CASE
A 46-year-old homeless man was transported to the emergency department (ED) after being found minimally responsive outside a public building. Upon evaluation in the ED, the patient was obtunded. He appeared to have sepsis with fever to 39.4°C and tachycardia to 168 bpm, but without hypotension. The patient had a full-thickness wound measuring 6 cm by 5 cm on the dorsal aspect of the distal left forearm with a pink wound bed and overlying purulent, yellowish exudate. The surrounding forearm was erythematous and edematous, with marked edema of the hand distal to the wound and decreased range of motion in the wrist and digits due to stiffness from swelling. There was no area of fluctuance, no bullae, no crepitus, and no exposed bone (Fig. 1). X ray of the extremity showed soft tissue swelling without bony erosion or subcutaneous gas.
FIG 1.

Wound on the dorsal left forearm.
The patient reported he injects heroin and methamphetamines intravenously, intramuscularly, and intradermally multiple times a day. He has a history of chronic untreated hepatitis C infection and reported the wound on his left dorsal forearm was chronic, having been present for over 10 years with associated chronic osteomyelitis of the underlying radius. The patient had declined deep/surgical culture on multiple occasions. Previous superficial swabs of the wound bed had grown methicillin-resistant Staphylococcus aureus, Streptococcus pyogenes, and diphtheroids.
At this encounter, exudative material in the wound bed was once again swabbed and sent for culture prior to starting empirical antibiotic therapy. No organisms or polymorphonuclear cells were observed on Gram stain of the exudate. Blood and urine cultures were negative for growth, and the suspected source of sepsis was superinfection of the chronic left upper extremity wound.
The patient was treated empirically with intravenous vancomycin and cefazolin, which he received for 48 h. He had a brisk clinical improvement with normalization of his mental status and marked decrease in edema and erythema of the left upper extremity. He was discharged with prescriptions for doxycycline and cephalexin to complete a planned 14-day course of therapy. Cultures of the exudate collected from the wound grew equal quantities of S. aureus, S. pyogenes, and a diphtheroid (Fig. 2). Previous studies have found an association between S. aureus, S. pyogenes, and the presence of Corynebacterium diphtheriae in wounds; therefore, our standard laboratory practice is to identify diphtheroids, of any quantity, from wound cultures mixed with S. aureus and S. pyogenes (1). The diphtheroid was identified as Corynebacterium diphtheriae by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Bruker MALDI Biotyper, BDAL library with 7,311 main spectra [MSP]). Additional testing performed at the Washington State Public Health Laboratories confirmed the identification using traditional biochemical methods, and the Centers for Disease Control and Prevention (CDC) determined this was a nontoxigenic strain. Isolate MICs determined by Epsilometer test (Etest) revealed susceptibility to vancomycin (0.5 μg/ml), tetracycline (0.25 μg/ml), and clindamycin (0.5 μg/ml) and intermediate susceptibility to ceftriaxone (2 μg/ml). No MIC result was reported for penicillin. These data were not available at the time of patient discharge. The patient could not recall his vaccination status, but Washington State vaccination records and electronic health records from 6 treating institutions showed no history of diphtheria toxoid vaccination during adulthood.
FIG 2.
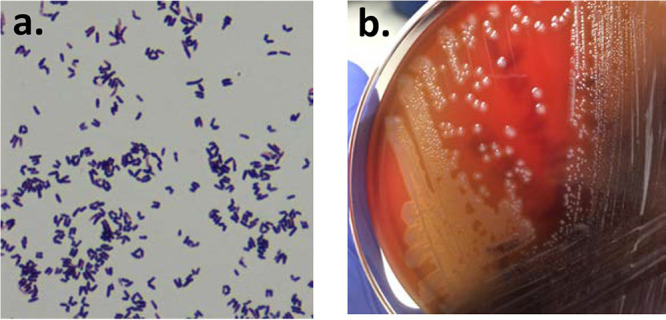
Gram stain and culture of the purified Corynebacterium diphtheriae isolate. (a) Gram stain revealed club-shaped Gram-positive rods, typical of Corynebacterium species. (b) Purified isolate producing a narrow zone of beta-hemolysis on sheep blood agar.
DISCUSSION
Diphtheria is a bacterial infection caused by Corynebacterium diphtheriae, a nonencapsulated, club-shaped Gram-positive rod. There are two classic forms of diphtheria: respiratory (nasopharyngeal) and cutaneous. Respiratory diphtheria is characterized by the development of an adherent pseudomembrane composed of fibrin, bacteria, and inflammatory cells in the upper respiratory tract. Respiratory diphtheria can be life-threatening. The pseudomembrane can cause difficulty swallowing and airway obstruction, and dissemination of the diphtheria toxin can cause necrosis at distant sites (myocarditis and neuritis) (2).
