LETTER TO THE EDITOR
Over 26,000 people will face a new diagnosis of acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) in 2020 in the U.S. alone, and only a minority will be long-term survivors.[1] Among the new therapeutics that have become available over the last several years to improve these outcomes are two antibody-drug conjugates (ADCs) delivering a toxic calicheamicin (CLM)-derivative to leukemia cells expressing either CD33 (gemtuzumab ozogamicin [GO])[2] or CD22 (inotuzumab ozogamicin [IO]).[3] While effective in some patients, however, many do not derive lasting benefit from these drugs for poorly-understood reasons. Thus, better understanding of the mechanisms of resistance relevant for CLM-based ADCs will be crucial to improve treatment outcomes via combination therapy and/or appropriate patient selection.
Impaired intrinsic apoptosis through expression of anti-apoptotic BCL-2 proteins such as BCL-2, BCL-XL, and MCL-1 has emerged as an important mechanism of leukemia resistance.[4] Pharmacologic inhibition of BCL-2 family members is now clinically exploited to induce leukemia cell death directly and/or to increase sensitivity to other anti-leukemia therapies. The small molecule BCL-2 inhibitor venetoclax, recently approved for use in AML,[5] and the BCL-2/BCL-XL/BCL-W inhibitor navitoclax are examples of current efforts. How BCL-2 family proteins might impact the anti-leukemia activity of CLM-based ADCs is unclear, with 2 studies investigating the role of BCL-2 family proteins on GO/CLM-induced cytotoxicity reaching opposite conclusions.[6,7] These conflicting reports prompted our interest in carefully studying the impact of anti-apoptotic BCL-2 family proteins on the anti-leukemia activity of the CLM-based ADCs GO and IO, using in vitro assays with genetically-engineered acute leukemia cell lines and primary samples from AML patients.
For our studies, we generated lentiviral expression constructs containing an IRES-EGFP cassette and human BCL-2, BCL-XL or MCL-1 sequences (template DNA provided by Drs. Daciana H. Margineantu and David M. Hockenbery [Fred Hutch]). Viral particles were packaged as described previously.[8] Sublines of human lymphoid REH and RS4;11 and myeloid ML-1 and HL-60 cells overexpressing human BCL-2 family proteins were generated with lentiviral particles at a multiplicity of infection (MOI) of 25. Frozen aliquots of Ficoll-isolated mononuclear cells from pretreatment peripheral blood or bone marrow specimens from adults with AML were obtained from an institutional repository. Samples were collected under protocols approved by the Fred Hutch Institutional Review Board. Drug-induced cytotoxicity in cell lines and primary human acute leukemia cells was determined as previously described[9] using various concentrations of GO, IO (both Pfizer, New York, NY), calicheamicin-γ1 (AdooQ Bioscience, Irvine, CA), venetoclax (ABT-199; Active Biochem, Hong Kong, China), navitoclax (ABT-263; Selleck Chemicals, Houston, Tx), and/or AZD5991 (ChemieTek, Indianapolis, IN). After 72 hours, cell numbers and drug-induced cytotoxicity, using 4’,6-diamidino-2-phenylindole (DAPI) to detect non-viable cells, were determined by flow cytometry and analyzed with FlowJo (Ashland, OR). For BH3 profiling, thawed aliquots of primary AML patient specimens were treated with FcR blocking agent (Miltenyi Biotec, San Diego, CA) and stained with the following antibodies: CD45-V450, CD3-APC, and CD20-APC (BD Bioscience, San Jose, CA). After permeabilization with digitonin, specimens were incubated with JC-1 mitochondrial dye and peptides comprising the BH3 domains of Bim (100 μM and 0.1 μM), Puma (10 μM), Noxa (100 μM), Bad (100 μM), Hrk (100 μM), Bid (1 μM), and MS-1 (50 μM). Peptide sequences have been described previously[10,11] and were synthesized by New England Peptide (Gardner, MA). Specimens were also incubated individually with dimethyl sulfoxide (DMSO [(1%]) or carbonyl cyanide m-chlorophenyl hydrazone (CCCP; 10 μM). Samples were analyzed via flow cytometry and FACS Diva software. The quantifiable propensity of a pro-apoptotic peptide to induce mitochondrial depolarization was calculated relative to DMSO as background (negative control) and CCCP as positive control:[12]
Statistical analyses were performed with Prism 7 (GraphPad Software, La Jolla, CA) and Spotfire (TIBCO Software, Palo Alto, CA).
