Abstract
Staphylococcus aureus enhances neutrophil extracellular vesicle (EV) production. To investigate whether S. aureus viability influences EV biogenesis, EVs were isolated from human neutrophils incubated with viable bacteria (bEVs) or heat-killed bacteria (heat-killed EVs). Protein analysis, nanoparticle tracking and transmission electron microscopy showed comparable EV production between subsets, and both viable and nonviable bacteria were also detected in respective EV subsets. As anticipated, S. aureus, as well as bEVs with viable bacteria, were proinflammatory, and killing bacteria with gentamicin reduced cytokine production to baseline levels. Although heat-killed bacteria induced macrophage IL-6 production, heat-killed EVs did not. Additionally, we found that human and bacterial DNA associated with bEVs, but not heat-killed EVs, and that the DNA association could be partially decreased by disrupting electrostatic interactions. We investigated the potential for DNA isolated from EVs (EV-DNA) or EVs to cause inflammation. Although liposomal encapsulation of EV-DNA increased IL-6 production from baseline by 7.5-fold, treatment of bEVs with DNase I had no effect on IL-6 and IL-1β production, suggesting that the DNA did not contribute to the inflammatory response. Filtered EVs, which lacked DNA and associated bacteria, exhibited less proinflammatory activity relative to bEVs, and enhanced macrophage expression of CD86 and HLA-DR. Ultimately, we show that bEVs isolated by differential centrifugation co-purify with bacteria and DNA, and studying their concerted activity and relative contribution to immune response is important to the study of host-pathogen interactions.
Keywords: CD86 and HLA-DR, ectosomes, exosomes, extracellular DNA, microparticles, MRSA
1 |. INTRODUCTION
Infections with Staphylococcus aureus represent a major healthcare problem, causing conditions ranging from mild skin and soft-tissue infection, to invasive and life-threatening disease.1 Community-associated methicillin-resistant S. aureus (CA-MRSA) is especially problematic, as strains are more likely to sicken apparently healthy individuals, are resistant to many antibiotic therapies, and are more likely to cause severe disease.2 CA-MRSA evades innate immune responses, inhibits pro-resolving pathways which results in cellular and tissue damage, and along with certain bacterial virulence factors, creates an amplification loop of deleterious inflammation.2,3 Given the increase in CA-MRSA prevalence,4 pinpointing additional molecular events that drive inflammation, or prevent resolution, and those which could bolster immunity and augment existing antibiotic therapy, is increasingly urgent.
Neutrophils are among the first responders in staphylococcal infection, and are critical for a successful host response to S. aureus.5 However, despite their helpful contribution to innate immunity, neutrophils may be detrimental in some settings.5,6 In response to S. aureus, neutrophils can perpetuate inflammation in the following ways: (i) neutrophils release elastase-containing neutrophil extracellular traps (NETs) that damage host tissues and (ii) neutrophils fail to kill the entire ingested inoculum and eventually lyse, releasing pathogen- and damage-associated molecular patterns (PAMPS and DAMPS, respectively).6,7 Neutrophils are known to produce extracellular vesicles (EVs), and their production is increased in response to many agonists, including S. aureus,8–10 but their role in inflammatory signaling and immunity remains undefined.
EVs are small, membrane-bound vesicles that carry proteins, lipids, and nucleic acids.11,12 EVs participate in intercellular signaling, but EV cargo depends on the originating cell and agonist, and their activity depends on the recipient cell.13 EVs from S. aureus-challenged neutrophils (termed bEVs) associate with bacteria and cause their aggregation.9,10 Because EVs are well known for their ability to deliver signals to neighboring cells and can contribute to host defense and inflammation,14,15 further characterization of this EV subset would be useful. First, we set out to characterize whether viable bacteria were required for bEV production. Then, given that S. aureus is a potent inducer of NET-associated DNA release from neutrophils, and that several types of EVs associate with extravesicular DNA,9,16–20 we sought to characterize EV-DNA, and probed whether EVs or associated DNA could contribute to staphylococcal-driven inflammation or immunity.
2 |. MATERIALS AND METHODS
2.1 |. Neutrophil and macrophage isolation
Venous blood was obtained from healthy donors in accordance with a protocol approved by the Institutional Review Board at Central Michigan University (Mount Pleasant, MI, USA). Neutrophils and macrophages were isolated as described in Greenlee-Wacker et al.7
2.2 |. S. aureus culture
USA300 LAC was cultured in tryptic soy broth overnight at 37°C, at 200 rpm. A subculture at optical density (OD)550 of 0.05 was grown to mid-log phase for 3 h (OD550 of 0.4–1.0). Bacteria were pelleted and resuspended in 20 mM HEPES in HBSS. To heat-kill bacteria, S. aureus was incubated at 80°C for 30 min. All bacteria were opsonized in 10% serum at 37°C for 20 min, then washed twice at 2800× g for 5 min at 4°C using RPMI 1640 without phenol red.
2.3 |. EV isolation
Neutrophils were mixed with either viable or heat-killed S. aureus at a 10:1 multiplicity of infection (bacteria: cell) at 37°C for 20 min, followed by 10 min on ice. Bacteria and neutrophils were pelleted twice at 4000× g for 20 min at 4°C. In some experiments, EVs in supernatants were either lysed with 0.05% Triton X-100 (TX-100) and vortexed for 30 s,21 or passed through a 0.45 μm polyethersulfone (PES) filter. EVs were concentrated at 160,000× g for 51 min at 4°C, pooled, re-pelleted, resuspended in PBS, aliquoted and flash-frozen. After EV isolation, colony forming units (CFU) were enumerated from serial dilutions plated onto tryptic soy agar (TSA) overnight at 37°C.
2.4 |. Protein and particle analysis
EV protein concentration was measured by bicinchoninic acid (BCA) based on manufacturer instructions. Nanoparticle tracking was performed using NanoSight ML10 (Salisbury, U.K.) and analysis was conducted with nanoparticle tracking analysis (NTA) 2.3 software.
2.5 |. Transmission electron microscopy (TEM)
Visualization through positive-negative staining was performed as described in Théry et al. 200622 on a Hitachi 7700 transmission electron microscope (Tokyo, Japan). Methods for immunogold labeling are included in supplemental materials.
2.6 |. DNA isolated from EVs (EV-DNA) isolation and analysis
DNA was isolated from 25–200μL EVs using DNeasy Blood and Tissue Kit (Qiagen, Inc., Hilden, Germany) and either visualized on a 0.7% agarose gel or a 5% polyacrylamide tris-borate-EDTA gel stained with Gel Red, and quantified by Nanodrop. Thereafter, DNA was used in macrophage experiments with or without N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTAP), or sequenced commercially (GeneWiz, Inc., South Plainfield, NJ). Paired end sequencing was completed on an Illumina MiSeq with 2 × 150 bp configuration and v3 chemistry. De novo assembles of the paired-end sequencing reads were performed using Ray (v2.3.123) with a k-mer value of 31. Using a 99% cutoff value, fragments were filtered into three components via basic local alignment search tool (BLAST)n search (Genbank; http://ncbi.nlm.nih.gov), and mapped back to genomes to determine coverage using Bowtie 2 (v 2.3.524).
