Domestic arthropod-borne viruses (arboviruses) are single-stranded RNA viruses, the most common of which include the mosquito-borne West Nile virus, St. Louis encephalitis virus, La Crosse virus, Jamestown Canyon virus, and eastern equine encephalitis virus, as well as the tick-borne Powassan virus. Previously considered rare infections, they have been detected with increasing frequency over the past 2 decades. Here, we present an overview of the domestic arboviruses listed above and describe the modalities employed to diagnose infection.
KEYWORDS: eastern equine encephalitis virus, Jamestown Canyon virus, La Crosse virus, Powassan virus, St. Louis encephalitis virus, West Nile virus, arbovirus, encephalitis
ABSTRACT
Domestic arthropod-borne viruses (arboviruses) are single-stranded RNA viruses, the most common of which include the mosquito-borne West Nile virus, St. Louis encephalitis virus, La Crosse virus, Jamestown Canyon virus, and eastern equine encephalitis virus, as well as the tick-borne Powassan virus. Previously considered rare infections, they have been detected with increasing frequency over the past 2 decades. Here, we present an overview of the domestic arboviruses listed above and describe the modalities employed to diagnose infection. Global arboviruses, including dengue virus, Zika virus, and chikungunya virus, have also been increasingly detected in the United States within the last 5 years but are not a focus of this minireview. Typical manifestations of arbovirus infection range from no symptoms, to meningitis or encephalitis, to death. Serologies are the standard means of diagnosis in the laboratory, since most viruses have a short period of replication, limiting the utility of molecular tests. The interpretation of serologies is confounded by antibody cross-reactivity with viruses belonging to the same serogroup and by long-lasting antibodies from prior infections. Next-generation assays have improved performance by increasing antigen purity, selecting optimal epitopes, and improving interpretive algorithms, but challenges remain. Due to cross-reactivity, a positive first-line serology test requires confirmation by either a plaque reduction neutralization test or detection of seroconversion or a 4-fold rise in virus-specific IgM or IgG antibody titers from acute- and convalescent-phase sera. The use of molecular diagnostics, such as reverse transcription PCR or unbiased metagenomic sequencing, is limited to the minority of patients who present with ongoing viremia or central nervous system replication. With the continued expansion of vector range, the diagnosis of domestic arboviruses will become an increasingly important task for generalists and specialists alike.
INTRODUCTION
Arthropod-borne viruses (arboviruses) are increasingly frequent causes of disease in the United States. The most common domestic arboviruses, which will be reviewed here, include the mosquito-borne viruses West Nile virus (WNV), St. Louis encephalitis virus (SLEV), La Crosse virus (LACV), Jamestown Canyon virus (JCV), and eastern equine encephalitis virus (EEE) as well as the tick-borne Powassan virus (POWV). Many arboviruses are nationally notifiable and are tracked by the Centers for Disease Control and Prevention (CDC) through ArboNET, a passive surveillance system established in 2000 (1). In 2018, a total of 2,813 arboviral infections were reported in the United States, 64% of which were neuroinvasive and 6% of which were fatal (2). However, these figures likely underestimate incidence and overestimate severity, because clinical testing is more commonly performed for patients with severe or neuroinvasive disease. Arboviruses can be challenging to diagnose, because patients usually present with nonspecific symptoms and because diagnostic assays require the expertise of centralized laboratories. Therefore, it is important for clinicians to be aware of the arboviruses circulating in their region and understand how to order and interpret diagnostic tests for them.
Natural history.
Arboviruses that are endemic to the United States generally exist in enzootic cycles in which birds and small mammals serve as reservoirs, and there is occasional spillover into humans as incidental “dead end” hosts after transmission by a mosquito or tick. In contrast, the most common arboviruses worldwide, such as dengue and chikungunya, are vectored by anthropophilic Aedes species mosquitoes, and humans serve as amplification hosts that are essential for infecting new vectors and furthering the spread of disease.
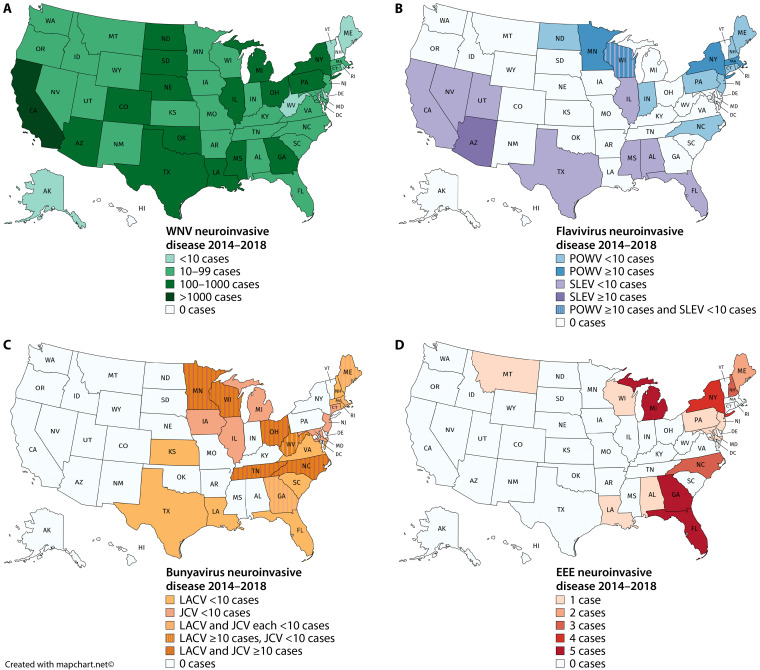
Vector activity drives seasonal patterns of arbovirus infections; more than 90% of cases are reported between April and September, and there is a negligible number between January and March (2). However, the specific seasonality differs slightly between viruses. For example, in 2018, similar to prior years, 92% of symptomatic WNV cases reported to ArboNET occurred between July and September, while POWV was frequently detected in the spring (April-June) and SLEV in the fall (October-December) (2). Some arboviruses have characteristic geographic distribution (Fig. 1), although overlapping distributions and patient travel may limit the usefulness of this information. Interestingly, many arbovirus infections show a male predominance; in 2018, 62% of WNV cases, 85% of JCV cases, and 67% of POWV cases were in men (2).
FIG 1.
Geographic distribution of the most common domestic arboviruses: WNV (A), other flaviviruses POWV and SLEV (B), bunyaviruses LACV and JCV (C), and alphavirus EEE (D). Maps reflect the total number of neuroinvasive cases reported from 2014 to 2018 (2, 89–92). (The maps were created with mapchart.net.)
Clinical manifestations.
Domestic arboviruses share several common clinical features. Following exposure to a mosquito or tick, patients experience an incubation period ranging from a few days to 2 weeks. Initial symptoms can include fever, gastrointestinal symptoms, and sometimes rash. Notably, unlike prevalent arboviruses worldwide (dengue virus and yellow fever virus), the arboviruses endemic to the United States do not cause hemorrhagic disease. However, a proportion of patients develop neurological disease, such as meningitis or encephalitis. In these patients, cerebrospinal fluid (CSF) parameters are generally similar to those of other viral infections of the central nervous system, with lymphocytic pleocytosis (generally less than 1,000 white blood cells/μl) and elevated protein (generally less than 200 mg/dl) (3, 4). Interestingly, patients with JCV may have normal CSF parameters, as described in 2 out of 6 patients in one case series (5) and 3 out of 9 in another (6).
