The rise of antimicrobial-resistant pathogens can be attributed to the lack of a rapid pathogen identification (ID) or antimicrobial susceptibility testing (AST), resulting in delayed therapeutic decisions at the point of care. Gonorrhea is usually empirically treated, with no AST results available before treatment, thus contributing to the rapid rise in drug resistance. Here, we present a rapid AST platform using RNA signatures for Neisseria gonorrhoeae. Transcriptome sequencing (RNA-seq) followed by bioinformatic tools was applied to explore potential markers in the transcriptome profile of N. gonorrhoeae upon minutes of azithromycin exposure.
KEYWORDS: Neisseria gonorrhoeae, RNA markers, RNA-seq, antimicrobial susceptibility testing, azithromycin
ABSTRACT
The rise of antimicrobial-resistant pathogens can be attributed to the lack of a rapid pathogen identification (ID) or antimicrobial susceptibility testing (AST), resulting in delayed therapeutic decisions at the point of care. Gonorrhea is usually empirically treated, with no AST results available before treatment, thus contributing to the rapid rise in drug resistance. Here, we present a rapid AST platform using RNA signatures for Neisseria gonorrhoeae. Transcriptome sequencing (RNA-seq) followed by bioinformatic tools was applied to explore potential markers in the transcriptome profile of N. gonorrhoeae upon minutes of azithromycin exposure. Validation of candidate markers using quantitative real-time PCR (qRT-PCR) showed that two markers (arsR [NGO1562] and rpsO) can deliver accurate AST results across 14 tested isolates. Further validation of our susceptibility threshold in comparison to MIC across 64 more isolates confirmed the reliability of our platform. Our RNA markers combined with emerging molecular point-of-care systems has the potential to greatly accelerate both ID and AST to inform treatment.
INTRODUCTION
The use of conventional clinical methods for pathogen identification (ID) and antimicrobial susceptibility testing (AST) is a time-consuming process that contributes to the rise of antimicrobial resistance and may, in turn, increase mortality rates (1). Thus, rapid diagnostic methods for timely and directed therapy is an urgent clinical need to be addressed.
Neisseria gonorrhoeae, the causative agent of gonorrhea, is an increasingly common sexually transmitted infection, with more than 550,000 cases reported annually in the United States (2). Gonorrhea is usually seen in female cervicitis and male urethritis (3). Untreated gonorrhea infections can lead to major complications, such as heart and nervous system infections, infertility, pelvic inflammatory disease, and newborn blindness (4, 5). Gonorrhea is often empirically treated based on a clinical syndromic diagnosis or after laboratory molecular detection with nucleic acid amplification testing (NAAT). NAAT has largely replaced diagnostic culture methods because it is faster and automated, with higher sensitivity to allow for cost-effective diagnosis (6). Treatments often proceed without AST results. Neisseria gonorrhoeae AST requires laborious and time-consuming (at least 1 to 2 days) culture methods and is undertaken only in cases of treatment failure (7, 8).
N. gonorrhoeae can rapidly develop resistance to antimicrobial agents due to innate mechanisms for acquiring resistance genes (9). Treatment is more challenging due to rapidly increasing resistance to all of the most commonly used antimicrobials, including sulfonamides, penicillin, tetracyclines, and fluoroquinolones, such as ciprofloxacin (10). Azithromycin is a macrolide antimicrobial that binds to the 23S rRNA component of the 50S ribosome, thereby inhibiting protein synthesis (11, 12). Azithromycin has been shown to be an effective treatment against gonococci, with prolonged levels in tissues and cells. Following oral administration, azithromycin concentrates in tissues, including genital sites (12). However, high levels of azithromycin resistance have been progressively reported worldwide (13, 14). Some of the known mechanisms of resistance to azithromycin include overexpression of the efflux pump (mtrR), 23S rRNA mutation in azithromycin binding sites (C2611T and A2059G), and ribosomal target modification by methylase (ermC and ermB) (3).
The CDC reported antimicrobial-resistant N. gonorrhoeae as one of the three most urgent threats to public health and currently recommends dual therapy with ceftriaxone and azithromycin for the treatment of uncomplicated gonorrhea (15). Despite recommended dual therapy, azithromycin monotherapy could be still used to treat uncomplicated gonorrhea in cephalosporin-allergic patients (16). In this study, azithromycin has been selected for our phenotypic AST development for gonorrhea as a proof of concept; however, our approach has the potential to be extended to other antimicrobials.
Given the time-consuming conventional AST, increasing level of resistance, and challenge of low growth rate (doubling time, 60 min) of N. gonorrhoeae (17), development of a novel AST that can guide initial treatment at the point of care is critically needed. DNA-based antimicrobial resistance tests are inherently limiting, as they require precise genetic knowledge of antibiotic resistance. They lack comprehensiveness, with many still-undiscovered markers for resistance (8). We have previously adopted the use of DNA for molecular phenotypic AST with quantitative PCR (qPCR) by quantifying changes in genomic DNA copy number in response to antimicrobial treatment (18). But given the slower-growing nature of N. gonorrhoeae, the time to result for DNA-based AST may still be prolonged. Recently, transcriptome sequencing (RNA-seq) has been effectively applied in discovery of reliable genomic biomarkers to develop clinical diagnosis (19). Previous work in our lab described a novel and accelerated AST workflow based on RNA-seq in Klebsiella pneumoniae upon exposure to ciprofloxacin (20). This method is independent of cell division and can be applied to distinguish between susceptible and resistant pathogens regardless of mechanism of resistance (20, 21).
