Abstract
Objective
Tobacco smoke exposure has negative impacts on the lung health of children with cystic fibrosis (CF), yet evidence-based strategies for smoking cessation have not been tested with or tailored to CF caregivers. This qualitative study identified barriers and facilitators of smoking cessation in this population and outlined potential interventional approaches.
Methods
We conducted semi-structured interviews with CF familial caregivers who were current or former smokers, and with members of the CF care team. We asked about experiences, practices, and prerequisites for a successful program. Interviews were recorded, transcribed verbatim, and coded by two investigators. Analysis used a thematic approach guided by the PRECEDE model, which identifies predisposing (intrapersonal), reinforcing (interpersonal), and enabling (structural) factors relevant to health behaviors and programs.
Results
Seventeen interviews were conducted—eight with familial caregivers and nine with CF team members. Whereas caregivers provided greater insight into internal difficulties and motivators to quit smoking, clinicians offered more extensive input on barriers and solutions related to the clinical environment. Based on study recommendations, a successful tobacco cessation program should include (a) family education about the harms of smoke exposure for children with CF; (b) screening for exposure, ideally with biochemical verification; (c) access to trained tobacco counselors; (d) affordable pharmacotherapy; and (e) outpatient follow-up of those undergoing tobacco treatment.
Conclusion
This qualitative study revealed intrapersonal, interpersonal, and structural barriers to eliminating tobacco smoke exposure in children with CF, outlined opportunities to address these barriers, and made recommendations for a comprehensive tobacco cessation strategy.
Keywords: cystic fibrosis, qualitative methods, second-hand smoke, smoke exposure, smoking cessation, tobacco
1 |. BACKGROUND
Cystic fibrosis (CF) is the most common life-shortening autosomal recessive genetic disorder among Caucasians and the second most common overall, affecting 1 in 3400 live births in the United States.1 Median age at death is 30.7 years, with an annual mortality rate of 1.3 deaths per 100 patients.2 Fifteen percent of deaths occur in children less than 20 years old.3 Death is typically from respiratory failure. The great variability in outcomes and survival, even among people with the same genotype, points to the importance of non-genetic contributors such as tobacco smoke exposure.4–8
The deleterious effect of tobacco smoke for lung health in pediatric CF is well-described.7,9–11 Lung damage attributable to smoke exposure begins early. By age 6, smoke-exposed children have 4.7% lower lung function than unexposed children, and this deficit is sustained through age 18 even after adjusting for covariates.12 Similar decreases of lung function attributable to smoke exposure have been reported by other studies.10,13 Despite these adverse consequences, approximately one-third of US children with CF are smoke-exposed, per caregiver self-report.12,13 Exposure is higher in specific geographic locations and socioeconomic strata. For instance, at our pediatric CF Center at the University of Alabama at Birmingham/Children’s of Alabama (UAB/COA), 44% of patients aged ≤6 years are smoke-exposed based on parent/caregiver self-report. The risk of exposure is two-fold higher in families with annual household income less than $50 000 and three-fold higher for children whose parents do not have college education.14 Finally, it is known that self-reports underestimate actual exposure rates. For example, 63% of infants and children age less than 10 years in two CF Centers were smoke-exposed based on elevated hair nicotine concentrations.15 These data indicate that addressing smoke exposure in pediatric CF merits prioritization, and highlight the need to revisit current practices aimed at eliminating exposure in this population.
Importantly, tobacco smoke exposure includes both second-hand exposure to smoke from burning tobacco products or exhaled by a smoker16 and third-hand exposure to smoke residue through skin contact with polluted objects and surfaces, ingestion, and inhalation of suspended house dust.15 The distinction between the two types of exposure is important because it has implications for interventions: whereas second-hand exposure can be minimized with indoor smoking bans, third-hand exposure can only be prevented with smoking cessation. Indoor smoking bans not only do not eliminate third-hand exposure, they may unintentionally support the belief that smoking outdoors is sufficient to protect children with CF from the harms of tobacco smoke. For example, in the CF Foundation Patient Registry, 15% of caregivers who responded affirmatively to the question, “Does anyone in the patient’s household smoke cigarettes?,” also responded that their child is “Never” exposed to smoke.12 However, children in homes with complete indoor smoking bans still have five to seven times more nicotine exposure than children in homes without any smokers,17 with particularly high nicotine levels among toddlers due to hand-to-mouth behaviors.18–22 Thus, eliminating indoor smoking is not enough to protect children from nicotine exposure, especially in homes with high occupant smoking levels.20,21,23
Clinical practice guidelines from the CF Foundation for infants24 and preschoolers25 with CF focus primarily on preventing secondhand exposure and recommend “tobacco smoke exposure avoidance education.” However, standardized educational content is not provided. In the early 2000s, a learning collaborative of seven CF Centers explored quality improvement approaches to smoke exposure because “interventions of proven effectiveness to meet those goals are currently used in an inconsistent manner.”26 Nearly two decades later, interventional studies of smoking cessation targeting CF parents/caregivers still have not been conducted. Given the lack of tobacco cessation strategies tailored to this population and the paucity of best practices to eliminate smoke exposure in pediatric CF, we conducted a qualitative study to identify barriers to and opportunities for such programs.
