Abstract
Purpose:
The utility of topical antibiotic prophylaxis for routine oculofacial plastic surgery is not well-established. Given concerns such as contact dermatitis, antibiotic resistance, and healthcare costs, in conjunction with a low baseline rate of surgical site infections, the investigators sought to determine the frequency of infection with and without the use of topical antibiotic prophylaxis.
Design:
Randomized, controlled, unmasked clinical trial.
Participants:
Adult patients undergoing routine periocular surgery without prior history of periocular surgical site infection, need for perioperative oral or parenteral antibiotics, or allergy to all study medications.
Methods:
Participants were randomized pre-operatively to receive either antibiotic or placebo (mineral oil/petrolatum-based) ointment after surgery. Outcomes were measured at the first post-operative visit. The two-tailed Fisher’s exact test was used to compare outcomes between groups.
Main Outcome Measures:
The primary outcome was the incidence of surgical site infections. The secondary outcomes included stratification of infections by patient risk characteristics, incidence of allergic contact dermatitis, and incidence of wound complications.
Results:
Four hundred and one participants were enrolled and randomized, and 13 participants did not proceed with surgery or were lost to follow-up. High-risk features for infection were identified in 24% of the placebo group and 21% of the antibiotic group. Surgical site infections were more common in the placebo group (2.7% vs. 0.0%; p=0.025). The rate of contact dermatitis was similar (0.5% vs. 0.5%; p=1.00), as was the rate of wound dehiscence (2.7% vs. 3.5%; p=0.77). Among the placebo group, the incidence of infections in the low- and high-risk participants was 2.9% and 2.2%, respectively. Infections were treated with oral or topical antibiotics and resolved without complication, except in one case that required two subsequent surgeries to address the sequelae.
Conclusion:
After routine oculofacial plastic surgery, patients treated with a topical antibiotic ointment had a lower risk of surgical site infection compared with patients treated with a non-antibiotic ointment.
The current standard of care among most oculofacial plastic surgeons is to provide patients with postoperative topical antibiotics in order to prevent surgical site infections (SSIs). For instance, a 2014 survey of oculofacial plastic surgeons from 43 countries revealed that while rates of prophylactic oral and perioperative intravenous antibiotic use varied considerably, topical antibiotic use was common across all geographic regions (85.2%).1 However, the rate of postoperative infection from clean and clean-contaminated surgical wounds, including those from periocular surgery, are known to be low.2–4 Presumably, the rich vascularity of the periocular region and relatively short case lengths of periocular surgeries contribute to this low infection rate.5
As antibiotic resistance,6,7 antibiotic-related adverse events,8,9 and healthcare costs are of increasing concern, antibiotic stewardship for oculofacial plastic surgery is imperative. The use of mineral oil/petrolatum-based topical ointments lacking antibiotic has been suggested as an alternative to traditional regimens, and has been studied for many types of procedures without a clear consensus10,11; however, to the investigators’ knowledge, this topic has not been addressed in the oculofacial plastic literature. Therefore, this study aimed to test the hypothesis that topical antibiotic prophylaxis does not significantly reduce the rate of infection after oculofacial plastic surgery, relative to a non-antibiotic ointment.
Methods
Study Design and Setting
The study was an investigator-initiated, randomized controlled trial approved by the University of California, San Francisco (UCSF) Institutional Review Board (IRB#:17-22309). The trial was registered at http://www.clinicaltrials.gov (Identifier - NCT03199911), and the protocol was submitted for online access. Patients undergoing periocular surgery were recruited from the Oculofacial Plastic Surgery Clinic at UCSF. The first participant was enrolled on October 4, 2017, and the last observation was recorded on November 14, 2019 after meeting the pre-specified enrollment target. Written informed consent was obtained from all participants and the study was performed in compliance with the provisions of the United States of America Health Insurance Portability and Accountability Act of 1996 and adhered to the World Medical Association’s ethical principles for medical research involving human subjects outlined in the Declaration of Helsinki as amended in 2013.
Selection of Participants
The study population consisted of participants who were 18 years or older undergoing periocular surgery in the operating room or office-based minor procedure room, including blepharoplasty (upper and/or lower eyelid); ectropion repair; entropion repair; external levator resection; internal levator resection; external dacryocystorhinostomy; wedge excision; eyelid lesion removal and/or biopsy; tarsorrhaphy; eyelid reconstruction after Mohs surgery; and orbitotomy requiring periocular incisions. The exclusion criteria included prior history of SSI at the site of surgery; contaminated wound resulting from trauma, human, or animal bite; use of oral or parenteral antibiotic within 10 days prior to or during the procedure; chalazion removal; and history of allergy to all of the study medications.
