To the Editor,
Diabetes, which is one of the leading causes of mortality and morbidity worldwide with increasing prevalence, is a well-known risk factor for various infections, post-infection complications, and increased mortality secondary to infections [1]. Diabetes has now been shown to be among the most common medical conditions in patients who develop coronavirus disease 2019 (COVID-19) [2] and has been associated with higher mortality in patients with this disease [3]. Zheng et al. reported that diabetes is associated with an almost fourfold greater risk for severe disease and death in patients with COVID-19 (odds ratio (OR) = 3.68, 95% confidence interval (CI) [2.68–5.03]; P < 0.001) [4]. However, although a significant association was observed between diabetes and disease severity (including severe and critical conditions and mortality) among COVID-19 patients based on the data of unadjusted effect estimates (hazard ratio (HR)) in the study by Cummings et al., this disappeared based on the data of the adjusted effect estimates [5], which suggest that several factors such as age, gender, and underlying diseases might modulate the relationship between diabetes and COVID-19 disease severity. Therefore, it was evident that the association between diabetes and severe COVID-19 disease needed to be investigated via a quantitative meta-analysis based on the data of adjusted effect estimates.
A systematic literature search was conducted for studies published from January 1, 2020, to July 25, 2020, in the PubMed, Chinese National Knowledge Infrastructure (CNKI), and Web of Science databases. According to the indices of the various databases, we used the search terms “coronavirus disease 2019,” “2019-nCoV, SARS-CoV-2,” “COVID-19,” and “diabetes,” and “diabetes mellitus.” Only articles reporting adjusted effect estimates (adjusted OR or HR) for diabetes and severity of disease in COVID-19 patients were considered eligible. There was no restriction on country or location. All calculations were carried out with Stata 11. 2 software. The pooled OR and pooled HR with their corresponding 95% CI were applied to evaluate the risk of severity in diabetic patients with COVID-19. The choice of the appropriate effects model was based on the analysis results, as follows: the fixed effect model was used if I2 was < 50% and the random-effects model was used if I2 was ≥ 50% [6]. Sensitivity analysis was conducted to evaluate the robustness of the results. Publication bias among the included studies was assessed by employing Begg’s funnel plot and Egger’s test.
A total of 1057 studies were identified using the search algorithm. Twenty-three studies [5, 7–28], comprising a total of 22,359 patients, were considered to be eligible for inclusion (Table 1). The median age of the patients ranged from 44 to 71 years; 4407 (20%) of them had diabetes. Among the 23 included articles, there were 19 retrospective studies and four prospective studies.
Table 1.
Characteristics of the included studies
| Author | Location | Case | Age (years) | Male (%) | Study design | DM | Unadjusted effect estimate (95% CI) | Adjusted effect estimate (95% CI) | Confounding factors |
|---|---|---|---|---|---|---|---|---|---|
|
Mo P PMID: 32173725 |