2 |. METHODS
2.1 |. Theoretical framework
Barriers and facilitators to smoking cessation, particularly approaches that target parents and caregivers of children with CF, may exist on multiple levels.27 Our interview questions and data analysis were therefore guided by the PRECEDE model,28 which identifies predisposing (intrapersonal), reinforcing (interpersonal), and enabling (structural) factors relevant to health behaviors and programs. Predisposing factors involve the knowledge, beliefs, and values affecting a health behavior. Reinforcing factors include the influence of family, friends, and others. Enabling factors include the social, economic, organizational, and policy factors influencing a health behavior. The PRECEDE model has been used successfully in interventions for human papillomavirus vaccine uptake,29 asthma self-management,30 and diabetes self-care.31
2.2 |. Study design and sampling
We conducted semi-structured interviews with familial caregivers (parents and grandparents of children with CF) who are current or former smokers, and with members of the multidisciplinary CF healthcare team. We used purposive sampling, with planned enrollment of 12 caregivers and 8 clinicians. Familial caregivers were recruited at the UAB/COA Pediatric CF Center. Potential participants were identified through the CF Patient Registry based on the patient’s smoke exposure status reported annually by caregivers; exposure data were verified by the CF Nurse Coordinator. Eligible caregivers (current or former smokers) were approached during a routine CF clinic visit. Their current smoking status was verified with the question, “Are you a current or a former smoker?” Those who agreed to participate were consented at that time. Interviews were conducted by telephone after the clinic visit, on a day and at time that participants indicated would be convenient. Clinicians were recruited across three pediatric CF centers (South, Southwest, and Mid-Atlantic). They were offered participation via email, and interviews were conducted face-to-face. All participants provided informed consent. The study was approved bythe Institutional Review Board of the University of Alabama at Birmingham (Protocol IRB-300001586).
To explore caregivers’ and clinicians’ perspectives regarding approaches to eliminate tobacco exposure in children with CF, we asked about their experiences, current practices, and the prerequisites for a successful smoking cessation program. A semi-structured guide (Table 1) was developed to prompt participants about challenges to quitting, factors that facilitate cessation, and strategies to help CF families quit. Interviews were recorded, transcribed verbatim, and deidentified for analysis. Data were collected from August to November 2018 and analyzed in January to April 2019.
TABLE 1.
Semi-structured interview guide
| Questions | Parents/caregivers | Clinicians |
|---|---|---|
| Introductory | Are you a current or a former smoker? How long have you smoked? Have you made attempts to quit? If yes, did you make attempts to quit after you had a child/dependent with CF? | What is your role on the CF care team? How long have you provided care for children with CF? |
| Overarching | If we designed a program to help parents and caregivers of children with CF quit smoking, what are some things that it should include? | What are the barriers to smoking cessation in CF, and what are the prerequisites for a successful smoking cessation program in your CF Center? |
| Probing | 1. Do you remember when and by whom you were first told that smoke exposure is bad for your child with CF? Can you tell me more about it? 2. Please tell me about the information you have received from the CF team about smoking? Was it helpful? Was it understandable? Was it received early or frequent enough? 3. Can you tell me how tobacco smoke affects the health of your child with CF? 4. Let’s talk about your experience with quitting. Do you feel it is/it was hard to quit? Why or why not? 5. What are some things that make it/made it harder for you to quit? What helped/would help you quit? 6. If the CF Center offered help to quit, what would be most helpful for you? 7. What else would motivate you to quit? |
1. How harmful do you think it is for a child with CF to be exposed to tobacco smoke? 2. In your view, how important is smoking cessation compared to other components of CF care (eg, infection control, airway clearance, nutrition, treatment adherence)? 3. Do you feel that you have sufficient knowledge about smoking cessation? 4. How do you address smoking in your clinical practice? 5. Do you think smoke exposure is addressed adequately in CF care? Why or why not? 6. What are some things that make it hard to address smoking cessation in your CF Center? 7. What can you tell me about clinical care guidelines and protocols for smoke exposure in CF? What smoking cessation resources are available in your CF Center? 8. Do you think there is a need for smoking cessation intervention/program in your CF Center? 9. What needs to be in place for a successful smoking cessation program in your CF Center. 10. What do you believe are some key elements of a successful smoking cessation program? |
Abbreviation: CF, cystic fibrosis
2.3 |. Data analysis
Data analysis was guided by the PRECEDE model, which identifies predisposing (intrapersonal), reinforcing (interpersonal), and enabling (structural) factors relevant to health behaviors and programs. We utilized a thematic analysis,32 an interpretative research approach that uses a purely qualitative account of data rather than frequency of codes for theme development.33 A constant comparative method34 was employed to generate categories, patterns, and themes. Transcriptions were coded independently by two investigators (CO and SJN), then codes were discussed by the team (GRO, SBR, CO, and SJN), and the final coding scheme was decided jointly. Discussions contributed to the iterative data analysis and acted as respondent validation,35 helping achieve trustworthiness through investigator triangulation.36 Analyses were conducted with NVivo 11.
3 |. RESULTS
Twenty participants (12 caregivers and 8 clinicians) were consented for the study. Of them, four caregivers could not be interviewed (two withdrew and two could not be reached for an interview). One additional clinician interview was conducted to ensure we have reached saturation, or the point at which no new themes are observed.37 Thus, 17 interviews were conducted in total: eight with familial caregivers and nine with clinical team members.
Familial caregivers were primarily female (88%) and included parents (50%) and grandparents (50%), 75% of them current smokers. Clinica! team members were 66% temale and included physicians (56%), social workers (22%), nurse practitioners (11%), and respiratory therapists (11%). Interview discussions identified predisposing (intrapersonal), reinforcing (interpersonal), and enabling (structural) barriers and facilitators to eliminating smoke exposure in CF patients and proposed features of a successful smoking cessation program in this population. The themes are presented in Table 2, and representative quotations (Q1-Q64) are provided in Tables S3a–c.
TABLE 2.
