Abstract
Purpose:
To describe associations between symptoms and signs of dry eye disease (DED) and meibomian gland (MG) morphology.
Methods:
Cross-sectional study utilizing data from the Dry Eye Assessment and Management (DREAM) Study. Readers graded MG features in the middle third of upper and lower lids on infrared meibography images. Associations with signs and symptoms of DED were evaluated with adjustment for age and sex.
Results:
Among 268 patients, no MG features were associated with symptom scores (p>0.08). Among 394 upper eyelids, better tear break-up times (<2, >2- <3.2and ≤3.2 seconds) were associated with more tortuous glands (mean (SD) 0.58(0.95), 0.83(1.2) and 1.14(1.4), p=0.01) and with higher scores on a composite score of MG features (21.90 (9.76), 23.29 (9.50), 26.26 (10.27); p=0.02). Longer Schirmer test wetting lengths (0-5, >5 −10, and >10 mm) were associated with increasing composite scores (22.02 (9.29), 23.80 (10.34), 24.96 (9.96), p=0.03). Patients with Sjogren syndrome compared to other patients had fewer distorted MGs (mean 3.4(2.3) vs 4.3(2.3), p=0.03) and fewer ghost glands (mean 0.33(0.88) vs 0.89 (1.8), p=0.006) in the upper lid.
Conclusion:
In the DREAM study, most MG morphologic features were not associated with the severity of DED symptoms or signs. Tortuous glands and a higher composite score for MG features were associated with longer tear break-up times and longer Schirmer test length in the upper eyelid only. Patients with Sjogren syndrome had fewer distorted and ghost glands.
Keywords: Dry eye disease, Meibography, Ocular Surface Disease Index, Tear function tests
INTRODUCTION
Dry eye disease (DED) has been broadly classified as aqueous deficient dry eye and evaporative dry eye.1 While both can occur together, evaporative dry eye is more common than aqueous deficient dry eye and is frequently associated with meibomian gland disease (MGD), considered to be the leading cause of dry eye in clinic and population-based studies.2,3 The diagnosis of DED is reliant on the patient-reported symptoms and ocular signs. The Ocular Surface Disease Index (OSDI; Allergan, Inc, Irvine, California) is a questionnaire that has been commonly used to assess the severity and range of symptoms related to DED.4,5 Ocular surface disease signs include tear film instability, damage to the epithelial cells of the cornea and conjunctiva, low tear production and high osmolarity. Patients with blepharitis and MGD may have several eyelid signs: lid margin irregularity, vascular engorgement, anterior or posterior displacement of the mucocutaneous junction, MG orifice plugging, abnormal expressibility and decreased secretion quality of meibum, and low blink rate. However, the associations between ocular symptoms and signs in DED are low and inconsistent. There is a need to identify additional reliable, objective measures to strengthen the clinical assessment of DED and to determine the efficacy of various current and evolving treatments employed in combating DED.
Non-invasive, infrared photography of meibomian glands (MG) allows detailed assessment of the morphological features of these glands. In a previous publication, we enumerated and defined various morphological features that were observed in the upper lids (UL) and lower lids (LL) of patients with moderate to severe DED, including some features that had been reported in earlier publications.6 However, there have been no studies that have investigated the association of ocular symptoms and DED related test results with a standardized, comprehensive grading system of MG morphological features.
The Dry Eye Assessment and Management (DREAM) study was a multi-center, randomized, double-masked clinical trial to evaluate the safety and efficacy of omega-3 fatty acids for the treatment of DED. Approximately 40% of the clinical centers obtained meibography images. This large well-characterized cohort allowed us to study the associations between structural variations of the MGs observed in meibography images with symptoms and DED signs at baseline in patients enrolled in the DREAM Study.
1. METHODS
1.1. Study population
The study design and the primary results of the DREAM were previously reported.7,8 Participants (⩾ 18 years) with ocular symptoms of DED for at least 6 months were recruited from 27 clinical centers in the United States from October 2014 through July 2016. On the screening and the eligibility confirmation visits, the patients needed to have a mean OSDI score between 23 and 80. Additionally, the patients needed to have at least two of the following four signs in at least one eye: a corneal fluorescein staining score of 4 or more (range 0–15, higher scores indicate greater abnormality), a conjunctival lissamine-green staining score of 1 or more (range 0–6, higher scores indicate greater abnormality), a tear break-up time of ≤7 s, and a result on Schirmer’s test with anesthesia of 1–7 mm in 5 min. The qualifying signs needed to be the same signs in the same eye at each of the visits. Eligibility criteria excluded pregnant or nursing mothers, patients with history of contact lens wear during 30 days before the screening visit, ocular surgery within 6 months of screening visit, using glaucoma medications and having eyelid abnormalities. The study was approved by the institutional review board associated with each center and the research followed the tenets of the Declaration of Helsinki. Each patient signed an informed consent statement.
1.2. Meibography
Ten of the 27 DREAM centers had the Oculus Keratograph 5M (Oculus Inc, Wetzlar, Germany) required for study imaging, so only 292 (55%) patients of the total 535 enrolled were eligible for meibography. Staff from each clinical site received training on meibography imaging using a standardized protocol that had instructions on taking the images after eversion of the upper and lower lids. Images from both eyes were uploaded to a secure central server for grading.
1.3. Grading of meibography images
A detailed description of the grading methodology has been reported previously.6 Briefly, a grading protocol was developed that included a comprehensive list of features of MGs with images of representative examples of each, selected from the study image database. Specific features of the MGs such as distorted, tortuous, hooked, dropout glands, shortened, thickened, thinned, overlapping, ghost, tadpoling, abnormal gap, fluffy areas (amorphous white substance occupying locations where normal MGs should have been present and where the normal architecture of individual MGs cannot be visualized), and no extension of MGs into the lid margin were included in the protocol for grading. Three experienced non-physician readers were trained by the ophthalmologist reading center director (ED) to grade meibography images. After they were adequately trained and certified as DREAM graders, two readers graded each lid meibography image independently using Adobe Photoshop (Adobe, Inc. San Jose, CA) using a template to enumerate and record each of the morphological abnormalities within three similarly spaced sections (lateral, middle and medial). Along with assessing each individual MG feature, they also used the lasso tool in Adobe® Photoshop to measure both total lid area and the area of gland drop out to calculate the percentage of drop out areas in each lid. Discrepancies in the grading between the two readers were adjudicated by the reading center director. The specific features of MGs in the middle third of the lid was used in the analysis. Area measurements (total area, dropout areas including and excluding fluffy areas and ghost glands) (Supplemental Figure A) from the whole lid were used for analysis.
1.4. Measurement of signs of dry eye disease:
All assessments of baseline signs were performed by DREAM certified clinicians or technicians prior to treatment in the randomized trial. Tear break-up time (TBUT) was measured thrice as the time between the last blink and the appearance of the first discontinuity in the fluorescein stained tear film. Evaluation of non-invasive tear film break-up time (NIKBUT) was performed with the Oculus Keratograph 5M without fluorescein and the first break up time was recorded. Tear meniscus height also was measured with the Keratograph along the eyelid with the built-in ruler at three points: directly below the 5, 6, and 7 o’clock positions relative to the cornea. Lissamine green staining of the interpalpebral conjunctiva was graded 1-2 minutes after instillation 5 μL of 1% lissamine green. The nasal-bulbar and temporal-bulbar conjunctiva were scored 0-3 (0= no coloration, 1= some punctations, 2= well defined punctations, 3= many punctations). Corneal fluorescein staining was graded using the cobalt blue filter of the slit lamp 2.5 minutes after fluorescein instillation and scored 0-3 in each of 5 areas of the cornea. Schirmer test was done bilaterally 5 minutes after anesthetic drops were installed. Test strips were kept in place for 5 minutes and the wetted portion from the fold was measured in millimeters.
1.5. Composite Score of MG features
The development of a composite score from MG features has been reported earlier.6 Briefly, 3 ophthalmologists independently scored each of the MG features on a severity scale of 0-10, where 10 was the most severe feature to be perceived to contribute to DED. We used 13 features, all the features in the tables except total glands and both of the percent dropout areas. The mean score from these three values for each morphological feature was used as the individual point for each morphological feature. The total composite score for each lid was calculated by adding the points from each feature that was present in that lid. This score did not take into account multiple appearances of the same morphological feature in the same lid. In order to account for the multiple occurrences we added 2 more points for each morphological feature that occurred more than once in the same lid.
