Zinc concentrations in cation-adjusted Mueller-Hinton broth (caMHB) from different manufacturers have been found to differ. Here, we evaluated the impact of utilizing different brands and lots of commercially available caMHB on the classification of the antimicrobial susceptibility of metallo-β-lactamase (MBL)-harboring Enterobacteriaceae. We also evaluated the addition of EDTA to caMHB as a means of achieving zinc-limited media. Fifteen clinical Enterobacteriaceae isolates (harboring NDM [n = 7], VIM [n = 3], IMP [n = 2], or KPC [n = 3]) and nine different commercial lots from three caMHB manufacturers (Becton, Dickinson; Oxoid; and Sigma-Aldrich) were utilized.
KEYWORDS: antimicrobial susceptibility testing, Enterobacteriaceae, metallo-β-lactamase, zinc
ABSTRACT
Zinc concentrations in cation-adjusted Mueller-Hinton broth (caMHB) from different manufacturers have been found to differ. Here, we evaluated the impact of utilizing different brands and lots of commercially available caMHB on the classification of the antimicrobial susceptibility of metallo-β-lactamase (MBL)-harboring Enterobacteriaceae. We also evaluated the addition of EDTA to caMHB as a means of achieving zinc-limited media. Fifteen clinical Enterobacteriaceae isolates (harboring NDM [n = 7], VIM [n = 3], IMP [n = 2], or KPC [n = 3]) and nine different commercial lots from three caMHB manufacturers (Becton, Dickinson; Oxoid; and Sigma-Aldrich) were utilized. Zinc-limited media were prepared by the addition of EDTA at concentrations ranging from 3 to 300 μg/ml. Meropenem MICs were determined in triplicate for each lot of conventional caMHB and zinc-limited media by broth microdilution. The zinc concentration in each lot of conventional caMHB was determined by inductively coupled plasma mass spectrometry. Up to 8-fold differences in meropenem MICs were observed between the commercial lots, resulting in different classifications of susceptibility among MBL-harboring isolates. Mean zinc concentrations were highest among conventional Becton, Dickinson caMHB lots relative to those for Oxoid and Sigma-Aldrich broth. Among MBL-harboring isolates, the impact of EDTA on MICs was dependent on the lot, correlating with initial zinc availability (i.e., less MIC reduction with higher initial zinc concentrations), while MICs for KPC-harboring isolates were unchanged. In summary, zinc variability was observed among commercial lots of caMHB, resulting in different classifications of susceptibility among MBL-harboring Enterobacteriaceae. The addition of EDTA at concentrations of ≥30 μg/ml was sufficient to provide a zinc-limited medium, resulting in MICs that reflect in vivo meropenem activity.
INTRODUCTION
Antimicrobial susceptibility testing (AST) has long been established as a critical component of patient care and microbiology. Susceptibility data are used by clinicians to predict the likelihood of treatment success and the potential for failure as well as for epidemiological surveillance purposes (1, 2). Current phenotypic AST methods routinely incorporate a variety of solid and liquid culturing media to provide nutrient conditions for the organism to be evaluated (3, 4).
The influence of medium composition on AST has been known for decades, resulting in several revisions to the manufacturing and preparation processes over the years. For example, prior to the standardization of cation-adjusted Mueller-Hinton broth (caMHB), historic MHB contained only trace amounts of calcium and magnesium cations, resulting in in vitro-in vivo discordance upon aminoglycoside susceptibility testing of Pseudomonas aeruginosa. Data suggested that MHB required Ca2+ and Mg2+ supplementation to attain physiologic levels, but this recommendation was revised to match free, unbound cation concentrations after the use of total concentrations was found to be suboptimal (5–8). In addition to concerns with aminoglycosides, susceptibility testing with several antimicrobial agents, such as colistin/polymyxin B, daptomycin, tigecycline, and cefiderocol, against certain bacterial strains has been shown to be influenced by cation concentrations, requiring medium modifications (9–13).
We have previously evaluated the impact of zinc concentrations on mediating metallo-β-lactamase (MBL) resistance (14). Relative to MICs determined in conventional caMHB (BD BBL; Becton, Dickinson and Company, NJ, USA), those determined in zinc-depleted broth (caMHB plus EDTA) provided better correlation with in vivo meropenem activity against MBL-harboring Enterobacteriaceae in an animal infection model (14). While calcium and magnesium concentrations are standardized per CLSI and EUCAST (ISO) guidelines and the medium is referred to as caMHB, zinc concentrations have been shown to differ across different batches of media (11, 15–18). Given the dependence of MBLs on zinc to hydrolyze β-lactams (19), understanding the impact of differences in zinc concentration across different commercially available broths is important to MBL AST and has significant implications for the clinic and drug development.
