Abstract
Background:
Most post-concussion eye movement (EM) research involves predominantly male samples. We evaluated pro- (PRO; reflexive shift of visual attention to target) and anti- (ANTI; executive control of visual attention away from target) computer-based saccade task performance among female, collegiate athletes with recent concussion (CON) versus healthy-control athletes (HC). We evaluated the relationship between EM performance and post-concussion outcomes. We hypothesized ANTI performance would differ among CON and HC due to greater executive control demands, and that EM performance (both tasks) would be associated with clinical outcomes in CON.
Methods:
16 CON (assessed 4–10 days post-injury [M=6.87, SD=2.15 days]) and 16 age-matched HC athletes were recruited. General linear mixed modeling and Pearson’s correlations were used.
Results:
On ANTI, CON demonstrated higher error rate [F(1,2863)=12.650, p<.001] and shorter latency on error trials [F(1,469)=5.976, p=.015] relative to HC. Multiple EM measures were associated with clinical outcomes: PRO duration predicted days to symptom remission (r=.44, p<.05); ANTI error rate was associated with symptom burden on the day of testing (r=.27, p<.05).
Conclusion:
This study demonstrates promising utility of EM measures to detect cognitive control and sensorimotor effects of concussion among female athletes and their use as a prognostic indicators of recovery.
Keywords: sports-related concussion, eye movement, oculomotor, saccade
Introduction
Athletes, particularly contact- and collision-sport athletes, are at risk of sustaining concussion (1). A concussion is a brain injury induced by biomechanical forces, “caused either by a direct blow to the head, face, neck, or elsewhere on the body with an impulsive force transmitted to the head”; this injury results in temporary impairment of neurological function, such as loss of consciousness, alteration of consciousness, disorientation, or amnesia (2). Sports-related concussion (SRC) is of particular interest at the collegiate level as there are approximately 460,000 varsity athletes and at least two million club athletes who compete each year, with club athletes often receiving less surveillance and monitoring by collegiate healthcare and sports medicine providers (3,4). The percentage of college athletes who sustain a concussion in one season of play ranges from 0.7% to 13.8%, depending on study and sport (5–8).
Visual system functioning abnormalities are reported in up to 65% of pediatric and adult patients following concussion (9–11). As such, eye movement testing has gained increasing attention as a reliable and objective assessment and monitoring tool among individuals who sustain head trauma (12–14). Among a variety of oculomotor measures, saccade-based eye movement testing is the most frequently used eye movement measure in concussion research (15). Saccades are rapid eye movements from one point to another and can be initiated reflexively (i.e., when a novel stimulus appears) or voluntarily (16). Laboratory-based saccadic eye movement tasks have been used to probe brain dysfunction as the underlying neural circuitry involved in the execution of saccades is well delineated by human and animal anatomical and physiological research (16). Various eye movement paradigms allow for non-invasive, reliable, and valid measurement of sensorimotor and cognitive functioning (17,18). Widely used saccadic eye movement tasks include the prosaccade (also referred to as visually-guided saccade) task in which the participant reflexively looks towards a novel target (also termed the “visual grasp reflex” (19)) and the anti-saccade task, in which the participant inhibits the reflex to look towards a target and instead directs gaze to the mirrored location of the target (20,21). Eye movement tasks with increased executive demands, such as the anti-saccade task, may be particularly sensitive to the effects of concussion (12). Performance on such tasks that require executive control involve a diffuse network of cortical and subcortical regions, including regions within the frontal cortex, the parietal cortex, visual cortex, cerebellum, thalamus, basal ganglia, superior colliculus, and reticular formation (16). The distributed nature of brain areas associated with performance on the anti-saccade task render the task a promising tool to detect effects following the diffuse neural changes that can occur acutely post-concussion (22).
Multiple studies have used saccadic eye movement testing to evaluate group differences in performance among individuals with a recent history of SRC versus controls. A recent systematic review and meta-analysis of the use of eye movement technology and SRC revealed increased inhibitory errors on the anti-saccade task among athletes in the acute post-concussion phase (<30 days following injury) relative to controls (14). However, the studies examining classic anti-saccade task performance in the meta-analysis were comprised of a maximum of 33.3% female athletes and no sex stratified analyses were conducted (14,23,24). Given that female athletes comprise at least 44% of the collegiate athlete population (25), may be at greater risk for sustaining concussion (26–28), and may experience more adverse outcomes following concussion (29–31), it is important to study female athletes in the investigation of the use of eye movement technology among athletes with SRC. To address the underrepresentation of females with concussion in this body of literature, in this pilot study we employed eye movement testing, specifically laboratory-based pro- and anti-saccade tasks, to assess the effects of recent concussion in a female, collegiate athlete sample.
The goal of our study was to evaluate pro- and anti-saccade task performance among female athletes with and without concussion. Compared to healthy-control female athletes (HC), we expected that female athletes with recent concussion (CON) would perform worse than controls on the more challenging, executive, anti-saccade task evident by higher error rate, reflecting decreased inhibitory control, and longer latency on correctly performed trials, reflecting longer time to successfully inhibit a reflexive response and generative a voluntary directed response. Although other studies examining individuals with a recent concussion and controls have typically failed to detect group differences on the prosaccade task (23,24,32), we nonetheless explored whether CON athletes perform worse than HC on the prosaccade task (specifically, longer latency and duration, slower velocity, and poorer spatial accuracy) in this female sample given the lack of inclusion of post-concussion female athletes in prior studies. Second, among CON athletes, we expected that there would be a significant relationship between eye movement performance (i.e., longer latency and duration, slower velocity, poorer spatial accuracy, and higher anti-saccade error rates) and clinical outcome variables such as time to symptom resolution, time to clearance for full return to play, symptom severity around the time of injury, and elevated symptom and mood ratings at the time of assessment.
