Abstract
Rationale:
Co-use of cannabis and nicotine is common among adolescents/young adults and is associated with poorer psychological and physical outcomes, compared to single substance use. Little is known about the impact of co-use on the developing brain.
Objectives:
Preliminary investigation of the effects of nicotine on white matter (WM) cerebral blood flow (CBF) in adolescents/young adults and its potential moderation by cannabis use.
Methods:
Adolescent/young adult (16–22 years-old) nicotine and tobacco product users (NTP; N=37) and non-nicotine users (non-NTP; N=26) underwent a neuroimaging session comprised of anatomical, optimized pseudo-continuous arterial spin labeling, and diffusion tensor imaging scans. Groups were compared on whole-brain WM CBF estimates and their relation to past-year cannabis use. Follow-up analyses assessed correlations between identified CBF clusters and corresponding fractional anisotropy (FA) values.
Results:
Group by cannabis effects were observed in five clusters (voxel-wise alpha<0.001, cluster-wise alpha<0.05; ≥11 contiguous voxels): non-NTP exhibited positive correlations between CBF and cannabis use in all clusters; whereas no significant relationships were observed for NTP. Greater CBF extracted from one cluster (including portions of right superior longitudinal fasciculus) was associated with reduced FA for non-NTP group only.
Conclusions:
This is the first investigation of WM health as indexed by CBF, and its association with FA, in adolescents/young adults with nicotine and/or cannabis use. Results suggest that cannabis use by itself may be related to increased CBF in WM fiber tracts demonstrating poorer structural intergrity, yet the occurrence of even infrequent NTP use (greater than once per month) appears to diminish this relationship.
Keywords: Cerebral blood flow, white matter, nicotine, cannabis, adolescence, young adults
INTRODUCTION
The growing popularity of electronic nicotine delivery device use among adolescents and young adults has contributed to sharp changes in use trends of cannabis and nicotine (Johnston et al. 2020) (Schulenberg et al. 2019). While past-month use rates of cannabis and tobacco cigarettes remained relatively stable in 2018 (22% and 8% of 12th graders, respectively; 25% and 6.8% of college students), vaping of both of these products has doubled and tripled for some high school grades (Johnston et al. 2020) and college students (Schulenberg et al. 2019) from 2017–2018. In fact, increases in vaping of these substances represent the largest increase in behavior observed for the Monitoring the Future Study which has been surveying US adolescent substance use since 1975.
The risk of using cannabis and/or nicotine and tobacco products (NTPs) increases with initiation of either substance during adolescence and co-use of both substances is common (estimated between 18–52% of tobacco users; Agrawal et al. 2012; Nicksic et al. 2020; Tucker et al. 2019). Many youth may be unknowingly exposed to nicotine during cannabis consumption (e.g., hollowed out cigars, spliffs; Bélanger et al. 2013). The co-use of cannabis and NTPs, as compared to single substance use, has been associated with adverse educational outcomes (Stiby et al. 2015), poorer physical and emotional functioning (Tucker et al. 2019), differential neural activation patterns and cognitive performance (Filbey et al. 2018; Schuster et al. 2016), and increased risk for substance use disorders and addiction severity symptoms (Bélanger et al. 2013; Ramo et al. 2013). Yet, there still remains very limited knowledge on how co-use (as compared to single substance use) may result in unique metabolic changes that affect neural tissue development (Agrawal et al. 2009; Fairman 2015; Ream et al. 2008).
The developmental stage of adolescence is marked by substantial dynamic cellular and morphological changes in the brain, including an increase in neural circuitry interconnectedness and specialization (Giedd 2015). During normative development, white matter fiber tracts show the most protracted development throughout adolescence and into young adulthood (mid-20s), possibly reflecting the enhancement of efficient neuronal communication (Bava et al. 2010; Giedd 2004; Lebel et al. 2012; Pfefferbaum et al. 2018; Simmonds et al. 2014; Willing and Juraska 2015). Given that white matter tissue integrity is essential for cortical connectivity in reward circuitry and optimal cognitive functioning (Fields 2008; Filley and Fields 2016; Fjell et al. 2011), external influences, such as substance use, during these ongoing micro and macrostructural changes may lead to increased risk for psychopathologies including addiction (Giedd et al. 2008).
Arterial spin labeling (ASL) is a technique commonly used to estimate brain health via measurement of cerebral blood flow (CBF). CBF is tightly coupled with glucose metabolism (Jueptner and Weiller 1995) and brain function (Raichle et al. 1976). Acute exposure to Δ9-tetrahydrocannabinol (THC; the primary psychoactive component of cannabis) appears to cause an increase in regional grey matter CBF, whereas chronic use is associated with a decline of CBF, particularly in the prefrontal cortex (for a review see Ogunbiyi et al. 2020). Similarly, chronic nicotine smoking in adults is associated with a decline of CBF (Chaarani et al. 2019; Vafaee et al. 2015); however, the acute effects of nicotine are less clear with reports of regional increases and/or decreases (Domino et al. 2000; Rose et al. 2003; Wennmalm 1982), potentially driven by study differences in dose-related effects, lengths of abstinence at testing, or the technique used to quantify CBF (e.g., positron emission tomography versus magnetic resonance imaging). However, the estimation of CBF is very limited in studies assessing adolescent/young adult substance use on brain health and has traditionally been limited to gray matter structures only. This is in part due to the lower and more heterogeneous perfusion and long transit times of the labelled bolus in white matter (van Gelderen et al. 2008; Wu et al. 2013). Recent advances in the field have enabled more precise estimates of CBF in white matter (Lu et al. 2009). Results suggest that CBF in white matter varies considerably across regions (Aslan et al. 2011), implying these improved ASL methods may be useful for the assessment of substance use effects on white matter function.
Insights into white matter structural health can be gleaned from diffusion tensor imaging (DTI) studies that assess subtle differences in tissue architecture and fiber tract coherence (Lebel et al. 2012; Mills et al. 2016). Fractional anisotropy (FA) is a metric commonly used to estimate the integrity of white matter tissue and provides a quantitative measure on the degree to which water molecules are directionally restricted within a voxel (Le Bihan 2003; Mori and Zhang 2006). DTI studies have provided important insights on associations between myelination changes and neurobehavioral development (Lebel and Deoni 2018). For example, an increase in FA is typically observed from early childhood through adolescence, suggesting an increase in axonal packing and myelination (Geeraert et al. 2019). Yet, while the vast majority of studies focus on FA as the primary measure of white matter integrity, FA is sensitive to the complex geometry of white matter, including the crossing and merging of fiber tracts which can result in lower anisotropy estimates; thus, comprised tissue integrity cannot be unequivocally linked to reduced FA values (Pierpaoli et al. 2001). Regular nicotine use in adolescent samples is typically associated with an increase in FA (Jacobsen et al. 2007; van Ewijk et al. 2015; Yu et al. 2016), although not exclusively (Kangiser et al. 2019), whereas regular cannabis use in adolescence is largely associated with reduced FA (Gruber et al. 2014; Jacobus et al. 2013b) or no effect (Cousijn et al. 2012; Orr et al. 2016; Thayer et al. 2017) when comparing cannabis users to controls. Daily cigarette smoking in adults has also been associated with an increase in FA; however, greater nicotine dependence and smoking history in this sample was shown to be associated with reduced FA in regions such as the internal capsule and anterior cingulum (Hudkins et al. 2012). These results suggest that the impact of nicotine on estimates of white matter health is likely dependent on ones’ stage of development and duration of nicotine use severity into adulthood.