1.6. Statistical Methods:
All analyses were performed separately for the upper and lower lids. Associations between continuous measures of MG features and signs were evaluated with linear regression, where the MG feature was the dependent variable. Associations between binary measures of MG features were evaluated with logistic regression where the MG feature was the dependent variable. All regression models involving signs measured on a continuous scale and symptoms were calculated using the continuous values as independent variables. Regression models were adjusted for age and sex, using generalized estimating equations to accommodate the correlation among lids in the same person.9 For descriptive purposes in the tables, the mean value of the MG feature measure was displayed for the eyes in each tertile of symptoms or signs. Inter-eye correlation was assessed with the intraclass correlation coefficient for continuous measures and with the Kappa statistic for categorical measures. The Benjamini-Hochberg procedure was used to adjust P-values for multiple comparisons of 13 MG features within each sign or symptom.10 P-values after adjustment for multiple comparisons are denoted PMC. Calculations were not performed for MG features present on less than 5% of lids. All statistical analyses were performed with SAS software version 9.4 (SAS, Inc, Cary, NC).
2. RESULTS
The baseline characteristics of the patients is given in Table 1. Patients had a mean (SD) age of 56.5 (14.1) years (range 18-87 years), were predominantly female (78%) and white (71 %). Nine percent had Sjogren Syndrome (SS) and 11 % reported a history of rheumatoid arthritis. After excluding images with poor lid eversion or poor image quality, 468 (87%) LLs (right and left eye) and 370 (69%) ULs from 268 patients were available for analysis. Inter-eye correlation was 0.30 or lower for most features, with the highest inter-eye correlations for the percent dropout including fluffy areas as dropped out (0.56 for upper lid, 0.53 for lower lid). None of the MG features in either lid were associated with the OSDI score (PMC > 0.08), Table 2.
Table 1.
Baseline characteristics of patients analyzed in the study
| Characteristic | 268 Patients |
|---|---|
| Age (years): mean ± SD, (range) | 56.5±14.1 (18-87) |
| Gender – n (%) | |
| Female | 208 (77.6) |
| Male | 60 (22.4) |
| Race – n (%) | |
| White | 189 (70.5) |
| Black | 23 (8.6) |
| Asian | 12 (4.5) |
| Native American/Alaskan | 1 (0.4) |
| More than one race category | 6 (2.2) |
| Unknown | 37 (13.8) |
| Ethnicity – n (%) | |
| Hispanic/Latino | 54 (20.1) |
| Not Hispanic/Latino | 214 (79.9) |
| Systemic disease, n (%)* | |
| Sjogren syndrome | 24 (9.0) |
| Rheumatoid arthritis | 29 (10.8) |
| Thyroid disease | 52 (19.4) |
| None of the above | 180 (67.2) |
| Total Ocular Surface Disease Index score, mean (SD) | 42.2 (15.5) |
| Conjunctival staining score, mean (SD) | 2.9 (1.4) |
| Corneal staining score, mean (SD) | 3.6 (2.8) |
| Tear break-up time (seconds), mean (SD) | 2.9 (1.4) |
| Schirmer’s test, mm/5 min, mean (SD) | 10.5 (7.2) |
15 patients had more than one of the listed systemic diseases, total does not add to 268.
Table 2.
Association of Meibomian gland features with Ocular Surface Disease Index (OSDI) in upper and lower lids
| Upper Lid |
Lower Lid |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ocular Surface Disease Index (OSDI) |
||||||||||||
| Meibomian gland feature | Total n=394 | >20-32 (Good) n=129 | >32-47.5 n=126 | >47.5 n=139 | * p-value | **PMC | Total n=498 | >20-32 (Good) n=167 | >32-47.5 n=170 | >47.5 n=161 | * p-value | **PMC |
| Total, mean (SD) | 8.98 (2.82) | 8.94 | 9.11 | 8.89 | 0.68 | 0.96 | 7.20 (1.89) | 7.28 | 7.14 | 7.18 | 0.98 | 0.98 |
| Distorted, mean (SD) | 4.20 (2.28) | 4.04 | 4.26 | 4.29 | 0.32 | 0.96 | 1.56 (1.45) | 1.65 | 1.38 | 1.66 | 0.78 | 0.98 |
| Tortuous, mean (SD) | 0.83 (1.20) | 0.85 | 0.95 | 0.70 | 0.35 | 0.96 | 0.06 (0.30) | 0.06 | 0.09 | 0.04 | 0.91 | 0.98 |
| Hooked, mean (SD) | 0.36 (0.71) | 0.38 | 0.35 | 0.36 | 0.96 | 0.96 | 0.05 (0.26) | 0.07 | 0.04 | 0.03 | 0.09 | 0.98 |
| Short, mean (SD) | 2.97 (2.22) | 2.95 | 2.74 | 3.21 | 0.23 | 0.96 | 1.66 (1.78) | 1.58 | 1.60 | 1.80 | 0.69 | 0.98 |
| Thick, mean (SD) | 0.16 (0.57) | 0.14 | 0.16 | 0.19 | 0.39 | 0.96 | 0.03 (0.20) | 0.02 | 0.01 | 0.05 | · | · |
| Thin, mean (SD) | 0.33 (0.69) | 0.36 | 0.31 | 0.33 | 0.96 | 0.96 | 0.08 (0.34) | 0.06 | 0.09 | 0.08 | 0.97 | 0.98 |
| Overlapping, mean (SD) | 0.46 (0.71) | 0.44 | 0.44 | 0.50 | 0.72 | 0.96 | 0.06 (0.25) | 0.06 | 0.06 | 0.05 | 0.86 | 0.98 |
| Ghost, mean (SD) | 0.86 (1.72) | 0.83 | 0.73 | 0.99 | 0.68 | 0.96 | 0.55 (1.37) | 0.72 | 0.40 | 0.53 | 0.31 | 0.98 |
| Dropout gland, mean (SD) | 0.07 (0.45) | 0.07 | 0.10 | 0.04 | 0.40 | 0.96 | 0.17 (0.54) | 0.16 | 0.12 | 0.25 | 0.41 | 0.98 |
| Tadpoling, n(%) | 24 (6%) | (9%) | (5%) | (4%) | 0.14 | 0.96 | 5 (1%) | (1%) | (1%) | (1%) | · | · |
| Abnormal gap, n(%) | 106 (27%) | (29%) | (25%) | (26%) | 0.65 | 0.96 | 2 (0%) | (1%) | (0%) | (1%) | · | · |
| No extension to lid margin, n(%) | 16 (4%) | (7%) | (2%) | (3%) | 0.03 | 0.46 | 27 (5%) | (5%) | (4%) | (7%) | 0.35 | 0.98 |
| Fluffy areas, n(%) | 43 (11%) | (16%) | (9%) | (9%) | 0.47 | 0.96 | 32 (6%) | (5%) | (8%) | (6%) | 0.80 | 0.98 |
| Composite score, mean (SD) Percent dropout area, mean (SD) | 23.7 (10.0) | 24.31 | 22.82 | 23.88 | 0.84 | 0.96 | 13.1 (8.4) | 13.38 | 12.19 | 13.82 | 0.97 | 0.98 |
| Including ghost glands and fluffy areas | 0.23 (0.20) | 0.24 | 0.20 | 0.24 | 0.71 | 0.96 | 0.32 (0.19) | 0.34 | 0.31 | 0.32 | 0.86 | 0.98 |
| Excluding ghost glands and fluffy areas | 0.11 (0.11) | 0.12 | 0.10 | 0.11 | 0.70 | 0.96 | 0.19 (0.14) | 0.18 | 0.19 | 0.20 | 0.60 | 0.98 |
Adjusted for age and sex; p-values not adjusted for multiple comparisons
Adjusted for multiple comparisons
2.1. Associations of Meibomian Gland Features with Ocular Surface Staining
Associations of the MGs with corneal staining are given in Table 3. Distorted MGs were less in the LLs than in the ULs (mean 4.20 vs. 1.56, difference −2.64 (−2.89, −2.39), p<0.001). In the UL, the number of tortuous MGs and the number of hooked glands decreased with more severe corneal staining scores but the associations were not statistically significant after adjustment for multiple comparisons (PMC ≥ 0.17). A similar result for tortuous glands was seen in the LLs with the number decreasing with more severe corneal staining scores, but not to a statistically significant degree (PMC = 0.19). The mean composite score in the LL increased with worse corneal staining scores (PMC =0.054); however, this trend was not present in the UL. The association of all of the other MG features with corneal staining were not statistically significant after adjustment for multiple comparisons.
Table 3.
