Abstract
Converging evidence suggests opioid abuse can increase the incidence and severity of post-traumatic stress disorder (PTSD) in clinical populations. Interestingly, opioid withdrawal alone can produce symptoms similar to those of PTSD. Despite this association, the neural mechanisms underlying the relationship of opioid abuse, withdrawal, and PTSD is poorly understood. Our laboratory has investigated the neurobiological underpinnings of stress enhanced fear learning (SEFL), an animal model of PTSD-like symptoms. We have previously shown that, in SEFL, a severe footshock induces an increase in dorsal hippocampal (DH) interleukin-1β (IL-1β) and subsequent fear learning is blocked by DH IL-1 receptor antagonism (IL-1RA). Given that opioids and stress engage similar neuroimmune mechanisms, the present experiments investigate whether the same mechanisms drive heroin withdrawal to induce a PTSD-like phenotype. First, we tested the effect of a chronic escalating heroin dose and withdrawal regimen on fear learning and found it produces enhanced future fear learning. Heroin withdrawal also induces a time-dependent, region-specific increase in IL-1β and glial fibrillary acidic protein (GFAP) immunoreactivity within the dentate gyrus of the DH. IL-1β was significantly colocalized with GFAP, indicating astrocytes may be involved in increased IL-1β. Moreover, intra-DH infusions of IL-1RA 0, 24, and 48 hours into heroin withdrawal prevents the development of enhanced fear learning but does not alter withdrawal-induced weight loss. Collectively, our data suggests heroin withdrawal is sufficient to produce enhanced fear learning, astrocytes may play a role in heroin withdrawal-induced IL-1β, and DH IL-1 signaling during withdrawal mediates the development of heroin withdrawal-enhanced fear learning.
Keywords: glia, heroin, neuroimmunology, opioid, PTSD, withdrawal
Introduction
Post-traumatic stress disorder (PTSD) is a complex and devastating mental disorder with a myriad of psychological and physiological consequences, including dysregulation of immune systems (Gill et al. 2009; Xia et al. 2013). At-risk populations include active military personnel, emergency responders, and abused individuals (Thomas et al. 2010). Importantly, evidence indicates significant comorbidity between PTSD and opioid use disorders (Jacobsen et al. 2001; McCauley et al. 2012; Meier et al. 2014; Patel et al. 2017). Heroin use disorder is associated with increased anxiety diagnoses and can increase severity of PTSD symptoms in clinical populations (Jacobsen et al. 2001; McCauley et al. 2012; Meier et al. 2014; Patel et al. 2017). Generally, in the clinical population, opioid withdrawal symptoms begin 6 hours following the last dose of drug, and peak within 24–72 hours following the last dose of drug. Moreover, there is often a shared symptomology between opioid withdrawal and PTSD (Patel et al. 2017). In the clinical population, the most severe symptoms of opioid withdrawal are present 24–48 hours following the last dose of drug. While it is generally thought that PTSD precedes, and therefore is a risk factor for, substance use disorders, there is also evidence that a prior history of substance use exacerbates the development and symptoms of PTSD (Bremner et al. 1996; Holmes et al. 2012; Jacobsen et al. 2001; McCauley et al. 2012; Meier et al. 2014; Patel et al. 2017). This suggests the adverse consequences of opioid abuse leave individuals more biologically vulnerable to the impact of severe stress and therefore the development of PTSD.
Depression and anxiety disorders have been associated with altered cytokine expression in both human and rodent models (Crews et al. 2015; Gill et al. 2009; Koo and Duman 2009; Silverman et al. 2007; Stepanichev et al. 2014). The pro-inflammatory cytokine interleukin-1β (IL-1β) is associated with PTSD (Gill et al. 2009; Gola et al. 2013; Guo et al. 2012; Passos et al. 2015) and is thought to be a potential biomarker for PTSD susceptibility (Cohen et al. 2011). IL-1β is also critical for normal learning and memory processing to occur, and either excessive or insufficient amounts of IL-1β impair memory formation (Goshen et al. 2007). Consistent with this, we have identified a novel mechanism involving dorsal hippocampal (DH) IL-1 signaling in the development of stress-enhanced fear learning (SEFL) (Rau et al. 2005), an animal model of PTSD-like symptoms. While it is difficult to incorporate all the symptoms of PTSD in a preclinical animal model, SEFL effectively demonstrates greater susceptibility to future fear learning, a key component of human PTSD. Exposure to a severe stressor in this model (inescapable footshocks) induces a time-dependent, region-specific increase in IL-1β immunoreactivity within the dentate gyrus (DG) (Jones et al. 2015). Critically, disruption of intra-DH IL-1 signaling, using interleukin-1 receptor antagonist (IL-1RA), following severe stress exposure prevents the development of SEFL (Jones et al. 2017). While multiple cell types express IL-1β (Bland et al. 2009; Hanisch 2002), we found that astrocytes are predominantly responsible for the DH IL-1β expression involved in SEFL. Astrocytes directly alter neuronal function and synaptic plasticity through the release of gliotransmitters, and astrocyte-derived cytokines (Lacagnina et al. 2018), including IL-1β, have been increasingly implicated in stress response mechanisms (Goshen and Yirmiya 2009). This suggests that DH neuroimmune processes mediate the response to severe stress that potentiates future fear learning in the SEFL model.
Given that heroin withdrawal can produce similar physiological responses to those induced by stress, heroin withdrawal may enhance fear learning through similar neuroimmune mechanisms. Furthermore, studies have shown IL-1β expression and astrocyte reactivity, indicated by expression of glial fibrillary acidic protein (GFAP), is altered following opioid use and withdrawal (Garcia-Perez et al. 2015; Liu et al. 2011), which supports the idea that IL-1β signaling could be a common underlying mechanism between severe stress and heroin withdrawal that mediates enhanced fear learning. The current studies test the overarching hypothesis that heroin withdrawal will produce enhanced fear learning and similar DH neuroimmune processes mediate this effect. To this end, Experiment 1 determined whether heroin withdrawal can produce enhanced fear learning. We identified that chronic escalating heroin administration does indeed produce a reliable heroin withdrawal and that this model of withdrawal will produce enhanced future fear learning. Subsequently, Experiment 2 examined the neuroimmune consequences of 24-hour heroin withdrawal on DH IL-1β, GFAP, and, microglial activation marker, ionized calcium binding adaptor molecule 1 (IBA-1), expression. We showed that withdrawal-induced increases in IL-1β and GFAP immunoreactivity, and identified astrocytes as the predominant glial cell expressing IL-1β specifically in the DG in our model. Experiment 3 tested whether blocking DH IL-1 signaling with IL-1RA 0, 24, and 48 hours into heroin withdrawal prevents the development of enhanced fear learning and withdrawal induced weight loss. Collectively, these novel experiments are the first to test whether heroin withdrawal can sensitize future fear learning, DH IL-1 signaling is critical to the development of withdrawal-enhanced fear learning, and whether astrocytes may be involved in heroin-withdrawal induced IL-1β expression.
Materials & Methods
Animals
Adult male Sprague Dawley rats (225–250g, Charles River Laboratories, Raleigh, NC) were individually housed under a reversed 12-hour light-dark cycle, given ad libitum access to food and water, and handled regularly throughout experimentation. All procedures were conducted in accordance with federal guidelines and with approval from the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Experiment 1: Effect of Heroin Withdrawal on Fear Learning
Chronic Escalating Heroin Administration:
Animals were randomly assigned to receive three daily subcutaneous (SC) injections of heroin or saline (control). Heroin [diacetylmorphine hydrochloride, National Institute on Drug Abuse (NIDA) Drug Supply Program, Bethesda, MD, USA] was dissolved in sterile 0.9% saline to produce 1.0, 2.5, 5.0, 7.5, or 10.0 mg/mL solutions and stored at 4°C until time of injection. The administration schedule of chronic escalating heroin (adapted from Kreek and colleagues (Zhou et al. 2013)) is 10 days of three injections over 24-hour periods (at 15:00, 21:00, and 9:00 the next day, UTC-05:00) and a dose increase every other day (Figure 1a). Animal weight change, a quantifiable and reliable measure of opioid withdrawal (Gellert and Holtzman 1978), was measured at every dose increase and subsequent withdrawal time point. Weight change was measured throughout the heroin administration paradigm to ensure that animals experience measurable heroin withdrawal and that during the heroin administration procedure animals are not experiencing withdrawal between dosing. This is indicative of the fact that animals are not losing weight throughout the heroin administration paradigm, and weight loss is specific to the withdrawal timepoint.