To the authors’ knowledge, only two studies have directly investigated the relationship between CBF and FA, with one reporting a negative association (Aslan et al. 2011) and the other a positive association (Giezendanner et al. 2016), suggesting perfusion changes may contribute to altered neural tissue properties of white matter tissue and thus functional integrity. However, both studies were conducted in healthy adults (ages 20–46), implying very little is known about white matter CBF in adolescents and young adults, especially concerning the impact of substance use.
In sum, extant research suggests nicotine or cannabis use during adolescence/young adulthood leads to altered white matter health; however, the potential interactive effects from co-use of nicotine and cannabis on white matter remain unknown. Animal models suggest the presence of a functional interaction between THC and nicotine (Valjent et al. 2002), which has potential implications for the additional synergistic and/or interactive effects of the co-use of these substances on protracted brain health and development (e.g., white matter tissue). Thus, the present preliminary analysis aimed to investigate the effects of nicotine on white matter CBF in adolescents and young adults (ages 16–22 years-old) and whether this relationship was moderated by cannabis use. Given the mixed state of the literature and novelty of the white matter CBF assessment in co-using adolescents and young adults, no directional hypotheses were stated; however, we predicted that any altered white matter CBF observed would be associated with poorer structural integrity of white matter fibers (i.e., FA) in corresponding regions.
MATERIALS AND METHODS
Participants & Procedures
Data for this preliminary report were culled from an ongoing study on the structural and functional neural effects of nicotine and cannabis co-use in adolescence/young adulthood. Participants (N=63; ages 16–22) were recruited from San Diego County via flyers posted physically and electronically at high schools, community colleges, four-year universities, and social media sites. To ensure our enrollment allowed for examination of single substance and co-use of cannabis and NTP products, participants were required to meet criteria for one of four group classifications at study enrollment. Groups were based on past six-month cannabis and NTP use patterns including: 1) frequent (≥1x/week on average) cannabis use only, 2) frequent NTP use only, 3) frequent cannabis and NTP use, and 4) minimal to no use of either substance (≤15 episodes of cannabis and of NTP use in the past 6 months). For this investigation, participants were re-categorized into NTP and non-NTP using groups, with NTP users reporting an average of >1 NTP use episode per month during the previous year. This resulted in two groups that did not overlap in their past-year NTP use history. NTP use in this analysis was defined as the use of any of the following: tobacco cigarettes, tobacco pipe, hookah with tobacco, cigars (including blunts, spliffs), electronic cigarettes (e.g., vape pens, Juul, e-hookah), smokeless tobacco, chew, snuff, snus, and/or nicotine replacement (e.g., patches, gum, nasal sprays, inhalers, lozenges).
Exclusion criteria for enrollment included current or past DSM-5 psychiatric disorder other than cannabis and/or tobacco use disorder, any lifetime illicit substance use >10 times, acute influence of alcohol or cannabis use at study visit (confirmed with breathalyzer, urine, and oral fluid toxicology), major psychiatric or medical issues, use of medications affecting the brain, MRI contraindications (e.g., implanted metal, metal braces, etc.), or history of developmental disability or prenatal substance exposure.
All participants came in for a single, four-hour visit in the laboratory and provided written informed consent in accordance with the University of California, San Diego Human Research Protections Program. Following consent, participants underwent a comprehensive demographic, mental health, and substance use interview, neurocognitive assessment, and magnetic resonance imaging scan session. Participants were asked to refrain from using cannabis within 12 hours prior to the appointment to avoid acute effects of intoxication. Urine, oral fluid, and breathalyzer for alcohol corroborated self-reported substance use and ruled out cannabis use within the past 12 hours. Oral fluid samples examined Δ9- tetrahydrocannabinol (THC) using the Drager DrugTest® 5000 (Dräger; Desrosiers et al. 2012) for onsite detection of recent cannabis use (≥5 μg/L THC). Urine samples were sent to Redwood Toxicology to quantify cotinine (nicotine metabolite) and THC metabolite 11-nor-9-carboxy-THC (THCCOOH) normalized to creatinine for all substance users and confirm no other presence of illicit substances. To avoid withdrawal effects during data acquisition, participants were not required to abstain from NTP use. While they could engage in NTP use ad libitum, time of last use was documented (NTP group range: 0.28 – 720 hours, median = 48 hours; see Table 1).
Table 1.
Sample demographics.
| Variable | |||
|---|---|---|---|
| Non-Nicotine | Nicotine | Sig | |
| Total N | 26 | 37 | |
| Age | 18.31 (1.52) [16–22] | 19.03 (1.32) [17–22] | 0.05 |
| % Male | 57.69% | 72.97% | 0.21 |
| % White | 50.00% | 45.90% | 0.75 |
| % Hispanic | 50.00% | 40.50% | 0.46 |
| Education Years Completed | 12.08 (1.55) [9–16] | 12.59 (1.19) [10–15] | 0.14 |
| DASS-21 Depression Standard Score | 2.04 (2.07) [0–7] | 4.24 (4.73) [0–20] | 0.03 |
| DASS-21 Anxiety Standard Score | 2.12 (2.03) [0–7] | 4.00 (3.15) [0–14] | 0.01 |
| DASS-21 Stress Standard Score | 2.46 (2.64) [0–10] | 5.22 (4.00) [0–14] | <0.01 |
| Age of Onset of Nicotine Use | 16.50 (1.60) [13–18] n=8 | 16.38 (1.91) [11–19] | 0.87 |
| Days Since Last Nicotine Use | 68.50 (78.86) [3–254] n=8 | 7.19 (9.19) [0–30] | <0.01 |
| Nicotine Use Episodes Previous Year | 1.54 (3.04) 1 [0–10] | 1109.16 (2289.36) [17–10272] | 0.02 |
| Age Onset Cannabis Use | 16.45 (2.73) [10–19] n=11 | 15.70 (1.75) [11–18] | 0.28 |
| Age Onset Regular Cannabis Use | 17.75 (1.04) [16–19] n=8 | 17.15 (1.70) [13–21] n=33 | 0.35 |
| Days Since Last Cannabis Use | 12.82 (22.90) [0–66] n=11 | 77.76 (332.68) [0–2016] | 0.52 |
| Cannabis Use Episodes Previous Year | 37.69 (82.12) [0–374] | 312.73 (380.08) [3–1855] | <0.01 |
| Age Onset Alcohol Use | 16.06 (2.41) [8–18] n=16 | 15.59 (2.41) [9–20] | 0.52 |
| Days Since Last Alcohol Use | 62.56 (178.75) [1–730] n=16 | 16.49 (37.96) [0–210] | 0.14 |
| Alcohol Drinks Per Drinking Day Previous Year | 3.38 (7.06) [0–35] | 5.03 (4.02) [2–25] | 0.25 |
| Alcohol Drinking Days Per Month Previous Year | 1.92 (3.32) [0–13] | 6.11 (5.74) [0–21] | 0.01 |
Measures
A demographic and psychosocial interview was conducted to assess background information on race/ethnicity, socioeconomic status, education, and medical history. The 21 item version of the DASS was administered to assess depression, anxiety, and stress symptomology (Lovibond and Lovibond 1995). The Customary Drinking and Drug Use Record structured interview (Brown et al. 1998), modified to include additional cannabis and nicotine questions (Jacobus et al. 2018; Karoly et al. 2019a; Karoly et al. 2019b), was used to assess history of substance use and substance-related problems. For the purposes of this study, current nicotine use was defined as the use of an NTP more than once per month during the previous year.