Treatment Assignment
A study investigator (OOI) randomized participants meeting selection criteria pre-operatively into the topical antibiotic or placebo groups on a 1:1 basis using a simple randomization scheme without matching, blocking, or stratification. The process was performed using the Research Electronic Database Capture (REDCap) tools (version 9.1.23) hosted at UCSF.12,13
The antibiotic group received 0.5% erythromycin ophthalmic ointment (Akorn, Lake Forest, IL), bacitracin zinc 500 unit/gram ophthalmic ointment (Bausch+Lomb, Bridgewater, NJ), or bacitracin zinc-polymyxin B sulfate 500-10,000 unit/gram ophthalmic ointment (Bausch+Lomb, Bridgewater, NJ) depending on pharmacy availability, patient allergies, and surgeon preference. Participants in the placebo group received any of the following ophthalmic lubricant ointments composed of mineral oil and petrolatum: Refresh PM (Allergan, Irvine, CA), Refresh Lacri-lube (Allergan, Irvine, CA), Pura-Lube (Paddock Laboratories, Minneapolis, MN) or GenTeal Tears Severe (Alcon Laboratories, Elkridge, MD). Each participant was instructed to apply the assigned medication on the surgical site(s) four times a day for seven days following surgery. Neither the participants nor investigators were masked during the study.
Surgical Preparation
Participants undergoing surgery in the operating room were prepared in typical fashion for oculofacial plastic surgery. A pre-operative instruction brochure recommended bathing with soap and water prior to arriving at the surgery center. Surgeons used a 10% povidone-iodine surgical skin scrub to sterilize the operative site; the surgeons do not use chlorhexidine in the periocular region due to concern regarding ocular toxicity.14–16 The surgical team employed typical surgical gloves, gowns, and drapes to maintain surgical asepsis.
For participants undergoing surgery in the minor procedure room, surgeons selected the intensity of surgical preparation based on the characteristics of the planned surgery. Surgeons practiced clean technique for surgeries involving only the superficial skin without the need for suturing, such as biopsy of marginal eyelid lesions. Clean technique involved the use of sterile instruments but non-sterile gloves, and did not include a surgical skin preparation.17 Surgeons practiced typical sterile technique as described above if the surgery involved deeper layers or suturing.
Outcome Measures
The primary outcome was the frequency of surgical site infection (SSI), both superficial or deep incisional, of clean and clean-contaminated wounds at the first post-operative visit occurring approximately 7-14 days after surgery. Superficial and deep incisional SSI were defined per modified CDC surgical site infection criteria18 (Figure S1, available at http://www.aaojournal.org), and were evaluated by one of the two senior investigators (MRV or RCK).
Secondary outcomes were the frequency of allergic contact dermatitis and wound dehiscence, as well as subgroup analysis of the infection rate among high-versus low-risk for infection participants. Investigators classified participants as high-risk for infection based on a medical history consistent with immunosuppression or a history of smoking.
Participants were asked to take note of medication compliance and adverse events. Throughout the study, any signs or symptoms of adverse events were recorded on a case report form, graded for severity and assessed for their relationship to the study’s medications.
Data Collection
Co-investigators (DCA, OOI, ST, and TSC) collected and stored data at each study visit using the REDCap tool hosted at UCSF.
Evaluation by Clinical Visit
Participants were enrolled at the time of their decision to pursue oculofacial plastic surgery. Investigators collected data on demographics, medical history, medication history, drug allergies, history of SSI, and a routine eye examination. Outcomes were assessed on the first post-operative visit, occurring approximately 7-14 days after surgery; this visit occurred earlier than scheduled in the event that the participant sought care for a perceived adverse event. Data collection included: adverse events (via examination and history), compliance deviations, changes in medical/medication history, routine eye examination, presence of SSI, and evidence of drug allergy or wound dehiscence. Infections were treated with oral or topical antibiotics, per the surgeon’s clinical judgment. In the event of an SSI, patients were followed until infection resolution. At each subsequent visit, data was collected on adverse events, medication compliance, eye examination, and treatment response. Investigators performed a retrospective chart review following study completion to identify evidence of delayed-onset infections occurring after the prospective post-operative evaluation.
Early Withdrawal Visit
In the case of a visit where a patient was withdrawn early from the study, data was collected on adverse events, medication compliance, eye examination, and changes to medications.
Safety Evaluations
Adverse events were reported according to the UCSF IRB reporting requirements. Serious adverse events were monitored on an ongoing basis throughout the study. Study data from each subsequent set of 25 enrolled were reviewed regularly by a Data Safety Monitoring Committee (DSMC). The DSMC received regular updates, including information such as: number of patients screened, number of participants enrolled, number of participants randomized/receiving treatment, number of participants lost to follow-up, number of participants meeting the outcome definition, and number of participants with an adverse event.
