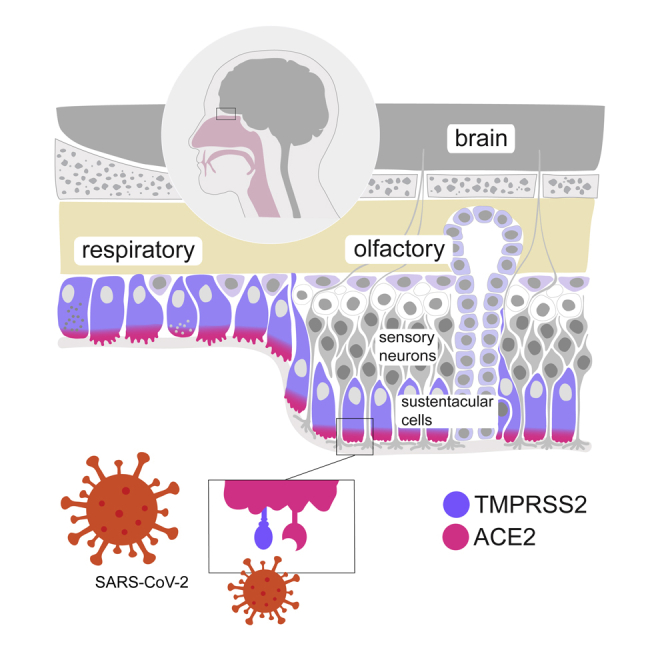
Summary
Reports indicate an association between COVID-19 and anosmia, as well as the presence of SARS-CoV-2 virions in the olfactory bulb. To test whether the olfactory neuroepithelium may represent a target of the virus, we generated RNA-seq libraries from human olfactory neuroepithelia, in which we found substantial expression of the genes coding for the virus receptor angiotensin-converting enzyme-2 (ACE2) and for the virus internalization enhancer TMPRSS2. We analyzed a human olfactory single-cell RNA-seq dataset and determined that sustentacular cells, which maintain the integrity of olfactory sensory neurons, express ACE2 and TMPRSS2. ACE2 protein was highly expressed in a subset of sustentacular cells in human and mouse olfactory tissues. Finally, we found ACE2 transcripts in specific brain cell types, both in mice and humans. Sustentacular cells thus represent a potential entry door for SARS-CoV-2 in a neuronal sensory system that is in direct connection with the brain.
Subject Areas: Biological Sciences, Microbiology, Virology, Cell Biology, Bioinformatics, Omics, Transcriptomics
Graphical Abstract
Highlights
-
•
SARS-CoV-2 receptors ACE2 and TMPRSS2 are expressed in olfactory neuroepithelia
-
•
ACE2 and TMPRSS2 are coexpressed in supporting sustentacular cells
-
•
A subset of neuronal and non-neuronal cells in the brain transcribe ACE2
Biological Sciences; Microbiology; Virology; Cell Biology; Bioinformatics; Omics; Transcriptomics
Introduction
A novel virus from the Coronaviridae family, termed SARS-CoV-2, which emerged in December 2019 in East Asia, is currently expanding on the planet. Its exact history is unknown, but its genomic sequence suggests that it was transmitted from bats to humans via an intermediate animal host (Zhou et al., 2020). It is now transmitted from human to human (Chan et al., 2020) and from human to cat (Shi et al., 2020). Infection by SARS-CoV-2 is associated in our species with a severe respiratory syndrome called COVID-19 and is characterized by a substantial mortality rate (Huang et al., 2020; Wang et al., 2020a; Zhou et al., 2020).
Entry of SARS-CoV-2 into target cells depends on the Spike protein (S), which is present on the virus capsid (Letko et al., 2020; Walls et al., 2020). Viral attachment involves an interaction between S and the angiotensin-converting enzyme-2 (ACE2), located on the surface of the target cell. One reported mechanism facilitating virus entry consists, in association with ACE2, in the priming of S by the cellular serine protease TMPRSS2, also attached to the cellular membrane, which eventually leads to the fusion between the cellular and the viral membranes (Hoffmann et al., 2020; Zhou et al., 2020). Expectedly, the main targets of SARS-CoV-2, respiratory cells that line the respiratory airways, coexpress ACE2 and TMPRSS2 (Bertram et al., 2012). Other proteins have been proposed to mediate SARS-CoV-2 internalization, in particular CD147 (Wang et al., 2020c) as an alternative receptor, and cathepsin (Ou et al., 2020) and furin-like proteases (Shang et al., 2020; Walls et al., 2020) as internalization activators.
Starting with anecdotal reports, both from SARS-CoV-2-infected patients and medical staff that suggested an association between viral infection and alterations of olfactory perception (Giacomelli et al., 2020; Perrigo, 2020) (and also taste sensitivity), the link between SARS-CoV-2 infection and olfactory dysfunction is today clearly established (Heidari et al., 2020; Hopkins et al., 2020; Luers et al., 2020; Moein et al., 2020; Spinato et al., 2020; Xydakis et al., 2020). A smartphone app recording self-reported olfactory symptoms is even used to predict potential COVID-19 (Menni et al., 2020). The olfactory perturbations, which range from mild to more severe, are usually reversible and affect up to 95% of patients with COVID-19 depending on the report. Whether this olfactory perturbation results from a deterioration of the nasal sensor or of a more central perturbation is unclear, but the second hypothesis is not to be discarded since neurological manifestations of brain origin also appear to be associated with COVID-19 infection (Gutierrez-Ortiz et al., 2020; Moriguchi et al., 2020; Paterson et al., 2020; Poyiadji et al., 2020; Wang et al., 2020b), and since SARS-CoV-2 virions have been found in the olfactory bulb (Nampoothiri et al., 2020).
The mammalian nasal cavity can be divided into two areas: the respiratory and the olfactory areas, which are anatomically, cellularly, and functionally different (Smith and Bhatnagar, 2019) (Figure 1A). In humans, the respiratory part covers the major part of the nasal cavity. It includes the turbinates and is lined with a ciliated pseudostratified columnar epithelium. Its function is to humidify, cool, or warm the incoming air, and to trap small particles before they get into the lungs. The second nasal area corresponds to the olfactory neuroepithelium. In our species, it is located in the very dorsal part of the cavity. There, it contacts volatile chemicals entering the nose, an interaction that represents the first step in the process that leads to the identification of a given smell. This epithelium is pseudostratified and includes Bowman's glands, olfactory sensory neurons, sustentacular cells, microvillar cells, globose, and horizontal basal cells (cells that keep dividing during adult life and replenish the pool of sensory neurons) (Moulton and Beidler, 1967). Each sensory neuron extends a single axon toward the olfactory bulb, which crosses the cribriform plate before reaching the olfactory bulb in the brain. On its apical side, the olfactory neuron extends a dendrite, which ends in multiple and long specialized cilia in contact with the outside world. These are covered with odorant receptors and bath in the mucus that lines the nasal cavity. Olfactory sensory neuron dendrites are enwrapped inside specialized cells, termed sustentacular cells (Liang, 2018), whose nuclei and cell bodies line the external layer of the thick neuroepithelium (although they remain attached to the basal lamina). The role played by the latter in maintaining the integrity and function of the neuroepithelium is critical, in a very similar way that Sertoli cells support germ cell development and survival. Indeed, contact of the olfactory mucosa with various drugs (such as 3-methylindole [Miller and O'Bryan, 2003], the anti-thyroid drug methimazole [Bergstrom et al., 2003], or nickel sulfate [Jia et al., 2010]) to which sustentacular cells are very sensitive leads to transient anosmia.