As proof of concept, in this study, we focused on developing an ultrarapid molecular phenotypic AST for N. gonorrhoeae. RNA-seq followed by bioinformatic analysis was used to identify candidate diagnostic RNA markers to determine susceptibility upon a short exposure to azithromycin. Further validation of selected markers was performed through quantitative real-time PCR (qRT-PCR) using 14 isolates, followed by validation of our cutoff in comparison to reported MICs using 64 more isolates to determine susceptibility.
MATERIALS AND METHODS
Microorganisms and culturing.
Reference strains of azithromycin-susceptible and -resistant N. gonorrhoeae, SPL-4 and SPJ-15, were obtained from the CDC. Clinical isolates of N. gonorrhoeae were obtained from Johns Hopkins Medicine, Department of Pathology, Division of Medical Microbiology (see Table S1 in the supplemental material). Isolates were cultured from glycerol stocks on GC agar (BD 228950; Becton, Dickinson, Cockeysville, MD) supplemented with 1% IsoVitaleX (BD 211876; Becton, Dickinson). Plates were incubated at 35°C in 5% CO2 for 24 h. A single colony of each isolate was resuspended in GW broth (22) and incubated overnight at 37°C, 5% CO2, and 200 rpm to an optical density equal to that of a 0.5 MacFarland standard.
Antimicrobial susceptibility testing.
The Etest (bioMérieux, Durham, NC) or agar dilution method was used to determine the MIC of azithromycin (Astatech, Inc., Bristol, PA) (23). The agar dilution method was performed using GC agar supplemented with 1% IsoVitaleX described by the Clinical and Laboratory Standards Institute (CLSI). Briefly, 2-fold dilutions of azithromycin (range, 0.03 to 256 μg/ml) was added to CG medium (24). Each plate was inoculated with a 0.5 McFarland standard of bacterial suspension. Inoculated plates were incubated at 35°C in 5% CO2 for 24 h. Measurements were performed in triplicate, and positive and negative controls were included for each MIC test. Following incubation, MICs were determined as the lowest concentrations of azithromycin that inhibited visible growth of bacteria. According to the guidelines set forth by the CLSI, isolates with MICs of ≤2 μg/ml are classified as azithromycin susceptible (25).
RNA extraction and sequencing.
Bacterial culture (2 ml) was prepared as described in “Microorganisms and culturing” above. Samples were exposed to azithromycin (2 μg/ml) and incubated at 37°C in 5% CO2 for 10 min and 60 min (see workflow in Fig. 1). Bacteria without exposure to azithromycin were also incubated under the same conditions as a control (water was added to the controls). Samples were collected and preserved in 4 ml of RNAprotect reagent (Qiagen, Valencia, CA) at each time point. RNA extraction was conducted using an RNeasy minikit (Qiagen; 74524) according to the manufacturer’s instructions. Extracted RNA was subjected to DNase treatment (Turbo DNase complete kit; Life Technologies; AM1907). The concentration of RNA was measured using a QIAxpert spectrophotometer (Qiagen, USA). To consider biological variability, triplicate RNA samples were prepared from susceptible and resistant isolates.
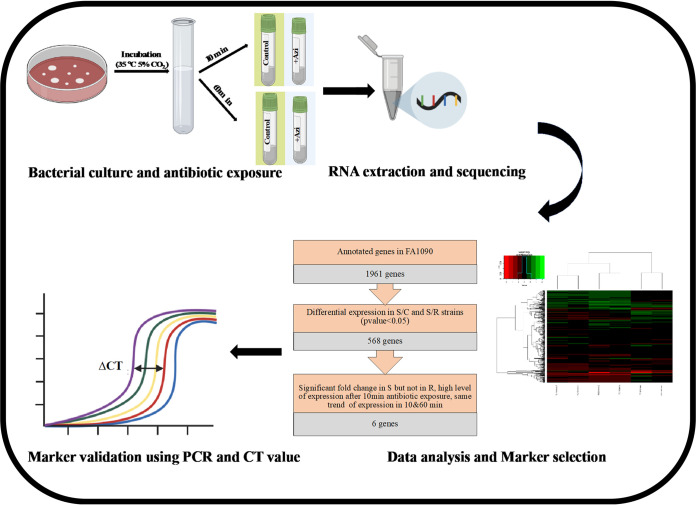
FIG 1.
Summarized workflow for generation of candidate RNA markers of the proposed molecular AST. N. gonorrhoeae was cultured and exposed to azithromycin for 10 and 60 min along with a control without azithromycin. Samples were collected for the RNA-seq library and sequencing. Data analysis was conducted to find differentially expressed genes, followed by marker selection steps. Selected markers were validated by qRT-PCR and ΔCT calculation. This figure was created with https://biorender.com/ and PowerPoint.
Sample preparation for sequencing was performed using the ScriptSeq complete kit for bacteria with rRNA depleted (Illumina Inc.; BB1224, RSBC10948, and SSIP1202). Samples were bioanalyzed at Stanford Functional Genomics Facility (SFGF), and libraries were sequenced at 75 pair-end reads on Illumina MiSeq. Quality control on raw reads from the sequenced libraries was conducted to remove the low-quality reads (using FastQC) (26), remove PCR duplicates (using SuperDeduper) (27), and trim the adaptor sequences (using Trim Galore, version 0.4.5) (https://github.com/FelixKrueger/TrimGalore). Finally, reads were aligned to N. gonorrhoeae FA 1090 (GenBank accession number NC_002946.2).
Differential expression analysis.
