Subarachnoid hemorrhage (SAH), intracerebral hemorrhage (ICH), and intraventricular hemorrhage (IVH) constitute the majority of hemorrhagic stroke patients. Development of hydrocephalus (HCP) post-hemorrhagic stroke leads to worse outcomes and necessitates surgical management with ventricular shunts which has its own set of potential complications. Hydrocephalus linked to ICH, SAH, and IVH occurs at a higher frequency when blood products extend to the subarachnoid and intraventricular spaces. Though it is known that the choroid plexus (CP) produces cerebrospinal fluid (CSF) and that CSF is re-absorbed through the arachnoid granulations, the mechanism of how blood products lead to HCP is not well understood. In an attempt to understand this mechanism, we have developed an intra-cerebro-ventricular (ICV) injection model where CSF from SAH and ICH patients is injected into rat and mouse ventricles. With the utilization of this unique human-animal hybrid model, we have identified hemoglobin degradation products, such as iron and perioxiredoxin-2 (PRX-2), as major players in the formation of HCP in hemorrhagic stroke patients.
Iron, a degradation product of hemoglobin via the enzymatic function of heme oxygenase-1, has been shown to lead to ventricular enlargement and cause ventricular wall damage in a rodent model. Such deleterious effects can be reversed with the use of deferoxamine, an iron chelator [1]. PRX-2, an abundant intracellular protein within RBCs, has also been shown to contribute to IVH-induced hydrocephalus when it is released into the extracellular compartment following RBC lysis [2]. PRX-2 administration within the intraventricular space of rats leads to hydrocephalus formation; however, co-administration of PRX with its inhibitor, conoidin A, leads to blockade of HCP formation and concomitant inhibition of macrophage activation (Figure 1). Furthermore, intraventricular injection of thrombin, which is produced during clot formation, was also found to have similar effects of macrophage activation and hydrocephalus induction [3]. Iron, PRX-2, and thrombin are speculated to induce injury through inflammatory responses involving macrophages within the CP.
Figure 1.
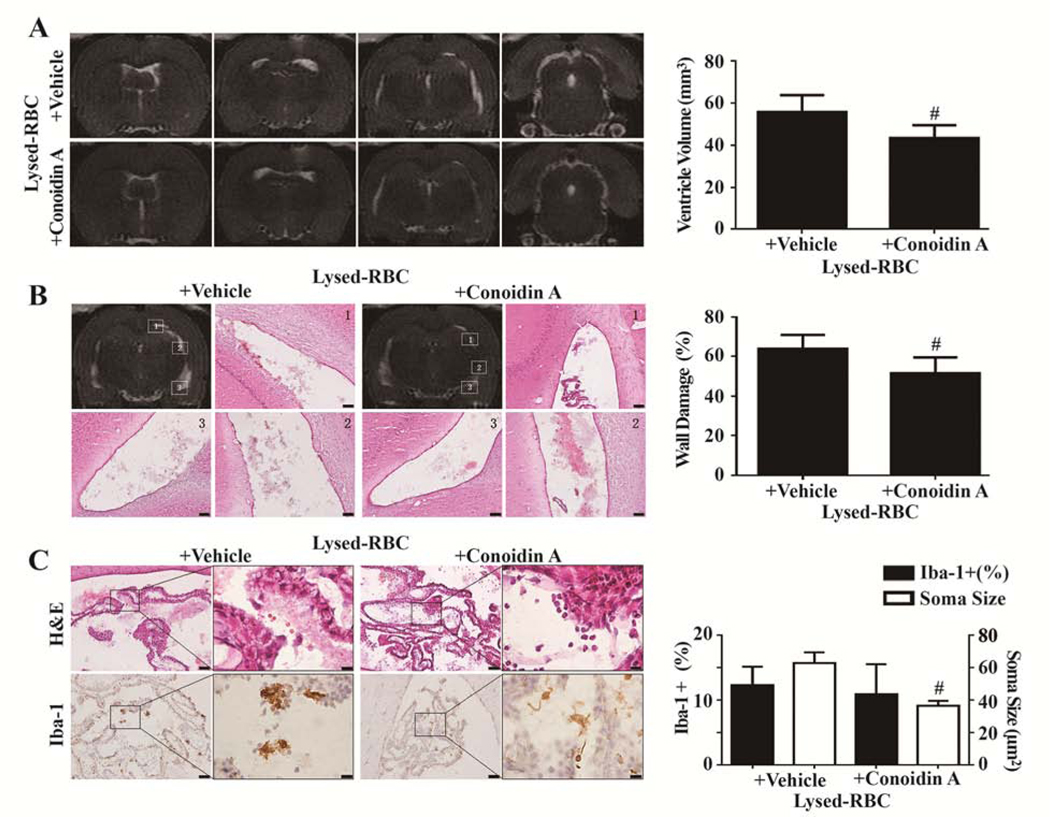
Effects of conoidin A on lysed red blood cell (RBC)–induced hydrocephalus, ventricular wall damage, and choroid plexus inflammation. (A) T2 MRIs 1 d after intraventricular injection of 30 μL lysed RBC with vehicle or conoidin A and quantification of ventricle volume. (B) Hematoxylin and eosin (H&E) staining in the same 2 groups showing ventricular wall damage and quantification of that damage. (C) Ionized calcium binding adaptor molecule 1 (Iba-1) immunohistochemistry at the choroid plexus and corresponding H&E sections 1 d after intraventricular injection of 30 μL lysed RBC with vehicle or conoidin A. The number of Iba-1–positive cells and soma size were quantified. Values are mean±SD; n=8; #P<0.01 vs lysed RBC+vehicle group. Scale bar=50 μm at low magnification, 10 μm at high magnification. (Reproduced with permission, Tan et al. Stroke, 2020;51:1578–1586)
Given the importance of inflammation in HCP formation, Kolmer epiplexus cells, which are intraventricular macrophages located in close proximity to the ependyma, may play a significant role by interacting with iron, PRX-2, or thrombin[4]. The number of epiplexus cells was noted to increase in the rat model from day of birth up to 17 days of age, indicating that there may be a more prominent role for these cells based on age. The phagocytic role of these epiplexus cells is reinforced by the presence of large numbers of lysosomes [4]. In studies examining the mechanism of inflammation in hemorrhagic stroke, Kolmer cells have been noted to be activated in rodent