Statistical Analysis and Sample Size Determination
The investigators set the recruitment target as the approximate institutional surgical volume of eligible patients for two years (400), aiming to identify whether a clinically relevant difference would be present on that time scale. For clean wounds, an infection rate greater than 5% is considered unacceptable19–21; therefore, this sample size allowed 87% power at a 95% confidence level to detect a difference between an expected infection rate of 0.2% with antibiotics versus a maximum acceptable rate of 5% without antibiotics. Descriptive statistics were used to report the baseline characteristics. Analyses were intention to treat. The investigators estimated exact binomial proportion 95% confidence intervals (CI) for the frequency of each outcome. Outcomes were compared between the two arms using the two-tailed Fisher’s exact test, and risk ratios were calculated. All analyses were conducted in R version 4.0.0 (R Core Team, 2020).22
Results
Characteristics of Study Subjects
The investigators recruited 401 participants from the UCSF Oculofacial Plastic Surgery Clinic, with 193 randomized to the control (placebo) group and 208 to the experimental (antibiotic) group (Figure 1). The investigators excluded 13 participants (6 in the placebo group and 7 in the antibiotic group) after randomization because the participants did not proceed with surgery or were lost to follow-up. Investigators collected data for the primary and secondary outcomes on all remaining participants. The two groups were similar at baseline in terms of age, gender, ethnicity, type of surgery, surgery setting, surgeon, smoking status, and proportion deemed to be at higher risk for infection (Table 1). Participants ranged in age from 18 to 101 years old, with a mean of 63.5 ± 16.0 years. Among the antibiotic cohort, 170 (83%) of participants received ointment containing erythromycin, whereas 36 (17%) received ointment containing bacitracin. A minority of participants in both groups underwent surgery in the minor procedure room, and in all cases the type of surgery was eyelid lesion removal and/or biopsy.
Figure 1. Flow of Participants.
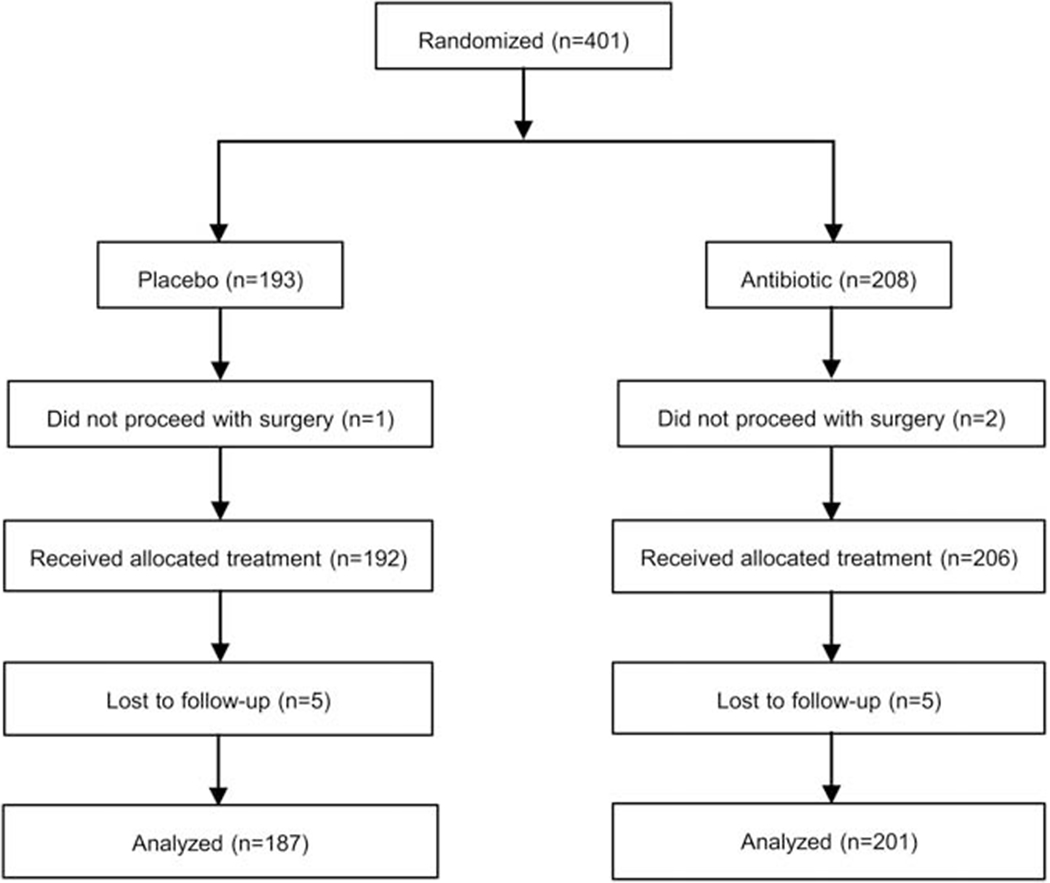
CONSORT flow diagram of participants randomized to receive topical antibiotic ointment or placebo ointment after surgery.
Table 1.