Quantification of transcript abundance and differential expression analyses were carried out using Rockhopper (28). Spearman correlation between samples/replicates from the normalized transcript counts obtained from Rockhopper were calculated using the cor function in R (29). The multidimensional scaling (MDS) that shows the dissimilarity of the replicates/samples by projecting them into two dimensions was also done using the cmdscale function.
To further analyze the differentially expressed genes under azithromycin treatment, the list was cut down to 568 differentially expressed genes with a P value of <0.05 in susceptible compared to control (SvC) and resistant (SvR) strains. Heat maps were generated using the heatmap.2 function to represent the log fold change (logFC) between these comparisons.
Marker selection and validation.
Candidate markers were selected from the RNA-seq data set based on the following criteria: (i) significant fold change in susceptible but not in resistant organisms after 10 min of drug exposure, (ii) high level of expression after 10 min of drug exposure (high level of expression for each gene was defined relative to average gene expression of all the genes), and (iii) same trend of expression in 10- and 60-min drug exposures (either up- or downregulated at both time points). Selected candidate markers were further evaluated using qRT-PCR.
Validation of final markers in clinical isolates was performed using qRT-PCR. Primer-BLAST was used to design primers for conserved regions of selected genes (Table S2). Quantification of gene expression level was conducted with the Rotor-Gene SYBR green qRT-PCR kit (Qiagen; catalog no. 204174) on a Rotor-Gene-Q PCR cycler (Qiagen). The concentrations of components used in the final 25-μl PCR mixture were as follows: 1× SYBR green master mix, 1 mM forward primer, 1 mM reverse primer, 0.05 U/μl of Rotor Gene RT mix, 0.2 U/μl of SUPERase-in RNase inhibitor, and 8% RNA template.
Expression of each candidate marker was quantified in the control and treated samples. A threshold of 0.1 was set to obtain accurate cycle threshold (CT) values for each isolate. This threshold is a point at which amplification plot reaches a fluorescence intensity above background level (30). To calculate the ΔCT value, a housekeeping gene was used to normalize potential differences between the control and treated samples. From our RNA-seq results, the atpA gene was selected as an internal control, as its expression level was not affected significantly upon antimicrobial treatment for the tested isolates in this study. Finally, fold change (FC) in gene expression between the control and treated samples was calculated as follows: FC = 2−ΔΔCT (18).
Accession number(s).
RNA-seq data are archived in the GenBank Sequence Read Archive (SRA) (BioProject identifier PRJNA627416).
RESULTS
Shift in transcriptome response following azithromycin exposure.
To identify markers that significantly differentiate susceptible and resistant isolates of N. gonorrhoeae, RNA-seq was used to compare their transcriptome responses after 10 and 60 min of azithromycin exposure. A summary of the workflow is shown in Fig. 1.
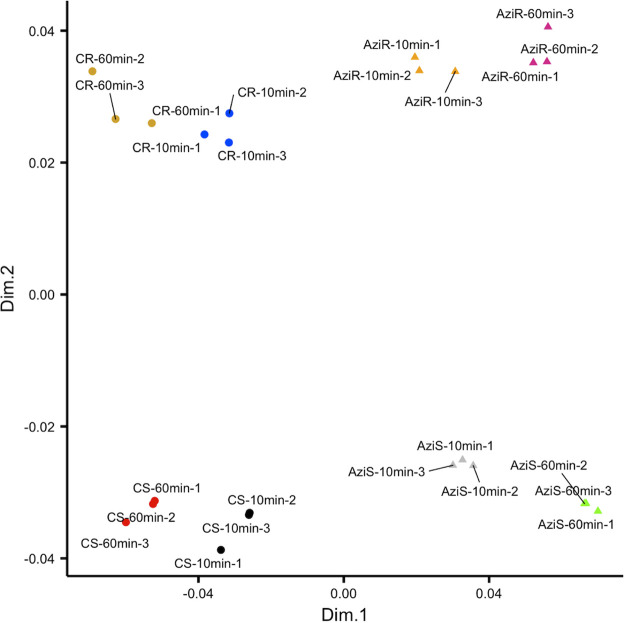
Multidimensional scaling analysis of the expression profile of the biological replicates, treatment conditions, and the two strains revealed strong reproducibility among replicates and dissimilarity between the conditions and strains (Fig. 2). MDS plot was also used to provide insights into the association between transcriptional profile and time of exposure (10 and 60 min) to azithromycin. Biological replicates of control and treated samples clustered very closely, indicating high correlation among replicates and high reproducibility of library preparation. The MDS plot demonstrated four distinct transcriptional clusters within the first two dimensions (dim1 and dim2), an indication of remarkable transcriptional changes upon antimicrobial exposure. Good separation of the control and treated samples in the first dimension showed that the sequencing data were qualified for identification of differentially expressed genes. Additionally, a significant diversity between the gene expressions of the 10-min- and 60-min-treated samples was observed, suggesting that distinct gene expression profiles are triggered by azithromycin in a time-dependent manner.
FIG 2.
MDS plot to display differences in the azithromycin-induced gene expression profile at 10 and 60 min. Dissimilarity in the expression profiles between the replicates, strains, and conditions was calculated and plotted. CS, control susceptible strain; AziS, azithromycin-treated susceptible strain; CR, control resistant strain; AziR, azithromycin-treated resistant strain.