Unifying themes, subthemes, and representative codes with quotationsa
| Representative codes |
|||
|---|---|---|---|
| Theme | Subtheme | Caregivers | Clinicians |
| Barriers | Predisposing (Intrapersonal) | • Stress: Q1, Q2 • Addiction: Q3, Q4 • Weight gain: Q5 • Perceived lack of harm to child: Q6 |
• Addiction: Q15 • Stress: Q16 • Not ready to quit: Q17, Q18 • Unwilling to disclose smoking status • Lack of caregiver awareness of harm: Q19 |
| Reinforcing (Interpersonal) | • Lack of understanding and support from family and friends: Q7 • Presence of other smokers • Shaming into quitting: Q8, Q9 |
• Smoking is a social norm: Q20, Q21 • Shaming into quitting: Q22 |
|
| Enabling (Structural) | • Cost of cessation aids: Q10, Q11 • Side effects of cessation aids: Q12, Q13 • No CF-specific information about the harms of smoke exposure: Q14 |
• Not a priority in CF care: Q23, Q24 • Lack of guidance and protocols • Time constrains: Q25 • Lack of provider knowledge/training: Q26 • Inability to prescribe to and treat adults: Q27 • Lack of CF educational materials/tools: Q28, Q29 |
|
| Facilitators | Predisposing (Intrapersonal) | • Desire to quit: Q30 • The health of one’s child: Q31 |
• Alternative ways to cope with stress: Q37 • Quitting for child’s sake: Q38 |
| Reinforcing (Interpersonal) | • Supportive family and friends: Q32 • Child’s awareness that smoking harms: Q33 • Nonjudgmental clinical providers: Q34 |
• Educating the extended family about smoke exposure: Q39 • Nonjudgmental attitude of providers: Q40 |
|
| Enabling (Structural) | • Information about the harms of smoke exposure to CF children: Q35 • Incentives for reaching goals: Q36 |
• Research to develop evidence: Q41 • CF Foundation guidelines for cessation: Q42 • Standardized protocols: Q43 • Educational materials/resources: Q44 • Biochemical verification • Prescription of pharmacotherapy: Q45, Q46 • Incentives for reaching goals: Q47 |
|
| Solutions | Predisposing (Intrapersonal) | • Change routines • Use counseling for motivation and to avoid triggers: Q48 • Treat self for reaching goals: Q49 |
• Offer positive reinforcement: Q52 |
| Reinforcing (Interpersonal) | • Engage the extended family: Q50 | • Provide letter and materials for extended family: Q53 • Local peer support group: Q54 |
|
| Enabling (Structural) | • Provide information and education • Provide access to cessation aids • Provide access to professional counselors: Q51 |
• Develop the evidence base: Q55 • Develop educational resources: Q56-Q59 • Include in center performance review: Q60 • Develop clinical protocols: Q61 • Train staff • Select clinical point person: Q62 • Offer professional counseling: Q63 • Provide tailored follow-up • Routine screening as part of care: Q64 |
|
Abbreviation: CF, cystic fibrosis
Representative quotations provided in Tables S3a (barriers), S3b (facilitators), and S3c (solutions).
3.1 |. Barriers: Caregiver perspective (quotations in Table S3a)
3.1.1 |. Predisposing (intrapersonal) barriers
One of the most frequent barriers to smoking cessation was stress. Caregivers discussed how people smoke because it reduces stress and anxiety (Q1). The stress-relieving function of cigarettes was particularly salient in the context of caring for a child with a serious chronic condition such as CF (Q2). Caregivers discussed the addictive nature of nicotine and compared the difficulties of quitting cigarettes to those of overcoming drug dependence (Q3). The over-whelming cravings and withdrawal symptoms associated with quitting were seen as a great challenge (Q4). Caregivers also discussed concerns about weight gain as a deterrent to quitting (Q5). Some expressed the opinion that their smoking is not harmful for their child or grandchild with CF (Q6).
3.1.2 |. Reinforcing (interpersonal) barriers
The lack of understanding from family and friends, the lack of support when trying to quit, and the presence of other smokers were acknowledged challenges (Q7). CF providers’ attitude of shaming caregivers into quitting was also seen as counterproductive, thus acting as a barrier rather than a facilitator of giving up tobacco (Q8, Q9).
3.1.3 |. Enabling (structural) barriers
Caregivers discussed the prohibitive cost of nicotine-replacement therapy as an impediment to quitting (Q10, Q11). They also expressed a frustration with the side effects of cessation aids. The experiences of discomfort while using various cessation aids were perceived by some as ineffectiveness (Q12, Q13). Some caregivers shared that they have not received enough information about the harm of second- and third-hand smoke exposure on the lungs of a child with CF. They expressed the opinion that the clinical team should do more to help people quit (Q14).
3.2 |. Barriers: Clinical team perspective (quotations in Table S3a)
3.2.1 |. Predisposing (intrapersonal) barriers
The addictive nature of nicotine was discussed not only by caregivers but by the clinical team as well (Q15). Clinicians also recognized that the stress and burden of caring for a child with CF hinders quitting (Q16). Several clinical providers emphasized that some caregivers are simply not ready to quit and pushing them is counterproductive (Q17, Q18). They also thought that caregivers are often not forthcoming about their smoking status, and this unwillingness to disclose smoke exposure hinders addressing the issue. Other clinicians thought that, compared to chronic respiratory diseases such as asthma, there is a general lack of awareness among CF families about the detrimental impact of smoke exposure (Q19).
3.2.2 |. Reinforcing (interpersonal) barriers
The clinical team discussed the “culture of smoking,” or an environment in which smoking is a social norm, and acknowledged it as a huge impediment to quitting (Q20, Q21). Clinicians recognized that conversations about quitting are difficult, and acknowledged that shaming people into quitting is not a successful strategy (Q22).