China | 155 | 54 (42–66) | 86 (55.5) | R | 15 (9.7) | NR |
OR 2.138 (0.483–9.471) |
Age, male, CVD, fever, shortness of breath, anorexia, blood test, chest CT or X-ray, treatment |
|
Hu L PMID: 32361738 |
China | 323 | 61 (23–91) | 166 (51.4) | R | 47 (14.6) | NR |
OR 3.109 (1.155–8.373) |
Age, smoking, hypnotics, diagnosis of critical status, hypersensitive troponin I, WBC, neutrophil count |
|
Huang R PMID: 32384078 |
China | 202 | 44.0 (33.0–54.0) | 116 (57.4) | R | 19 (9.4) |
OR 8.145 (2.842–23.342) |
OR 4.326 (1.059–17.668) |
Age, gender, BMI, HTN, smoking, WBC, neutrophils, lymphocyte, Hb, PLT, ALT, LDH, Tbil, ALB, CR, CRP, PT |
|
Shi S PMID: 32391877 |
China | 671 | 63 (50–72) | 322 (48.0) | R | 97 (14.5) | NR |
HR 1.16 (0.47–2.85) |
Age, gender, HTN, CHD, chronic renal disease, CHD, cerebrovascular diseases, PCT, cTnI, myoglobin; CRP; NT-proBNP; MYO, CK-MB |
|
Yu X PMID: 32351037 |
China | 333 | 50 (35–63) | 172 (51.7) | R | 28 (8.4) | NR |
OR 1.1 (0.3–3.6) |
Age, gender, heart disease, HTN, respiratory disease |
|
Cummings MJ PMID: 32442528 |
USA | 257 | 62 (51–72) | 171 (67) | P | 92 (36) |
HR 1.65 (1.11–2.44) |
HR 1.31 (0.81–2.10) |
Age, gender, symptom duration before hospital presentation, HTN, chronic cardiac disease, COPD or interstitial lung disease, CKD, BMI, interleukin-6, D-dimer |
|
Zhang Y PMID: 32446795 |
China | 258 | 64 (56–70) | 138 (53.5) | R | 63 (24.4) | NR |
HR 2.840 (1.01–8.01) |
Age, CVD, CKD |
|
Phipps MM PMID: 32473607 |
USA | 2273 | 65 (52–76) | 1297 (57) | R | 886 (39) |
OR 1.65 (1.34–2.02) |
OR 1.30 (1.02–1.68) |
Age, peak ALT, BMI, HTN, intubation, renal replacement therapy |
|
Galloway JB PMID: 32479771 |
UK | 1157 | 71 (57–82) | 666 (57.6) | R | 408 (35.3) | NR |
HR 1.20 (0.97–1.48) |
Age, gender |
|
Zhao M PMID: 32499448 |
China | 1000 | 61 (46–70) | 466 (46.6) | R | 118 (11.8) | NR |
HR 0.962 (0.576–1.608) |
Age |
|
Lim JH PMID: 32503180 |
Korea | 160 | NR | 86 (53.8) | R | 50 (31.3) |
HR 1.55 (0.85–2.83) |
HR 1.35 (0.72–2.56) |
Age, gender, HTN |
|
Lala A PMID: 32517963 |
USA | 2736 | 66.4 | 1630 (59.6) | R | 719 (26.3) | NR |
OR 1.01 (0.80–1.27) |
Age, gender, troponin strata, race, ethnicity, coronary artery disease, heart failure, HTN, atrial fibrillation, CKD, clinical variables |
|
Cen Y PMID: 32526275 |
China | 1007 | 61 (49–68) | 493 (49.0) | P | 119 (11.8) |
HR 2.920 (2.224–3.835) |
HR 1.816 (1.351–2.442) |
Age, gender, smoking history, HTN, chronic obstructive lung disease, coronary artery disease, duration of antiviral therapy |
|
Jang JG PMID: 32537954 |
Korea | 110 | 56.9 (± 17.0) | 48 (43.6) | R | 29 (26.4) |
OR 7.47 (2.73–20.04) |
OR 19.15 (1.90–193.42) |
Age, gender, HTN, body temperature, peripheral oxygen saturation, albumin, Tbil, CK-MB |
|
Rath D PMID: 32537662 |
Germany | 123 | 68 (±15) | 77 (62.6) | P | 30 (24.4) | NR |
HR 3.65 (1.06–12.63) |
Age, arterial HTN, LVEF, RV-function, tricuspid regurgitation > 1 |
|
Bertin D PMID:32564467 |
France | 56 | NR | 33 (58.9) | P | 10 (17.9) |
OR 0.33 (0.06–1.35) |
OR 0.21 (0.02–1.85) |
Gender, duration of symptoms, aCL IgG, CHD, HTN, chronic respiratory disease |
|
Yu C PMID: 32564974 |
China | 1464 | 64.0 (51.0–71.0) | 736 (50.3) | R | 211 (14.4) |
OR 3.77 (2.70–5.28) |
OR 2.34 (1.45–3.76) |
Age, gender, HTN, lymphopenia, ALT, LDH, D-dimer, PCT |