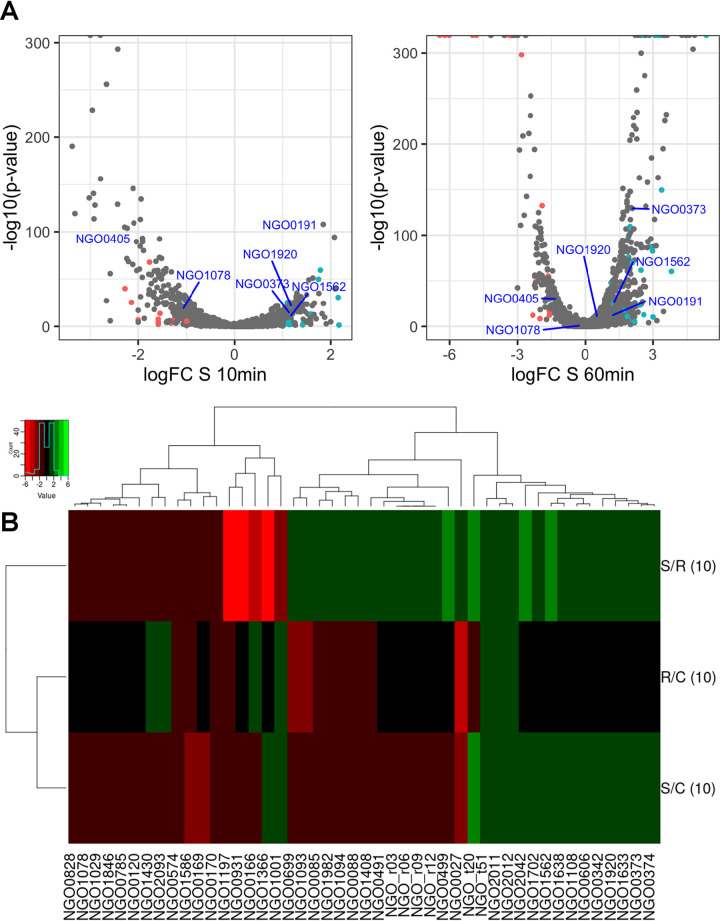
Subsequently, statistical analysis of differentially expressed genes following exposure to azithromycin was performed using logFC (gene expression ratio of treated compared to the control) against P values of <0.05 (Fig. 3A). Consistent with MDS results, a global shift in SvC and SvR was observed as early as 10 min of azithromycin exposure, although distribution of gene expression profile illustrated a higher magnitude of fold change at 60 min.
FIG 3.
(A) Volcano plot to show that the gene expression profile induced by azithromycin varied significantly at 10 and 60 min between the treated and untreated susceptible strains. Colored points indicate genes that are up- or downregulated in the SvC as well as SvR comparisons at each time point. (B) Heat map for differentially expressed genes (P value < 0.05, with an FC of ≥1) in SvC and SvR induced by 10 min of azithromycin exposure.
Marker selection and validation of candidate markers in N. gonorrhoeae clinical isolates.
Differential expression analyses of the susceptible strain revealed more down- than upregulated genes at 10 min (185:129). At 60 min, however, there were 386 and 212 genes up- and downregulated, respectively (Table S3). Since we are interested in markers that will successfully determine susceptibility upon a short antibiotic exposure, we further screened for genes that were differentially expressed between SvC and SvR by 10 min of azithromycin exposure. A P value cutoff of 0.05 resulted in 568 genes (Fig. S1), which was narrowed down to 48 when screening for abs(logFC) of ≥1 under both conditions (where abs indicates absolute value) (Fig. 3B).
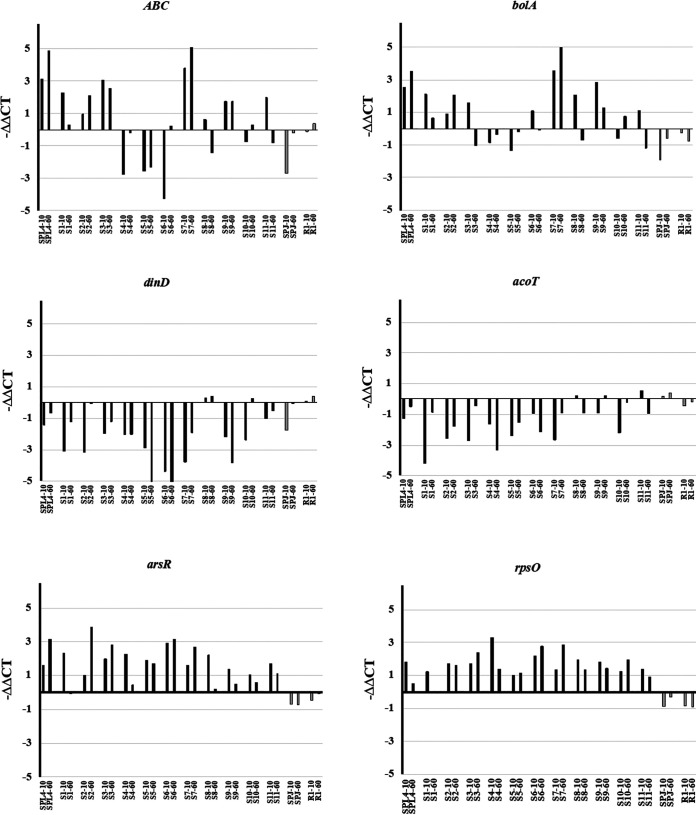
Among the differentially expressed genes with significant logFC, two more criteria, including high level of expression and same trend of expression at 10 and 60 min (see Materials and Methods), were applied to select the candidate markers. The initially selected markers include NGO0373, NGO1920, NGO1562, NGO0191, NGO0405, and NGO1078, here referred to as ABC, bolA, arsR, rpsO, dinD, and acoT, respectively (Table S2). These were tested in one susceptible and one resistant strain to confirm the diagnostic potential of markers to characterize azithromycin susceptibility using qRT-PCR. All six candidate RNA markers are highlighted in Table S4, among which ABC, bolA, arsR, and rpsO were upregulated while dinD and acoT were downregulated. Further validation of markers was conducted in 11 susceptible and 1 resistant clinical isolate of N. gonorrhoeae to confirm that RNA-seq-nominated markers can be applied in different strains (Fig. 4). We calculated −ΔΔCT values for samples following 10 min and 60 min of exposure to azithromycin and compared them between resistant and susceptible isolates for each marker. Overall, −ΔΔCT values of all six selected candidate markers were significantly different across resistant and susceptible isolates, indicating qualification of these candidate markers to be applied in our new AST platform. Among six tested markers, arsR and rpsO demonstrated consistent upregulation trends across all tested isolates. Although ABC and bolA were also validated as significantly upregulated RNA markers in most tested isolates, downregulation of these two markers was observed in some of the tested susceptible isolates, as shown in Fig. 4. On the other hand, dinD and acoT were validated as significantly downregulated RNA markers; however, inconsistency of results in some of the tested susceptible isolates was also found. According to the validation results of six selected markers, further analysis was conducted using arsR and rpsO as our final markers.