Although the mortality rate is low for many arboviruses, patients with neuroinvasive disease can have persistent and debilitating symptoms for years (3–5). There are no human vaccines in use and no specific treatments for arboviral infections. Case reports have described improvement in a patient with JCV treated with ribavirin (7) and mixed results for patients with EEE treated with intravenous immunoglobulin (8, 9). High-dose steroids may be considered for patients with WNV, especially for treatment of postinfectious proinflammatory symptoms (10). Despite the lack of specific treatments, making a timely diagnosis of arboviral infection is critical to avoid unnecessary empirical treatments, provide diagnostic and prognostic information to patients and families, and encourage practices to minimize future vector exposure.
SPECIFIC ARBOVIRUSES ENDEMIC TO THE UNITED STATES
Arboviruses cross a wide range of virus families, the most common of which are flaviviruses (WNV, SLEV, and POWV) and bunyaviruses (LACV and JCV). Understanding which family a virus belongs to is important in interpreting serologic cross-reactivity, especially for viruses with overlapping geographic ranges.
Flaviviruses.
Flaviviruses are enveloped, positive-strand RNA viruses with approximately 10-kb genomes; they include the most common arboviruses worldwide, such as dengue virus, Zika virus, yellow fever virus, and Japanese encephalitis virus. Within the United States, West Nile virus (WNV) is responsible for the vast majority of arbovirus infections each year. WNV is transmitted primarily by Culex species mosquitoes from bird reservoirs. It was first detected in the United States in New York in 1999, led to a large number of cases, including spread to the Midwest in 2002 to 2003 (11), and is now responsible for approximately 2,000 reported infections per year. In addition to mosquito-borne transmission, WNV can also be transmitted by organ transplantation and blood transfusion (although donated blood now undergoes screening), and transplacental and laboratory-acquired infections have been reported (1).
WNV is distributed throughout the continental United States, with the largest number of cases reported in California (Fig. 1). It is estimated that 80% of WNV infections are asymptomatic (1, 12). When symptoms do arise, following an incubation period of up to 1 week, individuals can develop fever and, sometimes, rash. There is progression to neurological disease in 40 to 60% of symptomatic patients, and the incidence of neuroinvasive disease increases with age (1). Neurological syndromes include meningitis, encephalitis, and acute flaccid paralysis; notable features can include parkinsonism, tremors, and myoclonus, with compatible magnetic resonance imaging (MRI) findings of bilateral lesions in basal ganglia, thalamus, and pons (3).
St. Louis encephalitis virus (SLEV) is another flavivirus that is also transmitted by Culex species mosquitoes and is primarily found in the southern United States. Powassan virus (POWV) is a flavivirus that is primarily transmitted by Ixodes scapularis ticks in the northeast and north-central United States from small-mammal reservoirs, such as the white-footed mouse. In patients with POWV, MRI can show the involvement of the cerebellum, basal ganglia/thalami, and brainstem (4). Both SLEV and POWV have higher reported fatality rates than WNV (Table 1), but this is likely at least in part due to underdetection of less severe cases. SLEV seroprevalence has been reported between 1 and 13% in regions with known disease activity (13, 14). Estimates of POWV seroprevalence among patients with tick exposure range from 0.4% in Maine (15) to 9% in Wisconsin (16).
TABLE 1.
Epidemiological characteristics of specific arboviruses in the United States
| Virus | Family | Vector | No. of cases per yra | % Neuroinvasivea | % Fatala |
|---|---|---|---|---|---|
| WNV | Flaviviridae | Culex species mosquitoes | 2,175 | 63 | 6 |
| SLEV | Flaviviridae | Culex species mosquitoes | 10 | 62 | 9 |
| POWV | Flaviviridae | Ixodes species ticks | 21 | 95 | 14 |
| JCV | Bunyaviridae | Multiple mosquito species | 15 | 55 | 0 |
| LACV | Bunyaviridae | Aedes/Ochlerotatus triseriatus | 63 | 95 | 0 |
| EEE | Togaviridae | Multiple mosquito species | 6 | 100 | 40 |
Orthobunyaviruses.
Orthobunyaviruses are enveloped viruses with segmented, negative-sense RNA genomes approximately 12 kb in length. Most orthobunyaviruses transmitted in the United States belong to the California serogroup, and serologic diagnosis can be complicated by antibody cross-reactivity. La Crosse virus (LACV) stands out among domestic arboviruses because it primarily infects children; in 2018, 94% of LACV cases occurred in individuals under the age of 18 (2). LACV, which is commonly found in the Appalachian region, is transmitted by the aggressive daytime biting mosquito Aedes (Ochlerotatus) triseriatus from chipmunk and squirrel reservoir species. Clinical features that may point toward LACV include hyponatremia and periodic lateralizing discharges on electroencephalogram, similar to those found with herpes simplex virus (17).
Jamestown Canyon virus (JCV) is another California serogroup orthobunyavirus that is primarily found in adults. JCV can be transmitted by multiple mosquito species and has a widespread geographic distribution, with most cases in recent years occurring in the northeast and north-central United States. Both JCV and LACV have high rates of neuroinvasive disease but very low fatality rates (Table 1). As for other arboviruses, many infections are likely unrecognized: JCV seroprevalence has been reported between 15 and 30% (18, 19) and LACV seroprevalence up to 18%, with higher rates in populations with greater outdoor exposure (20).
Other California encephalitis serogroup viruses (e.g., California encephalitis virus and Snowshoe Hare virus) are rarely reported, with a total of 4 cases in 2018 (2). Keystone virus has been identified in Aedes species mosquitoes and in human serological surveys in the southeast, but the first acute case was not reported until 2019 in a patient with fever and papular rash (21). Cache Valley virus is a rarely detected bunyavirus that is not part of the California serogroup. A total of 5 cases were reported in the eastern United States between 1995 and 2018 (22); all reported cases were neuroinvasive, and 60% were fatal.
Alphaviruses.
Alphaviruses are enveloped, positive-sense RNA viruses that include multiple globally important arboviruses, such as chikungunya and Ross River virus, as well as eastern equine encephalitis virus (EEE) in the United States. EEE is generally maintained in an enzootic cycle between Culiseta melanura mosquitoes and birds and causes spillover infection in humans (and horses) when vectored by large-mammal-biting mosquito genera, such as Aedes and Culex. Although there are generally only a few cases of EEE reported each year, larger outbreaks periodically occur, likely due to environmental perturbations. As a recent example, there were a total of 36 cases and 14 deaths in 2019 (23). This outbreak was most prominent in the northeast and in Michigan; however, EEE is believed to circulate primarily in Florida and the southeastern United States, which serve as a reservoir for distribution to other locations (24). EEE transmission has also been reported from organ transplantation (25). Nearly all reported cases of EEE are neuroinvasive, and its mortality rate is generally 15 to 30%. However, as for other arboviruses, a large number of unrecognized infections of lower severity likely occur. EEE can be associated with elevated opening pressure on lumbar puncture (22), and MRI may show the involvement of the midbrain, thalamus, and basal ganglia (26), although these imaging findings can also be seen in WNV and POWV.
Other viruses, all tick-borne.
Colorado tick fever virus is a coltivirus that is transmitted by Dermacentor andersoni ticks, predominantly in the western United States at high elevations. There were 75 cases reported between 2002 and 2012, with one fatality (27). Clinically, Colorado tick fever virus causes nonspecific febrile symptoms that may include rash and often includes a biphasic fever. Heartland virus is a phlebovirus that is transmitted by Amblyomma americanum ticks in the midwestern and southern United States, with 40 reported cases (28). In addition to nonspecific febrile symptoms, patients can have leukopenia, thrombocytopenia, and liver function test elevation. Bourbon virus is a thogotovirus that has been detected in Amblyomma americanum ticks and a few human cases (28). It is found in the midwestern and southern United States, and symptoms can include maculopapular rash as well as thrombocytopenia and leukopenia.