Figure 1. Chronic escalating heroin administration produces withdrawal behaviors.
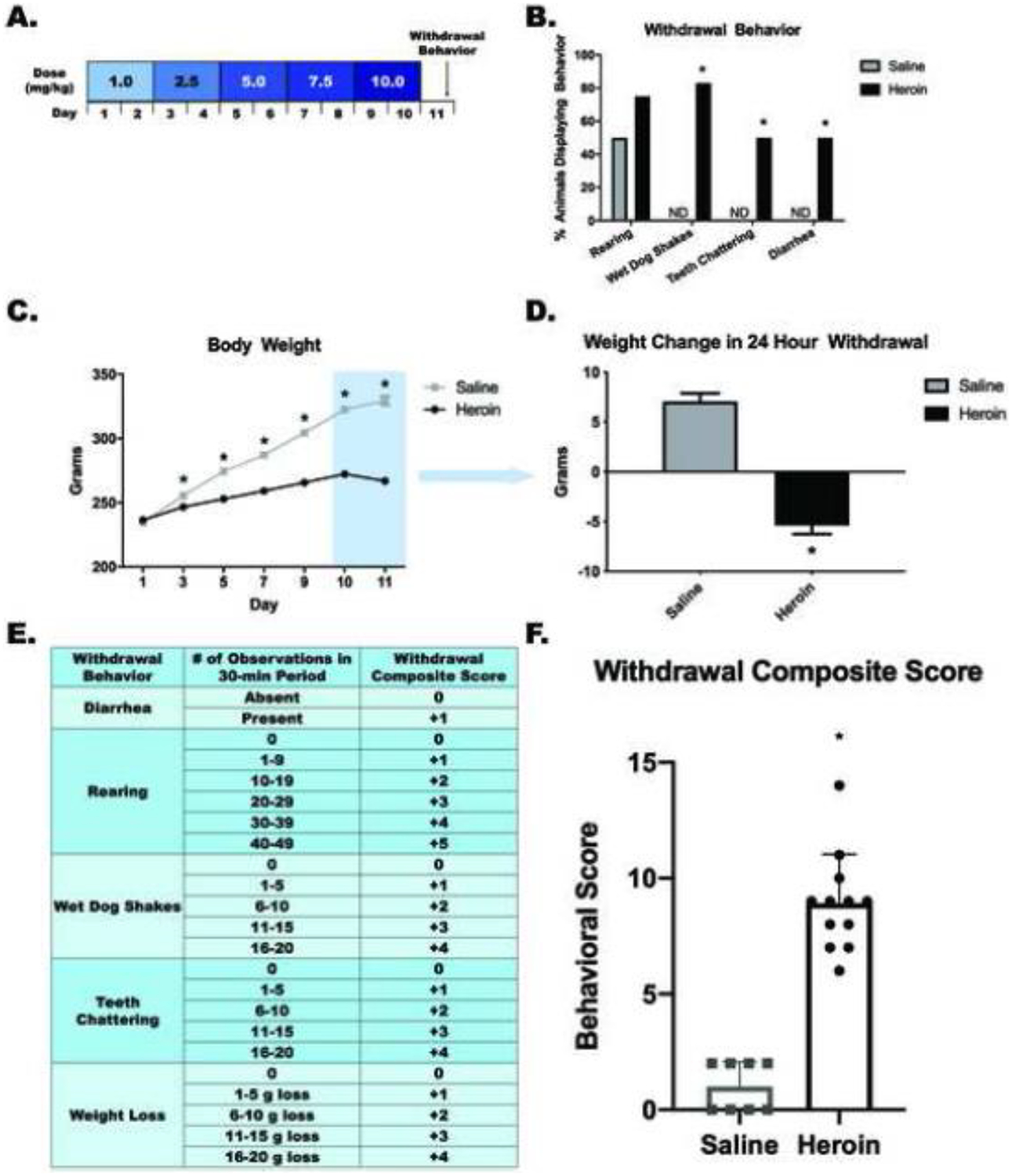
Chronic escalating heroin administration schematic (A). Withdrawal behavior (B). Overall body weight across days of heroin administration (C) and weight change in 24-hour withdrawal (D) Withdrawal composite score explanation chart (E). Heroin animals have a higher withdrawal composite score (range between 6 and 14) than saline animals (range between 0 and 2) (F). *, statistically significant difference relative to respective control. Error bars indicate SEM.
Heroin Withdrawal-Enhanced Fear Learning (HW-EFL):
We used a modified version of the SEFL model previously described (Jones et al. 2015; Rau et al. 2005). Rather than a severe footshock, animals underwent heroin withdrawal in the home cage. Control animals remained in home cage without receiving saline injections. Seven days after the start of withdrawal, animals were placed into a novel context for 15 min and video recorded for baseline activity (Test Day B). On Day 8, animals were placed into the same context for a single scrambled footshock (1mA, 1s) at 3 min, 12 s. On days 9, 10, 15, 22 (Test Days 1, 2, 7, and 14) animals are placed into the same context for 8 min, 32 s and were video recorded to analyze freezing behavior, a measure of learned fear. Automated video tracking and analysis software was used to quantify percent freezing behavior in all sessions (Ethovision XT, Activity Analysis Feature with Threshold=10, Noldus Information Technology Inc., Netherlands).
Experiment 1a quantified withdrawal behaviors following the chronic escalating heroin administration. Animals were randomly assigned to receive heroin (n=12) or saline (n=8) throughout the procedure. Twenty-four hours into withdrawal, animals were placed into open-field Plexiglas boxes (42cm × 42cm × 30cm). Withdrawal behaviors (rearing, wet dog shakes, teeth chattering, and diarrhea) were video-recorded for 30 min. Experimenters were trained on withdrawal behaviors, until there was 98% agreement in observation scores, and scored behaviors blind to treatment condition. Positive withdrawal behavior was recorded if the behavior occurred at least once in the 30 min observation period. A heroin withdrawal score was calculated for each behavior based on the number of behaviors observed during the 30 min observation period, and these individual behavior scores were summed to obtain the composite withdrawal score (Figure 1e). The twenty-four hour withdrawal assessment timepoint was chosen to assess withdrawal based on literature suggesting this is the best timepoint to observe behavioral changes from a spontaneous opioid withdrawal in rodents (Gellert and Holtzman 1978). Additionally, this timepoint is clinically relevant, as opioid withdrawal symptoms in a clinical population begin 6 hours following the last dose of drug, and generally peak within 24–72 hours following the last dose of drug. A thirty-minute session of observation and scoring on the withdrawal severity score scale was chosen to mimic the observations performed in the literature. Experiment 1b examined the effect of heroin withdrawal on enhanced fear learning. Animals were randomly assigned to receive heroin (n=15) or saline (n=16) and underwent the chronic escalating heroin administration and subsequently exposed to HW-EFL.
Statistical analysis:
Withdrawal behaviors were analyzed using a χ2 test. Body weight on Day 1 was analyzed using a one-way (Heroin, Saline) ANOVA, and on Days 3–12 using a 2 (Drug Treatment) × 6 (Day) repeated measures ANOVA. Weight change and withdrawal composite score was analyzed using an unpaired, two-tailed Student’s t-test. A one-way (Heroin, Saline) ANOVA was used to analyze baseline freezing data. A 2 (Heroin, Saline) × 4 (Test Day) repeated measures ANOVA was used to analyze freezing behavior across test days.
Experiment 2: Analysis of IL-1β, GFAP, and IBA-1 Immunoreactivity and Colocalization Following 24-hour Heroin Withdrawal
Animals (n=8–10) were randomly assigned to drug (heroin or saline) treatment and heroin withdrawal time point (0- or 24-hour) and underwent chronic escalating heroin administration. Animals were sacrificed via transcardial perfusion at 1 or 24 hours following their last injection. Animals perfused 1 hour following the last heroin injection were considered to have heroin in their system and therefore are in the 0-hour withdrawal group, having undergone no withdrawal. Animals perfused at 24 hours following the last injection are considered to be in the 24-hour withdrawal group. Animals were terminally anesthetized with 9:1 (vol:vol) ketamine hydrochloride (100 mg/mL) mixed with xylazine (100 mg/mL), and transcardially perfused with ice cold phosphate buffer (PB; pH=7.4) and 4% paraformaldehyde in 0.1 M PB. Brains were extracted, post-fixed in 4% paraformaldehyde for 6 hrs, and cryoprotected in 30% sucrose with 0.1% sodium azide at 4°C. Once the brains were saturated with sucrose, brains were cut into 40 μm coronal sections on a cryostat (Leica CM 3050 S, Leica Microsystems, Buffalo Grove, IL, USA).
