BACKGROUND
A new complexing protein-free botulinum toxin Type A (CBoNT) with the same mechanism of action as the botulinum toxin complex onabotulinumtoxinA (OBoNT) and complexing protein-free incobotulinumtoxinA (IBoNT) was recently developed.
OBJECTIVE
To compare the local paresis and chemodenervation efficacy of 3 different botulinum toxin Type A preparations in mice.
MATERIALS AND METHODS
Efficacy and duration of action of CBoNT, OBoNT, and IBoNT after a single intramuscular injection to the right gastrocnemius was evaluated by digit abduction score (DAS) and compound muscle action potential (CMAP) assays.
RESULTS
Mouse DAS and CMAP responses were comparable between CBoNT and OBoNT, indicating similar paresis and chemodenervation efficacy, as well as duration of action. Both botulinum toxins showed significantly higher efficacy and longer duration of action than IBoNT. Similarly, mean DAS potency of CBoNT (ED50: 3.85 ± 0.34 U/kg) and OBoNT (ED50: 4.13 ± 0.07 U/kg) were significantly higher compared with IBoNT (ED50: 6.70 ± 0.83 U/kg).
CONCLUSION
CBoNT displays the same efficacy as OBoNT as shown by their comparable chemodenervation and local paretic effects, and demonstrates superior efficacy and duration of action compared with IBoNT. Likewise, CBoNT has comparable DAS potency to OBoNT and is superior to IBoNT.
Botulinum neurotoxins (BoNT) are produced by anaerobic bacterium Clostridium botulinum and related species, and are the most potent protein neurotoxins known so far.1 Botulinum neurotoxins causes botulism, a rare but potentially fatal illness characterized by progressive flaccid paralysis.2 There are 7 antigenically distinct BoNT serotypes (A to G). Botulinum neurotoxins Types A, B, D, and F have been associated with human botulism.3 Botulinum neurotoxins disrupt the fusion of synaptic vesicles and plasma membrane in peripheral cholinergic nerve terminals by cleaving SNAREs (soluble N-ethylmaleimide sensitive factor attachment protein receptors) and prevent the release of acetylcholine (ACh) neurotransmitters at neuromuscular junctions. This mechanism of action has made BoNT a highly suitable therapeutic agent for spasticity-related muscle disorders.4,5
Botulinum toxin Type A (BoNT/A) is the first and most widely used BoNT serotype approved for clinical and cosmetic purposes.5 The first reported clinical application of BoNT/A was for treatment of strabismus as an alternative to surgical correction.6 Since then, its therapeutic application has expanded to various indications related to muscle spasticity, such as blepharospasm, hemifacial spasm, cervical dystonia, chronic migraine, acquired equinus deformity, sailorrhea, and urinary incontinence. BoNT/A is also used to temporarily correct moderate-to-severe glabellar, lateral canthal, and forehead lines, as well as auxiliary hyperhidrosis.7,8 Unit potency of different BoNT/A products are generally not interchangeable because of underlying differences in the manufacturing process, formulation, and testing method used.9
The 3 leading BoNT/A products—onabotulinumtoxinA (OBoNT, BOTOX/BOTOX Cosmetic; Allergan Inc., Irvine, CA), abobotulinumtoxinA (ABoNT; Dysport/Azzalure; Ipsen Ltd., Wrexham, United Kingdom), and incobotulinumtoxinA (IBoNT; Xeomin/Bocouture; Merz Pharmaceuticals GmbH, Frankfurt, Germany) are manufactured using C. botulinum Type A strain. OBoNT and ABoNT contain neurotoxin complexes, whereas IBoNT contains complexing-protein-free neurotoxin.10 Another complexing protein-free BoNT/A formulation marketed under the name Coretox (CBoNT; Medytox Inc.) was recently approved by South Korea's Ministry of Food and Drug Safety for the temporary improvement of glabellar wrinkles after completing the Phase 3 clinical trial (NCT03908008) in 2014.
The present study compares the pharmacodynamics of CBoNT, OBoNT, and IBoNT using mouse models that evaluate chemodenervation and local paresis. This investigation was conducted to establish performance comparability between CBoNT and the other 2 BoNT/A formulations.