3.2.3 |. Enabling (structural) barriers
Providers discussed the lack of emphasis on smoking cessation in clinical practice compared to other components of CF care. They suggested that smoke exposure is not a high priority in the CF clinic (Q23, Q24). All providers referred to the lack of concrete guidance regarding smoking cessation with CF families. In their opinion, the absence of uniformity and specific protocols contributed to the issue of smoke exposure not being addressed effectively, if at all. Limited time during clinic visit was often cited as a barrier to addressing smoke exposure and cessation (Q25). Some providers felt that they did not have the necessary knowledge and training to address smoking cessation (Q26). Inability to prescribe medications and treat the adult caregivers of their pediatric CF patients was also acknowledged as a barrier to helping families quit (Q27). The lack of CF-specific educational materials and tools that can aid providers in addressing tobacco smoke exposure and smoking cessation was also seen as a barrier (Q28, Q29).
3.3 |. Barriers: Summary of caregiver and clinician perspectives
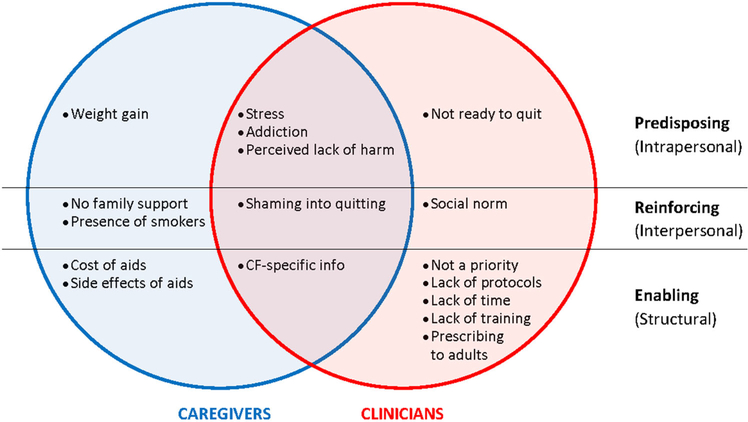
Figure 1 presents a summary of caregiver and clinician perspectives on barriers, showing overlapping and distinct barriers identified by caregivers and healthcare providers.
FIGURE 1.
Summary of barriers
3.4 |. Facilitators: Caregiver perspective (quotations in Table S3b)
3.4.1 |. Predisposing (intrapersonal) facilitators
Caregivers recognized that one of the most important steps to cessation is being internally motivated to quit (Q30). One of the strongest motivators to quit was the health of one’s own child (Q31).
3.4.2 |. Reinforcing (interpersonal) facilitators
Caregivers believed that family can be a positive force in quitting (Q32). Others shared that their child’s awareness that smoking is harmful both to their parent and themselves fuels their resolve to stay quit (Q33). They also expressed that nonjudgmental support from providers would help with quitting (Q34).
3.4.3 |. Enabling (structural) facilitators
Caregivers believed that more information about the effects of smoke exposure on their children with CF would be helpful. They also stressed that such education should cater to the needs of people of varied backgrounds (Q35). Others shared that incentives for reaching goals can encourage quitting (Q36).
3.5 |. Facilitators: Clinical team perspective (quotations in Table S3b)
3.5.1 |. Predisposing (intrapersonal) facilitators
Clinicians suggested that helping families cope with the stress and anxiety of caring for a child with a progressive chronic disease could replace the stress-relieving function of cigarettes and facilitate smoking cessation (Q37). Some providers thought that approaching smoking cessation as something a caregiver should consider for the sake of their child rather than for one’s own health is more productive (Q38).
3.5.2 |. Reinforcing (interpersonal) facilitators
Providers shared that educating the extended family about the harms of smoke exposure would be more effective coming from the CF team rather than the child’s parents (Q39). Providers were aware of their responsibility to help caregivers eliminate smoke exposure, and acknowledged that being persistent but compassionate and less disapproving actually helps (Q40).
3.5.3 |. Enabling (structural) facilitators
Clinicians emphasized the need for more research to provide evidence for the effect of smoke exposure in pediatric CF (Q41). They expressed the opinion that recommendations and guidance from the Cystic Fibrosis Foundation about smoking cessation strategies would help them address the issue more effectively (Q42). Clinicians also believed that having a systematic approach and establishing clinical protocols for addressing smoke exposure would greatly facilitate their work (Q43). CF-specific educational tools were widely believed to be necessary and helpful for addressing smoke exposure with CF families (Q44). Opportunities for biochemical verification of child’s smoke exposure were also discussed, as was the potential of such screening to raise awareness of the harms of smoke exposure and help families limit their child’s exposure. Providers believed that the ability to prescribe pharmacotherapy would be beneficial for CF families because of the frequent and regular contact they have with their child’s CF care team (Q45, Q46). Finally, clinicians suggested that financial incentives for reaching goals may be helpful, but were concerned about potential misuse (Q47).
