et al. 2014).
Sources of gut bacterial population in early life
An important source of gut bacteria for the infant is the mother, in a concept known as vertical transmission (Wang et al. 2020). The microbiota can be vertically transmitted from the maternal faecal microbiota, the vaginal microbiota and breast milk. In the faecal samples of vaginally born infants, it was found that over half the bacterial species present in the infant gut on one day of life originated from the mother, with the majority from the mother's gut but also from the vagina, oral cavity and skin in the 25 Italian infants cohort (Ferretti et al. 2018), and the mother–infant shared species proportion reached up to 72% in the 98 Swedish infants cohort in the first few days of life (Bäckhed et al. 2015).
Species from Bacteroides, Parabacteroides, Escherichia and Bifidobacterium were also shown to be vertically transmitted from the maternal faecal microbiota to the infant. Jost et al. (2012) have shown that strains of Bacteroides fragilis and Bacteroides stercoris can be isolated from maternal faecal samples and the corresponding infant stool. Yassour et al. (2018) made a particularly interesting observation in that strain diversity within faecal species was higher in the mother than the infant. However, when it came to vertical transmission, it was not always the dominant strain (identified as typically at least 70% relative abundance of that species) that was transferred to the infant. In the case of Bacteroides dorei, the secondary strain was more likely to be found in the infant faecal sample. The authors also noted that strains transmitted from the mother were more likely to persist in the infant than strains of the same species from elsewhere.
Bifidobacteria are also commonly vertically transferred from mother to infant, perhaps unsurprisingly given their dominance in the early infant gut. The bifidobacterial species transmitted from mothers’ stool or milk to infants in the first month were identified as B. bifidum, B. adolescentis, Bifidobacterium dentium, B. breve, B. longum spp. and B. pseudocatenulatum (Duranti et al. 2017). Likewise, in a separate study, strains of B. breve and B. bifidum were found in maternal and infant faecal samples, 10 days after birth (Avershina et al. 2016).
The vertical transmission of bacteria from breast milk to infants occurs during lactation. In the first month of life, infants who were mainly breastfed received 27.7% of their gut bacterial operational taxonomic units (OTUs) from breast milk (Pannaraj et al. 2017), and identical strains of B. breve and Lactobacillus plantarum were identified in both breast milk and the corresponding infant stool using a culture-dependent method (Murphy et al. 2017). Identical strains of B. bifidum, Coprococcus comes and Ruminococcus bromii were identified in breast milk and the corresponding infant stool in an Italian cohort with shotgun metagenomic sequencing (Asnicar et al. 2017). Aside from vertical transmission, breast milk can also promote the development of the infant microbiota via HMOs. A number of commensal members of the gut microbiota have been shown to utilise HMOs, including bifidobacteria and Bacteroides (Marcobal et al. 2011; Egan et al. 2014; Duranti et al. 2017). The genomes of bifidobacteria (in particular B. longum subsp. infantis) contain specific genes that are responsible for encoding enzymes that can metabolise HMOs (e.g. fucosidase, β-hexosaminidase, sialidase and β-galactosidase) and elements for internalisation of HMOs (e.g. permeases for ABC transport system) (Sela et al. 2008; Turroni et al. 2018). The utilisation of HMOs by Bacteroides (e.g. B. fragilis and Bacteroides thetaiotaomicron) involves the upregulation of mucin glycan degradation pathways (Marcobal et al. 2011).
In a separate study, the presence of Lactobacillus and Prevotella in the newborn's gut was ascribed to these being the predominant genera in the vaginal microbiota. (Dominguez-Bello et al. 2010). Similarly, Lactobacillus and bifidobacterial OTUs were also found to be shared between the vaginal microbiota and infant faecal samples (Yassour et al. 2016).
The hypothesis of in utero colonisation and a placental microbiota is relatively recent but also controversial. Bacteria have been found in the placenta, amniotic fluid and the umbilical cord (Wang et al. 2013; Aagaard et al. 2014; Collado et al. 2016). One study identified several genera that were present in the placenta and amniotic fluid as well as in the meconium (the first stool from newborn after birth) of the infant, indicating vertical transmission between placenta and infant (Collado et al. 2016). However, the validity of such research has been questioned, due to the low biomass present and potential contaminations of the sequenced samples (Lauder et al. 2016).
Maternal administration of probiotics is another source of beneficial bacteria in the infant gut. In a small cohort study with six mother–infant dyads, all four infants delivered vaginally and in one of two C-section-born infants, Lactobacillus rhamnosus GG was present in infant faecal samples at 1 and 6 months of age when mothers were taking L. rhamnosus GG during late pregnancy (Schultz et al. 2004). With a larger cohort, L. rhamnosus GG was found in maternal (74 of 116 subjects at 3 months after delivery) and infant (50 of 129 subjects at 10 days; 56 of 122 at 3 months) stool samples following intake by the mother from 36 weeks of gestation up to 3 months postnatally (Dotterud et al. 2015).
The infant gut virome
The infant gut virome, which includes eukaryotic and prokaryotic viruses (bacteriophage), has not been as intensively studied as the bacterial portion of the infant gut microbiota. One of the main difficulties in analysis of the infant gut virome is that a large number of sequences cannot be assigned to a taxonomic group (Reyes et al. 2015). The diversity of the infant gut virome is lower than that of adults (Breitbart et al. 2008; Pannaraj et al. 2018), but recent studies have shown that similar to bacteria, the infant gut virome is dynamic and changes throughout infancy, including an increase in alpha diversity (Reyes et al. 2015). In an early study of the infant gut virome 1 week after birth, it was found that more than half the viral genotypes present in infant stool could no longer be detected just 1 week later (Breitbart et al. 2008). In terms of alpha diversity, bacteriophage decrease from birth to 24 months, but eukaryotic species richness increases (Lim et al. 2015). In the first months after birth, Caudovirales are predominant, followed by a shift to Microviridae at 24 months, a similar time to the shift of the infant microbiota towards that of an adult. Eukaryotic viruses are low in abundance in early life (1 month) but increase thereafter, with Anelloviruses being dominant (Lim et al. 2015). In a second study of 20 twin pairs, the Anelloviridae, which targets eukaryotic hosts, were highly abundant until 15 to 18 months, after which it decreased (Reyes et al. 2015). In terms of bacteriophage, their abundance and diversity are inversely proportional to that of the bacterial population in the infant gut. In twins, the microbiota shifted from high bacteriophage-low bacterial diversity in the first month to high bacterial diversity-low bacteriophage by 2 years of age. The authors suggested that the low bacterial diversity in the first month of life leads to a contraction of the bacteriophage population, which subsequently allows the bacterial population to flourish (Lim et al. 2015). Pannaraj et al. (2018) found shared viruses between breast milk and corresponding infant stool in the first week of life, suggestive of vertical transmission.
The Siphoviridae family of the Caudovirales order includes bacteriophages that target bifidobacteria (Lugli et al. 2016). When looking at bifidobacterial prophage, ‘bifido(pro)phages’, it was found that bifidobacterial species such as B. longum that were high in relative abundance at a certain sampling point were then decreased at the next time point, which corresponded to an increase in the relative abundance in the corresponding bifido(pro)phage. Metatransriptomic analysis confirmed that the prophage were actively replicating and killing their hosts (Lugli et al. 2016). Duranti et al. (2017) later proved that such bifido(pro)phages could be vertically transmitted from mother to infant. A B. longum phage was identified in a mother's breast milk and the corresponding infant stool sample at two separate time points. A second B. longum phage was identified in a mother's faecal sample and in the corresponding faecal sample of the infant (Duranti et al. 2017).
The Bacteroides genus is also targeted by members of the gut virome. The Alpavirinae subfamily of the Microviridae is associated with the Bacteroides genus (Krupovic and Forterre 2011), while crAssphage, a Podoviridae bacteriophage first identified in 2014 has also been identified in the infant gut virome and predates Bacteroides (Dutilh et al. 2014; Lim et al. 2015; McCann et al. 2018).
The infant gut mycobiome
The gut mycobiota encompasses all the fungi present in the gastrointestinal tract. Similar to the virome, research into the infant gut mycobiota has gained traction in recent years. The fungal population in the infant gut tends to have a low biomass, which makes sequencing technologies such as shotgun sequencing difficult. Instead, amplicon-based techniques using the 18S or 23S rRNA gene sequence or internal transcribed spacer regions or culture-dependent approaches are utilised (Ward, Knights and Gale 2017).
In a study of faecal samples of 11 infants whose age range was not defined, Candida albicans was the dominant fungal species, followed by Candida parapsilosis, Candida krusei and Leptosphaerulina (Heisel et al. 2015). In a separate study of 111 participants that included 8 infants under 2 years of age, infants and children were found to have a higher fungal richness compared with adults. Penicillium, Aspergillus, Candida, Debaryomyces, Malassezia, Ascomycota, Eurotiomycetes, Tremellomycetes, Nectriaceae and Trichosporon were the dominant genera, with Penicillium being significantly more abundant in infants compared with adults (Strati et al. 2016). In contrast, in a study of 70 healthy control infants at 3 months of age, Saccharomycetales, Penecillium and Aspergillus were the dominant genera (Arrieta et al. 2018).
Candida spp., which are highly abundant in the maternal vaginal and skin microbiota, have also been shown to be vertically transmitted between maternal and corresponding infant faecal samples (Bliss et al. 2008).