Both toxigenic and nontoxigenic C. diphtheriae can be found in circulation. Toxigenic strains harbor a lysogenic corynephage that carries the structural gene for diphtheria toxin. Diphtheria toxin inhibits eukaryotic protein synthesis, leading to cell death. Any biovar of C. diphtheriae (and rarely the related species Corynebacterium ulcerans and Corynebacterium pseudotuberculosis) can be lysogenized by the corynephage. Infections from nontoxigenic strains are generally less severe, but rarely, nontoxigenic strains may cause invasive disease (1). Cutaneous diphtheria may be caused by toxigenic or nontoxigenic strains. Infection appears as chronic, nonhealing sores or shallow ulcers and may develop in a preexisting wound (2). Frequently, cultures from these wounds are polymicrobial. The most commonly isolated organisms are Staphylococcus aureus and Streptococcus pyogenes, possibly due to shared risk factors for carriage of these organisms and acquisition of C. diphtheriae, such as living homeless and intravenous substance use disorders (1). In toxigenic cutaneous diphtheria, the ulcer may be covered by a blue-gray pseudomembrane, resembling the findings in nasopharyngeal disease (3). The prevalence of toxigenic diphtheria syndromes has dropped since the introduction of the diphtheria toxoid vaccine, widely implemented in the United States in the 1940s (4). However, reports increasingly describe colonization with nontoxigenic strains in healthy, asymptomatic people and cutaneous infection with nontoxigenic strains in patients with homelessness, poor hygiene, injection drug use, or immunocompromised states (1, 5). Seattle, this patient’s city of residence, is home to some of the largest nontoxigenic cutaneous diphtheria outbreaks in recent history. Three clusters totaling over 1,000 infections (86% of which were cutaneous) occurred in the 1970s and 1980s (5) and were attributed to poor hygiene, crowding, season, contaminated fomites, underlying skin disease, hyperendemic streptococcal skin infections, and homelessness. In April 2019, Seattle and King County issued a public health advisory describing a new cluster of 5 cases of nontoxigenic cutaneous diphtheria among persons experiencing homelessness (6).
On standard blood agar plates, C. diphtheriae colonies have a diphtheroid-like appearance and are not easily discerned from other white, round, weakly hemolytic to nonhemolytic colonies. On selective and differential cysteine tellurite agar plates, C. diphtheriae forms gray to black colonies surrounded by a dark halo. Using traditional biochemical tests, C. diphtheriae are catalase, nitrate, and cystinase positive but urease, pyraminidase, and CAMP test negative (3). The semiautomated API Coryne system (bioMérieux) and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) have also been used successfully to identify C. diphtheriae and C. ulcerans to the species level. The API Coryne system is limited by poor performance for certain Corynebacterium species and long processing time. MALDI-TOF MS has shown superior accuracy, with >98% accuracy in multiple studies of C. diphtheriae identification. Both systems show less accuracy with Corynebacterium tuberculostearicum. 16S rRNA gene sequencing has been less successful due to a high degree of similarity among certain Corynebacterium species, with one study showing only 81% accuracy for identification of C. diphtheriae (2).
Unlike nasopharyngeal infection, cutaneous diphtheria is not reported to the National Notifiable Diseases Surveillance System in the United States. However, clinical microbiology laboratories that isolate C. diphtheriae in culture from any site should notify their state health department. Clinical laboratories may be required to submit the isolate to state public health laboratories to confirm the organism identification. Once confirmed, the Diphtheria Laboratory, National Center for Immunization and Respiratory Diseases (NCIRD), CDC can determine if the strain is toxigenic. CDC recommends phenotypic testing using an immunoprecipitation assay, the Elek test. PCR for the toxin gene is available but is not considered a definitive test, as organisms may harbor nonfunctional toxin genes (3).
To test for toxin production using the Elek test, test isolates and controls are struck across an agar plate in straight lines (Fig. 3). A strip of filter paper containing diphtheria antitoxin is placed in the center of the agar plate, perpendicular to the test isolate streak lines. Antitoxin diffuses away from the strip of filter paper, and toxin produced by toxin-producing strains diffuses away from the line of growth. At the zone of equivalence, a precipitin line is formed. After 24 h of incubation at 37°C, the plate is examined with transmitted light for the presence of fine precipitin lines at a 45° angle to the streaks, indicating toxin production (3).
FIG 3.

Representation of the Elek test. Test isolates and controls are struck across an agar plate in straight lines. A strip of filter paper containing diphtheria antitoxin is placed in the center of the agar plate, perpendicular to the test isolate streak lines. Antitoxin diffuses away from the strip of filter paper, and toxin produced by toxin-producing strains diffuses away from the line of growth. At the zone of equivalence, a precipitin line is formed at a 45° angle to the streak of toxigenic C. diphtheriae.
