Abstract
Rationale:
Major depressive disorder is a leading cause of disability worldwide and is likely precipitated by chronic stress. Although many antidepressants are currently available, these drugs require weeks to months of daily administration before reduction of symptoms occur and many patients remain treatment resistant despite several courses of treatment. There is a pressing need for new treatments for stress-related disorders. Kappa opioid receptors (KORs) are a promising new therapeutic target for MDD and anhedonia because acute KOR blockade prevents many effects of stress in rodents.
Objectives:
The following study assessed whether repeated treatment with the selective KOR antagonist aticaprant (also known as JNJ-67953964, and previously LY-2456302 and CERC-501) was effective in reversing behaviors in rodents following exposure to unpredictable chronic mild stress (UCMS).
Methods:
Adult male C57BL/6J mice were exposed to four weeks of UCMS. After three weeks of stress, aticaprant (10 mg/kg) was administered daily for 11 treatments. Behavioral assessments included the sucrose preference test, nesting, forced swim test, hot plate test, light-dark test, and social interaction test.
Results:
Aticaprant significantly reversed stress-induced deficits produced by UCMS on the SPT, nesting, FST, and hot plate test. The effects of aticaprant persisted through a stress and treatment recovery period. Aticaprant was not effective at reversing behavioral effects caused by stress in the light-dark and social interaction tests.
Conclusions:
The results support further study of the role of KORs in regulating circuits related to reward, self-care, and cognition when they are disrupted by chronic stress. They are also consistent with the clinical development of aticaprant as a therapeutic for stress-related disorders targeted at anhedonia, such as depression and post-traumatic stress disorder.
Keywords: Aticaprant, JNJ-67953964, Chronic mild stress, Antidepressant, Kappa opioid receptor, anhedonia
Introduction
Major depressive disorder (MDD) is a leading cause of disability worldwide, imposing a substantial health and economic burden on society (WHO 2018). Although the pathogenesis of MDD is unclear, reoccurring or chronic stress exposure plays an integral role in susceptibility to the development of depression (Ménard et al. 2016; Pittenger and Duman 2008). A variety of antidepressant drugs are widely available based on monoaminergic mechanisms and are considered helpful for many patients (Cipriani et al. 2018). However, these drugs require weeks to months of daily administration before reduction of symptoms occur and many patients (approximately 30%) that fail to reach remission remain resistant to treatment despite several courses of antidepressant compounds (van Bronswijk et al. 2019). Because of these limitations in their clinical effectiveness, there is a pressing medical need for new treatments for mood and anxiety disorders.
A promising new therapeutic target for MDD is the endogenous opioid system (Browne et al. 2020; Browne and Lucki 2019). A target of particular interest is the kappa opioid receptor (KOR) because it is a key regulator of responses to stress (Lutz and Kieffer 2013). KOR activation promotes aversion, dysphoria, and depression- and anxiety-like behaviors. Conversely, KOR antagonists prevent many effects of stress and also counteract stress-induced disruptions of behavioral performance (reviewed in (Jacobson et al. 2020a)). Most preclinical investigations have utilized a limited set of prototypical KOR antagonists, such as nor-binaltorphimine (nor-BNI) and JDTic. However, these prototypical compounds exhibit slow onset of KOR antagonism and long durations of action lasting several weeks (Munro et al. 2012), and these effects are considered by some to limit their potential to advance to clinical trials. Aticaprant (also known as JNJ-67953964, and previously LY-2456302 and CERC-501), is a high-affinity selective KOR antagonist with a half-life suitable for clinical development (Lowe et al. 2014; Naganawa et al. 2016; Rorick-Kehn et al. 2014b). Aticaprant was the first compound that was tested in the newly implemented National Institute of Mental Health (NIMH) fast-fail initiative, which utilizes concepts from the Research Domain Criteria (RDoC) framework to place emphasis on confirmation of neurological biomarkers. In a phase IIa double-blind, placebo-controlled, randomized proof of mechanism study, aticaprant (10 mg) given daily for eight weeks improved anhedonia as measured using the Snaith-Hamilton Pleasure Scale (SHAPS) and performance on a reward anticipation test, and increased concurrent ventral striatal activation in individuals that met criteria for clinically significant anhedonia, which is a major symptom of MDD on most clinical rating scales (Krystal et al. 2020). Most participants also met the Diagnostic and Statistical Manual (DSM)-IV diagnostic criteria for MDD, but the study included others with a wide range of psychiatric disorders. Aticaprant is the only selective KOR antagonist currently in phase II clinical trials for MDD (NCT03559192). Yet, no preclinical studies have systematically evaluated this compound on the behavioral performance of animals exposed to chronic stress under circumstances that have been shown to measure improvement by chronic treatment with antidepressant drugs.
The current study investigated the ability of aticaprant to reverse behaviors in rodents considered relevant to depression and anxiety following exposure to an unpredictable chronic mild stress (UCMS) procedure in mice. UCMS utilizes repeated, randomized presentation of mild stress events over the course of several weeks to produce long-lasting behavioral deficits that are reversed only following chronic treatment with clinically effective antidepressants. This model of chronic stress is widely used in rodents because of its strong construct and predictive validity for depression (Antoniuk et al. 2019; Belzung et al. 2015; Pothion et al. 2004; Willner 2017; Willner et al. 1987). After presentation of UCMS for 3 weeks, aticaprant was administered during the last week of UCMS (week 4) and continued for several days following UCMS cessation for a total of 11 daily treatments. Several behavioral endpoints that are sensitive to stress and have been reversed by chronic administration of antidepressants were selected for assessment. Three test points were interrogated: 1) during and directly following UCMS cessation, 2) during aticaprant treatment, and 3) following a recovery period to evaluate potential long-lasting reversal of stress-induced deficits in mice following both UCMS and aticaprant cessation. The results provide evidence that aticaprant can produce reversal of several behavioral effects resulting from exposure to UCMS. Specifically, aticaprant reversed stress-induced behaviors related to the positive valence and arousal/regulation domains, but not under the negative valence and social domains of the RDoC framework.
Methods and materials
Animals
A total of 152 male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) at 7 weeks of age and were allowed one week to adjust to the vivarium prior to any experiments. Mice were group housed (4 or 5 per cage) with standard enrichment of cotton nestlets (Ancare, Bellmore, NY) and huts (Bio-Serv, Flemington, NJ) under a 12-h-light/dark cycle (lights on at 6:00 AM) in temperature and humidity controlled rooms. Food and water were provided ad libitum. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011), and were approved by the Uniformed Services University Institutional Animal Care and Use Committee.
Drugs
Aticaprant (also known as JNJ-67953964 (Janssen, Piscataway, NJ), formerly LY-2456302 and CERC-501), morphine (Spectrum Chemical, New Brunswick, NJ), and (±)-trans-U-U50,488 methane sulfonate salt (U50,488, Sigma-Aldrich, St. Louis, MO, D8040) were freshly prepared prior to use and injected intraperitoneally (i.p.) using a 10 mL/kg injection volume. Aticaprant was dissolved in vehicle (MilliQ water (Millipore, MA) and 1% lactic acid (Fisher Scientific, Waltham, MA, A162–500)), sonicated for 15 min and titrated to pH 5. Control mice were administered vehicle. Morphine was dissolved in saline, and control mice were administered saline. U50,488 was dissolved in vehicle (MilliQ water), and control mice were administered vehicle.
UCMS procedure
Fifty mice were exposed to a variable sequence of mild, unpredictable stressors for four weeks as previously described with minor modifications (Falcon et al. 2016). Stressors were physical or psychosocial and applied three times per day: in the morning (beginning at 9:30 hours), afternoon (beginning at 14:00 hours), and overnight in a dedicated procedure room. Mice were returned to the animal colony room after each stress application. Stressors included: light fluctuations, stroboscope, restraint, shaker, home cage switch, cage partner switch, rat odor, low bedding, wet bedding, cage tilt, cold exposure, food deprivation, enrichment deprivation, and static noise. The sequence of stressors is presented in Supplemental Table S1. During the last week of stress, mice were injected once daily with either vehicle or aticaprant (10 mg/kg, i.p.) and injections continued for 10 more days, which consisted of the last five days of stress and the next five days following stress cessation, for a total of 11 injections (refer to Figure 2A for the experimental timeline). During behavioral testing, aticaprant treatments occurred several hours following cessation of the behavioral test and subsequent testing occurred at least 16 hours later or there was at least one hour of rest until initiation of the overnight sucrose preference test. Animals were assigned to the stress condition (between cages) and aticaprant treatment condition (within cages) groups based on body weights and baseline performance for sucrose preference and nest building (NS/Vehicle, n=13; NS/Aticaprant, n=12; UCMS/Vehicle, n=12; UCMS/Aticaprant, n=13). All behavioral tests except for the sucrose preference test were conducted during the light cycle.
Figure 2.