To that end, the objectives of this study were (i) to determine the impact of utilizing different brands and lots of commercially available MHB on antimicrobial susceptibility testing of MBL-harboring Enterobacteriaceae and (ii) to evaluate the addition of various EDTA concentrations to caMHB as a means of achieving zinc-limited media.
MATERIALS AND METHODS
Bacterial isolates.
A total of 15 clinical Enterobacteriaceae (Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae) isolates harboring a variety of β-lactamases, including MBLs (NDM [n = 7], VIM [n = 3], and IMP [n = 2]) and serine-based carbapenemases (KPC [n = 3]), were utilized in the study. Seven of these isolates were obtained from the FDA-CDC Antimicrobial Resistance Isolate Bank (AR Isolate Bank, Atlanta, GA, USA). The remaining isolates were obtained from the Center for Anti-Infective Research and Development (CAIRD) isolate repository. P. aeruginosa ATCC 27853 was tested as a quality control strain as per CLSI recommendations (20). Isolates were maintained at −80°C in skim milk prior to being subcultured twice on Trypticase soy agar with 5% sheep blood (BD Biosciences) and grown for 18 to 20 h at 37°C under 5% CO2.
Media and antimicrobial susceptibility.
In vitro testing of susceptibility to meropenem was compared using nine different commercial lots of dehydrated MHB from three manufacturers as follows: Becton, Dickinson and Company, NJ, USA (BD BBL caMHB II; product no. 212322, lot no. 9141514 [lot A], 9239528 [lot B], and 9324795 [lot C]); Oxoid, Thermo Scientific, Basingstoke, United Kingdom (Oxoid; product no. CM0405, lot no. 2472852 [lot D], 2871198 [lot E], and 2871200 [lot F]; and Sigma-Aldrich, St. Louis, MO, USA (Sigma; product no. 90922, lot no. BCCB1508 [lot G], BCCC4989 [lot H], and BCCD4873 [lot I]). All BD and Sigma MHB lots were purchased as cation-adjusted powders, while the three Oxoid MHB lots required cation adjustment per CLSI recommendations (20 to 25 μg/ml calcium and 10 to 12.5 μg/ml magnesium) (20).
Zinc-limited broth was prepared by aseptic addition of EDTA (Sigma-Aldrich, St. Louis, MO, USA; product no. 324503, lot no. 3152151) to an aliquot of autoclaved BD, Oxoid, or Sigma caMHB. EDTA was added to achieve final zinc concentrations of 3 μg/ml, 10 μg/ml, 30 μg/ml, 100 μg/ml, and 300 μg/ml, resulting in a range (n = 5) of zinc-limited broths per caMHB lot.
Analytical-grade meropenem (Sigma-Aldrich, St. Louis, MO, USA; lot no. LRAB7853) was utilized for susceptibility testing (MIC tray range, 0.06 to 64 μg/ml). Meropenem MIC values were determined in triplicate and concurrently for each lot of conventional caMHB and zinc-limited media using the broth microdilution methodology outlined by CLSI, and modal MICs were reported (20). The relationship between MICs and broth (conventional and zinc limited) was evaluated. MIC values were log2 transformed so that a 1-unit increment in log value on the graph corresponds to a doubling of the MIC.
Measurement of zinc concentrations.
The zinc concentration in each lot of conventional caMHB was measured in triplicate by inductively coupled plasma mass spectrometry (ICP-MS). ICP-MS analysis was performed by PureHoney Technologies (Billerica, MA), using an Agilent 7500 CE instrument (Agilent Technologies, USA) with a lower limit of detection of 0.002 μg/ml.
RESULTS
Comparison of MIC values across distinct lots of commercial caMHB.