Materials and methods
Participants and recruitment
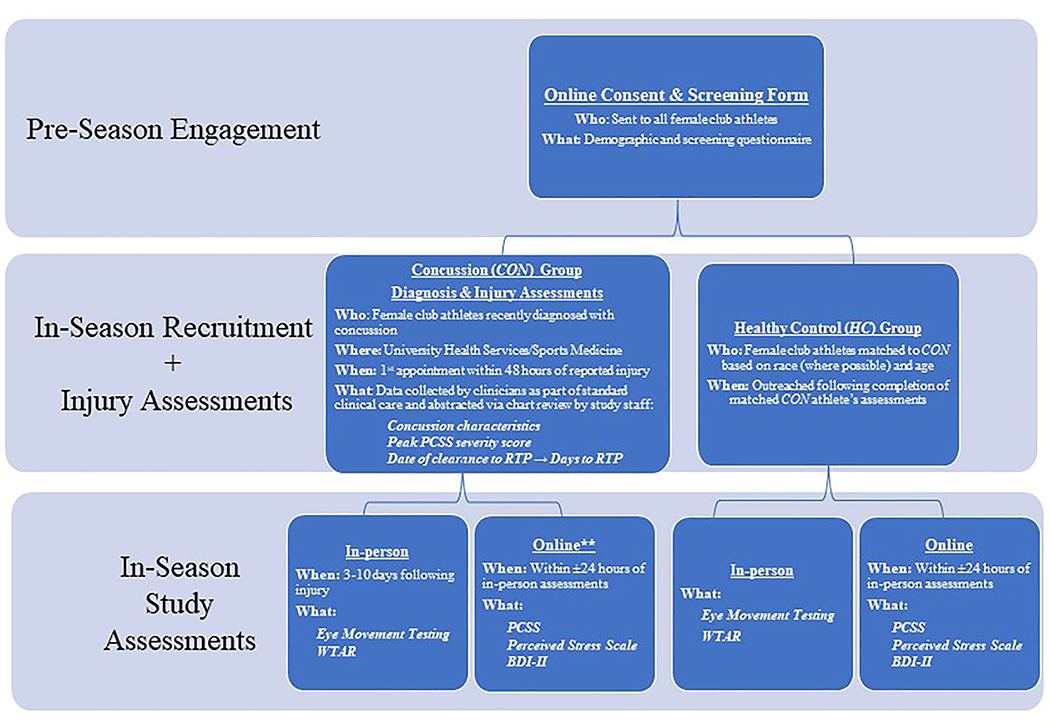
The study was part of a larger study examining brain-based biomarkers of concussion among female athletes; additional components, not discussed here, included neuroimaging assessments and menstrual cycle tracking and matching. See Figure 1 for recruitment and assessments overview. Prior to the start of the athletic season, all Northwestern University female club athletes between 18 and 25 years old were invited by e-mail to complete an online consent form and screening questionnaire for potential study enrollment should they suffer a concussion over the course of the season or as a control subject. Exclusion criteria for all study participants included factors known to influence eye movement performance such as a lifetime diagnosis of a psychotic disorder (33), a first-degree relative with a psychotic disorder (34), history of a seizure disorder or other neurological disorder (35), and self-reported vision or eye abnormalities (beyond corrected vision with glasses or contacts). Additional exclusion criteria for healthy controls included history of concussion within 6 months prior to study participation.
Figure 1. Study Recruitment and Assessments Overview.
**Study staff contacted athletes every 48 hours following their in-person study assessment to assess whether the athletes perceived that their symptoms had returned to baseline for at least 24 hours, yielding Days to Symptom Baseline variable. Abbreviations: BDI-II: Beck Depression Inventory, Second Edition; PCSS: Post-Concussion Symptom Scale; RTP: Return-to-play; WTAR: Wechsler Test of Adult Reading.
During the season, female club athletes newly diagnosed with concussion by a physician according to the criteria from the International Conference on Concussion in Sport (4th and 5th) (2,36) were approached by their treating sports medicine physician or athletic trainer regarding interest in study participation. Interested athletes who met study inclusion criteria were scheduled for in-person informed consent and assessments. Consistent with other studies of eye movement performance in the acute post-concussion phase (23,32,37–39), assessments occurred within 3–10 days following injury, to maximize the chance of detecting the neurocognitive and sensorimotor effects of concussion, as performance may normalize by two weeks post-injury (23,38).
After a CON athlete completed the protocol, a healthy female club athlete who qualified for study participation based on the pre-season screening form and who was matched with the CON athlete on age, race (where possible, 15/16 matches), and ethnicity was invited for in-person assessments. Screening information was reviewed and updated as needed with athletes if greater than one month elapsed between form completion and study enrollment. Note, as part of the larger protocol, HC and CON athletes were also matched on a) the use of hormonal contraception (yes versus no), b) menstrual cycle phase (follicular versus luteal) for non-hormonal contraceptive users, and c) active versus inactive phases of hormonal contraception for hormonal contraceptive users.
This study was approved by the Northwestern University Institutional Review Board and all participants provided informed consent prior to engagement in research procedures. Participants were reimbursed for their participation in the study, which required 4 – 5 hours to complete all study procedures (reimbursement rate = $20 per hour).
Assessments
Eye movement testing
Eye movements were measured in a window-less room using the Eyelink1000 system (SR Research Ltd, Ontario, Canada), an infrared video recording system that permits for high-resolution (1000 Hz; micro-saccade resolution of 0.05°) recording of eye position. A camera situated at the base of a computer monitor placed 76 cm from the participant recorded the reflection of an imperceptible light off the participant’s pupil while they viewed stimuli on the monitor. A non-restrictive chin and forehead rest stabilized the participant’s head position, maintaining a fixed position across subject to visual stimuli, and improved quality of eye trace. Pupil size and corneal reflection thresholds were adjusted for each subject to ensure consistent trace from both eyes. Saccades were automatically detected by the EyeLink system when eye position moved greater than 0.15 degrees from fixation and trace exceeded velocity and acceleration thresholds of 30 deg/sec and 8000 deg/sec2, respectively (40).
Primary eye movement measurements of interest (see Table 1) on the prosaccade task included a) latency: the time (in milliseconds [ms]) between the appearance of the stimuli and the initiation of a saccade, which reflects the speed of reflexive shifts of visual attention and response execution; b) gain: the spatial accuracy of the saccade to the target location, which is derived from the ratio of the saccade amplitude to the target amplitude (gain of 1 reflects perfect spatial accuracy, < 1 indicates participant undershot the target [hypometric], and > 1 indicates participant overshot the target [hypermetric]; c) accuracy: the spatial accuracy of the primary saccade, derived from the difference between the location of the target position (in x-coordinate pixels) and the location of the end point of the primary saccade; d) duration: the time (ms) taken to complete a saccade; and e) peak velocity: the highest velocity reached during the saccade, which is linearly related to the saccade duration (41). On the anti-saccade task, the primary eye movement measurements of interest were primary saccade latency, which reflects the speed of executive shifts of visual attention, and anti-saccade error rate (error trials/total trials), a measure of executive inhibition and cognitive control; an error trial occurred when the participant incorrectly looked ≥ 20% of the distance toward the peripheral target.
Table 1.
Eye movement tasks.