Global arboviruses.
Dengue virus, Zika virus, and chikungunya virus are transmitted by Aedes species mosquitoes and are most frequently detected in returning travelers, but endemic cases do occur. Dengue virus is a flavivirus that causes periodic outbreaks in Hawaii (29) but has also been reported in the continental United States, with 16, 2, and 1 case in Florida, Texas, and North Carolina, respectively, in 2019 (30). Zika virus is another flavivirus that caused a reported ∼230 endemic cases in 2016 to 2017 (mostly in Florida) (31). Chikungunya is an alphavirus that was associated with 12 endemic cases in 2014 (in Florida) and 1 in 2015 (in Texas) (32). Recent reviews have focused on diagnostic approaches to dengue (33), Zika (34), and chikungunya (35).
DIAGNOSTIC APPROACHES
Due to nonspecific symptoms and overlapping geographic distributions, arbovirus infections must be diagnosed by laboratory studies. Multiple modalities are available, summarized below and in Table 2.
TABLE 2.
Characteristics of laboratory tests used to diagnose arbovirus infections
| Modality | Strength(s) | Weakness(es) | Sensitivity (%) | Specificity (%) | Reference(s) |
|---|---|---|---|---|---|
| Serology | |||||
| ELISA | |||||
| IgM capture | Decreased rates of false positivity from nonspecific antibody binding Decreased rates of false negativity from competition with preexisting IgG |
Cross-reactivity with other members of the same family Persistence of IgM for >1 yr in some cases |
CDC, 84 | 94 | 39, 62 |
| Commercial assays, 98–100a | 92–97 | 24 | |||
| Indirect IgG | Fourfold rise in titer identifies recent infection in the presence of preexisting IgM | Cross-reactivity with other members of the same family Long-lasting IgG from prior infections |
CDC, 84 | 39 | |
| LDT,c 40 | 94 | 58 | |||
| Commercial, 100a | 83 | 42 | |||
| IgG avidity assay | Allows for differentiation of recent from past infection | No commercially available tests | |||
| PRNT | Quantifies neutralizing antibodies | Requires highly trained staff | |||
| Highly specific | Requires biosafety level 3 conditions | ||||
| Unaffected by history of prior infections | Slow turnaround time | ||||
| IFA | As sensitive as MAC ELISA | Subjective interpretation | IgM, 96–100b | 66–100 | 50, 66 |
| IgG, 92–100b | 42–90 | 42, 57 | |||
| MIA | Can be highly multiplexed | Not commercially available | 98 | 96 | 42, 57 |
| IHC | Useful for confirmatory diagnosis when tissue is available | Limited data on sensitivity and specificity | |||
| Molecular diagnostics | |||||
| qRT-PCR | High analytic sensitivity and specificity Rapid turnaround time High throughput |
Limited by short time period of viral replication | Serum, 14 | 100 | 70 |
| Whole blood, 87b | 100 | 71 | |||
| CSF, 57 | 100 | 70 | |||
| Urine, 44 | 100 | 72 | |||
| mNGS | No prior hypothesis on the target pathogen necessary | Low throughput | |||
| Limited by short time period of viral replication |
Relative to the CDC ELISA.
Relative to commercial or laboratory-developed ELISA.
LDT, laboratory-developed test.
General approach.
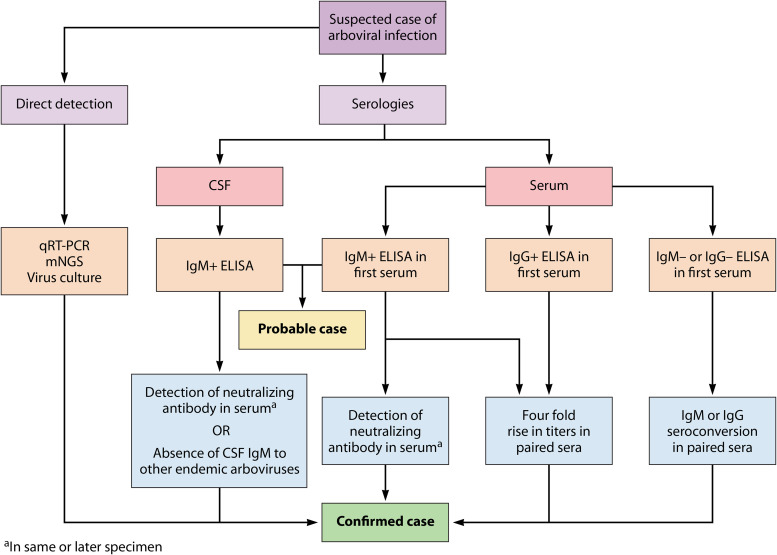
The CDC laboratory criteria for arboviral infection require direct detection of virus by culture, antigen, or nucleic acid; a 4-fold rise in antibody titers between acute- and convalescent-phase sera; or virus-specific IgM coupled with the presence of neutralizing antibodies (36). Neuroinvasive disease can also be diagnosed by the presence of IgM in CSF and the absence of IgM specific to other endemic viruses (Fig. 2). Testing should only be performed in patients with a compatible clinical presentation and is typically performed at commercial or governmental reference laboratories. The choice of test should be informed by the time from symptom onset. Direct detection is feasible in the minority of patients who present early enough to have ongoing viremia, but in most cases the diagnosis is made with serologies, as patients usually present outside this period. Positive IgM establishes a probable diagnosis and is often sufficient to stop diagnostic testing for other etiologies in a patient with compatible presentation and no history of arboviral infection. However, confirmation of infection for clinical diagnosis or reporting purposes requires identifying virus-specific neutralizing antibodies or testing convalescent-phase sera.
FIG 2.
Pathways to diagnose probable and confirmed arbovirus infection. For patients with suspected active viral replication, the direct detection of pathogen nucleic acid in serum or affected tissue or growth of virus in culture confirms diagnosis. Most patients present after the period of viremia, thereby requiring the use of serologies. A positive IgM ELISA from serum or CSF establishes a probable diagnosis. Confirmation of disease requires detection of serum-neutralizing antibodies, seroconversion, or a 4-fold increase in either IgM or IgG titers from paired sera. Neuroinvasive disease can be confirmed by the same methods or by negative CSF IgM ELISA testing against other endemic arboviruses that circulate in the same geographic area.
Below, we focus on tests for WNV, as it has the most developed set of diagnostic tools among the domestic arboviruses. Additional insights from other viruses are provided when substantially different from WNV. The following discussion does not apply to molecular tests that have been optimized for the screening of blood bank products and organ donors, as these are not meant for use in patients with high clinical suspicion of acute infection.
Serologic testing: first line.
The presence of IgM in serum or CSF is the primary laboratory criteria by which clinicians diagnose acute or recent arboviral infection. IgM for WNV appears a median of 4 to 10 days after the onset of symptoms (37) and typically lasts for 1 to 3 months. Thus, a negative IgM in a patient with high suspicion should be followed by a second sample obtained after day 5 and before day 15 postonset of illness. The presence of seroconversion confirms infection. For other arboviruses, such as EEE, SLE, and LACV, IgM is almost always present on the day of the onset of illness (38). For neuroinvasive disease, IgM in CSF can appear even earlier than in the serum (39) and indicates intrathecal synthesis, but a definitive diagnosis requires negative IgM to other viruses endemic to the geographic region where infection was thought to occur (36).