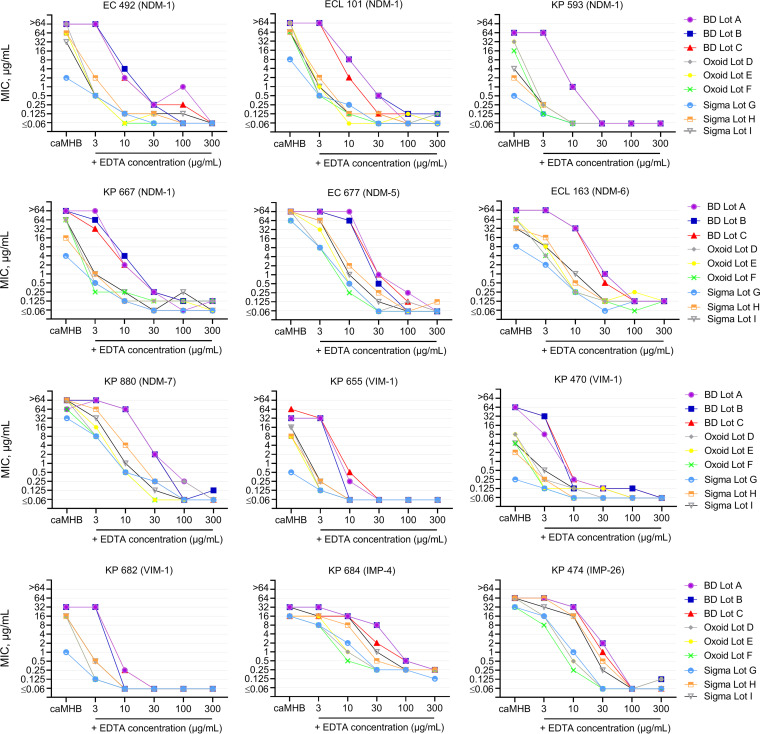
Susceptibility tests were performed for all clinical isolates across nine distinct lots of caMHB (Table 1). All 12 MBL-harboring Enterobacteriaceae demonstrated in vitro resistance to meropenem in each lot of BD (MIC range, 16 to ≥64 μg/ml) and Oxoid (MIC range, 4 to ≥64 μg/ml) broth. MIC values for each isolate were similar (±1 log2 dilution) across the three lots purchased from BD and from Oxoid. In contrast, there was marked MIC variation between the lots from Sigma; MICs ranged from 0.25 to 64 μg/ml, 2 to >64 μg/ml, and 4 to >64 μg/ml in lots G, H, and I, respectively. As a result, several MBL isolates tested as susceptible or intermediate per CLSI and EUCAST criteria. For example, KP 470 (VIM-1) was either susceptible, intermediate, or resistant to meropenem depending on the caMHB brand and lot utilized. Overall, MIC values were elevated and consistent between lots from BD and Oxoid, while lot-to-lot variation was observed with caMHB from Sigma. In addition, there was a trend toward lower MICs among VIM-harboring isolates than among isolates harboring NDM and IMP subtypes, irrespective of the caMHB lot utilized and suggestive of different zinc sensitivities.
TABLE 1.
Genotypic and phenotypic profiles of MBL- and serine carbapenemase-producing isolates determined in different commercial lots of cation-adjusted Mueller-Hinton broth
| Isolate ID (β-lactamase[s])a | MIC (μg/ml) of meropenem determined in caMHB from: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| BD |
Oxoid |
Sigma |
|||||||
| Lot A | Lot B | Lot C | Lot D | Lot E | Lot F | Lot G | Lot H | Lot I | |
| EC 492 (NDM-1, CTX-M-3, TEM) | >64 | >64 | >64 | >64 | 64 | 64 | 2 | 64 | 32 |
| ECL 101 (NDM-1, LAP-2, ACT-17, TEM-1) | >64 | >64 | >64 | >64 | >64 | 64 | 8 | 64 | 64 |
| KP 593 (NDM-1, SHV-11, CTX-M-15, OXA-1) | 64 | 64 | 64 | 32 | 32 | 16 | 0.5 | 2 | 4 |
| ECL 163 (NDM-6, SHV-12, TEM-OSBL, CTX-M-15) | >64 | >64 | >64 | 64 | 64 | 64 | 8 | 32 | 32 |
| KP 667 (NDM-1, CTX-M-15, OXA-1, TEM-1B)b | >64 | >64 | >64 | 64 | 64 | 64 | 4 | 16 | 64 |
| EC 677 (NDM-5, CTX-M-15) | >64 | >64 | >64 | >64 | >64 | 64 | 64 | >64 | >64 |
| KP 880 (NDM-7, SHV-12, TEM-OSBL, CTX-M-15) | 64 | >64 | >64 | >64 | >64 | 64 | 32 | 64 | >64 |
| KP 470 (VIM-1) | 64 | 64 | 64 | 8 | 8 | 4 | 0.25 | 2 | 4 |
| KP 655 (VIM-1, OXA-9, SHV-12, TEM-1A)c | 32 | 32 | 64 | 16 | 8 | 8 | 0.5 | 8 | 16 |
| KP 682 (VIM-1, SHV-30)d | 32 | 32 | 32 | 16 | 16 | 8 | 1 | 16 | 16 |
| KP 474 (IMP-26) | 64 | 64 | 64 | 64 | 64 | 32 | 32 | 64 | 64 |
| KP 684 (IMP-4, OKP-B-2, OXA-1, SFO-1, TEM-1B)e | 32 | 32 | 16 | 16 | 16 | 16 | 16 | 16 | 32 |
| KP 651 (KPC-2, TEM-1D)f | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 |
| KP 652 (KPC-3, OXA-9, TEM-1B)g | 64 | 64 | 64 | 64 | 64 | 32 | 64 | 64 | 64 |
| EC 548 (KPC-3, OXA-9, TEM-1A)h | 8 | 8 | 16 | 8 | 16 | 8 | 8 | 8 | 16 |
Isolate IDs begin with EC for E. coli, with KP for K. pneumoniae, and with ECL for E. cloacae. Isolate identification and corresponding meropenem MIC values as provided by the FDA-CDC Antimicrobial Resistance Isolate Bank for KP 667, KP 655, KP 682, KP 684, KP 651, KP 652, and EC 548 are listed in footnotes b to h.