| Task (Duration) | Overview | Primary measure(s) | Directions to Participant | Design |
|---|---|---|---|---|
| Prosaccade (10 minutes) | An automatic or reflexive attention task. | Primary saccade latency, gain, accuracy, duration, and peak velocity. | Participants are instructed to look at visual targets when they appear. | 96 trials that begin with a center fixation target that remains illuminated for 1.5–2.5 seconds before peripheral targets appear at 10° or 15° from center in the left or right visual field; three conditions (32 trials each) are conducted to manipulate the center fixation offset and peripheral target onset: gap, no gap, and overlap. |
| Anti-saccade (10 minutes) | An executive or voluntary attention task. | Anti-saccade error rate (error trials/total trials) and saccade latency. Errors occur when participants incorrectly look toward the peripheral target. | Participants are instructed to inhibit the automatic response to look towards the peripheral target and instead shift their gaze to the mirrored location (e.g. if target appears 3 inches to the left of center, participants look immediately 3 inches to the right of center). | 96 trials that begin with a center fixation target that remains illuminated for 1.5–2.5 seconds before peripheral targets appear at 10° or 15° from center in the left or right visual field; three conditions (32 trials each) are conducted to manipulate the center fixation offset and peripheral target onset: gap, no gap, and overlap. |
Each task consisted of 96 trials, with blocks of 32 trials for each of the following internal experimental manipulations of the duration between the offset of the central fixation and the onset of the target: gap, no gap, and overlap (33). In the no gap condition, participants fixated on a central target for 2000–3000 ms (variable to reduce anticipatory responses), followed by the extinction of the central target and simultaneous onset of one peripheral target, appearing 10 or 15 degrees to the left or right of center, yielding 8 trials for each target location (±10 and ±15 degrees), randomized across the block. In the gap condition the central fixation target was extinguished 200 ms before the peripheral target appeared, while in the overlap condition the central fixation remained illuminated for 200 ms after the appearance of the peripheral target. Typically, healthy individuals exhibit shorter latency in the gap condition versus the no-gap condition, often referred to as the “gap effect” (42). This is thought to occur because the attentional engagement system is released when the central fixation stimulus is extinguished, rendering the attentional engagement system ready and searching for a new stimulus (42,43). Among healthy individuals, the overlap condition results in longer latencies relative to the gap and no gap conditions, which is likely attributable to the attentional engagement system remaining occupied by the central fixation stimulus and, therefore, slowing down response to a new stimulus (44). Order of condition administration within the prosaccade and anti-saccade tasks was randomized by participant. Each task was preceded by six practice trials (anti-saccade task practice trials were overlap condition trials).
Questionnaires
Prior to in-person study activities, all participants completed a comprehensive, online, study questionnaire regarding demographic, health history (including concussion history), recent athletic participation, recent substance use (using the 30-day Timeline Followback form of recent alcohol and marijuana use (45–47)), educational background, and parental education and occupational history (yielding a total socioeconomic status score (48)); see Figure 1. In addition, all athletes completed several self-report measures within 24 hours of eye movement testing to assess the influence of physical, cognitive, and emotional symptoms on eye movement performance and recovery. The Post-Concussion Symptom Scale (PCSS) was used to assess the presence and severity of symptoms that occur as a result of a concussion (49). The PCSS yields two scores: 1) total symptom number (range: 0–22), and 2) symptom severity score (range: 0–132). The symptom severity score was used in main analyses. The Beck Depression Inventory-II (BDI-II) was used to assess the characteristic attitudes and symptoms of depression (50,51). The Perceived Stress Scale (PSS) was used to evaluate ability to handle stress (52,53). Given the high correlations among PCSS, PSS, and BDI-II scores among the total sample (r =.57–.68), a symptom composite score reflecting psychological, cognitive, and physical symptoms on the day of testing was created by transforming PCSS (on day of evaluation), PSS, and BDI-II scores into z-scores based on values from the total sample; the resulting z-scores were averaged per participant to create a symptom composite z-score (54).
The Wechsler Test of Adult Reading (WTAR) was administered in-person and age-adjusted standard scores were used to obtain an estimate of general intellectual functioning (55), given the high correlation between WTAR standard scores with estimated full-scale intellectual abilities (FSIQ) and with verbal intellectual abilities (VIQ) (56).
Concussion characteristics and outcomes
Injury characteristics.
Injury characteristics such as injury description, presence and duration of loss of consciousness, presence and duration of post-traumatic amnesia, and presence and results of neuroimaging related to the concussion were obtained by Northwestern University athletic training staff or sports medicine physicians as part of standard clinical care. The information was later abstracted from the patient’s chart and recorded by study staff.
Peak symptom severity.
As part of standard clinical care, CON athletes completed the PCSS with a physician or athletic trainer approximately 2 days following injury (median = 2.00 days, range = 1–6 days) and approximately every three to five days after the initial encounter until clearance for full return to play. The peak symptom severity score from the PCSS recorded by athletic training staff or sports medicine physicians throughout the recovery period was retrospectively abstracted from the patient’s chart by study staff and used in analyses.
Days to symptom baseline (Days to SxBL).
Study staff prospectively contacted athletes every 48 hours following their in-person study assessment to assess whether the athletes perceived that their symptoms had returned to baseline for at least 24 hours (note: two athletes reported being symptom free for 24 hours for the first time on the day of, or day before, in-person assessments). The date of injury was subtracted from the date of symptom resolution for at least 24 hours to yield Days to SxBL.
Days to full return-to-play (Days to RTP).
Prior to medical clearance for return to sport following concussion, athletes completed a standardized return-to-play protocol as part of their standard clinical care, which consisted of gradual increases in physical exertion, starting with light aerobic activity (e.g. stationary biking) and progressing to non-contact athletic participation. The final clearance for full-contact athletic participation was made by a sports medicine physician after the completion of the return-to-play protocol. The return-to-play protocol lasted a minimum of four days. Days to RTP was calculated by subtracting the number of days between final clearance by the physician and the date of injury.
Data analyses
Eye movement data scoring and cleaning
Eye movement data from the right eye only, for consistency across participants, were exported using the EyeLink Data Viewer software package (SR Research Ltd., version 3.1). For both the pro- and anti-saccade tasks, primary saccades were defined as the first saccade after the target appearance that originated within ± two visual degrees from center and amplitude ≥ 20% of the absolute distance toward the target measured in degrees of visual angle. Primary saccades were excluded from analyses if a blink occurred within 100 ms prior to the appearance of the target. If a primary saccade contained a blink during the saccade, the latency information was retained for analyses, but all other saccade measurements of interest were excluded from analyses. Unless otherwise stated, all analyses on eye movement measurements of interest were conducted on the primary saccade of the trial. Resulting saccade measurements were trimmed to exclude values below approximately the 2.5 percentile and above approximately the 97.5 percentile values (cut-off values were also set upon qualitative review of the distribution of the data with consensus among at least two research team members) across the sample to normalize the distribution of data.
On the anti-saccade task, saccades were coded as self-corrective saccades if they followed an anti-saccade error within 238.00–675.00 ms (parameters established upon review of data distribution) and the amplitude of the saccade was ≥ 40.00% of the absolute distance toward the correct position (i.e, reflecting a shift of gaze that passed center and towards the correct hemifield).
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (57). The Kolmogorov-Smirnov test of normality was used to evaluate the normality of continuous demographic, clinical history, and outcome variables among groups. Descriptive analyses were conducted to evaluate group differences in demographic, clinical history, and outcome variables; specific tests included one-way analyses of variance for parametric data, Mann-Whitney U-tests for non-parametric data, and chi-square tests.
General linear mixed modeling was used to evaluate group differences on eye movement measurements of interest within task. For prosaccade latency, anti-saccade latency (correct trials and incorrect trials analyzed separately), and anti-saccade error rate, main effects of interest included group (CON vs. HC), condition (gap, no gap, overlap), and a two-way interaction effect (group-by-condition). Given that the experimental manipulation of the offset of the central fixation target primarily affects latency and anti-saccade error rate, all other group analyses were conducted on no gap condition trials only. Therefore, for prosaccade gain, accuracy, duration, and peak velocity analyses, only no gap trials were analyzed to examine group differences.