Experiment 2a used fluorescent immunohistochemistry (IHC) to examine alterations in DH IL-1β, GFAP, and IBA-1 in the DG. We hypothesized a 24-hour heroin withdrawal would alter DH IL-1β, GFAP, and IBA-1 in the DG, either by increasing or decreasing immunoreactivity in comparison to saline. The IHC protocol used here has been described previously (Jones et al. 2015; Jones et al. 2017). The following primary antibodies were used: rabbit anti-IL-1β (1:500, Abcam, Cambridge, MA, Cat# Ab9722, USA), mouse anti-GFAP (1:1000, ThermoFisher Scientific, Waltham, MA, Cat #MS-1376P, USA), and rabbit anti-IBA-1-biotinylated (1:500, Wako, Richmond, VA, Cat#016–26461, USA). To visualize IL-1β and GFAP, Alexa Fluor conjugated secondary antibodies, goat anti-rabbit 488 (1:1000, ThermoFisher Scientific, Waltham, MA, Cat #A11008) and goat anti-mouse 594 (1:1000, ThermoFisher Scientific, Waltham, MA, Cat # A11005) were used. To visualize IBA-1, a streptavidin-conjugated Alexa Fluor 568 antibody (1:1000, ThermoFisher Scientific, Waltham, MA, Cat #S11226) was used. Sections were slide mounted (SuperFrost Plus, Fisher Scientific, Pittsburgh, PA, USA) using VECTASHIELD hard set mounting medium (Vector Laboratories, Burlingame, CA, USA). Fluorescent microscopy (Leica DM6000 B, Leica Microsystems, Buffalo Grove, IL, USA) was used to capture color images (20X), and immunoreactivity was quantified using ImageJ (NIH). Average DG immunoreactivity was calculated from 3–5 sections/animal. Tissue that yielded high nonspecific background from poor perfusions was dropped from analysis while blind to treatment group.
Experiment 2b examined colocalization of IL-1β with GFAP and IBA-1. The colocalization protocol used here has been described previously (Jones et al. 2015; Jones et al. 2017) for analysis of IL-1β/GFAP and IL-1β/IBA-1. Image acquisition and data analysis were completed blind to treatment group. Tissue was imaged using a Zeiss LSM800 confocal microscope with laser lines that excite at 405 nm, 488 nm, and 561 nm. Z-stacks of the DG (AP - 3.12 mm through −3.84 mm from bregma) were acquired (63X) with a 4-frame average, 1024 × 1024 frame, 16-bit resolution, and 0.8 μm step-size. Z-stacks were deconvolved using Bitplane AutoQuant X3 (10 iterations) and exported to the Bitplane Imaris software suite (Zurich, Switzerland). Background correction was done for each channel individually using manually set absolute intensity thresholds. In the colocalization module, voxels in both channels above the automatic threshold were included as colocalized voxels. A 2-D scatter plot was used to visually inspect the accuracy of colocalization thresholds. The following values were measured: % IL-1β colocalized with IBA-1, % IL-1β colocalized with GFAP, and the Pearson’s correlation coefficient for each pair of signals. These values represent the % IL-1β signal above threshold colocalized with IBA-1 signal, and the % IL-1β signal above threshold colocalized with GFAP signal. The Pearson’s correlation coefficient is calculated based on an algorithm developed by Costes and Lockett at the National Institute of Health, NCI/SAIC, which is based on the inclusion of intensity pairs that exhibit no correlation, and therefore the Pearson’s correlation per Z-stack represents the correlation between signals inside the colocalized region (Bitplane Imaris Software Suite; Zurich, Switzerland). Averages of the three values described above were calculated from 8 bi-lateral Z-stacks per animal.
Statistical analysis:
For Experiment 2a, planned comparisons using unpaired, two-tailed Student’s t-tests to determine specifically whether drug treatment altered IL-1β, GFAP or IBA-1 immunoreactivity between the 0-hour timepoint and 24-hour timepoints. In Experiment 2b, the % IL-1β colocalized and the Pearson’s correlation coefficient were analyzed using a 2 (Heroin, Saline) × 2 (GFAP, IBA-1) ANOVA. Planned comparisons were also made to compare GFAP/IL-1β colocalization parameters vs. IBA-1/IL-1β colocalization parameters.
Experiment 3: Effect of Intra-DH IL-1RA on the Development of Heroin-Withdrawal Enhanced Fear Learning and Withdrawal-Induced Weight Change
For stereotaxic surgery, animals were anesthetized with a 1.0 mL/kg intraperitoneal injection of 9:1 (vol:vol) ketamine hydrochloride (100 mg/mL) mixed with xylazine (100 mg/mL). Guide cannulae (26 Gauge, Plastics One, Roanoke, VA, USA) were directed bilaterally at the DH (AP −3.4 mm, ML ±3.1 mm, DV −2.2 mm, 15 degrees, relative to bregma). Animals were given two weeks for postoperative recovery prior to the start of experimental procedures. Animals (n=7–8) were randomly assigned to receive heroin or saline in the chronic escalating heroin administration and then either a saline or IL-1RA infusion treatment. IL-1RA (GenScript, Piscataway, NJ, USA) was reconstituted in sterile saline (2.5 μg/μL). Animals underwent the chronic heroin escalating administration and were given three infusions of IL-1RA or saline at 0, 24, and 48 hours into withdrawal. Forty-eight hours prior to first infusion, animals were given a sham microinjection to allow for habituation. Animals were microinfused with 1.25 μg of IL-1RA or saline vehicle per hemisphere at a rate of 0.25 μL/min, and injectors were left in place for 1 minute to allow for drug diffusion away from injection site. Animals were then exposed to the HW-EFL. Following experimentation, animals were sacrificed via rapid cervical dislocation and DH cannula placement was verified.
Statistical analysis:
A one-way (Heroin, Saline) ANOVA was used to analyze baseline freezing data. A 2 (Heroin, Saline) × 2 (IL-1RA, Saline) × 4 (Test Day) repeated measures ANOVA was used to analyze freezing behavior. A 2 (Heroin, Saline) × 2 (IL-1RA, Saline) × 5 (Time Point) repeated measures ANOVA was used to analyze weight change across withdrawal. Significant interactions for both freezing behavior and weight change were examined using Tukey’s post-hoc comparisons.
Results
Experiment 1: Heroin Withdrawal Produces Enhanced Fear Learning
The chronic escalating heroin administration (Figure 1a) produced withdrawal behaviors 24 hours after the last heroin dose. χ2 analysis revealed no difference between the heroin withdrawal and saline controls for rearing behavior (χ2(1,n=20)=1.319, p=.251). Importantly, χ2 analysis revealed significant increases in withdrawal behaviors between the heroin withdrawal and saline controls for wet dog shakes (χ2(1,n=20)=13.333, p<.001), teeth chattering (χ2(1,n=20)=5.714, p=.017), and diarrhea (χ2(1,n=20)=5.714, p=.017) (Figure 1b). A one-way ANOVA revealed no significant differences between heroin and saline animal weights on Day 1, (F(1,29)=.645, p=.428), indicating no pre-existing weight differences between groups. Importantly, a repeated measures ANOVA revealed a significant main effect of drug-treatment on weight between days 3–10, (F(1,29)=127.937, p<.001), indicating that heroin-treated animals have decreased weight compared to saline-treated controls. Although heroin-treated animals had decreased weight compared to saline-treated controls, heroin-treated animals on average did not lose weight, indicating that animals were not experiencing withdrawal during the 10 days of heroin administration. There was also a significant main effect across days (F(5,145)=705.236, p<.001) and significant day by drug treatment interaction (F(5,145)=189.873, p<.001) (Figure 1c).
Student’s t-test comparing weight change between Day 10 and Day 11 (first 24 hours of withdrawal) revealed that heroin-treated animals lost significantly more weight than saline-treated animals (t(29)=10.666, p<.001) (Figure 1d). Student’s t-test comparing composite withdrawal scores between heroin-treated animals and saline-treated animals revealed that heroin-treated animals had a significantly higher composite withdrawal score than saline-treated animals (t(18)=−9.754, p<.001) (Figure 1f). Furthermore, the range of heroin withdrawal scores was between 6 and 14, with no outliers. All animals but one animal displayed withdrawal scores between 6 and 11, suggesting that the all animals treated with the chronic escalating heroin administration experienced moderately similar withdrawal.