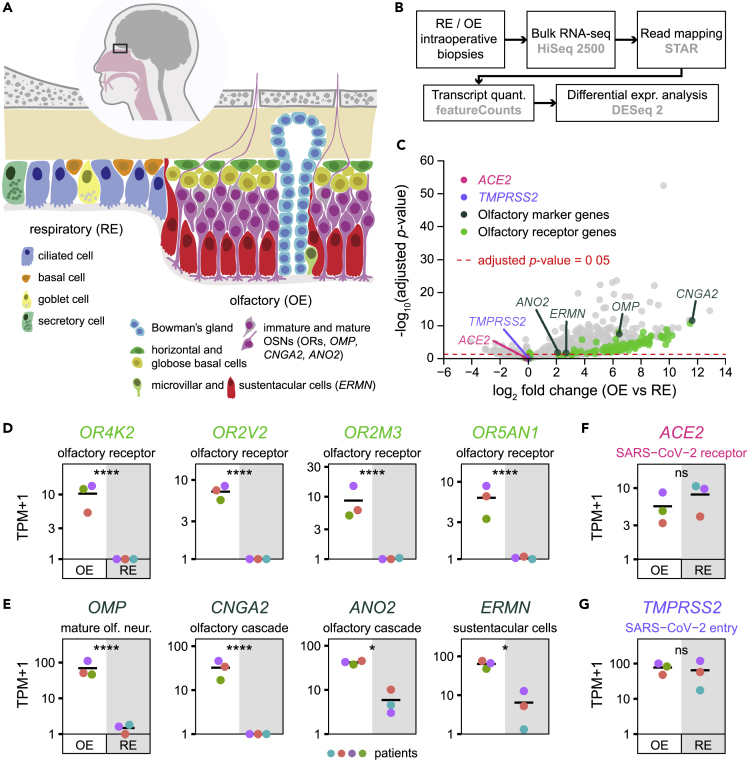
Figure 1.
ACE2 and TMPRSS2 Expression in Olfactory and Respiratory Nasal Epithelia
(A) Schematic of the human nasal respiratory and olfactory epithelia, with their respective cell types.
(B) Design of the bulk tissue transcriptome analysis. OE biopsies: n = 3, RE biopsies: n = 3.
(C) Volcano plot corresponding to the differential expression analysis, displaying the expression fold change and the associated adjusted p value (∗∗∗∗: p value ≤ 0.0001; ∗: 0.01 < p value ≤ 0.05; ns: non-significant, p value > 0.05) between olfactory and respiratory epithelia for each gene. OR genes, olfactory transduction cascade specific genes, and SARS-CoV-2 entry genes ACE2 and TMPRSS2 are highlighted with a color code.
(D–G) Transcript quantifications corresponding to (D) OR genes, (E) olfactory sensory epithelium markers and (F and G) SARS-CoV-2 entry proteins in olfactory and respiratory biopsies. A different color was attributed to the data of each patient (n = 4). Olfactory and respiratory epithelium sample data points are shown on a white and gray background, respectively. OE, olfactory epithelium; RE, respiratory epithelium.
Whether the apparent olfactory dysfunction associated with SARS-CoV-2 infection results from a general inflammation of the nasal cavity or from a more direct perturbation of the olfactory neuroepithelium (or olfactory bulb) is unclear (Cooper et al., 2020). In any case, it is critical to determine whether this virus disposes of a niche to replicate just under the cribriform plate of the ethmoid bone, a structure with large holes through which olfactory neuron axonal projections directly contact the olfactory bulb and offer a potential gateway to the brain. This latter, if expressing SARS-CoV2 receptors, could in turn become infected.
We asked whether specific cells present in the human olfactory neuroepithelium as well as cells in the brain may represent targets to SARS-CoV-2, by looking at the molecular players involved in infection, both at the RNA and protein levels.
Results
Expression of ACE2 and TMPRSS2 Human Sustentacular and Respiratory Ciliated Cells
We collected biopsies via nasal endoscopic surgery from four adult patients and explored the potential expression of ACE2 and TMPRSS2. Samples of both nasal respiratory and olfactory sensory epithelia were harvested. Bulk tissue RNA was extracted and libraries were generated and sequenced (Figure 1B).
To assess the specificity of our dissection, we performed a differential expression analysis between the respiratory and olfactory epithelia datasets (Figure 1B). As expected, olfactory-specific genes, including olfactory receptor genes, CNGA2 and ANO2, OMP, and ERMN (encoding critical elements of the olfactory transduction cascade, a specific marker of mature olfactory sensory neurons and a marker of sustentacular cells, respectively), were significantly enriched in olfactory samples (Figures 1C–1E). The presence of ACE2 and TMPRSS2 transcripts was then evaluated. We observed a mean of 7.1 and 63.7 TPMs in the respiratory epithelium samples for ACE2 and TMPRSS2, respectively (Figures 1F and 1G), reflecting the presence of ciliated cells, which represent targets of SARS-CoV-2. We found a mean of 4.6 and 76.1 TPMs in the sensory neuroepithelium samples for ACE2 and TMPRSS2, respectively (Figures 1F and 1G), indicating the presence in this tissue of cells that may express both genes, or of a mix of cells that express either TMPRSS2 or ACE2.
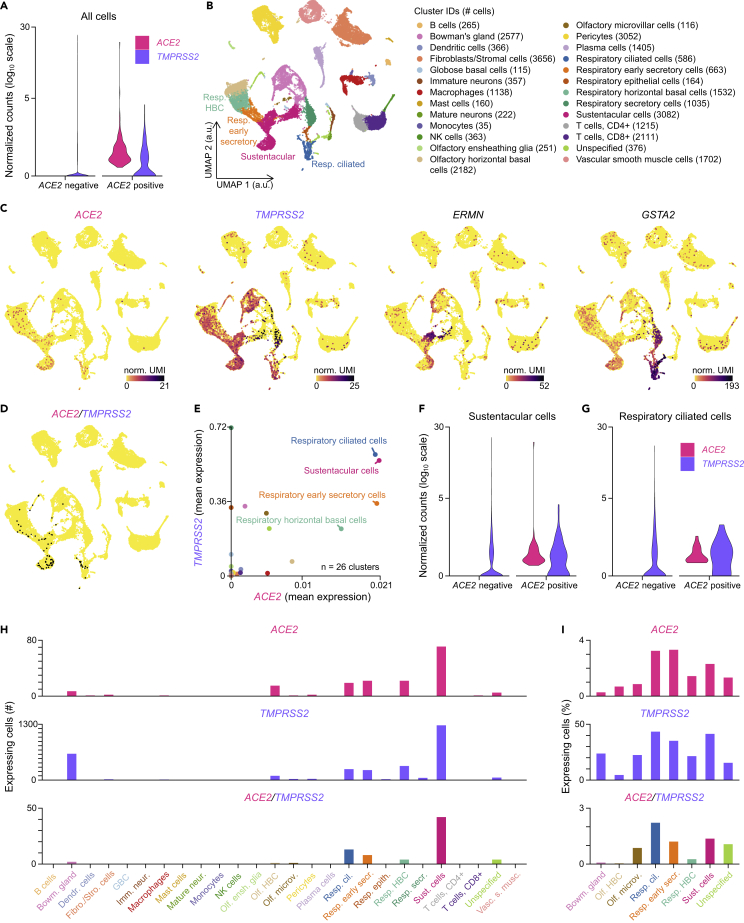
To identify putative viral targets transcribing both ACE2 and TMPRSS2 in the neuroepithelium, we took advantage of a very recently published dataset reported by Durante et al. (2020). This dataset contains the transcriptome of 28,726 single cells, collected during nasal endoscopic surgery of four adult patients. Prior to any cell type analysis, we monitored the existence of cells that would transcribe both ACE2 and TMPRSS2 (Figure 2A). We then performed an aggregate analysis of the 28,726 single cells and generated a UMAP dimensionality reduction plot, which allowed to display the clustering of the 26 different cell types reported in the original publication (Durante et al., 2020), including olfactory, respiratory, and immune cells (Figure 2B). We then identified all cells expressing ACE2, TMPRSS2, ERMN (an olfactory sustentacular cell marker) and GSTA2 (a marker of respiratory ciliated and epithelial cells) (Figures 2C, 2E, 2H, and 2I), and those coexpressing both ACE2 and TMPRSS2, respectively (Figures 2D, 2H, and 2I). Olfactory sensory neurons showed little or no expression of TMPRSS2 and ACE2, respectively (Figure 2C). In contrast, different types of respiratory cells, and also olfactory sustentacular cells, expressed ACE2. Many of these cells also coexpressed TMPRSS2. To better evaluate the coexpression levels of ACE2 and TMPRSS2 in the different cellular populations, we plotted the mean normalized expression levels of ACE2 and TMPRSS2 corresponding to each of these subpopulations (Figure 2E). Sustentacular cells and ciliated respiratory cells exhibited the highest levels of expression of both genes. Populations containing olfactory horizontal cells, microvillar cells, and Bowman's glands also showed transcription of both genes, although at lower levels. To better evaluate the different expression levels inside the two cell types expressing the highest levels of both ACE2 and TMPRSS2, we plotted the distribution of normalized transcript levels across all cells of both cell types (Figures 2F and 2G). Finally, the number (Figure 2H) and percentage (Figure 2I) of cells expressing ACE2, TMPRSS, or both genes were evaluated.