Imaging Acquisition and Processing
MRI.
Participants were imaged at the UCSD Center for Functional MRI on a 3.0 Tesla GE Discovery MR750 scanner with a 32-channel phased array head coil. Subjects were asked to remain still and awake for the duration of the scan. A high resolution T1-weighted anatomical fast spoiled gradient echo (FSPGR) scan was acquired with TI/TE/TR=1060/2/2500ms, flip angle=8˚, FOV=256 mm, 256×256 matrix, 1.0 mm3 voxels. Acquisition parameters were modeled after those used in the nationwide Adolescent Brain and Cognitive Development (ABCD) Study (Hagler et al. 2019).
Optimized pseudo-continuous arterial spin labeling (OptPCASL).
Resting CBF was measured using an OptPCASL method developed at the University of California, San Diego (Shin et al. 2012). This method offers a high level of sensitivity and robustness to make time-efficient measurements of CBF in gray and white matter tissue (Lu et al. 2009). The protocol consists of an optimized PCASL scan (FOV 240 mm, 64×64 matrix, 24 axial contiguous slices, 6 mm thick, single-shot spiral acquisition with TE = 3.2ms; TR = 4500ms, tag duration = 1900ms, post labeling delay = 1900ms, scan time 9:27 min) with background suppression and additional calibration (for CBF quantification) and field maps scans (Shin et al. 2012). The use of the short echo time spiral acquisition minimizes susceptibility related dropouts. The information from the field maps is used in the image reconstruction process to correct for susceptibility-induced distortions (Noll et al. 2005).
Both distortion correction and raw OptPCASL data to quantitative CBF map conversion were carried out using analysis pipelines found in Shin et al. (2016; 2013), which invokes relevant tools from different neuroimaging software (AFNI, Cox 1996; Freesurfer, Dale et al. 1999; FSL, Smith et al. 2004), and in-house Matlab scripts. A mean ASL image was formed from the average difference of the control and tag images from the resting ASL data, which was then corrected for coil inhomogeneities and converted to absolute units of CBF (mL 100g−1 min−1) using proton density weighted images acquired 30 seconds after the PCASL sequence.
The aligned high resolution and CBF images were warped to Talairach space using AFNI’s auto_tlrc function and resampled to a 4 × 4 × 4 mm resolution grid with AFNI’s adwarp. Voxels with negative intensities were replaced with zero (Brown et al. 2003). Data were visually screened for data quality and alignment. An average anatomical image was created across all participants and segmented using FSL’s FAST algorithm to define CSF, gray matter (GM) and white matter (WM) regions. The WM segmentation was used as a mask for participant’s CBF images in the statistical analyses.
Diffusion Tensor Imaging (DTI).
Diffusion data were acquired with a multi-shell 96-direction single-shot spin echo diffusion sequence with 4 b-values (500, 1000, 2000, and 3000 sec/mm2) and 6, 15, 15, and 60 unique diffusion directions, respectively, for each b-value (TE/TR=81.9/4100ms, 81 axial slices, FOV=240 mm, matrix=140×140, 1.7 mm3 voxels). Acquisition parameters were modeled after those used in the ABCD Study (Hagler et al. 2019). Data were collected with reversed phase-encode blips (A>P, P>A), resulting in pairs of images with distortions going in opposite directions. From these pairs the susceptibility-induced off-resonance field was estimated using a method similar to that described in Andersson et al. (2003) as implemented with the TOPUP tool in FSL (Smith et al. 2004) and the two images were combined into a single corrected one to correct for susceptibility-induced distortions (Graham et al. 2017). FSL’s Diffusion Toolbox (FDT) programs were applied to correct for eddy current distortion (EDDY), linear registration to standard space (FLIRT), and tensor decomposition to derive FA values for each subject (DTIFIT) as an estimate of white matter integrity for data analysis (Behrens et al. 2003).
Statistical Analysis
Independent t- or χ² tests were used to examine group differences on demographic variables with a statistical significance threshold set at p < .05. Similar to methods previously used by the authors (Courtney et al. 2019), AFNI 3dttest++ was used to contrast perfusion estimates between the NTP and non-NTP groups and examine the group by past year cannabis use interaction. Past year cannabis use episodes were entered in the model as a continuous covariate of interest as the majority of subjects endorsed some past-year cannabis use. Given the recommendations for more precise error control (Cox et al. 2017; Eklund et al. 2016), the Clustsim nonparametric randomization/permutation option was used with a voxel-wise alpha of 0.001 and cluster-wise alpha of 0.05, resulting in an estimated cluster size threshold of 11 contiguous voxels. The JHU White-Matter Tractography and JHU ICBM DTI-81 atlases were used to assist in region identification (Hua et al. 2008; Mori et al. 2005; Wakana et al. 2007).
The five clusters from the NTP group x cannabis use interaction effect that surpassed thresholding were used to form functional, cluster-specific masks. These cluster masks were then applied to the CBF and DTI images and the mean CBF and FA estimates were extracted from each cluster and tested for inhomogeneity of variance between the groups using the Levene’s test. CBF and FA values from each region were subjected to bivariate Pearson correlation tests (five correlations total; p-value threshold Bonferroni corrected at .05/5=.01) for each group separately. Correlations that showed statistical significance within a group were then compared across groups using Fisher r-to-z transformation. Exploratory follow-up correlational analyses examined the relationships between CBF and FA cluster estimates, DASS scores, age of substance use onset (nicotine and cannabis), and, in the case of FA, additional substance use metrics (past-year nicotine and cannabis use episodes; p-value threshold uncorrected at .05). Given that past-year NTP use, cannabis use, and alcohol use were not found to be highly correlated (rs: 0.11–0.28), a separate whole-brain model including NTP group, past-year cannabis use, and drinking days per month was estimated in order to investigate potential CBF effects due to alcohol use (thresholded at a voxel-wise alpha of 0.001, cluster-wise alpha of 0.05; ≥ 9 contiguous voxels).
RESULTS
Participants
The sample consisted largely of males (66.7%) and approximately 48% of the sample self-identified as White (see Table 1 for demographic information). The NTP group was comprised of individuals reporting a range of low (at least monthly) to heavy (>3 per day) NTP use episodes over the previous year (range: 17–10,272 episodes). Within the NTP group, 73.0% (n=27) endorsed greater vaping, versus smoking of combustible tobacco products, for nicotine delivery, and 20% (n=7) reported greater vaping of cannabis concentrate products, versus smoking of cannabis flower products. Within the non-NTP group, 64% (n=7) reported greater vaping of cannabis concentrate products, versus smoking of cannabis flower products. The NTP group reported an average of 2.28 more points on the DASS depression, anxiety, and stress subscales as compared to the non-NTP group; however, the NTP group means still fell within the normal to mild ranges per the cutoff scores provided by the scale authors (Lovibond and Lovibond 1995). As expected, the groups differed significantly on nicotine recency and past year use of nicotine, as well as past year use of cannabis. The groups also differed on drinking days per month, yet group differences in the number of drinks per drinking day did not meet statistical significance and additional exploratory analyses evinced no significant effect of alcohol use, nor its interaction with cannabis, on CBF metrics in these participants (thresholded at a voxel-wise alpha of 0.001, cluster-wise alpha of 0.05; ≥ 9 contiguous voxels).