Heroin withdrawal produced subsequent enhanced fear learning. There was no effect of drug treatment on baseline freezing to the context (F(1,16)=1.209, p=.288), indicating that there is no generalized fear to the novel context. Importantly, a 2 × 4 repeated measures ANOVA revealed a significant main effect of drug treatment (F(1,16)=7.389, p=.015), indicating the exciting finding that a history of heroin withdrawal enhances fear learning to a single shock experience. There was also a significant main effect of day (F(3,48)=14.222, p<.001), indicating that indicating that conditioned freezing behavior diminished over time. (Figure 2a).
Figure 2. Heroin withdrawal causes enhanced fear learning.
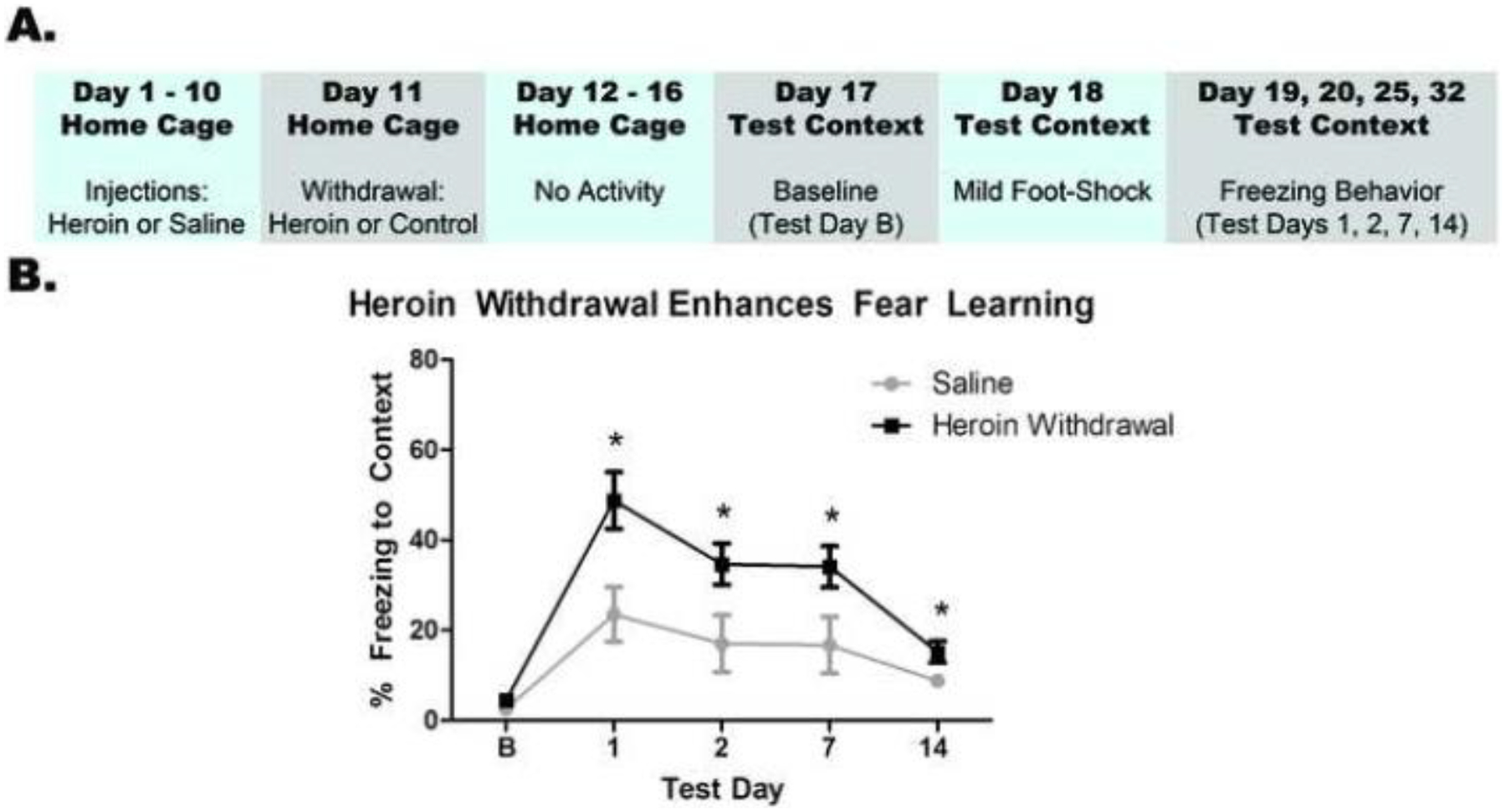
Experimental timeline (A). Heroin withdrawal significantly enhances contextual freezing behavior to a single mild footshock (B). *, statistically significant difference relative to respective control. Error bars indicate SEM.
Experiment 2: Heroin Withdrawal Increases IL-1β and GFAP Immunoreactivity and IL-1β Signal is Colocalized with GFAP
IL-1β and GFAP immunoreactivity were significantly enhanced by heroin withdrawal, but not IBA-1 immunoreactivity. IL-1β immunoreactivity was increased during 24-hour heroin withdrawal (t(16)=−2.179, p=.045), relative to saline controls, but no difference was observed at 0-hour withdrawal when heroin was still onboard (t(13)=−.277, p=.786). These results suggest that heroin withdrawal, and not simply a history of chronic heroin administration, is necessary for increased IL-1β immunoreactivity within the DH (Figure 3b). Similarly, GFAP immunoreactivity was increased during 24-hour heroin withdrawal relative to saline controls (t(16)=−2.402, p=.029), but was not increased at the 0-hour time point when heroin was still onboard (t(13)=−.301, p=.786). These results suggest that, as for DH IL-1β immunoreactivity, heroin withdrawal, not a history of chronic heroin administration, is necessary for increased DH GFAP immunoreactivity (Figure 3c). In contrast, IBA-1 immunoreactivity was not altered by either 0-hour withdrawal, (t(14)=1.503, p=.155) or 24-hour withdrawal, (t(16)=−.026, p=.980), in either heroin- or saline-treated animals (Figure 3d). Although there visually seems to be a reduction in IBA-1 immunoreactivity at the 0-hour withdrawal timepoint, this was not statistically significant. However, it may be interesting to investigate this reduction due to heroin’s immunosuppressive effects (Figure 3d). IL-1β signal was significantly colocalized with GFAP, with minimal IBA-1 colocalization. There was a significant main effect of cell-type analyzed in both the % IL-1β signal colocalized (F(1,26)=105.486, p<.001) (Figure 4b) and the Pearson’s correlation coefficient (F(1,26)=418.883, p<.001) (Figure 4c). Planned comparisons confirmed that there was significantly more colocalization of IL-1β with GFAP than IBA-1 for both measures (p<.001). There was no main effect of drug treatment on either the % IL-1β colocalized with GFAP or with IBA-1 (F(1,26)=.000, p=.994) or the Pearson’s correlation coefficient between signals(F(1,26)=.265, p=.611). These results suggest that IL-1β in the DG is colocalized with astrocytes and not microglia (Figure 5), and this does not change over the course of withdrawal.
Figure 3. Heroin withdrawal increases Il-1β and GFAP immunoreactivity in the dentate gyrus of the dorsal hippocampus.
