To the Editor,
Vitiligo is an autoimmune skin disease presenting with distinct, patchy areas of depigmentation that can appear anywhere on the body. These white patches occur when autoreactive, cytotoxic CD8+ T cells promote elimination of melanocytes, the pigment-producing cells of the epidermis (Frisoli et al. 2020). Vitiligo affects approximately 1% of the global population equally across races and sexes, and frequently presents before the age of 20 (Taieb and Picardo 2009).
Currently, there are no U.S Food and Drug Administration (FDA)-approved medical treatments to promote repigmentation in vitiligo, while topical immunosuppressants and phototherapy are moderately effective when used off-label (Frisoli et al. 2020). Recent studies report efficacy of the Janus kinase inhibitors (JAKi) tofacitinib and ruxolitinib in vitiligo patients through case reports and case studies, as well as ongoing clinical trials (Craiglow and King 2015; Harris et al. 2016; Kim et al. 2018; Liu et al. 2017). However, studies also reported rapid relapse of disease after stopping both conventional treatments (Cavalié et al. 2015) as well as JAKi (Harris et al. 2016; Liu et al. 2017).
We and others recently reported that autoreactive resident memory T cells (Trm) form in the lesions of vitiligo patients, and that these cells are responsible for disease relapse (Boniface et al. 2018; Cheuk et al. 2017; Richmond et al. 2018b). Trm function to recruit autoreactive T cells from the circulation, which participate in killing repigmenting melanocytes (Richmond et al. 2018a). Treatments that inhibit Trm function, but do not remove them from the skin, are not durable (Riding and Harris 2019). Therefore, we hypothesized that Trm numbers are largely unaffected by JAKi treatment based on the lack of durable responses in patients.
To test our hypothesis, we used JAKi in our mouse model of vitiligo, which is induced by adoptive transfer of melanocyte-specific CD8+ T cells, also known as premelanosome protein-specific T cells (PMEL), into recipient hosts with pigmented skin (Harris et al. 2012). We administered tofacitinib or ruxolitinib orally once daily to hosts prior to disease onset (prevention) or after stabilization (reversal) and measured their effect on extent of disease, as well as T cell populations within the epidermis and dermis by flow cytometry.