Materials and Methods
Animals
Six to 7-week-old female ICR/CD-1 mice were purchased from Orient Bio, Inc. (Sandaewon-dong, Seongnam-si, South Korea), housed in groups of 5 in a 12-hour light/dark cycle, and given free access to food and water. All protocols were approved by the Institutional Animal Care and Use Committee of Medytox Inc.
Toxin Preparation and Administration
OBoNT, IBoNT, and CBoNT were prepared and administered following the method described previously.11 Each unexpired 100 U vial of the BoNT/A formulations was reconstituted to 200 U/mL, then serially diluted to arrive at doses equivalent to 1.2, 3.3, 4.0, 9.8, 12, and 40 U/kg body weight using preservative-free normal saline vehicle.
Before BoNT/A injection, mice were anesthetized with an intraperitoneal (IP) dose of 60 mg/kg ketamine hydrochloride and 12 mg/kg xylazine, and the right hindlimb was shaved. Each BoNT/A preparation or vehicle was administered into the right gastrocnemius at 0.2 mL/kg using a 25-μL Hamilton syringe. Groups of 5 mice for OBoNT and IBoNT, and 15 mice for CBoNT were each given a single intramuscular (IM) dose of 3.3 or 9.8 U/kg of the BoNT/A formulations. Another 5 mice were given placebo as a negative control. For determining the median IM effective dose (ED50), a single IM injection of 1.2, 4.0, 12, or 40 U/kg of CBoNT, OBoNT, or IBoNT was each administered to groups of 6 mice, with the injections performed sequentially from low to high dosage. The average ED50 was obtained from 3 independent experiments.
Digit Abduction Score Assay
A modified digit abduction score (DAS) assay was used to evaluate local paresis on the injected leg.11,12 Briefly, mouse was suspended by the tail to elicit the characteristic startled response manifested by extension of the hindlimb followed by abduction of the hind digits. Digit abduction score of the right hindlimb was determined by 2 blinded observers using a 5-point scale from 0 (full digit abduction) to 4 (complete absence of digit abduction and leg extension). Digit abduction score was assessed before BoNT/A injection and daily for the first 3 days, then at various intervals thereafter up to 48 days.
Compound Muscle Action Potential Measurement
Change in compound muscle action potential (CMAP) was used to evaluate chemodenervation on the injected leg. Compound muscle action potential amplitude on the right hindlimb was measured using Nicolet VikingQuest (Viasys Healthcare, Madison, WI) following the method described previously.13 Briefly, mice were anesthetized, followed by electrical stimulation with 10 to 20 mA for 0.1 ms to the target muscle on the injected leg and recording of the CMAP amplitude. Compound muscle action potential measurement was performed before BoNT/A injection to establish the baseline CMAP amplitude (CMAPbaseline), then daily for the first 3 days, and at various intervals thereafter up to 132 days.
Data and Statistical Analyses
Mean and SD of DAS and CMAP amplitudes were obtained. Area under the curves for DAS (DASAUC) and CMAP (CMAPAUC) were calculated from DAS and CMAP response versus time curves, respectively. Area above the curve for CMAP (CMAPAAC) was calculated using the formula CMAPbaseline AUC−CMAPAUC, where CMAPbaseline AUC is the estimated CMAPAUC in the absence of BoNT/A treatment, extrapolated from the CMAPbaseline. ED50, which represents DAS potency, was calculated from the maximum DAS dose-response curves fitted using three-parameter logistic equation.14 Maximum DAS response (DASmax), lowest CMAP amplitude (CMAPmin), DASAUC, CMAPAAC and ED50 were compared using one-way analysis of variance (ANOVA) followed by post-hoc t-tests. Duration of action was compared using two-way ANOVA, followed by one-way ANOVA and t-test of the DAS and CMAP responses at different time points. A p < .05 was considered statistically significant.