The sporobiota in the infant gut
An endospore is a tough, dormant structure formed by certain types of bacteria, typically members of the phylum Firmicutes (Clostridiaceae and Lachnospiraceae families), which allows for greater resistance to otherwise inhospitable conditions such as extremes in temperature, oxygen, antibiotic exposure, UV radiation and nutrient and water deprivation. Such characteristics have led to challenges in health and disease, as the ability to form spores is linked to pathology, including persistent, chronic infection, resistance to antibiotics, relapses and spread of antimicrobial-resistance genes. Due to the significance of spore formers to human health and disease, it has been suggested that they should be considered as a separate grouping in microbiota studies. The term sporobiota has been suggested to cover the entirety of spore-forming bacteria in a population, while the term sporobiome has been used to define a collection of genomes of spore-forming bacteria related to a particular niche (Tetz and Tetz 2017).
The abundance of spore formers in the human gut microbiota is considered to be underrepresented in many metagenomic studies, for reasons including the resistance of endospores to traditional DNA isolation techniques (Filippidou et al. 2015), the high similarity between the 16S rRNA and housekeeping genes of otherwise unrelated spore formers (Wang et al. 2007) and the fact that spore formers tend to have larger genomes, resulting in fewer reads per gene per taxon (Galperin et al. 2012; Tetz and Tetz 2017). However, a study of the culturable bacteria of the adult gut microbiota found that 60% of the commensal bacteria in the gut are spore formers and 30% of the overall gut microbiota. Spore formers were found in several gut-associated families, including Lachnospiraceae, Ruminococcaceae and Clostridiaceae (Browne et al. 2016).
Spores are specialised for host–host transmission, which makes them ideal for colonisation of the developing infant gut microbiota (Tetz and Tetz 2017). The ability to form spores allows certain bacteria to colonise the infant gut early in low numbers and wait until conditions become more favourable, at which point they enter into a vegetative state and begin to multiply (Avershina et al. 2016).
Studies have also found that the Clostridium genus is present at low relative abundance (<1%) in breast milk (Jost et al. 2013; Murphy et al. 2017). For this reason, it is perhaps not surprising that studies have found a higher level of spore formers in formula-fed infants. In a study of 98 mother–infant pairs in Sweden, formula-fed infants had elevated levels of Clostridioides difficile in comparison to those who were breast-fed. Even in breastfed infants, the cessation of breastfeeding at 12 months also led to an increase in levels of Clostridium (Bäckhed et al. 2015). Another study of 107 mother–infant pairs in the United States found that the Erysipelotrichaceae family was prominent among non-exclusively breastfed infants (Pannaraj et al. 2017).
INFLUENCES OF PERINATAL FACTORS ON MICROBIOTA DEVELOPMENT AND MATURATION IN INFANTS
Maternal nutrition and gut microbiota
Maternal nutrient intakes during pregnancy and lactation have been linked to the acquisition and development of gut microbiota in the offspring (Chu et al. 2016b) (Fig. 1). In a human cohort with mother–infant dyads, where the mothers had a high-fat diet (>40%) within the third trimester of pregnancy based on a dietary questionnaire. An enrichment of species of Lactococcus, Granulicatella and Enterococcus, and a depletion of Bacteroides, Sutterella, Parabacteroides and Comamonas were observed in the gut microbiota of infants born to a maternal high-fat gestational diet, and this influence persisted to 6 weeks of age (Chu et al. 2016a). In addition, maternal fish and seafood intake during pregnancy increased the abundance of genus Streptococcus and decreased Bacteroides uniformis in infant gut at 6 weeks of age. Maternal dairy intake was positively associated with Clostridium neonatale, Clostridium butyricum and Staphylococcus, but decreased the abundance of Lachnospiraceae family. The abundance of genus Bifidobacterium was negatively associated with maternal fruit intake (Lundgren et al. 2018). The study additionally illustrated that this intergenerational nutritional influence on the gut microbiota of infants was in a delivery mode-dependent manner (Lundgren et al. 2018). In a non-human primate model [Japanese macaque (Macaca fuscata)], a maternal high-fat diet during pregnancy and lactation persistently shaped the gut microbiota in the offspring with reduced abundance of Camplyobacter species, and this impact could persist until adulthood at 1 year of age (Ma et al. 2014).
Figure 1.
Perinatal factors impact the health and development of offspring through modulating the foetal growth, and the gut microbiota in both mothers and infants, which leaves a lasting impression beyond the birth and weaning period until childhood and even adulthood.
The underlying biological mechanisms regarding how maternal gestational diet modifies the offspring gut microbiota are not entirely understood. During pregnancy, it is known that the molecular transfer of nutrients from mother to foetus is of critical importance for early life immune development (Macpherson, de Agüero and Ganal-Vonarburg 2017). Alternation in maternal diets may disrupt this process, and thus interfere with the interactions of the host immune system with early-life microbiota. Considering the microbial mother-to-infant transmission at delivery and/or postpartum (Ferretti et al. 2018; Yassour et al. 2018; Wang et al. 2020), the maternal microbiota altered by diet could be transmitted to the offspring. Thus, further studies recording the information of maternal gestational diet, and sampling maternal and infant microbiota are warranted to address these knowledge gaps.
Maternal antibiotic use and gut microbiota
Antibiotic treatment remains the major therapeutic strategy for many infectious diseases, however, increasing concerns have arisen due to its double-edged sword effect, in particular, the resulting risk of antibiotic resistance in the disease-causing microbes but also in beneficial commensal microbes (Fig. 1). Avoiding antibiotic use during the perinatal period is recommended when it is not mandatory, as increasing studies have identified associations between antibiotic use and the short- and long-term alternations of the microbiota and the health of the offspring.
The typical application of antibiotic administration during labour (referred to as intrapartum antibiotic prophylaxis, IAP) is for group B Streptococcus (GBS) positive women to prevent neonatal GBS infection and subsequent sepsis associated deaths (Al-Taiar et al. 2011). Meanwhile, changes of microbiota in newborns caused by IAP treatment have been disclosed by various approaches including routine microbiological culturing, molecular methods and next-generation sequencing in humans and animals. Using a molecular method of real-time polymerase chain reaction (PCR) for specific groups of bacteria, the absolute abundance of the Bifidobacterium genus in the gut microbiota of full-term and vaginally delivered newborns within 1 week after birth whose mothers were subjected to IAP treatment was decreased compared with newborns of mothers without antibiotics administered (Aloisio et al. 2014). Later, the author applied 16S amplicon sequencing to examine the effects of IAP on the gut microbiota of newborns in full-term and vaginally delivered newborns within 1 week after birth (Aloisio et al. 2016). In addition to confirmation of reduced bifidobacteria in newborns, the richness and diversity of the gut microbiota in newborns from the IAP group were decreased, with a lower abundance of Actinobacteria and Bacteroidetes as well as an overrepresentation of the Proteobacteria phylum and Enterobacteriaceae family (Aloisio et al. 2016). These findings were partially observed in other cohorts (Mazzola et al. 2016; Nogacka et al. 2017; Tapiainen et al. 2019). Within the first 6 months of life, differences in gut microbiota induced by IAP decreased gradually (Mazzola et al. 2016; Nogacka et al. 2017; Tapiainen et al. 2019). Bifidobacteria appeared to recover with a significant increase in the abundance similar to infants born normally but not for the Enterobacteriaceae family (Mazzola et al. 2016). Importantly, effects of postnatal antibiotic treatment to infants were comparable to that of IAP (Tapiainen et al. 2019).
Along with changes to the gut microbiota, enriched antimicrobial resistance genes (ARGs) in infants exposed to perinatal antibiotics have been noticed (Pärnänen et al. 2018; Tapiainen et al. 2019). These increased ARGs might result from, but are not limited to mother-to-infant transfer of maternal ARGs as there was a significant increase in the abundance of ARGs in the infants compared with their paired mothers (Pärnänen et al. 2018; Tapiainen et al. 2019), indicating that infants were at a high risk for the selection of antibiotic resistant strains once exposed to antibiotics, possibly due to the lesser richness or simpler composition of the gut microbiota in comparison to the adults (Pärnänen et al. 2018; Tapiainen et al. 2019).
The precise mechanism underlying the effects of maternal antibiotic intervention on the microbiota of offspring is still unknown. The response to antibiotics varies among species and the type and number of antibiotics administered. For example, the absolute abundance of Lactobacilllus, B. fragilis, C. difficile and Escherichia coli did not show any significant variation except for bifidobacteria in newborns of mothers exposed to IAP (Aloisio et al. 2014). The oral microbiota of offspring whose mothers received a cocktail of antibiotics clustered separately from those of mothers administered only a single antibiotic (Gomez-Arango et al. 2017). In addition, given that vertical transmission of microbes from mothers to infants is one of the critical sources for early-life microbiota (Ferretti et al. 2018; Yassour et al. 2018), perinatal antibiotic intervention in mothers may promote transmission of specific microbial strains to infants. Using a culture-dependent approach, a reduction of vaginal Lactobacillus transmission was observed from IAP-treated mothers to the infants’ oral cavity (Keski-Nisula et al. 2013), the result of which was later confirmed and expanded by 16S amplicon sequencing (Gomez-Arango et al. 2017). A high proportion (65%) of oral microbiota in newborns within 3 days after delivery was shared with maternal microbiota in the oral cavity; and maternal exposure to intrapartum antibiotics reduced the relative abundance of oral bacteria from families Micrococcaceae, Streptococcaceae, Gemellaceae and order Lactobacillales but increased the abundance of families Prevotellaceae, Bradyrhizobiaceae, Sphingomonadaceae, Comamonadaceae, Oxalobacteraceae and Neisseriaceae in the newborn (Gomez-Arango et al. 2017). Furthermore, perinatal antibiotic intervention can disrupt maternal microbiota, which may lead to an altered microbiota transferred to the offspring. This has been observed in a murine model (Miyoshi et al. 2017) but confirmation in humans still remains unexplored.