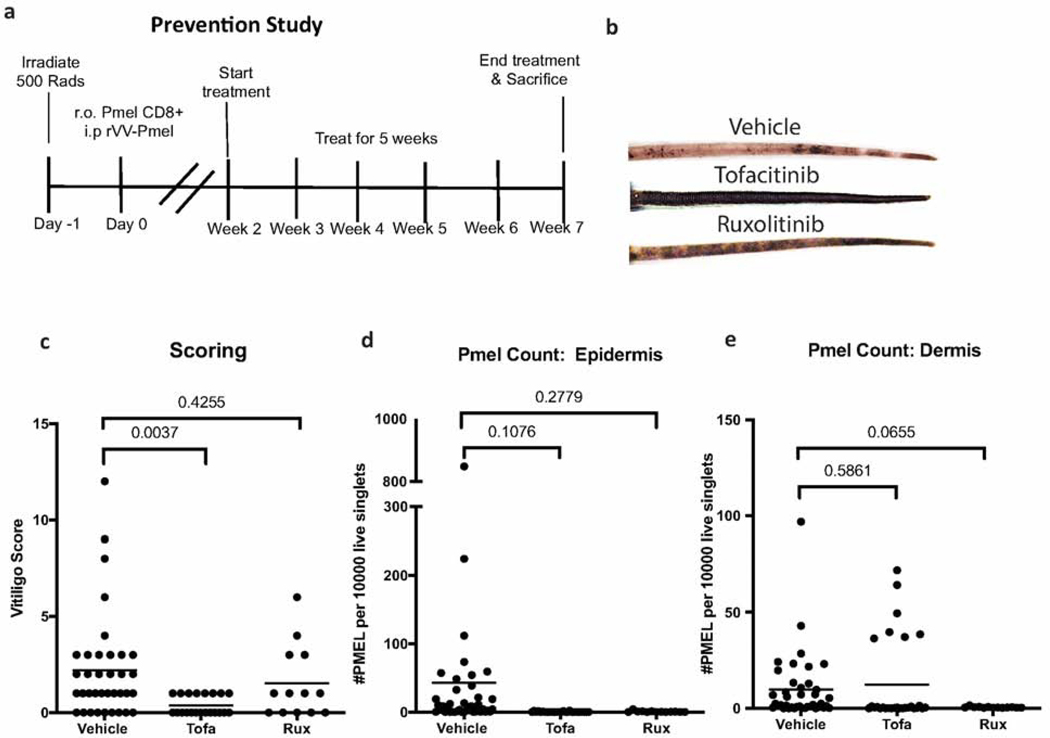
In the prevention model, hosts were treated daily with vehicle or JAKi from week two to seven post-induction of vitiligo (Fig 1A). We observed a mild, but statistically insignificant, decrease in the vitiligo score in hosts treated with ruxolitinib (p=0.4255), and a larger, statistically significant decrease in vitiligo score with tofacitinib treatment (p= 0.0037, n=36 vehicle, 21 tofacitinib, & 13 ruxolitinib mice; Fig 1B-C). Whether this indicates that ruxolitinib will be less effective at controlling active disease in humans is not clear, but should be monitored in clinical studies.
Figure 1. JAKi reduce vitiligo and prevent epidermal accumulation of autoreactive T cells in mice.
(a) Prevention experiments were completed according to the schedule above. (b) Sample photographs from mouse tails after 5 weeks of treatment. (c) Clinical scores of vitiligo in vehicle, tofacitinib (tofa), and ruxolitinib (rux)-treated mice. Total number of premelanosome protein specific T cells (PMEL) in the epidermis (d) and dermis (e) by flow cytometry (each dot represents one animal; n=36 vehicle, 21 tofacitinib and 13 ruxolitinib pooled from at least 3 separate experiments).
To understand how the JAKi were impacting disease, we examined the number of immune cells in the skin using flow cytometry. We found that hosts treated with either JAKi had fewer, though not statistically significant, PMELs in the epidermis (Fig 1D, tofacitinib vs vehicle p=0.1076, ruxolitinib vs vehicle p=0.2779). Ruxolitinib, but not tofacitinib, appeared to reduce PMEL in the dermis as well (Fig 1E, tofacitinib vs vehicle p=0.5861, ruxolitinib vs vehicle p=0.0655).
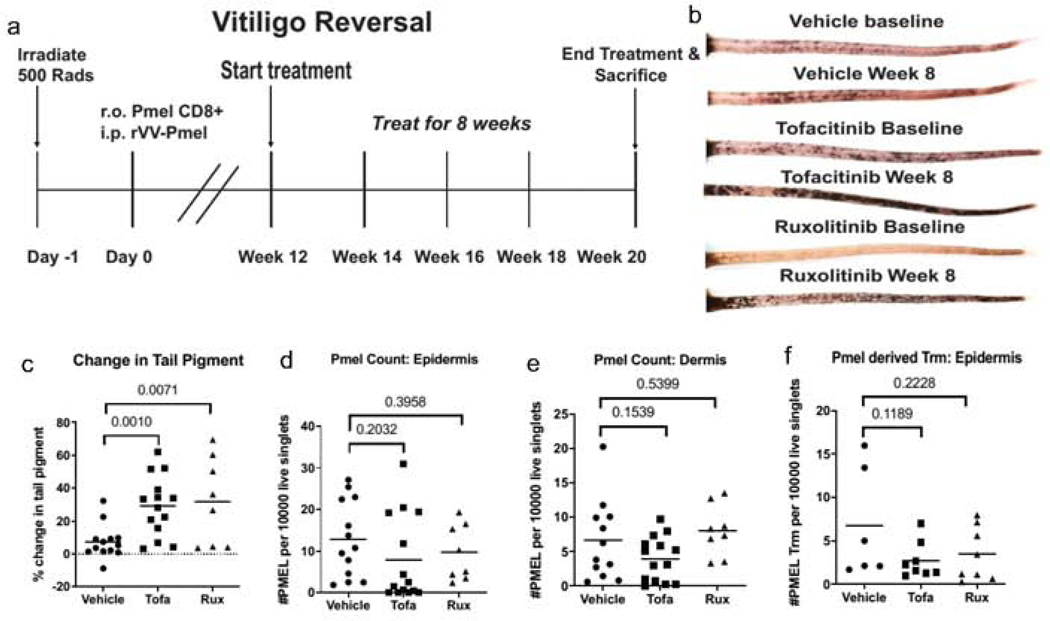
In the reversal model, we treated hosts twelve weeks post-induction with JAKi for eight more weeks (Fig 2A). Both tofacitinib and ruxolitinib significantly reversed disease as compared to vehicle controls (Fig 2B-C, p =0.0010 and 0.0071 respectively, n=13 vehicle, 14 tofacitinib and 8 ruxolitinib). Interestingly, in contrast to the prevention model, JAKi did not decrease the overall number of PMEL in the epidermis (Fig 2D, p=0.2032 and p=0.3958), dermis (Fig 2E, p= 0.1539 and p=0.5399), or specifically the Trm subpopulation, marked by expression of CD103 and CD69 (Fig 2F, p=0.1189; p=0.228). Thus, Trm populations remained in the skin during reversal, even after significant repigmentation was observed.