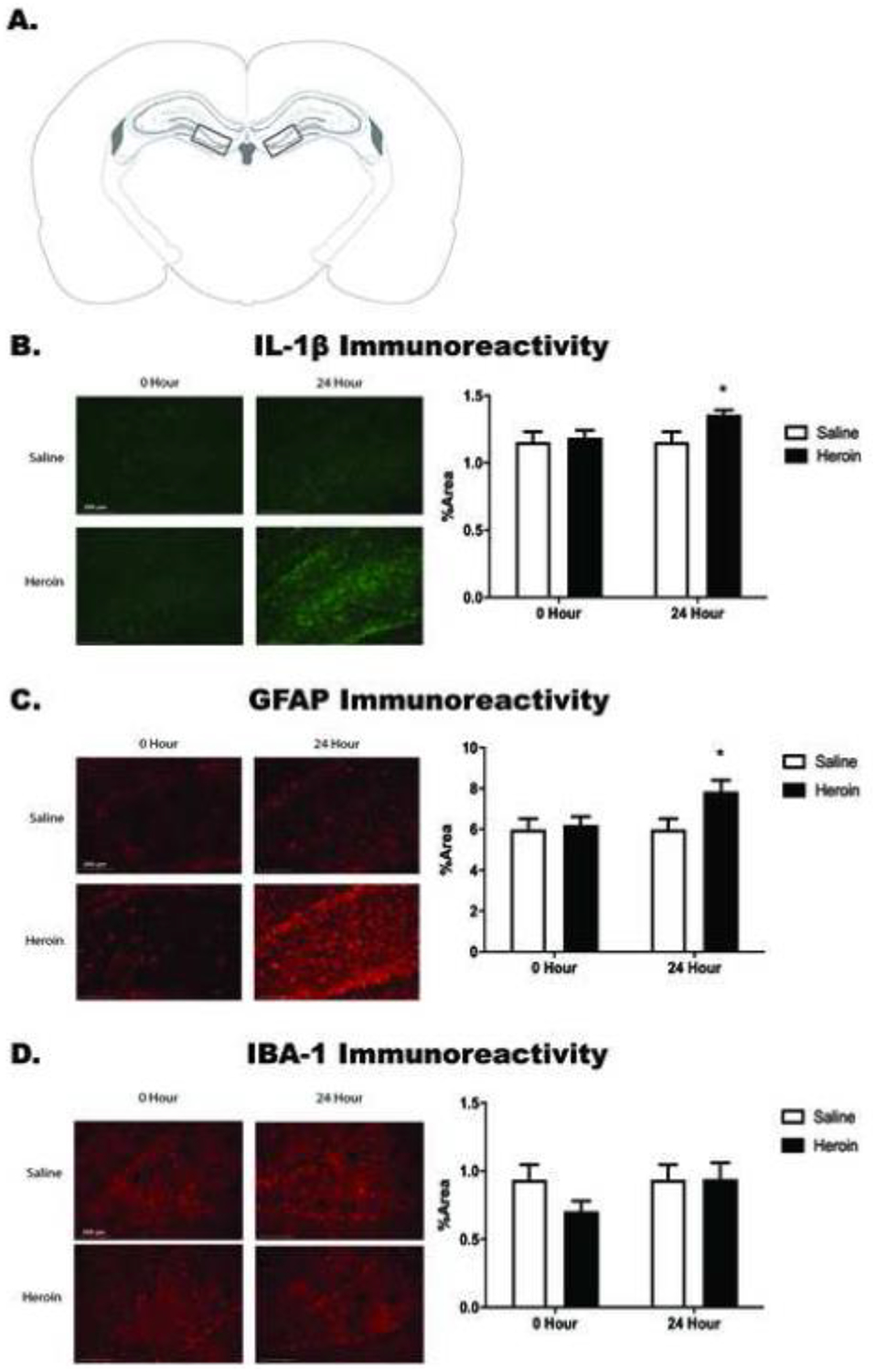
Paxinos and Watson (2007) schematic depicting bilateral image acquisition location. Each rectangular box represents the area in which images were taken within the DG of DH (A). Representative images (20X) for saline and heroin animals at both 0- and 24-hour time points (left) and quantification of positive fluorescence stain (right) of IL-1β (B), GFAP (C), and IBA-1 (D) taken within the DG of DH. *, statistically significant difference relative to respective control. Error bars indicate SEM.
Figure 4. IL-1β signal is colocalized with GFAP, and not with IBA-1, in the dentate gyrus of the dorsal hippocampus in heroin withdrawn and control animals.
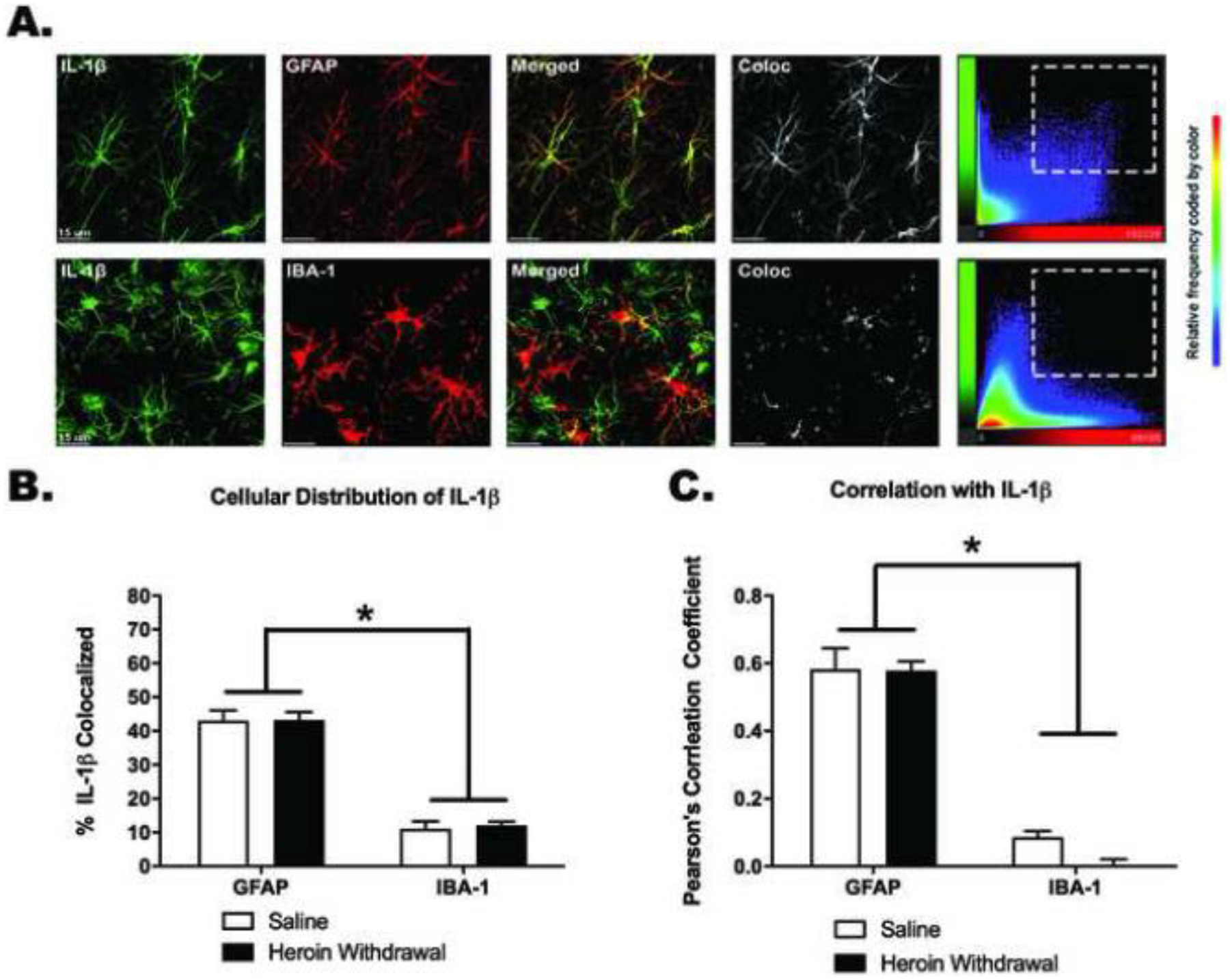
Representative 63X images of the DG. Images were taken in the area as seen in Figure 3A. Colocalization scatter plots show the signal intensity for each voxel in the Z-stack. (A). Colocalization of IL-1β signal with GFAP (top row) and IBA-1 (bottom row), respectively (B). Pearson’s correlation coefficient of colocalization of IL-1β signal intensity with GFAP and IBA-1 signal intensity, respectively (C). *, statistically significant difference relative to respective control. Error bars indicate SEM. Background signal was subtracted using Bitplane Imaris.
Figure 5. IL-1RA prevents the development of heroin withdrawal-enhanced fear learning but not weight loss.