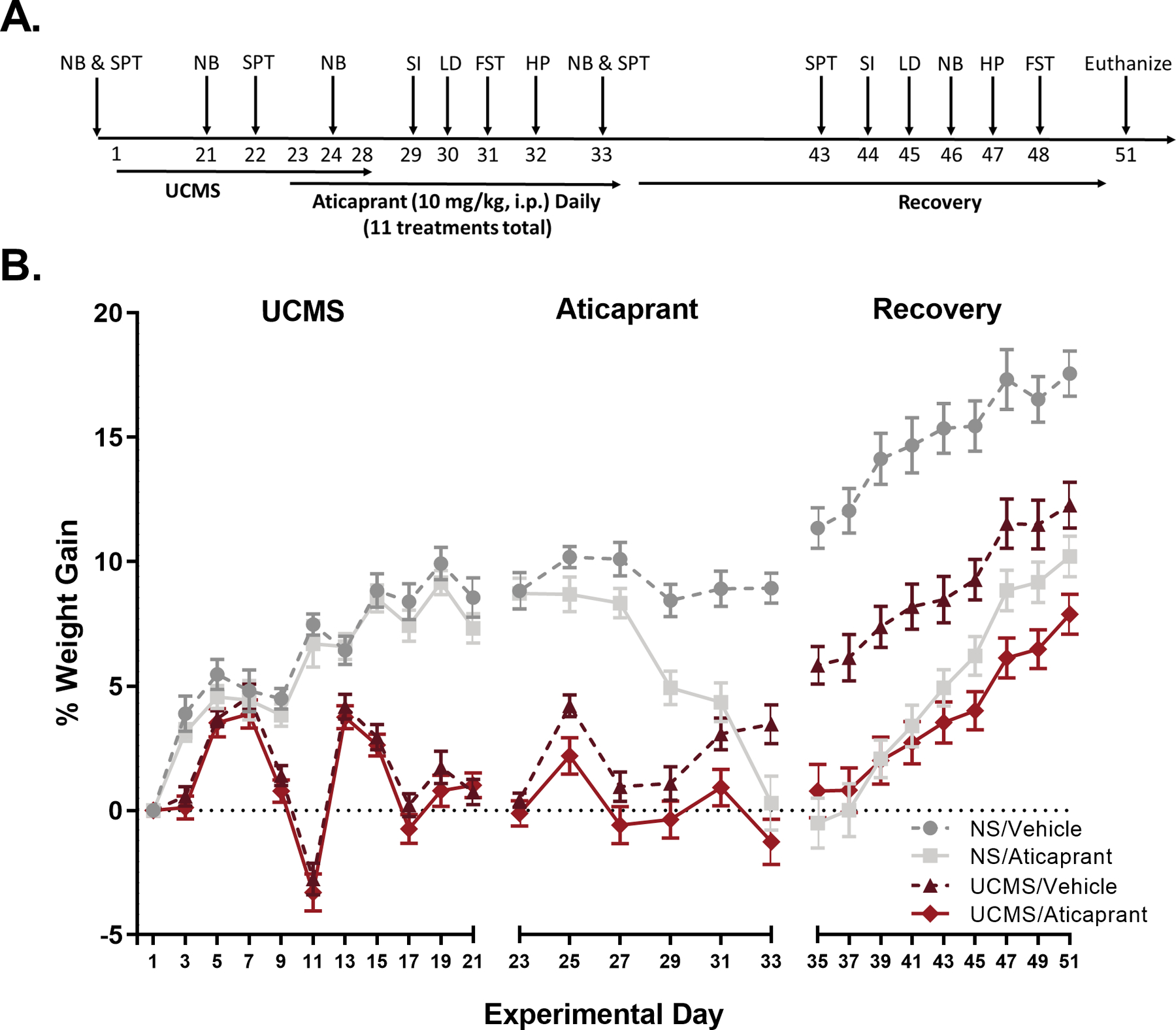
Experimental timeline (A) and percent body weight gain (B). Experimental timeline from commencement of the unpredictable chronic mild stress (UCMS) procedure to euthanasia. The UCMS procedure lasted for 28 days. The KOR antagonist aticaprant (10 mg/kg, i.p.) was administered daily 11 times. Treatment began at 23 days of UCMS and lasted five days post UCMS cessation during the first course of behavioral assessment. Abbreviations: nest building (NB); sucrose preference test (SPT); social interaction (SI); light-dark test (LD); forced swim test (FST); and hot plate test (HP). Body weight data is shown in two-day intervals separately for the UCMS only period (UCMS), aticaprant period (Aticaprant), and recovery from both the UCMS and aticaprant periods (Recovery). All groups were evenly matched in terms of body weight prior to stress exposure: (NS/Vehicle: n=13; 26.4 g ± 0.5; NS/Aticaprant: n=12; 25.6 g ± 0.5; UCMS/Vehicle: n=12; 25.6 g ± 0.7; UCMS/Aticaprant: n=13; 26.0 g ± 0.5).
Sucrose preference test
The sucrose preference test (SPT) was conducted by transferring mice from their colony to individual standard cages (7.5 x 11.5 x 5 inches, Ancare N10). Mice were given a free choice between two bottles, one with water and one with a 1% sucrose solution for 16 hours over the course of the dark cycle (17:00 – 9:00 hours). Bottle placement was rotated across sessions and subjects to avoid side preference. Animals were habituated to the test for five days prior to stress commencement, with the last session serving as the baseline. Fluid intake was determined by measuring the amount of liquid consumed from each of the bottles before and after the test. Sucrose preference was calculated as the percentage of sucrose consumed from the total amount of fluid consumed (sucrose + water).
Nest building
Nest building was assessed by transferring mice from their colony to individual standard cages (the same cages as used during the SPT). In brief, prior to stress commencement, mice were habituated to the test environment and assigned to individual test cages, which remained the same throughout testing, to allow for baseline assessment. The room temperature was 21±1°C. Mice were presented with one full 2.5 g pressed cotton 5 cm square nestlet identical to those given during weekly cage changes, for three hours. Animals were returned to their normal housing conditions following each session. Food and water were available during all sessions, but no additional enrichment was provided. Nests were visually inspected and photographed after three hours. The final nest score was taken after 3 hours of nesting. Scoring was performed by a rater that was blind to the treatment conditions using a scoring system described previously (Jacobson et al. 2020b), with scores ranging from 1 (no nest) – 5 (well-formed nest). To characterize the nest score, the amount of material torn up, the size and shape, wall height, and amount of nest material manipulated in a manner to produce “fluffy” pieces, were all considered.
Social interaction test
Animals were tested for social interaction (SI) behaviors in an open-field arena (40 x 40 x 34 cm) in which a wire-mesh cage was placed at the end of the arena, similarly to that previously described (Browne et al. 2018). Testing was conducted under 7 lux illumination. Each mouse was allowed to freely explore the arena and the wire-mesh cage for 180 s in the absence of a conspecific mouse. The mice were returned to their home cage briefly for ~60 s when the experimenter placed a conspecific C57BL/6J male mouse into the wire-mesh cage. A second 180 s session started immediately. Behavior was video-recorded and the time spent directly interacting with or near the wire-mesh cage during both trials was analyzed using the open source Behavioral Observation Research Interactive Software (BORIS) (Friard and Gamba 2016). Raters were blind to treatment condition of the mice. Interaction time during both time points were converted into a Discrimination Index (DI): DI = (TS – TO)/(TS + TO), where TS was the time spent investigating the cage when the conspecific was present, and TO was the time spent investigating the cage when the conspecific was absent. A higher DI represents an increase in social interaction. One NS/Aticaprant subject and one UCMS/Vehicle subject were removed from the first analysis because they interacted within the interaction zone during the initial session for less than 10 s total.
Light dark test
The light dark (LD) test consisted of a two chambered box made of Plexiglas with an opening (7 x 6.6 cm) for passage from one compartment to the other. One side was black, dark, and covered (40 x 19 cm), and the other side was clear (40 x 20.5 cm) and illuminated (3000 Kelvin). Mice were placed into the dark side of the compartment, and could freely explore for 10 min. The time spent in the light compartment (s) and the latency to enter the light compartment (s) were analyzed by raters blind to the treatment conditions using BORIS (Friard and Gamba 2016).
Forced swim test
The forced swim test (FST) was conducted as previously described (Browne et al. 2018; Jacobson et al. 2020b). Mice were individually placed in a cylinder of water (21 cm in diameter), filled with 15 cm of water (25 ± 1°C), for 6 min. Water was changed between each animal. A blind rater manually scored bouts of immobility during the full 6 min of the test from video recordings. Immobility was defined as the absence of movement, except that necessary to maintain the head above the water. Because the UCMS procedure can reduce the onset of immobility, the data are presented as immobility (s) during the total 6 min of the test.
Hot plate test
Animals were tested for latency to lick their hind paw or jump against the containing cylinder following placement on the hot plate (HP) (Ugo Basile). The hot plate was held at a constant 55°C for the morphine dose occupancy study and the UCMS experiment and at a constant 50°C for the U50,488 dose occupancy study. For the UCMS experiment, behavioral testing consisted of three exposures to the hot plate, each separated by a 30 min inter-trial-interval. The average of the response latency to the three exposures was calculated. Three animals were excluded from the analysis during both assessments (one from the NS/Vehicle group and two from the UCMS/Aticaprant group because they either jumped from the apparatus during every exposure or were significant outliers (> 2 standard deviations away from the average).
For the dose occupancy studies with morphine (n=55) or U50,488 (n=47), two separate groups of mice were injected with aticaprant (0, 1, 3, or 10 mg/kg) 24 h prior to testing on the hot plate. They were then exposed to the hot plate for two baseline measurements separated by a 30 min interval. Then, either morphine (20 mg/kg) or U50,488 (20 mg/kg) were administered, and responses on the hot plate were measured once again either 15 min later for the U50,488 experiment or 30 min later for the morphine experiment. For these studies, antinociception was measured by calculating the change in latency by subtracting the test response from the second baseline response. For all studies, a cutoff period of 60 s was used.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 for Windows (GraphPad Prism, San Diego, CA). The alpha was set at 0.05. A one-way ANOVA was used to analyze hot plate latency data with U50,488 or morphine. For the UCMS experiment, body weight gain was analyzed using three-way ANOVA (day*UCMS*aticaprant treatment) for three separate experimental time points (effect of UCMS exposure alone, effect of aticaprant treatment, and effect of a recovery period). Two-way ANOVAs were used to evaluate aticaprant treatment*UCMS interactions on behavior. Bonferroni multiple comparisons tests were used where appropriate. If variance was increased likely due to repeated testing during the recovery period, the false discovery rate (FDR) method for post hoc analysis was employed where specified. Values are presented as mean ± SEM.