Figure 2.
Coexpression of ACE2 and TMPRSS2 in Human Sustentacular and Respiratory Ciliated Cells
(A) Violin plots displaying ACE2 and TMPRSS2 normalized expression levels in all ACE2-positive (n = 169) and ACE2-negative (n = 28,557) cells.
(B) Visualization of the clustering results reported in Durante et al.(2020) on a uniform manifold approximation and projection (UMAP) plot. “Respiratory columnar cells” was renamed as “Respiratory early secretory cells” based on the expression of SERPINB3. a.u., arbitrary units.
(C) Normalized expression levels of ACE2, TMPRSS2, ERMN (a sustentacular cell gene marker), and GSTA2 (a respiratory ciliated cell gene marker), shown on the UMAP plot. norm. UMI, normalized unique molecular identifier counts.
(D) ACE2 and TMPRSS2 coexpressing cells, highlighted in black on the UMAP plot.
(E) Coexpression of ACE2 and TMPRSS2 in the different cell clusters (n = 26 clusters). Means of ACE2 and TMPRSS2 normalized expression levels are plotted.
(F and G) Violin plots displaying ACE2 and TMPRSS2 normalized expression levels in (F) ACE2-positive (n = 71) and ACE2-negative (n = 3,011) sustentacular cells, and in (G) ACE2 positive (n = 19) and ACE2 negative (n = 567) respiratory ciliated cells.
(H) Number of cells per cluster expressing ACE2 and/or TMPRSS2.
(I) Percentage of cells per cluster expressing ACE2 and/or TMPRSS2 in all coexpressing clusters (i.e., clusters with double-positive cells). Color gradient scales in (C) and y axis scales in (A), (F) and (G) are in log10.
Respiratory cells are thus not the only cells in contact with the outside world that exhibit the molecular keys involved in SARS-CoV-2 entry in the nose. Sustentacular cells, which lie at the interface between the central nervous system and the olfactory cavity, share the same characteristics.
ACE2 and TMPRSS2 Protein Expression in the Olfactory Neuroepithelium
Quantification of gene transcripts at the single cell level, a commonly used proxy of protein abundance, often leads to very inaccurate predictions, up to a complete discordance between real transcript and protein levels (Bauernfeind and Babbitt, 2017; Liu et al., 2016). Since it is the protein that matters here, and at the individual cell level, we investigated the expression of ACE2 and TMPRSS2 in tissues by immunohistochemistry. Given that ortholog tissue specificity is highly conserved between tetrapod species (Kryuchkova-Mostacci and Robinson-Rechavi, 2016), and in particular between mouse and humans (Liao and Zhang, 2006), we analyzed mouse tissues in parallel to human ones.
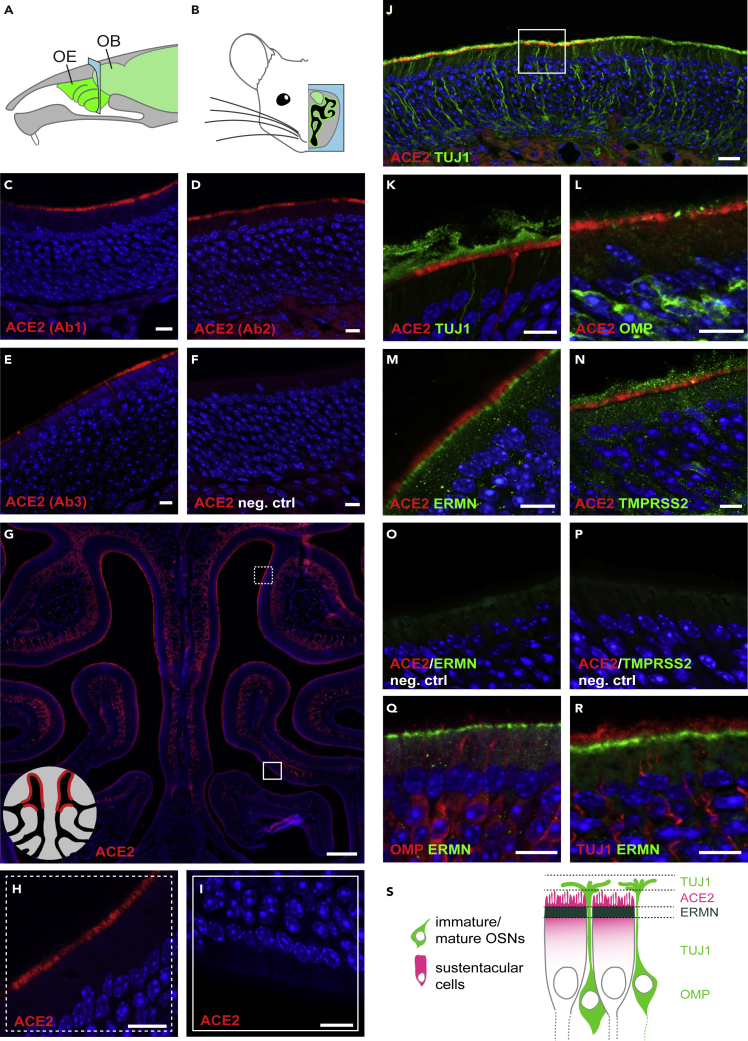
We first evaluated the potential ACE2 immunoreactivity of the mouse olfactory epithelium (Figures 3A and 3B). To assess antibody specificity, we used independently three different anti-ACE2 antibodies, which were raised against the extracellular part (Ab1 and Ab3 developed in goat and rabbit, respectively) or the intracellular part of ACE2 (Ab2, developed in rabbit). These antibodies were first evaluated to label colon and kidney tissues, which contain previously described ACE2-expressing cell types (Figures S1A–S1J). In the olfactory cavity, a strong and defined labeling was observed in the respiratory epithelium in the nasal cavity and nasopharyngeal duct (Figures S3A–S3H), and in a subset of sustentacular cells lining the nasal cavity (Figures 3C–3G and S3B). These were almost exclusively located in the dorsal part of the epithelium, with a relatively abrupt transition area (Figures 3H and 3I). All antibodies showed a labeling of the apical portion of sustentacular cells, except for a slight labeling of their somata with Ab2 (Figure 3D). To clearly differentiate olfactory sensory neurons from sustentacular cells, we colabeled the sections with TUJ1 (a marker of neurons), OMP (a marker of mature sensory neurons),and ERMN (a marker of sustentacular cells) (Figures 3J–3M, 3O, 3Q, and 3R). The localization of ACE2 was at the very luminal border of the sustentacular cells, sandwiched between the sensory neurons TUJ1-expressing cilia and ERMN, which labels the apical part of sustentacular cells (Figures 3J–3S). We also evaluated TMPRSS2 immunoreactivity in the mouse olfactory epithelium (Figures 3N and 3P). We tested two different antibodies, which were first evaluated on kidney tissue (Figure S2). In the mouse main olfactory epithelium, sustentacular cells were positive for TMPRSS2, and several other cell types showed weak expression (Figures 3N, S2A, S2B, S2E, and S2F).