FIG 4.
Validation of 6 RNA markers across 14 N. gonorrhoeae isolates upon 10 min of azithromycin exposure using qRT-PCR. Six RNA markers were selected based on the RNA-seq results and then validated using qRT-PCR, including ABC, bolA, arsR, and rpsO, which were upregulated, and dinD and acoT, which were downregulated. Black, susceptible isolates (SPL4 and S1 to S11); gray, resistant isolates (SPJ and R1).
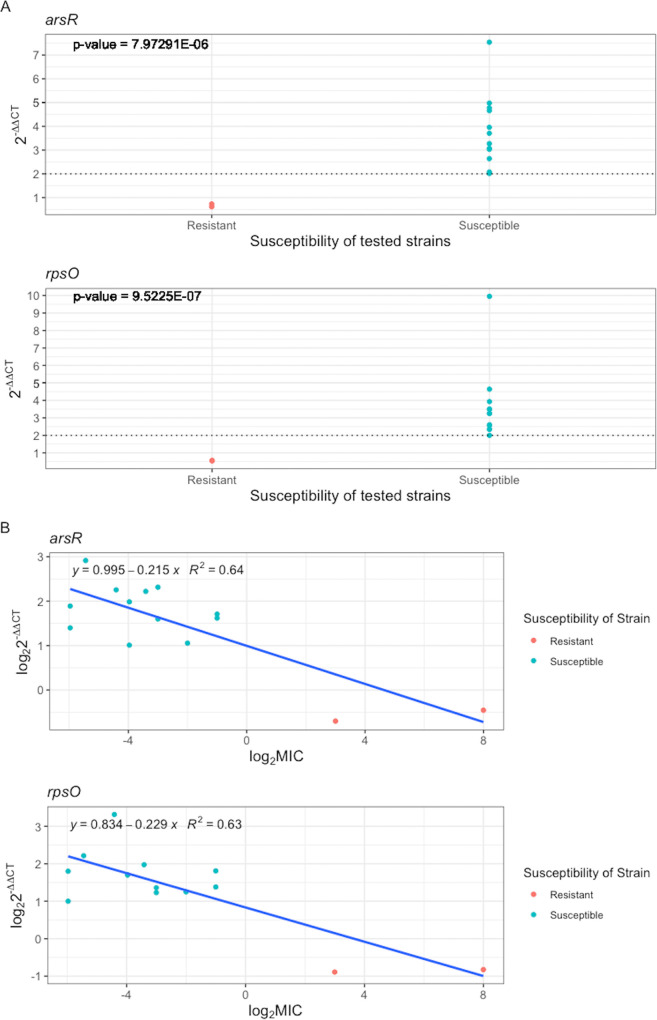
We examined the reliability of our method compared to the gold standard using the relationship between 2–ΔΔCT values of final markers and MIC in susceptible and resistant strains after 10 min of exposure to azithromycin (Fig. 5A). For both arsR and rpsO, 2–ΔΔCT values significantly changed between susceptible and resistant, and notably, no overlap in 2–ΔΔCT values was observed between two groups of susceptible and resistant strains. Additionally, a 2–ΔΔCT of 2 was defined as threshold susceptibility for susceptible and nonsusceptible isolates (2–ΔΔCT ≥ 2 for susceptible and 2–ΔΔCT < 2 for nonsusceptible). We also used a linear fitting to show how transcriptional responses of the final markers are associated with the MIC in susceptible and resistant strains. A strong negative relationship was observed between log2 2–ΔΔCT and log2 MIC values (R2 values, 0.64 and 0.63 for arsR and rpsO, respectively), meaning that the 2–ΔΔCT value in susceptible strains was significantly higher than that in resistant strains (Fig. 5B).
FIG 5.
(A) Determination of susceptibility threshold by correlation of MIC and our AST platform using arsR and rpsO across 14 tested N. gonorrhoeae isolates. (B) Linear fitting for arsR and rpsO to show how transcriptional response is associated with the MIC across 14 tested N. gonorrhoeae isolates (12 susceptible and 2 resistant).