Association of Meibomian gland features with corneal staining in upper and lower lids
| Upper Lid |
Lower Lid |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corneal Staining |
||||||||||||
| Meibomian gland feature | Total n=394 | 0-1 (Good) n=83 | 2-4 n=150 | >4(Bad) n=161 | * p-value | **PMC | Total n=498 | 0-1 (Good) n=142 | 2-4 n=181 | >4 (Bad) n=175 | * p-value | **PMC |
| Total, mean (SD) | 8.98 (2.82) | 8.86 | 8.92 | 9.09 | 0.45 | 0.93 | 7.20 (1.89) | 7.18 | 7.19 | 7.22 | 0.97 | 0.97 |
| Distorted, mean (SD) | 4.20 (2.28) | 4.16 | 4.29 | 4.13 | 0.12 | 0.93 | 1.56 (1.45) | 1.67 | 1.41 | 1.63 | 0.59 | 0.97 |
| Tortuous, mean (SD) | 0.83 (1.20) | 0.97 | 0.86 | 0.73 | 0.046 | 0.60 | 0.06 (0.30) | 0.15 | 0.04 | 0.02 | 0.01 | 0.19 |
| Hooked, mean (SD) | 0.36 (0.71) | 0.46 | 0.41 | 0.26 | 0.01 | 0.17 | 0.05 (0.26) | 0.05 | 0.05 | 0.05 | 0.76 | 0.97 |
| Short, mean (SD) | 2.97 (2.22) | 2.30 | 3.14 | 3.16 | 0.11 | 0.93 | 1.66 (1.78) | 1.26 | 1.82 | 1.81 | 0.02 | 0.26 |
| Thick, mean (SD) | 0.16 (0.57) | 0.26 | 0.15 | 0.12 | 0.29 | 0.93 | 0.03 (0.20) | 0.05 | 0.02 | 0.02 | · | · |
| Thin, mean (SD) | 0.33 (0.69) | 0.35 | 0.34 | 0.32 | 0.23 | 0.93 | 0.08 (0.34) | 0.08 | 0.09 | 0.07 | 0.07 | 0.73 |
| Overlapping, mean (SD) | 0.46 (0.71) | 0.56 | 0.50 | 0.39 | 0.33 | 0.93 | 0.06 (0.25) | 0.05 | 0.04 | 0.08 | 0.07 | 0.73 |
| Ghost, mean (SD) | 0.86 (1.72) | 0.75 | 0.94 | 0.83 | 0.93 | 0.93 | 0.55 (1.37) | 0.47 | 0.52 | 0.65 | 0.22 | 0.97 |
| Dropout gland, mean (SD) | 0.07 (0.45) | 0.02 | 0.08 | 0.08 | 0.53 | 0.93 | 0.17 (0.54) | 0.09 | 0.22 | 0.19 | 0.19 | 0.97 |
| Tadpoling, n(%) | 24 (6%) | (5%) | (9%) | (4%) | 0.56 | 0.93 | 5 (1%) | (1%) | (1%) | (1%) | · | · |
| Abnormal gap, n(%) | 106 (27%) | (28%) | (28%) | (25%) | 0.16 | 0.93 | 2 (0%) | (1%) | (1%) | (0%) | · | · |
| No extension to lid margin, n(%) | 16 (4%) | (4%) | (3%) | (5%) | 0.49 | 0.93 | 27 (5%) | (6%) | (5%) | (5%) | 0.91 | 0.97 |
| Fluffy areas, n(%) | 43 (11%) | (13%) | (13%) | (8%) | 0.87 | 0.93 | 32 (6%) | (6%) | (6%) | (7%) | 0.16 | 0.97 |
| Composite score, mean (SD) | 23.7 (10.0) | 23.78 | 25.12 | 22.27 | 0.04 | 0.54 | 13.1 (8.4) | 11.61 | 13.10 | 14.35 | 0.004 | 0.054 |
| Percent dropout area, mean (SD) Including ghost glands and fluffy areas | 0.23 (0.20) | 0.19 | 0.24 | 0.23 | 0.03 | 0.52 | 0.32 (0.19) | 0.30 | 0.32 | 0.34 | 0.15 | 0.97 |
| Excluding ghost glands and fluffy areas | 0.11 (0.11) | 0.09 | 0.12 | 0.11 | 0.02 | 0.32 | 0.19 (0.14) | 0.18 | 0.18 | 0.20 | 0.66 | 0.97 |
Adjusted for age and sex; p-values not adjusted for multiple comparisons
Adjusted for multiple comparisons
The associations of conjunctival staining scores with MG features are reported in Supplemental Table A. None of the features in either lid were associated with conjunctival staining (p ≥0.07 without adjustment for multiple comparisons; all PMC ≥0.47).
2.2. Associations of Meibomian Gland Features with Measures of Tears
MG associations with TBUT are reported in Table 4. In the UL, the number of tortuous glands increased with longer TBUT (PMC =0.01) (Figure 1) as did the composite severity score (PMC =0.02). However, such associations were not seen in the LL. The associations of all of the other MG features with TBUT were not statistically significant after adjustment for multiple comparisons. The associations of MG features with the Schirmer test are given in Table 5. The composite severity scores in the UL increased with greater length of wetting on the Schirmer test (PMC =0.03). This association was not present for the LL. The associations of all of the other MG features with Schirmer test results were not statistically significant after adjustment for multiple comparisons.
Table 4.
Association of Meibomian gland features with tear break-up time in upper and lower lids
| Upper Lid |
Lower Lid |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tear Break Up Time (TBUT) |
||||||||||||
| Meibomian gland feature | Total n=394 | <2* (Bad) n=141 | >2 - <3.2 n=136 | >=3.2 (Good) n=117 | ^PMC | ** p-value | Total n=498 | <2 (Bad) n=163 | >2 - <3.2 n=172 | >= 3.2 (Good) n=163 | ** p-value | ^PMC |
| Total, mean (SD) | 8.98 (2.82) | 8.65 | 9.31 | 8.98 | 0.95 | 0.86 | 7.20 (1.89) | 7.24 | 7.22 | 7.14 | 0.23 | 0.93 |
| Distorted, mean (SD) | 4.20 (2.28) | 3.91 | 4.39 | 4.32 | 0.95 | 0.66 | 1.56 (1.45) | 1.42 | 1.59 | 1.68 | 0.93 | 0.93 |
| Tortuous, mean (SD) | 0.83 (1.20) | 0.58 | 0.83 | 1.14 | 0.01 | <0.001 | 0.06 (0.30) | 0.05 | 0.03 | 0.11 | 0.14 | 0.93 |
| Hooked, mean (SD) | 0.36 (0.71) | 0.28 | 0.32 | 0.51 | 0.95 | 0.14 | 0.05 (0.26) | 0.02 | 0.03 | 0.10 | 0.14 | 0.93 |
| Short, mean (SD) | 2.97 (2.22) | 2.85 | 3.22 | 2.83 | 0.95 | 0.69 | 1.66 (1.78) | 1.72 | 1.58 | 1.67 | 0.30 | 0.93 |
| Thick, mean (SD) | 0.16 (0.57) | 0.15 | 0.22 | 0.12 | 0.95 | 0.30 | 0.03 (0.20) | 0.02 | 0.01 | 0.05 | · | · |
| Thin, mean (SD) | 0.33 (0.69) | 0.22 | 0.42 | 0.37 | 0.28 | 0.02 | 0.08 (0.34) | 0.06 | 0.05 | 0.12 | 0.20 | 0.93 |
| Overlapping, mean (SD) | 0.46 (0.71) | 0.43 | 0.46 | 0.51 | 0.95 | 0.83 | 0.06 (0.25) | 0.07 | 0.05 | 0.05 | 0.09 | 0.93 |
| Ghost, mean (SD) | 0.86 (1.72) | 0.80 | 0.91 | 0.86 | 0.95 | 0.92 | 0.55 (1.37) | 0.68 | 0.38 | 0.60 | 0.60 | 0.93 |
| Dropout gland, mean (SD) | 0.07 (0.45) | 0.08 | 0.04 | 0.08 | 0.95 | 0.95 | 0.17 (0.54) | 0.21 | 0.22 | 0.09 | 0.25 | 0.93 |
| Tadpoling, n(%) | 24 (6%) | (5%) | (6%) | (8%) | 0.95 | 0.12 | 5 (1%) | (1%) | (1%) | (1%) | · | · |
| Abnormal gap, n(%) | 106 (27%) | (27%) | (24%) | (30%) | 0.95 | 0.18 | 2 (0%) | (0%) | (1%) | (1%) | · | · |
| No extension to lid margin, n(%) | 16 (4%) | (3%) | (5%) | (4%) | 0.95 | 0.72 | 27 (5%) | (6%) | (6%) | (4%) | 0.50 | 0.93 |
| Fluffy areas, n(%) | 43 (11%) | (9%) | (8%) | (16%) | 0.48 | 0.03 | 32 (6%) | (6%) | (5%) | (9%) | 0.10 | 0.93 |
| Composite score, mean (SD) | 23.7 (10.0) | 21.90 | 23.29 | 26.26 | 0.02 | 0.001 | 13.1 (8.4) | 13.64 | 12.79 | 12.93 | 0.81 | 0.93 |
| Percent dropout area, mean (SD) | ||||||||||||
| Including ghost glands and fluffy areas | 0.23 (0.20) | 0.24 | 0.21 | 0.23 | 0.95 | 0.29 | 0.32 (0.19) | 0.34 | 0.30 | 0.33 | 0.39 | 0.93 |
| Excluding ghost glands and fluffy areas | 0.11 (0.11) | 0.12 | 0.11 | 0.10 | 0.95 | 0.85 | 0.19 (0.14) | 0.20 | 0.18 | 0.19 | 0.59 | 0.93 |
Seconds
Adjusted for age and sex; p-values not adjusted for multiple comparisons.