Figure 3.
ACE2 expression in the Mouse Olfactory Neuroepithelium
(A) Schematic representation of the mouse olfactory system. The sensory tissue and the brain are highlighted in green. The blue frame indicates the coronal section level corresponding to the next panels. OB, olfactory bulb; OE, olfactory epithelium.
(B) Schematic representation of a coronal section of the mouse olfactory system. The OE is highlighted in green, and the brain, including the olfactory bulb, in light green.
(C–F) Immunostainings on coronal sections of mouse olfactory neuroepithelium with three different anti-ACE2 antibodies (red) and a negative control. (C) ACE2 Ab1, polyclonal antibody against the extracellular domain of mouse ACE2. (D) ACE2 Ab2, polyclonal antibody against the intracellular domain of human ACE2 (that cross reacts with mouse ACE2). (E) ACE2 Ab3, monoclonal antibody against the extracellular domain of human ACE2 (that cross reacts with mouse ACE2). (F) Negative control immunostaining without primary antibody against ACE2.
(G) Immunostaining for ACE2 (red) on a coronal section of the mouse olfactory neuroepithelium. Scale bar, 0.2 mm. Squares indicate zones magnified in (H) and (I). The schematic on the lower left indicates that ACE2 expression is observed in the dorsal (H), but not in the ventral part (I) of the olfactory epithelium.
(J) Double immunostaining for ACE2 (red) and the neuronal marker TUJ1 (green) in the ACE2-positive part of the mouse olfactory neuroepithelium. The square indicates the portion of the MOE that is magnified in (K)–(R). Scale bar, 20 μm.
(K–N) Double immunostaining for ACE2 (red) together with (K) the neuronal marker TUJ1 (green), (L) the marker of mature olfactory sensory neurons OMP (green), (M) the marker for sustentacular cells ERMN (green) and (N) TMPRSS2 (green).
(O and P) Negative control immunostaining without primary antibody against (O) ERMN and (P) TMPRSS2.
(Q and R) Double immunostaining for the sustentacular cell marker ERMN (red) together with (Q) OMP and (R) TUJ1.
(S) Schematic of the external part of the olfactory epithelium, highlighting the relative positions of ACE2, TUJ1, ERMN, and OMP according to the immunostainings shown in (K)–(R). TUJ1 is present in the dendrites, axons, and somata of olfactory sensory neurons, OMP more concentrated in the cell body of olfactory sensory neurons, and ACE2 and ERMN in the apical portion of sustentacular cells. All sections with immunostaining were counterstained with DAPI (blue). When not indicated, scale bars are 10 μm. See also Figures S1–S3.
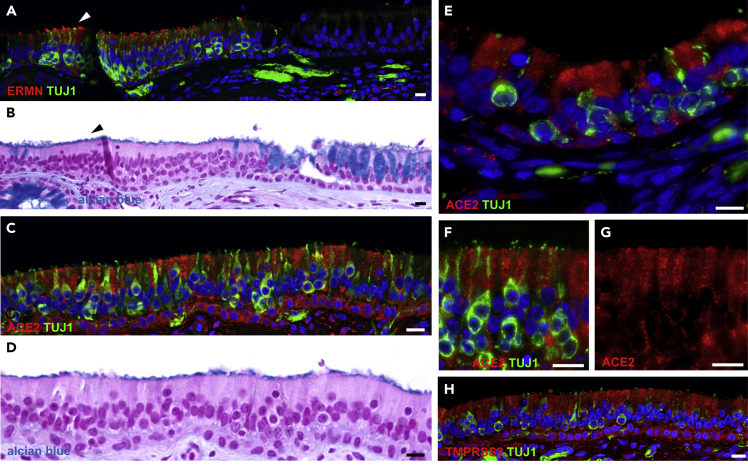
We then evaluated the expression of ACE2 in the human nasal cavity by immunohistochemistry. Similarly to what is observed in the mouse, the human neuroepithelium can be discriminated from the respiratory epithelium by the presence of TUJ1-positive olfactory sensory neurons (Figure 4A), ERMN-positive sustentacular cells along the apical border of the tissue (Figures 4A and S4C–S4E), as well as the absence of Alcian blue-labeled goblet cells (Figure 4B). In accordance with the single-cell data, a strong labeling of ACE2 was observed in some sustentacular cells of the sensory epithelium (Figures 4C–4G). However, the very apical localization of ACE2 observed in the mouse neuroepithelium was not recapitulated in humans, but ACE2 was rather enriched in the apical half of the cells. A similar somatic staining was also observed, with the same antibody, in proximal tubule cells of the mouse kidney (Figure S1D). Congruently with its pervasiveness in the single-cell transcriptomes, TMPRSS2 labeling was observed in many constituents of the nasal epithelium (Figures 4H, S4F, and S4G).
Figure 4.
ACE2 Expression in the Human Olfactory Neuroepithelium
(A and B) Section of human respiratory epithelium with (A) immunostaining for ERMN (red) and the neuronal marker TUJ1 (green), and (B) the same section stained with Alcian blue and counterstained with nuclear fast red, highlighting a transition between sensory (left) and respiratory (right, note the goblet cells) epithelium. ERMN is expressed in the apical part of sustentacular cells within the olfactory neuroepithelium (arrowheads).
(C, E) A portion of human olfactory neuroepithelium immunostained for ACE2 (red) and the neuronal marker TUJ1 (green), and (D) with Alcian blue (note the absence of goblet cells).
(F, G) Higher magnification of the external border of the human olfactory neuroepithelium. The same region is shown with (F) and without (G) TUJ1 staining. Nuclei were counterstained with DAPI (blue) or FastRed (red). Scale bars, 10 μm. See also Figures S4 and S5.
We sought to investigate whether the SARS-CoV-2 virus was present in sustentacular cells of patients infected with the virus. The olfactory sensory epithelium of two deceased SARS-CoV-2-infected individuals was analyzed. It was found to be highly damaged and largely detached from the basal lamina (Figure S5), hindering the potential identification of viral particles in sustentacular cells. Whether this damage was caused by the virus cannot be determined based on the few samples analyzed. However, a study of SARS-CoV-2-infected Syrian hamsters showed infection of sustentacular cells and a massive desquamation of the olfactory epithelium (Bryche et al., 2020).
SARS-CoV-2 Receptor Expression in the Brain
SARS-CoV (i.e., the virus responsible for the previous SARS epidemic in 2002), the genome of which is closely related to the one of SARS-CoV-2 and whose effects on human tissues appear similar to those observed with SARS-CoV-2, has been observed in human brains (Ding et al., 2004; Gu et al., 2005; Xu et al., 2005). Could SARS-CoV-2 replicate in the olfactory epithelium and could the infection spread to the brain? To explore the potential receptivity to SARS-CoV-2 infection of the various cell types in the brain, we evaluated, again, the potential coexpression of ACE2 and TMPRSS2 in neuronal and non-neuronal cell types in the central nervous system of both mice and humans.