2.7 |. EV flow cytometry
EVs were conjugated to aldehyde/sulfate latex beads as described in Németh et al.17 and stained with CellVue dye as per manufacturer instructions. EVs were incubated with 0.1 M or 1 M NaCl for 15 min at 20°C. EVs were stained with 5μL anti-CD63-PE antibody for 30 min on ice protected from light, or DNA was stained with 24.4 μg/mL propidium iodide (PI) per sample for 15 min prior to analysis. All flow cytometry was performed on a Beckman Coulter CytoFLEX FlowCytometer (Brea, CA).
2.8 |. Macrophage cytokine and surface protein analysis
Macrophages in RPMI 1640 with 10% serum were plated at 5 × 105 cells/well for ≥2 h at 37°C, and then washed extensively. EVs, EV DNA, or agonists were cocultured with macrophages for 16–24 h at 37°C in 5% CO2. For ELISA, supernatants were pelleted at 18,400× g for 10 min at 4°C. Supernatants were analyzed by ELISA according to manufacturer instructions. In some experiments, 200 ng EVs were treated with 5 units of DNase I for 1 h at 37°C, then inactivated with 10 mM EDTA for 10 min at 37°C. For analysis of protein expression, macrophages were treated with gentamicin (10 μg/mL) prior to activation, and then cultured with or without EVs. For flow cytometry, macrophages were harvested with trypsin-EDTA, stained with antibodies (2.5μL of anti-CD14-FITC, 5μL of anti-CD86-APC, and 5μL of anti-HLA-DR-PE), washed, and analyzed.
2.9 |. Statistical analysis
3 |. RESULTS AND DISCUSSION
3.1 |. Viable S. aureus is not required for EV biogenesis
To determine whether bacterial viability was a requirement for enhanced bEV production, we challenged neutrophils with viable or heat-killed S. aureus, recovered EV populations by differential centrifugation, and then characterized their quantity and appearance. Preparations of EVs isolated following coculture with heat-killed S. aureus (heat-killed EVs) had a higher protein concentration compared to bEVs (0.39 ± 0.04 mg/mL vs. 0.29 ± 0.05 mg/mL; Fig. 1A). More quantitative measurements obtained using NTA (Fig. 1B–F) showed particle concentration was nearly equivalent in the two EV subsets (3.07 × 1011 ± 0.27 × 1011 particles/mL for bEVs and 4.53 × 1011 ± 0.51 × 1011 particles/mL for heat-killed EVs). Additionally, no differences were observed in average EV size (171.93 ± 9.93 nm for bEVs and 201.09 ± 8.95 nm for heat-killed EVs) or mode EV size (137.60 ± 2.75 nm for bEVs and 151.50 ± 4.22 nm for heat-killed EVs).
FIGURE 1. Viable bacteria are not required for extracellular vesicle (EV) biogenesis.
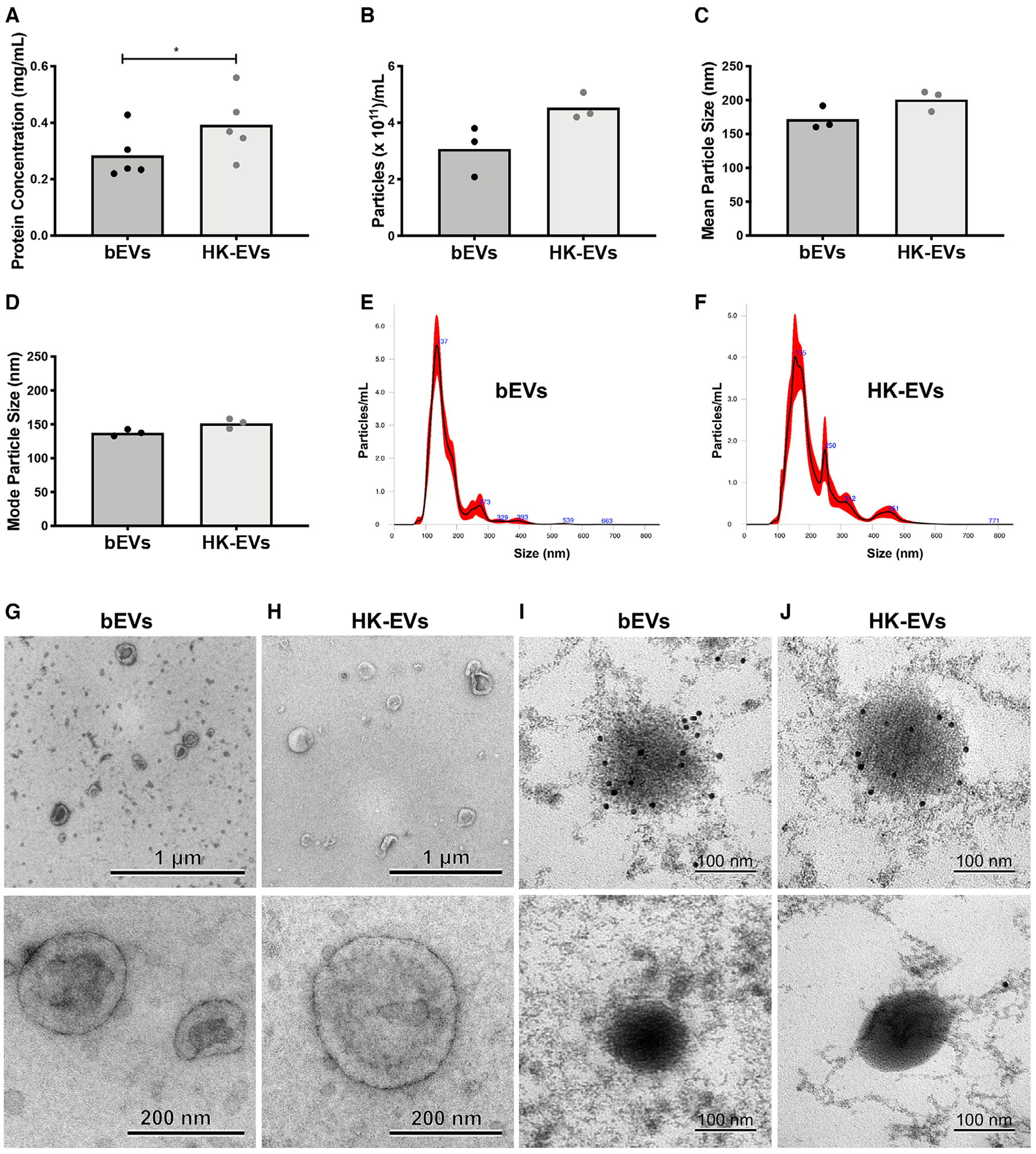
Protein concentration for the two EV subsets was determined by bicinchoninic acid (BCA) (A, n = 5). Nanoparticle tracking analysis (NTA) was used to determine particle concentration (B), mean particle size (C), and mode particle size (D) of EV samples (n = 3). Symbols represent individual data points and bars represent the mean. P-values were determined using a paired Student’s t-test (*P < 0.05). Representative histograms collected from NTA are shown in (E) and (F). Representative transmission electron microscopy micrographs are shown at 10,000× (top panels, scale bar = 1 μm) and 60,000× magnification (bottom panels, scale bar = 200 nm) for positive-negative stained bEV (G) and heat-killed EV (H) subsets (n = 3). For immunogold labeling, gp91 bound to primary antibody was labeled with secondary antibody-conjugated gold particles, then stained and visualized. Shown are representative images from two experiments (scale bar = 100 nm, I and J, top panels). In a single experiment, the primary antibody to gp91 was omitted to confirm specificity of immunogold staining (scale bar = 100 nm, I and J, bottom panels)
Although the majority of EVs were uniform in size, the presence of multiple peaks in the histograms illustrates the heterogeneity within EV preparations (Fig. 1E, F), a common feature of EVs isolated by differential centrifugation.12 The larger, less abundant particles detected in EV subsets could be larger EVs (e.g., ectosomes), S. aureus, or contaminating cellular debris.25
As a final means of characterization, we performed TEM. For each EV subset, sharply defined, rounded, or cup-shaped vesicles were visible at lower magnification, and consistent with the NTA, the quantity in each image was relatively equivalent between subsets (Fig. 1G, H—upper panels). Images also confirmed that although EVs were heterogeneous in size, the majority were below 250 nm. Additionally, no circular structures greater than 1 μm in diameter were found, confirming that cells and apoptotic bodies were absent from preparations. The physical appearance of EVs at higher magnification was more clearly defined, and consistent with images of EVs from other cell types,8,26 dark contrast at the membrane was visible (Fig. 1G, H—lower panels).