Whole-Brain White Matter CBF Results
Analysis of the whole-brain white matter contrast between NTP and non-NTP group perfusion estimates revealed no statistically significant main/group effects; however, five clusters surpassed thresholding within the interaction effect between groups and past year cannabis use (see Table 2, Figures 1 and 2). Examination of the whole-brain simple-main effects for each of the groups showed that the non-NTP group exhibited positive correlations between CBF and past year cannabis use in all five clusters (coordinates and z-scores nearly identical to significant clusters from the interaction effect in Table 2), whereas no statistically significant relationship was observed for the NTP group (thresholded at a voxel-wise alpha of 0.001, cluster-wise alpha of 0.05; ≥ 11 contiguous voxels). The variances of the mean cluster CBF estimates were found to be homogeneous between the groups (ps > .07). The interaction model was also re-run with recency of cannabis and nicotine use entered as covariates, with little effect on the results (cluster effects slightly diminished, yet still significant when thresholded at a voxel-wise alpha of 0.001, cluster-wise alpha of 0.05).
Table 2.
Clusters of significant cerebral blood flow (CBF) response from the interaction effect between nicotine group and past year cannabis use (thresholded at a voxel-wise alpha of 0.001, cluster-wise alpha of 0.05; ≥ 11 contiguous voxels).
| Region (peak) | Cluster Voxels | X | Y | Z | Z-Score | Alpha |
|---|---|---|---|---|---|---|
| Cluster 1: Right Superior Longitudinal Fasciculus/Inferior Fronto-Occipital Fasciculus/Forceps Major | 37 | 30.0 | 65.0 | 8.0 | 4.94 | <.01 |
| Cluster 2: Left Inferior/Superior Longitudinal Fasciculus | 25 | −58.0 | 29.0 | −24.0 | <.02 | |
| Cluster 3: Right Superior Corona Radiata | 17 | 26.0 | 1 | 36.0 | 3.44 | <.03 |
| Cluster 4: Left Superior Longitudinal Fasciculus | 12 | −46.0 | 450 | −8.0 | 4.04 | <.05 |
| Cluster 5: Right Inferior/Superior Longitudinal Fasciculus | 11 | 54.0 | 13.0 | −24.0 | 4.22 | <.06 |
Fig. 1.
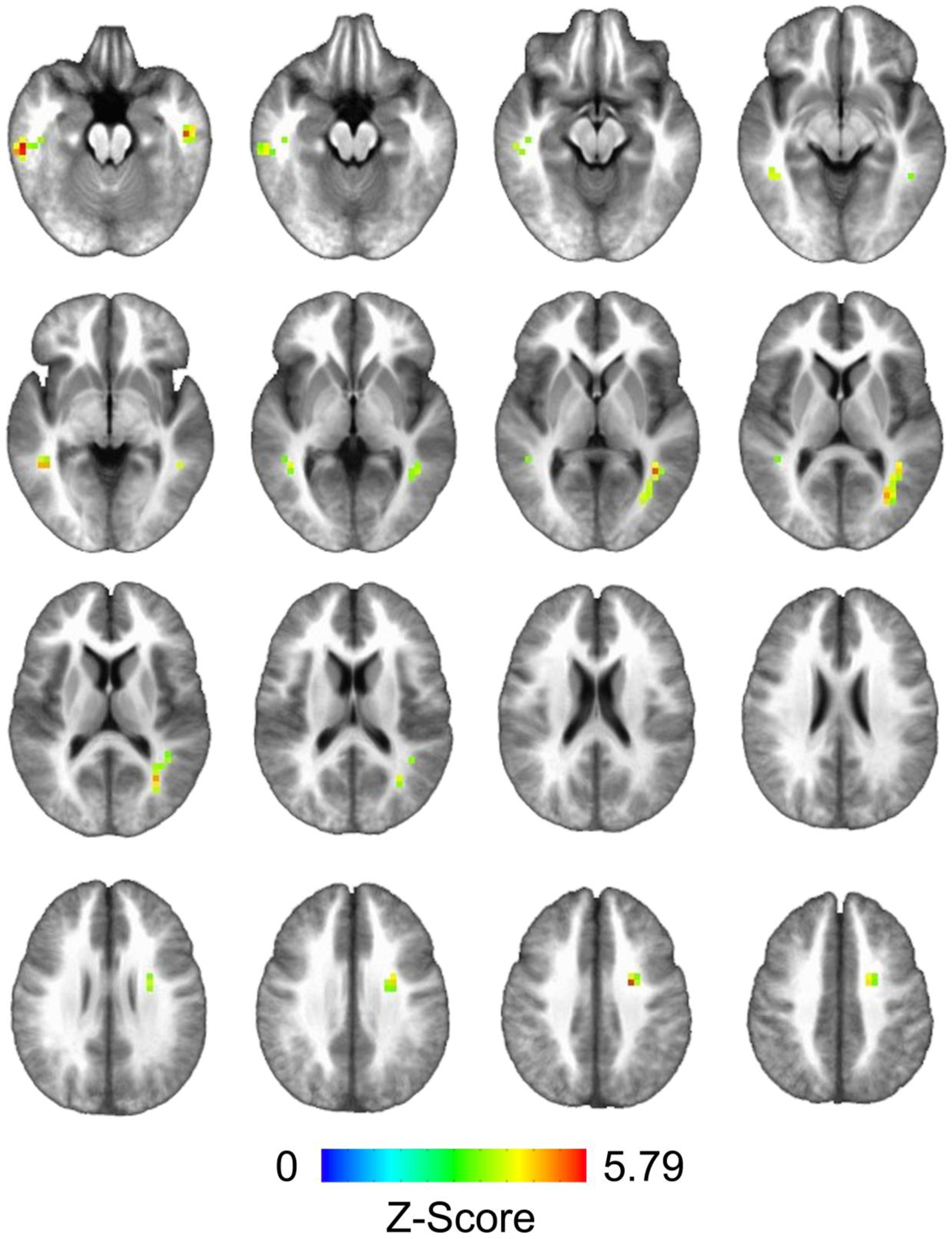
Clusters of significant cerebral blood flow (CBF) response obtained from the interaction effect between nicotine group and past year cannabis use (thresholded at a voxel-wise alpha of 0.001, cluster-wise alpha of 0.05; ≥ 11 contiguous voxels)
Fig. 2.