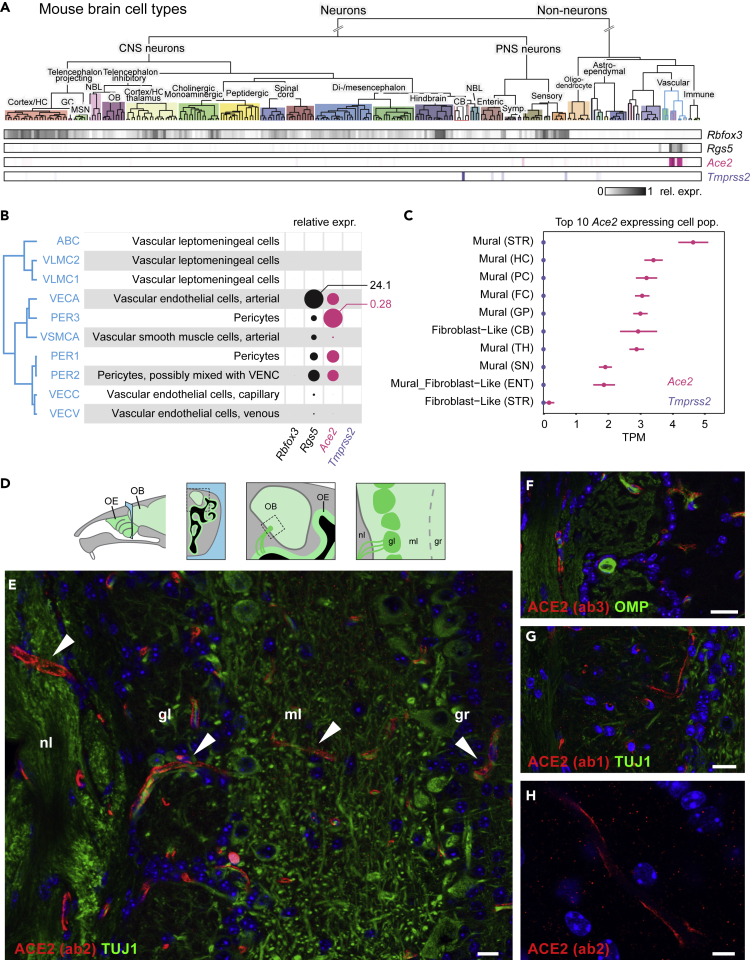
For the mouse, we took advantage of two available single-cell RNA sequencing (RNA-seq) datasets (Saunders et al., 2018; Zeisel et al., 2018), consisting in two broad collections of mouse brain cell types. In the first collection, published by Zeisel et al. (2018), which includes non-neuronal cells, we found a very limited number of cell types that expressed Ace2 (Figure 5A). These cells are related to pericytes and coexpress the mural cell marker Rgs5 (Figure 5B). Tmprss2, whose transcripts were barely detected, was expressed by even fewer cell types (Figure 5A), with Purkinje cells displaying the highest expression level. These observations were confirmed by the second mouse dataset (Saunders et al., 2018), in which mural cells were the main cell type that expressed Ace2 (Figure 5C). None of these cell populations coexpressed Tmprss2. Although our data showed substantial expression of ACE2 in the mouse brain, they did not replicate the widespread expression of ACE2 in the central nervous system previously reported by others, which found ACE2 in the motor cortex, raphe, and nuclei involved in the regulation of cardiovascular function (Doobay et al., 2007). To precisely evaluate the expression of ACE2 in the mouse brain, and in particular at the interface with the olfactory sensory neuroepithelium, we performed an immunostaining of mouse olfactory bulb sections with the three different anti-ACE2 antibodies. A strong labeling of capillary-associated cells was observed through the different layers of the olfactory bulb (Figures 5D–5H), in agreement with the single-cell RNA-seq observations.
Figure 5.
Ace2 and Tmprss2 Expression in the Mouse Brain
(A) Mouse brain cell type classification from Zeisel et al. (2018) was visualized via http://mousebrain.org/. Rows with shaded boxes below the tree indicate the gene mean expression levels for each cell type, relative to its maximum. Rbfox3, encoding the neuronal marker NEUN, and Rgs5, a marker of pericytes, are shown in comparison with Ace2 and Tmprss2.
(B) Details of the gene expression levels in pericytes (blue edges clade of the classification dendrogram in (A)). Discs represent mean relative expression levels. Maximum values of mean normalized expression levels for Rgs5 and Ace2 are connected to the corresponding circles.
(C) Ace2 and Tmprss2 expression levels in the top 10 Ace2-expressing cell populations from the http://dropviz.org/ dataset (Saunders et al., 2018). Abbreviations in parentheses denote the origin brain structure. STR, striatum; HC, hippocampus; PC, posterior cortex; FC, frontal cortex; GP, globus pallidus; CB, cerebellum; TH, thalamus; SN, substantia nigra; ENT, entopeduncular.
(D) Similar to Figure 3, subsequent panels show coronal sections (blue area) crossing the nasal cavity and the olfactory bulb. As represented in this schematic, olfactory sensory neurons from the olfactory epithelium send axonal projections to the olfactory bulb through the cribriform plate of the ethmoid bone, along the nerve layer. OB, olfactory bulb; OE, olfactory epithelium; nl, nerve layer; gl, glomerular layer; ml, mitral cell layer; gr, granule cell layer.
(E–G) Immunostaining for ACE2 (red) and for the neuronal marker TUJ1 (green) on sections of the mouse olfactory bulb. White arrowheads indicate the presence of ACE2 in the pericytes of capillaries. Scale bars, 20 μm.
(H) Magnification of capillary ACE2 immunostaining (red). Scale bar, 5 μm. All sections were counterstained with DAPI (blue).
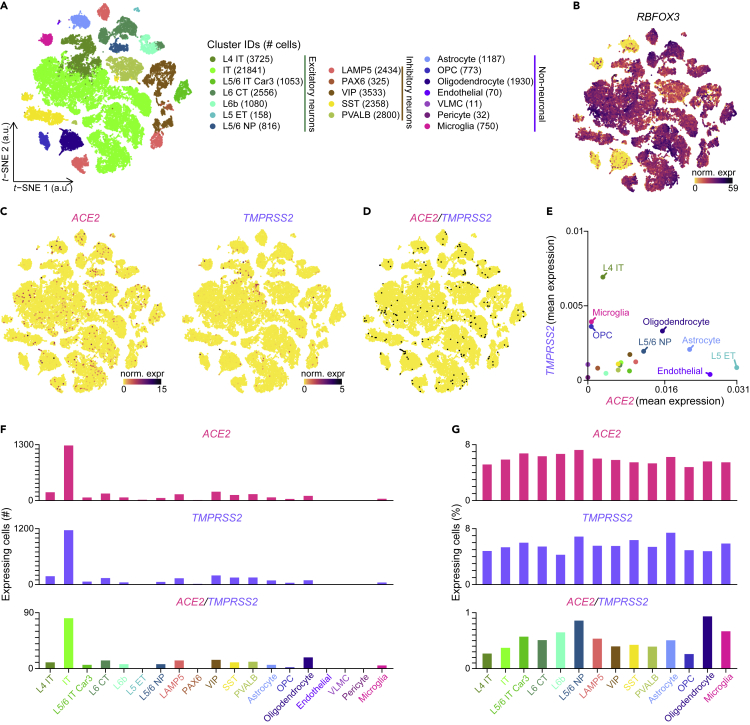
Finally, we explored two single-nucleus RNA-seq datasets to evaluate ACE2 expression in the human brain. In the first dataset (Lake et al. (2018)), we found neuronal and glial cell types, in particular Purkinje neurons and cerebellar astrocytes, that expressed ACE2 or TMPRSS2 (Figures S6A–S6E). In the second dataset, publicly available in the Allen Brain Map cell types database, we found expression of ACE2 (although at relatively limited levels) in various non-neuronal and neuronal types, in particular astrocytes, oligodendrocytes, and neurons from the cortical layer 5 (Figures 6A–6G), a portion of which coexpressed TMPRSS2 (Figures 6C–6E). ACE2 and TMPRSS2 are therefore expressed in a number of cell types in the human brain.