S. aureus can also produce EVs under very specific conditions.27 To confirm whether both bEVs and heat-killed EVs were neutrophil derived, we performed staining and immunogold labelling for gp91-phox, a subunit of the membrane-bound neutrophil NADPH oxidase. Although methods to embed and section samples disrupted membrane structure to some degree, dark, rounded vesicle-like particles falling inside the expected size range were visible. Of these particles, 96.55% were positive for immunogold (Fig. 1I, J; top panels, n > 50 particles in 5 images). No gold particle accumulation was observed when the primary antibody was omitted from staining (Fig. 1I, J—bottom panels). All together, these data support the conclusion that viable S. aureus is not required for EV biogenesis.
3.2 |. Differential centrifugation enriches for EVs, and to a lesser extent, bacteria
TEM was also used to investigate the larger, less abundant particles detected by NTA. With low frequency, structures resembling bacterial cocci were visible in both bEV and heat-killed EV preparations (Fig. 2A, B). Although we could not grow heat-killed bacteria, bacterial colonies grew when bEVs were plated on TSA overnight (6.4 × 106 ± 2.2 × 106 CFU/mL, n = 8), and this number of bacteria represented 0.04% of the original inoculum.
FIGURE 2. Differential centrifugation enriches for EVs and bacteria.
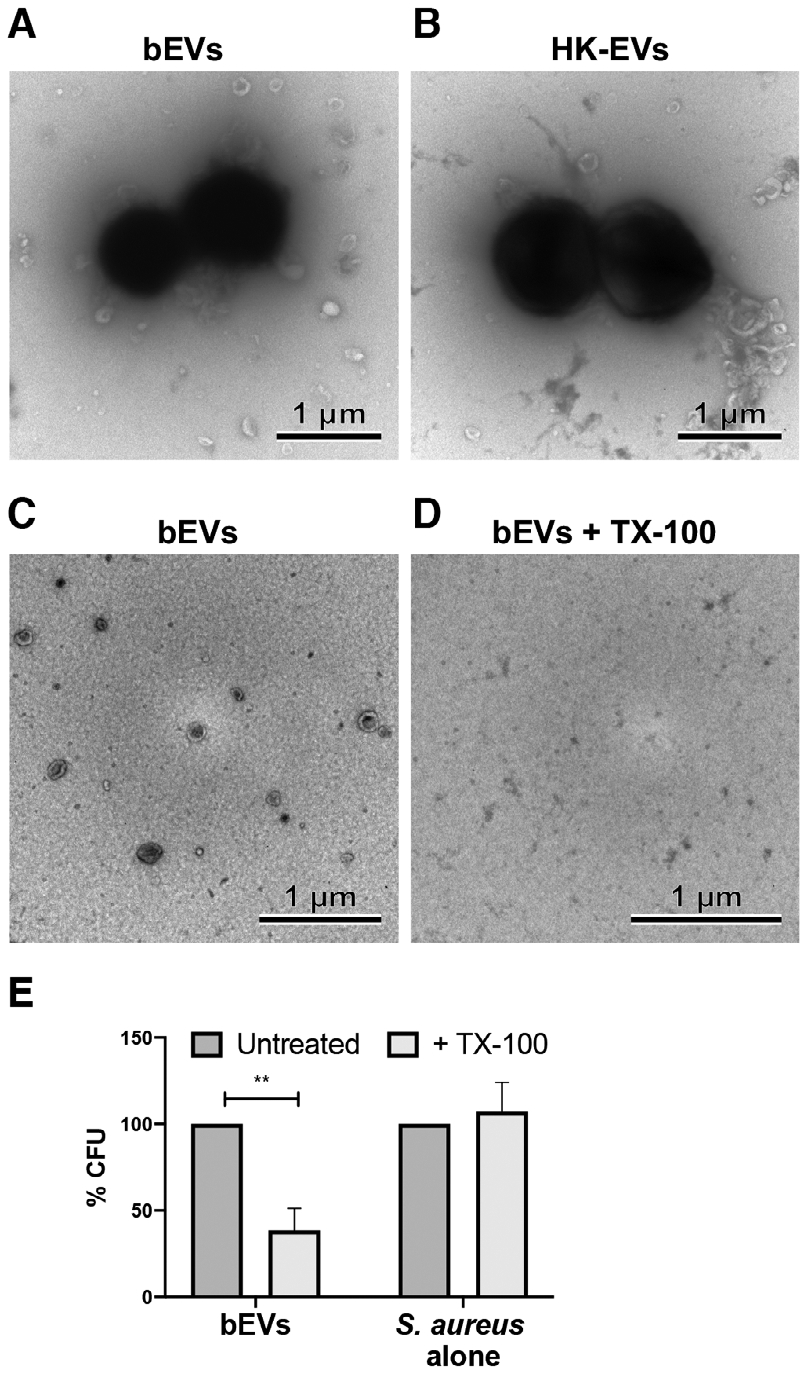
Shown are representative transmission electron microscopy (TEM) images from 3 experiments of bacteria in bEV (A) and heat-killed EV (B) preparations, as well as TEM images of paired preparations of bEVs (C) and Triton X-100 (TX-100) lysed bEVs (D) (10,000× magnification, scale bar = 1 μm). Colony forming unit were determined from both untreated and TX-100-treated bEVs, as well as S. aureus, and normalized to the paired untreated sample. Bars represent an average of at least three experiments ± SEM. P-values were determined for each group using a 2-way repeated measures ANOVA and Sidak’s multiple comparisons posttest (**P < 0.01; E)
Bacterial presence in EV preparations could result from EV-dependent bacterial aggregation.9,10 We sought to ascertain whether EVs influenced bacterial pelleting by lysing EV prior to ultracentrifugation.21 After detergent treatment, EVs disappeared (Fig. 2C, D); however, few bacterial cocci were still visible (data not shown). To quantify bacterial counts, bEVs and lysed-bEVs were plated overnight on TSA, and for each paired preparation of bEVs and lysed-bEVs, 61.50 ± 31.10% fewer bacteria were recovered for detergent-treated samples (Fig. 2E; Supporting Information Table S1). Because bacteria themselves were not susceptible to detergent-mediated lysis (Fig. 2E), these data suggest that EVs influence how bacteria are pelleted during differential centrifugation.