Results
Comparing Local Paretic Effects
Local paresis in mice induced by CBoNT, OBoNT, and IBoNT was evaluated by DAS. Mice injected with placebo displayed full digit abduction (DAS = 0) throughout the observation period (data not shown). In contrast, all 3 BoNT/A formulations displayed DASmax 2 ∼ 3 days after administration in both the 3.3 and 9.8 U/kg dose (Figure 1). As shown in Table 1, the 3-fold higher dosage of CBoNT, OBoNT, and IBoNT significantly increased DASmax (p < .01). At the 3.3 U/kg dose, there were no significant differences in DASmax, duration of action, and DASAUC among the 3 BoNT/A formulations. At the 9.8 U/kg dose, CBoNT and OBoNT showed comparable DASmax and duration of action, whereas IBoNT elicited a lower DASmax and shorter duration of action than the other 2 BoNT/A formulations. Similarly, the DASAUC of CBoNT (27.9 ± 15.5 DAS·day) was also comparable with OBoNT (27.2 ± 22.1 DAS·day) at this higher dosage, and was significantly higher than IBoNT (11.7 ± 5.3 DAS·day).
Figure 1.
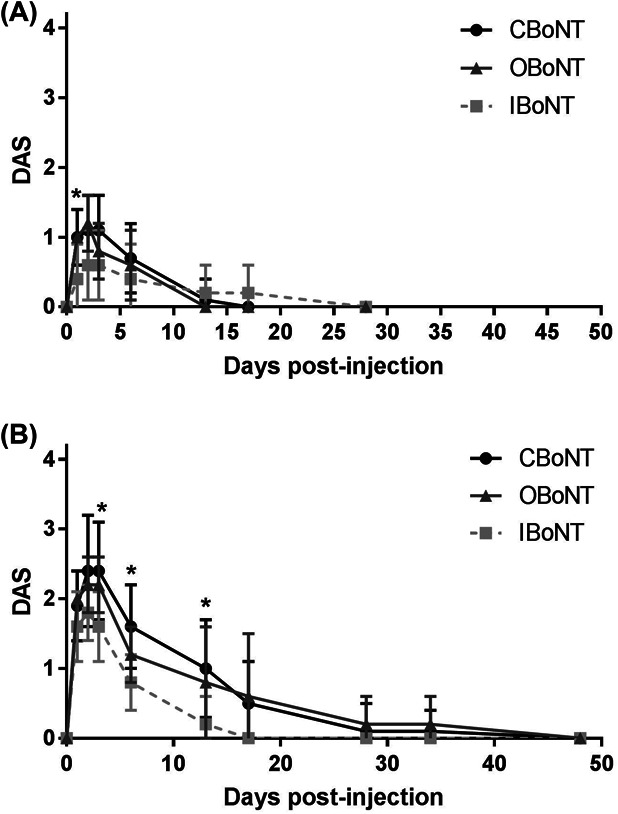
Level and duration of local paresis on the injected leg assessed by digit abduction score (DAS) assay. CBoNT, OBoNT, or IBoNT injected via intramuscular route to the right gastrocnemius at a dose of 3.3 U/kg (A) or 9.8 U/kg (B). All data points represent mean ± SD. Asterisks indicate significant differences between CBoNT and IBoNT (p < .01).
TABLE 1.
Digit Abduction Score (DAS) Response Observed on the Injected Leg After Intramuscular Administration of Different BoNT/A Preparations
| BoNT/A | Dose (U/kg) | No. of Animals (Responsive/Total) | DASmax | Duration of Action (d) | DASAUC (DAS·d) |
| CBoNT | 3.3 | 14/15 | 1.1 ± 0.5 | 10.5 ± 4.5 | 7.9 ± 4.2 |
| 9.8 | 15/15 | 2.5 ± 0.7 | 22.3 ± 9.8* | 27.9 ± 15.5* | |
| OBoNT | 3.3 | 5/5 | 1.2 ± 0.4 | 9.6 ± 4.8 | 6.8 ± 3.6 |
| 9.8 | 5/5 | 2.2 ± 0.4 | 23.8 ± 14.9 | 27.2 ± 22.1 | |
| IBoNT | 3.3 | 3/5 | 0.6 ± 0.5 | 9.4 ± 11.7 | 6.8 ± 9.3 |
| 9.8 | 5/5 | 1.8 ± 0.4 | 12.4 ± 4.0 | 11.7 ± 5.3 |
Data expressed as mean ± SD (p < .05 using 2-tailed t-test vs IBoNT).
DASmax, highest digit abduction score; DASAUC, total DAS response versus time.