AR Bank no. 0158 (MIC, >8 μg/ml).
AR Bank no. 0135 (MIC, 8 μg/ml).
AR Bank no. 0076 (MIC, 4 μg/ml).
AR Bank no. 0080 (MIC, 4 μg/ml).
AR Bank no. 0120 (MIC, >8 μg/ml).
AR Bank no. 0125 (MIC, >16 μg/ml).
AR Bank no. 0061 (MIC, 4 μg/ml).
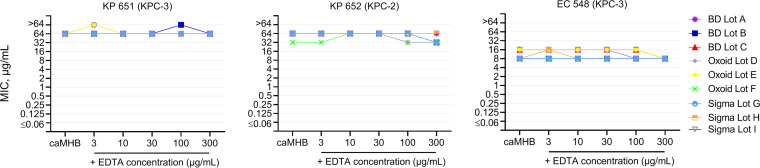
To serve as controls, serine carbapenemase-harboring isolates were examined. All three KPC isolates were meropenem resistant and resulted in similar MIC values (±1 log2 dilution) across all nine caMHB lots and manufacturer brands. P. aeruginosa ATCC 27853 was utilized as a quality control strain and was consistently within an acceptable susceptibility testing range across all lots.
Zinc concentrations.
Samples of BD, Oxoid, and Sigma conventional caMHB utilized in the AST studies were assayed to determine total zinc concentrations (Table 2). Zinc concentrations were highest among the lots purchased from BD, approximately 3- to 4-fold higher than zinc concentrations in Oxoid and Sigma lots. Zinc concentrations were generally comparable between lots from each caMHB manufacturer.
TABLE 2.
Total concentrations of zinc in commercial lots of cation-adjusted Mueller-Hinton broth
| caMHB manufacturer and lot | Total zinc concn (μg/ml) |
|---|---|
| BD | |
| Lot A | 1.181 ± 0.034 |
| Lot B | 1.318 ± 0.095 |
| Lot C | 1.262 ± 0.077 |
| Oxoid | |
| Lot D | 0.333 ± 0.007 |
| Lot E | 0.400 ± 0.005 |
| Lot F | 0.400 ± 0.002 |
| Sigma | |
| Lot G | 0.282 ± 0.013 |
| Lot H | 0.371 ± 0.005 |
| Lot I | 0.244 ± 0.007 |
Impact of EDTA on distinct lots of commercial MHB.
Because the utility of EDTA in creating zinc-limited media depends on initial total zinc availability, we evaluated the impact of the addition of fixed amounts of EDTA on different lots of caMHB. In vitro susceptibility testing of meropenem was conducted in a variety of zinc-limited media, reflecting the addition of EDTA to all nine distinct lots in order to assess MIC variability among MBL- and KPC-harboring isolates (Fig. 1). Across all isolates, the impact of EDTA on MICs was dependent on the lot, correlating with initial zinc availability. For example, at an EDTA concentration of 3 μg/ml, MIC values in BD lots (i.e., lots with the highest zinc concentrations) were unchanged, while substantial MIC reductions in Oxoid and Sigma broth were observed. Of note, the impact of EDTA on MIC reduction was also dependent on the MBL subtype. Indeed, at each EDTA concentration, the greatest reductions in MIC values were observed for VIM-harboring isolates, followed by NDM- and IMP-harboring isolates.
FIG 1.
Differences in meropenem MIC reduction among MBL-harboring Enterobacteriaceae upon the addition of EDTA to nine commercial lots of cation-adjusted Mueller-Hinton broth. Each data point represents a modal MIC (a minimum of 3 MIC replicates).