3.3 |. Viable bacteria in bEV preparations cause proinflammatory cytokine production
Given that the interaction between bacteria and bEVs would be relevant during infection, we explored the inflammatory activity of the bEV preparations. Unstimulated macrophages produced neither IL-6 nor IL-1β, but macrophages treated with bEVs produced both proinflammatory cytokines in a dose-dependent manner (Fig. 3A, B). Antibiotic inhibitors of bacterial protein synthesis, such as linezolid, can kill S. aureus and eliminate bacterial-dependent proinflammatory cytokine production.28 Based on this rationale, and to probe whether using an antibiotic could block cytokine production, we treated bEVs with 10 μg/ml of gentamicin, an antibiotic that inhibits bacterial protein synthesis and at a concentration that does not inhibit inflammasome activation.29 Addition of gentamicin to bEVs killed 100% of bacteria (n = 3), and drastically reduced bEV-induced macrophage production of IL-6 by 94.01 ± 5.94% and IL-1β by 90.46 ± 10.73% (Fig. 3A, B). Thus, we provide evidence that bacteria induce a proinflammatory response in the presence of bEVs, and that gentamicin abolishes this effect.
FIGURE 3. Viable bacteria in bEV preparations cause proinflammatory cytokine production.
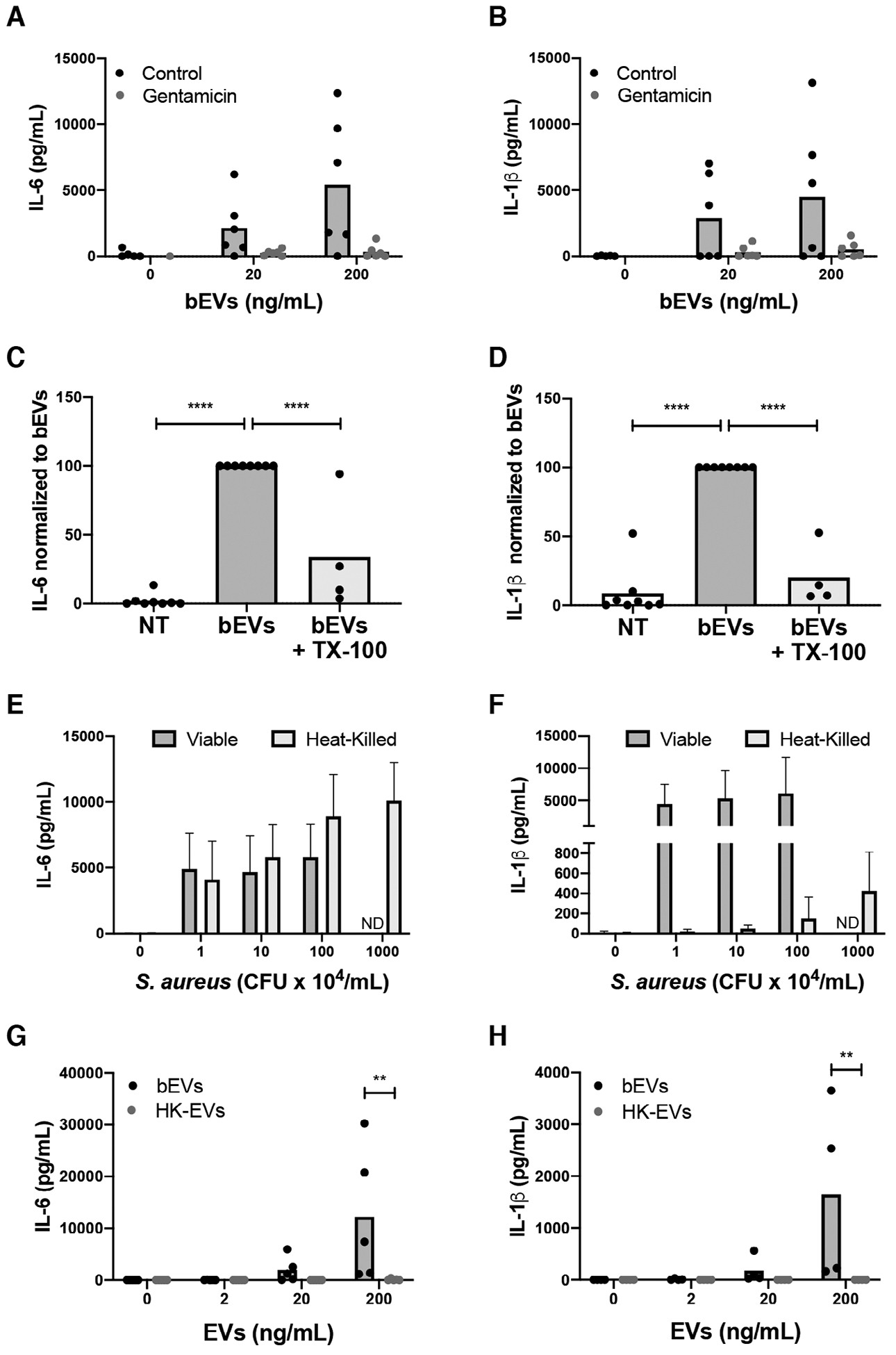
Macrophages were treated with vehicle, 20, or 200 ng/mL of bEVs with and without 10 μg/mL gentamicin. Supernatants were collected after 18 h at 37°C and analyzed by ELISA for IL-6 (A) and IL-1β (B). Symbols represent individual data points, and bars represent the mean (n = 6). Macrophages were treated with vehicle, bEVs (200 ng/mL), or lysed bEVs (200 ng/mL), and IL-6 (C) and IL-1β (D) in supernatants were analyzed by ELISA. Cytokine production was normalized to bEVs. Symbols represent individual data points, and bars represent the mean (n ≥ 4). P-values were determined using a 1-way ANOVA of values normalized to bEVs and Dunnett’s posttest (*P < 0.05 and **P < 0.01 vs. bEVs). Macrophages were cultured with buffer alone, 1–100 × 104 CFU/mL (colony forming unit) of viable S. aureus (n = 6), or 1–1000 × 104 CFU/mL heat-killed S. aureus (n = 5). After 18 h at 37°C, and IL-6 (E) and IL-1β (F) in supernatants were assayed by ELISA. Bars represent the average cytokine production ± SEM. After macrophages were treated with 0, 2, 20, and 200 ng/mL of bEVs and heat-killed extracellular vesicles (EVs) for 18 h at 37°C, supernatants were assayed by ELISA for IL-6 (G) and IL-1β (H). Symbols represent individual data points, and bars represent the mean (n ≥ 4). P-values were determined for each group using a 2-way repeated measures ANOVA and Sidak’s multiple comparisons posttest (**P < 0.01)
Next, we set out to investigate how EV lysis influenced cytokine production. Treatment of macrophages with bEVs induced the production of IL-6 and IL-1β by macrophages, whereas lysed-bEVs elicited a 66.35 ± 20.71% reduction in response for IL-6 and a 79.85 ± 10.95% reduction in response for IL-1β (Fig. 3C, D, raw values in Supporting Information Table S2). The reduction in cytokine production corresponded to the reduction in bacteria (Fig. 2E), suggesting that vesicles themselves were not a major factor affecting the macrophage proinflammatory cytokine profile; however, with viable bacteria as the dominant proinflammatory stimuli, it is plausible that EVs have a more subtle or priming effect on macrophages, which is not observed in this experimental system.