To evaluate the associations among eye movement performance and clinical outcomes (i.e., days to symptom baseline, days to clearance for full return to play, peak symptom severity, and symptom composite z-score on the day of testing), Pearson’s one-tailed correlation coefficients (r) were calculated between clinical outcome variables and participants’ eye movement performance on the prosaccade (latency: all conditions; gain, accuracy, duration, peak velocity: no gap condition only) and anti-saccade (latency, error rate: all conditions) tasks.
Results
Demographic, injury, and symptom data
Approximately 31.25% of CON participants were members of the ultimate frisbee team, 25.00% rugby, and 6.25% each for basketball, cheer, crew, lacrosse, soccer, triathlon, and volleyball teams; 37.50% of HC participants were members of the triathlon team, 18.75% crew, 18.75% ultimate frisbee, and 6.25% each lacrosse, tennis, track, and volleyball teams. CON did not differ from HC in hours of sports participation in 30 days prior to assessment (total sample M = 18.44, SD = 9.60 hours), total number of alcohol drinks in the 30 days prior to assessment (total sample M = 13.13, SD = 12.99 drinks), or total grams of marijuana consumed in the 30 days prior to assessment (total sample M = 0.41, SD = 1.11 grams). CON athletes were assessed between 4–10 days following injury (M = 6.87, SD = 2.15 days).
As demonstrated in Table 2, the CON group did not significantly differ from the HC group regarding demographic factors or health history (with the exception of prior concussion). Consistent with prior literature demonstrating increased risk of a subsequent concussion among athletes with previous concussion (58), CON participants had higher rates of previous concussion, excluding the current injury related to study participation, relative to HC. Post-hoc testing was conducted in which prior history of concussion was added to main analyses that yielded significant group findings; significance of group effects remained unchanged. There was no reported history of attention-deficit disorders, learning disabilities, or migraine headaches among the sample.
Table 2.
Sample demographic and health history information.
| Group | p | ||
|---|---|---|---|
| CON (n = 16) | HC (n = 16) | ||
| Age [M(SD)] | 19.94 (1.48) | 19.81 (1.28) | nsa |
| Race (% Caucasian) | 88% | 94% | ns |
| Ethnicity (% Hispanic or Latino) | 13% | 13% | ns |
| Socioeconomic status [Hollingshead 4-factor M(SD)] | 145.54 (27.46) | 139.47 (30.06) | ns |
| Years of education [M(SD)] | 13.13 (1.20) | 13.13 (1.09) | nsa |
| Weschler Test of Adult Reading standard score [M(SD)] | 113.75 (10.70) | 116.88 (7.70) | ns |
| Hx of anxiety or depression | 13% | 0% | ns |
| Hx of one or more previous concussions | 50% | 7% | 0.022 |
Note. Hx = history; M = mean; ns = group comparisons are not significant at the p < .05 level; p = statistical significance value for group comparisons (Pearson’s Chi-square for categorical data, analyses of variance tests for parametric continuous data, and
Mann-Whitney tests for non-parametric continuous data)
SD = standard deviation of the mean.
Within and between group symptom and outcome data is presented in Table 3. All CON athletes reported returning to symptom baseline in ≤ 25.00 days, with an average of less than two weeks.
Table 3.
Symptom and recovery data.
| Group | p | ||
|---|---|---|---|
| CON (n = 16) | HC (n = 16) | ||
| Symptoms on Day of Eye Movement Testing | |||
| Post-Concussion Symptom Scale (PCSS) | 21.69 (14.75) | 6.00 (5.88) | < .001a |
| Perceived Stress Scale (PSS) | 15.81 (5.52) | 8.87 (4.79) | .001 |
| Beck Depression Inventory-II (BDI-II) | 5.06 (5.74) | 1.56 (1.21) | .056a |
| Symptom Composite z-score | 0.51 (0.91) | −0.51 (0.42) | < .001 |
| Peak post-injury PCSS severity | 29.44 (17.88) | - | - |
| Days to SxBL | 13.50 (6.29) | - | - |
| Range: 5–25 | - | - | |
| Days to RTP | 22.40 (13.22) | - | - |
| Range: 10–66 | - | - | |
Note. Data presented as Mean (Standard Deviation of the Mean), unless otherwise noted. p = statistical significance value for group comparisons (analyses of variance tests for parametric continuous data, and
Mann-Whitney tests for non-parametric continuous data)
RTP = clearance to return to play; SxBL = symptom baseline; Symptom Composite z-score = average of participants’ z-scores transformed PCSS severity (on day of evaluation), PSS, and BDI-II total scores.
Eye Movement Results, CON vs. HC
Please see Supplementary Tables 1 and 2 for primary saccade descriptive data by group and condition.
Prosaccade task
On the reflexive prosaccade task, there were no main effects of group (CON vs. HC) on latency, gain, accuracy, duration, or peak velocity, nor were there group-by-condition (gap, no gap, overlap) interaction effects on prosaccade latency. There was an expected main effect of condition on latency [F(2, 2515) = 556.306, p < .001], with latency increasing on gap to no gap to overlap trials.
Anti-saccade task
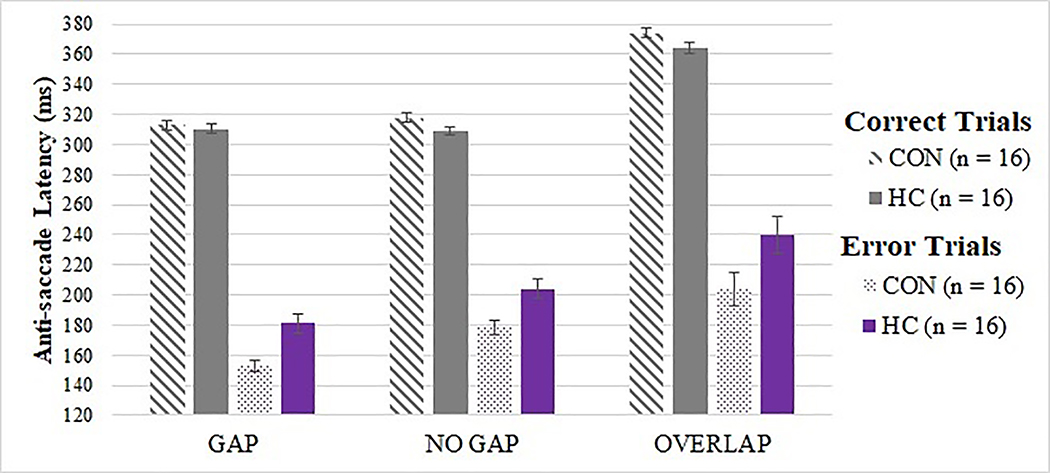
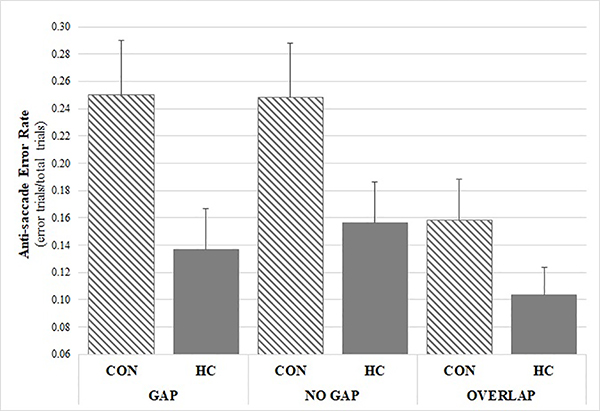
Tests of the two a priori hypotheses on the anti-saccade task were conducted using Bonferroni adjusted alpha levels of .025 (.05/2). On the executive, anti-saccade task, there was an expected main effect of condition on latency for correctly performed anti-saccade trials [F(2, 2093) = 28.110, p < .001], but no significant group or group-by-condition interaction effect (p > .05); see Figure 2. In an exploratory analysis of error trial latency, there was a main effect of group [F(1, 469) = 5.976, p = .015] and condition (p < .001), but no group-by-condition effect, whereby CON demonstrated shorter latency on error trials relative to HC; see Figure 2. There was also a main effect of group, although no group-by-condition interaction effect, on anti-saccade error rate (i.e., incorrect look towards the target when target appeared; see Figure 3) [F(1, 2863) = 12.650, p < .001], with CON committing more inhibitory errors than HC (OR = 1.906, 95% CI: 1.572, 2.310). There was also an expected main effect of condition on anti-saccade error rate [F(1, 2863) = 3.006, p = .050], with higher error rates on gap and no gap, relative to overlap, conditions; see Figure 3. There were no main effects of group or group-by-condition interaction effects on rates of self-correcting saccades after errors or latency of self-correcting saccades after errors.