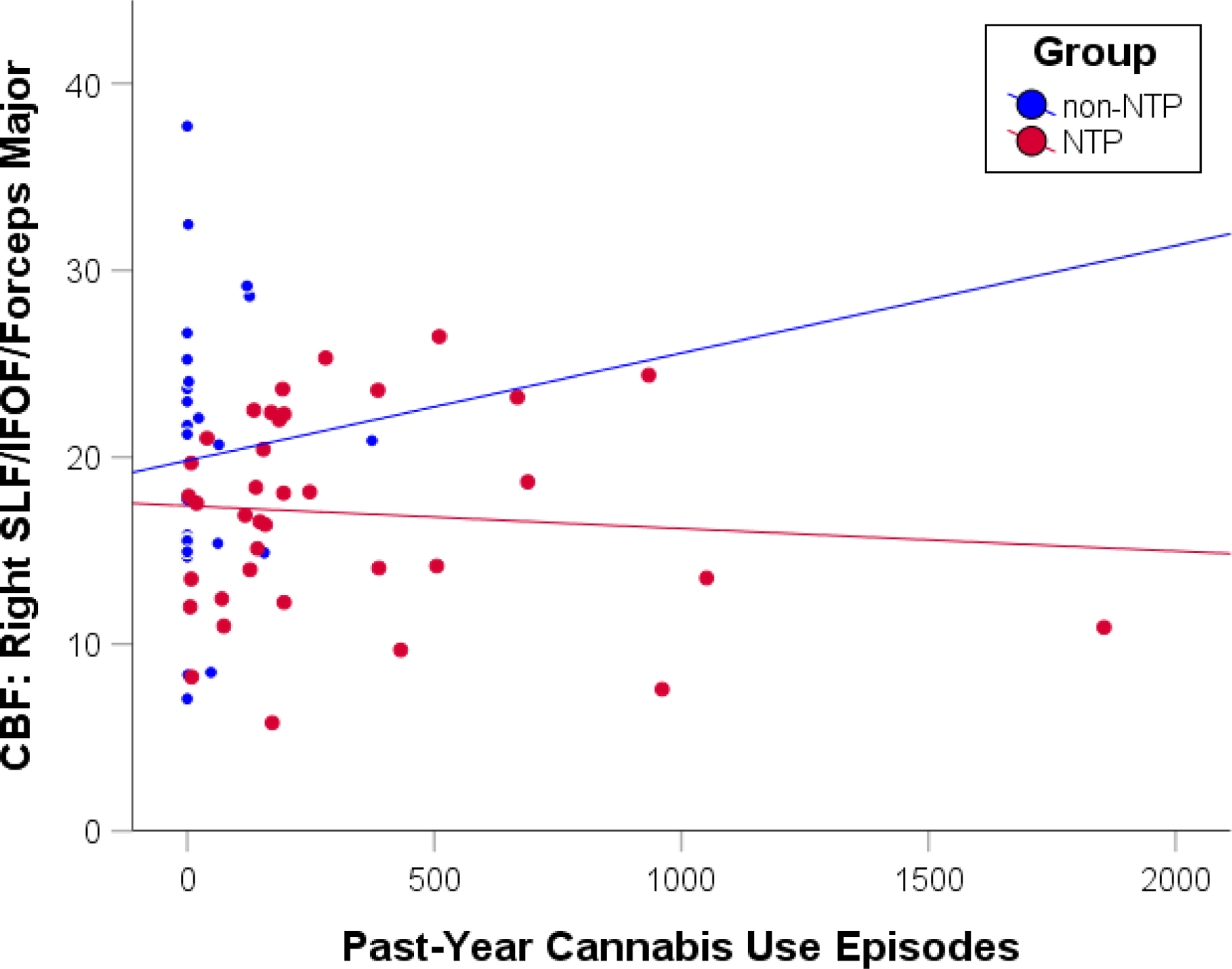
Scatterplot depicting the correlations between past-year cannabis use episodes and cerebral blood flow (CBF) extracted from the right superior longitudinal fasciculus (SLF)/inferior fronto-occipital fasciculus (IFOF)/forceps major cluster for the nicotine (NTP) and non-nicotine groups (non-NTP). Scatterplot presented for visualization purposes only - no statistics were calculated to avoid potential inflation of the correlation estimate
CBF Cluster Correlations with FA and DASS Scores
The correlations between CBF and FA estimates extracted from the five clusters identified in the interaction effect revealed one borderline statistically significant result within Cluster 1 (right superior longitudinal fasciculus/inferior fronto-occipital fasciculus/forceps major). Within this cluster, greater CBF was associated with reduced FA values for the non-NTP group (r = −0.49, p = .01), but no statistically significant relationship was observed for the NTP group (r = −0.12, p = .47; see Figure 3). Comparison of the two groups’ correlation coefficients confirmed that the groups statistically differed in their CBF and FA correlations (z = −2.44, p =.01).
Fig. 3.
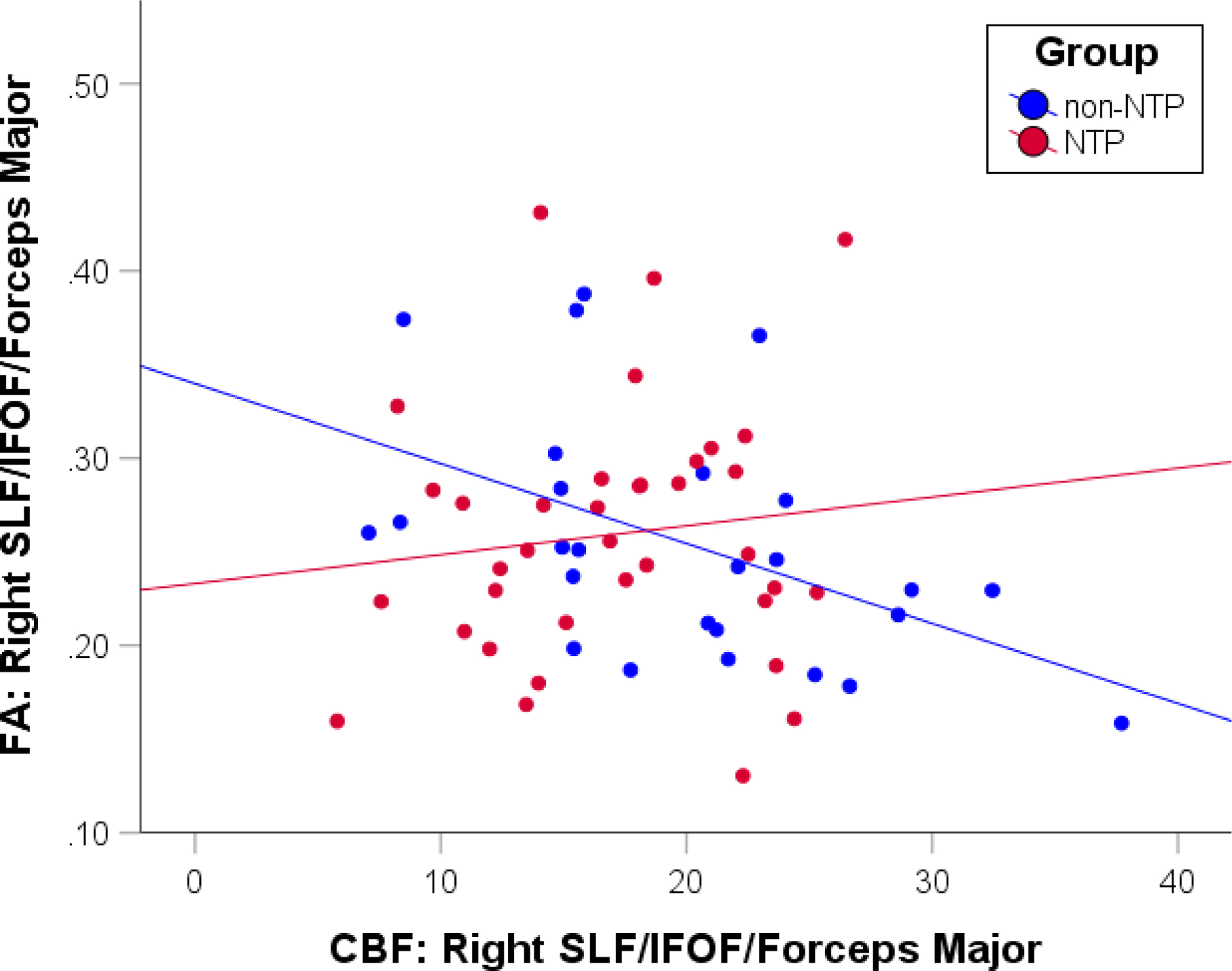
Scatterplot depicting the correlations between fractional anisotropy (FA) and cerebral blood flow (CBF) extracted from the right superior longitudinal fasciculus (SLF)/inferior fronto-occipital fasciculus (IFOF)/forceps major cluster for the nicotine (NTP: r = −0.12, p = .47) and non-nicotine (non-NTP: r = −0.49, p = .01) groups separately
Given the presence of significant group differences in the DASS subscale scores, exploratory follow-up analyses examining the correlations between DASS scores, CBF, and FA metrics were conducted. Statistically significant across and within-group correlations were observed between the DASS stress subscale scores and CBF estimates extracted from Cluster 4 (left superior longitudinal fasciculus) only (full sample: r=.34, p=.006; NTP: r=.43, p=.008; non-NTP: r=.33, p=.102); however, DASS stress scores did not significantly correlate with past-year NTP use (NTP: r=.12, p=.478). Furthermore, no significant correlations were found between CBF and age of substance use onset (ps > .07), or between FA cluster estimates and DASS subscale scores or substance use metrics (past-year NTP or cannabis use episodes) (ps > .10).