Results
Functional selectivity of aticaprant
U50,488 (20 mg/kg) administration produced antinociception on the hot plate (F(4,35)=14.49, p<0.0001) (Figure 1A) and increased the latency to respond relative to mice treated with vehicle (p<0.0001). U50,488-induced antinociception was blocked in mice pretreated 24 h earlier with 10 mg/kg aticaprant (p=0.93), but not with 1 mg/kg (p=0.0003) or 3 mg/kg (p=0.0001) aticaprant. Further, pretreatment with 10 mg/kg aticaprant significantly reduced the latency to respond relative to mice given U50,488 alone (p=0.0003). Another group of mice (n=7) served as an internal control where pretreatment with 10 mg/kg aticaprant alone (−3.61 s ± 1.37) had no effect on response latency relative to vehicle subjects (−2.07 s ± 4.51; ns (unpaired t-test)).
Figure 1.
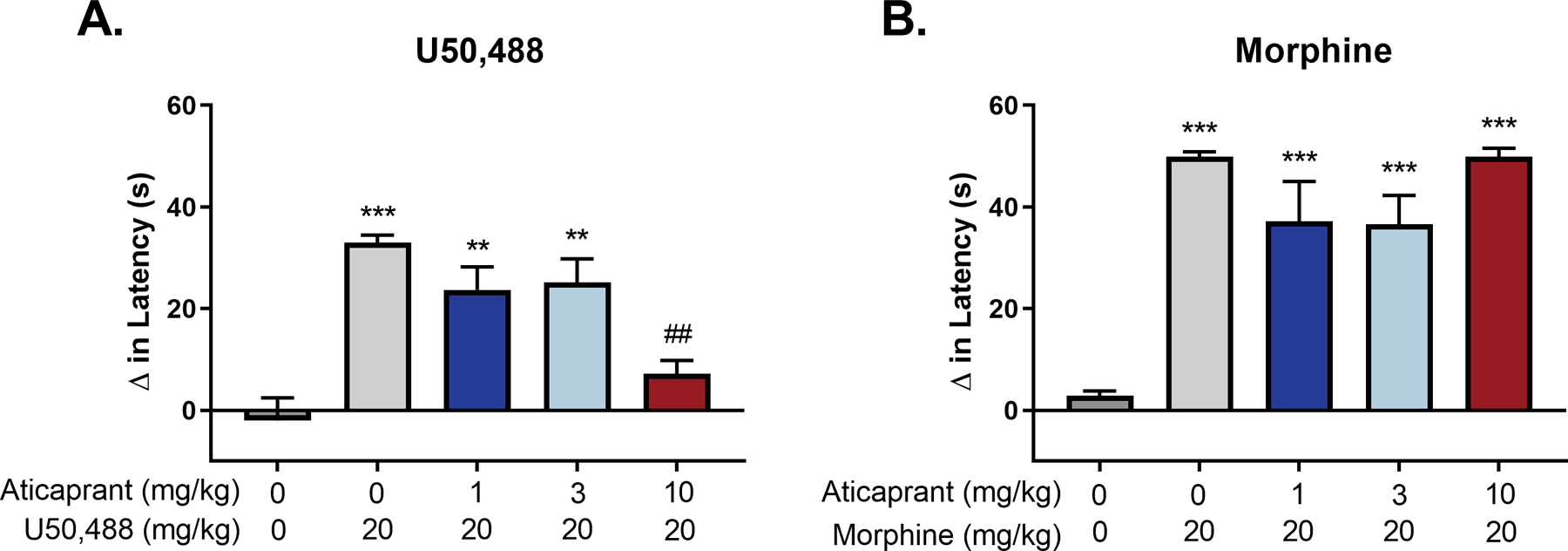
Effects of aticaprant (1, 3, or 10 mg/kg) on (A) U50,488 (20 mg/kg) and (B) morphine (20 mg/kg) induced antinociception on the hot plate assay. Aticaprant was injected at 24 hours, U50,488 at 15 min and morphine at 30 prior to testing. U50,488-induced antinociception (n=8 per group) was blocked only in mice pretreated with 10 mg/kg aticaprant. Morphine-induced antinociception was not blocked by any dose of aticaprant (vehicle, n=15; morphine, n=14; 1 mg/kg aticaprant, n=9; 3 mg/kg aticaprant, n=9; 10 mg/kg aticaprant, n=8). The * symbol denotes significant differences between the vehicle (0 mg/kg aticaprant/0 mg/kg U50,488 or 0 mg/kg morphine) group and all other groups (**p < 0.01; ***p < 0.0001). The # symbol denotes a significant difference between aticaprant-treated subjects and U50,488 only treated subjects (##p < 0.01).
Morphine (20 mg/kg) exposure also produced antinociception on the hot plate (F(4,50)=35.04, p<0.0001). In contrast to U50,488, however, this effect was not blocked by any doses of aticaprant (p<0.0001). These results support the in vivo functional selectivity of aticaprant for the kappa opioid receptor relative to the mu opioid receptor (Figure 1B).
Because 10 mg/kg aticaprant given 24 h prior to testing completely blocked U50,488-induced antinociception without effect on morphine-induced antinociception, and blocked U50,488-induced nesting suppression in a previous study (Jacobson et al. 2020b), this dose was chosen as the treatment for the UCMS experiment.
Body weight
The UCMS experimental timeline is illustrated in Figure 2A. The experiment was separated into three distinct time intervals, which included exposure to the UCMS procedure, the stage during which aticaprant was administered, and a post-treatment recovery period.
Body weight gain was differentially affected depending on the period interrogated (Figure 2B). The UCMS paradigm significantly attenuated weight gain in the stressed subjects relative to the nonstressed controls, as shown by a significant day*UCMS interaction (F(11,506)=66.31, p<0.0001). While nonstressed subjects showed the ordinary increase in body weight, body weight gain was consistently suppressed following exposure to stress after two weeks. During aticaprant treatment (F(4,184)=5.93, p=0.0002) and the recovery period (F(8,368)=5.29, p<0.0001), there were significant day*UCMS*aticaprant treatment interactions. Specifically, while nonstressed subjects given vehicle steadily maintained their body weight, nonstressed subjects treated with aticaprant experienced a loss in body weight. UCMS-exposed subjects treated with aticaprant maintained their decreased body weight level and did not lose any more weight. During recovery, all subjects’ body weight increased, suggesting revival of body weight over time. In the three groups that experienced weight attenuation, the UCMS-exposed subjects treated with vehicle recovered the quickest. Those stressed subjects given aticaprant continued to exhibit an attenuation of body weight gain following stress and treatment cessation relative to vehicle-treated controls.
Sucrose preference
Sucrose preference was assessed four times: final baseline prior to stress exposure, after three weeks of UCMS (day 22), following aticaprant treatment (day 33), and during the recovery period (day 43) (Figure 3). During baseline, all groups were equivalent (ns) and preferred sucrose > 70% (data not shown). However, following three weeks of stress, both UCMS-exposed groups exhibited a reduction of sucrose preference by about 30% compared to their respective nonstressed control groups (p≤0.01). Specifically, during the UCMS procedure, there was a significant effect of stress (F(1,46)=20.66, p<0.0001) and no treatment*stress interaction (ns). This shows that sucrose preference was affected equally in the two UCMS groups prior to treatment with aticaprant (Figure 3A).
Figure 3.
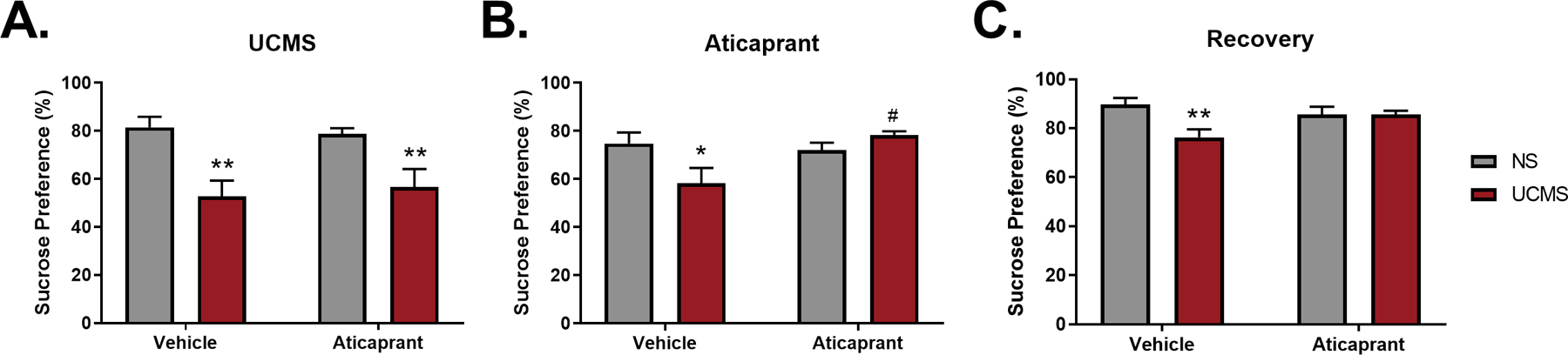
Sucrose preference during (A) Week three of UCMS (day 22), (B) following 11 days of aticaprant treatment (day 33), and (C) following a recovery period of 15 days post cessation of UCMS and 10 days post aticaprant treatment cessation (day 43). The percentage of sucrose preference (sucrose consumption/(sucrose + water consumption) did not differ at baseline (NS/Vehicle = 86.1 ± 1.1; NS/Aticaprant = 85.5 ± 1.4; UCMS/Vehicle = 85.1 ± 1.5; UCMS/Aticaprant = 85.9 ± 1.5). The * symbol denotes significant differences between UCMS-treated mice and their respective no stress (NS) controls (*p < 0.05; **p ≤ 0.01). The # symbol denotes significant differences between UCMS-treated mice given aticaprant or vehicle (#p < 0.05).