As expected, susceptibility testing in zinc-limited media had no impact on the serine carbapenemase-harboring isolates (Fig. 2). All KPC isolates were highly resistant to meropenem, with MIC values within a 1-fold dilution regardless of increasing concentrations of EDTA. No impact of EDTA was observed in the growth control wells for all isolates. Furthermore, the quality control strain P. aeruginosa ATCC 27853 consistently tested within the acceptable MIC range across all zinc-limited media, demonstrating no inhibition by EDTA at the concentrations evaluated.
FIG 2.
Differences in meropenem MIC reduction among serine carbapenemase-harboring Enterobacteriaceae upon the addition of EDTA to nine commercial lots of cation-adjusted Mueller-Hinton broth. Each data point represents a modal MIC (a minimum of 3 MIC replicates).
DISCUSSION
AST remains the cornerstone method for characterizing the in vitro relationship between an organism and an antibiotic under a set of standardized conditions. To be clinically meaningful, these conditions must be consistent and reproducible and must represent the in vivo environment in which the antibiotic, pathogen, and host would biologically interact. To that end, a set of harmonized and standardized guidelines exist for AST, including criteria for inoculum size, incubation time, and cation composition (specifically calcium and magnesium) (20). However, the current study confirms that the zinc content of commercially available media varies and, most importantly, that these differences in zinc concentration are of sufficient magnitude to result in different classifications of susceptibility among MBL-harboring Enterobacteriaceae. MIC shifts from resistant to intermediate/susceptible occurred in commercial lots with lower zinc concentrations, a finding concordant with expectations that a reduction in zinc cations available to MBL-harboring organisms mediates the magnitude of antimicrobial resistance. Notably, MIC variability in Sigma broth also suggests that as we approach lower zinc concentrations, noncationic broth components may impact susceptibility results.
To provide in vivo relevance, the isolates evaluated in this study were selected from clinical isolates utilized in previous studies of animal models infected with MBL- or KPC-harboring organisms (14, 21). In those studies, MBL-harboring isolates demonstrated in vitro meropenem resistance (MICs, 16 to >64 μg/ml) in conventional caMHB and in vitro susceptibility (MICs, ≤0.06 to 1 μg/ml) in two different types of zinc-depleted broth (EDTA broth and Chelex broth). In the corresponding animal infection model, meropenem showed no efficacy against KPC-harboring isolates that served as study controls while demonstrating >1-log bacterial killing of MBL-harboring isolates (14, 21). A similar magnitude of kill was observed for wild-type isolates, with meropenem MICs of ≤0.06 to 0.5 μg/ml (22, 23), suggesting that MICs for MBL-harboring isolates in conventional caMHB do not reflect the in vivo pharmacodynamic profile.
Lessons learned from the early development of AST methodology indicate that achieving physiologic free, unbound cation concentrations in media provides in vitro-in vivo parity (8). While total zinc concentrations in human serum range from 0.6 to 1.4 μg/ml, protein binding has been reported to be about 80 to 90%, effectively reducing the amount of zinc freely available to interact with cells (24–28), and in contrast to what is observed for Ca2+ and Mg2+ (i.e., macrominerals), the concentrations of free zinc (a trace mineral) are further reduced during an acute infection or inflammation (14, 29–31). The importance of these host factors cannot be overemphasized, as demonstrated in direct-from-blood-culture carbapenemase detection assays, where zinc has to be added to blood to improve MBL detection, further reinforcing the evidence that free zinc concentrations are low in vivo (32). Unsurprisingly, in the blood-modified carbapenem inactivation method assay, zinc supplementation is not required, given the extra dilution step in tryptic soy broth (33). Undoubtedly, reducing supraphysiologic zinc concentrations in media to match free physiologic concentrations will result in clinically relevant MBL AST results. To further highlight AST discrepancies, meropenem MIC values as reported by the FDA-CDC AR Isolate Bank for three of the four MBL-harboring CDC isolates included in this study are lower than the majority of the MICs we generated (Table 1), suggesting that without rapid molecular testing, MBL-harboring isolates are currently being treated solely on the basis of MICs that can potentially span all three susceptibility classifications.