Gestational age and microbiota
Prematurity strongly influences the initial gut microbiota in the newborn, and the subsequent trajectory of microbiota development and maturation (Fig. 1). Preterm infants are normally subjected to a microbiologically controlled environment (namely NICU) in the first few days of life or even longer depending on the newborn's health status. Exploring the development of the gut microbiota in the preterm infant is emerging as a critical research priority in the field of microbiology and paediatrics, due to the fact that perturbation of the gut microbiota during this key developmental window influences host physiology and disease risk (Groer et al. 2014). Up to now, our appreciation of gut microbiota in preterm infants is still relatively limited compared with full-term infants.
In the first few months, the richness of the gut microbiota colonising preterm infants accounted for only 10% of the bacterial species of the counterpart full-term infants (Gibson et al. 2016). The gut microbial composition in preterm infants was mainly comprised of microbes from classes of Bacilli, Gammaproteobacteria and Clostridia, accounting for >90% of gut microbiota, and each of the three classes represents the preponderance at different periods of life, with increasing proportions of Clostridia at the cost of Bacilli (La Rosa et al. 2014). In a large longitudinal study with 401 stool samples from 84 preterm infants aged from 1 week to 5 months, 6 bacterial species (Enterococcus faecalis, Enterobacter cloacae, Staphylococcus epidermidis, E. coli, Klebsiella pneumoniae and Klebsiella oxytoca) were consistently present in 99.8% samples from all preterm infants with high relative abundance (25–72%) (Gibson et al. 2016). The longitudinal pattern of gut microbiota development mentioned in La Rosa et al. (2014) was revealed to be driven primarily by species of Klebsiella, Escherichia and Enterobacter replacing Enteroccoccus and Staphylococcus as infants aged (Gibson et al. 2016). In addition, preterm infants have lower levels of Bifidobacterium and Bacteroides compared with full-term infants (Stewart et al. 2017; Chernikova et al. 2018). Sampling preterm infants at the early life stage is relatively undemanding, but there is a paucity of reports regarding the maturation of preterm infant gut microbiota in later life. A recent study following participants up to 4 years of age indicated that impact of gestational age at birth on the diversity and composition of gut microbiota in preterm children persisted at 1, 2 and 4 years, i.e. lower diversity, and discriminatory genera (Lactobacillus, Streptococcus and Carnobacterium) determined by linear discriminant analysis effect size for preterm children (Fouhy et al. 2019), indicating a delayed succession of microbial species in preterm infants (Arboleya et al. 2016).
The causes of preterm birth are complex (as discussed above), and a spectrum of vaginal microbiota is a significant contributor, particularly in women of African ancestry (Kindinger et al. 2017; Brown et al. 2018; Fettweis et al. 2019). The vaginal microbiota of women who gave preterm birth was characterised with low abundance of Lactobacillus crispatus and high levels of bacterial vaginosis-associated bacterium 1, Sneathia amnii, TM7-H1 and a group of Prevotella species (Fettweis et al. 2019). The preterm-associated bacteria generally expressed a series of proinflammatory cytokines into vaginal fluid, which were proposed to induce preterm birth (Fettweis et al. 2019). Combining these findings may provide a potential predictor for the risk of preterm birth early in pregnancy. However, causality still needs to be determined.
Developing strategies to prevent the adverse outcomes of preterm birth by intervening in the gut microbiota in early life is desirable. Feeding breast milk from own mothers or donors since the first days of life has been shown to stimulate the recovery and establishment of normal-like microbiota with gradually increased levels of bifidobacteria (Korpela et al. 2018; Parra-Llorca et al. 2018). In addition, supplementation of multispecies probiotics containing both Lactobacillus and Bifidobacterium species has been found to significantly reduce the risk of NEC in preterm infants (Kona and Matlock 2018).
Mode of delivery and gut microbiota
Influences of mode of delivery on the acquisition and colonisation of microbiota across multiple body sites (gut, skin, nares and oral cavity) in infancy ranging from birth up to 4 years have been well documented (Dominguez-Bello et al. 2010; Chu et al. 2017; Fouhy et al. 2019) (Fig. 1). The much more diverse set of microbiota residing within the gut following C-section birth compared with the other body sites is of particular relevance to various disorders including allergies, obesity and inflammatory bowel disease (Tamburini et al. 2016). Thus, the majority of emerging evidence regarding the abnormal microbiota related to mode of delivery (C-section versus vaginal delivery) is from the gastrointestinal tract, and more studies are necessary to address the effect of C-section on the other microbial niches in the human body.
While most studies report the significant influence of C-section on the gut microbiota immediately after birth, there are some inconsistencies between studies. For example, the phylogenetic diversity, richness and evenness of microbiota from C-section newborn in meconium were greater compared with vaginally delivered newborns (Bokulich et al. 2016), but not in Chu et al. (2017), and even the opposite result was observed in a small Chinese cohort (Shi et al. 2018). This perhaps is attributable to exact sampling points, sampling procedure, cohort size, genomic DNA extraction and sequencing approach, and other environmental and population variables. The C-section infants acquired gut microbial species typically on the skin surface, dominated by genera of Staphylococcus, Corynebacterium and Propionibacterium (Dominguez-Bello et al. 2010). This could be due to the different first major microbial exposures for C-section-born compared with vaginally born infants, as passing through the birth canal may be critical for establishing a healthy microbiota early in life (Dominguez-Bello et al. 2010).
As newborns grow to 1 month old, mode of delivery continues to be the dominant factor over breastfeeding and surrounding environment that affect the colonisation of the gut microbiota (Wampach et al. 2018; Shao et al. 2019). In this period, infants delivered by C-section are enriched with species of Enterococcus (E. faecalis, E. faecium), Staphylococcus (S. epidermis, S. saprophyticus, S. lugdunensis, S. aureus), Streptococcus (S. parasanguinis, S. australis), Klebsiella (K. oxytoca, K. pneumoniae), Enterobacter (E. cloacae, E. hormaechei, E. cancerogenus) and Clostridium (C. perfringens), Haemophilus (H. parainfluenzae, H. aegyptius, H. influenzae, H. haemolyticus), Veillonella (V. dispar, V. parvula), which are commonly associated with skin, oral cavity and the hospital environment, and partially are opportunistic pathogens (Bäckhed et al. 2015; Shao et al. 2019).
As infants grow, the abundance of species of Bacteroides that were undetectable in newborns delivered by C-section increased for the first time to enable detection at 6 months of age (Yassour et al. 2016), but was still less than vaginally born infants; conversely, Clostridiales and Enterobacteriaceae were more abundant in C-section-born infants. This difference remained until 1 year of age (Bäckhed et al. 2015; Bokulich et al. 2016). However, although nearly all of C-section-born infants from different cohorts lack members of the Bacteroides genus in their gut microbiota in the first few months of life, this feature still could not be confined solely to this group of infants. In a Finnish cohort, a subset (7/35) of vaginally born infants also had low abundance of Bacteroides; however these infants together with C-section-born infants exhibited high abundance of Bifidobacterium species. Functional analysis revealed that Bifidobacterium mainly contributed to HMOs breakdown in the low-Bacteroides group replacing Bacteroides species that were the main contributor in all normally delivered infants (Yassour et al. 2016). In such a case, in addition to confirmation of this hypothesis with larger cohorts, identification of the dominant degrader(s) of HMOs in those C-section-born infants with low relative abundance of Bifidobacterium and Bacteroides need to be addressed. However, it has to be mentioned that these observations were detected with the relative abundance of the microbial community and the absolute changes of these species were unclear.
This microbiota similarity between infants born vaginally or by C-section gradually increased after the first year of life (Bokulich et al. 2016; Fouhy et al. 2019), suggesting that both microbial ecosystems underwent maturation, resembling the adult-like gut microbiota. At 2 years of age, the discriminatory genera could be still identified for vaginally born infants, namely Parabacteroides and Ruminiclostridium, while Gordonibacter and Lachnospiracheae NC2004 group were discriminative of C-section-born infants. By 4 years of age, there were no discriminatory genera for C-section-born infants (Fouhy et al. 2019). The clinical consequences of the long-lasting and large-scale perturbations of early-life gut microbiota remain to be investigated.
Given that antibiotics normally are administered during pregnancy to mothers who give birth by C-section to prevent the risk of infections, in order to discriminate influences between mode of delivery and antibiotics, C-sections were specially performed with mice model without the use of antibiotics in the perinatal period. The maturation of gut microbiota in C-section-born mice was almost stagnated compared with the vaginally born mice showing a progressive approximation in microbial maturation during the first 6 weeks of life. The diversity of the gut microbiota in vaginally born animals decreased with age from weaning, which was also observed in C-section-born mice with lower diversity at weaning. In addition, C-section-born mice at weaning were enriched with Lactobacillus, and Erysipelotrichaceae with underrepresented taxa of Bacteroides, Ruminococcaceae, Lachnospiraceae and Clostridiales (Martinez et al. 2017). The research with the same hypothesis in humans is warranted.