Figure 2. Anti-saccade latency by trial type, group, and condition.
CON demonstrated shorter latency than HC on anti-saccade error trials (p = .015), but no significant group differences were observed on correct anti-saccade trials (p = .174). There was a main effect of condition on latency on both correct and error anti-saccade trials (p < .001).
Figure 3. Anti-saccade error rate by group and condition.
CON exhibited significantly higher error rate relative to HC (OR =1.906, 95% CI: 1.572, 2.310, p < .001).
Factors associated with clinical outcomes among CON group
Among CON athletes, peak symptom severity was not associated with days to symptom resolution or days to clearance for full return to play (p > .05).
Eye movement
Among the CON group, multiple aspects of saccade performance were associated with clinical outcomes (see Table 4). Specifically, on the prosaccade task, longer time to symptom remission was associated with decreased accuracy (trend effect, p = .100), longer saccade duration (p = .043), and slower peak velocity (trend effect, p = .060). Further, higher symptom composite scores, reflecting psychological, cognitive, and physical symptoms on the day of testing, were associated with longer prosaccade saccade duration (trend effect, p = .087) and higher anti-saccade error rate (averaged across all three conditions, p = .030). Of note, none of these correlations meets statistical significance when a Bonferroni correction for multiple comparisons (.05/20) is applied (p > .0025). Post-hoc testing revealed that higher symptom composite scores were associated with greater anti-saccade error rate on the gap (r(14) = 0.43, p = .047) and no gap (r(14) = 0.47, p = .034), but not the overlap (r(14) = –.17, p = .260), conditions. Latency on either task was not associated with clinical outcomes. There were no associations among eye movement measures and either time to return to play nor peak post-injury symptom scores.
Table 4.
Associations among eye movement performance and clinical outcomes among the Concussion group (n=16).
| Prosaccade Task—No Gap Only | Anti-saccade Error Rate | |||
|---|---|---|---|---|
| Accuracy | Duration | Peak Vel. | ||
| Days to SxBL | −.34* | .44** | −.41* | ns |
| Symptom Composite z-score | ns | .36* | ns | .27** |
Note. Data indicates Pearson’s correlational values for one-tailed correlations; ns = not significant (p > .05)
indicates trend effect (p ≤ .10)
indicates a significant effect, uncorrected for multiple comparisons (p < .05)
SxBL = symptom baseline; Symptom Composite z-score = average of participants’ z-score transformed PCSS severity (on day of evaluation), PSS, and BDI-II total scores. There were no associations among a) eye movement measures and days to return to play nor peak post-injury scores or b) latency (pro- and anti-saccade tasks) and clinical outcomes.
Discussion
In this pilot study evaluating the use of eye movement testing to detect impairments in sensorimotor and cognitive abilities following SRC among female athletes, our hypotheses were partially confirmed in that female athletes with recent concussion committed more anti-saccade errors relative to controls, meaning CON participants were more likely to incorrectly look toward the target when she was instructed not to. However, CON did not exhibit significantly longer latencies (i.e., reaction times) on the anti-saccade task (on correctly performed trials) relative to HC as we had expected. CON and HC did not differ on prosaccade task performance, consistent with prior literature among predominantly male participants(23,24,32). Consistent with our hypotheses, analyses revealed significant associations among eye movement performance and clinical outcomes such that symptom scores on the day of testing were associated with higher anti-saccade error rate and longer time to symptom remission was associated with longer prosaccade duration, though these relationships do not survive corrections for multiple comparisons.
Our finding that CON demonstrated increased inhibitory errors relative to HC (21.88% vs. 13.22% of anti-saccade trials, averaged across conditions, respectively) is consistent with prior studies comprised predominantly of male participants presenting to the emergency department (23,32,37,59). Increased errors on the anti-saccade task among CON—but similar performance to controls on the stimulus-driven, reflexive prosaccade task (e.g., latency, gain, accuracy, duration, and peak velocity)—suggests an acute, isolated, deficit in oculomotor executive control. That is, patients in the acute recovery phase following concussion exhibited increased difficulty inhibiting the automatic response to look towards the target. This finding is consistent with previous investigations of acute post-concussion functioning that reveal frontal networks dysfunction, which anatomically implicates the frontal and parietal cortices, as well as their subcortical connections and functionally implicates abilities such as executive functioning, problem solving, divided attention, sustained attention, and working memory (60–63). Anti-saccade errors may also be due to difficulty maintaining attention (more specifically, maintaining instructions in active working memory), suggesting deficits in the fronto-parietal working memory network (64); although the fact that CON and HC had comparable rates of self-correction of errors and latency of corrective saccades argues against this and suggest errors are likely attributable to reduced top-down executive control of attention.
On anti-saccade error trials, the CON group exhibited 14.55% shorter response (faster) time, averaged across conditions, relative to HC. The shorter latencies on error trials among CON relative to HC participants observed in the present study indicates increased disinhibition among the CON group, potentially suggesting increased difficulty inhibiting the saccade generating neurons in the frontal eye fields and superior colliculus (19). In contrast to our findings, previous studies found longer (32) or equivocal (37) latency on anti-saccade error trials among participants with recent concussion relative to controls.