|
Bravi F PMID: 32579597 |
Italy | 1603 | 58.0 (20.9) | 758 (47.3) | R | 194 (12.1) | NR |
OR 1.52 (1.05–2.18) |
Age, gender, HTN, CVD, cancer, COPD, renal disease |
|
Booth CM PMID: 12734147 |
Canada | 144 | 45 (34–57) | 56 (39) | R | 16 (11) | NR |
HR 3.1 (1.4–7.2) |
Age, comorbidity |
|
Han J PMID: 32580792 |
China | 185 | 44 (±17.88) | 95 (51.4) | R | 28 (15.1) |
OR 5.792 (2.366–14.176) |
OR 3.311 (1.093–10.031) |
Age, time from symptoms onset to treatment, PaO2/FiO2 on admission, NLR, PLT |
|
Hashemi N PMID: 32585065 |
USA | 363 | 63.4 (± 16.5) | 201 (55.4) | R | 117 (32.2) | NR |
OR 1.22 (0.74–2.00) |
Age, gender, HTN, obesity, cardiac diseases, hyperlipidemia, pulmonary disorders |
|
Ji W PMID: 32597048 |
Korea | 7541 | 47.05 (± 19.0) | 2970 (40.5) | R | 1043 (14.2) |
OR 4.646 (3.984–5.418) |
OR 1.247 (1.009–1.543) |
Comorbidity |
|
Pettit NN PMID: 32589784 |
USA | 238 | 58.5 (±17) | 113 (47.5) | R | 68 (28.6) |
OR 0.8 (0.3–2.2) |
OR 0.5 (0.2–1.7) |
Age, gender, HTN, obesity, pulmonary disease, CVD, kidney disease, cancer, stroke, hyperlipidemia, VTE |
All values are n (%), mean (standard deviation, SD), or median (interquartile range, IQR). USA, United States of America; NR, not reported; DM, diabetes mellitus; P, prospective; R, retrospective; HR, hazard ratio; OR, odds ratio; CVD, cardiovascular diseases; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; PCT, procalcitonin; COPD, chronic obstructive pulmonary diseases; HTN, hypertension; BMI, body mass index; CRP, C-reactive protein; CHD, coronary heart disease; WBC, white blood cell; PLT, platelet; Tbil, total bilirubin; ALB, albumin; CR, creatinine; PT, prothrombin time; Hb, hemoglobin; NT-proBNP, amino-terminal pro-brain natriuretic peptide; cTnI, cardiac troponin I; CK-MB, creatinine kinase-myocardial band; CKD, chronic kidney disease; LVEF, left ventricular ejection fraction; aCL: anti-cardiolipin antibodies; NLR, neutrophil-to-lymphocyte ratio; VTE: venous thromboembolism
The forest plot of the association between diabetes and the severity of COVID-19 symptoms is shown in Fig. 1 a and b. Diabetes was found to be associated with an increased risk of disease severity in COVID-19 patients on the basis of 14 studies reporting adjusted OR (OR = 1.44, 95% CI [1.14–1.82], I2 = 58.2%, random-effects model) (Fig. 1a) and nine studies reporting adjusted HR (HR = 1.37, 95% CI [1.19–1.57]; I2 = 29.2%, fixed-effects model) (Fig. 1b). In the 23 studies we included, only 11 studies reported both unadjusted and adjusted effect estimates (HR or OR) simultaneously. We calculated the pooled unadjusted and adjusted effect estimates (HR or OR) separately, and the pooled results based on unadjusted effect estimates showed that diabetes was associated with greater risk for disease severity in patients with COVID-19 compared to the pooled results based on adjusted effect estimates (HRunadjusted = 2.04 (95% CI: 1.30–3.19) and ORunadjusted = 2.98 (95% CI: 1.75–5.05); HRadjusted = 1.61 (95% CI: 1.28–2.04) and ORadjusted = 1.58 (95% CI: 1.07–2.32), respectively) (Fig. S1). Sensitivity analysis indicated that our results were robust and stable (Fig. 1c). There was no significant publication bias, as determined by Begg’s test (P = 0.224) and Egger’s test (P = 0.065).
Fig. 1.