Comparing Level and Duration of Chemodenervation
The same groups of animals administered with 3.3 and 9.8 U/kg of the BoNT/A formulations were evaluated by CMAP assay. In the placebo group, the average CMAP amplitude on the injected leg ranged between 47.58 and 48.94 millivolts (mV) throughout the observation period, with one of the animal showing the highest coefficient of variation of 4.7% (data not shown). This suggests that the impact of the procedure on the reproducibility of CMAP measurement is negligible, and that a full recovery from chemodenervation effects can be considered once the CMAP amplitude on the injected leg reaches 95% of the CMAPbaseline. The CMAPbaseline observed in the treatment groups averaged between 47.94 and 48.40 mV, which was within the normal range (data not shown). CBoNT, OBoNT, and IBoNT caused significant chemodenervation for both doses tested, with CMAPmin recorded within 3 days after administration (Figure 2). Similar to the DAS response, the higher dosage elicited a more significant decrease in CMAPmin (p < .01), as shown in Table 2. At the 9.8 U/kg dose, CBoNT and OBoNT showed comparable levels of chemodenervation with CMAPmin of 0.27 ± 0.09 mV and 0.24 ± 0.09 mV, respectively, whereas IBoNT (CMAPmin: 0.79 ± 0.19 mV) was significantly lower (p < .01). In addition, the duration of action of CBoNT (120.4 ± 12.5 days) was longer compared with OBoNT (116.2 ± 17.0 days) and IBoNT (104.2 ± 12.0 days). Moreover, the difference in the duration of action between CBoNT and IBoNT was statistically significant (p < .05). Based on their calculated CMAPAAC, the chemodenervation effects of both CBoNT (2,713.61 ± 336.70 mV·day) and OBoNT (2,657.01 ± 421.81 mV·day) was significantly higher than IBoNT (1854.75 ± 135.63 mV·day) at this higher dosage (p < .01).
Figure 2.
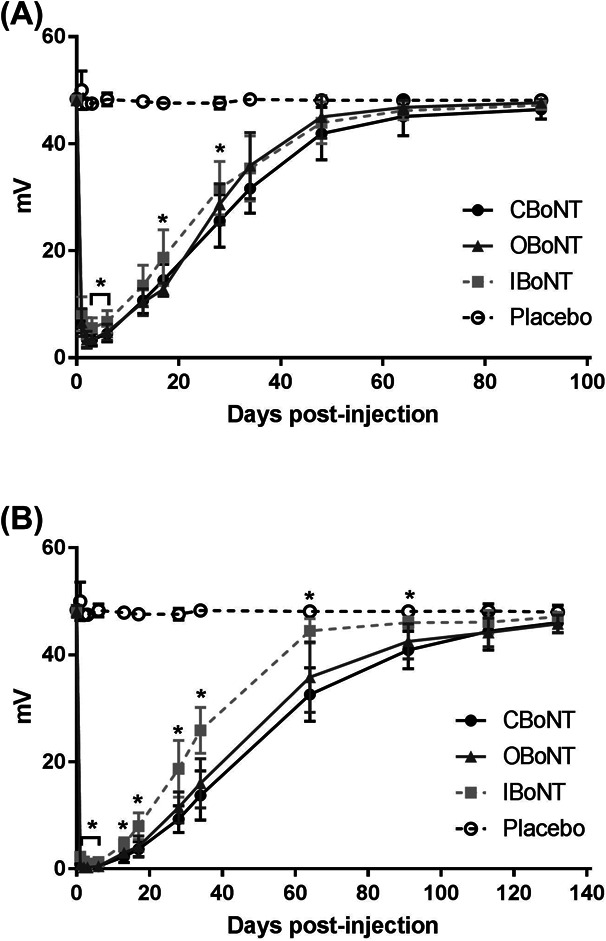
Level and duration of chemodenervation assessed by changes in compound muscle action potential (CMAP) amplitude of the injected leg muscle. CBoNT, OBoNT, or IBoNT injected via intramuscular route to the right gastrocnemius at a dose of 3.3 U/kg (A) or 9.8 U/kg (B). All data points represent mean ± SD. Asterisks indicate significant differences between CBoNT and IBoNT (p < .05).
TABLE 2.