Baseline Clinical and Demographic Characteristics
| Placebo N (%) | Antibiotic N (%) | |
|---|---|---|
| Age category, years | ||
| 18-25 | 4 (2) | 9 (4) |
| 26-35 | 7 (4) | 8 (4) |
| 36-45 | 12 (6) | 9 (4) |
| 46-55 | 29 (15) | 25 (12) |
| 56-65 | 44 (23) | 53 (26) |
| 66-75 | 54 (28) | 50 (24) |
| 76-85 | 32 (17) | 42 (20) |
| >85 | 10 (5) | 10 (5) |
| Gender | ||
| Female | 112 (58) | 127 (62) |
| Male | 80 (42) | 79 (38) |
| Ethnicity | ||
| Asian | 29 (15) | 33 (16) |
| African American | 3 (2) | 8 (4) |
| Hispanic | 12 (6) | 17 (8) |
| Caucasian | 144 (75) | 148 (72) |
| Other | 3 (2) | 0 (0) |
| Unknown | 1 (1) | 0 (0) |
| Smoking status | ||
| No | 178 (93) | 188 (91) |
| Yes | 13 (7) | 18 (9) |
| Unknown | 1 (1) | 0 (0) |
| High-risk for infection | ||
| No | 145 (76) | 162 (79) |
| Yes | 47 (24) | 44 (21) |
| High-risk etiology* | ||
| Asplenia | 0 (0) | 1 (2) |
| Autoimmune disease | 2 (4) | 6 (14) |
| Diabetes mellitus | 31 (66) | 24 (55) |
| HIV infection | 4 (9) | 0 (0) |
| Malignancy | 2 (4) | 0 (0) |
| Organ transplantation | 1 (2) | 0 (0) |
| Smoking | 13 (28) | 18 (41) |
| Type of surgery* | ||
| Blepharoplasty | 39 (20) | 48 (23) |
| Browplasty | 0 (0) | 3 (1) |
| Dacryocystorhinostomy | 6 (3) | 1 (1) |
| Ectropion/Entropion repair | 25 (12) | 25 (13) |
| Eyelid lesion removal and/or biopsy | 36 (17) | 36 (19) |
| Ptosis repair | 43 (21) | 41 (21) |
| Reconstruction after Mohs surgery | 22 (11) | 25 (13) |
| Orbitotomy | 11 (5) | 8 (4) |
| Tarsorrhaphy | 1 (1) | 3 (2) |
| Wedge excision | 15 (7) | 15 (8) |
| Surgery Location | ||
| Minor procedure room | 30 (15.6) | 21 (10.2) |
| Operating room | 162 (84.4) | 184 (89.8) |
| Surgeon | ||
| RCK | 161 (83.9) | 169 (82.4) |
| MRV | 31 (16.1) | 36 (17.7) |
Some participants are included in multiple categories.
HIV= Human Immunodeficiency Virus; MRV = M. Reza Vagefi; RCK = Robert C. Kersten;
The mean time to the first post-operative visit was 9.4 ± 6.2 and 8.6 ± 4.9 days in the placebo and antibiotic groups, respectively. Participants in the placebo group had an additional two or more months of follow-up in 75.4% of cases and three or more months in 71.1%. Participants in the antibiotic group had one or more additional months of follow-up in 74.6% of cases, two or more months in 73.1%, and three or more months in 68.2%.
Clinical Outcomes
Investigators identified surgical site infections in five participants from the placebo arm (2.7%, [95% CI 0.9%, 6.1%]) and zero participants from the antibiotic arm (0%, [95% CI 0%, 1.8%]; p=0.025 for difference from placebo) (Table 2). The type of surgery complicated by infection was: ptosis repair (2), reconstruction after Mohs surgery (1), and wedge resection (2), all of which had been performed in the operating room setting. Surgeons treated infections with oral antibiotics (3) or topical antibiotic ointment (2); participants without infection did not receive oral or parenteral antibiotics during the prospective study follow-up period. Retrospective assessment did not identify evidence of delayed-onset infections among the available follow-up data in the medical record.
Table 2.
Primary and Secondary Outcomes by Treatment Arm
| Placebo N (%) | Antibiotic N (%) | Risk Ratio [95% CI] | p-value | |
|---|---|---|---|---|
| Surgical site infection | 5 (2.7) | 0 (0.0) | 0.00 | 0.025* |
| Contact dermatitis | 1 (0.5) | 1 (0.5) | 0.93 [0.06, 14.77] | 1.00 |
| Wound dehiscence | 5 (2.7) | 7 (3.5) | 1.30 [0.42,4.03] | 0.77 |
Fisher’s exact test, two-tailed.
p < 0.05.
CI = Confidence interval
The rate of infection in the placebo arm was 2.2% and 2.9% among high-risk and low-risk participants, respectively. Investigators did not proceed with a planned comparison of infection rates between the two arms stratified by perceived risk for infection due to the lack of infections in the antibiotic arm. One participant in each group developed allergic contact dermatitis (0.5%; p=1.00) (Table 2). Investigators noted wound dehiscence among five participants in the placebo group (2.7%), and seven participants in the antibiotic group (3.5%; p=0.77) (Table 2); none of these participants had infections.