A number of studies have shown a correlation between C-section delivery and a higher abundance of the spore-forming Clostridium genus. In the INFANTMET study, the Clostridium genus was found to be more abundant at 1 week in C-section-born infants, both full term and preterm, as compared with vaginally born infants. However, at later samples, no significant difference was observed. Interestingly, the metabolomic analysis performed in this study revealed that bile acids were present in higher amounts in preterm urine, compared with full term. Clostridioides difficile spores germinate in response to primary bile salts (Shen 2015), although this study did not specify which bile salts were found (Hill et al. 2017). In a recent comprehensive study of 596 healthy full-term babies, the spore former Clostridium perfringens was also found to be enriched in the gut microbiota of C-section delivered infants during the first week of life (Shao et al. 2019). In a study of 13 C-section delivered infants in southern Spain, Clostridium was present in all time points, in all infants and adults. Two of the infants in this cohort were found to have Clostridium in their meconium samples, which were recovered at 100% identity with the corresponding maternal sample, indicative of vertical transmission from mother to infant, possibly in utero (Vallès et al. 2014)
Breastfeeding and microbiota
The early diet of infants in the first few months is dominated by the breast milk from their own mother or donor, or formula where breast milk is unavailable, followed by the introduction of solid food (Fig. 1). The early feeding pattern including the extent of breastfeeding, the timing of solid food introduction and other provided dietary ingredients such as prebiotics, probiotics and symbiotics may result in different trajectories of gut microbiota development.
Breast milk harbours >700 bacterial species at concentrations of ∼1000 colony-forming units (CFUs)/ml, and thus breastfed infants ingest up to ∼800 000 bacteria daily (Le Doare et al. 2018). The origins of the human milk microbiota may stem from maternal skin and the oral cavity of the infants or possibly translocate from the maternal gut by the entero-mammary pathway, although this theory is still controversial and needs to be confirmed (Rodríguez 2014). The microbial profile of the breast milk can change over the course of lactation from colostrum, to transition and mature breast milk. Species of Weissella and Leuconostoc, followed by Staphylococcus, Streptococcus and Lactococcus, dominate the microbial community in colostrum (Cabrera-Rubio et al. 2012). Afterwards, genera of Pseudomonas, Staphylococcus and Streptococcus constitute the main members of a core set of microbial species in breast milk, while the other members vary across studies, possibly attributed to the different stages of lactation, maternal health status and delivery mode (Cabrera-Rubio et al. 2012; Jost et al. 2013; Khodayar-Pardo et al. 2014; Murphy et al. 2017).
A close relationship between the infant's gut microbiota and mother's breast milk microbiota has been established, and it is dependent on the infant’s age. During the first week of life, studies showed that the feeding pattern, either exclusive breastfeeding or non-exclusive breastfeeding, did not affect the microbiota in the newborn's gut (Bäckhed et al. 2015; Shao et al. 2019). As newborns grow, breastfeeding becomes the most important factor shaping the gut microbiota (Stewart et al. 2018). Within 6 months, exclusively breastfed infants had increased levels of species of Lactobacillus (L. johnsonii, L.gasseri, L. paracasei, L. casei), and B. longum. In contrast, the relative abundance of Bacteroides, Eubacterium, Veillonella and Megasphaera in non-exclusively breastfed infants increased (Bäckhed et al. 2015; Ho et al. 2018).
In general, microbial diversity is higher in non-exclusively breastfed than exclusively breastfed infants in early life (Ho et al. 2018) and during 12 to 24 months of life (Bokulich et al. 2016). The maturation of microbiota in infants fed exclusively by breast milk occurs later than in infants who received breast milk and formula, reflected by the predicted microbial age (Bäckhed et al. 2015; Ho et al. 2018). An increase in gut microbial age is related to a shorter duration of exclusively breastfeeding (Ho et al. 2018). Breast-fed dominant infants aged from 12 to 24 months were enriched with species of Lactobacillus, Staphylococcus, Megasphaera and Actinobacteria, while genera of Clostridiales and Proteobacteria were more abundant in formula-fed dominant infants (Bokulich et al. 2016).
The influence of feeding pattern depends on the other perinatal factors, such as mode of delivery. A study showed that the impact of breastfeeding on the gut microbiota in infants became significant only after the first week (Shao et al. 2019). The effect size of breastfeeding was still smaller than mode of delivery, and importantly breastfeeding did not impact the gut microbiota differently according to mode of delivery during the first month of life (Reyman et al. 2019). Infants born by C-section and fed by breast milk did not gain the comparable abundance of Bifidobacterium at 1 week of life compared with vaginally born infants fed by formula (Reyman et al. 2019). As infants age to around 8 months, the influence of breastfeeding was increasing and becoming comparable to the impact of mode of delivery (Shao et al. 2019). Conversely, results from the TEDDY cohort indicated that breastfeeding dominated the microbial development as measured from 3 to 14 months of life compared with the other perinatal factors including mode of delivery that significantly influences the gut microbiota of infants (Stewart et al. 2018). The contradictory results might be attributable to the statistical analysis methods, the heterogeneity among populations such as the breastfeeding cessation in practice, and the clinical structure of the involved participants (e.g. the ratio of C-section to vaginally born infants, the extent of exclusive breastfeeding) (Shao et al. 2019).
Maternal stress and gut microbiota
With increasing evidence that the microbiota is one of the key regulators of the gut–brain axis (Cryan et al. 2019), influences of maternal stress during pregnancy on the offspring's psychological function and behaviour, and physical development and health have been linked to the maternal microbiota (Fig. 1). An altered maternal microbiota as a result of perinatal stress may be transmitted to the offspring, which impacts the maturation of an infant's immunity, and the hypothalamic-pituitary-adrenal axis (Zijlmans et al. 2015; Gensollen et al. 2016). Indeed, infants of mothers who suffered from high cumulative stress assessed by a combination of high reported stress and high cortisol concentrations during pregnancy had a higher microbial diversity, and were enriched in species of Proteobacteria (Escherichia, Enterobacter, Serratia) with lower abundances of lactic acid bacteria (Lactobacillus, Lactoccus, Aerococcus) and Actinobacteria (bifidobacteria, Collinsella, Eggerthella) during the first 4 months of life. The altered microbial colonisation pattern increased the risk of predisposing the infant to gastrointestinal symptoms and allergic reactions (Zijlmans et al. 2015). Later, as shown in mice, this early prenatal stress was proven to influence the acquisition and development of gut microbiota in a temporal and sex-specific manner via altering the maternal gut and vaginal microbiota (Jašarević et al. 2017), and lasted until adulthood in female mice (Gur et al. 2017), which needs to be confirmed further in humans.
ASSOCIATION OF MICROBIOTA IN EARLY LIFE WITH HEALTH
As discussed above, a number of intrinsic and extrinsic perinatal factors can affect the composition of the infant gut microbiota. The next logical question is what is the effect of these changes, particularly in relation to infant health and development. The hygiene hypothesis, although somewhat controversial, claims that the increasing incidence of allergic and auto-immune diseases in western countries can be explained by changes in early microbial exposure as colonisation of the infant gut is considered a critical factor in training the immune system's reactions to microorganisms (Penders et al. 2014).
Development of the immune system and risk of allergy
The gut microbiota plays a key role in the development and maturation of the infant immune system. Reduced diversity in the infant gut microbiota has been linked with a range of auto-immune diseases and allergies. Atopic disease relates to eczema (atopic dermatitis), allergic rhinitis (hay fever) or asthma and is generally defined by elevated levels of IgE in the serum. A lower bacterial diversity in early life (1 week to 1 month) has been linked with development of asthma and eczema later in childhood at 1 year (Ismail et al. 2012), one and half years (Wang et al. 2008) and 7 years of age (Abrahamsson et al. 2014). Lower bacterial richness at 3 months was also linked to an increased risk of food sensitisation at 1 year of age. However, there was no difference in bacterial richness or diversity between sensitised and non-sensitised infants at 1 year of age, which further highlights the importance of the development of the infant microbiota in the first months of life (Azad et al. 2015). A recent study also found that the numbers of bacterial cells by real-time PCR in faecal samples of infants with atopic dermatitis were lower than healthy controls at 6 months. Although this study did not find a compositional difference in gut microbiota between the two groups, at a functional level the relative abundance of microbial genes involved in oxidative phosphorylation (involving regulatory T cells activation) and nucleotide-binding oligomerisation domain (NOD)-like receptor signalling (being a function of sensing commensal microbiota and maintaining homeostasis) were higher in the control group (Lee et al. 2018).
Specific phyla and genera of bacteria have also been linked with increased susceptibility to allergies. A recent longitudinal study followed the development of the IgE-mediated allergies and compositional changes in the gut microbiota in 93 children from 4 months to 8 years. In children that developed allergies, Bacteroides were significantly underrepresented in early samples, which persisted to 8 years. Coprococcus and Prevotella were also underrepresented in allergic children. Interestingly and perhaps unexpectedly given their status as health-benefiting bacteria, bifidobacteria were enriched in allergic children compared to nonallergic children, along with OTUs from the Ruminococcaceae family. Roseburia and Clostridium, including C. difficile, were also consistently overrepresented in allergic children. This study hypothesised that the production of butyrate by Coprococcus plays a key role in preventing the development of allergic disease (Simonyté Sjödin et al. 2019). Butyrate is the preferred energy source of colonic epithelial cells while also playing a role in maintaining gut barrier function (Lopetuso et al. 2013).