The most common first-line assay is the IgM antibody capture enzyme-linked immunosorbent assay (MAC ELISA), which uses an anti-IgM antibody to specifically isolate IgM-class antibodies from the other isotypes and has been used for diagnosis since the 1990s. Compared to standard ELISAs, the MAC ELISA reduces competition from IgG and also reduces the risk of nonspecific antibody binding, improving both sensitivity and specificity (40). The CDC-developed MAC ELISA has an estimated sensitivity of 84% relative to WNV clinical case definitions (39) and a specificity of 94% when assessed against Japanese encephalitis complex viruses (41). Commercial MAC ELISAs have a sensitivity of >95% relative to the CDC assay (42, 43).
A newer generation of serologic tests using microsphere immunoassays (MIAs) coated with recombinant viral antigens provides accurate results with a short turnaround time while also being well suited for multiplexing (44–46). The latter is a significant benefit, given that overlapping clinical presentations and geography necessitates testing of multiple arboviruses in parallel. A large validation study of a 13-antigen multiplexed IgM and IgG MIA platform using 647 serum and CSF samples found an error rate of <5% relative to gold standard neutralization assays (46).
One challenge in the diagnosis of acute arbovirus infection is the persistence of IgM, which can last for up to 500 days in some patients with WNV (47). Thus, it is critical to simultaneously check IgG, which is expected to be negative in acute infection. The standard test for IgG is the indirect ELISA, which uses secondary antibodies to the patient’s own virus-specific antibodies. In some cases, IgG avidity assays may help in determining the timeline of infection by differentiating lower-affinity IgG generated in response to a recent infection from higher-affinity IgG targeting strains from an older infection (48), although this is not routinely performed in clinical practice.
Serologic testing: confirmation.
Another primary challenge with interpreting serologic tests for acute arbovirus infection is a high rate of false positivity due to cross-reactivity (49–53). Historically, the source of antigen in the assay represented a source of false positivity. First-generation ELISAs utilized antigens derived from virus-inoculated suckling mouse brain preparations or supernatants from infected cell cultures (54). These preparations contained a mixture of multiple viral antigens, making them highly sensitive but prone to cross-reaction. Later generation assays improved on this by using bacterial or eukaryotic expression vectors to produce large quantities of pure recombinant envelope (E) protein domains (55–58) or noninfectious virus-like particles containing the premembrane/membrane and E proteins (58–60). These strategies allowed for better standardization of assays, safer laboratory handling, reduced costs, and improved test specificity while still maintaining an analytic sensitivity comparable to that of earlier versions of tests. Further modifications to protocols and analytic methods for normalizing against the background rate of cross-reactivity led to additional improvements in specificity (40, 61, 62); however, cross-reactivity with related viruses remains a challenge, necessitating confirmatory testing.
The gold standard confirmatory assay is the plaque reduction neutralization test (PRNT). This method quantifies the degree to which cellular infection is inhibited by neutralizing antibodies present in serum or CSF. Results provide a direct estimate of the quantity of functional antibodies and are valuable not only for confirming recent infection but also for vaccine development. Different thresholds for positivity are used depending on the intended purpose. When used to diagnose active infection, the threshold is usually set at 90% plaque reduction (PRNT90) to optimize test specificity. Conversely, for vaccine studies where the intent is to detect low-level neutralizing titers from attenuated vaccine strains, the threshold is set at a reduction in plaque formation by 50% (PRNT50), which optimizes analytic sensitivity. The use of PRNT is restricted to reference laboratories, because it is a complex test that requires skilled staff who have experience handling live virus under biosafety level 3 conditions. It also has a slow turnaround time, ranging from 4 to 10 days to observe a cytopathic effect. Despite these drawbacks, PRNT remains the gold standard for diagnosis, because it is able to resolve ambiguity in settings where cross-reactivity and persistence of antibodies from prior infections are suspected or when it is not possible to obtain a convalescent-phase specimen (46). For example, PRNT is essential to confirm infection for JCV and LACV, which are both bunyaviruses with up to 20% serological cross-reactivity (63) and overlapping geographic regions (Fig. 1); in fact, it is estimated that much of the historically reported LACV infection may instead have been due to JCV (64). In addition, PRNT is needed to distinguish between patients with flaviviruses WNV, POWV, and dengue and patients with prior vaccination against yellow fever or Japanese encephalitis virus (65).
Alternatives to the PRNT include direct detection (described below) or the use of convalescent-phase samples, in which a 4-fold rise in virus-specific IgM or IgG titers above baseline is necessary to diagnose new infection (54).
Serologic testing: alternatives.
Immunofluorescence assays (IFAs) are an alternative approach that use fixed cells infected with a panel of arboviruses spotted onto a slide. Patient serum is added to wells, followed by fluorescently labeled anti-IgM or anti-IgG. The presence of bright spots against the counterstained background indicates the presence of virus-specific antibodies. IFAs are not widely used due to the subjectivity in interpreting fluorescence, but laboratory-developed and commercial assays for IgM have been shown to have performance that matches or exceeds that of MAC ELISA (66). A commercial two-tiered (ELISA and IFA) assay for POWV has recently been described (67).
Direct detection: PCR.
In some cases, serologic analyses may be negative due to blunted antibody responses in patients with intrinsic or acquired immunosuppression (68). Nucleic acid amplification assays may be useful in this setting as well as in patients suspected of having ongoing viral replication. Quantitative real-time reverse transcriptase PCR (qRT-PCR) assays have high analytic sensitivity and specificity and a broad dynamic range. When coupled with automated RNA extraction kits, they can be converted into high-throughput assays with rapid turnaround (69). qRT-PCR assays have been designed to identify the common lineages of WNV (I and II), with most targeting the nonstructural, envelope, and capsid genes or the 5′ untranslated region (UTR). Despite these benefits, the use of qRT-PCR in practice is limited because viremia is low-level and transient. Only 57% of CSF and 14% of serum samples from patients with neuroinvasive WNV in New York City during the 1999 epidemic had nucleic acid detected by qRT-PCR (70). WNV nucleic acid detection may be higher using whole blood (71) or urine (72); however, neither is routinely used in clinical practice. qRT-PCR tests for POWV (73, 74), EEE (75), LACV (76), JCV (77, 78), and SLE (79) have been described in the literature and primarily applied to vector samples or human brain tissue; there is insufficient data to estimate their clinical performance.
Direct detection: other.
There is substantial interest in the development of point-of-care molecular tests for some arboviral infections, such as a recently described CRISPR-Cas13 assay coupled with a simple heat and chemical inactivation step, which allowed for sensitive and specific detection for Zika and dengue viruses in <2 h using minimal equipment (80). The utility of such assays is debatable for WNV and the other domestic arboviruses due to the shorter period of viremia. Nevertheless, they show promise in overcoming some of the analytic and logistical barriers for global arbovirus diagnosis.
Unbiased metagenomic next-generation sequencing (mNGS) represents the last line of molecular testing for arbovirus infection and is typically reserved for instances in which all attempts using targeted diagnostics have failed to identify a pathogen. Examples of the successful use of mNGS include the diagnosis of neuroinvasive WNV (81), SLEV (82), and POWV (83) infections. In most of these cases, serologies were falsely negative due to blunted humoral responses in the setting of iatrogenic immunosuppression. In addition, mNGS can be crucial for identifying unexpected arboviruses; the first case of acute infection with Keystone virus in the United States was diagnosed by mNGS from a urine sample (21). Recently, attempts have been made to enhance the sensitivity of mNGS through use of enrichment methods such as hybrid capture (84) and spiked primer enrichment (85) for predefined pathogen subsets, including arboviruses. While labor-intensive and expensive, these methods offer a lifeline to patients, families, and clinicians seeking a microbiologic diagnosis where traditional methods have fallen short.