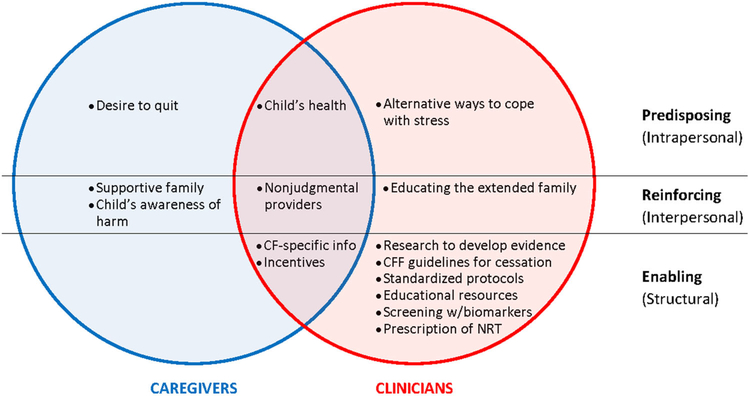
3.6 |. Facilitators: Summary of caregiver and clinician perspectives
Figure 2 presents a summary of caregiver and clinician perspectives on facilitators, showing overlapping and distinct facilitating factors identified by caregivers and healthcare providers.
FIGURE 2.
Summary of facilitators
3.7 |. Solutions: Caregiver perspective (quotations in Table S3c)
3.7.1 |. Predisposing (intrapersonal) strategies
Caregivers spoke of the importance of changing one’s routines. They discussed how a trained counselor can help those who are not motivated to quit to start thinking about cessation. Others shared how one-on-one counseling has been very effective in giving them tools to reduce cravings and avoid triggers (Q48). Caregivers considered positive reinforcement, or treating oneself for meeting small goals, as helpful (Q49). However, they were concerned that financial incentives to motivate people to quit may be abused by some.
3.7.2 |. Reinforcing (interpersonal) strategies
Caregivers recommended engaging the extended family in interventions to eliminate smoke exposure in patients with CF (Q50).
3.7.3 |. Enabling (structural) strategies
Caregivers recommended more information and use of CF-specific educational resources about the harm of smoke exposure. They suggested that resources should be offered in various formats that appeal to people of different educational backgrounds. Caregivers also recommended that they should have the opportunity to access various cessation aids, as not everyone finds success with the same aids. Finally, caregivers recommended that professional counseling should be offered to all (Q51).
3.8 |. Solutions: Clinical team perspective (quotations in Table S3c)
3.8.1 |. Predisposing (intrapersonal) strategies
Clinicians recommended incorporating sustained positive reinforcement during clinic visits for caregivers trying to quit smoking (Q52).
3.8.2 |. Reinforcing (interpersonal) strategies
Just like caregivers, clinicians recommended involving the extended family both in education about the importance of limiting smoke exposure, and in smoking cessation interventions. They recommended providing parents with a formal letter from the CF care team and educational materials that parents can give to their extended family (Q53). Providers suggested that a local peer support group of successful quitters could be made available to CF families (Q54).
3.8.3 |. Enabling (structural) strategies
Clinicians emphasized developing evidence for the harms of smoke exposure in children with CF so it can be shared with CF families (Q55). Development of educational tools for CF caregivers and their families was a central theme in clinicians’ interviews. They talked in detail about educational resources and made specific recommendations for the content, type, and format of such resources, from wall posters to handouts to videos running on clinic and hospital closed-circuit TVs (Q56, Q57). Providers stressed that education should be standardized but also provided in a variety of formats to meet the needs and preferences of all caregivers and families (Q58). It was recommended that education about smoke exposure should begin early, at CF diagnosis. Providers also acknowledged the need to tailor existing tobacco cessation approaches to CF and develop best practices for addressing smoking cessation with CF caregivers (Q59). Others suggested that smoking cessation should be part of the performance review of CF Centers, with financial incentives and accreditation implications by the CF Foundation (Q60). Providers recommended development and implementation of clinical protocols that addresses in a systematic way all aspects of smoke exposure and smoking cessation, including screening, education, referral, and follow-up (Q61). They recommended training for clinical staff in evidence-based approaches to tobacco treatment. They also proposed that there should be a point person to coordinate smoke exposure and cessation efforts, and that would help alleviate role strain in clinic (Q62). They agreed that professional counseling is most effective and recommended using a trained tobacco treatment specialist who can work with the families of children exposed to smoke (Q63). Follow-up with families, either by telephone or telemedicine tools, and referral to local resources was central to clinicians’ recommendations. Clinicians also pointed out that follow-up should be tailored to the needs and circumstances of each family. Screening for exposure as part of routine CF care was also recommended by several providers (Q64).
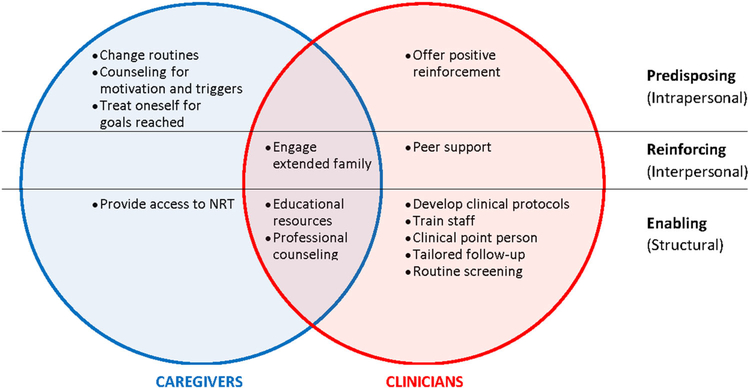
3.9 |. Solutions: Summary of caregiver and clinician perspectives
Figure 3 presents a summary of caregiver and clinician perspectives on solutions, showing overlapping and distinct recommendations by caregivers and healthcare providers.
FIGURE 3.
Summary of solutions
4 |. DISCUSSION
Guided by the PRECEDE model, we conducted semi-structured interviews with CF familial caregivers (parents and grandparents) who are current or former smokers, and with health professionals who provide care to children with CF. The goal was to identify barriers and facilitators to smoking cessation and gather insights about designing a smoking cessation program tailored to this population. Our findings outline both caregiver- and clinician-proposed recommendations for interventions to eliminate tobacco smoke exposure in pediatric CF.