Higher levels of Enterobacteriaceae and lower levels of Bacteroidaceae at 3 months of age were associated with an increased risk of food sensitisation (Azad et al. 2015). The KOALA study in the Netherlands identified a link between the presence of C. difficile and the development of atopic diseases such as asthma, eczema and sensitisation towards food allergies. This study identified not only C-section birth with increased risk of atopic diseases but also hospital birth. Vaginally born infants in hospital had an increased risk compared with those born at home, which correlated with increased colonisation by C. difficile (van Nimwegen et al. 2011).
Several studies have found that an underrepresentation of Bacteroides is linked with an increased risk of allergic disease. As described elsewhere in this review, this genus is significantly affected by mode of delivery, with a sustained decrease in relative abundance in C-section-born infants. C-section birth and the lower abundance of Bacteroides compared with vaginally born controls was associated with lower levels of Th1-associated chemokines in a study of 24 infants over the first 2 years of life, indicating a reduced Th1 immune response (Jakobsson et al. 2014). A low diversity in the Bacteroides genus at the first month of life was associated with IgE-mediated atopic eczema during the first 2 years (Abrahamsson et al. 2012). Another study found that the Bacteroidetes were 3-fold less abundant in infants with eczema at 18 months (Nylund et al. 2013). The Bacteroides genus was also found to be underrepresented in infants with IgE-mediated food allergies (Ling et al. 2014).
The presence of Lactobacillus and Bifidobacterium has been associated with a reduced susceptibility to allergic diseases (Sjögren et al. 2009a; Johansson et al. 2011). Lactobacillus strains can induce IL-12 and INF-γ, which results in the suppression of IgE (Shida et al. 1998). Increased bifidobacterial diversity is associated with increased IgA production and protection against allergy (Sjögren et al. 2009b).
Diabetes
Type I Diabetes (T1D) is an auto-immune disorder that results from T cell-mediated destruction of the insulin-producing β cells of the pancreatic islets. Although there is a genetic element to the development of T1D, it has been suggested that pathogenesis may be influenced by the gut microbiota (Wen et al. 2008). In Eastern Europe, T1D is prevalent in Finnish and Estonian children. In a study of 33 infants from Finland and Estonia who were genetically at risk for the development of T1D, it was found that there was a 25% decrease in alpha diversity in T1D-diagnosed individuals compared with those who had not developed the disease. At genus level, Ruminococcus and Streptococcus were overrepresented in T1D-diagnosed infants but the differences were not significant (Kostic et al. 2015). Another study in Eastern Europe linked the prevalence of T1D in Finland and Estonia to a higher relative abundance of Bacteroides in the infant gut microbiota. In comparison, infants from Russia had a lower relative abundance of Bacteroides and a lower prevalence of T1D (Vatanen et al. 2016). These results correlated with a wider European study that also found an increased abundance of Bacteroides in children younger than 2.9 years who developed T1D (de Goffau et al. 2014). A transatlantic longitudinal study described changes in the functional potential of the microbiota, correlating a decrease in fermentation pathways and the production of short-chain fatty acids with an increased incidence of T1D development in infants genetically at risk (Vatanen et al. 2018).
Obesity
Compositional changes in the infant gut microbiota have also been linked with a tendency towards obesity later in life. Obese adults typically have lower numbers of Bacteroidetes than normal-weight individuals (Kotzampassi, Giamarellos-Bourboulis and Stavrou 2014) and as described above C-section-born infants also lack this group. An early study using fluorescent in situ hybridization (FISH) with flow cytometry found that a microbiota population with an abundance of bifidobacteria and lower numbers of Staphylococcus aureus in early life (6 months) was shown to inversely correlate with obesity by the age of 7 (Kalliomäki et al. 2008). This was corroborated by a later study using 16S rRNA sequencing that found that high Bifidobacterium and Collinsella levels at 6 months of age corresponded with lower adiposity at 18 months. On the contrary, high levels of Streptococcus were associated with earlier gestational age and higher adiposity (Dogra et al. 2015).
Probiotic intervention studies
Probiotics are defined as ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’. Due to the adverse effects attributed to perturbations in the infant gut microbiota described above, probiotic intervention studies have increased among the scientific and medical communities. The majority of such studies utilise strains of bifidobacteria and lactobacilli, due to their technical robustness and generally safe status, but overall results have been mixed.
Lactobacillus rhamnosus GG has been widely used in such studies. As mentioned above, this strain has been shown to transfer to the infant following use by the mother (Schultz et al. 2004; Dotterud et al. 2015). This probiotic strain was also used in an intervention study in infants who were at risk of developing asthma in Northern Europe. Administration of the strain daily from birth to 6 months resulted in a distinct community composition including an increased relative abundance of Lactobacillaceae and Bifidobacteriaceae. The community composition was also more stable in the high L. rhamnosus GG group that the authors speculated could be a protective mechanism against pathogen overgrowth (Cox et al. 2010). Lactobacillus rhamnosus GG was also used in a probiotic mixture containing Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis BB-12 to investigate if maternal intake of probiotics could decrease the risk of atopic disease in infants. The probiotic was found to reduce the incidence of atopic dermatitis but there was no effect on the composition of the gut microbiota of the infant (Dotterud et al. 2010). A later investigation of the data by the same group suggested that the effect of the probiotic is dependent on the intrinsic microbiota of the infant (Avershina et al. 2017). Supplementation with B. longumsubsp. infantis EV001 was shown to reduce levels of faecal calprotectin in the first 2 months after birth (Henrick et al. 2019). High levels of faecal calprotectin are indicative of intestinal inflammation and an increased risk of atopic dermatitis and asthma later in life (Orivuori et al. 2015).
Prevention of atopic disease was also the primary objective of a longitudinal perinatal probiotic intervention study. In this study, pregnant mothers were given a capsule containing L. rhamnosus GG, L. rhamnosus, B. breve Bb99 and Propionibacterium freudenreichiissp.shermanii DSM 7076 and after birth their infants received the same probiotic daily for the first 6 months of life. The prevalence of atopic disease was measured at 2, 5 and 13 years. After 5 and 13 years, it was found that there was no significant difference in the overall cohort between the probiotic and placebo groups. However, in the C-section delivered subgroup who received the probiotic, there was a significant reduction in allergic disease and eczema (Kallio et al. 2019). A similar study investigated the administration of probiotic mixtures to mothers with allergic disease, prenatally and postnatally. The probiotic mixtures were L. rhamnosus LPR and B. longum BL999 or Lactobacillus paracasei ST11 and B. longum BL999. After 24 months, the risk of eczema in the infants was significantly reduced in both probiotic groups as compared with the control group (Rautava et al. 2012).
One particular area that has produced positive results in probiotic intervention studies is the prevention of NEC in preterm infants. The L. rhamnosus GG strain mentioned above was shown to reduce the incidence of NEC when used in conjunction with bovine lactoferrin (Manzoni et al. 2014). Another Lactobacillus strain, Lactobacillus reuteri DSM17938, was similarly effective in reducing the incidence of NEC (Hunter et al. 2012). In a comprehensive meta-analysis of 24 separate clinical trials, it was found that probiotic supplementation significantly reduced the rate of severe NEC and mortality. The studies utilised Lactobacillus, Bifidobacterium, Saccharomyces boulardii or a mixture of bacterial strains (AlFaleh and Anabrees 2014). However, a more recent trial using B. breve BBG-001 found no significant difference between probiotic and placebo groups (Costeloe et al. 2016). In preterm infants, oral supplementation with a probiotic mixture of B. bifidum and L. acidophilus was shown to restore the composition of the microbiome closer to that of healthy full-term infants (Alcon-Giner et al. 2019).
‘MISSING MICROBES’ FROM PERINATAL PERIOD
Some microbes have been observed (as described above) to be absent or at very low levels under certain circumstances, such as Bacteroides for C-section, Bifidobacterium and Bacteroides for preterm infants, Bifidobacterium for short-term breastfeeding or without breastfeeding (referred to as ‘missing microbes’). However, it has to be kept in mind that those missing microbes might be still present but cannot be detected with current approaches. For example, the missing Bacteroides from C-section-born infants gain prevalence in a few months of life, but the source of the recovered Bacteroides and the underlying mechanisms are still unknown. Similar to genetic mutations and modifications, the missing microbes are able to ‘mutate’ the profile of the initial members of the gut microbiota, resulting in a different trajectory of microbial development and host health in life. To this end, future research exploring the missing microbes as the ‘next-generation probiotics’ should be considered by leveraging the advances in sequencing technology and metagenomic analyses, together with improvements in culture-dependent techniques (Browne et al. 2016; Lagier et al. 2018), for example, Bacteroides species whose effect, by their presence or absence, is clearly fundamental in infant health and development (Tan, Zhai and Chen 2019).
On the other side, as the same functions can be shared by different species, it is possible that the other species with increased abundance in the ecosystem compensate for the functions left open by the missing microbes (Yassour et al. 2016). Thus, future research must incorporate more than the composition of the microbial community, and characterisation at functional level will be essential to uncover the functional interactions between commensal microbiota with the clinical consequence for the host.