Viral cultures are an alternative to molecular methods for direct detection and are generally performed in mammalian and mosquito cell lines, such as Vero E6, BHK, and C6/36 cells (86). Similarly to PRNT, all procedures must be performed under biosafety level 3 conditions, and it takes approximately 2 days to observe a cytopathic effect for EEE, 3 days for WNV, and 5 days for SLEV. Antigen tests for WNV, such as the VecTest (87) or RAMP assay (88), generally are reserved for surveillance of avian or mosquito populations. Although less sensitive than molecular methods, immunohistochemistry (IHC) can play an adjunct role in neuroinvasive disease when other testing modalities are negative but suspicion remains high. However, it is rarely used, as it requires a brain biopsy for tissue or is performed postmortem. Relative to serologic or molecular assays, the role of viral culture and tissue-based diagnostics for arbovirus infection is limited.
CONCLUSIONS
Infections with mosquito- and tick-borne viruses represent an increasingly recognized source of morbidity in the United States, and new arboviruses are being identified on a regular basis. Clinical presentations are nonspecific, often including fever and neurological symptoms that can be difficult to distinguish from other infectious and inflammatory processes. Thus, the understanding and appropriate use of laboratory diagnostic tests are essential to identify infection in individual patients and to evaluate nationwide patterns of disease. In most cases, arboviruses have limited duration of replication in blood and CSF, making serologies the mainstay of laboratory diagnosis. However, molecular testing should be considered for immunocompromised patients, especially those with impaired B-cell responses who may have falsely negative serology. Given the complexities in diagnosing these infections and the fact that many patients likely have unrecognized infection, patients should be reminded about the importance of preventative measures for tick and mosquito avoidance, primarily wearing long-sleeved clothing and using Environmental Protection Agency-registered mosquito repellents.
REFERENCES
- 1.Lindsey NP, Staples JE, Lehman JA, Fischer M, Centers for Disease Control and Prevention (CDC). 2010. Surveillance for human West Nile virus disease—United States, 1999–2008. MMWR Surveill Summ 59:1–17. [PubMed] [Google Scholar]
- 2.McDonald E, Martin SW, Landry K, Gould CV, Lehman J, Fischer M, Lindsey NP. 2019. West Nile virus and other domestic nationally notifiable arboviral diseases—United States, 2018. MMWR Morb Mortal Wkly Rep 68:673–678. doi: 10.15585/mmwr.mm6831a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, Fleischauer A, Leis AA, Stokic DS, Petersen LR. 2003. Neurologic manifestations and outcome of West Nile virus infection. JAMA 290:511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 4.Piantadosi A, Rubin DB, McQuillen DP, Hsu L, Lederer PA, Ashbaugh CD, Duffalo C, Duncan R, Thon J, Bhattacharyya S, Basgoz N, Feske SK, Lyons JL. 2016. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 62:707–713. doi: 10.1093/cid/civ1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinsella CM, Paras ML, Smole S, Mehta S, Ganesh V, Chen LH, McQuillen DP, Shah R, Chan J, Osborne M, Hennigan S, Halpern-Smith F, Brown CM, Sabeti P, Piantadosi A. 2020. Jamestown Canyon virus in Massachusetts: clinical case series and vector screening. Emerg Microbes Infect 9:903–912. doi: 10.1080/22221751.2020.1756697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matkovic E, Hoang Johnson DK, Staples JE, Mora-Pinzon MC, Elbadawi LI, Osborn RA, Warshauer DM, Wegner MV, Davis JP. 2019. Enhanced arboviral surveillance to increase detection of Jamestown Canyon virus infections, Wisconsin, 2011–2016. Am J Trop Med Hyg 100:445–451. doi: 10.4269/ajtmh.18-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savard M, Paradis A, Francoeur CL. 2018. Jamestown Canyon encephalitis with NORSE and electrographic response to ribavirin: a case report. Epilepsia Open 3:286–289. doi: 10.1002/epi4.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukerji SS, Lam AD, Wilson MR. 2016. Eastern equine encephalitis treated with intravenous immunoglobulins. Neurohospitalist 6:29–31. doi: 10.1177/1941874415578533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon IH, Ciarlini P, Santagata S, Ahmed AA, De Girolami U, Prasad S, Mukerji SS. 2017. Fatal eastern equine encephalitis in a patient on maintenance rituximab: a case report. Open Forum Infect Dis 4:ofx021. doi: 10.1093/ofid/ofx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai F, Thompson EA, Vig PJS, Leis AA. 2019. Current understanding of West Nile virus clinical manifestations, immune responses, neuroinvasion, and immunotherapeutic implications. Pathogens 8:193. doi: 10.3390/pathogens8040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarner J, Hale GL. 2019. Four human diseases with significant public health impact caused by mosquito-borne flaviviruses: West Nile, Zika, dengue and yellow fever. Semin Diagn Pathol 36:170–176. doi: 10.1053/j.semdp.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. 2001. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet 358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 13.Meehan PJ, Wells DL, Paul W, Buff E, Lewis A, Muth D, Hopkins R, Karabatsos N, Tsai TF. 2000. Epidemiological features of and public health response to a St. Louis encephalitis epidemic in Florida, 1990-1. Epidemiol Infect 125:181–188. doi: 10.1017/s0950268899004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisen WK, Chiles RE. 1997. Prevalence of antibodies to western equine encephalomyelitis and St. Louis encephalitis viruses in residents of California exposed to sporadic and consistent enzootic transmission. Am J Trop Med Hyg 57:526–529. doi: 10.4269/ajtmh.1997.57.526. [DOI] [PubMed] [Google Scholar]
- 15.Smith RP Jr, Elias SP, Cavanaugh CE, Lubelczyk CB, Lacombe EH, Brancato J, Doyle H, Rand PW, Ebel GD, Krause PJ. 2019. Seroprevalence of Borrelia burgdorferi, B. miyamotoi, and Powassan Virus in residents bitten by Ixodes ticks, Maine, USA. Emerg Infect Dis 25:804–807. doi: 10.3201/eid2504.180202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost HM, Schotthoefer AM, Thomm AM, Dupuis AP Jr, Kehl SC, Kramer LD, Fritsche TR, Harrington YA, Knox KK. 2017. Serologic evidence of Powassan virus infection in patients with suspected Lyme disease. Emerg Infect Dis 23:1384–1388. doi: 10.3201/eid2308.161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McJunkin JE, de los Reyes EC, Irazuzta JE, Caceres MJ, Khan RR, Minnich LL, Fu KD, Lovett GD, Tsai T, Thompson A. 2001. La Crosse encephalitis in children. N Engl J Med 344:801–807. doi: 10.1056/NEJM200103153441103. [DOI] [PubMed] [Google Scholar]