Our findings therefore challenge the appropriateness of commercial caMHB as a means of characterizing MBL resistance, without zinc content standardization. The nonphysiologic zinc concentrations and inconsistent brand-to-brand performance of the caMHB lots have significant implications for clinical microbiology and drug development laboratories that perform MBL AST and in vitro time-kill assays. The compositions of media within commercial microdilution systems and rapid automated methods will require similar examination and standardization to provide results consistent with those obtained by the reference broth microdilution standard (once optimized to mimic in vivo free zinc concentrations). In addition, the CLSI-recommended zinc concentration target (0.5 to 1 mg/liter) of iron-depleted, cation-adjusted Mueller-Hinton broth may require revision, since this target was based on zinc concentrations present in broth before cation depletion, and no formal studies were conducted (34). Robust clinical and microbiological studies are certainly needed to determine the ideal zinc concentration range appropriate for MBL AST. The impact of zinc content and medium components on other clinically relevant MBL-harboring bacterial species, MBL variants, and β-lactam antimicrobials also warrants investigation.
Zinc variations have implications for the development of zinc-limited media. Through resin binding and subsequent resupplementation with cations, the Chelex methodology for zinc reduction as described previously would be optimal (14); however, because EDTA is more frequently utilized as a means to reduce zinc availability for the evaluation of MBL activity, we also assessed the impact of the addition of fixed amounts of EDTA on the different lots of caMHB. EDTA sequesters several metal cations, including zinc, and influences the ratio of total to free cation without changing the total amount of cation in the medium. For each MBL-harboring isolate, the same amount of EDTA resulted in varied reductions in the MIC in the different lots of caMHB, indicative of different baseline zinc concentrations in each lot. It is worth noting that while MICs in zinc-limited media with high EDTA concentrations (i.e., 100 and 300 μg/ml) appear to plateau at 0.06 μg/ml, this is a function of the range of MICs (64 to 0.06 μg/ml) tested on the broth microdilution tray; thus, MIC values could potentially be lower. Nonetheless, reductions in MIC values appear to mirror reductions in freely available zinc concentrations in each of the lots, and for the majority of the MBL isolates evaluated, the clinically relevant restoration of meropenem susceptibility was observed at EDTA concentrations of ≥30 μg/ml. Other broth manufacturers, unfortunately, should be assumed to have different concentrations of zinc, and if these are higher than the concentrations observed in this study, they may require a different EDTA concentration to reproduce a zinc-limited environment.
It is also worth noting that the sequestration of other cations by EDTA is expected to impact the testing of susceptibility to specific drugs, i.e., tetracyclines and aminoglycosides; thus, this zinc-limiting approach should be limited to β-lactam susceptibility testing of MBL-harboring organisms until future studies suggest otherwise. We acknowledge the practical challenge of not being able to measure zinc in zinc-limited media in this study; ICP-MS analyses provide data on the total amount of zinc in the media and cannot distinguish between EDTA-bound zinc and zinc that is freely available to organisms (35, 36). Finally, a decision on the clinical relevance of MBL-harboring Enterobacteriaceae demonstrating susceptibility to meropenem must await supporting data from preclinical pharmacokinetic-pharmacodynamic and clinical outcome studies.
Conclusion.
In summary, these data demonstrate that variations in the zinc content of commercially available caMHB significantly influence MBL AST and the development of zinc-limited media using EDTA. As evidence of carbapenem efficacy against MBL-harboring Enterobacteriaceae in animal infection models continues to mount (14, 37, 38), a harmonized consensus on the appropriate amount of zinc that culture media should contain will be paramount to optimizing current antimicrobial agents and the development of novel therapeutics.
ACKNOWLEDGMENTS
We thank all team members at the Center for Anti-Infective Research and Development, Hartford, CT, for assistance with the conduct of the study.
This work was supported by internal funds from the Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT.
We have no potential conflicts of interest to declare.