Case Review of Surgical Site Infections
Case 1 involved a 77-year-old woman without high risk characteristics who had undergone ptosis repair by external levator resection in the operating room. On post-operative day 12, she developed erythema, swelling, and purulent discharge from the upper eyelid incision. The surgeon prescribed a 10-day course of oral azithromycin and the infection resolved without complication.
Case 2 involved a 63-year-old woman without high risk characteristics who had undergone ptosis repair by internal levator resection in the operating room. On post-operative day 3, she developed erythema, edema, and tenderness of the upper eyelid. The surgeon prescribed a two-week course of bacitracin-polymyxin B ointment and a 5-day course of oral azithromycin; the infection resolved without complication.
Case 3 involved a 41-year-old woman without high risk characteristics who had undergone reconstruction of a lower eyelid Mohs defect using a wedge excision of tarsal remnants followed by a myocutaneous advancement flap in the operating room. On post-operative day 3, her surgeon noted erythema, edema, and tenderness of her incision with purulent drainage. The surgeon prescribed bacitracin ointment and oral azithromycin. On post-operative day 5, the wound appeared less inflamed but she had persistent pain and wound cultures grew methicillin resistant Staphylococcus aureus insensitive to azithromycin; thus, the surgeon prescribed a 10-day course of trimethoprim/sulfamethoxazole. On post-operative day 20, the infection had resolved but the affected region exhibited dense cicatrix leading to a lateral ectropion. The surgeon treated the cicatrix with a series of intralesional triamcinolone injections, but ultimately, she required a wedge excision of the cicatrix at post-operative month three, followed by a lid retraction repair at post-operative year one.
Case 4 involved a 50-year-old woman with a history of smoking who had undergone wedge excision of a basal cell carcinoma in the operating room. On post-operative day 10, she developed increased pain, conjunctival injection, erythema, and purulent discharge. The surgeon prescribed a two-week course of tobramycin ointment, and the infection resolved without complication.
Case 5 involved an 84-year-old man without high risk characteristics who had undergone wedge excision of a basal cell carcinoma in the operating room. On post-operative day 4, the surgeon identified erythema, edema, and tenderness of the lower eyelid. The surgeon removed a gut suture centered in the infected region and prescribed a one-week course of bacitracin ointment, leading to resolution of the infection without complication.
Discussion
This study demonstrated a modestly higher incidence of SSI in the cohort treated with bland mineral oil/petrolatum ointment, as compared with the cohort treated with antibiotic ointment. The rate of SSI after oculofacial plastic surgery has generally been reported to be low, between 0.04%-0.2% for eyelid surgery,2,3 1-1.2% for external dacryocystorhinostomy,23,24 and 0.82% for orbitotomy4 in retrospective studies. Therefore, the frequency of infection in the placebo group was higher than the usual reported rates (exact 95% CI: 0.9% to 6.1%), whereas the frequency in the antibiotic group was consistent with previous reports (exact 95% CI: 0% to 1.8%). Taken together, these findings suggest a benefit to topical antibiotic therapy after oculofacial plastic surgery.
There is conflicting evidence regarding the efficacy of topical antibiotics for post-operative infection prophylaxis. Saco et al. reported no significant benefit to antibiotics in a meta-analysis of four randomized trials in dermatologic surgery.11 Notably, the risk ratio was 0.72 [95% CI of 0.44, 1.18], raising the question of whether a larger sample size might have demonstrated a small benefit to antibiotic therapy. Indeed, a more recent and larger meta-analysis by Heal et al. demonstrated a risk ratio of 0.61 [95% CI 0.42, 0.87] in favor of antibiotic therapy10; however, the applicability of their finding may be affected by their inclusion of a wide range of surgeries, some with higher baseline risk of infection than periocular surgery. A recent review on infection prophylaxis for periorbital Mohs surgery and reconstruction favored the use of non-antibiotic ointment based on the authors’ assessment of the literature.25 The findings of this present study instead contribute to the evidence base that topical antibiotic prophylaxis may be beneficial for oculofacial plastic surgery. In corroboration, a large retrospective cohort study of publicly available Medicare data demonstrated a decreased odds of infection among patients undergoing oculofacial plastic surgery who received post-operative oral antibiotics.26 An important consideration in the periocular region is the presence of eyelash and eyebrow hairs that have been demonstrated to harbor bacteria27,28, and furthermore to be difficult to account for with a variety of surgical preparation techniques.29,30 This may underlie the possible benefit of antibiotics for periocular surgery, as compared with other cutaneous surgeries in the dermatologic literature.