Per CF Foundation recommendations, caregivers of infants and preschoolers with CF should be instructed not to smoke indoors.24,25 While the prevalence of second-hand vs third-hand smoke exposure in children with CF is currently unknown, both caregivers and clinicians focused their discussion on smoking cessation rather than on limiting second-hand exposure from indoor smoking. There is not a safe level of tobacco smoke exposure for children,38 and children with CF are at particular risk. Therefore, further efforts should be focused on exposure elimination through smoking cessation interventions rather than on limiting exposure through indoor smoking bans.
Overall, both caregivers and clinicians addressed predisposing (intrapersonal), reinforcing (interpersonal), and structural (enabling) factors relevant to smoking cessation strategies in this population. One conclusion emerging from the perspectives of both groups is the need to intervene on multiple levels, ensuring access to nicotine-replacement therapy, help from professional tobacco treatment counselors, nonjudgmental follow-up and assistance from the CF care team, and support from family and friends. As related to program development, this indicates a need for comprehensive, multicomponent interventions that offer affordable pharmacotherapy and access to counseling, include the smoker’s extended family, and mobilize a supportive network. Such a multipronged approach is recommended in the existing tobacco literature.39 For example, it is well established that the combination of tobacco cessation counseling and first-line pharmacotherapy is more effective than either alone.40 As well, caregiver smokers are more likely to quit when their children are simultaneously enrolled in a tobacco prevention program.41 To maximize success, interventions may aim to enroll all family members of the smoke-exposed child with CF in a tobacco cessation and prevention program. At a minimum, CF-specific materials that illustrate the dangers of smoke exposure should be made available so that parents can readily and comfortably share them with relatives and friends on behalf of the child’s CF physician.
Another commonality between caregivers and clinicians was the need for CF-specific educational resources in a variety of formats to show how smoke exposure harms the lungs of children with CF. As smoking is a cultural norm in many caregiver environments, both groups voiced a concern over the lack of informational materials that address family, relatives, and friends. While there is a wealth of knowledge regarding the harms of smoke exposure on the developing lungs, there remains a gap in the literature regarding the specific dangers of exposing a child with CF to second- and third-hand smoke. Filling this gap would require research to expand the evidence base for the impact on nicotine on CF lung development and outcomes, and development of standardized, CF-specific educational resources that can aid CF care teams in their efforts to eliminate exposure.
Whereas caregivers provided greater insight into predisposing (intrapersonal) difficulties and motivators to quit smoking, clinicians offered more extensive input on enabling (structural) barriers and solutions related to the clinical environment, practice, and policy. The biggest barrier to program development, as identified by health professionals, was the lack of focus on smoking cessation in CF care. Eight of the nine interviewed health professionals practicing at three different CF Centers indicated that there are no clinical protocols in place for addressing smoking cessation with CF caregivers, including documentation, follow-up, dedicated clinical time, or trained staff. A tobacco-focused quality improvement initiative in seven CF care centers in the early 2000s documented that few providers consider intervening to reduce smoke exposure, and most are either unfamiliar with evidence-based tobacco treatment strategies or ambivalent about the need to develop a therapeutic relationship with smoking caregivers rather than the patient.26 Two decades later, these barriers persist. They are reinforced by lack of training, lack of support from the health systems, lack of funding to compensate for time spent or hire trained tobacco treatment specialists, and lack of consensus on the need to undertake an effort with a perceived low level of success. For a meaningful change across the CF center network, clinical care guidelines, policies, and dedicated resources from the CF Foundation will be necessary.
Health professionals acknowledged the barriers discussed by caregivers (addiction, stress, cravings) and the need for open and respectful communication. However, they also expressed that just talking to caregivers about cessation is not enough. Evidence-based tobacco cessation strategies tailored to this population are necessary. To date, no smoking cessation interventions have been tested in CF caregivers in the United States. This gap can be bridged by adopting successful interventional approaches from similar populations, for example, caregivers of children with asthma or other respiratory illnesses.42,43 Based on such evidence, a comprehensive intervention that includes motivational interviewing, CF-specific informational materials, nicotine-replacement therapy, telephone counseling, referral to the parents’ primary physician, and biomarker feedback may be successful in CF care. Additionally, smoke exposure screening and tobacco cessation protocols (which are not currently part of CF care) may be considered for inclusion as performance indicators for accredited CF care centers.
The American Academy of Pediatrics considers treating tobacco dependence of caregivers an important part of children’s health care and recommends that pediatricians provide such treatment, including prescription of parental tobacco cessation pharmacotherapy while following state regulations and institutional policies for documentation of care provided to caregivers to benefit the health of the child.44 Nevertheless, pediatric pulmonologists participating in the study expressed discomfort treating smoking adult caregivers. Potential solutions to this barrier include further training of pediatric providers, inclusion of trained tobacco treatment specialists in pediatric settings, and enhanced referral and coordination of tobacco treatment for caregivers.
Most parents expect pediatricians to address tobacco dependence and smoke exposure.45 Prior research shows that even brief advice from a physician can increase smoking cessation rates.46 It also corroborates the insight of clinicians in this study that smokers may be more willing to accept tobacco treatment for the sake of their children rather than their own health.47 This approach is particularly successful among caregivers of children with pre-existing conditions versus caregivers of healthy children, especially when interventions are coupled with biochemical feedback.42 Such strategies can be replicated in the context of pediatric CF care. As noted by both caregivers and clinicians, tobacco treatment should be appropriate to the caregiver’s readiness to change to be effective. This would require either training of CF providers or incorporation of tobacco treatment specialists in the multidisciplinary CF care team.