Given that nonviable staphylococci were present in heat-killed EV subsets, we reasoned that the bacteria present in both bEV and heat-killed EV preparations would cause proinflammatory cytokine production by activating multiple pattern recognition receptors (PRRs).30 To confirm that heat-killed bacteria were still proinflammatory, we treated human primary macrophages with 1–100 × 104 CFU/mL of S. aureus or 1–1000 × 104 CFU/mL heat-killed S. aureus. As expected, macrophages produced IL-6 and IL-1β in response to viable S. aureus. Heat-killed S. aureus also induced IL-6 production, but 100-fold more heat-killed bacteria were required to induce any IL-1β production (Fig. 3E, F). These data are consistent with a requirement for toxin-mediated inflammasome activation for IL-1β processing.31
Finally, we treated macrophages with both bEVs and heat-killed EVs to determine how heat-killed bacteria influenced EV activity. Again, unstimulated macrophages produced neither IL-6 nor IL-1β, whereas bEVs induced their dose-dependent production (Fig. 3G, H). At the concentration of 200 ng/mL of bEVs, approximately 1.84 × 104 CFU/mL of S. aureus were present. Although heat-killed bacteria induced macrophage IL-6 production, heat-killed EVs lacked any measurable proinflammatory activity relative to any of the bacterial concentrations previously tested (Fig. 3E, F). Consistent with EVs produced in response to sterile agonists,15 these data could suggest that heat-killed EVs are dampening macrophage proinflammatory cytokine production. As a means to begin to investigate the mechanism for this action, we wanted to determine what, if anything, was different between the two EV populations.
3.4 |. DNA is present and associated electrostatically with bEVs
Viable S. aureus is a potent inducer of neutrophil DNA release,32 and extracellular DNA can associate with EVs and promote coagulation and antigen presentation.16–19,33 Given these data, we assessed if DNA was present and associated with bEV and heat-killed EV subsets by gel electrophoresis. DNA isolated from bEVs resolved as a single, large band near 10.0 kb, whereas heat-killed EVs lacked any associated DNA (Fig. 4A). To determine if DNA was present on the outside of the EVs, we tested its susceptibility to DNase I. First, to confirm DNase I activity, we treated Escherichia coli DNA (EC-DNA) both alone and following liposomal encapsulation with DOTAP (Fig. 4B). EC-DNA alone was degraded upon DNase treatment, but the liposomally encapsulated EC-DNA was not. Pretreatment of bEVs with DNase completely removed the visible band near 10.0 kb, suggesting that DNA is present on the outer surface of EVs rather than enclosed within the vesicles.
FIGURE 4. DNA is present and associated electrostatically with bEVs, but does not contribute significantly to IL-6 and IL-1β production.
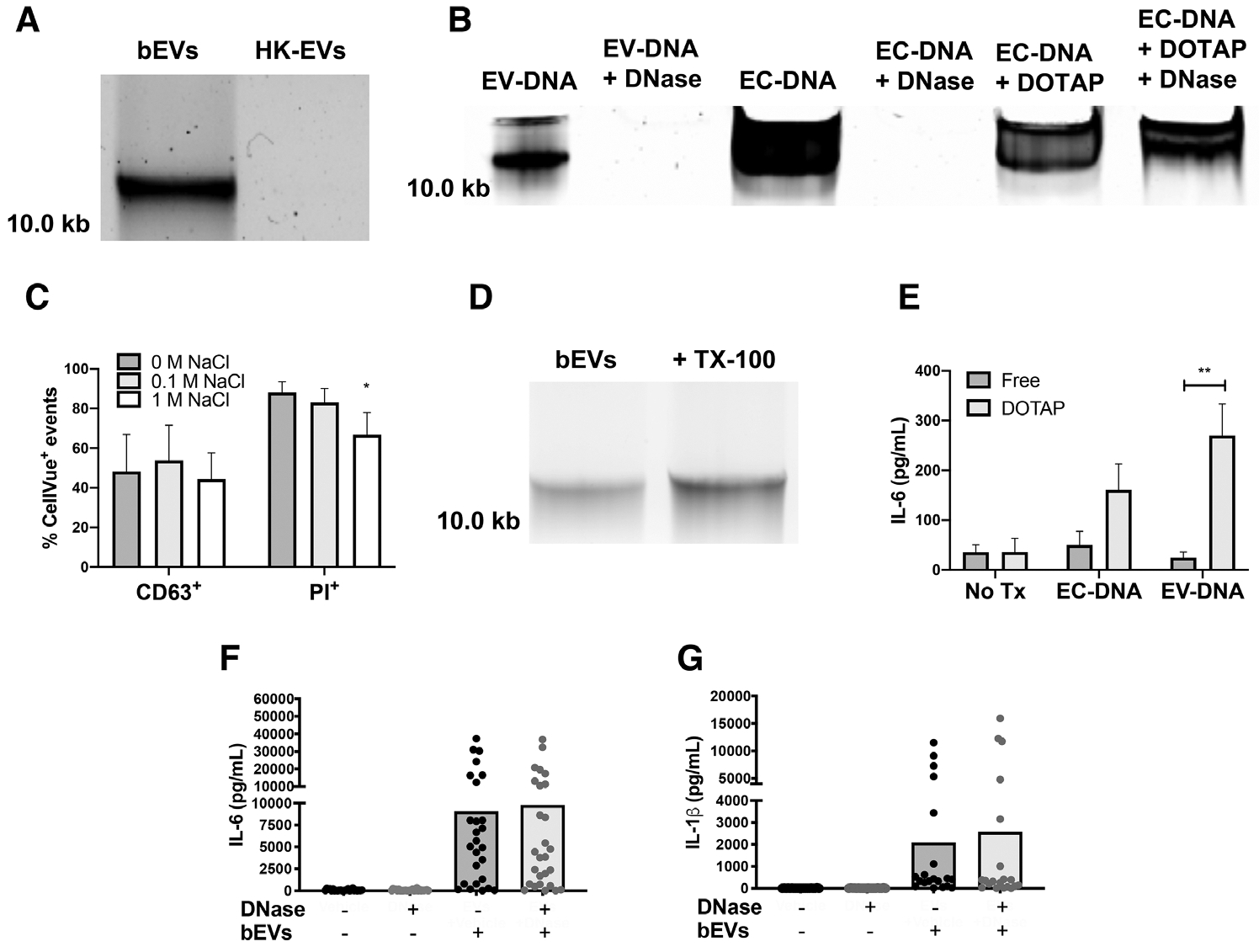
DNA extracted from 50μL of bEVs and heat-killed extracellular vesicles (EVs) was separated by electrophoresis on a 0.7% agarose gel stained with Gel Red (n = 3, A). E. coli DNA (EC-DNA, 1 μg), bEVs, or EC-DNA encapsulated in DOTAP were treated with PBS or DNase I (5 units), then DNA was resolved by gel electrophoresis (n = 3, B). EVs were conjugated to beads, stained with CellVue, and then treated with 0 M, 0.1 M, or 1 M NaCl. After washing, EVs were then stained with either propidium iodide or CD63 and analyzed by flow cytometry (n ≥ 3, C). Bars represent average percentage of positive events ± SEM. P-values were determined for each group using a 1-way ANOVA and Dunnett’s posttest (*P < 0.05 vs. 0 M NaCl). Extracted DNA from untreated or lysed bEVs was assessed as described in A (n = 6, D). Macrophages were treated with either free or DOTAP-encapsulated purified EV-DNA or EC-DNA (1 μg/mL) for 18 h at 37°C before supernatants were analyzed by ELISA for IL-6 (E). Bars represent average values ± SEM (n = 3). P-values were determined for each group using a 2-way ANOVA and multiple comparisons posttest (**P < 0.01). bEVs (200 ng/mL) were incubated with either vehicle or DNase I (5 units) for 1 h at 37°C followed by DNase inactivation with 5 mM EDTA. Macrophages were treated with vehicle or manipulated bEVs for 18–20 h, then supernatants were collected and analyzed by ELISA for IL-6 (F) and IL-1β (G). Symbols represent individual data points, and bars represent the average of at least 24 experiments. P-values were determined using a 1-way ANOVA and Dunnett’s posttest (*P < 0.05 and **P < 0.01 vs. bEVs + vehicle)
To determine the origin of the DNA, we sequenced the extravesicular DNA associated with bEVs. Assembled sequences of bEV DNA aligned with staphylococcal, human mitochondrial, and human nuclear DNA (Table 1). At the selected sequencing depth, we achieved nearly complete coverage of the S. aureus and mitochondrial genome, but coverage of the human genome was incomplete (data not shown). Although these data do not provide insights into the mechanism by which the three types of DNA are released extracellularly, evidence exists that neutrophils can release nuclear and mitochondrial DNA without lysis,32 and S. aureus can release its own DNA.34 Of particular interest is that viable S. aureus is a potent inducer of vital NETosis, a process by which DNA-containing NETs are released in the absence of cell death. Moreover, each type of DNA triggers a unique activation profile in recipient cells,35 and thus the interplay between DNA types and their association with EVs may influence immune cell behavior.