REFERENCES
- 1.Puttaswamy S, Gupta SK, Regunath H, Smith LP, Sengupta S. 2018. A comprehensive review of the present and future antibiotic susceptibility testing (AST) systems. Arch Clin Microbiol 9(3):83. doi: 10.4172/1989-8436.100083. [DOI] [Google Scholar]
- 2.Doern GV, Brecher SM. 2011. The clinical predictive value (or lack thereof) of the results of in vitro antimicrobial susceptibility tests. J Clin Microbiol 49:S11–S14. doi: 10.1128/JCM.00580-11. [DOI] [Google Scholar]
- 3.van Belkum A, Bachmann TT, Lüdke G, Lisby JG, Kahlmeter G, Mohess A, Becker K, Hays JP, Woodford N, Mitsakakis K, Moran-Gilad J, Vila J, Peter H, Rex JH, Dunne WM Jr, JPIAMR AMR-RDT Working Group on Antimicrobial Resistance and Rapid Diagnostic Testing. 2019. Developmental roadmap for antimicrobial susceptibility testing systems. Nat Rev Microbiol 17:51–62. doi: 10.1038/s41579-018-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews JM. 2001. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48(Suppl S1):5–16. doi: 10.1093/jac/48.suppl_1.5 (Erratum, 49:1049, 2002, doi:.). [DOI] [PubMed] [Google Scholar]
- 5.Reller LB, Schoenknecht FD, Kenny MA, Sherris JC. 1974. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis 130:454–463. doi: 10.1093/infdis/130.5.454. [DOI] [PubMed] [Google Scholar]
- 6.Casillas E, Kenny MA, Minshew BH, Schoenknecht FD. 1981. Effect of ionized calcium and soluble magnesium on the predictability of the performance of Mueller-Hinton agar susceptibility testing of Pseudomonas aeruginosa with gentamicin. Antimicrob Agents Chemother 19:987–992. doi: 10.1128/aac.19.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry AL, Miller GH, Thornsberry C, Hare RS, Jones RN, Lorber RR, Ferraresi R, Cramer C. 1987. Influence of cation supplements on activity of netilmicin against Pseudomonas aeruginosa in vitro and in vivo. Antimicrob Agents Chemother 31:1514–1518. doi: 10.1128/aac.31.10.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry AL, Reller LB, Miller GH, Washington JA, Schoenknect FD, Peterson LR, Hare RS, Knapp C. 1992. Revision of standards for adjusting the cation content of Mueller-Hinton broth for testing susceptibility of Pseudomonas aeruginosa to aminoglycosides. J Clin Microbiol 30:585–589. doi: 10.1128/JCM.30.3.585-589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezadi F, Ardebili A, Mirnejad R. 2018. Antimicrobial susceptibility testing for polymyxins: challenges, issues, and recommendations. J Clin Microbiol 57:e01390-18. doi: 10.1128/JCM.01390-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiegand I, Hilpert K, Hancock REW. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Mazarrasa C, Mazarrasa O, Calvo J, Del Arco A, Martínez-Martínez L. 2009. High concentrations of manganese in Mueller-Hinton agar increase MICs of tigecycline determined by Etest. J Clin Microbiol 47:827–829. doi: 10.1128/JCM.02464-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huband MD, Ito A, Tsuji M, Sader HS, Fedler KA, Flamm RK. 2017. Cefiderocol MIC quality control ranges in iron-depleted cation-adjusted Mueller–Hinton broth using a CLSI M23-A4 multi-laboratory study design. Diagn Microbiol Infect Dis 88:198–200. doi: 10.1016/j.diagmicrobio.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs PC, Barry AL, Brown SD. 2000. Daptomycin susceptibility tests: interpretive criteria, quality control, and effect of calcium on in vitro tests. Diagn Microbiol Infect Dis 38:51–58. doi: 10.1016/S0732-8893(00)00164-4. [DOI] [PubMed] [Google Scholar]
- 14.Asempa TE, Abdelraouf K, Nicolau DP. 2020. Metallo-β-lactamase resistance in Enterobacteriaceae is an artefact of currently utilized antimicrobial susceptibility testing methods. J Antimicrob Chemother 75:997–1005. doi: 10.1093/jac/dkz532. [DOI] [PubMed] [Google Scholar]
- 15.Washington JA, Snyder RJ, Kohner PC, Wiltse CG, Ilstrup DM, McCall JT. 1978. Effect of cation content of agar on the activity of gentamicin, tobramycin, and amikacin against Pseudomonas aeruginosa. J Infect Dis 137:103–111. doi: 10.1093/infdis/137.2.103. [DOI] [PubMed] [Google Scholar]
- 16.Walsh TR, Bolmström A, Qwärnström A, Gales A. 2002. Evaluation of a new Etest for detecting metallo-β-lactamases in routine clinical testing. J Clin Microbiol 40:2755–2759. doi: 10.1128/jcm.40.8.2755-2759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girardello R, Bispo PJM, Yamanaka TM, Gales AC. 2012. Cation concentration variability of four distinct Mueller-Hinton agar brands influences polymyxin B susceptibility results. J Clin Microbiol 50:2414–2418. doi: 10.1128/JCM.06686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. 2019. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev 32:e00115-18. doi: 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MI, Badarau A. 2008. The mechanisms of catalysis by metallo β-lactamases. Bioinorg Chem Appl 2008:576297. doi: 10.1155/2008/576297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute (CLSI). 2020. Performance standards for antimicrobial susceptibility testing, 30th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Asempa TE, Motos A, Abdelraouf K, Bissantz C, Zampaloni C, Nicolau DP. 2019. Efficacy of human-simulated epithelial lining fluid exposure of meropenem-nacubactam combination against class A serine β-lactamase-producing Enterobacteriaceae in the neutropenic murine lung infection model. Antimicrob Agents Chemother 63:e02382-18. doi: 10.1128/AAC.02382-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. 2017. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of Gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother 61:e01022-17. doi: 10.1128/AAC.01022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghazi IM, Crandon JL, Lesho EP, McGann P, Nicolau DP. 2015. Efficacy of humanized high-dose meropenem, cefepime, and levofloxacin against Enterobacteriaceae isolates producing Verona integron-encoded metallo-β-lactamase (VIM) in a murine thigh infection model. Antimicrob Agents Chemother 59:7145–7147. doi: 10.1128/AAC.00794-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Board of Internal Medicine. 2020. Laboratory test reference ranges. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/exam/laboratory-reference-ranges.pdf. Accessed 2 August 2020.