Figure 2. JAKi reverse depigmentation in vitiligo but do not clear Trm in the skin.
(a) Reversal experiments were completed according to the schedule above. (b) Photographs of mouse tails at the beginning and end of treatment after 8 weeks. (c) Change in tail pigment was calculated using before and after photos of each mouse. PMEL count within the epidermis (d) and dermis (e) was assessed at the end of treatment (each dot represents one animal; n=13 vehicle, 14 tofacitinib, and 8 ruxolitinib). (f) Resident memory T cells (Trm) were stained in the epidermis and quantified after treatment (n=6 vehicle, 8 tofacitinib, and 8 ruxolitinib pooled from 2 separate experiments).
Taken together, our data suggest that JAKi may prevent accumulation of T cells in the skin during disease progression, but do not affect T cell numbers once established. The observed efficacy of treatments to promote reversal of disease may instead be through inhibition of T cell function within the skin, rather than by decreasing their numbers. Both tofacitinib and ruxolitinib inhibit signaling through IFN-g (Damsky and King 2017), a cytokine produced by Trms and required for recruitment of cytotoxic cells from the circulation (Richmond et al. 2018a). While IFN-y signaling through JAKs appears to be necessary for depigmentation, it is dispensable for Trm maintenance in the skin, which supports clinical observations that JAK inhibition does not result in durable treatment responses.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Harris lab for technical assistance. We thank B.J. Longley for Krt14-Kitl* mice and N. Restifo for recombinant vaccinia virus.
Funding: JEH is supported by NIH R01 AR069114, R61 AR070302, the Hartford Foundation, and a Calder Research Scholar Award from the American Skin Association. JMR is supported by a Research Grant and Calder Research Scholar Award from the American Skin Association, a Career Development Award from the Dermatology Foundation, and a Target Identification in Lupus Award from the Lupus Research Alliance. Training grants supported JPS (T32s AI132152 and AI095213) and RLR (T32 AI007349). Flow cytometry equipment is maintained by UMMS Flow Cytometry Core Facility.
Footnotes
DATA AVAILABILITY
Primary data are available upon request.