The decision to use post-operative antimicrobial prophylaxis depends not only on efficacy, but on consideration of cost and adverse effects. A commonly cited risk of topical antibiotic therapy is allergic contact dermatitis. Many topical antibiotics are associated with drug allergies, and patch testing has identified neomycin allergy in 11.6% and bacitracin allergy in 9.1% of the population.31 Notably, the rate of clinically significant allergic contact dermatitis in the periocular area has been reported to be lower,32 and a large meta-analysis was unable to draw conclusions about the relative effect of topical antibiotic versus non-antibiotic ointment on this complication (relative risk 3.94 [95% CI 0.46, 34.00]).10 In the present study, this complication was rarely encountered in both the placebo and the antibiotic cohorts.
Costs to the healthcare system are another important consideration. Smack et al. performed a cost analysis on antibiotic versus bland petrolatum therapy and found that the healthcare system could save $8 to $10 million annually if all dermatologists switched to bland ointment after surgery.33 These savings are likely overestimated for oculofacial plastic surgery, due to the higher price of ophthalmic mineral oil/petrolatum-based ointments,34 though non-ophthalmic formulas are likely safe for most applications. Cost and adverse effects must be weighed against the likely modest protective effect of antibiotics against post-operative infection. Generally, SSIs after cutaneous surgery are mild and easily treated35; however, infection can be more severe and affect cosmesis,36 flap survival,37 or rarely be life-threatening.36,38 Indeed, the unfortunate course of the participant who developed a SSI after Mohs reconstruction illustrates the potential for harm.
This study’s strengths included the randomized design and good subject retention. The broad range of oculofacial plastic surgeries, permissive inclusion/exclusion criteria, and naturalistic design, aside from the intervention itself, provide good generalizability of the results to the overall population of patients undergoing oculofacial plastic surgery.
This study has several limitations. The primary outcome was sufficiently rare that it was not possible to conduct secondary subgroup analyses, such as comparison of high-versus low-risk participants or stratification by type of surgery. Similarly, while the study found no differences in the secondary outcomes between groups, it had limited power to identify small differences due to the rarity of the outcomes. Though adherence to assigned treatment was excellent, there was heterogeneity of the treatment medication within the cohorts. Formal dispensation of a uniform placebo and antibiotic medication by the institution’s pharmacy would have been cost-prohibitive, and therefore the study investigators instead pursued the prescription of medications subject to outpatient pharmacy availability and surgeon preference. Among patients who received placebo, this was unlikely to have any effect on study outcomes. Over the counter mineral oil/petrolatum formulations from different brands vary little; furthermore, the real-world use of any bland ophthalmic ointment improves generalizability of the results. On the contrary, the variety of antibiotics prescribed could potentially have differential effects on infection (due to spectrum) and allergic contact dermatitis; however, this was unlikely to have had an effect on this study’s outcomes given the lack of infections in the antibiotic group and the rarity of allergic contact dermatitis. Both participants and surgeons were unmasked to the treatment interventions. This may have led to unconscious bias in the determination of infection, leading to an overestimation of infection frequency among the placebo group. Prospectively mandated follow-up times were selected for the detection of typical bacterial SSIs, and would be less likely to identify atypical mycobacterial infections, which can present between 1 to 12 weeks after surgery.39 Retrospective review did not identify evidence of additional delayed-onset infections, but was limited by the non-prospective nature and lack of long-term follow-up data for a minority of participants; this limitation is balanced by the rarity of these atypical infections. Finally, while demographics of the study population generally reflect that of the United States, individuals of Asian descent were over-represented and individuals of African-American descent were under-represented, reflecting the demographics of the treating institution.
In conclusion, this study of patients undergoing routine oculofacial plastic surgery identified a modestly higher incidence of periocular SSI in patients receiving a topical mineral oil/petrolatum-based ointment, when compared to patients receiving a topical antibiotic ointment. This provides new evidence for the oculofacial plastic surgeon to consider when determining the use of topical antibiotic prophylaxis in his or her practice. Future research could involve multi-institutional collaboration to achieve a greater sample size in order to confirm the present findings and evaluate for patient or surgery characteristics that would differentially benefit from post-operative antibiotic ointment.
Supplementary Material
Figure S1. Modified Surgical Site Infection Criteria.
The criteria utilized by investigators to determine eligibility for the primary outcome. Investigators adapted the criteria from those published by the Center for Disease Control, shortening the post-operative period from 30 to 14 days and removing a criterion requiring exploration of the wound for superficial infections.
Figure S2. CONSORT Checklist.
Figure 2. Frequency of Primary and Secondary Outcomes by Treatment Arm.