Prior studies revealed anti-saccade error rates among those with recent concussion (injury 4–6 days prior to assessment) ranging from 27% (no gap condition) to 34% (overlap condition), and controls’ error rates ranging from 13% (overlap condition) to 19% (no gap condition) (23,32). The average error rates among our CON participants (24.80% no gap condition, 15.82% overlap condition) were lower than previously reported rates. This mild discrepancy could be attributable to multiple factors, including differences in premorbid cognitive abilities among samples. Our study is comprised of young, largely healthy, undergraduate students with above average estimated intelligence, which has been associated with lower anti-saccade error rate (65). Further, the elevated error rates reported in previous studies may be attributable to increased severity of brain injury in previously reported samples relative to the current sample. Specifically, in the Heitger et al. study (2004), 83% of participants had loss of consciousness and 100% of participants experienced post-traumatic amnesia associated with the head injury, whereas no participants in our sample experienced either of these conditions (23,32). It is also possible the average error rate among CON is lower in our sample versus others because the average time since injury at the time of assessment was 6.87 days in the current study but approximately 4.80 days in the Heitger and Webb studies (23,32). Longitudinal studies of eye movement testing following concussion show a positive relationship between time since injury and improvement on eye movement performance measures such as anti-saccade latency and anti-saccade error rate, which appear to normalize for the majority of participants post-injury by two to three weeks post-injury (38,66).
In contrast to our hypothesis, CON did not exhibit significantly longer latencies (i.e., reaction times) on correctly performed anti-saccade trials relative to HC, although results trended in this direction; CON latency was longer compared to HC to a small degree, (CON averaged 9 and 10 ms longer than HC on no gap and overlap conditions, respectively). Prior studies demonstrated longer latencies among CON relative to HC, ranging from 27 ms (no gap condition) (32), to 37–38 ms (gap condition) (37,59), and to 93 ms (overlap condition) (66). The decreased magnitude of group differences on anti-saccade latency in the current study versus previous post-concussion studies could be attributable to the discrepancy in average time since injury in this study (M = 6.87 days) versus previous studies (approximately 4.80 days) (23,32,37,59).
Consistent with the majority of prior studies (23,24,32), there were no group differences between CON and HC on eye movement performance on the reflexive, stimulus driven prosaccade task (i.e., look towards the new target when it appears). As Webb and colleagues (2018) suggested, the prosaccade task may not yield group differences due to the increased recruitment of more primitive, subcortical structures in this task, which are not typically implicated in concussive injuries (16,23). Furthermore, the prosaccade task may be too simple to recruit adequate neuronal effort to detect subtle effects from concussive injury (66), particularly among this highly educated sample.
In a prior study, we demonstrated that peak symptom severity is not a robust predictor of duration of symptoms following concussion among collegiate female athletes, although it may be a suitable predictor for duration of symptoms among male athletes (29). Similarly, in this study of female athletes, peak symptom severity was not associated with duration of symptoms or time to clearance for full return to play. However, several aspects of eye movement performance in this cohort were predictive of clinical outcomes, including trending and significant effects of prosaccade accuracy, duration, peak velocity, and anti-saccade error rate. Longer time to symptom remission was associated with trending decreased accuracy on the pro-saccade task, significantly longer saccade duration, and trending slower peak velocity. Additionally, higher symptom composite scores had a trending association with longer prosaccade saccade duration and significantly elevated anti-saccade error rate. If symptom severity at time of study and time to symptom remission are thought to genuinely reflect the severity of the underlying brain injury, then prosaccade accuracy, duration, and peak velocity, and anti-saccade error rate may reflect the severity of the underlying injury on distributed brain regions underlying oculomotor control. Alterations in accuracy, duration, and peak velocity may reflect alterations in functioning of the superior colliculus, cerebellum, basal ganglia, or the white matter connections among these regions and connections with cortical regions (16,67,68). Recent evidence suggests mild TBI sustained by athletes may significantly affect cerebellar white matter pathways in the acute period, which would have implications for spatial accuracy of saccades and, to a lesser extent, velocity and duration of saccades (69).
There are multiple limitations to this study. First, the small sample size in this pilot study may result in a Type II error, such that certain eye movement metrics may be able to reliably distinguish female collegiate athletes with recent concussion versus controls, but the analyses are under-powered to detect those effects. Second, the sample size limits our ability to conduct analyses that would clarify whether eye movement testing aids in diagnostic clarity of concussion by reliably distinguishing those with recent concussion from controls. Third, given the estimated intellectual functioning in this sample, the results may not generalize to other populations, although one may expect more pronounced effects on eye movement testing among those with relatively lower intellectual functioning compared to the current sample. Logical next steps in this research domain include a larger sample size, including male and female athletes to directly examine the role of sex, longitudinal assessments to track change over time, and use of neuroimaging to assess the associations among neural structure, connectivity, and functioning and eye movement performance in the course of recovery following concussion.
In sum, the results of this study suggest the following: a) anti-saccade error rate, which implicates frontal cortical structures such as the dorsolateral prefrontal cortex, may be useful in distinguishing individuals with concussion from those without among female club athletes and may reflect symptom severity at the time of testing and b) subcortical aspects of saccades, such as prosaccade accuracy, duration, and velocity, may reflect underlying severity of the brain injury to the extent that time between injury and return to symptom baseline reflects injury severity.
Supplementary Material
Acknowledgments
Declaration of Interest Statement
This work was funded by the Eleanor Wood-Prince Grant Initiative: A Project of the Woman’s Board of Northwestern Memorial Hospital, the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number F31NS106840, and data collection via REDCap was supported in part by a Clinical and Translational Science Award (CTSA) grant from the National Institutes of Health (NIH) under Award Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support for Drs. Hans Breiter and James Reilly was also supplied by the Northwestern University Warren Wright Adolescent Center.
References
- 1.Zuckerman SL, Kerr ZY, Yengo-Kahn A, Wasserman E, Covassin T, Solomon GS. Epidemiology of Sports-Related Concussion in NCAA Athletes From 2009–2010 to 2013–2014: Incidence, Recurrence, and Mechanisms. Am J Sports Med 2015;43:2654–2662. [DOI] [PubMed] [Google Scholar]
- 2.McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, Cantu RC, Cassidy D, Echemendia RJ, Castellani RJ, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med 2017; 51(11):838–847. [DOI] [PubMed] [Google Scholar]
- 3.National Collegiate Athletic Association (NCAA). Sport Sponsorship, Participation and Demographics Search [2012–2013]. [accessed 2017 Nov 1]. http://web1.ncaa.org/rgdSearch/exec/main.
- 4.Pennington B Rise of College Club Teams Creates a Whole New Level of Success. New York Times. 2008. December 1 [accessed 2017 May 29]. https://www.nytimes.com/2008/12/02/sports/02club.html.