TABLE 1.
EV-associated DNA aligns with sequences from the nucleus and mitochondria of neutrophils as well as the S. aureus genome*
| Total sequencing reads | 1,972,591 |
| Assembled contigs | 186,901 |
| S. aureus contigs | 6,243 |
| Human mitochondrial contigs | 37 |
| Human genomic contigs | 180,621 |
Sequence reads were assembled into overlapping contigs and compared with known sequences using a custom basic local alignment search tool (BLAST)n search.
First, to explore the mechanism by which DNA was bound to bEVs, we interrupted electrostatic interactions with NaCl, stained DNA with PI, and analyzed latex bead-conjugated and CellVue-stained EVs by flow cytometry. After EVs were gated as CellVue+ singlets of beads, we observed 88.1% ± 2.4% of EVs stained positive for PI. Addition of 1 M NaCl reduced PI+ events by 33.26% ± 5.01% (Fig. 4C). To confirm that NaCl did not disrupt EV membranes, we monitored CD63 staining. The percentage of CD63+ events remained unchanged in the presence 1 M NaCl. Thus, EV-DNA association is partially dependent on electrostatic interactions because 1 M NaCl reduced PI staining, but not CD63 staining.
Meanwhile, lysed bEVs still showed a large band near 10.0 kb (Fig. 4D), suggesting that EVs were not solely responsible for pelleting of DNA. However, given the electrostatic interaction established by flow cytometry (Fig. 4C), and that some DNA structures are large enough to be pelleted by ultracentrifugation, these data support that multiple molecular interactions, including electrostatic association, occur.
3.5 |. Degradation of bEV-DNA does not alter the macrophage proinflammatory cytokine profile
Bacterial DNA, and in some instances human DNA, act as agonists for multiple PRRs,30 and extracellular DNA is associated with increased mortality in multiple proinflammatory conditions, including sepsis.36 Therefore, we focused our attention on the role of bEV-associated DNA on macrophage proinflammatory cytokine production. First, to determine whether vesicle-associated DNA possessed any proinflammatory activity, we isolated bEV-associated DNA and encapsulated the purified EV-DNA in DOTAP. As a positive control, we included liposomal E. coli DNA,37 which caused increased production of IL-6 (Fig. 4E), but not IL-1β, by human macrophages (data not shown). Purified EV-DNA alone did not cause IL-6 production; however, treatment of macrophages with liposomal EV-DNA caused a significant, 10.9-fold increase in IL-6 production. Although transfected DNA may be taken up and processed differently than naturally occurring EV-DNA, these data show that bEV-associated DNA has the capacity to trigger IL-6 production by macrophages.
Next, we determined whether DNase I pretreatment of bEVs reduced cytokine production. Compared to unstimulated macrophages, bEVs caused a marked increase in macrophage proinflammatory cytokine production. Although DNA associates with bEVs, pretreatment of bEVs with DNase I did not alter IL-6 or IL-1β production by macrophages (Fig. 4F, G), suggesting that EV-associated DNA is not a significant proinflammatory agonist. Consistent with these results, human macrophages have previously been found to ingest NET DNA without increased proinflammatory cytokine production.37 All together, these data suggest that molecular patterns associated with bacteria, but not EV-associated human or bacterial DNA, caused macrophage proinflammatory cytokine production.
3.6 |. Filtered EVs are not proinflammatory and enhance expression of HLA-DR and CD86
Although our data suggest that bacteria are the primary determinants of the bEV-dependent proinflammatory response, another possibility is that bEV-associated DNA activates macrophages, and that the presence of viable bacteria obscures this activity from being resolved. To attempt to address the direct contribution of EVs and bEV-associated DNA to activation, we subjected bEVs to filtration. We selected a 0.45 μM PES filter, and as expected, filtration of bEVs significantly reduced bacterial presence by 99.1% ± 0.7% (Fig. 5A). TEM imaging confirmed the presence of round membrane-bound structures in the expected EV size range, with no cocci being located (Fig. 5B). Extracellular DNA was also removed by filtration (Fig. 5C), likely due to the DNA size or negative charge. Although we were unable to assess EVs and DNA together, filtered EVs (devoid of DNA and the majority of bacteria) did not cause an increase in IL-6 or IL-1β production compared to untreated macrophages (Fig. 5D, E), providing more evidence that the EVs themselves are not inherently proinflammatory in macrophages.