Compound Muscle Action Potential Evaluation on the Right Leg After Intramuscular Injection With Different BoNT/A Preparations
| BoNT/A | Dose (U/kg) | No. of Animals (Responsive/Total) | CMAPmin (mV) | Duration of Action (d) | CMAPAAC (mV·d) |
| CBoNT | 3.3 | 15/15 | 2.59 ± 0.57* | 65.1 ± 15.1 | 1,379.24 ± 155.08* |
| 9.8 | 15/15 | 0.27 ± 0.09† | 120.4 ± 12.5* | 2,713.61 ± 336.70† | |
| OBoNT | 3.3 | 5/5 | 2.80 ± 1.07 | 54.4 ± 8.8 | 1,228.05 ± 168.37 |
| 9.8 | 5/5 | 0.24 ± 0.09† | 116.2 ± 17.0 | 2,657.01 ± 421.81† | |
| IBoNT | 3.3 | 5/5 | 3.96 ± 1.80 | 63.0 ± 17.6 | 1,174.36 ± 120.94 |
| 9.8 | 5/5 | 0.79 ± 0.19 | 104.2 ± 12.0 | 1854.75 ± 135.63 |
Data expressed as mean ± SD (p < .05, using 2-tailed t-test vs IBoNT).
Data expressed as mean ± SD (p < .01 using 2-tailed t-test vs IBoNT).
CMAPmin, lowest CMAP amplitude; CMAPAAC, Total CMAP reduction versus time.
Comparing Digit Abduction Score Potency
DAS potency of the 3 BoNT/A formulations was determined by the average ED50 of the DAS response from 3 independent experiments. The maximum DAS response for each dose of CBoNT, OBoNT, and IBoNT, ranging from 1.2 to 40 U/kg, was plotted (Figure 3) and used to calculate ED50. CBoNT and OBoNT showed comparable DAS potency, with an ED50 of 3.85 ± 0.34 U/kg and 4.13 ± 0.07 U/kg, respectively. By comparison, the ED50 of IBoNT (6.70 ± 0.83 U/kg) was significantly higher than CBoNT (p < .01) and OBoNT (p < .05), indicating that IBoNT has a lower DAS potency compared with the other 2 BoNT/A formulations.
Figure 3.
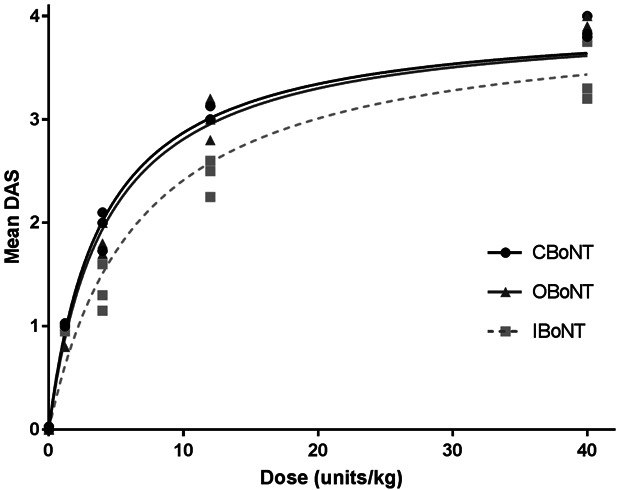
Dose-maximal digit abduction score (DAS) response curve. Dose is expressed in U/kg of body weight. All lines represent best-fit three-parameter logistic regression curves and mean values from 3 independent experiments. Digit abduction score potency based on the median effective dose (ED50) was derived from this graph.