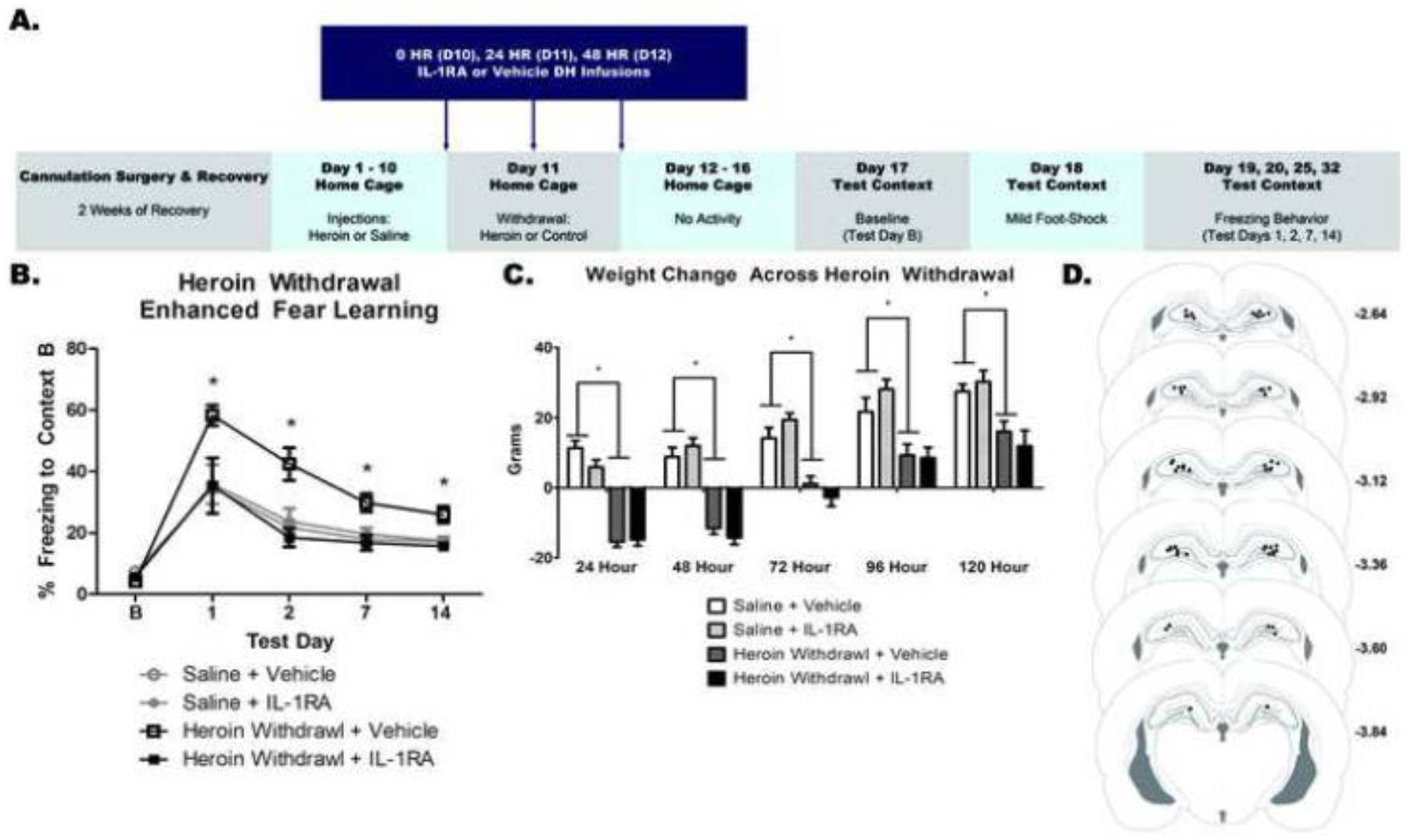
Experimental timeline (A). DH IL-1RA significantly attenuated HW-EFL (B). DH IL-1RA had no effect on weight change (C). Paxinos and Watson (2007) schematic with DH cannula placements shown. Each circle represents termination site of the cannula tract (D). *, statistically significant difference relative to respective control. Error bars indicate SEM.
Experiment 3: IL-1RA Prevents Heroin Withdrawal-Enhanced Fear Learning but not Weight Loss
Intra-DH IL-1RA prevented HW-EFL, but had no effect on withdrawal-induced weight change. There was no effect of heroin treatment or IL-1RA infusion on baseline contextual freezing (F(3,26)=1.066, p=.380), indicating that there is no generalized fear to the novel context. A 2 × 2 × 4 ANOVA revealed a significant main effect of heroin treatment (F(1,26)=5.379, p=.029) and a significant main effect of IL-1RA treatment (F(1,26)=11.3030, p=.002). These main effects of heroin treatment and IL-1RA treatment were on contextual freezing. There was also a significant effect of test day (F(3,78)=32.454, p<.001), indicating that conditioned freezing behavior diminished over time. Importantly, there was a significant heroin treatment by IL-1RA infusion interaction (F(1,26)=14.650, p=.001). Tukey’s post hoc comparisons revealed heroin withdrawn, vehicle-treated animals exhibited significantly higher freezing behavior compared to animals that were saline controls and vehicle-treated (p<.05), replicating HW-EFL. Heroin-withdrawn, IL-1RA treated animals exhibited significantly less freezing than withdrawn controls that received intra-DH vehicle (p<.05). Furthermore, heroin-withdrawn, IL-1RA-treated animals exhibited a comparable amount of freezing behavior (no significant difference) to both saline control groups (p>.05) (Figure 6b). These results indicate that intra-DH IL-1RA during heroin withdrawal prevented future enhanced fear learning.
A 2 × 2 × 5 repeated measures ANOVA revealed a significant main effect of heroin treatment on withdrawal weight change (F(1,26)=69.597, p<.001) but no significant effect of IL-1RA infusion (F(1,26)=.004, p=.953) indicating that heroin withdrawal decreases weight regardless of IL-1RA treatment. There was also an overall effect of withdrawal time (F(4,104)=162.489, p<.001), indicating over the course of withdrawal animals started to gain weight again. (Figure 5c). These results show that contrary to its effects on HW-EFL, IL-1RA had no effect on heroin withdrawal-induced weight loss.
Discussion
The current studies demonstrate for the first time that exposure to chronic heroin administration and withdrawal is capable of producing a PTSD-like phenotype in a rodent model, specifically the symptoms of greater susceptibility to future fear learning. We establish that HW-EFL is mediated by DH IL-1 neuroimmune signaling, revealing a mechanism for opioid-stress interactions. Specifically, heroin withdrawal induces both DH IL-1β and GFAP immunoreactivity within the DG, and blocking DH IL-1 signaling disrupts HW-EFL. Additionally, both control and heroin withdrawal induced IL-1β is primarily expressed in hippocampal astrocytes, suggesting this cell type may be involved in IL-1 signaling as astrocytes are the predominant glial cell type expression DH IL-1β. Collectively, these findings provide new evidence that heroin withdrawal-induced hippocampal IL-1β is a critical component for the development of future enhanced fear learning and is one mechanism by which opioids and opioid withdrawal can elicit a PTSD-like phenotype.
In the present study, exposure to heroin withdrawal is shown to produce a PTSD-like phenotype in a rodent model. PTSD, alone, is a severe psychological disorder, and to further exacerbate the consequences of PTSD, multiple lines of evidence indicate that opiate abuse leads to increased incidence and severity of PTSD. Consistently, opiate use disorder has emerged as a significant issue in clinical populations with PTSD due to prolonged pain treatments with opiates following active duty (Bennett et al. 2017; Finley et al. 2018). While the clinical PTSD incorporates many psychological and physiological consequences, our model only tests the greater susceptibility to future fear learning. Studies can also test other symptoms of PTSD in a pre-clinical model such as altered circadian cycle and physiological alterations, such as plasma corticosterone levels. However, future fear learning is a major component of human PTSD, and therefore the current findings provide strong evidence that heroin use and withdrawal can lead to a PTSD-like phenotype and provides a possible mechanism that may contribute to the similarities in symptomology and overwhelming comorbidity of opiate use disorders and PTSD.
Our finding that HW-EFL is driven by hippocampal IL-1 signaling is consistent with previously published work suggesting that both heroin use and PTSD engage neuroimmune signaling. Opioids directly modulate neuroimmune processes that can lead to long-lasting neural adaptations that increase vulnerability to health detriments associated with use (Hutchinson et al. 2008a; Hutchinson et al. 2008b). The present study demonstrates that heroin withdrawal induces DH IL-1β expression and that when blocked prevents future enhanced fear learning. Collectively, these data indicate that heroin withdrawal engages hippocampal IL-1 signaling to alter learning and memory processes.
We hypothesized that DH IL-1RA infusions would attenuate HW-EFL and heroin withdrawal-induced weight loss. Although DH IL-1RA did indeed prevent the development of HW-EFL, this effect did not extend to withdrawal-induced weight loss. This intriguing finding demonstrates a selective role for DH IL-1 signaling in the learning processes following withdrawal, but not withdrawal behaviors, per se. In particular, enhanced fear learning is tied to impaired memory formation. Hippocampal IL-1β is critical for normal learning and memory mechanisms and deviations from physiological levels impairs memory formation (Goshen et al. 2007). As our data show that heroin withdrawal alters DH IL-1β expression, we hypothesize that the IL-1β expression change has later implications for processes involved in fear learning. IL-1β has not been associated with withdrawal symptoms such as weight loss, however other cytokines, such as tumor necrosis factor alpha, TNF-α, have been implicated in modulating weight loss. Potentially targeting these cytokines in brain regions known to play a role in opiate withdrawal may show changes in heroin withdrawal induced weight loss. The present work focused on the DH, a region in the brain known to be involved in memory. However, further studies should investigate whether IL-1β expression in other brain regions involved in opioid abuse, such as the ventral tegmental area and locus coeruleus, which regulate symptoms of opiate dependence and withdrawal (Garcia-Perez et al. 2017; Kaufling and Aston-Jones 2015), may also contribute to HW-EFL and alter heroin withdrawal-induced weight changes.