In toxigenic C. diphtheriae infections (cutaneous or nasopharyngeal), infection control measures should be implemented to keep the patient in isolation. Contacts should be tested for nasopharyngeal colonization and offered postexposure prophylaxis with intramuscular benzathine penicillin or oral erythromycin. Unimmunized contacts should be vaccinated, and previously immunized contacts should receive a booster if it has been over 5 years.(4) In the laboratory, isolates of suspected C. diphtheriae should be handled in a safety cabinet during potential aerosol-generating procedures to minimize the risk of infection and subsequent transmission of a potentially toxigenic strain (3). Although infection prevention and safety measures must be undertaken when an isolate is first identified, they are intended to prevent the spread of toxigenic C. diphtheriae and are not needed for isolates that are determined to be nontoxigenic (4).
C. diphtheriae infections should be treated with penicillin or erythromycin for 14 days, and bacterial resistance to first-line therapy is rarely reported. The mainstay of treatment for toxigenic C. diphtheriae infections is rapid administration of antitoxin (4).
Consistent with previous reports of cutaneous diphtheria, our patient was both homeless and using injection drugs. Further investigation of diphtheroids in wound cultures also growing S. aureus and S. pyogenes helped lead to the diagnosis of cutaneous diphtheria in this case. The patient briefly received vancomycin and piperacillin-tazobactam and was discharged with therapies that are not considered first line for C. diphtheriae, although results of susceptibility testing suggested that doxycycline might be effective. Repeat cultures of his wound collected several months after this presentation did not isolate C. diphtheriae.
SELF-ASSESSMENT QUESTIONS
- What organisms have been found in mixed infections with C. diphtheriae (diphtheroids) in cases of cutaneous diphtheria?
-
a.Group B streptococcus and group A streptococcus
-
b.Gram-negative rods and S. aureus
-
c.S. aureus and group A streptococcus
-
d.S. epidermidis and Bacillus species
-
a.
- What statement best describes the Elek test?
-
a.A PCR-based test for the toxin subunit genes
-
b.An ELISA-based test measuring toxin antigen production
-
c.An immunoprecipitation test that detects toxin production
-
d.A test based on a pattern of sugar fermentation to identify C. diphtheriae
-
a.
- Vaccination programs targeting diphtheria have led to which of the following?
-
a.Decrease in toxigenic nasopharyngeal diphtheria
-
b.Decrease in nontoxigenic cutaneous diphtheria
-
c.Increase in diphtheria-associated myocarditis
-
d.Decrease in prevalence of circulating antibodies to diphtheria toxin
-
a.
Footnotes
For answers to the self-assessment questions and take-home points, see https://doi.org/10.1128/JCM.00507-20 in this issue.
REFERENCES
- 1.Lowe CF, Bernard KA, Romney MG. 2011. Cutaneous diphtheria in the urban poor population of Vancouver, British Columbia, Canada: a 10-year review. J Clin Microbiol 49:2664–2666. doi: 10.1128/JCM.00362-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajamani Sekar S, Veeraraghavan B, Anandan S, Devanga Ragupathi N, Sangal L, Joshi S. 2017. Strengthening the laboratory diagnosis of pathogenic Corynebacterium species in the vaccine era. Lett Appl Microbiol 65:354–365. doi: 10.1111/lam.12781. [DOI] [PubMed] [Google Scholar]
- 3.Efstratiou A, Engler KH, Mazurova IK, Glushkevich T, Vuopio-Varkila J, Popovic T. 2000. Current approaches to the laboratory diagnosis of diphtheria. J Infect Dis 181:S138–S145. doi: 10.1086/315552. [DOI] [PubMed] [Google Scholar]
- 4.Hamborsky J, Kroger A, Wolfe S (ed). 2015. Epidemiology and prevention of vaccine-preventable diseases: diphtheria, 13th ed Centers for Disease Control and Prevention, Washington, DC. [Google Scholar]
- 5.Harnisch JP, Tronca E, Nolan CM, Turck M, Holmes KK. 1989. Diphtheria among alcoholic urban adults. A decade of experience in Seattle. Ann Intern Med 111:71–82. doi: 10.7326/0003-4819-111-1-71. [DOI] [PubMed] [Google Scholar]
- 6.Seattle & King County Public Health. 15 April 2019. Cluster of non-toxigenic cutaneous diphtheria among persons experiencing homelessness. Healthcare advisories. Seattle & King County Public Health, Seattle, WA: https://kingcounty.gov/depts/health/communicable-diseases/health-care-providers/advisories/2019/15-april.aspx. Accessed 10 October 10 2019. [Google Scholar]