Figure 6.
Coexpression of ACE2 and TMPRSS2 in the Human Brain
(A) Visualization of the human brain cell-type subclasses on a t-distributed stochastic neighbor embedding (t-SNE) plot. a.u., arbitrary units.
(B and C) Normalized expression levels of (B) RBFOX3 (which encodes for the neuronal marker NEUN), (C) ACE2 and TMPRSS2, shown on the t-SNE plot. norm. expr, normalized expression levels.
(D) ACE2 and TMPRSS2 coexpressing cells, highlighted in black on the t-SNE plot.
(E) Coexpression of ACE2 and TMPRSS2 in the different subclasses (n = 19 subclasses). Means of ACE2 and TMPRSS2 normalized expression levels are plotted.
(F) Number of cells per subclass expressing ACE2 and/or TMPRSS2.
(G) Percentage of cells per subclass expressing ACE2 and/or TMPRSS2 in all coexpressing subclasses (i.e., subclasses with double-positive cells). Color gradient scales in (B) and (C) are in log10. L, cortical layer; IT, intratelencephalic; CT, corticothalamic; ET, extratelencephalic; NP, near-projecting; LAMP5, lysosomal-associated membrane protein family member 5; PAX6, paired box 6; VIP, vasoactive intestinal polypeptide; SST, somatostatin; PVALB, parvalbumin; OPC, oligodendrocyte precursor cell; VLMC, vascular leptomeningeal cell. See also Figure S6.
Discussion
Facing the current pandemic, worldwide efforts have been made to better understand the molecular bases of SARS-CoV-2 infection. Here, we aimed at investigating the distribution of the SARS-CoV-2 receptor ACE-2 in the human olfactory neuroepithelium and in the brain. Other groups, whose results are mostly in line with our findings, have also addressed parallel questions (Bilinska et al., 2020; Brann et al., 2020a, 2020b; Sungnak et al., 2020).
Taking a multidisciplinary approach, based both on our own and publicly available RNA-seq datasets, and on immunohistochemical staining of mouse and human tissues, we demonstrate here that a subset of olfactory sustentacular cells in the olfactory neuroepithelium, but not olfactory sensory neurons, expresses ACE2, the main player in the binding and entry of the SARS-CoV-2 into human cells. These cells were found to coexpress TMPRSS2, a serine protease known to facilitate viral entry. We also found ACE2 expression in specific cell types of the brain, including neuronal and non-neuronal cell types.
We first determined, using transcriptomic analyses of whole tissue and single cells from human olfactory epithelia, that a subset of sustentacular cells expresses ACE2. We recently reported this finding as a preprint on BioRxiv (Fodoulian et al., 2020), which is in agreement with another preprint on the same server (Brann et al., 2020a). We then confirmed this finding in mouse and human tissues by immunohistochemistry. In the mouse, in which the olfactory mucosa is particularly well organized both in terms of pseudostratified layers and in terms of its very strict separation from the respiratory epithelium, we observed (similarly to humans) a clear expression of ACE2 in the apical border of sustentacular cells (in agreement with a very recent report citing our BioRxiv article [Bilinska et al., 2020]). This distribution was, however, non-homogenous, since ACE2 was present in the most dorsally located sustentacular cells, but was entirely absent from more ventrally located ones. Such dichotomy between the dorsal and the ventral neuroepithelium is reminiscent, in the mouse, of the dorsally located olfactory neurons, which are known to share molecular markers that are dissimilar from those present in more ventral neurons (Gussing and Bohm, 2004). This known contrast is not limited to the neuronal identities, since the function and response to stress of these dorsally located neurons also appear to be different (Kobayakawa et al., 2007; Tuerdi et al., 2018). We now add a non-neuronal cell type to this dorsoventral neural dichotomy. In the human olfactory neuroepithelium, a similar labeling of ACE2 was observed in sustentacular cells, although the staining was mainly present in the somata. Again, as observed in the mouse, some of these cells were positive and others were negative for ACE2. These human ACE2-expressing sustentacular cells were found isolated or in small clusters, intermingled with sustentacular cells negative for ACE2, or even sometimes intermingled with respiratory epithelium, an expected observation given the relatively poor organization of the human olfactory neuroepithelium, in particular in aged individuals.
How likely is it that the coexpression of ACE2 in olfactory sustentacular cells is involved in the SARS-CoV-2-induced anosmia? And why are horizontal basal cells that also express ACE2 not prime candidates? First, given the slow rate of neuronal renewal in the main olfactory epithelium (a few months) and the apparent rapid development of SARS-CoV-2-triggered anosmia, a perturbation of the stem cell pool constituted by the horizontal basal cells appears unlikely to represent an immediate disturbance to the system. Second, the amounts of ACE2 transcripts as well as the intensity of the immunohistological staining we observed in sustentacular cells are in the same range as the ones observed in respiratory ciliated cells, suggesting at least that sustentacular cells play in the same league in terms of SARS-CoV-2 receptors. Third, sustentacular cells are in direct contact with the olfactory cavity and thus interact with everything that enters the nose. Fourth, sustentacular cells play a critical role in the maintenance of the olfactory neuroepithelium integrity: their loss or suffering would cause disaggregation or malfunctioning of the neuroepithelium, respectively, leading to anosmia. Supporting this, SARS-CoV-2 infection of golden hamsters via the nasal route leads to massive damage of the olfactory neuroepithelium (Bryche et al., 2020). Finally, given that the role played by ACE2 in sustentacular cells is unknown, the hijack of this receptor by SARS-CoV-2 may by itself perturb the functioning of the olfactory sensor. Taken together, and despite the fact that one cannot exclude inflammation and the infection of other non-neuronal cell types in the olfactory neuroepithelium as an origin of the SARS-CoV-2-induced anosmia, the link between the viral molecular entry tools expressed by olfactory sustentacular cells and the SARS-CoV-2-induced chemosensory alteration appears quite credible.
The idea that viruses may affect directly or indirectly the integrity and function of the sensory part of the olfactory system is not new. Some viruses indeed disturb the neuroepithelium in different ways, and often alter specific cell types, including neurons. For example, and among many others, olfactory sensory neurons and sustentacular cells were shown to be major portals of host entry of the Murid Herpesvirus-4 (MuHV-4) (Milho et al., 2012). Again, the direct contact of this latter cell type with the respiratory tract, as well as its phagocytic activity, may explain its particular sensitivity to viral infection. The cell type targeted by viruses matters, in particular to fight infection using drugs. For instance, in the case of a MuHV-4 challenge, olfactory sensory neurons are not responsive to interferon treatment (IFNγ), whereas sustentacular cells are (Jacobs et al., 2019).