Treatment with aticaprant reversed the reduction of sucrose preference in UCMS-exposed animals (Figure 3B), as supported by a significant stress*treatment interaction (F(1,46)=7.15, p=0.01). Sucrose preference remained significantly lower in stressed animals given vehicle (p=0.04), but stressed animals treated with aticaprant had significantly higher sucrose preference compared to stressed animals given vehicle (p=0.01).
During recovery, a significant stress*treatment interaction continued (F(1,46)=6.15, p=0.02) (Figure 3C). At this time point, stressed animals given vehicle continued to exhibit a reduction in sucrose preference. This was reflected in 15% less sucrose consumed compared with their nonstressed controls (p=0.006). Stressed animals treated with aticaprant continued to prefer sucrose at a comparable level to all nonstressed controls (p>0.99), illustrating a persistent reversal of UCMS-induced reduction of sucrose preference.
Nest scores
Nesting was assessed five times: final baseline prior to stress exposure, following three weeks of UCMS (day 21), following 1 aticaprant treatment (day 24), following 11 treatments with aticaprant (day 33), and during a recovery period (day 46) (Figure 3). During baseline, all groups were equal and exhibited high nest scores (ns). Stress exposure significantly reduced nest scores (F(1,46)=14.42, p=0.0004) (Figure 4A). There was no interaction (ns) or effect of treatment group (ns), ensuring the two UCMS groups had equally lower nest scores relative to their control groups (p<0.05) at this time point.
Figure 4.
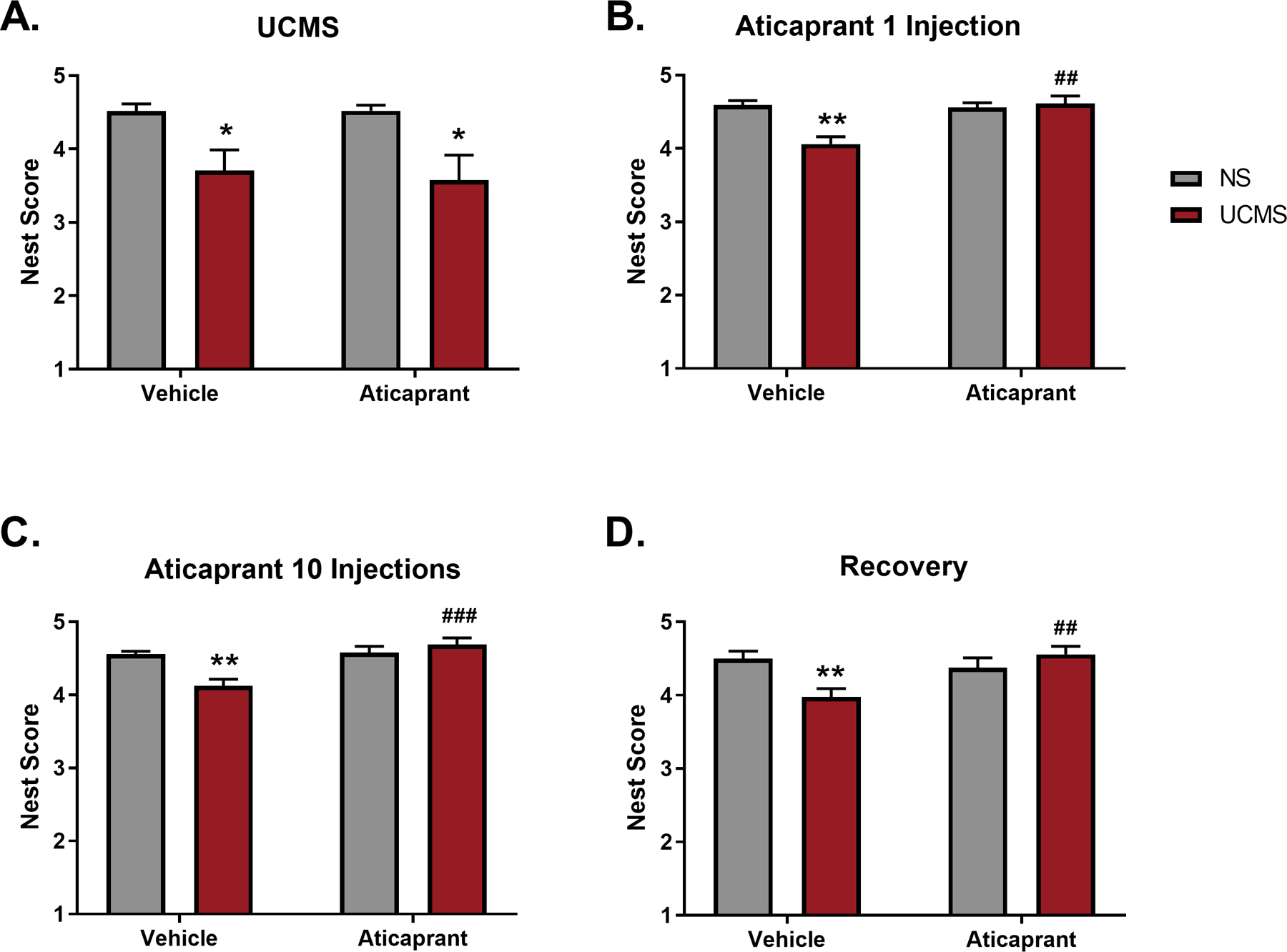
The final nest score following three hours of testing during (A) unpredictable chronic mild stress (UCMS) (day 21) (B) following one injection with aticaprant (day 24), (C) following 10 treatments with aticaprant (day 33), and (D) following a recovery period (day 46). During baseline, groups nested equally (NS/Vehicle = 4.5 ± 0.04; NS/Aticaprant = 4.5 ± 0.05; UCMS/Vehicle = 4.5 ± 0.04; UCMS/Aticaprant = 4.5 ± 0.06). The * symbol denotes significant differences between UCMS-treated mice and their respective no stress (NS) controls (*p < 0.05; **p ≤ 0.01). The # symbol denotes significant differences between UCMS-treated mice given aticaprant or vehicle (##p < 0.01; ###p < 0.0001).
A single injection with aticaprant during the third week of stress was sufficient to reverse UCMS-induced suppression of nesting (Figure 4B). There was a significant stress*treatment interaction (F(1,46)=12.19, p=0.001). Reversal of stress-induced suppression of nesting persisted following 10 daily treatments with aticaprant (Figure 4C), supported by a significant stress*treatment interaction (F(1,46)=11.97, p=0.001). Stressed subjects given vehicle continued to construct poorer quality nests relative to their nonstressed controls during the third week of stress after one injection (p=0.0003) (Figure 4B) and also following ten injections once stress had already concluded (p=0.002) (Figure 4C). Stressed animals treated with aticaprant just once (p=0.0002) and ten times (p<0.0001) had higher nest scores than stressed animals treated with vehicle.
Suppression of nesting due to stress exposure was long-lasting and evident following a recovery period, with a stress*treatment interaction (F(1,46)=9.30, p=0.004) (Figure 4D). Whereas stressed animals treated with aticaprant continued to nest more than stressed animals treated with vehicle (p=0.006), the stressed animals given vehicle also continued to nest less than the nonstressed controls (p=0.01).
Forced swim test
The FST was first assessed three days after stress cessation and following eight treatments with aticaprant (day 31). There was a significant stress*treatment interaction (F(1,46)=4.71, p=0.03) on total immobility (Figure 5A). The duration of immobility by vehicle-injected mice exposed to stress was 40% longer than when compared with nonstressed mice (p=0.0004). However, aticaprant reversed this increase in immobility in stressed animals as they were significantly less immobile relative to stressed animals not treated with aticaprant (p=0.02).
Figure 5.
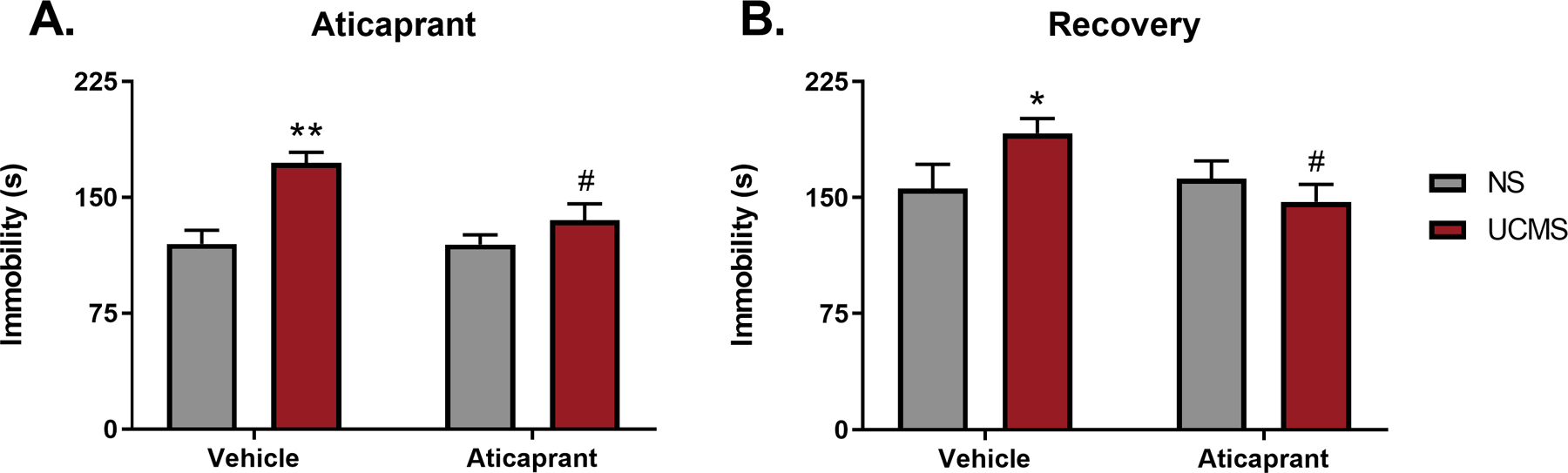
Immobility in the forced swim test (FST) following (A) eight aticaprant treatments (day 31) and (B) during the recovery period at 20 days post UCMS cessation and 15 days post treatment cessation (day 48). The * symbol denotes significant differences between UCMS-treated mice and their respective no stress (NS) controls (*p < 0.05; **p < 0.01). The # symbol denotes significant differences between UCMS-treated mice given aticaprant or vehicle (#p < 0.05).