DISCUSSION
This preliminary report sought to investigate the effects of nicotine and cannabis co-use on CBF markers of white matter health using an optimized PCASL scanning protocol. The results suggest that nicotine and cannabis use may interact to produce effects on white matter CBF estimates, such that increased cannabis use by itself is related to increased CBF in association (superior longitudinal fasciuculs) and projection (corona radiata) white matter fiber tracts, yet the occurrence of even infrequent NTP use (greater than once per month) appears to diminish this relationship. This result seems to be inconsistent with human and animal reports of a decline in gray matter CBF associated with the chronic effects of nicotine (Chaarani et al. 2019; Vafaee et al. 2015) and cannabis (for a review see Ogunbiyi et al. 2020); however, it is unclear whether the same relationships should hold for gray and white matter CBF, particularly in late adolescence/young adulthood during which gray and white matter neurodevelopment processes progress independently. Notably, these findings are unchanged after controlling for recency of nicotine and cannabis use.
NTP use has been associated with both vasoconstrictive and vasodilatory effects, but acute nicotine administration appears to have a dose-dependent vasodilation effect on cerebrovasculature (Iida et al. 1998; Toda 1975). Acute cannabis use has similarly been associated with cerebral vasodilation, yet concomitant vasoconstriction is also often observed (Richter et al. 2018). Chronic effects of either substance on cerebral vasculature are even more unclear, suggesting the presence of a complex relationship between dosage, recency of use, and region-specific effects. The observed relationship between greater CBF and less FA in the non-NTP group may represent increased metabolic demand for tissue that is less developed, coherent, tightly packed, and myelinated and therefore less efficient. For example, Aslan and colleagues (2011) found that, among healthy adults, fiber tracts with higher FA values had lower CBF values. The authors suggest that greater axonal diameter and myelination may contribute to receiving less blood flow, which is consistent with older adults showing decreased CBF blood flow in white matter (Lu et al. 2011).
One possible explanation is that nicotine may be functioning to counteract cannabis-induced deleterious effects on white matter in the NTP group. This hypothesis is partially supported by 1) the non-NTP group demonstrating greater CBF associated with more past year cannabis use across several white matter tracts and 2) the non-NTP group demonstrating greater CBF associated with poorer white matter integrity estimates sensitive to microstructural changes (decreased FA). Regular cannabis use has been consistently linked to decreased FA and other DTI-derived diffusion estimates (Gruber et al. 2014; Jacobus et al. 2013a; Jacobus et al. 2013b; Jakabek et al. 2016; Orr et al. 2016); however, this is less consistent among regular NTP users. In fact, several studies report increased FA among NTP users (Jacobsen et al. 2007; Kangiser et al. 2019; van Ewijk et al. 2015; Yu et al. 2016) and suggest that stimulation of acetylcholine receptors (nAChRs) via nicotine use may result in the promotion of some neurodevelopmental processes, including glial activity (Garrido et al. 2003; Liu et al. 2005), that support maturation of white matter (Dwyer et al. 2009). Further, nicotine may be distinct in how it acts on the adolescent brain as compared to earlier and later developmental stages, including how it interacts with other endogenous systems such as the endocannabinoid system (Scherma et al. 2016), which also plays a significant role in neurodevelopment (Meyer et al. 2018).
This preliminary study has a number of strengths. The well-characterized sample of adolescent/young adult substance users represents a significant strength as measurement of nicotine and cannabis use is exceedingly difficult given the increasing popularity and variety of new devices and delivery methods. The inclusion of all NTP-type use, as opposed to limiting the inclusion to tobacco cigarette use only, further increases the generalizability of these findings to the majority of adolescent/young adult nicotine users. The use of the optimized PCSAL protocol to enable more precise estimates of white matter CBF represents another strength and the results support the potential utility of this advanced technology for future white matter CBF investigations.
The present study is also not without limitations. Although attempts were made to match the groups based on other substance use and demographic variables, the NTP group did exhibit slightly elevated depression, anxiety, and stress, as well as greater frequency of alcohol and cannabis use. Follow-up analyses found support for the influence of stress on CBF estimates extracted from one of the five clusters; however, stress appears to be independent from past-year NTP use in this sample. Regardless, stress and anxiety levels are likely a confound to some degree given the generally higher levels of these factors in nicotine users. Whether these elevated levels represent antecedents or consequences of NTP use in this sample remains unknown. Additional exploratory analyses on these subjects were unable to detect alcohol-related effects on CBF markers, yet that does not preclude potential subthreshold alcohol-related interactions in the observed effects. Further, although explicitly modeled as a moderator the analyses, differences in cannabis use between the groups were large and could have impacted the results; thus future investigations are needed with large, non-using ‘control’ and ‘pure’ cannabis use groups to tease apart the individual contributions of nicotine and cannabis use on white matter health. In addition, the NTP group consisted of individuals reporting a range of low (at least monthly) to heavy (>20 per day) NTP use episodes over the previous year; yet, the majority of this sample reported lower frequencies of use (median use = 94 episodes past year). This limits the generalization of the results to frequent and heavy NTP users. However, the presence of NTP-related effects even while including individuals with lower use frequencies suggests heavy use levels may not be needed to detect meaningful nicotine induced effects on white matter health. Lastly, given the cross-sectional nature of the data, directional hypotheses relating substance co-use to future brain health cannot be tested.