Adjusted for multiple comparisons
Fig. 1.
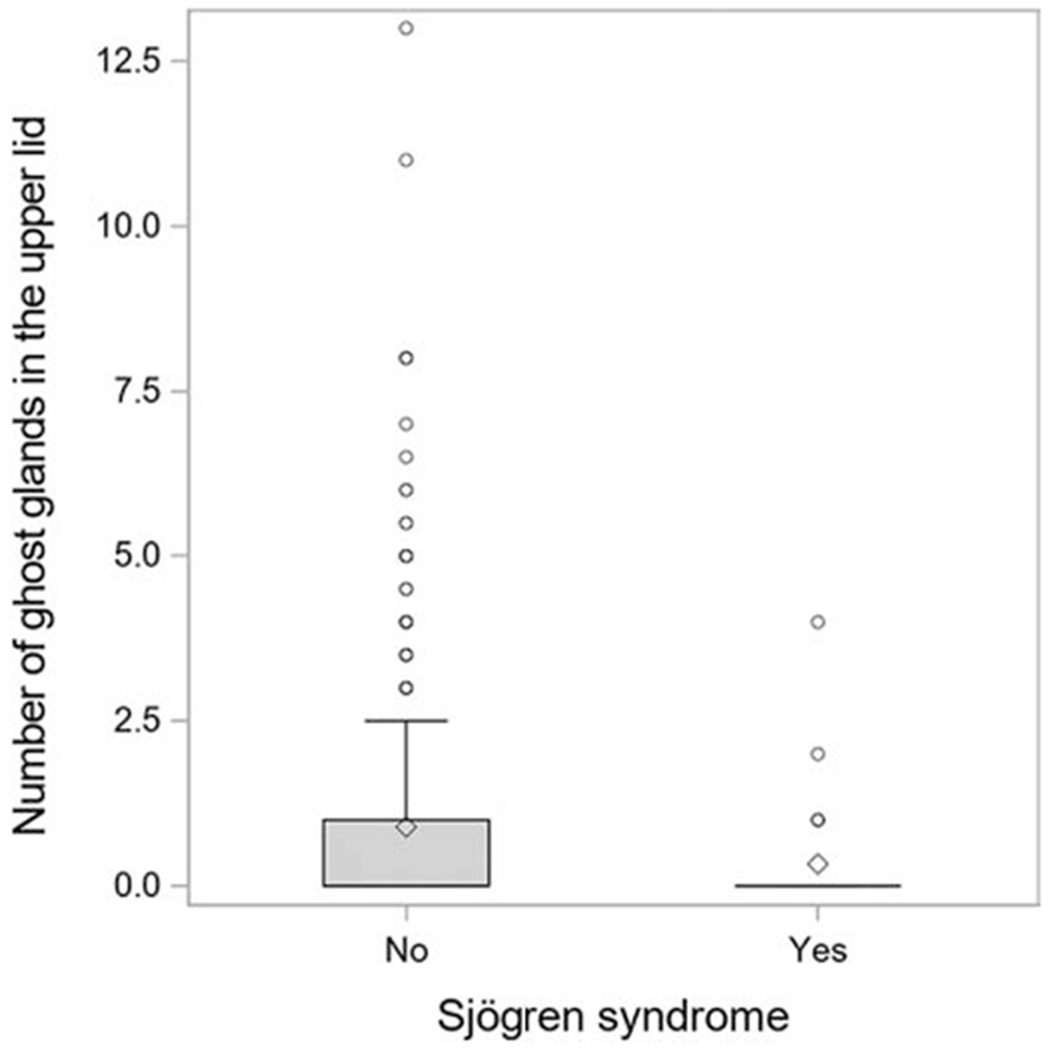
Box plot showing the number of meibomian glands present in the upper eye lid of dry eye subjects with and without Sjogren syndrome.
Table 5.
Association of Meibomian gland features with Schirmer test in upper and lower lids
| Upper Lid |
Lower Lid |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schirmer Test |
||||||||||||
| Meibomian gland feature | Total n=394 | 0-5 mm n=112 | >5-10 mm n=151 | >10 mm n=131 | * p-value | **PMC | Total n=498 | 0-5 mm n=139 | >5-10 mm n=187 | >10 mm n=172 | *p-value | **PMC |
| Total, mean (SD) | 8.98 (2.8) | 8.46 | 9.08 | 9.30 | 0.02 | 0.33 | 7.20 (1.9) | 7.05 | 7.33 | 7.18 | 0.95 | 0.95 |
| Distorted, mean (SD) | 4.20 (2.3) | 3.87 | 4.19 | 4.49 | 0.04 | 0.51 | 1.56 (1.5) | 1.61 | 1.55 | 1.53 | 0.84 | 0.95 |
| Tortuous, mean (SD) | 0.83 (1.2) | 0.67 | 0.96 | 0.81 | 0.72 | 0.87 | 0.06 (0.3) | 0.07 | 0.06 | 0.07 | 0.38 | 0.95 |
| Hooked, mean (SD) | 0.36 (0.7) | 0.30 | 0.37 | 0.40 | 0.87 | 0.87 | 0.05 (0.3) | 0.06 | 0.04 | 0.06 | 0.92 | 0.95 |
| Short, mean (SD) | 2.97 (2.2) | 2.82 | 3.08 | 2.97 | 0.24 | 0.87 | 1.66 (1.8) | 1.70 | 1.70 | 1.58 | 0.31 | 0.95 |
| Thick, mean (SD) | 0.16 (0.6) | 0.18 | 0.09 | 0.23 | 0.79 | 0.87 | 0.03 (0.2) | 0.03 | 0.02 | 0.03 | · | · |
| Thin, mean (SD) | 0.33 (0.7) | 0.23 | 0.40 | 0.34 | 0.64 | 0.87 | 0.08 (0.3) | 0.07 | 0.09 | 0.08 | 0.51 | 0.95 |
| Overlapping, mean (SD) | 0.46 (0.7) | 0.35 | 0.49 | 0.53 | 0.55 | 0.87 | 0.06 (0.3) | 0.05 | 0.05 | 0.06 | 0.72 | 0.95 |
| Ghost, mean (SD) | 0.86 (1.7) | 0.75 | 0.69 | 1.13 | 0.02 | 0.28 | 0.55 (1.4) | 0.45 | 0.61 | 0.57 | 0.54 | 0.95 |
| Dropout gland, mean (SD) | 0.07 (0.45) | 0.05 | 0.05 | 0.10 | 0.31 | 0.87 | 0.17 (0.54) | 0.15 | 0.21 | 0.15 | 0.75 | 0.95 |
| Tadpoling, n(%) | 24 (6%) | (4%) | (7%) | (7%) | 0.22 | 0.87 | 5 (1%) | (1%) | (1%) | (2%) | · | · |
| Abnormal gap, n(%) | 106 (27%) | (21%) | (29%) | (29%) | 0.08 | 0.87 | 2 (0%) | (0%) | (0%) | (1%) | · | · |
| No extension to lid margin, n(%) | 16 (4%) | (2%) | (6%) | (4%) | 0.71 | 0.87 | 27 (5%) | (5%) | (5%) | (6%) | 0.29 | 0.95 |
| Fluffy areas, n(%) | 43 (11%) | (17%) | (7%) | (11%) | 0.24 | 0.87 | 32 (6%) | (9%) | (5%) | (6%) | 0.42 | 0.95 |
| Composite score, mean (SD) | 23.7 (10.0) | 22.02 | 23.80 | 24.96 | 0.002 | 0.03 | 13.1 (8.4) | 12.76 | 13.59 | 12.88 | 0.85 | 0.95 |
| Percent dropout area, mean (SD) | ||||||||||||
| Including ghost glands and fluffy areas | 0.23 (0.2) | 0.23 | 0.20 | 0.25 | 0.31 | 0.87 | 0.32 (0.2) | 0.32 | 0.34 | 0.31 | 0.92 | 0.95 |
| Excluding ghost glands and fluffy areas | 0.11 (0.1) | 0.12 | 0.10 | 0.11 | 0.59 | 0.87 | 0.19 (0.1) | 0.19 | 0.20 | 0.18 | 0.69 | 0.95 |
Adjusted for age and sex; p-values not adjusted for multiple comparisons
Adjusted for multiple comparisons
The associations of MG features with NIKBUT, Keratographic tear meniscus height and Schirmer test are given in Supplemental Tables B, C, respectively. In the LL, the number of tortuous glands decreased with greater tear meniscus height, but this association was not statistically significant after adjustment for multiple comparisons (PMC = 0.10). The associations of all of the other MG features with tear meniscus height and NIKBUT in the UL and LL were not statistically significant after adjustment for multiple comparisons.