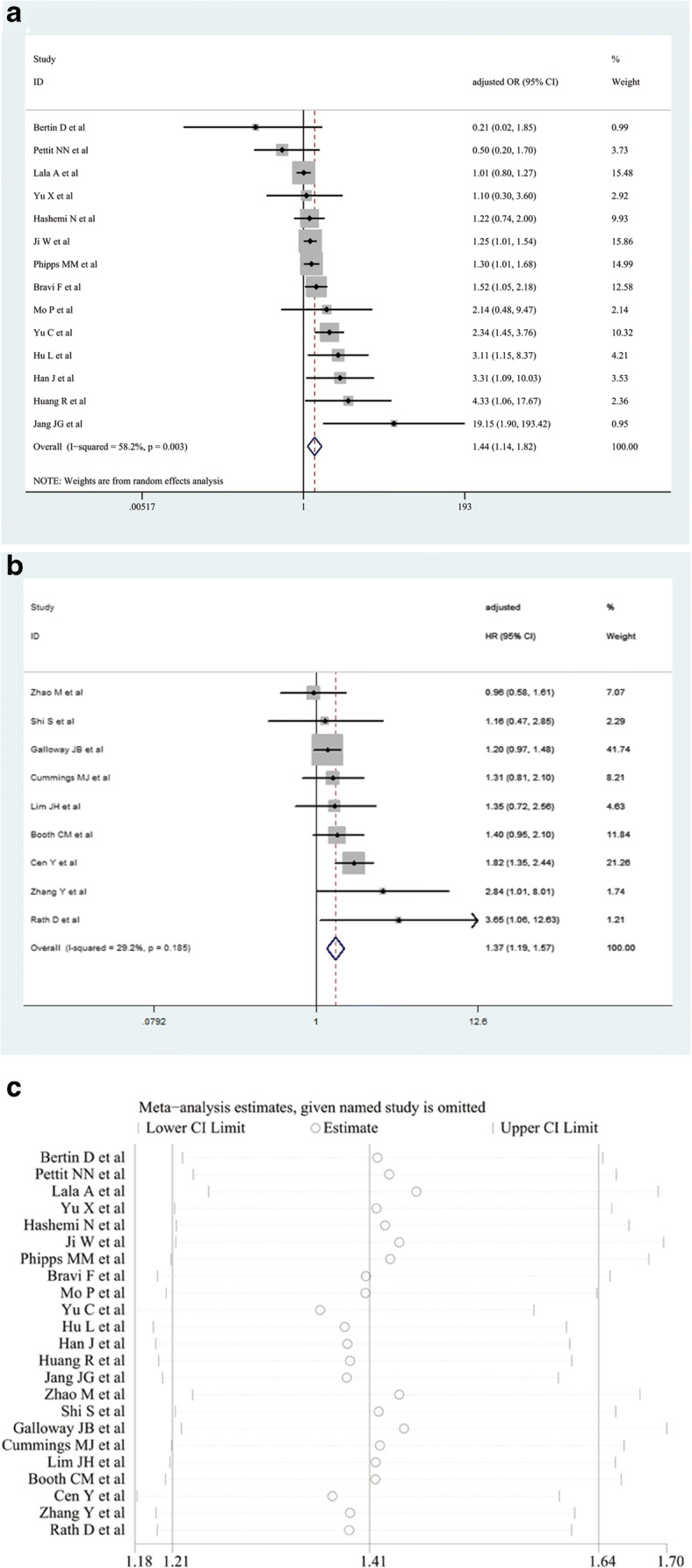
The pooled odds ratio (OR) (a), hazard ratio (HR) (b), and their 95% confidence interval (CI) of the relationship between diabetes and the risk of disease severity in patients with COVID-19. Sensitivity analysis for evaluating the relationship between diabetes and the risk of disease severity in patients with COVID-19 (c)
Although previous meta-analyses have demonstrated that diabetes was positively associated with an increased risk of severity and mortality in COVID-19 patients, these studies did not uniformly address the influences of several factors, including age, gender, and underlying diseases, on the results [4, 29–33]. Therefore, our present study investigated the relationship between diabetes and disease severity in COVID-19 patients based on adjusted effect estimates: the results demonstrated that diabetes was an independent predictor of COVID-19 disease severity.
Some limitations should be considered in our study. Firstly, the definitions of severity of COVID-19 varied among the included studies. Secondly, the type of diabetes and whether it was with good or with poor glycemic control are also unknown. Because the selected studies did not adequately present data on the treatment of diabetes and blood glucose control, these could not be evaluated. Finally, all selected studies presented adjusted effect estimates, but the adjusted confounders among the studies were not completely consistent: for example, the number and kinds of adjusted confounders are different among the included studies.