SARS-CoV-2-infection has not only been linked to the loss of a single chemosensory ability. Indeed, in addition to anosmia, ageusia has also been reported (Cooper et al., 2020; Giacomelli et al., 2020). Whether these reports truly reflect taste anomalies or rather olfactory perturbations that may drastically affect the flavor of food is unclear. However, this potential double effect on two chemosensory systems that share nothing at the periphery may suggest a more central alteration, involving, for example, a direct infection of the brain by SARS-CoV-2. But this would require potential targets. We identified various cell types in the brain that express ACE2, although at relatively low levels in humans. In the mouse, we detected ACE2 expression in brain vascular endothelial cells and pericytes, but not in neurons. In humans, we found ACE2 transcripts in non-neuronal cell types such as astrocytes and microglia, and also in neurons, in particular Purkinje and cortical layer 5 neurons. Whether these different cell types reflect differences in tissue specificity between the two mammalian species is unclear, since RNA-seq discordances may result from differences in the sequencing depth, sequencing protocols (single-nucleus versus single-cell RNA-seq), dissimilar mRNA half-lives, or variable cell type selection strategies between the different studies. But whatever the reason for the discordances, these data point to potential targets for SARS-CoV-2 infection in brain cells.
Similar to the infection of the olfactory neuroepithelium by SARS-CoV-2, the transport of viral particles from the olfactory mucosa to the brain would not be a first. In 1935 already, the olfactory route was hypothesized (because of its direct connection with the brain) to represent a portal for entry of viruses into the central nervous system (Flexner, 1936). We know today that the olfactory nerve route is indeed used by various viruses to reach the brain, including HSV1, poliovirus, MHV (a coronavirus), paramyxoviruses, Hendra virus, Nipah virus, influenza virus, adenoviruses, bunyaviruses, and VSV (Durrant et al., 2016).
Although most pathogenic microorganisms appear to access the CNS via the axons of olfactory sensory neurons, other routes of entry through the cribriform plate have been described. For instance, the ameba Naegleria fowleri penetrates the olfactory epithelium either via sustentacular cells or in a paracellular way and then travels along the nerve bundle through the cribriform plate (Jarolim et al., 2000). Moreover, the bacterium Burkholderia pseudomallei causes a widespread loss of OSNs, which leads to the degeneration of the olfactory nerves (St John et al., 2014). B. pseudomallei then migrates along the empty conduits of olfactory nerves toward the CNS. An even more convincing example may be the neurotropic MHV virus, which is found in high concentrations within the perineurium of the olfactory nerve and in the brain after nasal infection (Barthold, 1988) (olfactory sensory neurons lack the main MHV virus receptor CEACAM1 [Brann et al., 2020b; Miura et al., 2008).
SARS-CoV-2 virions have in fact been observed in the human olfactory bulb (Nampoothiri et al., 2020). SARS-CoV particles have also been found in the human brain (Ding et al., 2004; Gu et al., 2005; Xu et al., 2005). Adding to these observations, brain lesions were observed in a transgenic mouse model expressing the human ACE2 in the nose and infected intranasally with SARS-CoV (Netland et al., 2008). Moreover, a retrospective case study on 214 patients with COVID-19 reported neurological manifestations possibly correlated with the severity of the disease (Mao et al., 2020) (with the confounding factor that old people are more likely to develop severe disease). As worrying are suggestions that a subset of patients infected with the virus but lacking respiratory symptoms may exhibit neurologic symptoms (Wang et al., 2020b) and that they may be associated with encephalitis (Moriguchi et al., 2020; Poyiadji et al., 2020), Miller Fisher syndrome, and polyneuritis cranialis (Gutierrez-Ortiz et al., 2020). Given, as we showed here, the presence in the human brain of various neuronal and non-neuronal cell populations expressing ACE2, this certainly appears to be a line of investigation worth pursuing.
Limitations of the Study
We investigated whether the SARS-CoV-2 virus was present in sustentacular cells of patients infected with the virus. We analyzed the olfactory sensory epithelium of two deceased SARS-CoV-2-infected individuals. However, the epithelium was found to be highly damaged and largely detached from the basal lamina, hindering the potential identification of viral particles in sustentacular cells. Hence, we could not determine whether the SARS-CoV-2 virus is indeed present in sustentacular cells of infected patients. Whether the histological damage was caused by the virus cannot be determined based on the few samples analyzed.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ivan Rodriguez (ivan.rodriguez@unige.ch).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
All datasets generated during and/or analyzed during the current study have been deposited in Mendeley Data (https://doi.org/10.17632/4cynjrymgf.1) and NCBI GEO: GSE151973. All codes used for the analyses of the data reported in this manuscript are provided at the GitHub repository https://github.com/leonfodoulian/SARS_CoV_2_anosmia.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the iGE3 Genomics Platform at the University of Geneva for expert technical assistance during RNA-seq experiments. This research was supported by the University of Geneva and the Swiss National Science Foundation (grant numbers 31003A_172878 to A.C. and 310030_189153 to I.R.).
Author Contributions
L.F., J.T., M.B., D.R., C.K., and V.P. acquired and analyzed data. L.F. performed bulk, single-cell, and single-nucleus RNA-seq data analysis. J.T. performed bulk RNA-seq data analysis, mouse brain cell database mining, and confocal microscopy. D.R. generated the human bulk RNA-seq dataset. M.B. performed immunohistochemical staining and epifluorescence microscopy with help from C.K. and V.P. K.E., J.A.L., and B.N.L. collected human biopsies. L.F., J.T., M.B., D.R., A.C., and I.R. carried out the conceptualization and experimental design. A.C. and I.R. wrote the manuscript with comments from all the other authors.
Declaration of Interests
The authors declare no competing interests.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101839.
Contributor Information
Alan Carleton, Email: alan.carleton@unige.ch.
Ivan Rodriguez, Email: ivan.rodriguez@unige.ch.
Supplemental Information
References
- Barthold S.W. Olfactory neural pathway in mouse hepatitis virus nasoencephalitis. Acta Neuropathol. 1988;76:502–506. doi: 10.1007/BF00686390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind A.L., Babbitt C.C. The predictive nature of transcript expression levels on protein expression in adult human brain. BMC Genomics. 2017;18:322. doi: 10.1186/s12864-017-3674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom U., Giovanetti A., Piras E., Brittebo E.B. Methimazole-induced damage in the olfactory mucosa: effects on ultrastructure and glutathione levels. Toxicol. Pathol. 2003;31:379–387. doi: 10.1080/01926230390201101. [DOI] [PubMed] [Google Scholar]
- Bertram S., Heurich A., Lavender H., Gierer S., Danisch S., Perin P., Lucas J.M., Nelson P.S., Pohlmann S., Soilleux E.J. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7:e35876. doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem. Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D., Tsukahara T., Weinreb C., Logan D., Datta S. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. bioRxiv. 2020 doi: 10.1101/2020.03.25.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I.C., Chou H.J., Fletcher R.B. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020;6:eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryche B., St Albin A., Murri S., Lacôte S., Pulido C., Ar Gouilh M., Lesellier S., Servat A., Wasniewski M., Picard-Meyer E. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020;89:579–586. doi: 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.W., Brann D.H., Farruggia M.C., Bhutani S., Pellegrino R., Tsukahara T., Weinreb C., Joseph P.V., Larson E.D., Parma V. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020;107:219–233. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doobay M.F., Talman L.S., Obr T.D., Tian X., Davisson R.L., Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante M.A., Kurtenbach S., Sargi Z.B., Harbour J.W., Choi R., Kurtenbach S., Goss G.M., Matsunami H., Goldstein B.J. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat. Neurosci. 2020;23:323–326. doi: 10.1038/s41593-020-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant D.M., Ghosh S., Klein R.S. The olfactory bulb: an immunosensory effector organ during neurotropic viral infections. ACS Chem. Neurosci. 