intraventricular injections of blood, human aneurysmal CSF, thrombin and even rodent models of SAH [3, 5, 6]. Furthermore, it has been shown that inflammation likely plays a key role in hydrocephalus development, potentially secondary to hypersecretion of CSF from the CP epithelium [7]. It should also be noted that epiplexus macrophage activation is associated with spontaneous hydrocephalus development in rats [8]. Altogether, it appears that red blood cell components released into the intraventricular component following lysis can induce ventricular dilation and ventricular wall damage as well as activation of Kolmer epiplexus cells, which may be leading to inflammation and hypersecretion of CSF from the CP.
Given the global burden of hemorrhagic stroke and associated HCP, it is imperative to understand the mechanism of hydrocephalus formation if medical therapies for HCP prevention and treatment are to be developed. Chronic hydrocephalus requires permanent CSF diversion in the form of a ventriculoperitoneal shunt (VPS), which requires an invasive surgical procedure. Furthermore, this hardware is at high risk of failure and has the potential for leading to further revision operations, increasing the patient morbidity as well as financial burden. In addition, such surgical care is frequently not readily available to patients in other countries or from underprivileged socioeconomic backgrounds, creating a huge opportunity for advancement of HCP care globally. Medical therapies based on mechanistic understanding of HCP formation have the potential to treat HCP while obviating the need for VPS and thus preventing associated complications. Utilizing a unique hybrid model of injecting SAH patient CSF into rat and mice ventricles, we are studying a multitude of RBC metabolic products to delineate the mechanism of HCP formation. The inhibitors mentioned above, deferoxamine and conoidin A, are readily available, cheap and easy to administer, making them excellent potential treatments for HCP throughout the world. Though the work described here has made strides in understanding the underlying mechanism of hydrocephalus, safety of such therapy in humans needs further investigation.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gao C, et al. , Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab, 2014. 34(6): p. 1070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan X, et al. , Prx2 (Peroxiredoxin 2) as a Cause of Hydrocephalus After Intraventricular Hemorrhage. Stroke, 2020. 51(5): p. 1578–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan Y, et al. , Activation of epiplexus macrophages in hydrocephalus caused by subarachnoid hemorrhage and thrombin. CNS Neurosci Ther, 2019. 25(10): p. 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ling EA, Kaur C, and Lu J, Origin, nature, and some functional considerations of intraventricular macrophages, with special reference to the epiplexus cells. Microsc Res Tech, 1998. 41(1): p. 43–56. [DOI] [PubMed] [Google Scholar]
- 5.Wan S, et al. , Cerebrospinal Fluid from Aneurysmal Subarachnoid Hemorrhage Patients Leads to Hydrocephalus in Nude Mice. Neurocrit Care, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan Y, et al. , Effects of aging on hydrocephalus after intraventricular hemorrhage. Fluids Barriers CNS, 2020. 17(1): p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimy JK, et al. , Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med, 2017. 23(8): p. 997–1003. [DOI] [PubMed] [Google Scholar]
- 8.Gu C, et al. , Effects of minocycline on epiplexus macrophage activation, choroid plexus injury and hydrocephalus development in spontaneous hypertensive rats. J Cereb Blood Flow Metab, 2019. 39(10): p. 1963–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]