CONCLUSIONS
Associations between maternal perinatal factors and the health of the offspring have been confirmed by cohorts of various sizes. The impaired growth and development of the foetus due to aberrant perinatal elements such as maternal malnutrition and antibiotics, in particular affecting infant immunity, is proposed to leave a negative impression on infant health in early and later life; although, the underlying mechanisms of action are largely unknown. However, the gut microbiota has been indicated to be highly sensitive to a number of perinatal factors and could provide the missing link. Indeed, the microbiota is increasingly recognised as a biological bond between mothers and infants. Given the vital contribution of the maternal microbiota to the early-life microbiota, and the relationship between early-life microbiota and subsequent adverse health outcomes, inheriting an altered maternal microbiota could be detrimental to the offspring. Direct evidence combining the perinatal factors, maternal and infant microbiota, and health outcomes of the offspring is beginning to emerge, where emphasis on the longer term maturation of the offspring will be essential. In parallel, the discovery of key microbes that respond specifically to different perinatal factors represents an obvious strategy in the exploration of next-generation probiotics for therapeutic interventions that maintain/improve the health of mothers and children to ensure a good start in life.
Contributor Information
Shaopu Wang, APC Microbiome Ireland, Cork, Ireland, P12 YT20; Teagasc Food Research Centre, Moorepark, Fermoy, County Cork, Ireland, P61 C996.
Muireann Egan, APC Microbiome Ireland, Cork, Ireland, P12 YT20; Teagasc Food Research Centre, Moorepark, Fermoy, County Cork, Ireland, P61 C996.
C Anthony Ryan, APC Microbiome Ireland, Cork, Ireland, P12 YT20; Department of Paediatrics & Child Health, University College Cork, Cork, Ireland, T12 YN60.
Patrick Boyaval, DuPont Nutrition & Biosciences, Danisco France SAS - DuPont, 22, rue Brunel, F- 75017 Paris, France.
Eugene M Dempsey, APC Microbiome Ireland, Cork, Ireland, P12 YT20; Department of Paediatrics & Child Health, University College Cork, Cork, Ireland, T12 YN60.
R Paul Ross, APC Microbiome Ireland, Cork, Ireland, P12 YT20.
Catherine Stanton, APC Microbiome Ireland, Cork, Ireland, P12 YT20; Teagasc Food Research Centre, Moorepark, Fermoy, County Cork, Ireland, P61 C996.
FUNDING
This work was supported by the Science Foundation Ireland [grant number SFI/12/RC/2273] and by funding from DuPont Nutrition & Biosciences.
Conflicts of interest
None declared.
REFERENCES
- Aagaard K, Ma J, Antony KM et al. . The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenhaim HA, Tulandi T, Wilchesky M et al. . Effect of cesarean delivery on long-term risk of small bowel obstruction. Obstet Gynecol. 2018;131:354–9. [DOI] [PubMed] [Google Scholar]
- Abrahamsson TR, Jakobsson HE, Andersson AF et al. . Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–40. [DOI] [PubMed] [Google Scholar]
- Abrahamsson TR, Jakobsson HE, Andersson AF et al. . Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–50. [DOI] [PubMed] [Google Scholar]
- Ahern GJ, Hennessy A, Ryan CA et al. . Advances in infant formula science. Annu Rev Food Sci Technol. 2019;10:75–102. [DOI] [PubMed] [Google Scholar]
- Al-Taiar A, Hammoud MS, Thalib L et al. . Pattern and etiology of culture-proven early-onset neonatal sepsis: a five-year prospective study. Int J Infect Dis. 2011;15:e631–4. [DOI] [PubMed] [Google Scholar]
- Alcon-Giner C, Dalby MJ, Caim S et al. . Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome. bioRxiv. 2019;698092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid-Based Child Health. 2014;9:584–671. [DOI] [PubMed] [Google Scholar]
- Aloisio I, Mazzola G, Corvaglia LT et al. . Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol. 2014;98:6051–60. [DOI] [PubMed] [Google Scholar]
- Aloisio I, Quagliariello A, De Fanti S et al. . Evaluation of the effects of intrapartum antibiotic prophylaxis on newborn intestinal microbiota using a sequencing approach targeted to multi hypervariable 16S rDNA regions. Appl Microbiol Biotechnol. 2016;100:5537–46. [DOI] [PubMed] [Google Scholar]
- Andreas NJ, Kampmann B, Le-Doare KM. Human breast milk: a review on its composition and bioactivity. Early Hum Dev. 2015;91:629–35. [DOI] [PubMed] [Google Scholar]
- Arboleya S, Sánchez B, Solís G et al. . Impact of prematurity and perinatal antibiotics on the developing intestinal microbiota: a functional inference study. Int J Mol Sci. 2016;17:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta M-C, Arévalo A, Stiemsma L et al. . Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol. 2018;142:424–34.. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E et al. . Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnicar F, Manara S, Zolfo M et al. . Studying vertical microbiome transmission from mothers to infants by strain-level metagenomic profiling. mSystems. 2017;2:e00164–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avershina E, Lundgård K, Sekelja M et al. . Transition from infant- to adult-like gut microbiota. Environ Microbiol. 2016;18:2226–36. [DOI] [PubMed] [Google Scholar]
- Avershina E, Rubio RC, Lundgård K et al. . Effect of probiotics in prevention of atopic dermatitis is dependent on the intrinsic microbiota at early infancy. J Allergy Clin Immunol. 2017;139:1399–402.e8. [DOI] [PubMed] [Google Scholar]
- Azad MB, Konya T, Guttman DS et al. . Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45:632–43. [DOI] [PubMed] [Google Scholar]
- Bergström A, Skov TH, Bahl MI et al. . Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol. 2014;80:2889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betrán AP, Ye J, Moller A-B et al. . The increasing trend in caesarean section rates: global, regional and national estimates: 1990–2014. PLoS One. 2016;11:e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezold KY, Karjalainen MK, Hallman M et al. . The genomics of preterm birth: from animal models to human studies. Genome Med. 2013;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss JM, Basavegowda KP, Watson WJ et al. . Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr Infect Dis J. 2008;27:231–5. [DOI] [PubMed] [Google Scholar]
- Blumfield ML, Hure AJ, MacDonald-Wicks LK et al. . Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am J Clin Nutr. 2012;96:1032–41. [DOI] [PubMed] [Google Scholar]
- Bokulich NA, Chung J, Battaglia T et al. . Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8:343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart M, Haynes M, Kelley S et al. . Viral diversity and dynamics in an infant gut. Res Microbiol. 2008;159:367–73. [DOI] [PubMed] [Google Scholar]
- Browne HP, Forster SC, Anonye BO et al. . Culturing of ‘unculturable' human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533:543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RG, Marchesi JR, Lee YS et al. . Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Roswall J, Peng Y et al. . Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. [DOI] [PubMed] [Google Scholar]
- Cabrera-Rubio R, Collado MC, Laitinen K et al. . The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012;96:544–51. [DOI] [PubMed] [Google Scholar]
- Cappelletti M, Della Bella S, Ferrazzi E et al. . Inflammation and preterm birth. J Leukoc Biol. 2016;99:67–78. [DOI] [PubMed] [Google Scholar]
- Chen L-W, Tint M-T, Fortier MV et al. . Maternal macronutrient intake during pregnancy is associated with neonatal abdominal adiposity: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study. J Nutr. 2016;146:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernikova DA, Madan JC, Housman ML et al. . The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr Res. 2018;84:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AW, Jewesson PJ. Pharmacokinetics and safety of antimicrobial agents during pregnancy. Rev Infect Dis. 1985;7:287–313. [DOI] [PubMed] [Google Scholar]
- Chu DM, Antony KM, Ma J et al. . The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016a;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DM, Ma J, Prince AL et al. . Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23:314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DM, Meyer KM, Prince AL et al. . Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes. 2016b;7:459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Wang Q, O'Sullivan O et al. . Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res. 2010;38:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado MC, Rautava S, Aakko J et al. . Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costeloe K, Bowler U, Brocklehurst P et al. . A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial. Health Technol Assess. 2016;20:1–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons-Read ME, Lobel M, Carey JC et al. . The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun. 2012;26:650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MJ, Huang YJ, Fujimura KE et al. . Lactobacillus caseiabundance is associated with profound shifts in the infant gut microbiome. PLoS One. 2010;5:e8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crume TL, Brinton JT, Shapiro A et al. . Maternal dietary intake during pregnancy and offspring body composition: the Healthy Start Study. Am J Obstet Gynecol. 2016;215:609.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O'Riordan KJ, Cowan CS et al. . The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. [DOI] [PubMed] [Google Scholar]
- de Goffau MC, Fuentes S, van den Bogert B et al. . Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia. 2014;57:1569–77. [DOI] [PubMed] [Google Scholar]
- Del Chierico F, Vernocchi P, Petrucca A et al. . Phylogenetic and metabolic tracking of gut microbiota during perinatal development. PLoS One. 2015;10:e0137347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux G, Litonjua AA, Turner SW et al. . Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–9. [DOI] [PubMed] [Google Scholar]
- Dogra S, Sakwinska O, Soh S-E et al. . Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. mBio. 2015;6:e02419–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M et al. . Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotterud C, Storrø O, Johnsen R et al. . Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol. 2010;163:616–23. [DOI] [PubMed] [Google Scholar]
- Dotterud CK, Avershina E, Sekelja M et al. . Does maternal perinatal probiotic supplementation alter the intestinal microbiota of mother and child? J Pediatr Gastroenterol Nutr. 2015;61:200–7. [DOI] [PubMed] [Google Scholar]