- 18.Mayo D, Karabatsos N, Scarano FJ, Brennan T, Buck D, Fiorentino T, Mennone J, Tran S. 2001. Jamestown Canyon virus: seroprevalence in Connecticut. Emerg Infect Dis 7:911–912. doi: 10.3201/eid0705.017529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimstad PR, Calisher CH, Harroff RN, Wentworth BB. 1986. Jamestown Canyon virus (California serogroup) is the etiologic agent of widespread infection in Michigan humans. Am J Trop Med Hyg 35:376–386. doi: 10.4269/ajtmh.1986.35.376. [DOI] [PubMed] [Google Scholar]
- 20.Harding S, Greig J, Mascarenhas M, Young I, Waddell LA. 2018. La Crosse virus: a scoping review of the global evidence. Epidemiol Infect 147:1–13. doi: 10.1017/S0950268818003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lednicky JA, White SK, Stephenson CJ, Cherabuddi K, Loeb JC, Moussatche N, Lednicky A, Morris JG Jr. 2019. Keystone virus isolated from a Florida teenager with rash and subjective fever: another endemic arbovirus in the southeastern United States? Clin Infect Dis 68:143–145. doi: 10.1093/cid/ciy485. [DOI] [PubMed] [Google Scholar]
- 22.Gill CM, Beckham JD, Piquet AL, Tyler KL, Pastula DM. 2019. Five emerging neuroinvasive arboviral diseases: Cache Valley, eastern equine encephalitis, Jamestown Canyon, Powassan, and Usutu. Semin Neurol 39:419–427. doi: 10.1055/s-0039-1687839. [DOI] [PubMed] [Google Scholar]
- 23.Morens DM, Folkers GK, Fauci AS. 2019. Eastern equine encephalitis virus—another emergent arbovirus in the United States. N Engl J Med 381:1989–1992. doi: 10.1056/NEJMp1914328. [DOI] [PubMed] [Google Scholar]
- 24.Tan Y, Lam TT, Heberlein-Larson LA, Smole SC, Auguste AJ, Hennigan S, Halpin RA, Fedorova N, Puri V, Stockwell TB, Shilts MH, Andreadis T, Armstrong PM, Tesh RB, Weaver SC, Unnasch TR, Ciota AT, Kramer LD, Das SR. 2018. Large-scale complete-genome sequencing and phylodynamic analysis of eastern equine encephalitis virus reveals source-sink transmission dynamics in the United States. J Virol 92:e00074-18. doi: 10.1128/JVI.00074-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pouch SM, Katugaha SB, Shieh WJ, Annambhotla P, Walker WL, Basavaraju SV, Jones J, Huynh T, Reagan-Steiner S, Bhatnagar J, Grimm K, Stramer SL, Gabel J, Lyon GM, Mehta AK, Kandiah P, Neujahr DC, Javidfar J, Subramanian RM, Parekh SM, Shah P, Cooper L, Psotka MA, Radcliffe R, Williams C, Zaki SR, Staples JE, Fischer M, Panella AJ, Lanciotti RS, Laven JJ, Kosoy O, Rabe IB, Gould CV, Eastern Equine Encephalitis Virus Transplant Transmission Investigation Team. 2019. Transmission of eastern equine encephalitis virus from an organ donor to 3 transplant recipients. Clin Infect Dis 69:450–458. doi: 10.1093/cid/ciy923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. 1997. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med 336:1867–1874. doi: 10.1056/NEJM199706263362604. [DOI] [PubMed] [Google Scholar]
- 27.Yendell SJ, Fischer M, Staples JE. 2015. Colorado tick fever in the United States, 2002–2012. Vector Borne Zoonotic Dis 15:311–316. doi: 10.1089/vbz.2014.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodino KG, Theel ES, Pritt BS. 2020. Tick-borne diseases in the United States. Clin Chem 66:537–548. doi: 10.1093/clinchem/hvaa040. [DOI] [PubMed] [Google Scholar]
- 29.Lew RJ, Tsai WY, Wang WK. 2018. Dengue outbreaks in Hawai'i after WWII—a review of public health response and scientific literature. Hawaii J Med Public Health 77:315–318. [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. 2020. Dengue statistics and maps. https://www.cdc.gov/dengue/statistics-maps/. Accessed 2 July 2020.
- 31.Centers for Disease Control and Prevention. 2020. Zika virus statistics and maps. https://www.cdc.gov/zika/reporting. Accessed 2 July 2020.
- 32.Centers for Disease Control and Prevention. 2020. Chikungunya virus geographic distribution. https://www.cdc.gov/chikungunya/geo. Accessed 2 July 2020.
- 33.Muller DA, Depelsenaire AC, Young PR. 2017. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis 215:S89–S95. doi: 10.1093/infdis/jiw649. [DOI] [PubMed] [Google Scholar]
- 34.Waggoner JJ, Pinsky BA. 2016. Zika virus: diagnostics for an emerging pandemic threat. J Clin Microbiol 54:860–867. doi: 10.1128/JCM.00279-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Natrajan MS, Rojas A, Waggoner JJ. 2019. Beyond fever and pain: diagnostic methods for chikungunya virus. J Clin Microbiol 57:e00350-19. doi: 10.1128/JCM.00350-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. 2015. Arboviral diseases, neuroinvasive and non-neuroinvasive 2015 case definition. https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/. Accessed 26 May 2020.
- 37.Busch MP, Kleinman SH, Tobler LH, Kamel HT, Norris PJ, Walsh I, Matud JL, Prince HE, Lanciotti RS, Wright DJ, Linnen JM, Caglioti S. 2008. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis 198:984–993. doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]
- 38.Calisher CH. 1994. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev 7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tardei G, Ruta S, Chitu V, Rossi C, Tsai TF, Cernescu C. 2000. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection. J Clin Microbiol 38:2232–2239. doi: 10.1128/JCM.38.6.2232-2239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol 38:1823–1826. doi: 10.1128/JCM.38.5.1823-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin DA, Biggerstaff BJ, Allen B, Johnson AJ, Lanciotti RS, Roehrig JT. 2002. Use of immunoglobulin m cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin Diagn Lab Immunol 9:544–549. doi: 10.1128/cdli.9.3.544-549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogrefe WR, Moore R, Lape-Nixon M, Wagner M, Prince HE. 2004. Performance of immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays using a West Nile virus recombinant antigen (preM/E) for detection of West Nile virus- and other flavivirus-specific antibodies. J Clin Microbiol 42:4641–4648. doi: 10.1128/JCM.42.10.4641-4648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malan AK, Martins TB, Hill HR, Litwin CM. 2004. Evaluations of commercial West Nile virus immunoglobulin G (IgG) and IgM enzyme immunoassays show the value of continuous validation. J Clin Microbiol 42:727–733. doi: 10.1128/jcm.42.2.727-733.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson AJ, Cheshier RC, Cosentino G, Masri HP, Mock V, Oesterle R, Lanciotti RS, Martin DA, Panella AJ, Kosoy O, Biggerstaff BJ. 2007. Validation of a microsphere-based immunoassay for detection of anti-West Nile virus and anti-St. Louis encephalitis virus immunoglobulin M antibodies. Clin Vaccine Immunol 14:1084–1093. doi: 10.1128/CVI.00115-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson AJ, Noga AJ, Kosoy O, Lanciotti RS, Johnson AA, Biggerstaff BJ. 2005. Duplex microsphere-based immunoassay for detection of anti-West Nile virus and anti-St. Louis encephalitis virus immunoglobulin m antibodies. Clin Diagn Lab Immunol 12:566–574. doi: 10.1128/CDLI.12.5.566-574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basile AJ, Horiuchi K, Panella AJ, Laven J, Kosoy O, Lanciotti RS, Venkateswaran N, Biggerstaff BJ. 2013. Multiplex microsphere immunoassays for the detection of IgM and IgG to arboviral diseases. PLoS One 8:e75670. doi: 10.1371/journal.pone.0075670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roehrig JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, Campbell GL. 2003. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis 9:376–379. doi: 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levett PN, Sonnenberg K, Sidaway F, Shead S, Niedrig M, Steinhagen K, Horsman GB, Drebot MA. 2005. Use of immunoglobulin G avidity assays for differentiation of primary from previous infections with West Nile virus. J Clin Microbiol 43:5873–5875. doi: 10.1128/JCM.43.12.5873-5875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. 2008. False-positive results with a commercially available West Nile virus immunoglobulin M assay—United States. MMWR Morb Mortal Wkly Rep 58:458–460. [PubMed] [Google Scholar]