The study has several limitations. Although caregivers included current and former smokers and CF clinical team members represented various disciplines, data may not be applicable to all CF programs or geographic areas. As the interviews were retrospective, it may have been difficult for some to recall their experiences. Subjects were not compensated for participation, which may have biased the enrollment. Additionally, we did not interview CF stakeholders, such as representatives of the CF Foundation, hospital administration, and insurance providers. CF stakeholder studies may be beneficial, particularly for addressing structural barriers. Finally, while we acknowledge that our sample size is small, the sampling decision was based on evidence of data saturation.48,49 In developing evidence-based recommendations regarding sample sizes for interviews, Guest et al50 report that 70% and 92% of all themes are identified in the first 6 and 12 interviews, respectively. Morgan et al51 report that the first five to six interviews produce the majority of new information, and across four datasets, approximately 80% to 92% of all concepts were noted within the first 10 interviews.
This qualitative examination of caregiver and clinician perspectives regarding tobacco smoke exposure in pediatric CF indicates that successful programs to limit exposure will require a multipronged approach to address predisposing, reinforcing, and enabling factors. Both family-centered and health system interventions will be necessary to over-come existing barriers. Based on study recommendations, a successful tobacco cessation program in CF care should include (a) family education about the harms of smoke exposure for children with CF; (b) screening for smoke exposure, ideally with biochemical verification; (c) access to trained tobacco counselors for patients and familial caregivers; (d) free or low-cost pharmacotherapy; and (e) outpatient follow-up of those undergoing tobacco treatment. A rigorous research that generates evidence for the development, implementation, and dissemination of these strategies will be needed, as well as advocacy on policies that encourage smoking cessation.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the NIH (UL1TR001417, P30DK72482, K08HL140190, R03HL148467), the AHRQ (K12HS023009), and the CF Foundation (HARRIS19A0-KB, GUTIER14QIO, CC032-14).
Funding Information
Cystic Fibrosis Foundation, Grant/Award Numbers: CC032-14, GUTIER14QIO, HARRIS19A0-KB; Agency for Healthcare Research and Quality, Grant/Award Number: K12HS023009; National Institutes of Health, Grant/Award Numbers: K08HL140190, R03HL148467, P30DK72482, UL1TR001417
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Cystic Fibrosis Foundation. Patient Registry, 2017 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation; 2018. [Google Scholar]
- 2.Cutting GR. Cystic fibrosis In: Rimoin D, Pyeritz R, Korf B, eds. Emery and Rimoin’s principies and practice of medical genetics. 6th ed. Academic Press; 2013:1–54. [Google Scholar]
- 3.Cystic Fibrosis Foundation. Patient Registry: 2018 Annual Data Report to the Center Directors. Bethesda, MD: Cystic Fibrosis Foundation; 2019. [Google Scholar]
- 4.Balmer DF, Schall JI, Stallings VA. Social disadvantage predicts growth outcomes in preadolescent children with cystic fibrosis. J Cyst Fibros. 2008;7(6):543–550. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor GT, Quinton HB, Kneeland T, et al. Median household income and mortality rate in cystic fibrosis. Pediatrics. 2003;111(4): e333–e339. [DOI] [PubMed] [Google Scholar]
- 6.Schechter MS, Shelton BJ, Margolis PA, FitzSimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–1337. [DOI] [PubMed] [Google Scholar]
- 7.Sanders DB, Emerson J, Ren CL, et al. Early childhood risk factors for decreased FEV1 at age six to seven years in young children with cystic fibrosis. Ann Am Thorac Soc. 2015;12(8):1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR. Quantification of the relative contribution of environmental and genetic factors to variation in cystic fibrosis lung function. J Pediatr. 2010;157(5):802–807. e801–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega-García JA, López-Fernández MT, Llano R, et al. Smoking prevention and cessation programme in cystic fibrosis: integrating an environmental health approach. J Cyst Fibros. 2012;11(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collaco JM, Vanscoy L, Bremer L, et al. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA. 2008;299(4):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell PW 3rd, Parker RA, Roberts BT, Krishnamani MR, Phillips JA 3rd. Association of poor clinical status and heavy exposure to tobacco smoke in patients with cystic fibrosis who are homozygous for the F508 deletion. J Pediatr. 1992;120(2 Pt 1):261–264. [DOI] [PubMed] [Google Scholar]
- 12.Oates GR, Baker E, Rowe SM, et al. Tobacco smoke exposure and socioeconomic factors are independent predictors of pulmonary decline in pediatric cystic fibrosis. J Cyst Fibros. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ong T, Schechter M, Yang J, et al. Socioeconomic status, smoke exposure, and health outcomes in young children with cystic fibrosis. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oates GR, Zhu A, Stepanikova I. Impact of tobacco smoke exposure on pulmonary function in paediatric cystic fibrosis patients. J Cystic Fibrosis. 2018;17(Suppl. 3):9. [Google Scholar]