In conclusion, our findings indicated that diabetes is an independent risk factor for predicting COVID-19 disease severity in these patients. These results clearly underscore the necessity to increase our focus in clinical practice on COVID-19 patients with diabetes so as to prevent rapid deterioration of their condition.
Given the limited level of evidence, further well-designed studies with larger samples are needed to confirm our current results.
Supplementary Information
Forest plot of the pooled effects for the relationship between diabetes and the risk of disease severity in patients with COVID-19: (A) unadjusted HR, (B) adjusted HR, (C) unadjusted OR, (D) adjusted OR. (PNG 3695 kb)
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No. 81973105). The funder has no role in data collection and analysis, manuscript preparation, and decision to submission.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Frydrych LM, Bian G, O’Lone DE, Ward PA, Delano MJ. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol. 2018;104(3):525–534. doi: 10.1002/JLB.5VMR0118-021RR. [DOI] [PubMed] [Google Scholar]
- 2.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, Southern WN, Mantzoros CS. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metab Clin Exp. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W (2020) Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed]
- 5.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, Hochman BR, Salazar-Schicchi J, Yip NH, Brodie D, O'Donnell MR. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/s0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 7.Bertin D, Brodovitch A, Beziane A, Hug S, Bouamri A, Mege JL, Bardin N (2020) Anti-cardiolipin IgG autoantibodies are an independent risk factor of COVID-19 severity. Arthritis Rheum. 10.1002/art.41409 [DOI] [PMC free article] [PubMed]
- 8.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, Detsky AS. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 9.Bravi F, Flacco ME, Carradori T, Volta CA, Cosenza G, De Togni A, Acuti Martellucci C, Parruti G, Mantovani L, Manzoli L. Predictors of severe or lethal COVID-19, including angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, in a sample of infected Italian citizens. PLoS One. 2020;15(6):e0235248. doi: 10.1371/journal.pone.0235248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cen Y, Chen X, Shen Y, Zhang XH, Lei Y, Xu C, Jiang WR, Xu HT, Chen Y, Zhu J, Zhang LL, Liu YH (2020) Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019-a multi-centre observational study. Clin Microbiol Infect. 10.1016/j.cmi.2020.05.041 [DOI] [PMC free article] [PubMed]
- 11.Galloway JB, Norton S, Barker RD, Brookes A, Carey I, Clarke BD, Jina R, Reid C, Russell MD, Sneep R, Sugarman L, Williams S, Yates M, Teo J, Shah AM, Cantle F (2020) A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: an observational cohort study. J Infect. 10.1016/j.jinf.2020.05.064 [DOI] [PMC free article] [PubMed]
- 12.Han J, Shi LX, Xie Y, Zhang YJ, Huang SP, Li JG, Wang HR, Shao SF. Analysis of factors affecting the prognosis of COVID-19 patients and viral shedding duration. Epidemiol Infect. 2020;148:e125. doi: 10.1017/s0950268820001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW (2020) Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: a multicentre United States experience. Liver Int. 10.1111/liv.14583 [DOI] [PMC free article] [PubMed]
- 14.Hu L, Chen S, Fu Y, Gao Z, Long H, Wang JM, Ren HW, Zuo Y, Li H, Wang J, Xu QB, Yu WX, Liu J, Shao C, Hao JJ, Wang CZ, Ma Y, Wang Z, Yanagihara R, Deng Y (2020) Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed]