FIGURE 5. Filtered extracellular vesicles (EVs) are not proinflammatory in macrophages, and enhance macrophage expression of CD86 and HLA-DR.
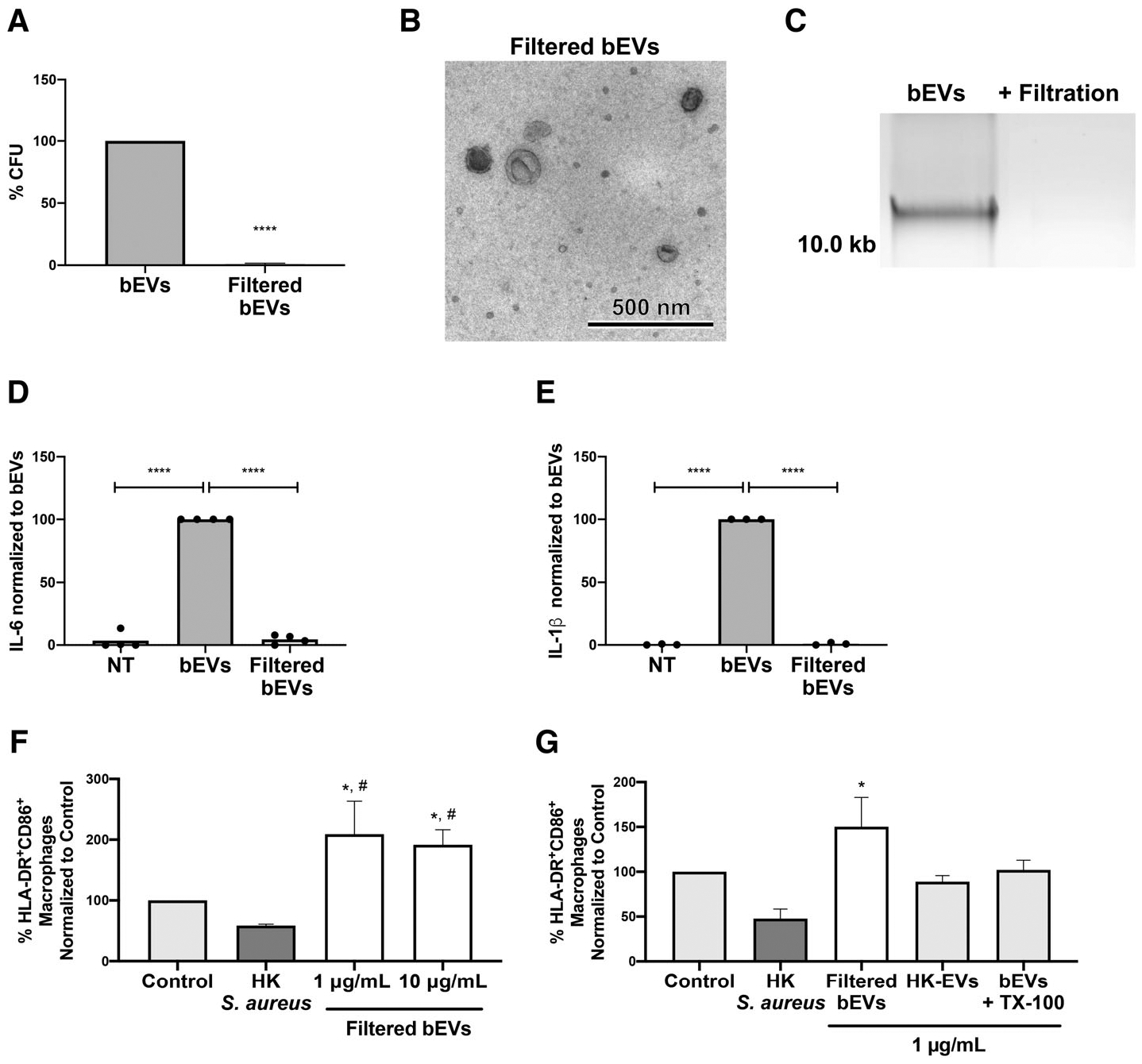
bEVs and filtered bEVs were plated on tryptic soy agar (TSA) overnight at 37°C and colony forming units were enumerated. For each preparation, bacterial quantity was normalized to bEVs and bars represent an average of four experiments. P-values were determined using an unpaired t-test (****P < 0.0001; A). A representative transmission electron microscopy micrograph of filtered bEVs is shown at 10,000× magnification (scale bar = 500 nm; n = 3; B). DNA from bEVs or filtered bEVs was separated on a 0.7% agarose gel stained with Gel Red (n = 3; C). Macrophages were treated with either PBS, bEVs, or filtered bEVs at 200 ng/mL, then IL-6 (D) and IL-1β (E) in supernatants were analyzed by ELISA. Cytokine production was normalized to bEVs. Symbols represent individual data points, and bars represent the mean (n ≥ 4). P-values were determined using a 1-way ANOVA of values normalized to bEVs and Dunnett’s posttest (*P < 0.05 and **P < 0.01 vs. bEVs). Macrophages were pretreated with gentamicin (10 μg/mL), and left in buffer alone (control) or treated with heat-killed S. aureus (75 μg/mL), filtered bEVs, EVs isolated from neutrophils stimulated with heat-killed S. aureus, or Triton X-100 (TX-100) lysed bEVs at indicated concentrations. CD14+macrophages were analyzed by flow cytometry for CD86 and HLA-DR (n ≥ 3, F, G). Bars represent average percentage of HLA-DR+CD86+ events of CD14+ ± SEM. P-values were determined using a 1-way ANOVA and Tukey’s posttest (*P < 0.05 vs. control and #P < 0.05 vs. heat-killed S. aureus)
Because S. aureus inhibits antigen presentation38 and EVs have been shown to enhance antigen presentation via up-regulation of CD80, CD86 and HLA-DR,39 we tested whether filtered bEVs similarly enhanced expression. As expected, macrophages treated with heat-killed S. aureus exhibited a reduction to 58.67 ± 2.29% in expression of both HLA-DR and CD8639 compared to unstimulated macrophages (control) (Fig. 5F). At higher concentrations of filtered EVs (1–10 μg/mL), gentamicin was included to keep cytokine production at baseline levels (data not shown), and upon treatment with filtered EVs, macrophages up-regulated expression of both CD80 and HLA-DR 2.09-fold for 1 μg/mL filtered bEVs and 1.92-fold for 10 μg/mL filtered bEVs. Next, we investigated whether this effect was driven by EVs themselves (Fig. 5G). Compared to control, filtered bEVs (1 μg/mL) produced a 1.5-fold increase in macrophage HLA-DR and CD86 co-expression, but in contrast, TX-100 lysed bEVs, as well as heat-killed EVs, failed to induce a similar up-regulation (Fig. 5H). Although heat-killed bacteria in heat-killed EV preparations may have mitigated the up-regulation of CD86 and HLA-DR, these data suggest that bEVs themselves mediate up-regulation, and that bEVs may ultimately play an important role in macrophage antigen presentation.