- 5.Kerr ZY, Roos KG, Djoko A, Dalton SL, Broglio SP, Marshall SW, Dompier TP. Epidemiologic Measures for Quantifying the Incidence of Concussion in National Collegiate Athletic Association Sports. J Athl Train. 2017;52:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putukian M, Echemendia R, Dettwiler-Danspeckgruber A, Duliba T, Bruce J, Furtado J, Murugavel M. Prospective Clinical Assessment Using Sideline Concussion Assessment Tool-2 Testing in the Evaluation of Sport-Related Concussion in College Athletes. Clin J Sport Med 2015;25:36–42. [DOI] [PubMed] [Google Scholar]
- 7.Marinides Z, Galetta KM, Andrews CN, Wilson JA, Herman DC, Robinson CD, Smith MS, Bentley BC, Galetta SL, Balcer LJ, et al. Vision testing is additive to the sideline assessment of sports-related concussion. Neurol Clin Pract. 2015;5:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCrea M, Guskiewicz K, Randolph C, Barr WB, Hammeke TA, Marshall SW, Powell MR, Ahn KW, Wang Y, Kelly JP. Incidence, Clinical Course, and Predictors of Prolonged Recovery Time Following Sport-Related Concussion in High School and College Athletes. J Int Neuropsychol Soc. 2013;19:22–33. [DOI] [PubMed] [Google Scholar]
- 9.Master CL, Scheiman M, Gallaway M, Goodman A, Robinson RL, Master SR, Grady MF. Vision Diagnoses Are Common After Concussion in Adolescents. Clin Pediatr (Phila). 2016;55:260–267. [DOI] [PubMed] [Google Scholar]
- 10.Howell DR, OʼBrien MJ, Raghuram A, Shah AS, Meehan WP. Near Point of Convergence and Gait Deficits in Adolescents After Sport-Related Concussion. Clin J Sport Med Off J Can Acad Sport Med. 2018;28:262–267. [DOI] [PubMed] [Google Scholar]
- 11.Capó-Aponte JE, Urosevich TG, Temme LA, Tarbett AK, Sanghera NK. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil Med. 2012;177:804–813. [DOI] [PubMed] [Google Scholar]
- 12.Mani R, Asper L, Khuu SK. Deficits in saccades and smooth-pursuit eye movements in adults with traumatic brain injury: a systematic review and meta-analysis. Brain Inj. 2018;32:1315–1336. [DOI] [PubMed] [Google Scholar]
- 13.McKee AC, Alosco ML. Assessing Subconcussive Head Impacts in Athletes Playing Contact Sports—The Eyes Have It. JAMA Ophthalmol. 2019;137:270–271. [DOI] [PubMed] [Google Scholar]
- 14.Snegireva N, Derman W, Patricios J, Welman KE. Eye tracking technology in sports-related concussion: a systematic review and meta-analysis. Physiol Meas. 2018; 39:12TR01. [DOI] [PubMed] [Google Scholar]
- 15.Hunt AW, Mah K, Reed N, Engel L, Keightley M. Oculomotor-Based Vision Assessment in Mild Traumatic Brain Injury: A Systematic Review. J Head Trauma Rehabil. 2016;31:252–261. [DOI] [PubMed] [Google Scholar]
- 16.Munoz D, Armstrong I, Coe B. Using eye movements to probe development and dysfunction In: Van Gompel R, Fischer M, Murray W, Hill R, editors. Eye Movements. Oxford: Elsevier; 2007. p. 99–124. [Google Scholar]
- 17.Klein C, Fischer B. Instrumental and test–retest reliability of saccadic measures. Biol Psychol. 2005;68:201–213. [DOI] [PubMed] [Google Scholar]
- 18.Samadani U, Li M, Qian M, Laska E, Ritlop R, Kolecki R, Reyes M, Altomare L, Sone JY, Adem A, et al. Sensitivity and specificity of an eye movement tracking-based biomarker for concussion. Concussion. 2015;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. [DOI] [PubMed] [Google Scholar]
- 20.Curtis CE, D’Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003;15:409–418. [DOI] [PubMed] [Google Scholar]
- 21.McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: Evidence from studies of humans. Brain Cogn. 2008;68:255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heitger MH, Anderson TJ, Jones RD. Saccade sequences as markers for cerebral dysfunction following mild closed head injury. Prog Brain Res. 2002;140:433–448. [DOI] [PubMed] [Google Scholar]
- 23.Webb B, Humphreys D, Heath M. Oculomotor Executive Dysfunction during the Early and Later Stages of Sport-Related Concussion Recovery. J Neurotrauma. 2018;35:1874–1881. [DOI] [PubMed] [Google Scholar]
- 24.Johnson B, Zhang K, Hallett M, Slobounov S. Functional neuroimaging of acute oculomotor deficits in concussed athletes. Brain Imaging Behav. 2014;1–10. [DOI] [PubMed] [Google Scholar]
- 25.National College Athletic Association (NCAA). NCAA Sports Sponsorship and Participation Rates Database. 2019. [Accessed 2020 Jun 20]. http://www.ncaa.org/about/resources/research/ncaa-sports-sponsorship-and-participation-rates-database.
- 26.Davis-Hayes C, Gossett JD, Levine WN, Shams T, Harada J, Mitnick J, Noble J. Sex-specific Outcomes and Predictors of Concussion Recovery. J Am Acad Orthop Surg 2017;25:818–828. [DOI] [PubMed] [Google Scholar]
- 27.Covassin T, Savage JL, Bretzin AC, Fox ME. Sex differences in sport-related concussion long-term outcomes. Int J Psychophysiol. 2018;132:9–13. [DOI] [PubMed] [Google Scholar]
- 28.Breck J, Bohr A, Poddar S, McQueen MB, Casault T. Characteristics and Incidence of Concussion Among a US Collegiate Undergraduate Population. JAMA Netw Open. 2019;2:e1917626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallagher VT, Kramer N, Abbott K, Alexander J, Breiter H, Herrold A, Lindley T, Mjaanes J, Reilly J.The effects of sex differences and hormonal contraception on outcomes following collegiate sports-related concussion. J Neurotrauma; 2018;35:1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sufrinko AM, Mucha A, Covassin T, Marchetti G, Elbin RJ, Collins MW, Kontos AP. Sex Differences in Vestibular/Ocular and Neurocognitive Outcomes After Sport-Related Concussion. Clin J Sport Med. 2017;27:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedict PA, Baner NV, Harrold GK, Moehringer N, Hasanaj L, Serrano LP, Sproul M, Pagnotta G, Cardone DA, Flanagan SR, et al. Gender and age predict outcomes of cognitive, balance and vision testing in a multidisciplinary concussion center. J Neurol Sci. 2015;353:111–115. [DOI] [PubMed] [Google Scholar]
- 32.Heitger MH, Anderson TJ, Jones RD, Dalrymple-Alford JC, Frampton CM, Ardagh MW. Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain. 2004. March 1;127(3):575–90. [DOI] [PubMed] [Google Scholar]
- 33.Reilly JL, Harris MS, Khine TT, Keshavan MS, Sweeney JA. Reduced Attentional Engagement Contributes to Deficits in Prefrontal Inhibitory Control in Schizophrenia. Biol Psychiatry. 2008;63:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reilly JL, Frankovich K, Hill S, Gershon ES, Keefe RS, Keshavan MS, Pearlson GD, Tamminga CA, Sweeney JA. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr Bull. 2014;40:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lunn J, Donovan T, Litchfield D, Lewis C, Davies R, Crawford T. Saccadic Eye Movement Abnormalities in Children with Epilepsy. PLOS ONE 2016;11:e0160508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCrory P, Meeuwisse WH, Aubry M, Cantu RC, Dvorak J, Echemendia RJ, Engebretsen L, Johnston KM, Kutcher JS, Raftery M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013;47:250–258. [DOI] [PubMed] [Google Scholar]