Finally, to validate our susceptibility threshold, we used MIC information of two panels of N. gonorrhoeae, including 64 clinical isolates from the CDC (Antibiotic Resistance Isolate Bank) on the equation for the line of best fit for our markers. Notably, arsR supported the delineated 2–ΔΔCT value by correctly classifying susceptibility of all 64 CDC isolates based on our 2–ΔΔCT threshold as 51 susceptible strains and 13 resistant strains, which was in agreement with the CLSI susceptibility category (MIC breakpoint of ≥2 μg/ml) (Table S5). rpsO also accurately identified the susceptibility of most tested isolates with a broad range of MICs, although susceptible isolates with a MIC of 1 μg/ml were classified as nonsusceptible isolates; however, this category is in agreement with EUCAST breakpoint values (MIC breakpoint of ≥1 μg/ml).
In summary, these observations confirmed the accurate application of 2–ΔΔCT values to categorize susceptibility of isolates as a replacement of MIC in traditional AST.
DISCUSSION
To stem the tide of multidrug-resistant N. gonorrhoeae, a rapid NAAT-based diagnostic with compatible molecular phenotypic AST capability is critically needed to inform initial treatment decisions. In this study, we have discovered azithromycin susceptibility RNA markers and demonstrated their early promise for ultrarapid AST in N. gonorrhoeae.
Transcriptome profiling of susceptible and resistant N. gonorrhoeae upon exposure to azithromycin revealed a significantly altered response as early as 10 min in SvC and SvR (Fig. 2 and 3). While significantly different transcriptional responses among N. gonorrhoeae isolates were previously reported after 60 min of azithromycin exposure (31), our main objective was to discover the earliest susceptibility RNA markers to minimize AST time. Therefore, two time points, 10 min and 60 min, were included in azithromycin treatment to be able to identify consistent and reliable RNA markers as part of early cellular stress responses to antimicrobials. Unlike the previous reported studies in which sublethal concentrations of drugs were used (8, 31), we studied bacterial transcriptomic profile following a high concentration of azithromycin (2 μg/ml) to enhance the discovery of differentially expressed genes (20). Six candidate markers were initially identified through RNA-seq and subsequently tested in 14 isolates with qRT-PCR. arsR and rpsO were chosen as our two final markers, as they were able to consistently determine susceptibility based on CT value only after 10 min of antimicrobial exposure (Fig. 4).
The arsR gene (NGO1562) encodes a transcriptional regulatory protein that has been shown to respond to environmental stimuli, such as iron. Upregulation of arsR may also play a role in anaerobic growth of NG (32–34). Our study is the first to associate arsR with antimicrobial susceptibility. Transcript rpsO, encoding 30S ribosomal protein S15, also forms a bridge to the 50S subunit to contact the 23S rRNA (35). Upregulation of ribosomal protein-encoding transcripts such as rpsO upon antimicrobial exposure has been shown previously (31). Notably, previously reported RNA markers for N. gonorrhoeae upon antimicrobial exposure included a different panel of markers (except rpsO, which is in common in both studies), suggesting that drug concentration, antimicrobial exposure time, path of marker selection, and bioinformatic pipelines could result in different outcomes and should be considered in further application of this approach.
In order to use any novel AST platform in clinical decision making, it is necessary to compare it to the gold standard AST method, MIC, which has been in used for decades (36). However, current phenotypic assays still mistakenly classify some resistant strains as susceptible, resulting in failed clinical antimicrobial therapy (37). In this study, the measured changes in transcription level, 2–ΔΔCT, were correlated with MIC and translated to the susceptibility category, resulting in a 2–ΔΔCT threshold of ≥2 for susceptibility (Fig. 5). This threshold is further supported by the clustering of 64 susceptible and resistant CDC isolates with the 14 experimentally tested strains using their known MICs and calculated ΔΔCT values (Table S5). However, when isolates with a MIC of 1 μg/ml, categorized as susceptible based on CLSI breakpoint, were tested using rpsO linear fitting, they were categorized as nonsusceptible based on our 2–ΔΔCT threshold. Of note, this classification is in agreement with EUCAST susceptibility breakpoints, where an isolate with a MIC of ≥1 μg/ml is called resistant. This result highlights the difficulties underlying azithromycin susceptibility across N. gonorrhoeae isolates due to lack of a universal breakpoint interpretation in conventional AST, confirming the critical need for a new AST with a reliable susceptibility breakpoint (24).
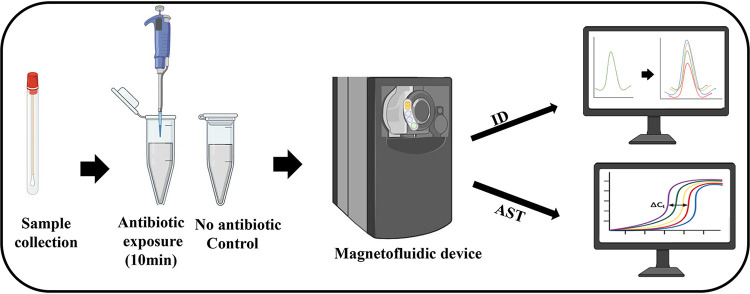
Overall, we have demonstrated the potential for antimicrobial susceptibility RNA marker discovery through combined application of NGS, bioinformatics, and validation assays. A larger-scale experimental validation of these putative RNA markers by testing additional N. gonorrhoeae strains with different mechanisms of resistance to azithromycin is still needed to better assess test accuracy and reproducibility. We envision translation of our ultrarapid susceptibility markers into an NAAT-based diagnostic with combined ID and AST capability for point-of-care use (Fig. 6). We have previously developed a palm-sized magnetofluidic platform with combined bacterial ID and AST directly from swab samples in less than 2.5 h, of which AST required 2 h of antimicrobial incubation to assess for bacterial doubling (38). Integrating our growth-independent, ultrarapid AST markers on this platform would compress total assay time to 40 min, drastically shifting the current paradigm for diagnosing and treating N. gonorrhoeae infections.