Our study provided very high power (>99%) to detect medium (0.30) to strong (0.50) correlations, yet provided low power (<50%) to detect small correlations (0.10).
2.3. Associations of Meibomian Gland Features with Sjogren Syndrome
The associations of the MGs with SS is given in Table 6. In the UL, when compared to patients without SS, patients with SS had less distorted glands (mean 3.4 vs 4.3, p=0.03), less ghost glands (mean 0.33 vs 0.89, p=0.006) (Figure 2) and lower composite score (19 vs 24, p=0.004). In the LL, patients with SS had less shortened glands than patients without SS (mean 1.14 vs 1.71, p=0.02).
Table 6.
Association of Meibomian gland features with Sjogren’s disease in upper and lower lids
| Upper Lid | Lower Lid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sjogren’s Disease |
||||||||||
| Meibomian gland feature | Total n=394 | No n=367 | On-going n=27 | * p-value | **PMC | Total n=498 | No n=455 | On-going n=43 * | * p-value | **PMC |
| Total, mean (SD) | 8.98 (2.8) | 9.00 | 8.59 | 0.39 | 0.83 | 7.20 (1.9) | 7.22 | 7.01 | 0.81 | 0.99 |
| Distorted, mean (SD) | 4.20 (2.3) | 4.26 | 3.39 | 0.03 | 0.37 | 1.56 (1.5) | 1.59 | 1.22 | 0.32 | 0.99 |
| Tortuous, mean (SD) | 0.83 (1.2) | 0.85 | 0.57 | 0.22 | 0.83 | 0.06 (0.3) | 0.06 | 0.06 | 0.99 | 0.99 |
| Hooked, mean (SD) | 0.36 (0.7) | 0.36 | 0.43 | 0.53 | 0.83 | 0.05 (0.3) | 0.05 | 0.02 | 0.45 | 0.99 |
| Short, mean (SD) | 2.97 (2.2) | 2.94 | 3.43 | 0.38 | 0.83 | 1.66 (1.8) | 1.71 | 1.14 | 0.02 | 0.29 |
| Thick, n(%) | 38 (10%) | (10%) | (0%) | · | · | 10 (2%) | (2%) | (2%) | · | · |
| Thin, mean (SD) | 0.33 (0.7) | 0.34 | 0.26 | 0.48 | 0.83 | 0.08 (0.3) | 0.07 | 0.14 | 0.47 | 0.99 |
| Overlapping, mean (SD) | 0.46 (0.7) | 0.47 | 0.43 | 0.83 | 0.83 | 0.06 (0.3) | 0.05 | 0.07 | 0.60 | 0.99 |
| Ghost, mean (SD) | 0.86 (1.7) | 0.89 | 0.33 | 0.006 | 0.07 | 0.55 (1.4) | 0.54 | 0.66 | 0.58 | 0.99 |
| Dropout gland, mean (SD) | 0.07 (0.5) | 0.06 | 0.11 | 0.59 | 0.83 | 0.17 (0.5) | 0.17 | 0.19 | 0.74 | 0.99 |
| Tadpoling, n(%) | 24 (6%) | (7%) | (0%) | · | · | 5 (1%) | (1%) | (2%) | · | · |
| Abnormal gap, n(%) | 106 (27%) | (27%) | (22%) | 0.31 | 0.83 | 2 (0%) | (0%) | (0%) | · | · |
| No extension to lid margin, n(%) | 16 (4%) | (4%) | (0%) | · | · | 27 (5%) | (6%) | (0%) | · | · |
| Fluffy areas, n(%) | 43 (11%) | (11%) | (7%) | 0.52 | 0.83 | 32 (6%) | (6%) | (9%) | 0.60 | 0.99 |
| Composite score, mean (SD) | 23.7 (10.0) | 24.01 | 19.25 | 0.004 | 0.051 | 13.1 (8.4) | 13.13 | 12.92 | 0.76 | 0.99 |
| Percent dropout area, mean (SD) | ||||||||||
| Including ghost glands and fluffy areas | 0.23 (0.2) | 0.23 | 0.18 | 0.31 | 0.83 | 0.32 (0.2) | 0.32 | 0.34 | 0.84 | 0.99 |
| Excluding ghost glands and fluffy areas | 0.11 (0.1) | 0.11 | 0.13 | 0.28 | 0.83 | 0.19 (0.1) | 0.19 | 0.19 | 0.99 | 0.99 |
Adjusted for age and sex; p-values not adjusted for multiple comparisons
Adjusted for multiple comparisons
Fig. 2.
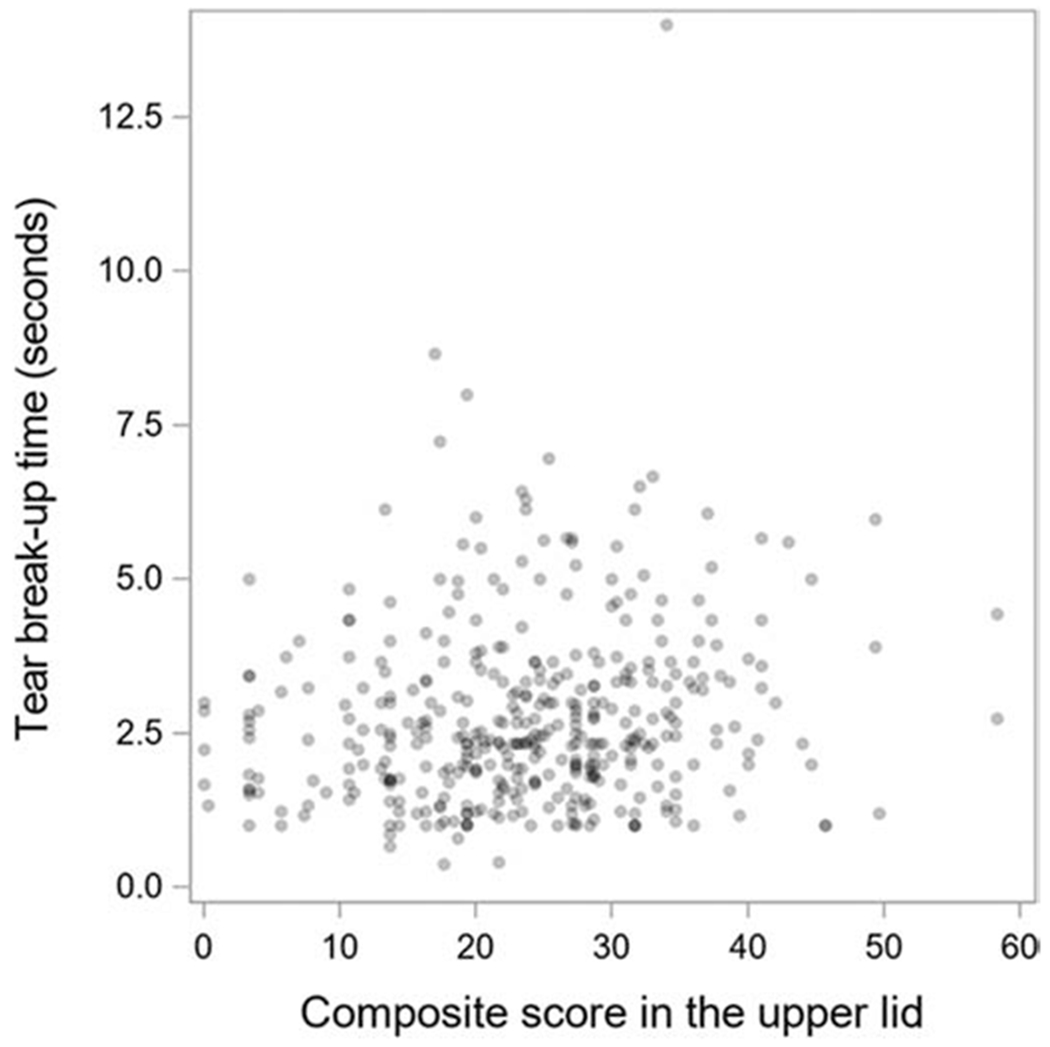
Scatter plot showing the association between composite score in the upper lid and tear break up time.