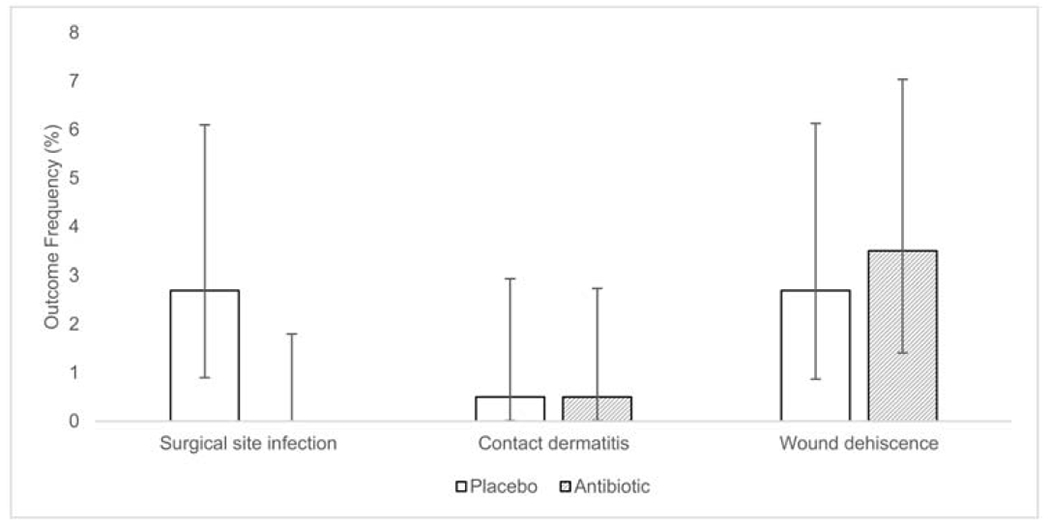
The frequency of the primary and secondary outcomes for participants randomized to receive topical antibiotic ointment or placebo ointment after surgery. Error bars correspond to the 95% exact binomial proportion confidence interval for the respective frequencies.
In this study, patients who underwent routine oculofacial plastic surgery had a higher rate of postoperative infection when randomized to administer a topical mineral oil/petrolatum-based ointment post-operatively, rather than a topical antibiotic ointment.
Acknowledgments
Financial Support: Supported in part by NIH-NEI EY002162–Core Grant for Vision Research and by an unrestricted grant from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting presentation: Presented in part at the 2019 ARVO Annual Meeting, held in Vancouver, Canada, April 28 - May 2, 2019.
Conflict of interest: The authors have no conflict of interests.
References
- 1.Fay A, Nallasamy N, Bernardini F, et al. Multinational Comparison of Prophylactic Antibiotic Use for Eyelid Surgery. JAMA Ophthalmol 2015;133:778–784. [DOI] [PubMed] [Google Scholar]
- 2.Carter SR, Stewart JM, Khan J, et al. Infection after blepharoplasty with and without carbon dioxide laser resurfacing. Ophthalmology 2003;110:1430–1432. [DOI] [PubMed] [Google Scholar]
- 3.Lee EW, Holtebeck AC, Harrison AR. Infection rates in outpatient eyelid surgery. Ophthal Plast Reconstr Surg 2009;25:109–110. [DOI] [PubMed] [Google Scholar]
- 4.de Keizer ROB, Kozdras G, Wubbels R, et al. Retrospective study in 608 cases on the rate of surgical site infections after orbital surgery without prophylactic systemic antibiotics. Br J Ophthalmol 2019;103:1466–1468. [DOI] [PubMed] [Google Scholar]
- 5.Kaoutzanis C, Gupta V, Winocour J, et al. Incidence and Risk Factors for Major Surgical Site Infections in Aesthetic Surgery: Analysis of 129,007 Patients. Aesthet Surg J 2017;37:89–99. [DOI] [PubMed] [Google Scholar]
- 6.Williamson DA, Carter GP, Howden BP. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin Microbiol Rev 2017;30:827–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Rosso JQ, Webster GF, Rosen T, et al. Status Report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society. J Clin Aesthetic Dermatol 2016;9:18–24. [PMC free article] [PubMed] [Google Scholar]
- 8.Gehrig KA, Warshaw EM. Allergic contact dermatitis to topical antibiotics: Epidemiology, responsible allergens, and management. J Am Acad Dermatol 2008;58:1–21. [DOI] [PubMed] [Google Scholar]
- 9.Gette MT, Marks JG, Maloney ME. Frequency of postoperative allergic contact dermatitis to topical antibiotics. Arch Dermatol 1992;128:365–367. [PubMed] [Google Scholar]
- 10.Heal CF, Banks JL, Lepper PD, et al. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst Rev 2016;11:CD011426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saco M, Howe N, Nathoo R, Cherpelis B. Topical antibiotic prophylaxis for prevention of surgical wound infections from dermatologic procedures: a systematic review and metaanalysis. J Dermatol Treat 2015;26:151–158. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bever GJ, Brodie FL, Hwang DG. Corneal Injury from Presurgical Chlorhexidine Skin Preparation. World Neurosurg 2016;96:610.e1–610.e4. [DOI] [PubMed] [Google Scholar]
- 15.Tabor E, Bostwick DC, Evans CC. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA 1989;261:557–558. [DOI] [PubMed] [Google Scholar]
- 16.Phinney RB, Mondino BJ, Hofbauer JD, et al. Corneal edema related to accidental Hibiclens exposure. Am J Ophthalmol 1988;106:210–215. [DOI] [PubMed] [Google Scholar]
- 17.Rietz A, Barzin A, Jones K, Mounsey A. PURLs: Sterile or non-sterile gloves for minor skin excisions? J Fam Pract 2015;64:723–727. [PMC free article] [PubMed] [Google Scholar]
- 18.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 19.Cruse PJ, Foord R. The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds. Surg Clin North Am 1980;60:27–40. [DOI] [PubMed] [Google Scholar]
- 20.Culver DH, Horan TC, Gaynes RP, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med 1991;91:152S–157S. [DOI] [PubMed] [Google Scholar]