- 25.Hennigar SR, Lieberman HR, Fulgoni VL, McClung JP. 2018. Serum zinc concentrations in the US population are related to sex, age, and time of blood draw but not dietary or supplemental zinc. J Nutr 148:1341–1351. doi: 10.1093/jn/nxy105. [DOI] [PubMed] [Google Scholar]
- 26.Lu J, Stewart AJ, Sadler PJ, Pinheiro TJT, Blindauer CA. 2008. Albumin as a zinc carrier: properties of its high-affinity zinc-binding site. Biochem Soc Trans 36:1317–1321. doi: 10.1042/BST0361317. [DOI] [PubMed] [Google Scholar]
- 27.Foote JW, Delves HT. 1984. Albumin bound and α2-macroglobulin bound zinc concentrations in the sera of healthy adults. J Clin Pathol 37:1050–1054. doi: 10.1136/jcp.37.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coverdale JPC, Khazaipoul S, Arya S, Stewart AJ, Blindauer CA. 2019. Crosstalk between zinc and free fatty acids in plasma. Biochim Biophys Acta Mol Cell Biol Lipids 1864:532–542. doi: 10.1016/j.bbalip.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 31.Hennigar SR, McClung JP. 2016. Nutritional immunity: starving pathogens of trace minerals. Am J Lifestyle Med 10:170–173. doi: 10.1177/1559827616629117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meier M, Hamprecht A. 2019. Systematic comparison of four methods for detection of carbapenemase-producing Enterobacterales directly from blood cultures. J Clin Microbiol 57:e00709-19. doi: 10.1128/JCM.00709-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sfeir MM, Satlin MJ, Fauntleroy KA, Jenkins SG, Westblade LF. 2019. Blood-modified carbapenem inactivation method: a phenotypic method for detecting carbapenemase-producing Enterobacteriaceae directly from positive blood culture broths. J Clin Microbiol 58:e01377-19. doi: 10.1128/JCM.01377-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clinical and Laboratory Standards Institute (CLSI). 2020. AST meeting files and resources. Summary minutes, Subcommittee on Antimicrobial Susceptibility Testing, Tempe, AZ, 10 to 12 January 2016. https://clsi.org/meetings/ast-file-resources/. Accessed 18 September 2020.
- 35.Pröfrock D, Prange A. 2012. Inductively coupled plasma-mass spectrometry (ICP-MS) for quantitative analysis in environmental and life sciences: a review of challenges, solutions, and trends. Appl Spectrosc 66:843–868. doi: 10.1366/12-06681. [DOI] [PubMed] [Google Scholar]
- 36.D’Orazio M, Mastropasqua MC, Cerasi M, Pacello F, Consalvo A, Chirullo B, Mortensen B, Skaar EP, Ciavardelli D, Pasquali P, Battistoni A. 2015. The capability of Pseudomonas aeruginosa to recruit zinc under conditions of limited metal availability is affected by inactivation of the ZnuABC transporter. Metallomics 7:1023–1035. doi: 10.1039/c5mt00017c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roujansky A, de Lastours V, Guérin F, Chau F, Cheminet G, Massias L, Cattoir V, Fantin B. 2020. Analysis of paradoxical efficacy of carbapenems against carbapenemase-producing Escherichia coli in a murine model of lethal peritonitis. Antimicrob Agents Chemother 64:e00853-20. doi: 10.1128/AAC.00853-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss WJ, Pulse ME, Nguyen P, Growcott EJ. 2019. In vivo efficacy of novel monobactam LYS228 in murine models of carbapenemase-producing Klebsiella pneumoniae infection. Antimicrob Agents Chemother 63:e02214-18. doi: 10.1128/AAC.02214-18. [DOI] [PMC free article] [PubMed] [Google Scholar]