4 |. SUMMARY
We showed that viable S. aureus was not required for EV biogenesis, however viable S. aureus was isolated along with bEVs, triggered production of bEV-associated DNA, and elicited proinflammatory cytokine production from human macrophages. Although EV-associated DNA possessed proinflammatory activity when delivered liposomally, DNase I had no effect on bEV-induced cytokine production. Viable bacteria were required to produce neutrophil-derived EVs that, upon filtration, could enhance antigen presentation. Ultimately, characterizing bEVs and heat-killed EVs is a first step toward clinical application, and our hypothesis going forward is that bEVs without associated bacteria and heat-killed EVs might improve the immune response toward S. aureus.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Justin Camiller, Maham Khan, Jenna Owens, Nicholas DeMattei, and Phil Oshel (Central Michigan University) for their excellent technical assistance. The authors also thank Dr. Jeffery Schorey and Li Li (University of Notre Dame) for their help with NTA analysis, Dr. Frank R. DeLeo (Rocky Mountain Laboratories, NIAID) and Dr. Alex Horswill (University of Colorado, Anschutz Medical Campus) for bacteria, and Dr. William Nauseef (University of Iowa) for his advice in the creation of the manuscript. Research in this publication was supported by funds (M.C.G.-W.) awarded by Central Michigan University, as well as the National Institute of General Medical Sciences of the National Institutions of Health under award number R15GM132992. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- BCA
bicinchoninic acid
- bEVs
EVs isolated from neutrophils stimulated with viable Staphylococcus aureus
- BLAST
basic local alignment search tool
- CA-MRSA
community-associated methicillin-resistant Staphylococcus aureus
- CD
cluster of differentiation
- CFU
colony forming unit
- DAMP
damage-associated molecular pattern
- DOTAP
N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride
- EC-DNA
Escherichia coli DNA
- EV-DNA
DNA isolated from EVs
- EVs
extracellular vesicles
- MRSA
methicillin-resistant Staphylococcus aureus
- NET
neutrophil extracellular trap
- NTA
nanoparticle tracking analysis
- OD
optical density
- PAMP
pathogen-associated molecular pattern
- PES
polyethersulfone
- PI
propidium iodide
- PRR
pattern recognition receptor
- TEM
transmission electron microscopy
- TSA
tryptic soy agar
- TX-100
Triton X-100
Footnotes
DISCLOSURES
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care. 2017;21(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am. 2009;23:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011;19:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monaco M, Pimentel de Araujo F, Cruciani M, Coccia EM, Pantosti A. Worldwide epidemiology and antibiotic resistance of Staphylococcus aureus. Curr Top Microbiol Immunol. 2017;409:21–56. [DOI] [PubMed] [Google Scholar]
- 5.Mölne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun. 2000;68:6162–6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolaczkowska E, Jenne CN, Surewaard BG, et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J Immunol. 2014;192:4709–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasser O, Hess C, Miot S, Deon C, Sanchez JC, Schifferli JA. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res. 2003;285:243–257. [DOI] [PubMed] [Google Scholar]
- 9.Timár CI, Lorincz AM, Csépányi-Kömi R, et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood. 2013;121:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann IK, Bertazzo S, O’Callaghan DJ, et al. Differentiating sepsis from non-infectious systemic inflammation based on microvesicle-bacteria aggregation. Nanoscale. 2015;7(32):13511–13520. [DOI] [PubMed] [Google Scholar]
- 11.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalli J, Montero-Melendez T, Norling LV, et al. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol Cell Proteomics. 2013;12(8):2205–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buzas EI, György B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10(6):356–364. [DOI] [PubMed] [Google Scholar]
- 15.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Luo L, Braun OÖ, et al. Neutrophil extracellular trapmicroparticle complexes enhance thrombin generation via the intrinsic pathway of coagulation in mice. Sci Rep. 2018;8:4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Németh A, Orgovan N, Sódar BW, et al. Antibiotic-induced release of small extracellular vesicles (exosomes) with surface-associated DNA. Sci Rep. 2017;7:8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer S, Cornils K, Speiseder T, et al. Indication of horizontal DNA gene transfer by extracellular vesicles. PLoS ONE. 2016;11:e0163665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torralba D, Baixauli F, Villarroya-Beltri C, et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat Commun. 2018;9(1):2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinkmann V, Laube B, Abed UA, Goosmann C, Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp. 2010:e1724 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.György B, Módos K, Pállinger E, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117(4): e39–48. [DOI] [PubMed] [Google Scholar]
- 22.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 23.Boisvert S, Raymond F, Godzaridis E, Laviolette F, Corbeil J. Ray meta: scalable de novo metagenome assembly and profiling. Genome Biol. 2012;13:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momen-Heravi F, Balaj L, Alian S, et al. Alternative methods for characterization of extracellular vesicles. Front Physiol. 2012;3:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butin-Israeli V, Houser MC, Feng M, et al. Deposition of microparticles by neutrophils onto inflamed epithelium: a new mechanism to disrupt epithelial intercellular adhesions and promote transepithelial migration. FASEB J. 2016;30(12):4007–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Thompson CD, Weidenmaier C, Lee JC. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat Commun. 2018;9(1):1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf AJ, Arruda A, Reyes CN, et al. Phagosomal degradation increases TLR access to bacterial ligands and enhances macrophage sensitivity to bacteria. J Immunol. 2011;187(11):6002–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenlee-Wacker MC, Nauseef WM. IFN-γ targets macrophage-mediated immune responses toward Staphylococcus aureus. J Leukoc Biol. 2017;101(3):751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Askarian F, Wagner T, Johannessen M, Nizet V. Staphylococcus aureus modulation of innate immune responses through Toll-like (TLR), (NOD)-like (NLR) and C-type lectin (CLR) receptors. FEMS Microbiol Rev. 2018;42:656–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greaney AJ, Leppla SH, Moayeri M. Bacterial exotoxins and the inflammasome. Front Immunol. 2015;6:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilsczek FH, Salina D, Poon KK, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. [DOI] [PubMed] [Google Scholar]
- 33.Shelke GV, Jang SC, Yin Y, Lässer C, Lötvall J. Human mast cells release extracellular vesicle-associated DNA. Matters Zürich. 2016. [Google Scholar]
- 34.Sugimoto S, Sato F, Miyakawa R, et al. Broad impact of extracellular DNA on biofilm formation by clinically isolated Methicillin-resistant and -sensitive strains of Staphylococcus aureus. Sci Rep. 2018;8:2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhagirath VC, Dwivedi DJ, Liaw PC. Comparison of the proinflammatory and procoagulant properties of nuclear, mitochondrial, and bacterial DNA. Shock. 2015;44(3):265–271. [DOI] [PubMed] [Google Scholar]
- 36.Dwivedi DJ, Toltl LJ, Swystun LL, et al. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Crit Care. 2012;16(4):R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191(5):2647–2656. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Roderiquez G, Norcross MA. Control of adaptive immune responses by Staphylococcus aureus through IL-10, PD-L1, and TLR2. Sci Rep. 2012;2:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez-Jiménez VD, Leyva-Paredes K, García-Martínez M, et al. Extracellular vesicles released from Mycobacterium tuberculosis-infected neutrophils promote macrophage autophagy and decrease intracellular mycobacterial survival. Front Immunol. 2018;9:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