- 21.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132; quiz 133–134; discussion 96. [PubMed] [Google Scholar]
- 22.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available at: https://www.R-project.org/. [Google Scholar]
- 23.Yazici B, Meyer DR. Selective antibiotic use to prevent postoperative wound infection after external dacryocystorhinostomy. Ophthal Plast Reconstr Surg 2002;18:331–335; discussion 335. [DOI] [PubMed] [Google Scholar]
- 24.Dulku S, Akinmade A, Durrani OM. Postoperative infection rate after dacryocystorhinostomy without the use of systemic antibiotic prophylaxis. Orbit Amst Neth 2012;31:44–47. [DOI] [PubMed] [Google Scholar]
- 25.DelMauro MA, Kalberer DC, Rodgers IR. Infection prophylaxis in periorbital Mohs surgery and reconstruction: a review and update to recommendations. Surv Ophthalmol 2020;65:323–347. [DOI] [PubMed] [Google Scholar]
- 26.Olds C, Spataro E, Li K, et al. Postoperative Antibiotic Use Among Patients Undergoing Functional Facial Plastic and Reconstructive Surgery. JAMA Facial Plast Surg 2019;21:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu M, Cheng C, Yi H, et al. Quantitative Analysis of the Bacteria in Blepharitis With Demodex Infestation. Front Microbiol 2018;9:1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nattis A, Perry HD, Rosenberg ED, Donnenfeld ED. Influence of bacterial burden on meibomian gland dysfunction and ocular surface disease. Clin Ophthalmol Auckl NZ 2019;13:1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers DL, Chheda L, Ford C, et al. The effect of surgical preparation technique on the bacterial load of surgical needles and suture material used during strabismus surgery. J AAPOS Off Publ Am Assoc Pediatr Ophthalmol Strabismus 2011;15:230–233. [DOI] [PubMed] [Google Scholar]
- 30.Peral A, Alonso J, Garcia-Garcia C, et al. Importance of Lid Hygiene Before Ocular Surgery: Qualitative and Quantitative Analysis of Eyelid and Conjunctiva Microbiota. Eye Contact Lens 2016;42:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks JG, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol 1998;38:911–918. [DOI] [PubMed] [Google Scholar]
- 32.Moore NA, Czyz CN, Carter TD, et al. Neomycin, polymyxin B, and dexamethasone allergic reactions following periocular surgery. J Ophthalmic Inflamm Infect 2017;7 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5468176/ [Accessed April 15, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. A randomized controlled trial. JAMA 1996;276:972–977. [PubMed] [Google Scholar]
- 34.Waduthantri S, Yong SS, Tan CH, et al. Cost of Dry Eye Treatment in an Asian Clinic Setting. PLoS ONE 2012;7 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3372539/ [Accessed April 15, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirschmann JV. When Antibiotics are Unnecessary. Dermatol Clin 2009;27:75–83. [DOI] [PubMed] [Google Scholar]
- 36.Klapper SR, Patrinely JR. Management of Cosmetic Eyelid Surgery Complications. Semin Plast Surg 2007;21:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen RW, Parkin JL. Skin flap survival. Influence of infection, anemia, and tubing. Arch Otolaryngol Chic Ill 1960 1976;102:727–728. [DOI] [PubMed] [Google Scholar]
- 38.Suñer IJ, Meldrum ML, Johnson TE, Tse DT. Necrotizing fasciitis after cosmetic blepharoplasty. Am J Ophthalmol 1999;128:367–368. [DOI] [PubMed] [Google Scholar]
- 39.Kheir WJ, Sheheitli H, Abdul Fattah M, Hamam RN. Nontuberculous Mycobacterial Ocular Infections: A Systematic Review of the Literature. BioMed Res Int 2015;2015. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4461732/ [Accessed June 3, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Modified Surgical Site Infection Criteria.
The criteria utilized by investigators to determine eligibility for the primary outcome. Investigators adapted the criteria from those published by the Center for Disease Control, shortening the post-operative period from 30 to 14 days and removing a criterion requiring exploration of the wound for superficial infections.
Figure S2. CONSORT Checklist.