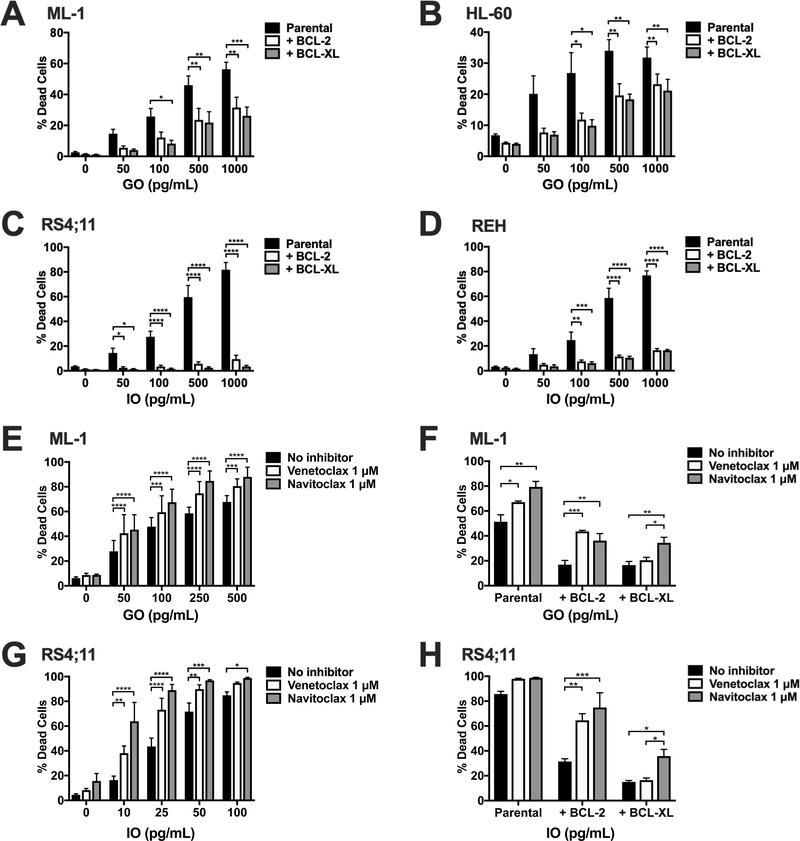
To address the impact of anti-apoptotic BCL-2 family proteins on the sensitivity of human acute leukemia cells to CLM-based ADCs, we used human AML (ML-1, HL-60) and ALL (RS4;11, REH) cell lines overexpressing BCL-2 and BCL-XL and treated parental and engineered cell lines with GO (for ML-1 cells) or IO (for REH cells). In both cell types, BCL-2 and BCL-XL overexpression reduced ADC-mediated cytotoxicity at all doses tested (Figure 1A–D). BCL-2 and BCL-XL also caused relative resistance to commercially-obtained calicheamicin-γ1, which is closely related but not identical to the CLM derivative used in GO and IO (which cannot be obtained commercially; Supplementary Figure 1). The magnitude of resistance conferred by calicheamicin-γ1 appeared similar to that observed with GO or IO, consistent with the notion anti-apoptotic BCL-2 family members reduce the anti-leukemia efficacy of CLM-based ADCs by inducing resistance to CLM rather than impacting antibody-mediated intracellular delivery of the CLM moiety.
Figure 1. Overexpression and pharmacologic inhibition of BCL-2 and BCL-XL modulates in vitro sensitivity of AML and ALL cell lines to CLM-based ADCs.
Human AML ([A,E] ML-1, [B] HL-60, [F] NB4) and ALL ([C, G] RS4;11, [D, H] REH) cell lines were treated with increasing concentrations of GO (for AML cells) or IO (for ALL cells) for 72 hours, and the percentage of dead cells measured flow cytometrically via DAPI staining. In A-D, parental and sublines lentivirally engineered to overexpress BCL-2 or BCL-XL were treated. In E and G, parental cells were co-treated with venetoclax (inhibitor specific for BCL-2) or navitoclax (inhibitor specific for BCL-2, BCL-XL, and BCL-W) at a dose of 1 μM each and compared with no inhibitor control. In F and H, parental (F) (ML-1) and (H) ALL (RS4;11) cells or sublines overexpressing BCL-2 or BCL-XL were treated with GO (ML-1) or IO (RS4;11) at a dose of 500 pg/mL with or without venetoclax or navitoclax at a dose of 1 μM each. Results are shown as mean ± SEM from at least 3 separate experiments. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001 compared to correspondingly treated parental cells or no inhibitor control as appropriate.