2016;7:464–469. doi: 10.1021/acschemneuro.6b00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexner S. Respiratory versus gastro-intestinal infection in poliomyelitis. J. Exp. Med. 1936;63:209–226. doi: 10.1084/jem.63.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodoulian L., Tuberosa J., Rossier D., Boillat M., Kan C., Pauli V., Egervari K., Lobrinus J.A., Landis B.N., Carleton A. SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. bioRxiv. 2020 doi: 10.1101/2020.03.31.013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A., Rizzardini G. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin. Infect. Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussing F., Bohm S. NQO1 activity in the main and the accessory olfactory systems correlates with the zonal topography of projection maps. Eur. J. Neurosci. 2004;19:2511–2518. doi: 10.1111/j.0953-816X.2004.03331.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Ortiz C., Mendez A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Manas R., de Aragon-Gomez F., Benito-Leon J. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- Heidari F., Karimi E., Firouzifar M., Khamushian P., Ansari R., Mohammadi Ardehali M., Heidari F. Anosmia as a prominent symptom of COVID-19 infection. Rhinology. 2020;58:302–303. doi: 10.4193/Rhin20.140. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C., Surda P., Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020;58:295–298. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Zeippen C., Wavreil F., Gillet L., Michiels T. IFN-lambda decreases murid herpesvirus-4 infection of the olfactory epithelium but fails to prevent virus reactivation in the vaginal mucosa. Viruses. 2019;11:757. doi: 10.3390/v11080757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolim K.L., McCosh J.K., Howard M.J., John D.T. A light microscopy study of the migration of Naegleria fowleri from the nasal submucosa to the central nervous system during the early stage of primary amebic meningoencephalitis in mice. J. Parasitol. 2000;86:50–55. doi: 10.1645/0022-3395(2000)086[0050:ALMSOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Jia C., Roman C., Hegg C.C. Nickel sulfate induces location-dependent atrophy of mouse olfactory epithelium: protective and proliferative role of purinergic receptor activation. Toxicol. Sci. 2010;115:547–556. doi: 10.1093/toxsci/kfq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa K., Kobayakawa R., Matsumoto H., Oka Y., Imai T., Ikawa M., Okabe M., Ikeda T., Itohara S., Kikusui T. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Kryuchkova-Mostacci N., Robinson-Rechavi M. Tissue-specificity of gene expression diverges slowly between orthologs, and rapidly between paralogs. PLoS Comput. Biol. 2016;12:e1005274. doi: 10.1371/journal.pcbi.1005274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake B.B., Chen S., Sos B.C., Fan J., Kaeser G.E., Yung Y.C., Duong T.E., Gao D., Chun J., Kharchenko P.V. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 2018;36:70–80. doi: 10.1038/nbt.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F. Olfactory receptor neuronal dendrites become mostly intra-sustentacularly enwrapped upon maturity. J. Anat. 2018;232:674–685. doi: 10.1111/joa.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao B.-Y., Zhang J. Evolutionary conservation of expression profiles between human and mouse orthologous genes. Mol. Biol. Evol. 2006;23:530–540. doi: 10.1093/molbev/msj054. [DOI] [PubMed] [Google Scholar]
- Liu Y., Beyer A., Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165:535–550. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Luers J.C., Rokohl A.C., Loreck N., Wawer Matos P.A., Augustin M., Dewald F., Klein F., Lehmann C., Heindl L.M. Olfactory and gustatory dysfunction in coronavirus disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71:2262–2264. doi: 10.1093/cid/ciaa525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Wang M., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Li Y., Jin H. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv. 2020 doi: 10.1101/2020.02.22.20026500. [DOI] [Google Scholar]
- Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., Ganesh S., Varsavsky T., Cardoso M.J., El-Sayed Moustafa J.S. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milho R., Frederico B., Efstathiou S., Stevenson P.G. A heparan-dependent herpesvirus targets the olfactory neuroepithelium for host entry. PLoS Pathog. 2012;8:e1002986. doi: 10.1371/journal.ppat.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.A., O'Bryan M.A. Ultrastructural changes and olfactory deficits during 3-methylindole-induced olfactory mucosal necrosis and repair in mice. Ultrastruct. Pathol. 2003;27:13–21. doi: 10.1080/01913120309944. [DOI] [PubMed] [Google Scholar]
- Miura T.A., Travanty E.A., Oko L., Bielefeldt-Ohmann H., Weiss S.R., Beauchemin N., Holmes K.V. The spike glycoprotein of murine coronavirus MHV-JHM mediates receptor-independent infection and spread in the central nervous systems of Ceacam1a-/- Mice. J. Virol. 2008;82:755–763. doi: 10.1128/JVI.01851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton D.G., Beidler L.M. Structure and function in the peripheral olfactory system. Physiol. Rev. 1967;47:1–52. doi: 10.1152/physrev.1967.47.1.1. [DOI] [PubMed] [Google Scholar]
- Nampoothiri S., Sauve F., Ternier G., Fernandois D., Coelho C., Imbernon M., Deligia E., Perbet R., Florent V., Baroncini M. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis. bioRxiv. 2020 doi: 10.1101/2020.06.08.139329. [DOI] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrigo B. Time; 2020. Why Losing Your Sense of Smell Could Be a Symptom of COVID-19. [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A., Macosko E.Z., Wysoker A., Goldman M., Krienen F.M., de Rivera H., Bien E., Baum M., Bortolin L., Wang S. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018;174:1015–1030.e16. doi: 10.1016/j.cell.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, J., Wan, Y., Luo, C., Ye, G., Geng, Q., Auerbach, A., and Li, F. (2020). Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., He X., Shuai L., Sun Z. Science; 2020. Susceptibility of Ferrets, Cats, Dogs, and Other Domesticated Animals to SARS-Coronavirus 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.D., Bhatnagar K.P. Anatomy of the olfactory system. Handb. Clin. Neurol. 2019;164:17–28. doi: 10.1016/B978-0-444-63855-7.00002-2. [DOI] [PubMed] [Google Scholar]
- Spinato G., Fabbris C., Polesel J., Cazzador D., Borsetto D., Hopkins C., Boscolo-Rizzo P. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323:2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John J.A., Ekberg J.A., Dando S.J., Meedeniya A.C., Horton R.E., Batzloff M., Owen S.J., Holt S., Peak I.R., Ulett G.C. Burkholderia pseudomallei penetrates the brain via destruction of the olfactory and trigeminal nerves: implications for the pathogenesis of neurological melioidosis. mBio. 2014;5:e00025. doi: 10.1128/mBio.00025-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M., Talavera-Lopez C., Maatz H., Reichart D., Sampaziotis F. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerdi A., Kikuta S., Kinoshita M., Kamogashira T., Kondo K., Iwasaki S., Yamasoba T. Dorsal-zone-specific reduction of sensory neuron density in the olfactory epithelium following long-term exercise or caloric restriction. Sci. Rep. 2018;8:17300. doi: 10.1038/s41598-018-35607-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-Y., Li X.-L., Yan Z.-R., Sun X.-P., Han J., Zhang B.-W. Potential neurological symptoms of COVID-19. Ther. Adv. Neurol. Disord. 2020;13 doi: 10.1177/1756286420917830. 1756286420917830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P., Gong L., Zhang Y., Cui H.-Y., Geng J.-J. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 doi: 10.1101/2020.03.14.988345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., Li Z., Deng P., Zhang J., Zhong N. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., Geisthoff U.W., Bauer C., Hautefort C., Herman P., Manley G.T., Lyon D.M., Hopkins C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect. Dis. 2020;20:1015–1016. doi: 10.1016/S1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A., Hochgerner H., Lonnerberg P., Johnsson A., Memic F., van der Zwan J., Haring M., Braun E., Borm L.E., La Manno G. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014.e22. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated during and/or analyzed during the current study have been deposited in Mendeley Data (https://doi.org/10.17632/4cynjrymgf.1) and NCBI GEO: GSE151973. All codes used for the analyses of the data reported in this manuscript are provided at the GitHub repository https://github.com/leonfodoulian/SARS_CoV_2_anosmia.