Following a recovery period (day 48), there was increased variability in the nonstressed animals, and subjects were more immobile overall, likely due to retesting. A significant stress*treatment interaction persisted (F(1,46)=4.14, p=0.04) (Figure 5B). FDR post-hoc analysis supported the observation that UCMS-exposed animals were still significantly more immobile relative to their non-stressed controls (p=0.05), but UCMS-exposed animals treated with aticaprant exhibited lower immobility scores relative to UCMS mice treated with vehicle (p=0.02).
Antinociception
During aticaprant treatment (day 32) there was a significant effect of stress (F(1,43)=5.12, p=0.03), a trend for a stress*treatment interaction (F(1,43)=3.67, p=0.06), and no effect of aticaprant treatment (F(1,43)=3.02, p=0.10) (Figure 6A) on responses measured on the hot plate. Stressed subjects given vehicle remained on the hot plate apparatus for roughly 2.5 seconds longer than nonstressed controls (p=0.03). This finding appears to demonstrate an instance of stress-induced analgesia, and it was blocked in stressed mice administered aticaprant (p>0.99).
Figure 6.
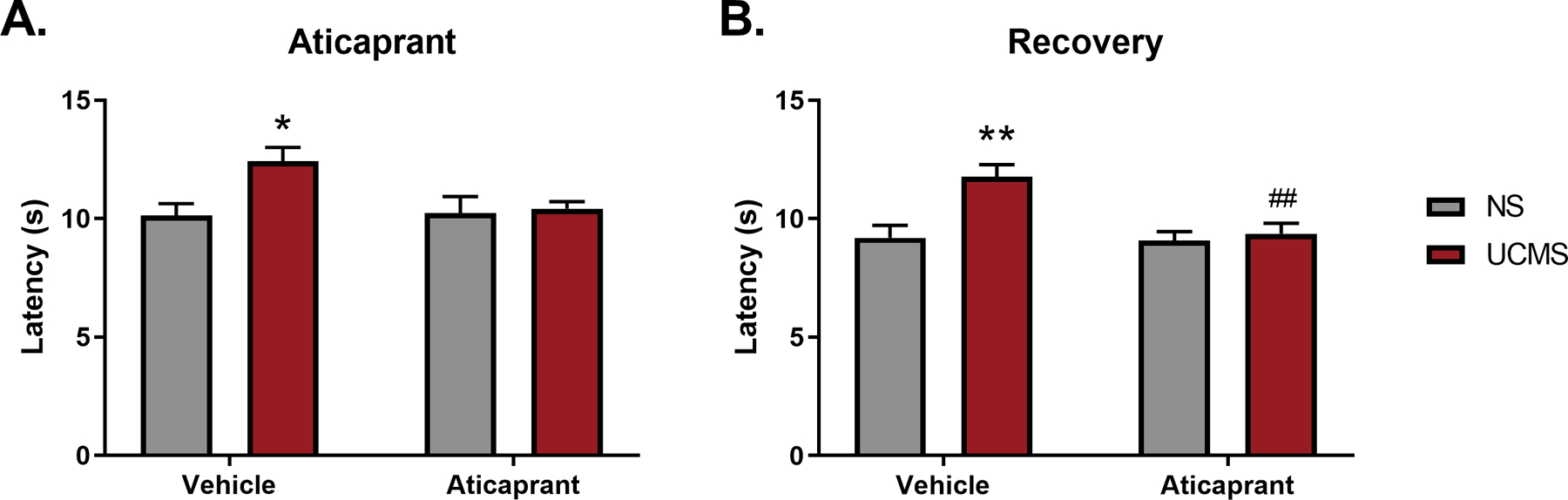
Average latency to respond during three exposures to the hot plate apparatus in animals exposed to unpredictable chronic mild stress (UCMS) or non-stressed controls (NS) (A) after 9 treatments with aticaprant (day 32) and (B) after a recovery period of 19 days post UCMS cessation and 14 days post aticaprant treatment cessation (day 47). (NS/Vehicle, n=12; NS/Aticaprant, n=12; UCMS/Vehicle, n=12; UCMS/Aticaprant, n=11). The * symbol denotes significant differences between UCMS-treated mice and their respective non-stressed (NS) controls (*p < 0.05; **p < 0.01). The # symbol denotes significant differences between UCMS-treated mice given aticaprant or vehicle (##p < 0.01).
Stress-induced analgesia was more pronounced following a recovery period (day 47); there was a significant stress*aticaprant treatment interaction (F(1,43)=6.01, p=0.02) (Figure 6B). While stressed subjects given vehicle had a longer latency to respond on the hot plate relative to nonstressed controls (p=0.002), the administration of aticaprant in stressed animals blocked this enhancement relative to the other stressed animals (p=0.005).
Social interaction
Stressed mice exhibited less interest in social interaction illustrated by a lower discrimination index (DI) during aticaprant treatment (day 29), and a statistically significant effect of stress (F(1,44)=17.12, p=0.0002) (Figure 7A). There was no interaction (ns) or effect of aticaprant treatment (ns). Both UCMS groups regardless of treatment with vehicle (p=0.01) or aticaprant (p=0.009) had lower DI scores relative to controls.
Figure 7.
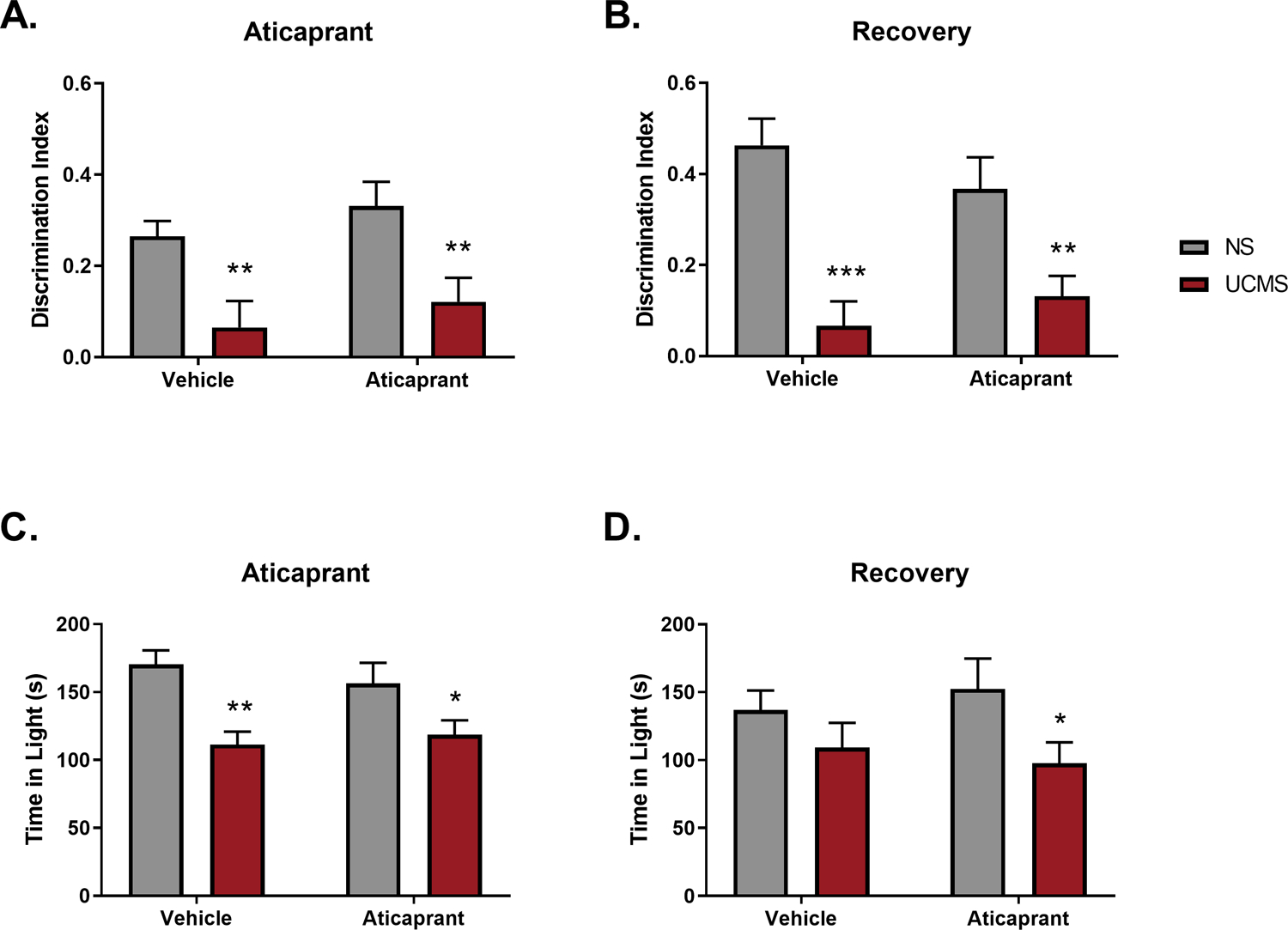
Social interaction was expressed as a discrimination index and was reduced by exposure to UCMS during (A) aticaprant treatment (day 29) and (B) a recovery period (day 44). Performance on the light dark test was also expressed as time in the light during (C) aticaprant treatment (day 30) and (D) a recovery period (day 45). (NS/Vehicle, n=13; NS/Aticaprant, n=11; UCMS/Vehicle, n=11; UCMS/Aticaprant, n=13). The * symbol denotes significant differences between UCMS-treated mice and their respective no stress (NS) controls (*p ≤ 0.05; **p < 0.01; *** p < 0.0001).