Having shown that overexpression of BCL-2 and BCL-XL confers resistance to CLM-containing ADCs and free calicheamicin-γ1, we next investigated whether the clinically exploited inhibitors of BCL-2 and BCL-XL, venetoclax and navitoclax, overcome this resistance and increase leukemia cell sensitivity to CLM-based ADCs. As shown in Figure 1E and 1G, and Supplemental Figure 2, non-toxic doses of venetoclax and navitoclax (1 μM each) also increased GO- and IO-induced cytotoxicity against parental human AML and ALL cell lines, with the magnitude of sensitization substantially greater for IO compared to GO. Furthermore, in BCL-2- and BCL-XL-overexpressing engineered acute leukemia cell lines, venetoclax partially reversed BCL-2- but not BCL-XL-mediated resistance to GO and IO, whereas navitoclax partially reversed resistance mediated by BCL-2 and BCL-XL (Figure 1F and 1H), consistent with the target specificities of these inhibitors.
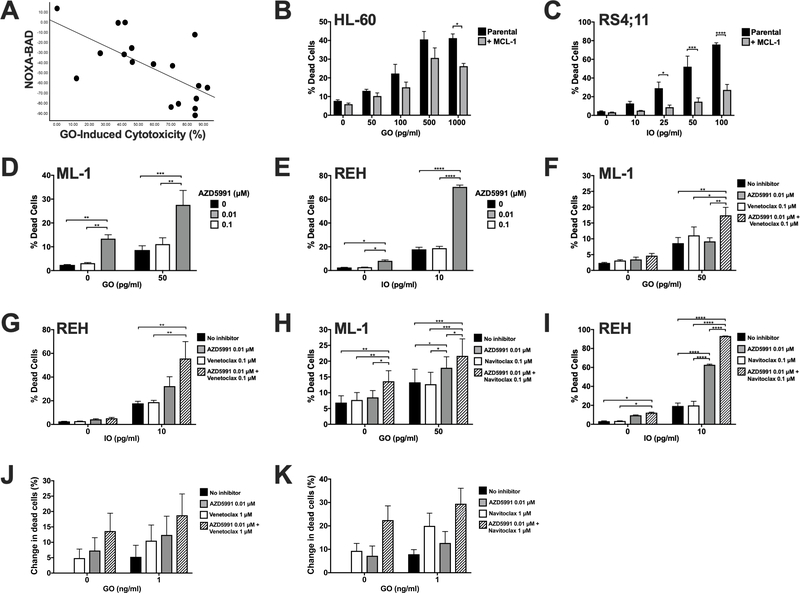
The anti-apoptotic proteins BCL-2, BCL-XL, and MCL-1 mediate pro-survival and resistance to anti-cancer therapies by binding to their pro-apoptotic counterpart BH3 proteins. BH3 profiling can be used to indirectly measure the occurrence of complexes of BCL-2, BCL-XL, or MCL-1 and readouts from BH3 profiling can predict cancer cell therapy response.[12] Here, we used BH3 profiling to determine whether primary human AML cells depend on BCL-2 family proteins beyond BCL-2 and BCL-XL to resist apoptosis when exposed to GO. The relative priming determined by BH3 profiling was measured in 18 AML patient samples (17 peripheral blood, 1 bone marrow) and correlated to in vitro cytotoxicity response to GO monotherapy. These samples were obtained at diagnosis (n=10) or relapse/treatment-refractoriness (n=8) from 9 men and 9 women, median age 55 (range: 24–79) years with cytogenetically favorable (n=3), intermediate (n=12) and adverse (n=3) disease, using modified MRC/NCRI criteria to denote cytogenetic risk.[13] Among these 18 samples, median viability at the time of thawing was 92% (range: 24–99%), with samples comprising a median of 88% (range: 70–97%) blasts. Median expression level of CD33 on blasts was 907 (range: 6–3,294) arbitrary fluorescence units. Analysis for the single nucleotide polymorphism rs12459419[2] showed the CC genotype in 4 specimens, CT genotype in 11 specimens, and TT genotype in 3 specimens, respectively. There was no significant linear correlation between GO-induced cytotoxicity and the individual readouts of peptides that are selective for BCL-2, BCL-XL or MCL-1 (BAD-HRK, HRK, and NOXA, respectively) or BCL-2 & BCL-XL (BAD; Supplementary Figure 3). However, we found a statistically significant negative relationship between GO-induced cytotoxicity and NOXA (MCL-1-specific priming) relative to BAD (BCL-2- & BCL-XL-specific priming) (Figure 2A). In other words, high MCL-1 priming relative to other anti-apoptotic proteins was associated with lower sensitivity to GO. This is consistent with MCL-1 dependence underlying BCL-2- and BCL-XL- mediated resistance.