DISCUSSION
Within DREAM Study patients with moderate to severe DED, there were no morphological features of MGs observed in both ULs and LLs that were associated with the severity of symptoms as measured by the OSDI. When the associations between MG features and signs of DED were evaluated, most features were not associated with the signs of ocular surface staining, TBUT, Schirmer test, NIKBUT, or tear meniscus height. Tortuous glands showed some consistency in association with corneal staining and TBUT; however, the association was that having more of tortuous glands was associated with less severe signs. Eyes of patients with SS, generally had fewer MG morphological features.
A large study of 538 patients having MGD reported a similar lack of association of MG features with OSDI scores.11 A smaller study with 20 patients showed an association between OSDI and MG bent angle of the LL and with MG thickness of the UL. However, these results were not adjusted for age and sex.12
Although not statistically significant after adjustment for multiple comparisons in all cases, in our study there was some consistency with an association of a higher number of tortuous MGs with better TBUT and less corneal staining. Other studies have reported on tortuous MGs but with slightly different definitions and methods. One study measured the angle of the most bent MG in degrees and found the UL to have more severely bent tortuous MGs than the LL and were negatively correlated with NIKBUT.12 In our study tortuous MGs of the UL were associated with better TBUT but not with NIKBUT. An automated study on meibography images reported MG irregularity of the UL (defined as shape dissimilarity of each gland from a standardized regular gland) was correlated with TBUT and OSDI. High MG irregularity correlated with the Schirmer test and inversely correlated with corneal fluorescein staining.13
Tortuous MGs have also been associated with contact lens (CL) wear. Curled MGs, defined as those MGs that appeared curly compared to other glands, were found to be significantly higher in CL wearers than in those who had never used CLs.14 These curled glands appear to be similar to the distorted, tortuous and hooked glands in our study. In the DREAM study, we excluded DED patients if they had worn CL during the 30 days prior to the screening visit. An inverse correlation between UL meibograde score (amount of MG dropout) and MG tortuosity (defined as a gland having at least 1 angle greater than 45 degrees) was found in a study on MGD patients, with increasing amounts of tortuous MGs when the meibograde score was zero.11
MG tortuosity (defined as being present when two or more MGs exhibited a curled or twisted appearance) has been more commonly observed in Caucasian lids compared to Asian eye lids among children aged between 5 and 18 years.15 Male children have more tortuous MGs.16 In a large study on the ULs of Chinese children with no signs and symptoms of DED, tortuous MGs were negatively correlated with lipid layer thickness (LLT) giving rise to possibility that MG distortion may affect the excretion of meibum, leading to a decrease in LLT values.17 The DREAM study took into account the age and sex of patients during analysis but did not enroll children and most patients were Caucasian.
Chronic irritation, apart from CLs appears to influence the presence of tortuous MGs. Patients with perennial allergic conjunctivitis have more distorted glands compared to normal controls.18 CL wearers having contact lens-related allergic conjunctivitis have more distorted MGs than those who do not have CL related allergic conjunctivitis.19 Children with physiological conjunctival follicles and patients with severe obstructive sleep apnea syndrome have increased tortuous MGs.17,20 An inflammatory etiopathogenesis has been implied in these patients with lids manifesting MG tortuosity.
Among DREAM patients, all of the other morphological features investigated were either too infrequent for meaningful analysis or lost their significant associations after accounting for multiple comparisons. In relatively smaller studies, thin or attenuated MGs have been associated with worse expressibility of meibum15 and mean width of MGs has been associated with TBUT,21 MG thickening has been associated with tear osmolarity;11 Inter-glandular space (defined as space between 2 adjacent MGs of the UL) increased with progressive MG loss:11 MG duct length has been associated with meibum expression, corneal staining, DED symptoms, being Asian and elderly.14,22, Ghost glands were significantly decreased in patients with on-going SS when compared to patients without SS. Ghost glands were also associated with better Schirmer test results. In an earlier report we showed ghost MGs to be associated with thick or scanty meibomian secretions.6 A study which investigated a feature similar to our "ghost glands” termed it "MG vagueness”.23 Areas of vagueness had glands that were narrow, fading and difficult to identify. Severe vagueness was associated with severe symptoms (OSDI) and signs of the ocular surface, lower stability of tear film (TBUT) and worse MG function (expressibility and meibum quality). Our study was not able to corroborate their findings on OSDI and TBUT. However, the better Schirmer test association supports the possibility of an increase in tear fluid production that likely compensates for the functional loss of meibomian glands in individuals with MGD.24
Most of the studies investigating morphological features of MGs and their associations with the signs and symptoms of DED had small numbers or failed to adjust for age, sex and ethnicity. For making reasonably accurate comparisons between studies several other methodological issues need to be addressed. First, it is important to evaluate MGs in the UL and LL as two different entities. Even at a basic anatomical level, MG structural variations of UL are different from LL. Associations and their direction vary between the two eyelids. Often only LL MGs are examined because of the ease of everting the LL relative to the UL. Second, the areas of MG evaluation should be defined. Ideally this would be the entire exposed palpebral conjunctival area of a well everted lid but this may not be possible in pediatric populations and in over-sensitive patients. Third, there must be consensus on the terminology in describing the morphological forms of MGs. Distortion, curled, tortuous and hooked have been used to describe essentially the same morphological feature; ghost glands and MG vagueness appear to be similar. Fourth, with the data available, we cannot assume that similar looking morphological features are increasingly severe forms of the same MG morphology. Longitudinal studies will help in finding answers to these questions.
There are some limitations to our study. Meibography images were procured only from sites that had the meibography equipment. Only the mid 1/3 of the portion of the lid was used in the analysis because a consistent evaluation of the MGs in a substantial number of meibograph images was possible only in this area due to incomplete lid eversion and/or photographic angle. Our analysis may have underestimated morphological features that might have a predilection to be in the lateral and medial areas of the lid.
In conclusion, certain morphological features of MGs were associated with some ocular surface signs of DED. These associations are not very strong and are complex and unique to the lid in which they appear. Some morphological features such as tortuous MGs that we initially thought might be abnormalities indicating pathology, were actually found to be associated with better signs of DED. This information is important for future efforts in formulating severity composite scores of MG morphology.
Supplementary Material
Acknowledgments
Financial Support:
Grants U10EY022881, U10EY022879 and R01 EY026972 from the National Eye Institute; Supplemental funding from the Office of Dietary Supplements, National Institutes of Health; Prevention of blindness. The funding agencies had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Credit Roster for the DRy Eye Assessment And Management (DREAM) Trial
Certified Roles at Clinical Centers: Clinician (CL); Clinic Coordinator (CC), Data Entry Staff (DE) Principal Investigator (PI), Technician (T).
Milton M. Hom (Azusa, CA): Milton M. Hom, OD FAAO (PI); Melissa Quintana (CC/T); Angela Zermeno (CC/T).
Pendleton Eye Center (Oceanside, CA): Robert Pendleton, MD, PhD. (PI); Debra McCluskey (CC); Diana Amador (T); Ivette Corona (CC/T); Victor Wechter, MD (CL).
University of California School of Optometry, Berkeley (Berkeley, CA): Meng C. Lin, OD PhD FAAO (PI); Carly Childs (CC); Uyen Do (CC); Mariel Lerma (CC); Wing Li, OD (T); Zakia Young (CC); Tiffany Yuen, OD (CC/T).
Clayton Eye Center (Morrow, GA): Harvey Dubiner, MD (PI); Heather Ambrosia, OD (C); Mary Bowser (CC/T); Peter Chen, OD (CL); Helen Dubiner, PharmD, CCRC (CC/T); Cory Fuller (CC/T); Kristen New (DE); Tu Vy Nguyen (C); Ethen Seville (CC/T); Daniel Strait, OD (CL); Christopher Wang (CC/T); Stephen Williams (CC/T); Ron Weber, MD (CL).
University of Kansas (Prairie Village, KS) John Sutphin, MD (PI); Miranda Bishara, MD (CL); Anna Bryan (CC); Asher Ertel (CC/T); Kristie Green (T); Gloria Pantoja, Ashley Small (CC); Casey Williamson (T).