In our experiments, astrocytes were the primary glial cell colocalized with IL-1β, in both control and heroin-withdrawal conditions. We found this specifically within the dentate gyrus of the dorsal hippocampus, and heroin withdrawal itself upregulated DH GFAP expression. We hypothesized that the colocalization between IL-1β and GFAP would increase following heroin withdrawal. While the current experiments did not confirm this hypothesis here, there may be a potential explanation for our observed lack of this effect. The colocalization measure reveals that the percent of IL-1β signal colocalized with GFAP signal is significantly greater than the percent of IL-1β signal colocalized with IBA-1 signal. We believe since we reported an increase in both IL-1β and GFAP immunoreactivity following heroin withdrawal, we did not see an increase in colocalization due to the % increase in both proteins. Although this suggests that there may be a relationship between astrocytes and IL-1β, both in basal levels and following heroin withdrawal, this does not imply a direct causal link between astrocyte reactivity and IL-1β expression. However, this does demonstrate that heroin administration and withdrawal may directly alter astrocyte reactivity, as heroin withdrawal itself increases DH GFAP expression, and suggests that astrocyte signaling may have a role in heroin withdrawal and enhanced future fear learning. Multiple studies suggest astrocyte-dependent signaling is important in stress and anxiety-related behaviors, such as PTSD (Barres 2008). For example, our laboratory has previously established that chemogenetic stimulation of astroglial Gi-signaling is sufficient to attenuate SEFL (Jones et al. 2018). This, coupled with our finding that DH GFAP increases in heroin withdrawal while DH IBA-1 does not, supports our hypothesis that astrocytes in the dorsal hippocampus, may play a role in the withdrawal-induced DH neuroimmune signaling that promotes enhanced fear learning.
Opioid withdrawal has been shown to upregulate neuroinflammatory genes, and astrocytes in particular are highly active during the withdrawal period (O’Sullivan et al. 2019). Although microglia-derived cytokines are an established model for many stress-related diseases (Block et al. 2007), our data suggests that hippocampal microglia levels remain stable through heroin withdrawal. Consistent with these results, multiple studies have shown that morphine does not induce microglial pro-inflammatory cytokines in vitro (El-Hage et al. 2006; Turchan-Cholewo et al. 2009), and morphine alone has no effect on NF-kB, a precursor to microglial activation and production of proinflammatory cytokines (Gessi et al. 2016), suggesting, that specifically hippocampal microglia may not play a major role in opiate dependence and withdrawal. However, there is extensive literature on the interaction between microglia and opioid dependence and withdrawal that shows microglia, particularly in the spinal cord, is involved in regulating multiple cytokines and drug seeking behaviors (Bland et al. 2009; Grace et al. 2016; Hutchinson et al. 2009; Reiss et al. 2020). It would be interesting to determine whether chronic escalating heroin administration can be employed to study heroin withdrawal-induced changes in microglia in the spinal cord both across heroin administration and during withdrawal.
Although we report IL-1β is highly colocalized with astrocytes in the DH, the current study does not directly demonstrate a causal link between astrocyte signaling, IL-1, and HW-EFL. We show that blocking IL-1 signaling can attenuate future enhanced fear learning, however this is not directly linked to astrocyte signaling. Studies can employ chemogenetic tools under the control of a GFAP promoter to test if astrocyte signaling during withdrawal is causally linked to IL-1 production and subsequent fear learning. Furthermore, additional studies could examine the development of HW-EFL using an astrocyte-specific IL-1 knockout animal strain or test whether pharmacological astrocyte ablation influences HW-EFL development. In addition to astrocyte signaling, changes in astrocyte physiology can be a major indicator of whether astrocyte signaling is involved in a behavior. We report increases in GFAP immunoreactivity during withdrawal, and although GFAP is one of the most widely used astrocyte markers in the field, it only accounts for 15% of total astrocyte volume (Benediktsson et al. 2005). To examine astrocyte physiology in rich detail, we can employ innovative strategies developed by Reissner and colleagues. This methodology uses a membrane-tagged GFP under the control of a GFAP promoter to visualize and quantify individual astrocyte morphology its entirety throughout a three-dimensional reconstruction (Testen et al. 2020). These strategies will expand this line of research and identify how astrocyte signaling and physiology is altered during heroin withdrawal to promote future fear learning.
While to sole focus on the dentate gyrus of the dorsal hippocampus is well justified given previous work supporting the importance of IL-1 signaling in the dorsal hippocampus (Jones et al. 2015; Jones et al. 2017; Jones et al. 2018), it is important to note that the hippocampus is not acting alone in regards to contextual fear learning. There are multiple other regions, such as the nucleus accumbens, amygdala, frontal cortex, and cingulate cortex that have been implicated in contextual and cued fear conditioning. Studies to examine the significance of heroin-withdrawal induced IL-1 signaling in these brain regions during HW-EFL would provide more information regarding broader circuity associated with HW-EFL.
Here, our focus was on IL-1 signaling, as previous work supports the significance of IL-1 signaling in stress responses (Jones et al. 2015; Jones et al. 2017), however it is important to note that IL-1β is not acting alone, and it’s very possible that other proinflammatory cytokines play additional roles in regards to both heroin withdrawal and enhanced fear learning. For example, both IL-6 and TNF-α have been shown to be upregulated in stress-related disorders, especially PTSD (Maes et al. 1999) and following morphine withdrawal (Mansouri et al. 2020). Studies to examine IL-6 and TNF-α, along with other cytokines, in heroin-withdrawal-induced immune signaling would provide more information regarding the neuroimmune mechanisms of HW-EFL.
In summary, our findings demonstrate that exposure to chronic heroin administration and withdrawal is capable of producing a PTSD-like phenotype in rats. Furthermore, heroin withdrawal increases expression of certain neuroimmune proteins, such as IL-1β and GFAP, and blockade of DH IL-1 signaling disrupts future enhanced fear learning. Collectively, these data provide important new evidence that chronic heroin administration and withdrawal alters hippocampal neuroimmune signaling and downstream learning and memory processes.