FIG 6.
Proposed workflow for combined ID and AST using novel molecular point-of-care systems such as a magnetofluidic device (38). Clinical samples are collected and exposed to antimicrobial for 10 min. Control and antimicrobial-treated samples are loaded into the magnetofluidic device for further ID and AST. This figure was created with https://biorender.com/ and PowerPoint.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for funding support from the National Institutes of Health (NIH R01AI137272 and R01AI138978).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Shin DJ, Andini N, Hsieh K, Yang S, Wang T-H. 2019. Emerging analytical techniques for rapid pathogen identification and susceptibility testing. Annu Rev Anal Chem (Palo Alto Calif) 12:41–67. doi: 10.1146/annurev-anchem-061318-115529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voter AF, Callaghan MM, Tippana R, Myong S, Dillard JP, Keck JL. 2020. Antigenic variation in Neisseria gonorrhoeae occurs independently of RecQ-mediated unwinding of the pilE G quadruplex. J Bacteriol 202:e00607-19. doi: 10.1128/JB.00607-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shigemura K, Osawa K, Miura M, Tanaka K, Arakawa S, Shirakawa T, Fujisawa M. 2015. Azithromycin resistance and its mechanism in Neisseria gonorrhoeae strains in Hyogo. Antimicrob Agents Chemother 59:2695–2699. doi: 10.1128/AAC.04320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett AM, Anderson CP, Zwank MD. 2012. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med 30:1114–1117. doi: 10.1016/j.ajem.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2000. Gonorrhea—United States, 1998. MMWR Morb Mortal Wkly Rep 49:538–542. [PubMed] [Google Scholar]
- 6.Pond MJ, Hall CL, Miari VF, Cole M, Laing KG, Jagatia H, Harding-Esch E, Monahan IM, Planche T, Hinds J, Ison CA, Chisholm S, Butcher PD, Sadiq ST. 2016. Accurate detection of Neisseria gonorrhoeae ciprofloxacin susceptibility directly from genital and extragenital clinical samples: towards genotype-guided antimicrobial therapy. J Antimicrob Chemother 71:897–902. doi: 10.1093/jac/dkv432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Shin DJ, Zheng S, Melendez JH, Gaydos CA, Wang T-H. 2018. Direct-qPCR assay for coupled identification and antimicrobial susceptibility testing of Neisseria gonorrhoeae. ACS Infect Dis 4:1377–1384. doi: 10.1021/acsinfecdis.8b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khazaei T, Barlow JT, Schoepp NG, Ismagilov RF. 2018. RNA markers enable phenotypic test of antibiotic susceptibility in Neisseria gonorrhoeae after 10 minutes of ciprofloxacin exposure. Sci Rep 8:11606. doi: 10.1038/s41598-018-29707-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarantonelli L, Borthagaray G, Lee EH, Veal W, Shafer WM. 2001. Decreased susceptibility to azithromycin and erythromycin mediated by a novel mtrR promoter mutation in Neisseria gonorrhoeae. J Antimicrob Chemother 47:651–654. doi: 10.1093/jac/47.5.651. [DOI] [PubMed] [Google Scholar]
- 10.Ma KC, Mortimer TD, Hicks AL, Wheeler NE, Sánchez-Busó L, Golparian D, Taiaroa G, Rubin DH, Wang Y, Williamson DA, Unemo M. 2020. Increased antibiotic susceptibility in Neisseria gonorrhoeae through adaptation to the cervical environment. bioRxiv 2020:896696. doi: 10.1101/2020.01.07.896696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinos GP, Michelinaki M, Kalpaxis DL. 2001. Insights into the mechanism of azithromycin interaction with an Escherichia coli functional ribosomal complex. Mol Pharmacol 59:1441–1445. doi: 10.1124/mol.59.6.1441. [DOI] [PubMed] [Google Scholar]
- 12.Zarantonelli L, Borthagaray G, Lee E-H, Shafer WM. 1999. Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations. Antimicrob Agents Chemother 43:2468–2472. doi: 10.1128/AAC.43.10.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, Morgan M, Newnham R, Golparian D, Unemo M, Crook DW, Peto TE, Hughes G, Cole MJ, Fifer H, Edwards A, Andersson MI. 2018. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England. Euro Surveill 23:1800323. doi: 10.2807/1560-7917.ES.2018.23.27.1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan C, Li Y, Le W-J, Liu Y-R, Li S, Wang B-X, Rice PA, Su X-H. 2018. Increasing resistance to azithromycin in Neisseria gonorrhoeae in eastern Chinese cities: resistance mechanisms and genetic diversity among isolates from Nanjing. Antimicrob Agents Chemother 62:e02499-17. doi: 10.1128/AAC.02499-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines. MMWR Recommend Rep 64:1–137. [Google Scholar]
- 16.Centers for Disease Control and Prevention. 2011. Neisseria gonorrhoeae with reduced susceptibility to azithromycin—San Diego County, California, 2009. MMWR Morb Mortal Wkly Rep 60:579–581. [PubMed] [Google Scholar]