Clinical Eye Research of Boston (Boston, MA): Jack Greiner, MS OD DO, PhD (PI); EveMarie DiPronio (CC/T); Michael Lindsay (CC/T); Andrew McPherson (CC/T); Paula Oliver (CC/T); Rina Wu (T).
Mass Eye & Ear Infirmary (Boston, MA): Reza Dana, MD (PI); Tulio Abud (T): Lauren Adams (T); Marissa Arnofsky (T); Jillian Candlish, COA (T); Pranita Chilakamarri (DE); Joseph Ciolino, MD (CL); Naomi Crandall (T); Antonio Di Zazzo (T); Merle Fernandes (T); Mansab Jafri (T); Britta Johnson (T); Ahmed Kheirkhah (T); Sally Kiebdaj (CC/T); Andrew Mullins (CC/T); Milka Nova (T); Vannarut Satitpitakul (T); Chunyi Shao (T); Kunal Suri (T); Vijeeta Tadla (CC); Saboo Ujwala (T); Jia Yin MD, PhD (T); Man Yu (T).
Kellogg Eye Center, University of Michigan (Ann Arbor, MI): Roni Shtein, MD (PI); Christopher Hood, MD (CL); Munira Hussain, MS, COA, CCRP (CC/T); Erin Manno, COT (T); Laura Rozek, COT (T/DE).
Minnesota Eye Consultants (Bloomington, MN): David R. Hardten, MD FACS (PI); Kimberly Baker (T); Alex Belsaas (T); Erich Berg (CC/T); Alyson Blakstad, OD (CL); Ken DauSchmidt (T); Lindsey Fallenstein (CC/T); Ahmad M. Fahmy OD (CL); Mona M. Fahmy OD FAAO (CL); Ginny Georges (T); Deanna E. Harter (CL); Scott G. Hauswirth, OD (CL); Madalyn Johnson (T); Ella Meshalkin (T); Rylee Pelzer (CC/T); Joshua Tisdale (CC/T); JulieAnn C. Wick (CL).
Tauber Eye Center (Kansas City, MO): Joseph Tauber, MD, PHD (PI); Megan Hefter (CC/T).
Silverstein Eye Centers (Kansas City, MO): Steven Silverstein, MD (PI); Cindy Bentley (CC/T); Eddie Dominguez (CC/T); Kelsey Kleinsasser, OD (CL).
Icahn School of Medicine at Mt. Sinai, (New York, NY): Penny Asbell, MD, FACS, MBA (PI); Brendan Barry (CC/T); Eric Kuklinski (CC/T); Afsana Amir (CC/T); Neil Chen (CC/T); Marko Oydanich (CC/T); Viola Spahiu (CC/T); An Vo, MD (T); Matthew Weinstein, DO (T).
University of Rochester Flaum Eye Institute (Rochester, NY): Tara Vaz, OD (PI); Holly Hindman, MD (PI); Rachel Aleese (CC/T); Andrea Czubinski (CC/T); Gary Gagarinas, COMT CCRA (CC/T); Peter McDowell (CC); George O’Gara (DE); Kari Steinmetz (CC/T).
University of Pennsylvania Scheie Eye Institute (Philadelphia, PA): Vatinee Bunya, MD (PI); Michael Bezzerides (CC/T); Dominique Caggiano (CC/T); Sheri Drossner (T); Joan Dupont (CC); Marybeth Keiser (CC/T); Mina Massaro, MD (CL); Stephen Orlin, MD (CL); Ryan O’Sullivan (CC/T).
Southern College of Optometry (Memphis, TN): Michael Christensen, OD PhD (PI); Havilah Adkins (CC); Randy Brafford (CC/T); Cheryl Ervin (CL); Rachel Grant OD (CL); Christina Newman (CL).
Shettle Eye Research (Largo, FL): Lee Shettle, DO (PI); Debbie Shettle (CC).
Stephen Cohen, OD, PC (Scottsdale, AZ): Stephen Cohen, OD (PI); Diane Rodman (CC/T).
Case Western Reserve University (Cleveland, OH): Loretta Szczotka-Flynn, OD PhD (PI); Tracy Caster (T); Pankaj Gupta MD MS (CL); Sangeetha Raghupathy (CC/T); Rony Sayegh, MD (CL).
Mayo Clinic Arizona (Scottsdale, AZ): Joanne Shen, MD (PI); Nora Drutz, CCRC (CC); Lauren Joyner, COA (T); Mary Mathis, COA (T); Michaele Menghini, CCRP (CC); Charlene Robinson, CCRP (CC).
Wolston & Goldberg Eye Associates (Torrance, CA): Damien Goldberg, MD (PI); Lydia Jenkins (T); Brittney Rodriguez (CC/T); Jennifer Picone Jones (CC/T); Nicole Thompson (T), Barry Wolstan, MD (CL).
Northeast Ohio Eye Surgeons (Stow, OH): Marc Jones, MD (PI); April Lemaster (CC/T); Julie Ransom-Chaney (T); William Rudy, OD (CL).
Tufts Medical Center (Boston, MA): Pedram Hamrah, MD (PI); Mildred Commodore (CC); Christian Iyore (T); Lioubov Lazarev (T): Leah Mullen (T); Nicholas Pondelis (T); Carly Satsuma (CC).
University of Illinois at Chicago (Chicago, IL): Sandeep Jain, MD (PI); Peter Cowen (CC/T); Joelle Hallak (CC);Christine Mun (CC/T); Roxana Toh (CC).
The Eye Centers of Racine & Kenosha (Racine, WI): Inder Singh, MD (PI); Pamela Lightfield (CC/T); Eunice Lowery (T); Sarita Ornelas (T); R. Krishna Sanka, MD (CL); Beth Saunders (T).
Mulqueeny Eye Centers (St. Louis, MO): Sean P. Mulqueeny, OD (PI); Maggie Pohlmeier (CC/T).
Oculus Research at Garner Eyecare Center (Raleigh, NC): Carol Aune, OD (PI); Hoda Gabriel (CC); Kim Major Walker, RN MS (CC/T); Jennifer Newsome (CC/T).
Resource Centers
Chairman’s Office (Icahn School of Medicine at Mount Sinai, New York, NY): Penny Asbell, MD, FACS, MBA (Study Chair); Brendan Barry (Clinical Research Coordinator); Eric Kuklinski (Clinical Research Coordinator); Shir Levanon (Clinical Research Coordinator); Michael Farkouh, MD FRCPC, FACC, FAHA (Medical Safety Monitor); Seunghee Kim-Schulze, PhD (Consultant); Robert Chapkin, PhD, MSc. (Consultant); Giampaolo Greco, PhD (Consultant); Artemis Simopoulos, MD (Consultant); Ines Lashley (Administrative Assistant); Peter Dentone, MD (Clinical Research Coordinator); Neha Gadaria-Rathod, MD (Clinical Research Coordinator); Morgan Massingale, MS (Clinical Research Coordinator); Nataliya Antonova (Clinical Research Coordinator).
Coordinating Center (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA): Maureen G. Maguire, PhD (PI); Mary Brightwell-Arnold, SCP (Systems Analyst) John Farrar, MD PhD (Consultant); Sandra Harkins (Staff Assistant); Jiayan Huang, MS (Biostatistician); Kathy McWilliams, CcRp (Protocol Monitor); Ellen Peskin, mA, CCRP (Director); Maxwell Pistilli, MS, MEd (Biostatistician); Susan Ryan (Financial Administrator); Hilary Smolen (Research Fellow); Claressa Whearry (Administrative Coordinator); Gui-Shuang Ying, PhD (Senior Biostatistician) Yinxi Yu (Biostatistician).
Biomarker Laboratory (Icahn School of Medicine at Mount Sinai, New York, NY): Yi Wei, PhD, DVM (co-Director, Biomarker Laboratory); Neeta Roy, PhD (co-Director, Biomarker Laboratory); Seth Epstein, MD (Former co-Director; Biomarker Laboratory); Penny A. Asbell, MD, FACS, MBA (Director and Study Chair).
Investigational Drug Service (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA): Kenneth Rockwell, Jr., PharmD MS (Director).
Peroxisomal Diseases Laboratory at the Kennedy Krieger Institute, Johns Hopkins University Baltimore MD: Ann Moser (Co-Director/Consultant); Richard O. Jones, PhD (Co-Director/Consultant)
Meibomian Gland Reading Center (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA): Ebenezer Daniel, MBBS, MpH, PhD, (PI); E. Revell Martin (Image Grader); Candace Parker Ostroff, (Image Grader); Eli Smith (Image Grader); Pooja Axay Kadakia (Student Researcher).