- Duranti S, Lugli GA, Mancabelli L et al. . Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome. 2017;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilh BE, Cassman N, McNair K et al. . A highly abundant bacteriophage discovered in the unknown sequences of human faecal metagenomes. Nat Commun. 2014;5:4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M, Motherway MOC, Ventura M et al. . Metabolism of sialic acid by Bifidobacterium breve UCC2003. Appl Environ Microbiol. 2014;80:4414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M, van Sinderen D. Carbohydrate metabolism in Bifidobacteria. : Mattarelli P, Biavati B, Holzapfel WH. et al (). The Bifidobacteria and Related Organisms: Biology, Taxonomy, Applications. London, UK: Academic Press, 2018, 145–64. [Google Scholar]
- Ferretti P, Pasolli E, Tett A et al. . Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 2018;24:133–45.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettweis JM, Serrano MG, Brooks JP et al. . The vaginal microbiome and preterm birth. Nat Med. 2019;25:1012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippidou S, Junier T, Wunderlin T et al. . Under-detection of endospore-forming Firmicutes in metagenomic data. Comput Struct Biotechnol J. 2015;13:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JE, Özaltin E, Canning D. The association of maternal age with infant mortality, child anthropometric failure, diarrhoea and anaemia for first births: evidence from 55 low-and middle-income countries. BMJ Open. 2011;1:e000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouhy F, Watkins C, Hill CJ et al. . Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10:1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin MY, Mekhedov SL, Puigbo P et al. . Genomic determinants of sporulation in Bacilli and Clostridia: towards the minimal set of sporulation-specific genes. Environ Microbiol. 2012;14:2870–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganal-Vonarburg SC, Hornef MW, Macpherson AJ. Microbial–host molecular exchange and its functional consequences in early mammalian life. Science. 2020;368:604–7. [DOI] [PubMed] [Google Scholar]
- Gensollen T, Iyer SS, Kasper DL et al. . How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CM, Wendt A, Peters S et al. . The impact of early age at first childbirth on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26:259–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MK, Wang B, Ahmadi S et al. . Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat Microbiol. 2016;1:16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD et al. . Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Arango LF, Barrett HL, McIntyre HD et al. . Antibiotic treatment at delivery shapes the initial oral microbiome in neonates. Sci Rep. 2017;7:43481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gallego C, Garcia-Mantrana I, Salminen S et al. . The human milk microbiome and factors influencing its composition and activity. Semin Fetal Neonatal Med. 2016;21:400–5. [DOI] [PubMed] [Google Scholar]
- Groer MW, Luciano AA, Dishaw LJ et al. . Development of the preterm infant gut microbiome: a research priority. Microbiome. 2014;2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurol-Urganci I, Bou-Antoun S, Lim C et al. . Impact of caesarean section on subsequent fertility: a systematic review and meta-analysis. Hum Reprod. 2013;28:1943–52. [DOI] [PubMed] [Google Scholar]
- Gur TL, Shay L, Palkar AV et al. . Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav Immun. 2017;64:50–8. [DOI] [PubMed] [Google Scholar]
- Heisel T, Podgorski H, Staley CM et al. . Complementary amplicon-based genomic approaches for the study of fungal communities in humans. PLoS One. 2015;10:e0116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrick BM, Chew S, Casaburi G et al. . Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr Res. 2019;86:749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CJ, Brown JR, Lynch DB et al. . Effect of room temperature transport vials on DNA quality and phylogenetic composition of faecal microbiota of elderly adults and infants. Microbiome. 2016;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CJ, Lynch DB, Murphy K et al. . Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho NT, Li F, Lee-Sarwar KA et al. . Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun. 2018;9:4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C, Dimaguila MAV, Gal P et al. . Effect of routine probiotic, Lactobacillus reuteri DSM 17938, use on rates of necrotizing enterocolitis in neonates with birthweight < 1000 grams: a sequential analysis. BMC Pediatr. 2012;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail IH, Oppedisano F, Joseph SJ et al. . Reduced gut microbial diversity in early life is associated with later development of eczema but not atopy in high-risk infants. Pediatr Allergy Immunol. 2012;23:674–81. [DOI] [PubMed] [Google Scholar]
- Jakobsson HE, Abrahamsson TR, Jenmalm MC et al. . Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–66. [DOI] [PubMed] [Google Scholar]
- Javaid MK, Crozier SR, Harvey NC et al. . Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. [DOI] [PubMed] [Google Scholar]
- Jašarević E, Howard CD, Misic AM et al. . Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep. 2017;7:44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MA, Sjögren YM, Persson J-O et al. . Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS One. 2011;6:e23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost T, Lacroix C, Braegger C et al. . Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br J Nutr. 2013;110:1253–62. [DOI] [PubMed] [Google Scholar]
- Jost T, Lacroix C, Braegger CP et al. . New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One. 2012;7:e44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomäki M, Carmen Collado M, Salminen S et al. . Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8. [DOI] [PubMed] [Google Scholar]
- Kallio S, Kukkonen AK, Savilahti E et al. . Perinatal probiotic intervention prevented allergic disease in a Caesarean-delivered subgroup at 13-year follow-up. Clin Exp Allergy. 2019;49:506–15. [DOI] [PubMed] [Google Scholar]
- Keag OE, Norman JE, Stock SJ. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med. 2018;15:e1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Nisula L, Kyynäräinen HR, Kärkkäinen U et al. . Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth. Acta Paediatr. 2013;102:480–5. [DOI] [PubMed] [Google Scholar]
- Khodayar-Pardo P, Mira-Pascual L, Collado M et al. . Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J Perinatol. 2014;34:599–605. [DOI] [PubMed] [Google Scholar]
- Kindinger LM, Bennett PR, Lee YS et al. . The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kona SK, Matlock DN. Probiotics, prebiotics, and synbiotics for preterm neonates. NeoReviews. 2018;19:e654–63. [Google Scholar]
- Korpela K, Blakstad EW, Moltu SJ et al. . Intestinal microbiota development and gestational age in preterm neonates. Sci Rep. 2018;8:2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Gevers D, Siljander H et al. . The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzampassi K, Giamarellos-Bourboulis EJ, Stavrou G. Obesity as a consequence of gut bacteria and diet interactions. ISRN Obes. 2014;2014:651895. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Krupovic M, Forterre P. Microviridae goes temperate: microvirus-related proviruses reside in the genomes of Bacteroidetes. PLoS One. 2011;6:e19893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhle S, Tong O, Woolcott C. Association between caesarean section and childhood obesity: a systematic review and meta-analysis. Obes Rev. 2015;16:295–303. [DOI] [PubMed] [Google Scholar]
- Lagier J-C, Dubourg G, Million M et al. . Culturing the human microbiota and culturomics. Nat Rev Microbiol. 2018;16:540–50. [DOI] [PubMed] [Google Scholar]
- La Rosa PS, Warner BB, Zhou Y et al. . Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci USA. 2014;111:12522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder AP, Roche AM, Sherrill-Mix S et al. . Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean SC, Derricott H, Jones RL et al. . Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doare K, Holder B, Bassett A et al. . Mother's milk: a purposeful contribution to the development of the infant microbiota and immunity. Front Immunol. 2018;9:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-J, Kang M-J, Lee S-Y et al. . Perturbations of gut microbiome genes in infants with atopic dermatitis according to feeding type. J Allergy Clin Immunol. 2018;141:1310–9. [DOI] [PubMed] [Google Scholar]
- Lewis A, Austin E, Galbally M. Prenatal maternal mental health and fetal growth restriction: a systematic review. J Dev Orig Health Dis. 2016;7:416–28. [DOI] [PubMed] [Google Scholar]
- Li H, Zhou Y, Liu J. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes. 2013;37:893–9. [DOI] [PubMed] [Google Scholar]
- Lim ES, Zhou Y, Zhao G et al. . Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med. 2015;21:1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Li Z, Liu X et al. . Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol. 2014;80:2546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londero AP, Rossetti E, Pittini C et al. . Maternal age and the risk of adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. 2019;19:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetuso LR, Scaldaferri F, Petito V et al. . Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli GA, Milani C, Turroni F et al. . Prophages of the genus Bifidobacterium as modulating agents of the infant gut microbiota. Environ Microbiol. 2016;18:2196–213. [DOI] [PubMed] [Google Scholar]
- Lundgren SN, Madan JC, Emond JA et al. . Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome. 2018;6:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, de Agüero MG, Ganal-Vonarburg SC. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol. 2017;17:508–17. [DOI] [PubMed] [Google Scholar]
- Ma J, Prince AL, Bader D et al. . High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5:3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni P, Meyer M, Stolfi I et al. . Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: a randomized clinical trial. Early Hum Dev. 2014;90:S60–5. [DOI] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Sonnenburg ED et al. . Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 2011;10:507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez KA, Devlin JC, Lacher CR et al. . Increased weight gain by C-section: functional significance of the primordial microbiome. Sci Adv. 2017;3:eaao1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola G, Murphy K, Ross RP et al. . Early gut microbiota perturbations following intrapartum antibiotic prophylaxis to prevent group B streptococcal disease. PLoS One. 2016;11:e0157527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann A, Ryan FJ, Stockdale SR et al. . Viromes of one year old infants reveal the impact of birth mode on microbiome diversity. PeerJ. 2018;6:e4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AA, Gilboa SM, Werler MM et al. . Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205:51.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi J, Bobe AM, Miyoshi S et al. . Peripartum antibiotics promote gut dysbiosis, loss of immune tolerance, and inflammatory bowel disease in genetically prone offspring. Cell Rep. 2017;20:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DJ. Drug disposition in mother and foetus. Clin Exp Pharmacol Physiol. 1997;24:869–73. [DOI] [PubMed] [Google Scholar]