- 15.Huang R, Zhu L, Xue L, Liu L, Yan X, Wang J, Zhang B, Xu T, Ji F, Zhao Y, Cheng J, Wang Y, Shao H, Hong S, Cao Q, Li C, Zhao XA, Zou L, Sang D, Zhao H, Guan X, Chen X, Shan C, Xia J, Chen Y, Yan X, Wei J, Zhu C, Wu C. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi-center study. PLoS Negl Trop Dis. 2020;14(5):e0008280. doi: 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang JG, Hur J, Choi EY, Hong KS, Lee W, Ahn JH. Prognostic factors for severe coronavirus disease 2019 in Daegu, Korea. J Korean Med Sci. 2020;35(23):e209. doi: 10.3346/jkms.2020.35.e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji W, Huh K, Kang M, Hong J, Bae GH, Lee R, Na Y, Choi H, Gong SY, Choi YH, Ko KP, Im JS, Jung J. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020;35(25):e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V, Mount Sinai Covid Informatics C (2020) Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed]
- 19.Lim JH, Park SH, Jeon Y, Cho JH, Jung HY, Choi JY, Kim CD, Lee YH, Seo H, Lee J, Kwon KT, Kim SW, Chang HH, Kim YL (2020) Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J Clin Med 9(6). 10.3390/jcm9061718 [DOI] [PMC free article] [PubMed]
- 20.Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, Xiong Y, Cheng Z, Gao S, Liang K, Luo M, Chen T, Song S, Ma Z, Chen X, Zheng R, Cao Q, Wang F, Zhang Y (2020) Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed]
- 21.Pettit NN, MacKenzie EL, Ridgway J, Pursell K, Ash D, Patel B, Pho MT (2020) Obesity is associated with increased risk for mortality among hospitalized patients with COVID-19. Obesity (Silver Spring, Md). 10.1002/oby.22941 [DOI] [PMC free article] [PubMed]
- 22.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC (2020) Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large US cohort. Hepatology. 10.1002/hep.31404 [DOI] [PMC free article] [PubMed]
- 23.Rath D, Petersen-Uribe A, Avdiu A, Witzel K, Jaeger P, Zdanyte M, Heinzmann D, Tavlaki E, Muller K, Gawaz MP (2020) Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol. 10.1007/s00392-020-01683-0 [DOI] [PMC free article] [PubMed]
- 24.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu C, Lei Q, Li W, Wang X, Liu W, Fan X, Li W (2020) Clinical characteristics, associated factors, and predicting COVID-19 mortality risk: a retrospective study in Wuhan, China. Am J Prev Med. 10.1016/j.amepre.2020.05.002 [DOI] [PMC free article] [PubMed]
- 26.Yu X, Sun X, Cui P, Pan H, Lin S, Han R, Jiang C, Fang Q, Kong D, Zhu Y, Zheng Y, Gong X, Xiao W, Mao S, Jin B, Wu H, Fu C (2020) Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai, China. Transbound Emerg Dis. 10.1111/tbed.13604 [DOI] [PMC free article] [PubMed]
- 27.Zhang Y, Cui Y, Shen M, Zhang J, Liu B, Dai M, Chen L, Han D, Fan Y, Zeng Y, Li W, Lin F, Li S, Chen X, Pan P, medical team from Xiangya Hospital to support Hubei C Association of diabetes mellitus with disease severity and prognosis in COVID-19: a retrospective cohort study. Diabetes Res Clin Pract. 2020;165:108227. doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao M, Wang M, Zhang J, Gu J, Zhang P, Xu Y, Ye J, Wang Z, Ye D, Pan W, Shen B, He H, Liu M, Liu M, Luo Z, Li D, Liu J, Wan J. Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging. 2020;12(11):10070–10086. doi: 10.18632/aging.103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantovani A, Byrne CD, Zheng MH, Targher G (2020) Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 10.1016/j.numecd.2020.05.014 [DOI] [PMC free article] [PubMed]
- 32.Palaiodimos L, Chamorro-Pareja N, Karamanis D, Li W, Zavras PD, Mathias P, Kokkinidis DG (2020) Diabetes is associated with increased risk for in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis comprising 18,506 patients. medRxiv. 10.1101/2020.05.26.20113811 [DOI] [PMC free article] [PubMed]
- 33.Wu ZH, Tang Y, Cheng Q (2020) Diabetes increases the mortality of patients with COVID-19: a meta-analysis. Acta Diabetol. 10.1007/s00592-020-01546-0 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot of the pooled effects for the relationship between diabetes and the risk of disease severity in patients with COVID-19: (A) unadjusted HR, (B) adjusted HR, (C) unadjusted OR, (D) adjusted OR. (PNG 3695 kb)