Figure 2. Expression or pharmacologic inhibition of MCL-1 with or without inhibitors of other BCL-2 family proteins modulates leukemia cell response to CLM-ADCs.
A. Mitochondrial priming with the BH3 peptide BAD was subtracted from the same readout using NOXA as the BH3 priming agent for each of 18 primary AML patient samples. The resulting difference was then correlated to GO-induced cytotoxicity for the same patient sample (using GO at a dose of 10 ng/mL); p=0.002 (Spearman’s rank correlation).
B, C. Human AML (HL-60, [A]) and ALL (RS4;11, [B]) cells were lentivirally transduced to overexpress MCL-1, and parental and engineered cells were exposed to increasing concentrations of GO (HL-60) or IO (RS4;11). D, E. Human AML (ML-1, [D]) and ALL (REH, [E]) cells were treated with GO and IO as appropriate in combination with the MCL-1 inhibitor AZD5991 at the indicated doses. F-I. ML-1 cells (F, H) and REH cells (G, I) were treated with venetoclax (F, G) or navitoclax (H, I) alone or in combination with GO (ML-1 cells) or IO (REH) cells and AZD5991 as shown. J, K. 5 AML patient specimens were treated with venetoclax (J) or venetoclax (K) alone or in combination with GO and AZD5991 as shown. Mean ± SEM of the 5 patient specimens is shown. In all cytotoxicity experiments, dead cells were enumerated flow cytometrically after 72 hours via DAPI staining. For cell line experiments, results are shown as mean ± SEM values from at least three separate experiments. *p<0.05, **p<0.01, *** p<0.001, and ****p<0.0001.
Having shown that MCL-1 may modulate AML cell response to GO, we then examined whether overexpression of MCL-1 conferred resistance to CLM-based ADCs. We therefore engineered human AML (HL-60) and ALL (RS4;11) cells to overexpress MCL-1. As depicted in Figure 2B and 2C, cells overexpressing MCL-1 were indeed relatively resistant to GO and IO. Conversely, the MCL-1 inhibitor AZD5991 significantly sensitized human ALL cells to IO-induced cytotoxicity whereas an additive effect was found with GO-treated AML cells (Figure 2D, E). Having demonstrated that individual BH3 mimetics can sensitize human acute leukemia cell to CLM-based ADCs, we tested whether combined use of such inhibitors, a strategy which has shown remarkable pre-clinical anti-leukemia efficacy,[14] could sensitize to GO or IO. Indeed, GO- and, in particular, IO-induced cytotoxicity was significantly increased by the combination of AZD5991 with either venetoclax (Figure 2F, G) or navitoclax (Figure 2H, I). Finally, in 5 AML patient specimens, the combination of venetoclax (Figure 2J) or navitoclax (Figure 2K) with GO and/or AZD5991 resulted in increased cytotoxicity in an additive fashion, though this increase did not reach statistical significance.
In summary, our data demonstrate that anti-apoptotic BCL-2 family members reduce the cytotoxicity of CLM-based ADCs. This finding provides the scientific basis to explore GO and, perhaps in particular, IO with one or more small-molecule inhibitors of BCL-2 family proteins to improve the anti-leukemia efficacy of these ADCs (or, vice versa, the small molecule inhibitors). The availability of such inhibitors allows for immediate translation of our findings to the clinic. Ideally, early clinical trials would include assessments of the relative contribution each anti-apoptotic BCL-2 family member has in modulating the efficacy of CLM-based ADC therapy to further refine and optimize – and possibly individualize – exact treatment regimens.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the Leukemia & Lymphoma Society (Translational Research Program, grant 6489–16) and the National Institutes of Health/National Cancer Institute (NIH/NCI) Cancer Center Support Grant (P30-CA015704). C.D.G. is supported by a fellowship training grant from the National Heart, Lung, and Blood Institute (NHLBI)/NIH (T32-HL007093) and an institutional K12 grant from the National Cancer Institute (K12-CA076930). S.R.J. and M.H.C. receive support from the NIH/NCI Small Business Innovation Research (SBIR) grant R44-CA203610.