- 50.Koraka P, Zeller H, Niedrig M, Osterhaus ADME, Groen J. 2002. Reactivity of serum samples from patients with a flavivirus infection measured by immunofluorescence assay and ELISA. Microbes Infect 4:1209–1215. doi: 10.1016/S1286-4579(02)01647-7. [DOI] [PubMed] [Google Scholar]
- 51.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE. 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 52.Kerkhof K, Falconi-Agapito F, Van Esbroeck M, Talledo M, Arien KK. 2020. Reliable serological diagnostic tests for arboviruses: feasible or utopia? Trends Microbiol 28:276–292. doi: 10.1016/j.tim.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Rathore APS, St John AL. 2020. Cross-reactive immunity among flaviviruses. Front Immunol 11:334. doi: 10.3389/fimmu.2020.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunsperger E. 2015. Arboviruses, p 1644–1659. In Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 11th ed, vol 2 ASM Press, Washington, DC. [Google Scholar]
- 55.Purdy DE, Noga AJ, Chang GJ. 2004. Noninfectious recombinant antigen for detection of St. Louis encephalitis virus-specific antibodies in serum by enzyme-linked immunosorbent assay. J Clin Microbiol 42:4709–4717. doi: 10.1128/JCM.42.10.4709-4717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beasley DW, Holbrook MR, Travassos Da Rosa AP, Coffey L, Carrara AS, Phillippi-Falkenstein K, Bohm RP Jr, Ratterree MS, Lillibridge KM, Ludwig GV, Estrada-Franco J, Weaver SC, Tesh RB, Shope RE, Barrett AD. 2004. Use of a recombinant envelope protein subunit antigen for specific serological diagnosis of West Nile virus infection. J Clin Microbiol 42:2759–2765. doi: 10.1128/JCM.42.6.2759-2765.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alonso-Padilla J, Jimenez de Oya N, Blazquez AB, Loza-Rubio E, Escribano JM, Saiz JC, Escribano-Romero E. 2010. Evaluation of an enzyme-linked immunosorbent assay for detection of West Nile virus infection based on a recombinant envelope protein produced in Trichoplusia ni larvae. J Virol Methods 166:37–41. doi: 10.1016/j.jviromet.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Chao DY, Galula JU, Shen WF, Davis BS, Chang GJ. 2015. Nonstructural protein 1-specific immunoglobulin M and G antibody capture enzyme-linked immunosorbent assays in diagnosis of flaviviral infections in humans. J Clin Microbiol 53:557–566. doi: 10.1128/JCM.02735-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberson JA, Crill WD, Chang GJJ. 2007. Differentiation of West Nile and St. Louis encephalitis virus infections by use of noninfectious virus-like particles with reduced cross-reactivity. J Clin Microbiol 45:3167–3174. doi: 10.1128/JCM.01143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holmes DA, Purdy DE, Chao DY, Noga AJ, Chang GJ. 2005. Comparative analysis of immunoglobulin M (IgM) capture enzyme-linked immunosorbent assay using virus-like particles or virus-infected mouse brain antigens to detect IgM antibody in sera from patients with evident flaviviral infections. J Clin Microbiol 43:3227–3236. doi: 10.1128/JCM.43.7.3227-3236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welch RJ, Anderson BL, Litwin CM. 2008. Evaluation of a new commercial enzyme immunoassay for the detection of IgM antibodies to West Nile virus using a ratio method to eliminate nonspecific reactivity. J Clin Lab Anal 22:362–366. doi: 10.1002/jcla.20271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin DA, Noga A, Kosoy O, Johnson AJ, Petersen LR, Lanciotti RS. 2004. Evaluation of a diagnostic algorithm using immunoglobulin M enzyme-linked immunosorbent assay to differentiate human West Nile Virus and St. Louis encephalitis virus infections during the 2002 West Nile Virus epidemic in the United States. Clin Diagn Lab Immunol 11:1130–1133. doi: 10.1128/CDLI.11.6.1130-1133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pastula DM, Hoang Johnson DK, White JL, Dupuis AP Jr, Fischer M, Staples JE. 2015. Jamestown Canyon virus disease in the United States-2000–2013. Am J Trop Med Hyg 93:384–389. doi: 10.4269/ajtmh.15-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grimstad PR. 2001. Jamestown Canyon virus In Service M. (ed), Encyclopedia of arthropod-transmitted infections of man and domesticated animals. CABI, Wallingford, United Kingdom. [Google Scholar]
- 65.Davis LE, Beckham JD, Tyler KL. 2008. North American encephalitic arboviruses. Neurol Clin 26:727–757. doi: 10.1016/j.ncl.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malan AK, Stipanovich PJ, Martins TB, Hill HR, Litwin CM. 2003. Detection of IgG and IgM to West Nile virus. Development of an immunofluorescence assay. Am J Clin Pathol 119:508–515. doi: 10.1309/WJJ7-UE42-DFHT-TF1X. [DOI] [PubMed] [Google Scholar]
- 67.Thomm AM, Schotthoefer AM, Dupuis AP Jr, Kramer LD, Frost HM, Fritsche TR, Harrington YA, Knox KK, Kehl SC. 2018. Development and validation of a serologic test panel for detection of Powassan virus infection in U.S. patients residing in regions where Lyme disease is endemic. mSphere 3:e00467-17. doi: 10.1128/mSphere.00467-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zanoni F, Alfieri C, Moroni G, Passerini P, Regalia A, Meneghini M, Messa P. 2020. Delayed diagnosis of West Nile virus infection in a kidney transplant patient due to inaccuracies in commonly available diagnostic tests. Exp Clin Transplant 18:385–389. doi: 10.6002/ect.2018.0107. [DOI] [PubMed] [Google Scholar]
- 69.Shi PY, Kauffman EB, Ren P, Felton A, Tai JH, Dupuis AP Jr, Jones SA, Ngo KA, Nicholas DC, Maffei J, Ebel GD, Bernard KA, Kramer LD. 2001. High-throughput detection of West Nile virus RNA. J Clin Microbiol 39:1264–1271. doi: 10.1128/JCM.39.4.1264-1271.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M, West Nile Outbreak Response Working Group. 2001. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 71.Lustig Y, Mannasse B, Koren R, Katz-Likvornik S, Hindiyeh M, Mandelboim M, Dovrat S, Sofer D, Mendelson E. 2016. Superiority of West Nile virus RNA detection in whole blood for diagnosis of acute infection. J Clin Microbiol 54:2294–2297. doi: 10.1128/JCM.01283-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barzon L, Pacenti M, Franchin E, Pagni S, Martello T, Cattai M, Cusinato R, Palu G. 2013. Excretion of West Nile virus in urine during acute infection. J Infect Dis 208:1086–1092. doi: 10.1093/infdis/jit290. [DOI] [PubMed] [Google Scholar]