National Eye Institute, National Institutes of Health, Department of Health and Human Services: Maryann Redford, DDS, MPH (Program Officer).
Office of Dietary Supplements/National Institutes of Health, Department of Health and Human Services
Committees
Executive Committee (Members from all terms of appointment): Penny Asbell, MD FACS, MBA (Chair); Brendan Barry, MS; Munira Hussain, MS, COA, CCRP; Jack Greiner, MS OD PhD; Milton Hom, OD, FAAO; Holly Hindman, MD, MPH; Eric Kuklinski, BA; Meng C. Lin OD, PhD. FAAO; Maureen G. Maguire, PhD; Kathy McWilliams, CCRP; Ellen Peskin, MA, CCRP; Maryann Redford, DDS, MPH; Roni Shtein, MD, MS; Steven Silverstein, MD; John Sutphin, MD.
Operations Committee: Penny Asbell, MD FACS, MBA (Chair); Brendan Barry, MS; Eric Kuklinski, bA; Maureen G. Maguire, PhD; Kathleen McWilliams, CCRP, Ellen Peskin, MA, CCRP; Maryann Redford, DDS, MPH.
Clinic Monitoring Committee: Ellen Peskin, MA, CCRP (Chair); Mary Brightwell-Arnold, SCP, Maureen G. Maguire, PhD; Kathleen McWilliams, CCRP.
Data and Safety Monitoring Committee: Stephen Wisniewski, PhD (Chair); Tom Brenna, PhD; William G. Christen Jr, SCD, OD, PhD; Jin-Feng Huang, PhD; Cynthia S. McCarthy, DHCE, MA; Susan T. Mayne, PhD; Mari Palta, PhD; Oliver D. Schein, MD, MPH, MBA.
Industry Contributors of Products and Services
Access Business Group, LLC (Ada, MI) Jennifer Chuang, PhD. CCRP; Maydee Marchan, M.Ch.E; Tian Hao, PhD; Christine Heisler; Charles Hu, PhD; Clint Throop, Vikas Moolchandani, PhD.
Compounded Solutions in Pharmacy (Monroe, CT)
Letter’s (San Jose, CA)
Immco Diagnostics Inc. (Buffalo NY
OCULUS Inc. (Arlington, WA)
RPS Diagnostics, Inc. (Sarasota, FL)
TearLab Corporation (San Diego, CA)
TearScience Inc. (Morrisville, NC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Presentation to be given at ARVO May 2020
Disclosures:
Dr. Asbell: Consultant for Sun Pharma, Dompe, Novaliq, Senju, Santen, Shire, Alcon, Kala, CLAO, Allakon, Medscape and Regeneron. Dr Bunya: Grant recipient from Bausch &Lomb/Immco Diagnostics; consultant for Celularity and Verily. Dr Massaro-Giordano: consultant for Verily, Lynthera, Dompe Celularity and PRN. Ebenezer Daniel, Maureen Maguire, Maxwell Pistilli have no conflicts of interest.
References:
- 1.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276–283. [DOI] [PubMed] [Google Scholar]
- 2.Viso E, Gude F, Rodriguez-Ares MT. The association of meibomian gland dysfunction and other common ocular diseases with dry eye: a population based study in Spain. Cornea 2011. ;30:1e6. [DOI] [PubMed] [Google Scholar]
- 3.Tong L, Chaurasia SS, Mehta JS, Beuerman RW. Screening for meibomian gland disease: its relation to dry eye subtypes and symptoms in a tertiary referral clinic in Singapore. Invest Ophthalmol Vis Sci 2010;51:3449e54. [DOI] [PubMed] [Google Scholar]
- 4.Walt J Ocular Surface Disease Index (OSDI) Administration and Scoring Manual.Irvine, CA: Allergan, Inc; 2004. [Google Scholar]
- 5.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. [DOI] [PubMed] [Google Scholar]
- 6.Daniel E, Maguire MG, Pistilli M, Bunya VY, Massaro-Giordano GM, Smith E, et al. Dry Eye Assessment and Management (DREAM) Study Research Group. Grading and baseline characteristics of meibomian glands in meibography images and their clinical associations in the Dry Eye Assessment and Management (DREAM) study. Ocul Surf. 2019;17:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Dry Eye Assessment and Management Study Research Group. n-3 fatty acid supplementation for treatment of dry eye disease. N Engl J Med 2018;378:1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asbell PA, Maguire MG, Peskin E, Kuklinski E, Bunya VT, the DREAM Study Research Group. The Dry Eye Assessment and Management (DREAM©) Study: Study design and baseline characteristics. Contemp Clin Trials 2018; 71:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health 1993;14:43–68 [DOI] [PubMed] [Google Scholar]
- 10.Hochberg Y, Benjamini Y. More Powerful Procedures for Multiple Significance Testing. Stat Med 1990;9:811–8. [DOI] [PubMed] [Google Scholar]
- 11.Adil MY, Xiao J, Olafsson J, Chen X, Lagali NS, Reader S, et al. Meibomian Gland Morphology Is a Sensitive Early Indicator of Meibomian Gland Dysfunction. Am J Ophthalmol. 2019;200:16–25. [DOI] [PubMed] [Google Scholar]
- 12.Pult H, Riede-Pult BH, Nichols JJ. Relation between upper and lower lids’ meibomian gland morphology, tear film, and dry eye. Optom Vis Sci. 2012;89:E310–5. [DOI] [PubMed] [Google Scholar]
- 13.Llorens-Quintana C, Rico-Del-Viejo L, Syga P, Madrid-Costa D, Iskander DR. Meibomian Gland Morphology: The Influence of Structural Variations on Gland Function and Ocular Surface Parameters. Cornea. 2019;38:1506–1512. [DOI] [PubMed] [Google Scholar]
- 14.Uçakhan Ö, Arslanturk-Eren M. The Role of Soft Contact Lens Wear on Meibomian Gland Morphology and Function. Eye Contact Lens. 2019. ;45:292–300. [DOI] [PubMed] [Google Scholar]
- 15.Kim JS, Wang MTM, Craig JP. Exploring the Asian ethnic predisposition to dry eye disease in a pediatric population. Ocul Surf. 2019;17:70–77 . [DOI] [PubMed] [Google Scholar]
- 16.Gupta PK, Stevens MN, Kashyap N, Priestley Y. Prevalence of Meibomian Gland Atrophy in a Pediatric Population. Cornea. 2018;37:426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Chen S, Wang S, Chen Y, Li J, Fu Y, et al. The significance of meibomian gland changes in asymptomatic children. Ocul Surf. 2018;16:301–305. [DOI] [PubMed] [Google Scholar]
- 18.Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Tomidokoro A, et al. Meibomian gland duct distortion in patients with perennial allergic conjunctivitis. Cornea. 2010;29:858–60. [DOI] [PubMed] [Google Scholar]
- 19.Arita R, Itoh K, Maeda S, Maeda K, Tomidokoro A, Amano S. Association of contact lens-related allergic conjunctivitis with changes in the morphology of meibomian glands. Jpn J Ophthalmol. 2012;56:14–19. [DOI] [PubMed] [Google Scholar]
- 20.Karaca I, Yagci A, Palamar M, Tasbakan MS, Basoglu OK. Ocular surface assessment and morphological alterations in meibomian glands with meibography in obstructive sleep apnea Syndrome. Ocul Surf. 2019. pii:S1542-0124(19)30061-8. [DOI] [PubMed] [Google Scholar]
- 21.Liang Q, Pan Z, Zhou M, Zhang Y, Wang N, Li B, et al. Evaluation of Optical Coherence Tomography Meibography in Patients With Obstructive Meibomian Gland Dysfunction. Cornea. 2015;34:1193–9. [DOI] [PubMed] [Google Scholar]
- 22.Ban Y, Shimazaki-Den S, Tsubota K, Shimazaki J. Morphological evaluation of meibomian glands using noncontact infrared meibography. Ocul Surf. 2013;11:47–53. [DOI] [PubMed] [Google Scholar]
- 23.Yin Y, Gong L. The quantitative measuring method of meibomian gland vagueness and diagnostic efficacy of meibomian gland index combination. Acta Ophthalmol.2019;97:e403–e409. [DOI] [PubMed] [Google Scholar]
- 24.Arita R, Morishige N, Koh S, Shirakawa R, Kawashima M, Sakimoto T, et al. Increased Tear Fluid Production as a Compensatory Response to Meibomian Gland Loss: A Multicenter Cross-sectional Study. Ophthalmology. 2015;122:925–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