CONFLICT OF INTEREST
JEH has acted as consultant and received grant funding from Pfizer and Incyte. JMR, JPS and JEH are inventors on patent application #62489191, “Diagnosis and Treatment of Vitiligo” which covers targeting IL-15 and Trm for the treatment of vitiligo. JEH is scientific founder of Villaris Therapeutics, Inc, which is focused on developing treatments for vitiligo. JMR and JEH are inventors on patent application #15/851,651, “Anti-human CXCR3 antibodies for the Treatment of Vitiligo” which covers targeting CXCR3 for the treatment of vitiligo.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Boniface K, Jacquemin C, Darrigade A-S, Dessarthe B, Martins C, Boukhedouni N, et al. Vitiligo Skin Is Imprinted with Resident Memory CD8 T Cells Expressing CXCR3. J. Invest. Dermatol 2018;138(2):355–64 Available from: 10.1016/j.jid.2017.08.038 [DOI] [PubMed] [Google Scholar]
- Cavalié M, Ezzedine K, Fontas E, Montaudié H, Castela E, Bahadoran P, et al. Maintenance Therapy of Adult Vitiligo with 0.1% Tacrolimus Ointment: A Randomized, Double Blind, Placebo-Controlled Study. J. Invest. Dermatol. Elsevier; 2015;135(4):970–4 Available from: http://www.sciencedirect.com/science/article/pii/S0022202X15371815 [DOI] [PubMed] [Google Scholar]
- Cheuk S, Schlums H, Gallais Sérézal I, Martini E, Chiang SC, Marquardt N, et al. CD49a Expression Defines Tissue-Resident CD8+ T Cells Poised for Cytotoxic Function in Human Skin. Immunity. 2017;46(2):287–300 Available from: 10.1016/j.immuni.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craiglow BG, King BA. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol. 2015; 151(10):1110–2 Available from: 10.1001/jamadermatol.2015.1520 [DOI] [PubMed] [Google Scholar]
- Damsky W, & King BA (2017). JAK inhibitors in dermatology: The promise of a new drug class. Journal of the American Academy of Dermatology, 76(4), 736–744. doi: 10.1016/j.jaad.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoli ML, Essien K, & Harris JE (2020). Vitiligo: Mechanisms of Pathogenesis and Treatment. Annual Review of Immunology. doi: 10.1146/annurev-immunol-100919-023531 [DOI] [PubMed] [Google Scholar]
- Harris JE, Harris TH, Weninger W, John Wherry E, Hunter CA, Turka LA. A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFN-y for Autoreactive CD8+ T-Cell Accumulation in the Skin. J. Invest. Dermatol 2012; 132(7): 1869–76 Available from: http://www.sciencedirect.com/science/article/pii/S0022202X15358188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Rashighi M, Nguyen N, Jabbari A, Ulerio G, Clynes R, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J. Am. Acad. Dermatol 2016;74(2):370–1 Available from: 10.1016/j.jaad.2015.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Heaton H, Liu LY, King BA. Rapid Repigmentation of Vitiligo Using Tofacitinib Plus Low-Dose, Narrowband UV-B Phototherapy. JAMA Dermatol. 2018;154(3):370–1 Available from: 10.1001/jamadermatol.2017.5778 [DOI] [PubMed] [Google Scholar]
- Liu LY, Strassner JP, Refat MA, Harris JE, King BA. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J. Am. Acad. Dermatol 2017;77(4):675–82.e1 Available from: 10.1016/j.jaad.2017.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JM, Strassner JP, Rashighi M, Agarwal P, Garg M, Essien KI, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J. Invest. Dermatol 2018a; Available from: 10.1016/j.jid.2018.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JM, Strassner JP, Zapata L Jr, Garg M, Riding RL, Refat MA, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci. Transl. Med 2018b;10(450) Available from: 10.1126/scitranslmed.aam7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riding RL, Harris JE. The Role of Memory CD8+ T Cells in Vitiligo. J. Immunol. Am Assoc Immnol; 2019;203(1):11–9 Available from: 10.4049/jimmunol.1900027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb A, Picardo M. Clinical practice. Vitiligo. N. Engl. J. Med 2009;360(2):160–9 Available from: 10.1056/NEJMcp0804388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.