The present report represents the first investigation of white matter health as indexed by CBF, and its association with FA, in adolescent/young adult users of nicotine and/or cannabis products. The results support the presence of important interactions of these two substances on markers of brain health which may differentially impact future psychological and behavioral outcomes of young co-users, as compared to single substance users. Future investigations of the nuanced effects of nicotine and cannabis co- versus single substance use on white matter CBF and diffusion metrics, as well as the roles of pre-existing characteristics, are planned for the larger sample. Cannabis use has been associated with alterations in white matter development trajectories (Becker et al. 2015), thus additional longitudinal studies are also greatly needed to model these brain health estimates and clinical outcomes as a function of co-substance use during this neurologically vulnerable period.
Acknowledgments:
Research supported by the National Institute on Drug Abuse grants U01 DA041089 and R21 DA047953 and the California Tobacco-Related Disease Research Grants Program Office of the University of California grants 580264 and T30IP0962.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest Statement:
On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
- Agrawal A, Budney AJ, Lynskey MT (2012) The co-occurring use and misuse of cannabis and tobacco: a review. Addiction 107:1221–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, Madden PA, Pergadia ML, Bucholz KK, Heath AC (2009) Simultaneous cannabis and tobacco use and cannabis-related outcomes in young women. Drug Alcohol Depend 101:8–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20:870–88 [DOI] [PubMed] [Google Scholar]
- Aslan S, Huang H, Uh J, Mishra V, Xiao G, van Osch MJ, Lu H (2011) White matter cerebral blood flow is inversely correlated with structural and functional connectivity in the human brain. Neuroimage 56:1145–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF (2010) Longitudinal characterization of white matter maturation during adolescence. Brain Res 1327:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M (2015) Longitudinal changes in white matter microstructure after heavy cannabis use. Dev Cogn Neurosci 16:23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003) Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50:1077–88 [DOI] [PubMed] [Google Scholar]
- Bélanger RE, Marclay F, Berchtold A, Saugy M, Cornuz J, Suris JC (2013) To what extent does adding tobacco to cannabis expose young users to nicotine? Nicotine Tob Res 15:1832–8 [DOI] [PubMed] [Google Scholar]
- Brown GG, Eyler Zorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB (2003) BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J Cereb Blood Flow Metab 23:829–37 [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW (1998) Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. J Stud Alcohol 59:427–438 [DOI] [PubMed] [Google Scholar]
- Chaarani B, Spechler PA, Ivanciu A, Snowe M, Nickerson JP, Higgins ST, Garavan H (2019) Multimodal Neuroimaging Differences in Nicotine Abstinent Smokers Versus Satiated Smokers. Nicotine Tob Res 21:755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Infante MA, Brown GG, Tapert SF, Simmons AN, Smith TL, Schuckit MA (2019) The relationship between regional cerebral blood flow estimates and alcohol problems at 5-year follow-up: the role of level of response. Alcohol Clin Exp Res 43:812–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE (2012) Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage 59:3845–51 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–73 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017) FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect 7:152–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–94 [DOI] [PubMed] [Google Scholar]
- Desrosiers NA, Lee D, Schwope DM, Milman G, Barnes AJ, Gorelick DA, Huestis MA (2012) On-site test for cannabinoids in oral fluid. Clin Chem 58:1418–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, Zubieta JK (2000) Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse 38:313–21 [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM (2009) The dynamic effects of nicotine on the developing brain. Pharmacol Ther 122:125–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016) Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 113:7900–7905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman BJ (2015) Cannabis problem experiences among users of the tobacco-cannabis combination known as blunts. Drug Alcohol Depend 150:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31:361–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Gohel S, Prashad S, Biswal BB (2018) Differential associations of combined vs. isolated cannabis and nicotine on brain resting state networks. Brain Struct Funct 223:3317–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM, Fields RD (2016) White matter and cognition: making the connection. J Neurophysiol 116:2093–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien IK, Walhovd KB (2011) Reduced white matter integrity is related to cognitive instability. J Neurosci 31:18060–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido R, King-Pospisil K, Son KW, Hennig B, Toborek M (2003) Nicotine upregulates nerve growth factor expression and prevents apoptosis of cultured spinal cord neurons. Neurosci Res 47:349–55 [DOI] [PubMed] [Google Scholar]
- Geeraert BL, Lebel RM, Lebel C (2019) A multiparametric analysis of white matter maturation during late childhood and adolescence. Hum Brain Mapp 40:4345–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN (2004) Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci 1021:77–85 [DOI] [PubMed] [Google Scholar]
- Giedd JN (2015) Adolescent neuroscience of addiction: A new era. Dev Cogn Neurosci 16:192–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Keshavan M, Paus T (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giezendanner S, Fisler MS, Soravia LM, Andreotti J, Walther S, Wiest R, Dierks T, Federspiel A (2016) Microstructure and Cerebral Blood Flow within White Matter of the Human Brain: A TBSS Analysis. PLoS One 11:e0150657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MS, Drobnjak I, Jenkinson M, Zhang H (2017) Quantitative assessment of the susceptibility artefact and its interaction with motion in diffusion MRI. PLoS One 12:e0185647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Lukas SE (2014) Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology (Berl) 231:1455–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, Sutherland MT, Casey BJ, Barch DM, Harms MP, et al. (2019) Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage 202:116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S (2008) Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39:336–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudkins M, O’Neill J, Tobias MC, Bartzokis G, London ED (2012) Cigarette smoking and white matter microstructure. Psychopharmacology (Berl) 221:285–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida M, Iida H, Dohi S, Takenaka M, Fujiwara H (1998) Mechanisms underlying cerebrovascular effects of cigarette smoking in rats in vivo. Stroke 29:1656–65 [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, Jackowski MP, Constable RT, Mencl WE (2007) Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci 27:13491–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF (2013a) White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res 214:374–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF (2013b) White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci 3:396–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Taylor CT, Gray KM, Meredith LR, Porter AM, Li I, Castro N, Squeglia LM (2018) A multi-site proof-of-concept investigation of computerized approach-avoidance training in adolescent cannabis users. Drug Alcohol Depend 187:195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakabek D, Yücel M, Lorenzetti V, Solowij N (2016) An MRI study of white matter tract integrity in regular cannabis users: effects of cannabis use and age. Psychopharmacology (Berl) 233:3627–37 [DOI] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME (2020) Monitoring the Future national survey results on drug use 1975–2019: Overview, key findings onadolescent drug use. Institute for Social Research, University of Michigan, Ann Arbor [Google Scholar]
- Jueptner M, Weiller C (1995) Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage 2:148–56 [DOI] [PubMed] [Google Scholar]