During the recovery period (day 44), stressed mice continued to exhibit less interest in social interaction (F(1,46)=30.89, p<0.0001) (Figure 7B). There was no interaction (ns) or effect of aticaprant treatment (ns). Both UCMS groups regardless of treatment with vehicle (p<0.0001) or aticaprant (p=0.01) had lower DI scores relative to controls. Total distance travelled by mice during both assessments did not differ between groups (ns), suggesting no effects of stress or aticaprant on general locomotion, at least during this behavioral assay. Overall, these data indicate that stress exposure caused a persistent reduction of social interaction that was insensitive to remediation by aticaprant.
Light dark test
Subjects were assessed on the light dark test two days following stress cessation and after seven treatments with aticaprant. During treatment (day 30), all stressed mice spent less time in the light compartment as shown by a significant effect of UCMS (F(1,46)=17.47, p=0.0001) (Figure 7C), but with no interaction (ns) or effect of treatment (ns). Stressed mice also took longer to enter the light compartment (F(1,46)=5.06, p=0.03). Irrespective of vehicle (p=0.001) or aticaprant (p=0.05) treatment, stressed animals spent significantly less time in the light compartment relative to the nonstressed groups (Figure 7C).
Following a recovery period (day 45), stressed subjects continued to spend less time in the light compartment relative to nonstressed subjects (F(1,46)=5.47, p=0.02) (Figure 7D). Stressed subjects also took significantly longer to enter the light compartment relative to nonstressed controls (F(1,46)=12.73, p=0.0009). Overall, these data indicate that aticaprant did not block the anxiogenic effects of stress in this behavioral test.
Additional physiological and molecular measures
During euthanasia, the adrenal glands, thymus, spleen, and heart were cleaned of fat and weighed (Supplemental Figure S1). The weight of the adrenal glands (F(1,46)=12.07, p=0.001) and the thymus (F(1,46)=19.45, p<0.0001) were significantly higher after UCMS exposure. There were no effects of aticaprant treatment or interactions detected (ns). The spleen and heart weighed similarly in all groups, with no significant effects or interactions (ns). Corticosterone levels measured from the serum taken at the time of euthanasia did not differ among the groups (Supplemental Figure S2D). There were no significant effects or interactions (ns) for the phosphorylation of JNK (Supplemental Figure S2A), ERK (Supplemental Figure S2B), or p38 (Supplemental Figure S2C) in the medial prefrontal cortex (mPFC).
Discussion
The goal of the present study was to investigate the ability of the selective KOR antagonist aticaprant to reverse behaviors considered relevant to depression, pain, and anxiety in mice following chronic exposure to stress. Utilizing UCMS as a validated procedure for inducing deficits in several behavioral domains relevant to MDD, it was established that aticaprant treatment reversed deficits in anhedonia, pain, and coping with inescapable stress, but not anxiety and social interaction (summarized in Supplemental Table S2). These effects persisted through a stress and treatment recovery period, illustrating sustained remission following aticaprant treatment cessation. Taken together, these data provide preclinical evidence that supports recent clinical studies of aticaprant specifically suggesting further interrogation of KOR antagonism for the treatment of anhedonia.
Aticaprant reversed behaviors relevant to depression
Behaviors relevant to depression, including reductions in sucrose preference, poor nest construction, and increased immobility on the forced swim test, were induced in stressed mice and verified the effectiveness of the UCMS procedure. Both UCMS groups were equally affected by the stress procedure prior to treatment based on the responses on these assays and these effects were all robustly reversed in stressed mice treated with aticaprant. First, a reduction of sucrose preference is widely accepted as a measure of anhedonia in rodents following UCMS (Willner 2017), although it does not distinguish reward-related functions. It has also been shown that other KOR antagonists normalize deficits in sucrose preference following exposure to chronic stress (Dogra et al. 2016; Falcon et al. 2016; Williams et al. 2018). Second, nesting, an innate spontaneously occurring behavior, is an ethologically relevant indicator of self-care and an animal’s overall well-being. An impairment in nesting may represent an anhedonic state, lack of motivation to engage in a rewarding species-typical behavior, or loss of overall energy/arousal (Cryan and Holmes 2005; Deacon 2012; Deacon 2006; Gaskill et al. 2013; Jacobson et al. 2020b; Jirkof 2014). Previous studies have also illustrated a suppression of nesting following chronic stress exposure (Dournes et al. 2013; Newman et al. 2019; Otabi et al. 2016; Woo et al. 2018), but to date, no studies had illustrated that KOR antagonism can rescue a stress-induced deficit in nesting. Just one injection with aticaprant was sufficient to reverse UCMS-induced suppression of nesting, and this effect was maintained throughout the remainder of testing. Third, the FST is a widely used, reliable, and validated assay to evaluate antidepressant drugs, and the immobility is thought to represent a measure of passive coping with inescapable stress (Commons et al. 2017). The FST has also been used to illustrate the effectiveness of KOR antagonists to reverse chronic stress-induced increase in immobility (Browne et al. 2018; Brüning et al. 2014; Dogra et al. 2016; Falcon et al. 2016; Haj-Mirzaian et al. 2019). Overall, stress-induced behavioral deficits that appeared on several assays were reversed by aticaprant treatment and this rescue was maintained throughout a recovery period.
Aticaprant did not reverse behaviors relevant to anxiety
In contrast to the efficacy of aticaprant in behavioral measures relevant to depression, aticaprant failed to normalize the effects of stress on exploratory behaviors in the light dark box or deficits in social interaction. Previously, our lab group has shown that the mixed opioid receptor antagonist buprenorphine reversed stress-induced effects on the light dark test following UCMS (Falcon et al. 2016) and social interaction deficits following social defeat stress (Browne et al. 2018). Aticaprant, however, did not reverse social interaction deficits following social defeat stress (Browne et al. 2018). Buprenorphine is a KOR antagonist and MOR partial agonist (Lutfy and Cowan 2004). Thus, it has been suggested that reversal of anxiety-relevant behaviors following stress exposure are MOR dependent (Browne et al. 2018).
It is also possible that aticaprant may alleviate behaviors related to anxiety arising from a different context or measured with different assays. Specifically, aticaprant diminished the frequency of withdrawal symptoms from exposure to nicotine (Jackson et al. 2015) and alcohol (Domi et al. 2018) in mice and rats, including a reduction of open arm time in the elevated plus maze (EPM) (Domi et al. 2018; Jackson et al. 2015). Additionally, reduction of open arm time in the EPM in adult prenatally stressed rats was reversed in subjects treated just once with aticaprant two hours prior to testing (Peters et al. 2011). Increased time in the open arms of the EPM was also observed in naïve mice given aticaprant 30 minutes prior to testing, although the dose was much higher (30 mg/kg, i.p.) (Wang et al. 2017).
It should also be noted that a few reports indicated no effect of KOR antagonists on behavioral deficits produced by stress, depending on when the KOR antagonist was administered in relationship to stress exposure [reviewed in Jacobson 2020a]. Specifically, it has been suggested that KOR antagonists are most efficacious when administered prior to stress exposure. For example, the short-acting KOR antagonist AZ-MTAB restored stress-induced deficits on the FST, EPM, and in social interaction, only when it was given daily prior to three days of social defeat, but not when it was given just once prior to behavioral assessment [Williams 2018]. However, it was difficult to conclude whether this effect was due to the number of injections [Williams 2018], and in the current study, aticaprant was indeed efficacious in reversing other behaviors. Regardless, it is possible that aticaprant may be efficient at reversing behaviors relevant to anxiety if it were administered prophylactically. Further research should explore whether there are time-dependent effects related to treatment efficacy, and whether aticaprant can reverse behaviors related to anxiety on different types of tests or conditions than those already tested.
Aticaprant reversed stress-induced analgesia
It is not uncommon for stress to elicit pain relief or increase pain tolerance in both humans and rodents (Adler and Gattaz 1993; Bär et al. 2005; Butler and Finn 2009; Thompson et al. 2017). Rodents exposed to UCMS exhibited increased latencies to respond on several pain assays, including the hot plate, tail flick, or von Frey tests (Lian et al. 2017; Pinto-Ribeiro et al. 2004; Shi et al. 2010a; Shi et al. 2010b). In the current study, stressed mice had longer response latencies on the hot plate relative to nonstressed mice, and this effect was normalized to control levels by aticaprant. In line with this finding, repeated swim stress-induced antinociception on the tail withdrawal assay was blocked by nor-BNI treatment and also in prodynorphin knockout mice (McLaughlin et al. 2004). Taken together, these data support a role for KORs in mediating stress-induced analgesia in the context of chronic stress.