Acknowledgements:
This research was supported by National Institute on Drug Abuse (NIDA) grants R01 DA034721 (DTL), T32 DA007244 (SVP, JEP, CLL), and F31 DA047054 (JEP), a National Science Foundation grant DGE-1144081 (CLL), a Richard A. King Research Excellence Award (SVP), and a Behavioral Neuroscience Graduate Student Opportunity Fund (SVP).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Statement: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease Neuron 60:430–440 doi: 10.1016/j.neuron.2008.10.013 [DOI] [PubMed] [Google Scholar]
- Benediktsson AM, Schachtele SJ, Green SH, Dailey ME (2005) Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures J Neurosci Methods 141:41–53 doi: 10.1016/j.jneumeth.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Bennett AS, Elliott L, Golub A, Wolfson-Stofko B, Guarino H (2017) Opioid-Involved Overdose Among Male Afghanistan/Iraq-Era U.S. Military Veterans: A Multidimensional Perspective Subst Use Misuse 52:1701–1711 doi: 10.1080/10826084.2017.1306563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Hutchinson MR, Maier SF, Watkins LR, Johnson KW (2009) The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release Brain Behav Immun 23:492–497 doi: 10.1016/j.bbi.2009.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms Nat Rev Neurosci 8:57–69 doi: 10.1038/nrn2038 [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Darnell A, Charney DS (1996) Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse Am J Psychiatry 153:369–375 doi: 10.1176/ajp.153.3.369 [DOI] [PubMed] [Google Scholar]
- Cohen M, Meir T, Klein E, Volpin G, Assaf M, Pollack S (2011) Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents International journal of psychiatry in medicine 42:117–131 [DOI] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, Vetreno RP (2015) Neuroimmune Function and the Consequences of Alcohol Exposure Alcohol Res 37:331–341, 344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N et al. (2006) HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines Glia 53:132–146 doi: 10.1002/glia.20262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley EP, Schneegans S, Tami C, Pugh MJ, McGeary D, Penney L, Sharpe Potter J (2018) Implementing prescription drug monitoring and other clinical decision support for opioid risk mitigation in a military health care setting: a qualitative feasibility study J Am Med Inform Assoc 25:515–522 doi: 10.1093/jamia/ocx075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez D, Laorden ML, Milanes MV (2015) Regulation of Pleiotrophin, Midkine, Receptor Protein Tyrosine Phosphatase beta/zeta, and Their Intracellular Signaling Cascades in the Nucleus Accumbens During Opiate Administration Int J Neuropsychopharmacol 19 doi: 10.1093/ijnp/pyv077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez D, Laorden ML, Milanes MV (2017) Acute Morphine, Chronic Morphine, and Morphine Withdrawal Differently Affect Pleiotrophin, Midkine, and Receptor Protein Tyrosine Phosphatase beta/zeta Regulation in the Ventral Tegmental Area Mol Neurobiol 54:495–510 doi: 10.1007/s12035-015-9631-2 [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG (1978) Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions J Pharmacol Exp Ther 205:536–546 [PubMed] [Google Scholar]
- Gessi S, Borea PA, Bencivenni S, Fazzi D, Varani K, Merighi S (2016) The activation of muopioid receptor potentiates LPS-induced NF-kB promoting an inflammatory phenotype in microglia FEBS Lett 590:2813–2826 doi: 10.1002/1873-3468.12313 [DOI] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, Page G (2009) PTSD is associated with an excess of inflammatory immune activities Perspect Psychiatr Care 45:262–277 doi: 10.1111/j.1744-6163.2009.00229.x [DOI] [PubMed] [Google Scholar]
- Gola H et al. (2013) Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells BMC psychiatry 13:40 doi: 10.1186/1471-244X-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I et al. (2007) A dual role for interleukin-1 in hippocampal-dependent memory processes Psychoneuroendocrinology 32:1106–1115 doi: 10.1016/j.psyneuen.2007.09.004 [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R (2009) Interleukin-1 (IL-1): a central regulator of stress responses Frontiers in neuroendocrinology 30:30–45 doi: 10.1016/j.yfrne.2008.10.001 [DOI] [PubMed] [Google Scholar]
- Grace PM et al. (2016) Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation Proc Natl Acad Sci U S A 113:E3441–3450 doi: 10.1073/pnas.1602070113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Liu T, Guo JC, Jiang XL, Chen F, Gao YS (2012) Study on serum cytokine levels in posttraumatic stress disorder patients Asian Pacific journal of tropical medicine 5:323–325 doi: 10.1016/S1995-7645(12)60048-0 [DOI] [PubMed] [Google Scholar]
- Hanisch UK (2002) Microglia as a source and target of cytokines Glia 40:140–155 doi: 10.1002/glia.10161 [DOI] [PubMed] [Google Scholar]
- Holmes A et al. (2012) Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding Nat Neurosci 15:1359–1361 doi: 10.1038/nn.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR et al. (2008a) Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia Brain Behav Immun 22:1178–1189 doi: 10.1016/j.bbi.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR et al. (2009) Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun 23:240–250 doi: 10.1016/j.bbi.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR et al. (2008b) Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia Brain Behav Immun 22:1248–1256 doi: 10.1016/j.bbi.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR (2001) Substance use disorders in patients with posttraumatic stress disorder: a review of the literature Am J Psychiatry 158:1184–1190 doi: 10.1176/appi.ajp.158.8.1184 [DOI] [PubMed] [Google Scholar]
- Jones ME, Lebonville CL, Barrus D, Lysle DT (2015) The role of brain interleukin-1 in stress-enhanced fear learning Neuropsychopharmacology 40:1289–1296 doi: 10.1038/npp.2014.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Lebonville CL, Paniccia JE, Balentine ME, Reissner KJ, Lysle DT (2017) Hippocampal interleukin-1 mediates stress-enhanced fear learning: A potential role for astrocyte-derived interleukin-1β Brain, Behavior, and Immunity doi: 10.1016/j.bbi.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Paniccia JE, Lebonville CL, Reissner KJ, Lysle DT (2018) Chemogenetic manipulation of dorsal hippocampal astrocytes protects against the development of stress-enhanced fear learning Neuroscience doi: 10.1016/j.neuroscience.2018.07.015 [DOI] [PubMed] [Google Scholar]
- Kaufling J, Aston-Jones G (2015) Persistent Adaptations in Afferents to Ventral Tegmental Dopamine Neurons after Opiate Withdrawal J Neurosci 35:10290–10303 doi: 10.1523/JNEUROSCI.0715-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS (2009) Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory Neurosci Lett 456:39–43 doi: 10.1016/j.neulet.2009.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Rivera PD, Bilbo SD (2018) Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse Neuropsychopharmacology 42:156–177 doi: 10.1038/npp.2016.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR (2011) Naloxone-precipitated morphine withdrawal behavior and brain IL-1beta expression: comparison of different mouse strains Brain Behav Immun 25:1223–1232 doi: 10.1016/j.bbi.2011.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, Bosmans E (1999) Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events Biol Psychiatry 45:833–839 [DOI] [PubMed] [Google Scholar]
- Mansouri MT, Naghizadeh B, Ghorbanzadeh B, Amirgholami N, Houshmand G, Alboghobeish S (2020) Venlafaxine inhibits naloxone-precipitated morphine withdrawal symptoms: Role of inflammatory cytokines and nitric oxide Metab Brain Dis 35:305–313 doi: 10.1007/s11011-019-00491-4 [DOI] [PubMed] [Google Scholar]
- McCauley JL, Killeen T, Gros DF, Brady KT, Back SE (2012) Posttraumatic Stress Disorder and Co-Occurring Substance Use Disorders: Advances in Assessment and Treatment Clin Psychol (New York) 19 doi: 10.1111/cpsp.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, Lambert-Harris C, McGovern MP, Xie H, An M, McLeman B (2014) Co-occurring prescription opioid use problems and posttraumatic stress disorder symptom severity Am J Drug Alcohol Abuse 40:304–311 doi: 10.3109/00952990.2014.910519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SJ et al. (2019) Single-Cell Glia and Neuron Gene Expression in the Central Amygdala in Opioid Withdrawal Suggests Inflammation With Correlated Gut Dysbiosis Front Neurosci 13:665 doi: 10.3389/fnins.2019.00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos IC et al. (2015) Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression The lancet Psychiatry 2:1002–1012 doi: 10.1016/S2215-0366(15)00309-0 [DOI] [PubMed] [Google Scholar]
- Patel RS, Elmaadawi A, Nasr S, Haskin J (2017) Comorbid Post-Traumatic Stress Disorder and Opioid Dependence Cureus 9:e1647 doi: 10.7759/cureus.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS (2005) Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder Neuroscience and biobehavioral reviews 29:1207–1223 doi: 10.1016/j.neubiorev.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Reiss D, Maduna T, Maurin H, Audouard E, Gaveriaux-Ruff C (2020) Mu opioid receptor in microglia contributes to morphine analgesic tolerance, hyperalgesia, and withdrawal in mice J Neurosci Res doi: 10.1002/jnr.24626 [DOI] [PubMed] [Google Scholar]
- Silverman MN, Macdougall MG, Hu F, Pace TW, Raison CL, Miller AH (2007) Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice Mol Psychiatry 12:408–417 doi: 10.1038/sj.mp.4001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanichev M, Dygalo NN, Grigoryan G, Shishkina GT, Gulyaeva N (2014) Rodent models of depression: neurotrophic and neuroinflammatory biomarkers Biomed Res Int 2014:932757 doi: 10.1155/2014/932757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testen A, Kim R, Reissner KJ (2020) High-Resolution Three-Dimensional Imaging of Individual Astrocytes Using Confocal Microscopy Curr Protoc Neurosci 91:e92 doi: 10.1002/cpns.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Wilk JE, Riviere LA, McGurk D, Castro CA, Hoge CW (2010) Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq Arch Gen Psychiatry 67:614–623 doi: 10.1001/archgenpsychiatry.2010.54 [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Gupta S, Keller JN, Knapp PE, Hauser KF, Bruce-Keller AJ (2009) Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation J Neurochem 108:202–215 doi: 10.1111/j.1471-4159.2008.05756.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Zhai M, Wang L, Miao D, Zhu X, Wang W (2013) FGF2 blocks PTSD symptoms via an astrocyte-based mechanism Behav Brain Res 256:472–480 doi: 10.1016/j.bbr.2013.08.048 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Ho A, Kreek MJ (2013) Suppression of hypothalamic-pituitary-adrenal axis by acute heroin challenge in rats during acute and chronic withdrawal from chronic heroin administration Neurochem Res 38:1850–1860 doi: 10.1007/s11064-013-1091-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