Discussion
The active substance in all BoNT/A formulations is the 150 kDa BoNT/A holotoxin. This holotoxin is synthesized as a single polypeptide chain post-translationally nicked to yield the 100 kDa Heavy Chain (HC) and 50 kDa Light Chain (LC) segments, covalently bound by a disulfide bridge. During synthesis, the holotoxin forms multi-protein complexes with nontoxic nonhemagglutinin protein and hemagglutinin components HA-70, HA-33, and HA-17 to generate different molecular size complex, the largest of which is the 900 kDa 19 S LL-PTC (progenitor toxin complex).15–17
The manufacturing process for all commercial BoNT/A differs among manufacturers, this includes the strain of C. botulinum Type A used, purification method and choice of excipients, resulting in different formulations.9,18,19 The formulation of OBoNT contains the 900 kDa neurotoxin complex, whereas IBoNT and CBoNT formulations contain only the purified 150 kDa botulinum neurotoxin protein.20–23 The mouse LD50 assay is the standard method used for determining BoNT/A potency based on their intraperitoneal median lethal dose. However, the conditions for this assay that could significantly affect potency such as the strain, sex and age of mice, composition of the reconstitution buffer and diluent, and injection volume have not been harmonized.24–26 Thus, the dose units indicated on the label of different BoNT/A products are generally not interchangeable, and that any BoNT/A formulation should be used according to the guidelines of their respective manufacturer.9,19,22
Several clinical studies comparing BoNT/A dose potencies have generally agreed on the noninferiority of IBoNT to OBoNT in therapeutic performance and patient satisfaction.21,27–30 Others, however, have reported either lower clinical efficacy or shorter duration of action for IBoNT compared with OBoNT.31–33 Similarly, nonclinical studies comparing the potency or efficacy of OBoNT and IBoNT have disagreed on their dose equivalence. Some have demonstrated comparable dose potencies between the 2 BoNT/A products using the mouse LD50 or CMAP assays,34,35 whereas others have reported lower potencies for IBoNT compared to OBoNT when the same dose units are tested using the mouse LD50 assay.36,37 Interestingly, nonclinical studies that evaluated the paretic effects of BoNT/A in mice have reported lower dose efficacy of IBoNT compared with OBoNT, including the study that asserted comparable dose potencies between the 2 BoNT/A formulations based on CMAP.35,37,38 The present study similarly observed lower efficacy for IBoNT compared with OBoNT based on DAS response and DAS potency, whereas their relative CMAP reduction were comparable to the results for similar doses tested in a previous study.35
A clinical study comparing the chemodenervation effects of CBoNT and OBoNT on the extensor digitorum brevis in healthy volunteers reported comparable efficacies between these 2 BoNT/A formulations.23 Similarly, the present nonclinical study, which evaluated DAS and CMAP responses of mice after BoNT/A injection, have shown comparable efficacy and duration of action between CBoNT and OBoNT, suggesting that these 2 BoNT/A formulations are equi-efficacious. In contrast, CBoNT displayed more effective chemodenervation and local paresis than IBoNT, with a longer duration of action. In addition, CBoNT and OBoNT showed comparable DAS potency, whereas IBoNT displayed lower DAS potency than the other 2 BoNT/A formulations. Based on the DAS potency test, a dose conversion ratio for the nonclinical efficacy between OBoNT, CBoNT, and IBoNT is estimated to be 1.0:0.93:1.62.
Complexing proteins play an important role in protecting the core neurotoxin from the harmful acidic environment of the gut and ensure successful translocation of BoNT/A across the intestinal mucosa.15,39,40 However, they are not involved in the mode of action of BoNT/A, whereas their role in maintaining the stability of the manufactured holotoxin is debatable.41 Conversely, the presence of complexing proteins in BoNT/A formulations has been associated with an increased risk of secondary nonresponse, defined as the diminishing or complete loss of response to subsequent BoNT/A injections. Secondary nonresponse can be attributed to many factors, one of which is the formation of neutralizing antibodies (NAbs) that bind to the BoNT/A holotoxin and block its mode of action.42 Although there is no direct evidence linking the complexing proteins with NAbs formation, one study had shown that patients who had developed NAbs-related secondary nonresponse from OBoNT or ABoNT that eventually waned before injection with IBoNT did not show formation of NAbs as a response to the subsequent injection.43 Because of this, BoNT/A formulations devoid of complexing proteins has been suggested to offer a therapeutic advantage over BoNT/A complexes.10,41,44
Other factors may contribute to differences in potency and efficacy of BoNT/A formulations. The comparative pharmacodynamics study described here was performed using only 2 different batches of the BoNT/A formulations and thus recommends testing other batches to establish their relative potency. Also, the dose conversion ratio reported in this study is based on the relative DAS potency of the BoNT/A formulations in mice, and may not necessarily reflect the equivalent therapeutic efficacy in human patients.
Footnotes
The study was sponsored by Medytox, Inc. All authors are employees of Medytox, Inc.
The authors have indicated no significant interest with commercial supporters.