Body weight alterations
A significant reduction in body weight of subjects in the nonstressed control group treated with aticaprant was measured after one week of treatment. This effect coincided with the start of behavioral assessments. The reduction in body weight in the nonstressed control group did not correspond with any noticeable behavioral alterations on any assessment relative to the nonstressed controls given vehicle. Body weight appeared to recover following treatment cessation. As anticipated, stressed mice exhibited an attenuation of weight gain during the UCMS procedure. Importantly, treatment with aticaprant did not induce any additional weight loss in stressed subjects. The physiological mechanisms underlying body weight loss in healthy subjects are currently unknown and should be evaluated in future studies. There is evidence that opioid agonists can increase food intake whereas opioid antagonists can reduce food intake and body weight in both rodents and humans (Cintron-Colon et al. 2019; de Zwaan and Mitchell 1992; Greenway et al. 2009). We hypothesize this effect was not directly due to a reduction in feeding as food consumption measured during the sucrose consumption tests did not differ between groups (data not shown). Weight gain has been repeatedly reported in both rodent studies and the clinical population following treatment with antidepressants (Alonso-Pedrero et al. 2019; Fava 2000).
Effects of aticaprant are related to KOR antagonism
Aticaprant has been demonstrated to be a potent and selective KOR antagonist in previous publications (Rorick-Kehn et al. 2014a; Rorick-Kehn et al. 2014b; Urbano et al. 2014). However, it is important to consider whether the behavioral actions of aticaprant observed here may have been mediated in part by mu opioid receptor (MOR) blockade. Functional studies showed that the 10 mg/kg dose of aticaprant was the lowest effective dose that blocked U50,488-induced antinociception on the hot plate test and exerted KOR occupancy for a full 24 hours after injection. Further, the 10 mg/kg dose fully blocked U50,488-induced suppression of nesting in both male and female mice while having no effect on locomotion in a previous study (Jacobson et al. 2020b). In vivo functional selectivity of aticaprant for KORs relative to MORs was illustrated in this current study by the failure of aticaprant to block antinociception in response to the MOR agonist morphine. It has been previously reported that doses up to 30 mg/kg aticaprant in mice did not result in measurable MOR or delta opioid receptor occupancy (Rorick-Kehn et al. 2014a). Therefore, it is reasonable to conclude that the behavioral effects observed in the current study are associated with KOR blockade. However, it should be noted that assessments regarding functional selectivity and absorption were a result of single drug exposures, and it is possible that repeated treatment with aticaprant may result in compounding drug availability, which could have affected functional selectivity. Currently, only two other preclinical studies have assessed effects in response to repeated treatment with aticaprant; following either four daily treatments (10 mg/kg, oral administration) in rats (Rorick-Kehn et al. 2014b) or seven daily treatments (1 mg/kg, i.p.) in mice (Browne et al. 2018). Both studies reported no adverse effects, and Rorick-Kehn and colleagues (2014b) reported no tolerance following repeated treatments of the ability of aticaprant to significantly reduce ethanol intake. To our knowledge, the current study was the first to assess the effects of repeated treatment of aticaprant in mice.
Future directions and limitations
Future research is needed to interrogate the mechanisms underlying the efficacy of aticaprant in the reversal of stress-induced behaviors. The current study was not designed with the intent to investigate the mechanisms because both the UCMS procedure and aticaprant treatment were discontinued several weeks before euthanasia. Thus, it was also not surprising that there was no change in basal corticosterone levels. In humans, four daily treatments with aticaprant modestly increased circulating adrenocorticotropic hormone and cortisol levels while having no effect on serum prolactin levels (Reed et al. 2018). The molecular mechanisms associated with KOR antagonists involve transcription factor cAMP-response element binding protein (CREB) and MAP kinase signaling cascades including extracellular signal-regulated kinase (ERK), p38, and JNK (Bruchas and Chavkin 2010; Bruchas et al. 2011; Bruchas et al. 2007; Pliakas et al. 2001). Although these markers were interrogated in the mPFC, no significant alterations were identified. The mPFC was assessed as it is known to be affected by chronic stress and is involved in the functioning of several of the behaviors that were interrogated (McEwen and Morrison 2013). It is possible that neurochemical changes at earlier time points or in other brain regions may be associated with the behavioral effects of aticaprant. In humans, aticaprant engages the ventral striatum to alleviate anhedonia in stress-related disorders and MDD in particular (Krystal et al. 2020). Going forward, there is a strong rationale for the preclinical interrogation of the ventral striatum, specifically the subregions of the nucleus accumbens, and reward-related behaviors to build a translational bridge to the human studies and determine molecular and neurochemical mediators underlying aticaprant’s behavioral effects.
Collectively these results illustrated that aticaprant reversed several behaviors in male mice which emerged because of chronic stress exposure. Currently approved antidepressants require weeks to months of daily administration before symptom reduction (van Bronswijk et al. 2019). The current study provides data suggesting that aticaprant has the potential to rapidly alleviate symptoms of depression as it ameliorated behavioral deficits within days of treatment onset. Additionally, the reversal of stress-induced behaviors by aticaprant persisted following discontinuation (findings summarized in Supplemental Table S2). A limitation of this study was that it was conducted only in male mice and the effects of aticaprant following stress exposure remain to be demonstrated in females. Emerging evidence has suggested females are less sensitive to the effects of KOR alterations, and whether this carries over to reversal from UCMS-induced behaviors will need to be addressed. Nevertheless, our laboratory has previously shown that the 10 mg/kg dose of aticaprant was effective in blocking U50,488-induced suppression of nesting in females (Jacobson et al. 2020b).
Conclusion
The results of the current study support further investigation into the therapeutic potential of aticaprant for psychiatric conditions. Although UCMS was developed as a preclinical model of depression with the intent of modeling symptoms according to traditional psychiatric criteria (Willner 2017), the behavioral deficits evoked by UCMS and their reversal can also be interpreted using the behavioral dimension framework of the RDoC and applied to a broader spectrum of psychiatric disorders (Cuthbert and Insel 2013; Dillon et al. 2014). The ability of aticaprant to reverse behavioral effects of stress related to anhedonia in constructs of positive valence and arousal/regulatory systems in mice, and in humans with clinically significant anhedonia (Krystal et al. 2020), encourage further investigations into the clinical effects of KOR antagonists relevant to anhedonic-related symptoms that may be applicable to MDD and other types of psychiatric disorders.
Supplementary Material
Acknowledgements
This work was supported by US Public Health (USPHS) grant R01 MH105623. Aticaprant was a gift from Eli Lily. The authors gratefully acknowledge the valuable contributions of discussions with Drs. Linda Rorick-Kehn and Jeffrey Witkin. We thank Dr. Rema Santhappan, Amanda Namchuk, and Kaitlyn Gouty for their contributions to the molecular analyses. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or U.S. Government.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Declaration of competing interest
The authors declare no competing financial interests.
References
- Adler G, Gattaz WF (1993) Pain perception threshold in major depression. Biological psychiatry 34: 687–9. [DOI] [PubMed] [Google Scholar]
- Alonso-Pedrero L, Bes-Rastrollo M, Marti A (2019) Effects of antidepressant and antipsychotic use on weight gain: A systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity 20: 1680–1690. [DOI] [PubMed] [Google Scholar]
- Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J (2019) Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neuroscience and biobehavioral reviews 99: 101–116. [DOI] [PubMed] [Google Scholar]
- Bär KJ, Brehm S, Boettger MK, Boettger S, Wagner G, Sauer H (2005) Pain perception in major depression depends on pain modality. Pain 117: 97–103. [DOI] [PubMed] [Google Scholar]
- Belzung C, Willner P, Philippot P (2015) Depression: from psychopathology to pathophysiology. Current opinion in neurobiology 30: 24–30. [DOI] [PubMed] [Google Scholar]
- Browne CA, Falcon E, Robinson SA, Berton O, Lucki I (2018) Reversal of Stress-Induced Social Interaction Deficits by Buprenorphine. The international journal of neuropsychopharmacology 21: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Jacobson ML, Lucki I (2020) Novel Targets to Treat Depression: Opioid-Based Therapeutics. Harvard review of psychiatry 28: 40–59. [DOI] [PubMed] [Google Scholar]
- Browne CA, Lucki I (2019) Targeting opioid dysregulation in depression for the development of novel therapeutics. Pharmacology & therapeutics 201: 51–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology 210: 137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C (2011) Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 71: 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C (2007) Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. The Journal of biological chemistry 282: 29803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüning CA, Gai BM, Soares SM, Martini F, Nogueira CW (2014) Serotonergic systems are implicated in antinociceptive effect of m-trifluoromethyl diphenyl diselenide in the mouse glutamate test. Pharmacology, biochemistry, and behavior 125: 15–20. [DOI] [PubMed] [Google Scholar]
- Butler RK, Finn DP (2009) Stress-induced analgesia. Progress in neurobiology 88: 184–202. [DOI] [PubMed] [Google Scholar]
- Cintron-Colon R, Johnson CW, Montenegro-Burke JR, Guijas C, Faulhaber L, Sanchez-Alavez M, Aguirre CA, Shankar K, Singh M, Galmozzi A, Siuzdak G, Saez E, Conti B (2019) Activation of Kappa Opioid Receptor Regulates the Hypothermic Response to Calorie Restriction and Limits Body Weight Loss. Current biology : CB 29: 4291–4299.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet (London, England) 391: 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, Ehlinger DG (2017) The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS chemical neuroscience 8: 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Holmes A (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nature reviews Drug discovery 4: 775–90. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR (2013) Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine 11: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zwaan M, Mitchell JE (1992) Opiate antagonists and eating behavior in humans: a review. Journal of clinical pharmacology 32: 1060–72. [PubMed] [Google Scholar]