- Mueller NT, Whyatt R, Hoepner L et al. . Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes. 2015;39:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Curley D, O'Callaghan TF et al. . The composition of human milk and infant faecal microbiota over the first three months of life: a pilot study. Sci Rep. 2017;7:40597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Kobayashi T, Tanaka S et al. . Aberrant structures of fecal bacterial community in allergic infants profiled by 16S rRNA gene pyrosequencing. FEMS Immunol Med Microbiol. 2011;63:397–406. [DOI] [PubMed] [Google Scholar]
- Neuman H, Forsythe P, Uzan A et al. . Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol Rev. 2018;42:489–99. [DOI] [PubMed] [Google Scholar]
- Nogacka A, Salazar N, Suárez M et al. . Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates. Microbiome. 2017;5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund L, Satokari R, Nikkilä J et al. . Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC Microbiol. 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orivuori L, Mustonen K, de Goffau M et al. . High level of fecal calprotectin at age 2 months as a marker of intestinal inflammation predicts atopic dermatitis and asthma by age 6. Clin Exp Allergy. 2015;45:928–39. [DOI] [PubMed] [Google Scholar]
- Örtqvist AK, Lundholm C, Halfvarson J et al. . Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: a population-based study. Gut. 2019;68:218–25. [DOI] [PubMed] [Google Scholar]
- Pannaraj PS, Li F, Cerini C et al. . Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannaraj PS, Ly M, Cerini C et al. . Shared and distinct features of human milk and infant stool viromes. Front Microbiol. 2018;9:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Llorca A, Gormaz M, Alcántara C et al. . Preterm gut microbiome depending on feeding type: significance of donor human milk. Front Microbiol. 2018;9:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Gerhold K, Thijs C et al. . New insights into the hygiene hypothesis in allergic diseases: mediation of sibling and birth mode effects by the gut microbiota. Gut Microbes. 2014;5:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters LL, Thornton C, de Jonge A et al. . The effect of medical and operative birth interventions on child health outcomes in the first 28 days and up to 5 years of age: a linked data population-based cohort study. Birth. 2018;45:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol. 2017;41:387–91. [DOI] [PubMed] [Google Scholar]
- Pärnänen K, Karkman A, Hultman J et al. . Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nat Commun. 2018;9:3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautava S, Kainonen E, Salminen S et al. . Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol. 2012;130:1355–60. [DOI] [PubMed] [Google Scholar]
- Reali A, Ximenes A, Cuzzolin L et al. . Antibiotic therapy in pregnancy and lactation. J Chemother. 2005;17:123–30. [DOI] [PubMed] [Google Scholar]
- Reyes A, Blanton LV, Cao S et al. . Gut DNA viromes of Malawian twins discordant for severe acute malnutrition. Proc Natl Acad Sci USA. 2015;112:11941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyman M, van Houten MA, van Baarle D et al. . Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10:4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JM. The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr. 2014;5:779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salone LR, Vann Jr WF, Dee DL. Breastfeeding: an overview of oral and general health benefits. J Am Dent Assoc. 2013;144:143–51. [DOI] [PubMed] [Google Scholar]
- Sandall J, Tribe RM, Avery L et al. . Short-term and long-term effects of caesarean section on the health of women and children. Lancet. 2018;392:1349–57. [DOI] [PubMed] [Google Scholar]
- Sawyer KM, Zunszain PA, Dazzan P et al. . Intergenerational transmission of depression: clinical observations and molecular mechanisms. Mol Psychiatry. 2019;24:1157–77. [DOI] [PubMed] [Google Scholar]
- Schultz M, Göttl C, Young RJ et al. . Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38:293–7. [DOI] [PubMed] [Google Scholar]
- Sela DA, Chapman J, Adeuya A et al. . The genome sequence of Bifidobacteriumlongum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci USA. 2008;105:18964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Forster SC, Tsaliki E et al. . Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A. A gut odyssey: the impact of the microbiota on Clostridium difficile spore formation and germination. PLoS Pathog. 2015;11:e1005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida K, Makino K, Morishita A et al. . Lactobacillus casei inhibits antigen-induced IgE secretion through regulation of cytokine production in murine splenocyte cultures. Int Arch Allergy Immunol. 1998;115:278–87. [DOI] [PubMed] [Google Scholar]
- Shi Y-C, Guo H, Chen J et al. . Initial meconium microbiome in Chinese neonates delivered naturally or by cesarean section. Sci Rep. 2018;8:3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyté Sjödin K, Hammarström ML, Rydén P et al. . Temporal and long-term gut microbiota variation in allergic disease: a prospective study from infancy to school age. Allergy. 2019;74:176–85. [DOI] [PubMed] [Google Scholar]
- Sjögren YM, Jenmalm MC, Böttcher MF et al. . Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009a;39:518–26. [DOI] [PubMed] [Google Scholar]
- Sjögren YM, Tomicic S, Lundberg A et al. . Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses: gut microbiota and immune responses. Clin Exp Allergy. 2009b;39:1842–51. [DOI] [PubMed] [Google Scholar]
- Starling AP, Sauder KA, Kaar JL et al. . Maternal dietary patterns during pregnancy are associated with newborn body composition. J Nutr. 2017;147:1334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensballe LG, Simonsen J, Jensen SM et al. . Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr. 2013;162:832–8.e3. [DOI] [PubMed] [Google Scholar]
- Stewart CJ, Ajami NJ, O'Brien JL et al. . Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CJ, Embleton ND, Clements E et al. . Cesarean or vaginal birth does not impact the longitudinal development of the gut microbiome in a cohort of exclusively preterm infants. Front Microbiol. 2017;8:1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati F, Di Paola M, Stefanini I et al. . Age and gender affect the composition of fungal population of the human gastrointestinal tract. Front Microbiol. 2016;7:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222–31. [PMC free article] [PubMed] [Google Scholar]
- Talati A, Odgerel Z, Wickramaratne PJ et al. . Brain derived neurotrophic factor moderates associations between maternal smoking during pregnancy and offspring behavioral disorders. Psychiatry Res. 2016;245:387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburini S, Shen N, Wu HC et al. . The microbiome in early life: implications for health outcomes. Nat Med. 2016;22:713–22. [DOI] [PubMed] [Google Scholar]
- Tan H, Zhai Q, Chen W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res Int. 2019;116:637–44. [DOI] [PubMed] [Google Scholar]
- Tapiainen T, Koivusaari P, Brinkac L et al. . Impact of intrapartum and postnatal antibiotics on the gut microbiome and emergence of antimicrobial resistance in infants. Sci Rep. 2019;9:10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz G, Tetz V. Introducing the sporobiota and sporobiome. Gut Pathog. 2017;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turroni F, Milani C, Duranti S et al. . Glycan utilization and cross-feeding activities by Bifidobacteria. Trends Microbiol. 2018;26:339–50. [DOI] [PubMed] [Google Scholar]
- Vallès Y, Artacho A, Pascual-García A et al. . Microbial succession in the gut: directional trends of taxonomic and functional change in a birth cohort of Spanish infants. PLos Genet. 2014;10:e1004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nimwegen FA, Penders J, Stobberingh EE et al. . Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–55.e3. [DOI] [PubMed] [Google Scholar]
- Vatanen T, Franzosa EA, Schwager R et al. . The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562:589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanen T, Kostic AD, d'Hennezel E et al. . Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinturache AE, Gyamfi-Bannerman C, Hwang J et al. . Maternal microbiome—a pathway to preterm birth. Semin Fetal Neonatal Med. 2016;21:94–9. [DOI] [PubMed] [Google Scholar]
- Wampach L, Heintz-Buschart A, Fritz JV et al. . Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat Commun. 2018;9:5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-T, Lee F-L, Tai C-J et al. . Comparison of gyrB gene sequences, 16S rRNA gene sequences and DNA–DNA hybridization in the Bacillus subtilis group. Int J Syst Evol Microbiol. 2007;57:1846–50. [DOI] [PubMed] [Google Scholar]
- Wang M, Karlsson C, Olsson C et al. . Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121:129–34. [DOI] [PubMed] [Google Scholar]
- Wang S, Ryan CA, Boyaval P et al. . Maternal vertical transmission affecting early-life microbiota development. Trends Microbiol. 2020;28:28–45. [DOI] [PubMed] [Google Scholar]
- Wang X, Buhimschi CS, Temoin S et al. . Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS One. 2013;8:e56131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward TL, Knights D, Gale CA. Infant fungal communities: current knowledge and research opportunities. BMC Med. 2017;15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY et al. . Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization) Appropriate technology for birth. Lancet. 1985;2:436–7. [PubMed] [Google Scholar]
- WHO (World Health Organization) Preterm Birth: Fact Sheet. https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth (26 November 2019, date last accessed).
- Yassour M, Jason E, Hogstrom LJ et al. . Strain-level analysis of mother-to-child bacterial transmission during the first few months of life. Cell Host Microbe. 2018;24:146–54.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassour M, Vatanen T, Siljander H et al. . Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8:343ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ et al. . Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Feenstra B, Bacelis J et al. . Genetic associations with gestational duration and spontaneous preterm birth. N Engl J Med. 2017;377:1156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Su H, Cheng J et al. . Prenatal antibiotic use and risk of childhood wheeze/asthma: a meta-analysis. Pediatr Allergy Immunol. 2015;26:756–64. [DOI] [PubMed] [Google Scholar]
- Zijlmans MA, Korpela K, Riksen-Walraven JM et al. . Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–45. [DOI] [PubMed] [Google Scholar]