- 15.Kopp BT, Thompson R, Kim J, et al. Secondhand smoke alters arachidonic acid metabolism and inflammation in infants and children with cystic fibrosis. Thorax. 2019;74(3):237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Public Health Service. Office of the Surgeon General. The Health Consequences of Smoking−-50 Years of Progress: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 17.Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matt GE, Quintana PJ, Zakarian JM, et al. When smokers move out and non-smokers move in: residential thirdhand smoke pollution and exposure. Tob Control. 2011;20(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matt GE, Quintana PJE, Zakarian JM, et al. When smokers quit: exposure to nicotine and carcinogens persists from thirdhand smoke pollution. Tob Control. 2016;26(5):548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Northrup TF, Jacob P 3rd, Benowitz NL, et al. Thirdhand smoke: state of the science and a call for policy expansion. Public Health Rep. 2016; 131(2):233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahabee-Gittens EM, Merianos AL, Hoh E, Quintana PJ, Matt GE. Nicotine on children’s hands: limited protection of smoking bans and initial clinical findings. Tob Use Insights. 2019;12:1179173 1179173 X18823493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahl V, Jacob P 3rd, Havel C, Schick SF, Talbot P. Thirdhand cigarette smoke: factors affecting exposure and remediation. PLoS One. 2014; 9(10):e108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Northrup TF, Matt GE, Hovell MF, Khan AM, Stotts AL. Thirdhand smoke in the homes of medically fragile children: assessing the impact of indoor smoking levels and smoking bans. Nicotine Tob Res. 2016; 18(5):1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cystic Fibrosis Foundation, Borowitz D, Robinson KA, et al. Cystic fibrosis foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(6 Suppl):S73–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahiri T, Hempstead SE, Brady C, et al. Clinical practice guidelines from the Cystic Fibrosis Foundation for preschoolers with cystic fibrosis. Pediatrics. 2016;137(4). [DOI] [PubMed] [Google Scholar]
- 26.Schechter MS, Margolis P. Improving subspecialty healthcare: lessons from cystic fibrosis. J Pediatr. 2005;147(3):295–301. [DOI] [PubMed] [Google Scholar]
- 27.Cox NS, Oliveira CC, Lahham A, Holland AE. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: a systematic review using the Theoretical Domains framework. J Physiother. 2017;63 (2):84–93. [DOI] [PubMed] [Google Scholar]
- 28.Green L, M K. Health program planning: an educational and ecological approach. 4th ed. New York, NY: McGrawhill; 2005. [Google Scholar]
- 29.Dilley SE, Peral S, Straughn JM Jr., Scarinci IC. The challenge of HPV vaccination uptake and opportunities for solutions: lessons learned from Alabama. Prev Med. 2018;113:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey WC, Richards JM Jr., Manzella BA, Windsor RA, Brooks CM, Soong SJ. Promoting self-management in adults with asthma: an overview of the UAB program. Health Educ Q. 1987;14(3):345–355. [DOI] [PubMed] [Google Scholar]
- 31.Dizaji MB, Taghdisi MH, Solhi M, et al. Effects of educational intervention based on PRECEDE model on self care behaviors and control in patients with type 2 diabetes in 2012. J Diabetes Metab Disord. 2014;13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398–405. [DOI] [PubMed] [Google Scholar]
- 33.Vaismoradi M, Snelgrove S. Theme in qualitative content analysis and thematic analysis. Forum: Qual Social Res. 2019;20(3). [Google Scholar]
- 34.Strauss A, Corbin J. Basics of qualitative research: procedures and techniques for developing grounded theory. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- 35.Bloor M Techniques of validation in qualitative research: a critical commentary. Context Method Qualitative Res. 1997:37–50. [Google Scholar]
- 36.Shenton AK. Strategies for ensuring trustworthiness in qualitative research projects. Educ Inf. 2004;22(2):63–75. [Google Scholar]
- 37.Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52(4):1893–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United States Public Health Service. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General; 2006. [Google Scholar]
- 39.Behbod B, Sharma M, Baxi R, Roseby R, Webster P. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2018;1: CD001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiore M Treating Tobacco Use and Dependence: 2008 Update. Tobacco Use and Dependence Guideline Panel. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 41.Caldwell AL, Tingen MS, Nguyen JT, et al. Parental smoking cessation: impacting children’s tobacco smoke exposure in the home. Pediatrics. 2018;141(Suppl 1):S96–S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borrelli B, McQuaid EL, Tooley EM, et al. Motivating parents of kids with asthma to quit smoking: the effect of the teachable moment and increasing intervention intensity using a longitudinal randomized trial design. Addiction. 2016;111(9):1646–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winickoff JP, Hillis VJ, Palfrey JS, Perrin JM, Rigotti NA. A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the Stop Tobacco Outreach program. Pediatrics. 2003;111(1):140–145. [DOI] [PubMed] [Google Scholar]
- 44.Farber HJ, Walley SC, Groner JA, Nelson KE. Section on tobacco control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136(5):1008–1017. [DOI] [PubMed] [Google Scholar]
- 45.Winickoff JP, Tanski SE, McMillen RC, Klein JD, Rigotti NA, Weitzman M. Child health care clinicians’ use of medications to help parents quit smoking: a national parent survey. Pediatrics. 2005; 115(4):1013–1017. [DOI] [PubMed] [Google Scholar]
- 46.Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;5:CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen LJ, Noach MB, Winickoff JP, Hovell MF. Parental smoking cessation to protect young children: a systematic review and meta-analysis. Pediatrics. 2012;129(1):141–152. [DOI] [PubMed] [Google Scholar]
- 48.Tuckett AG. Qualitative research sampling: the very real complexities. Nurse Res. 2004;12(1):47–61. [DOI] [PubMed] [Google Scholar]
- 49.Sandelowski M Real qualitative researchers do not count: the use of numbers in qualitative research. Res Nurs Health. 2001;24(3): 230–240. [DOI] [PubMed] [Google Scholar]
- 50.Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006; 18(1):59–82. [Google Scholar]
- 51.Morgan M, Fischoff B, Bostrom A, Atman C. Risk communication: a mental models approach. New York, NY: Cambridge University Press; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.