Financial support: Supported by grants from the Leukemia & Lymphoma Society (Translational Research Program, grant 6489–16) and the National Institutes of Health/National Cancer Institute (NIH/NCI; Cancer Center Support Grant P30-CA015704 and Small Business Innovation Research grant R44-CA203610). C.D.G. is supported by a fellowship training grant from the NIH/National Heart, Lung, and Blood Institute (NHLBI; T32-HL007093) and an institutional K12 grant from the NIH/NCI (K12-CA076930).
Footnotes
Conflict of interest disclosure: S.R.J. and M.H.C. are employees of Eutropics Pharmaceuticals, Inc. R.B.W. received laboratory research grants and/or clinical trial support from Actinium Pharmaceuticals, Agios, Amgen, Aptevo Therapeutics, Arog, BioLineRx, Jazz, Pfizer, Seattle Genetics, and Selvita; has ownership interests with Amphivena Therapeutics; and is (or has been) a consultant to Agios, Amphivena Therapeutics, Astellas, BiVictrix, Boehringer Ingelheim, Covagen, Emergent Biosolutions/Aptevo Therapeutics, Jazz, Kite, Pfizer, and Seattle Genetics. The other authors declare no competing financial interests.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020. January;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Godwin CD, Gale RP, Walter RB. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia. 2017. September;31(9):1855–1868. [DOI] [PubMed] [Google Scholar]
- 3.Wynne J, Wright D, Stock W. Inotuzumab: from preclinical development to success in B-cell acute lymphoblastic leukemia. Blood Adv. 2019. January 8;3(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu H, Medeiros LJ, Young KH. Apoptosis signaling and BCL-2 pathways provide opportunities for novel targeted therapeutic strategies in hematologic malignances. Blood Rev. 2018. January;32(1):8–28. [DOI] [PubMed] [Google Scholar]
- 5.Mei M, Aldoss I, Marcucci G, et al. Hypomethylating agents in combination with venetoclax for acute myeloid leukemia: Update on clinical trial data and practical considerations for use. Am J Hematol. 2019. March;94(3):358–362. [DOI] [PubMed] [Google Scholar]
- 6.Walter RB, Raden BW, Cronk MR, et al. The peripheral benzodiazepine receptor ligand PK11195 overcomes different resistance mechanisms to sensitize AML cells to gemtuzumab ozogamicin. Blood. 2004. June 1;103(11):4276–84. [DOI] [PubMed] [Google Scholar]
- 7.Cianfriglia M, Mallano A, Ascione A, et al. Multidrug transporter proteins and cellular factors involved in free and mAb linked calicheamicin-gamma1 (gentuzumab ozogamicin, GO) resistance and in the selection of GO resistant variants of the HL60 AML cell line. Int J Oncol. 2010. June;36(6):1513–20. [DOI] [PubMed] [Google Scholar]
- 8.Laszlo GS, Harrington KH, Gudgeon CJ, et al. Expression and functional characterization of CD33 transcript variants in human acute myeloid leukemia. Oncotarget. 2016. July 12;7(28):43281–43294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laszlo GS, Beddoe ME, Godwin CD, et al. Relationship between CD33 expression, splicing polymorphism, and in vitro cytotoxicity of gemtuzumab ozogamicin and the CD33/CD3 BiTE(R) AMG 330. Haematologica. 2019. February;104(2):e59–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006. May;9(5):351–65. [DOI] [PubMed] [Google Scholar]
- 11.Foight GW, Ryan JA, Gullá SV, et al. Designed BH3 peptides with high affinity and specificity for targeting Mcl-1 in cells. ACS Chem Biol. 2014. September 19;9(9):1962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierceall WE, Kornblau SM, Carlson NE, et al. BH3 profiling discriminates response to cytarabine-based treatment of acute myelogenous leukemia. Mol Cancer Ther. 2013. December;12(12):2940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010. July 22;116(3):354–65. [DOI] [PubMed] [Google Scholar]
- 14.Moujalled DM, Pomilio G, Ghiurau C, et al. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia. 2019. April;33(4):905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.