- 73.Cavanaugh CE, Muscat PL, Telford SR III, Goethert H, Pendlebury W, Elias SP, Robich R, Welch M, Lubelczyk CB, Smith RP. 2017. Fatal deer tick virus infection in Maine. Clin Infect Dis 65:1043–1046. doi: 10.1093/cid/cix435. [DOI] [PubMed] [Google Scholar]
- 74.Tavakoli NP, Wang H, Dupuis M, Hull R, Ebel GD, Gilmore EJ, Faust PL. 2009. Fatal case of deer tick virus encephalitis. N Engl J Med 360:2099–2107. doi: 10.1056/NEJMoa0806326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sherwood JA, Brittain DC, Howard JJ, Oliver J. 2015. Antibody and viral nucleic acid testing of serum and cerebrospinal fluid for diagnosis of eastern equine encephalitis. J Clin Microbiol 53:2768–2772. doi: 10.1128/JCM.00647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambert AJ, Nasci RS, Cropp BC, Martin DA, Rose BC, Russell BJ, Lanciotti RS. 2005. Nucleic acid amplification assays for detection of La Crosse virus RNA. J Clin Microbiol 43:1885–1889. doi: 10.1128/JCM.43.4.1885-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H, Nattanmai S, Kramer LD, Bernard KA, Tavakoli NP. 2009. A duplex real-time reverse transcriptase polymerase chain reaction assay for the detection of California serogroup and Cache Valley viruses. Diagn Microbiol Infect Dis 65:150–157. doi: 10.1016/j.diagmicrobio.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang C, Campbell W, Grady L, Kirouac I, LaForce FM. 1999. Diagnosis of Jamestown Canyon encephalitis by polymerase chain reaction. Clin Infect Dis 28:1294–1297. doi: 10.1086/514789. [DOI] [PubMed] [Google Scholar]
- 79.Re V, Spinsanti L, Farias A, Diaz A, Vazquez A, Aguilar J, Tenorio A, Contigiani M. 2008. Reliable detection of St. Louis encephalitis virus by RT-nested PCR. Enferm Infecc Microbiol Clin 26:10–15. doi: 10.1157/13114389. [DOI] [PubMed] [Google Scholar]
- 80.Myhrvold C, Freije CA, Gootenberg JS, Abudayyeh OO, Metsky HC, Durbin AF, Kellner MJ, Tan AL, Paul LM, Parham LA, Garcia KF, Barnes KG, Chak B, Mondini A, Nogueira ML, Isern S, Michael SF, Lorenzana I, Yozwiak NL, MacInnis BL, Bosch I, Gehrke L, Zhang F, Sabeti PC. 2018. Field-deployable viral diagnostics using CRISPR-Cas13. Science 360:444–448. doi: 10.1126/science.aas8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson MR, Zimmermann LL, Crawford ED, Sample HA, Soni PR, Baker AN, Khan LM, DeRisi JL. 2017. Acute West Nile virus meningoencephalitis diagnosed via metagenomic deep sequencing of cerebrospinal fluid in a renal transplant patient. Am J Transplant 17:803–808. doi: 10.1111/ajt.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chiu CY, Coffey LL, Murkey J, Symmes K, Sample HA, Wilson MR, Naccache SN, Arevalo S, Somasekar S, Federman S, Stryke D, Vespa P, Schiller G, Messenger S, Humphries R, Miller S, Klausner JD. 2017. Diagnosis of fatal human case of St. Louis encephalitis virus infection by metagenomic sequencing, California, 2016. Emerg Infect Dis 23:1964–1968. doi: 10.3201/eid2310.161986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piantadosi A, Kanjilal S, Ganesh V, Khanna A, Hyle EP, Rosand J, Bold T, Metsky HC, Lemieux J, Leone MJ, Freimark L, Matranga CB, Adams G, McGrath G, Zamirpour S, Telford S III, Rosenberg E, Cho T, Frosch MP, Goldberg MB, Mukerji SS, Sabeti PC. 2018. Rapid detection of Powassan virus in a patient with encephalitis by metagenomic sequencing. Clin Infect Dis 66:789–792. doi: 10.1093/cid/cix792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaudin M, Desnues C. 2018. Hybrid capture-based next generation sequencing and its application to human infectious diseases. Front Microbiol 9:2924. doi: 10.3389/fmicb.2018.02924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng X, Achari A, Federman S, Yu G, Somasekar S, Bartolo I, Yagi S, Mbala-Kingebeni P, Kapetshi J, Ahuka-Mundeke S, Muyembe-Tamfum JJ, Ahmed AA, Ganesh V, Tamhankar M, Patterson JL, Ndembi N, Mbanya D, Kaptue L, McArthur C, Munoz-Medina JE, Gonzalez-Bonilla CR, Lopez S, Arias CF, Arevalo S, Miller S, Stone M, Busch M, Hsieh K, Messenger S, Wadford DA, Rodgers M, Cloherty G, Faria NR, Theze J, Pybus OG, Neto Z, Morais J, Taveira NR, Hackett JJ, Chiu CY. 2020. Metagenomic sequencing with spiked primer enrichment for viral diagnostics and genomic surveillance. Nat Microbiol 5:443–454. doi: 10.1038/s41564-019-0637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sambri V, Capobianchi MR, Cavrini F, Charrel R, Donoso-Mantke O, Escadafal C, Franco L, Gaibani P, Gould EA, Niedrig M, Papa A, Pierro A, Rossini G, Sanchini A, Tenorio A, Varani S, Vazquez A, Vocale C, Zeller H. 2013. Diagnosis of West Nile virus human infections: overview and proposal of diagnostic protocols considering the results of external quality assessment studies. Viruses 5:2329–2348. doi: 10.3390/v5102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panella NA, Burkhalter KL, Langevin SA, Brault AC, Schooley LM, Biggerstaff BJ, Nasci RS, Komar N. 2005. Rapid West Nile virus antigen detection. Emerg Infect Dis 11:1633–1635. doi: 10.3201/eid1110.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burkhalter KL, Horiuchi K, Biggerstaff BJ, Savage HM, Nasci RS. 2014. Evaluation of a rapid analyte measurement platform and real-time reverse-transcriptase polymerase chain reaction assay West Nile virus detection system in mosquito pools. J Am Mosq Control Assoc 30:21–30. doi: 10.2987/13-6386.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burakoff A, Lehman J, Fischer M, Staples JE, Lindsey NP. 2018. West Nile virus and other nationally notifiable arboviral diseases–United States, 2016. MMWR Morb Mortal Wkly Rep 67:13–17. doi: 10.15585/mmwr.mm6701a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curren EJ, Lehman J, Kolsin J, Walker WL, Martin SW, Staples JE, Hills SL, Gould CV, Rabe IB, Fischer M, Lindsey NP. 2018. West Nile virus and other nationally notifiable arboviral diseases—United States, 2017. MMWR Morb Mortal Wkly Rep 67:1137–1142. doi: 10.15585/mmwr.mm6741a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krow-Lucal E, Lindsey NP, Lehman J, Fischer M, Staples JE. 2017. West Nile virus and other nationally notifiable arboviral diseases—United States, 2015. MMWR Morb Mortal Wkly Rep 66:51–55. doi: 10.15585/mmwr.mm6602a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lindsey NP, Lehman JA, Staples JE, Fischer M. 2015. West Nile virus and other nationally notifiable arboviral diseases—United States, 2014. MMWR Morb Mortal Wkly Rep 64:929–934. doi: 10.15585/mmwr.mm6434a1. [DOI] [PubMed] [Google Scholar]