- Deacon R (2012) Assessing burrowing, nest construction, and hoarding in mice. Journal of visualized experiments : JoVE: e2607. [DOI] [PMC free article] [PubMed]
- Deacon RM (2006) Assessing nest building in mice. Nature protocols 1: 1117–9. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, Killgore WD, Rauch SL, Pizzagalli DA (2014) Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depression and anxiety 31: 233–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra S, Kumar A, Umrao D, Sahasrabuddhe AA, Yadav PN (2016) Chronic Kappa opioid receptor activation modulates NR2B: Implication in treatment resistant depression. Scientific reports 6: 33401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domi E, Barbier E, Augier E, Augier G, Gehlert D, Barchiesi R, Thorsell A, Holm L, Heilig M (2018) Preclinical evaluation of the kappa-opioid receptor antagonist CERC-501 as a candidate therapeutic for alcohol use disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43: 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dournes C, Beeské S, Belzung C, Griebel G (2013) Deep brain stimulation in treatment-resistant depression in mice: comparison with the CRF1 antagonist, SSR125543. Progress in neuro-psychopharmacology & biological psychiatry 40: 213–20. [DOI] [PubMed] [Google Scholar]
- Falcon E, Browne CA, Leon RM, Fleites VC, Sweeney R, Kirby LG, Lucki I (2016) Antidepressant-like Effects of Buprenorphine are Mediated by Kappa Opioid Receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41: 2344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M (2000) Weight gain and antidepressants. The Journal of clinical psychiatry 61 Suppl 11: 37–41. [PubMed] [Google Scholar]
- Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7: 1259–1424. [Google Scholar]
- Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP (2013) Impact of nesting material on mouse body temperature and physiology. Physiology & behavior 110–111: 87–95. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, Gadde KM, Gupta AK, O’Neil P, Schumacher D, Smith D, Dunayevich E, Tollefson GD, Weber E, Cowley MA (2009) Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring, Md) 17: 30–9. [DOI] [PubMed] [Google Scholar]
- Haj-Mirzaian A, Nikbakhsh R, Ramezanzadeh K, Rezaee M, Amini-Khoei H, Haj-Mirzaian A, Ghesmati M, Afshari K, Haddadi NS, Dehpour AR (2019) Involvement of opioid system in behavioral despair induced by social isolation stress in mice. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 109: 938–944. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Jackson A, Carroll FI, Damaj MI (2015) Effects of orally-bioavailable short-acting kappa opioid receptor-selective antagonist LY2456302 on nicotine withdrawal in mice. Neuropharmacology 97: 270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson ML, Browne CA, Lucki I (2020a) Kappa Opioid Receptor Antagonists as Potential Therapeutics for Stress-Related Disorders. Annual review of pharmacology and toxicology 60: 615–636. [DOI] [PubMed] [Google Scholar]
- Jacobson ML, Wulf HA, Tsuda MC, Browne CA, Lucki I (2020b) Sex differences in the modulation of mouse nest building behavior by kappa opioid receptor signaling. Neuropharmacology. [DOI] [PMC free article] [PubMed]
- Jirkof P (2014) Burrowing and nest building behavior as indicators of well-being in mice. Journal of neuroscience methods 234: 139–46. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Pizzagalli DA, Smoski M, Mathew SJ, Nurnberger J Jr., Lisanby SH, Iosifescu D, Murrough JW, Yang H, Weiner RD, Calabrese JR, Sanacora G, Hermes G, Keefe RSE, Song A, Goodman W, Szabo ST, Whitton AE, Gao K, Potter WZ (2020) A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nature medicine. [DOI] [PMC free article] [PubMed]
- Lian YN, Chang JL, Lu Q, Wang Y, Zhang Y, Zhang FM (2017) Effects of fluoxetine on changes of pain sensitivity in chronic stress model rats. Neuroscience letters 651: 16–20. [DOI] [PubMed] [Google Scholar]
- Lowe SL, Wong CJ, Witcher J, Gonzales CR, Dickinson GL, Bell RL, Rorick-Kehn L, Weller M, Stoltz RR, Royalty J, Tauscher-Wisniewski S (2014) Safety, tolerability, and pharmacokinetic evaluation of single- and multiple-ascending doses of a novel kappa opioid receptor antagonist LY2456302 and drug interaction with ethanol in healthy subjects. Journal of clinical pharmacology 54: 968–78. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Cowan A (2004) Buprenorphine: a unique drug with complex pharmacology. Current neuropharmacology 2: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL (2013) Opioid receptors: distinct roles in mood disorders. Trends in neurosciences 36: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH (2013) The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79: 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Myers LC, Zarek PE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Chavkin C (2004) Prolonged kappa opioid receptor phosphorylation mediated by G-protein receptor kinase underlies sustained analgesic tolerance. The Journal of biological chemistry 279: 1810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard C, Hodes GE, Russo SJ (2016) Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 321: 138–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro TA, Berry LM, Van’t Veer A, Béguin C, Carroll FI, Zhao Z, Carlezon WA, Jr., Cohen BM (2012) Long-acting κ opioid antagonists nor-BNI, GNTI and JDTic: pharmacokinetics in mice and lipophilicity. BMC pharmacology 12: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganawa M, Dickinson GL, Zheng MQ, Henry S, Vandenhende F, Witcher J, Bell R, Nabulsi N, Lin SF, Ropchan J, Neumeister A, Ranganathan M, Tauscher J, Huang Y, Carson RE (2016) Receptor Occupancy of the κ-Opioid Antagonist LY2456302 Measured with Positron Emission Tomography and the Novel Radiotracer 11C-LY2795050. The Journal of pharmacology and experimental therapeutics 356: 260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) The National Academies Collection: Reports funded by National Institutes of Health Guide for the Care and Use of Laboratory Animals. National Academies Press (US) Copyright © 2011, National Academy of Sciences., Washington (DC) [Google Scholar]
- Newman EL, Covington HE 3rd, Suh J, Bicakci MB, Ressler KJ, DeBold JF, Miczek KA (2019) Fighting Females: Neural and Behavioral Consequences of Social Defeat Stress in Female Mice. Biological psychiatry 86: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otabi H, Goto T, Okayama T, Kohari D, Toyoda A (2016) Subchronic and mild social defeat stress alter mouse nest building behavior. Behavioural processes 122: 21–5. [DOI] [PubMed] [Google Scholar]
- Peters MF, Zacco A, Gordon J, Maciag CM, Litwin LC, Thompson C, Schroeder P, Sygowski LA, Piser TM, Brugel TA (2011) Identification of short-acting κ-opioid receptor antagonists with anxiolytic-like activity. European journal of pharmacology 661: 27–34. [DOI] [PubMed] [Google Scholar]
- Pinto-Ribeiro F, Almeida A, Pêgo JM, Cerqueira J, Sousa N (2004) Chronic unpredictable stress inhibits nociception in male rats. Neuroscience letters 359: 73–6. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 33: 88–109. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA Jr. (2001) Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience 21: 7397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C (2004) Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behavioural brain research 155: 135–46. [DOI] [PubMed] [Google Scholar]
- Reed B, Butelman ER, Fry RS, Kimani R, Kreek MJ (2018) Repeated Administration of Opra Kappa (LY2456302), a Novel, Short-Acting, Selective KOP-r Antagonist, in Persons with and without Cocaine Dependence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43: 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witcher JW, Lowe SL, Gonzales CR, Weller MA, Bell RL, Hart JC, Need AB, McKinzie JH, Statnick MA, Suico JG, McKinzie DL, Tauscher-Wisniewski S, Mitch CH, Stoltz RR, Wong CJ (2014a) Determining pharmacological selectivity of the kappa opioid receptor antagonist LY2456302 using pupillometry as a translational biomarker in rat and human. The international journal of neuropsychopharmacology 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, Forster BM, Wong CJ, Li X, Crile RS, Shaw DB, Sahr AE, Adams BL, Quimby SJ, Diaz N, Jimenez A, Pedregal C, Mitch CH, Knopp KL, Anderson WH, Cramer JW, McKinzie DL (2014b) LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology 77: 131–44. [DOI] [PubMed] [Google Scholar]
- Shi M, Qi WJ, Gao G, Wang JY, Luo F (2010a) Increased thermal and mechanical nociceptive thresholds in rats with depressive-like behaviors. Brain research 1353: 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Wang JY, Luo F (2010b) Depression shows divergent effects on evoked and spontaneous pain behaviors in rats. The journal of pain : official journal of the American Pain Society 11: 219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JY, Byrne C, Williams MA, Keene DJ, Schlussel MM, Lamb SE (2017) Prognostic factors for recovery following acute lateral ankle ligament sprain: a systematic review. BMC musculoskeletal disorders 18: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano M, Guerrero M, Rosen H, Roberts E (2014) Antagonists of the kappa opioid receptor. Bioorganic & medicinal chemistry letters 24: 2021–32. [DOI] [PubMed] [Google Scholar]
- van Bronswijk S, Moopen N, Beijers L, Ruhe HG, Peeters F (2019) Effectiveness of psychotherapy for treatment-resistant depression: a meta-analysis and meta-regression. Psychological medicine 49: 366–379. [DOI] [PubMed] [Google Scholar]
- Wang J, Song Q, Xu A, Bao Y, Xu Y, Zhu Q (2017) Design, synthesis and biological evaluation of aminobenzyloxyarylamide derivatives as selective κ opioid receptor antagonists. European journal of medicinal chemistry 130: 15–25. [DOI] [PubMed] [Google Scholar]
- WHO (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AV, Laman-Maharg A, Armstrong CV, Ramos-Maciel S, Minie VA, Trainor BC (2018) Acute inhibition of kappa opioid receptors before stress blocks depression-like behaviors in California mice. Progress in neuro-psychopharmacology & biological psychiatry 86: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P (2017) The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiology of stress 6: 78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93: 358–64. [DOI] [PubMed] [Google Scholar]
- Woo H, Hong CJ, Jung S, Choe S, Yu SW (2018) Chronic restraint stress induces hippocampal memory deficits by impairing insulin signaling. Molecular brain 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