- Kangiser MM, Thomas AM, Kaiver CM, Lisdahl KM (2019) Nicotine Effects on White Matter Microstructure in Young Adults. Arch Clin Neuropsychol 35:10–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Schacht JP, Jacobus J, Meredith LR, Taylor CT, Tapert SF, Gray KM, Squeglia LM (2019a) Preliminary evidence that computerized approach avoidance training is not associated with changes in fMRI cannabis cue reactivity in non-treatment-seeking adolescent cannabis users. Drug Alcohol Depend 200:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Schacht JP, Meredith LR, Jacobus J, Tapert SF, Gray KM, Squeglia LM (2019b) Investigating a novel fMRI cannabis cue reactivity task in youth. Addict Behav 89:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D (2003) Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4:469–80 [DOI] [PubMed] [Google Scholar]
- Lebel C, Deoni S (2018) The development of brain white matter microstructure. Neuroimage 182:207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C (2012) Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60:340–52 [DOI] [PubMed] [Google Scholar]
- Liu JJ, Mohila CA, Gong Y, Govindarajan N, Onn SP (2005) Chronic nicotine exposure during adolescence differentially influences calcium-binding proteins in rat anterior cingulate cortex. Eur J Neurosci 22:2462–74 [DOI] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF (1995) Manual for the Depression Anxiety & Stress Scales (2 Ed.). Psychology Foundation, Sydney [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC (2011) Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 21:1426–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Liu T, Wong E, Jung Y (2009) Regional white matter perfusion measurement using an optimized pseudo-continuous ASL MRI 17th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Honolulu, HI, pp 1521 [Google Scholar]
- Meyer HC, Lee FS, Gee DG (2018) The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain Development. Neuropsychopharmacology 43:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Herting MM, Meuwese R, Blakemore SJ, Crone EA, Dahl RE, Guroglu B, Raznahan A, Sowell ER, et al. (2016) Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. Neuroimage 141:273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM (2005) MRI Atlas of Human White Matter. Elsevier, Amsterdam, The Netherlands [Google Scholar]
- Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–39 [DOI] [PubMed] [Google Scholar]
- Nicksic NE, Do EK, Barnes AJ (2020) Cannabis legalization, tobacco prevention policies, and Cannabis use in E-cigarettes among youth. Drug Alcohol Depend 206:107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll DC, Fessler JA, Sutton BP (2005) Conjugate phase MRI reconstruction with spatially variant sample density correction. IEEE Trans Med Imaging 24:325–36 [DOI] [PubMed] [Google Scholar]
- Ogunbiyi MO, Hindocha C, Freeman TP, Bloomfield MAP (2020) Acute and chronic effects of Delta(9)-tetrahydrocannabinol (THC) on cerebral blood flow: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry:109900. [DOI] [PubMed] [Google Scholar]
- Orr JM, Paschall CJ, Banich MT (2016) Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. Neuroimage Clin 12:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, Brown SA, Colrain IM, Baker FC, Prouty D, et al. (2018) Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. Am J Psychiatry 175:370–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P (2001) Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13:1174–85 [DOI] [PubMed] [Google Scholar]
- Raichle ME, Grubb RL Jr., Gado MH, Eichling JO, Ter-Pogossian MM (1976) Correlation between regional cerebral blood flow and oxidative metabolism. In vivo studies in man. Arch Neurol 33:523–6 [DOI] [PubMed] [Google Scholar]
- Ramo DE, Delucchi KL, Hall SM, Liu H, Prochaska JJ (2013) Marijuana and tobacco co-use in young adults: patterns and thoughts about use. J Stud Alcohol Drugs 74:301–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream GL, Benoit E, Johnson BD, Dunlap E (2008) Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend 95:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JS, Quenardelle V, Rouyer O, Raul JS, Beaujeux R, Gény B, Wolff V (2018) A Systematic Review of the Complex Effects of Cannabinoids on Cerebral and Peripheral Circulation in Animal Models. Front Physiol 9:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE (2003) PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiatry 160:323–33 [DOI] [PubMed] [Google Scholar]
- Scherma M, Muntoni AL, Melis M, Fattore L, Fadda P, Fratta W, Pistis M (2016) Interactions between the endocannabinoid and nicotinic cholinergic systems: preclinical evidence and therapeutic perspectives. Psychopharmacology (Berl) 233:1765–77 [DOI] [PubMed] [Google Scholar]
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME (2019) Monitoring the Future national survey results on drug use, 1975–2018: Volume II, College students and adults ages 19–60. Institute for Social Research, The University of Michigan, Ann Arbor [Google Scholar]
- Schuster RM, Mermelstein RJ, Hedeker D (2016) Ecological momentary assessment of working memory under conditions of simultaneous marijuana and tobacco use. Addiction 111:1466–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DD, Liu TT, Wong EC, Shankaranarayanan A, Jung Y (2012) Pseudocontinuous arterial spin labeling with optimized tagging efficiency. Magn Reson Med 68:1135–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DD, Ozyurt IB, Brown GG, Fennema-Notestine C, Liu TT (2016) The Cerebral Blood Flow Biomedical Informatics Research Network (CBFBIRN) data repository. Neuroimage 124:1202–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DD, Ozyurt IB, Liu TT (2013) The Cerebral Blood Flow Biomedical Informatics Research Network (CBFBIRN) database and analysis pipeline for arterial spin labeling MRI data. Front Neuroinform 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds D, Hallquist MN, Asato M, Luna B (2014) Developmental Stages and Sex Differences of White Matter and Behavioral Development through Adolescence: A Longitudinal Diffusion Tensor Imaging (DTI) Study. Neuroimage 92:356–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208–19 [DOI] [PubMed] [Google Scholar]
- Stiby AI, Hickman M, Munafo MR, Heron J, Yip VL, Macleod J (2015) Adolescent cannabis and tobacco use and educational outcomes at age 16: birth cohort study. Addiction 110:658–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer RE, YorkWilliams S, Karoly HC, Sabbineni A, Ewing SF, Bryan AD, Hutchison KE (2017) Structural neuroimaging correlates of alcohol and cannabis use in adolescents and adults. Addiction 112:2144–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N (1975) Nicotine-induced relaxation in isolated canine cerebral arteries. J Pharmacol Exp Ther 193:376–84 [PubMed] [Google Scholar]
- Tucker JS, Pedersen ER, Seelam R, Dunbar MS, Shih RA, D’Amico EJ (2019) Types of cannabis and tobacco/nicotine co-use and associated outcomes in young adulthood. Psychol Addict Behav 33:401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaee MS, Gjedde A, Imamirad N, Vang K, Chakravarty MM, Lerch JP, Cumming P (2015) Smoking normalizes cerebral blood flow and oxygen consumption after 12-hour abstention. J Cereb Blood Flow Metab 35:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R (2002) Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol 135:564–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk H, Groenman AP, Zwiers MP, Heslenfeld DJ, Faraone SV, Hartman CA, Luman M, Greven CU, Hoekstra PJ, Franke B, et al. (2015) Smoking and the developing brain: altered white matter microstructure in attention-deficit/hyperactivity disorder and healthy controls. Hum Brain Mapp 36:1180–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen P, de Zwart JA, Duyn JH (2008) Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn Reson Med 59:788–95 [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, et al. (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36:630–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennmalm A (1982) Effect of cigarette smoking on basal and carbon dioxide stimulated cerebral blood flow in man. Clin Physiol 2:529–35 [DOI] [PubMed] [Google Scholar]
- Willing J, Juraska JM (2015) The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience 301:268–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Lin SC, Wang DJ, Chen KL, Li YD (2013) Measurement of cerebral white matter perfusion using pseudocontinuous arterial spin labeling 3T magnetic resonance imaging--an experimental and theoretical investigation of feasibility. PLoS One 8:e82679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Yuan K, Zhang B, Liu J, Dong M, Jin C, Luo L, Zhai J, Zhao L, Zhao Y, et al. (2016) White matter integrity in young smokers: a tract-based spatial statistics study. Addict Biol 21:679–87 [DOI] [PubMed] [Google Scholar]