- 17.Tobiason DM, Seifert HS. 2006. The obligate human pathogen, Neisseria gonorrhoeae, is polyploid. PLoS Biol 4:e185. doi: 10.1371/journal.pbio.0040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andini N, Hu A, Zhou L, Cogill S, Wang T-H, Wittwer CT, Yang S. 2018. A “culture” shift: broad bacterial detection, identification, and antimicrobial susceptibility testing directly from whole blood. Clin Chem 64:1453–1462. doi: 10.1373/clinchem.2018.290189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao YH, Qin XL, Yang JY, Liao YW, Wu XZ, Zheng HP. 2019. Identification and expression analysis of ceftriaxone resistance-related genes in Neisseria gonorrhoeae integrating RNA-Seq data and qRT-PCR validation. J Glob Antimicrob Resist 16:202–209. doi: 10.1016/j.jgar.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Hashemi MM, Andini N, Li MM, Kuang S, Carroll KC, Wang T-H, Yang S. 19 March 2020. RNA markers for ultra-rapid molecular antimicrobial susceptibility testing in fluoroquinolone-treated Klebsiella pneumoniae. J Antimicrob Chemother doi: 10.1093/jac/dkaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diallo K, Coulibaly MD, Rebbetts LS, Harrison OB, Lucidarme J, Gamougam K, Tekletsion YK, Bugri A, Toure A, Issaka B, Dieng M, Trotter C, Collard J-M, Sow SO, Wang X, Mayer LW, Borrow R, Greenwood BM, Maiden MCJ, Manigart O, MenAfriCar Consortium. 2018. Development of a PCR algorithm to detect and characterize Neisseria meningitidis carriage isolates in the African meningitis belt. PLoS One 13:e0206453. doi: 10.1371/journal.pone.0206453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wade JJ, Graver MA. 2007. A fully defined, clear and protein-free liquid medium permitting dense growth of Neisseria gonorrhoeae from very low inocula. FEMS Microbiol Lett 273:35–37. doi: 10.1111/j.1574-6968.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 23.Gose S, Kong CJ, Lee Y, Samuel MC, Bauer HM, Dixon P, Soge OO, Lei J, Pandori M. 2013. Comparison of Neisseria gonorrhoeae MICs obtained by Etest and agar dilution for ceftriaxone, cefpodoxime, cefixime and azithromycin. J Microbiol Methods 95:379–380. doi: 10.1016/j.mimet.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 24.McAuliffe GN, Smith M, Cooper G, Forster RF, Roberts SA. 2019. Variability in azithromycin susceptibility results for Neisseria gonorrhoeae obtained using gradient MIC strip and agar dilution techniques. J Clin Microbiol 57:e01353-19. doi: 10.1128/JCM.01353-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2020. M100 performance standards for antimicrobial susceptibility testing, 30th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Andrew S. 2010. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 27.Petersen KR, Streett DA, Gerritsen AT, Hunter SS, Settles ML. 2015. Super deduper, fast PCR duplicate detection in fastq files, p 491–492. In Proceedings of the 6th ACM Conference on Bioinformatics, Computational Biology and Health Informatics. Association for Computing Machinery, New York, NY. [Google Scholar]
- 28.Tjaden B. 2015. De novo assembly of bacterial transcriptomes from RNA-seq data. Genome Biol 16:1. doi: 10.1186/s13059-014-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 30.Applied Biosystems. 2004. Guide to performing relative quantitation of gene expression using real-time quantitative PCR. Applied Biosystems, Foster City, CA. [Google Scholar]
- 31.Wadsworth CB, Sater MR, Bhattacharyya RP, Grad YH. 2019. Impact of species diversity on the design of RNA-based diagnostics for antibiotic resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 63:e00549-19. doi: 10.1128/AAC.00549-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isabella VM, Clark VL. 2011. Deep sequencing-based analysis of the anaerobic stimulon in Neisseria gonorrhoeae. BMC Genomics 12:51. doi: 10.1186/1471-2164-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu C, McClure R, Nudel K, Daou N, Genco CA. 2016. Characterization of the Neisseria gonorrhoeae iron and fur regulatory network. J Bacteriol 198:2180–2191. doi: 10.1128/JB.00166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isabella V, Wright LF, Barth K, Spence JM, Grogan S, Genco CA, Clark VL. 2008. Cis- and trans-acting elements involved in regulation of norB (norZ), the gene encoding nitric oxide reductase in Neisseria gonorrhoeae. Microbiology (Reading) 154:226–239. doi: 10.1099/mic.0.2007/010470-0. [DOI] [PubMed] [Google Scholar]
- 35.Marzi S, Myasnikov AG, Serganov A, Ehresmann C, Romby P, Yusupov M, Klaholz BP. 2007. Structured mRNAs regulate translation initiation by binding to the platform of the ribosome. Cell 130:1019–1031. doi: 10.1016/j.cell.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Taylor TH, Pettus K, Trees D. 2014. Assessment of Etest as an alternative to agar dilution for antimicrobial susceptibility testing of Neisseria gonorrhoeae. J Clin Microbiol 52:1435–1440. doi: 10.1128/JCM.02131-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutgring JD, Limbago BM. 2016. The problem of carbapenemase-producing-carbapenem-resistant-Enterobacteriaceae detection. J Clin Microbiol 54:529–534. doi: 10.1128/JCM.02771-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee PW, Chen L, Trick AY, Athamanolap P, Chen FE, Wang TH. 2019. Palm-sized magnetofluidic platform for bacterial identification and antimicrobial susceptibility testing of infected wounds In 23rd International Conference on Miniaturized Systems for Chemistry and Life Sciences. Chemical and Biological Microsystems Society, San Diego, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.