References
- 1.Poulain B, Popoff MR. Why are botulinum neurotoxin-producing bacteria so diverse and botulinum neurotoxins so toxic? Toxins (Basel) 2019;11:E34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev 2017;69:200–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franciosa G, Pourshaban M, De Luca A, Buccino A, et al. Identification of type A, B, E, and F botulinum neurotoxin genes and of botulinum neurotoxigenic clostridia by denaturing high-performance liquid chromatography. Appl Environ Microbiol 2004;70:4170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binz T, Sikorra S, Mahrhold S. Clostridial neurotoxins: mechanism of SNARE cleavage and outlook on potential substrate specificity reengineering. Toxins (Basel) 2010;2:665–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsui JK. Botulinum toxin as a therapeutic agent. Pharmacol Ther 1996;72:13–24. [DOI] [PubMed] [Google Scholar]
- 6.Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology 1980;87:1044–9. [DOI] [PubMed] [Google Scholar]
- 7.Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry 2004;75:951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guida S, Farnetani F, Nistico SP, Mariarosaria CG, et al. New trends in botulinum toxin use in dermatology. Dermatol Pract Concept 2018;8:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics 2014;8:227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frevert J. Content of botulinum neurotoxin in botox®/vistabel®, dysport®/azzalure®, and xeomin®/bocouture®. Drugs R D 2010;10:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SB, Ban B, Jung KS, Yang GH. A pharmacodynamic comparison study of different botulinum toxin type A preparations. Dermatol Surg 2013;39:150–4. [DOI] [PubMed] [Google Scholar]
- 12.Aoki KR. A comparison of the safety margins of botulinum neurotoxin serotypes A, B, and F in mice. Toxicon 2001;39:1815–20. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Kim SB, Yang GH, Rhee CH. Mouse compound muscle action potential assay: an alternative method to conduct the LD50 botulinum toxin type A potency test. Toxicon 2012;60:341–7. [DOI] [PubMed] [Google Scholar]
- 14.Donald S, Elliott M, Gray B, Hornby F, et al. A comparison of biological activity of commercially available purified native botulinum neurotoxin serotypes A1 to F1 in vitro, ex vivo, and in vivo. Pharmacol Res Perspect 2018;6:e00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam KH, Jin R. Architecture of the botulinum neurotoxin complex: a molecular machine for protection and delivery. Curr Opin Struct Biol 2015;31:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amatsu S, Sugawara Y, Matsumura T, Kitadokoro K, et al. Crystal structure of Clostridium botulinum whole hemagglutinin reveals a huge triskelion-shaped molecular complex. J Biol Chem 2013;288:35617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu S, Jin R. Assembly and function of the botulinum neurotoxin progenitor complex. Curr Top Microbiol Immunol 2013;364:21–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schantz EJ, Johnson EA. Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol Rev 1992;56:80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D 2015;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frevert J. Xeomin is free from complexing proteins. Toxicon 2009;54:697–701. [DOI] [PubMed] [Google Scholar]
- 21.Jost WH, Blumel J, Grafe S. Botulinum neurotoxin type A free of complexing proteins (XEOMIN) in focal dystonia. Drugs 2007;67:669–83. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari A, Manca M, Tugnoli V, Alberto L. Pharmacological differences and clinical implications of various botulinum toxin preparations: a critical appraisal. Funct Neurol 2018;33:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh HM, Park JH, Song DH, Chung ME. Efficacy and safety of a new botulinum toxin type A free of complexing proteins. Toxins (Basel) 2015;8:E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EDQM. Botulinum toxin type A for injection. In: European Pharmacopoeia (5th ed) Strasbourg: Council of Europe; 2005; pp. 1117–1119. [Google Scholar]