- 37.Ting WK, Schweizer TA, Topolovec-Vranic J, Cusimano MD. Antisaccadic Eye Movements Are Correlated with Corpus Callosum White Matter Mean Diffusivity, Stroop Performance, and Symptom Burden in Mild Traumatic Brain Injury and Concussion . Front Neurol. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffer ME, Balaban C, Szczupak M, Buskirk J, Snapp H, Crawford J, Wise S, Murphy S, Marshall K, Pelusso C, et al. The use of oculomotor, vestibular, and reaction time tests to assess mild traumatic brain injury (mTBI) over time. Laryngoscope Investig Otolaryngol. 2017;2:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balaban C, Hoffer ME, Szczupak M, Snapp H, Crawford J, Murphy S, Marshall K, Pelusso C, Knowles S, Kiderman A. Oculomotor, Vestibular, and Reaction Time Tests in Mild Traumatic Brain Injury . PLOS ONE. 2016;11:e0162168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.SR Research. EyeLink 1000 User Manual, Version 1.5.2. Mississauga, Ontario, Canada. [Google Scholar]
- 41.Cui H, Liu XH, Wang KY, Zhu CY, Wang C, Xie XH. Association of Saccade Duration and Saccade Acceleration/Deceleration Asymmetry during Visually Guided Saccade in Schizophrenia Patients. PLOS ONE. 2014;9:e97308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pratt J, Bekkering H, Abrams RA, Adam J. The Gap effect for spatially oriented responses. Acta Psychol (Amst) 1999;102:1–12. [DOI] [PubMed] [Google Scholar]
- 43.Drew AS, Langan J, Halterman C, Osternig LR, Chou LS, van Donkelaar P. Attentional disengagement dysfunction following mTBI assessed with the gap saccade task. Neurosci Lett. 2007;417:61–65. [DOI] [PubMed] [Google Scholar]
- 44.Kalesnykas RP, Hallett PE. The differentiation of visually guided and anticipatory saccades in gap and overlap paradigms. Exp Brain Res. 1987;68:115–121. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen ER, Grow J, Duncan S, Neighbors C, Larimer ME. Concurrent Validity of an Online Version of the Timeline Followback Assessment. Psychol Addict Behav. 2012;26:672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28:154–162. [DOI] [PubMed] [Google Scholar]
- 47.Sobell LC, Agrawal S, Sobell MB, Leo GI, Young LJ, Cunningham JA, Simco ER. Comparison of a quick drinking screen with the timeline followback for individuals with alcohol problems. J Stud Alcohol. 2003;64:858–861. [DOI] [PubMed] [Google Scholar]
- 48.Hollingshead AB. Four Factor Index of Social Status 1975. Available from: http://ubir.buffalo.edu/xmlui/handle/10477/1879.
- 49.Lovell MR, Iverson GL, Collins MW, Podell K, Johnston KM, Pardini D, Pardini J, Norwig J, Maroon JC. Measurement of Symptoms Following Sports-Related Concussion: Reliability and Normative Data for the Post-Concussion Scale. Appl Neuropsychol 2006;13:166–174. [DOI] [PubMed] [Google Scholar]
- 50.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J Pers Assess. 1996;67:588–597. [DOI] [PubMed] [Google Scholar]
- 51.Sprinkle SD, Lurie D, Insko SL, Atkinson G, Jones GL, Logan AR, Bissada NN. Criterion Validity, Severity Cut Scores, and Test-Retest Reliability of the Beck Depression Inventory-II in a University Counseling Center Sample. J Couns Psychol 2002;49:381–385. [Google Scholar]
- 52.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 53.Lee E-H. Review of the Psychometric Evidence of the Perceived Stress Scale. Asian Nurs Res. 2012;6:121–127. [DOI] [PubMed] [Google Scholar]
- 54.Song MK, Lin FC, Ward SE, Fine JP. Composite Variables: When and How . Nurs Res. 2013;62:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wechsler D Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- 56.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary, 3rd ed. New York, NY, US: Oxford University Press; 2006. [Google Scholar]
- 57.IBM SPSS Statistics of Windows. Armonk, NY: IBM Corp.; 2017. [Google Scholar]
- 58.Abrahams S, Mc Fie S, Patricios J, Posthumus M, September AV. Risk factors for sports concussion: an evidence-based systematic review. Br J Sports Med. 2014;48:91–97. [DOI] [PubMed] [Google Scholar]
- 59.Landry AP, Ting WK, Zador Z, Sadeghian A, Cusimano MD. Using artificial neural networks to identify patients with concussion and postconcussion syndrome based on antisaccades. J Neurosurg. 2018;1:1–8. [DOI] [PubMed] [Google Scholar]
- 60.Dettwiler A, Murugavel M, Putukian M, Cubon V, Furtado J, Osherson D. Persistent Differences in Patterns of Brain Activation after Sports-Related Concussion: A Longitudinal Functional Magnetic Resonance Imaging Study . J Neurotrauma. 2013;31:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karr J, Areshenkoff C, Garcia-Barrera M. The Neuropsychological Outcomes of Concussion: A Systematic Review of Meta-Analyses on the Cognitive Sequelae of Mild Traumatic Brain Injury . Neuropsychology. 2014;28:321–336. [DOI] [PubMed] [Google Scholar]
- 62.Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, Gold T, Shifteh K, Ardekani BA, Branch CA. Diffusion-Tensor Imaging Implicates Prefrontal Axonal Injury in Executive Function Impairment Following Very Mild Traumatic Brain Injury . Radiology. 2009;252:816–824. [DOI] [PubMed] [Google Scholar]
- 63.McDonald BC, Saykin AJ, McAllister TW . Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging Behav 2012;6:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology. 2006;43:302–313. [DOI] [PubMed] [Google Scholar]
- 65.Klein C, Rauh R, Biscaldi M. Cognitive correlates of anti-saccade task performance. Exp Brain Res. 2010;203:759–764. [DOI] [PubMed] [Google Scholar]
- 66.Webb B, Humphreys D, Heath M. Oculomotor Executive Dysfunction during the Early and Later Stages of Sport-Related Concussion Recovery. J Neurotrauma 2018;35:1874–1881. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto M, Ohtsuka K. Transcranial magnetic stimulation over the posterior cerebellum during visually guided saccades in man. Brain. 1995;118:1185–1193. [DOI] [PubMed] [Google Scholar]
- 68.Smalianchuk I, Jagadisan UK, Gandhi NJ. Instantaneous Midbrain Control of Saccade Velocity. J Neurosci. 2018;38:10156–10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mallott JM, Palacios EM, Maruta J, Ghajar J, Mukherjee P. Disrupted white matter microstructure of the cerebellar peduncles in scholastic athletes after concussion. Front Neurol 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.