- 25.Samizadeh S, De Boulle K. Botulinum neurotoxin formulations: overcoming the confusion. Clin Cosmet Investig Dermatol 2018;11:273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLellan K, Das RE, Ekong TA, Sesardic D. Therapeutic botulinum type A toxin: factors affecting potency. Toxicon 1996;34:975–85. [DOI] [PubMed] [Google Scholar]
- 27.Kane MA, Gold MH, Coleman WP, III, Jones DH, et al. A randomized, double-blind trial to investigate the equivalence of IncobotulinumtoxinA and OnabotulinumtoxinA for glabellar frown lines. Dermatol Surg 2015;41:1310–9. [DOI] [PubMed] [Google Scholar]
- 28.Banegas RA, Farache F, Rancati A, Chain M, et al. The South American Glabellar Experience Study (SAGE): a multicenter retrospective analysis of real-world treatment patterns following the introduction of incobotulinumtoxinA in Argentina. Aesthet Surg J 2013;33:1039–45. [DOI] [PubMed] [Google Scholar]
- 29.Benecke R, Jost WH, Kanovsky P, Ruzicka E, et al. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology 2005;64:1949–51. [DOI] [PubMed] [Google Scholar]
- 30.Scaglione F. Conversion ratio between Botox®, Dysport®, and Xeomin® in clinical practice. Toxins (Basel) 2016;8:E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chundury RV, Couch SM, Holds JB. Comparison of preferences between onabotulinumtoxinA (Botox) and incobotulinumtoxinA (Xeomin) in the treatment of benign essential blepharospasm. Ophthalmic Plast Reconstr Surg 2013;29:205–7. [DOI] [PubMed] [Google Scholar]
- 32.Yeilding RH, Fezza JP. A prospective, split-face, randomized, double-blind study comparing OnabotulinumtoxinA to IncobotulinumtoxinA for upper face wrinkles. Plast Reconstr Surg 2015;135:1328–35. [DOI] [PubMed] [Google Scholar]
- 33.Wilson AJ, Chang B, Taglienti AJ, Chin BC, et al. A quantitative analysis of OnabotulinumtoxinA, AbobotulinumtoxinA, and IncobotulinumtoxinA: a randomized, double-blind, prospective clinical trial of comparative dynamic strain reduction. Plast Reconstr Surg 2016;137:1424–33. [DOI] [PubMed] [Google Scholar]
- 34.Dressler D, Mander G, Fink K. Measuring the potency labelling of onabotulinumtoxinA (Botox®) and incobotulinumtoxinA (Xeomin®) in an LD50 assay. J Neural Transm (Vienna) 2012;119:13–5. [DOI] [PubMed] [Google Scholar]
- 35.Chung ME, Song DH, Park JH. Comparative study of biological activity of four botulinum toxin type A preparations in mice. Dermatol Surg 2013;39:155–64. [DOI] [PubMed] [Google Scholar]
- 36.Hunt T, Clarke K. Potency evaluation of a formulated drug product containing 150-kd botulinum neurotoxin type A. Clin Neuropharmacol 2009;32:28–31. [DOI] [PubMed] [Google Scholar]
- 37.Brown M, Nicholson G, Ardila MC, Satorius A, et al. Comparative evaluation of the potency and antigenicity of two distinct BoNT/A-derived formulations. J Neural Transm (Vienna) 2013;120:291–8. [DOI] [PubMed] [Google Scholar]
- 38.Kutschenko A, Manig A, Reinert MC, Monnich A, et al. In-vivo comparison of the neurotoxic potencies of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA. Neurosci Lett 2016;627:216–21. [DOI] [PubMed] [Google Scholar]
- 39.Eisele KH, Fink K, Vey M, Taylor HV. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon 2011;57:555–65. [DOI] [PubMed] [Google Scholar]
- 40.Lam TI, Stanker LH, Lee K, Jin R, et al. Translocation of botulinum neurotoxin serotype A and associated proteins across the intestinal epithelia. Cell Microbiol 2015;17:1133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frevert J, Dressler D. Complexing proteins in botulinum toxin type A drugs: a help or a hindrance? Biologics 2010;4:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellows S, Jankovic J. Immunogenicity associated with botulinum toxin treatment. Toxins (Basel) 2019;11:E491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dressler D, Pan L, Adib Saberi F. Antibody-induced failure of botulinum toxin therapy: re-start with low-antigenicity drugs offers a new treatment opportunity. J Neural Transm (Vienna) 2018;125:1481–6. [DOI] [PubMed] [Google Scholar]
- 44.Naumann M, Boo LM, Ackerman AH, Gallagher CJ. Immunogenicity of botulinum toxins. J Neural Transm (Vienna) 2013;120:275–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


