Abstract
Introduction:
Hematopoietic cell transplantation (HCT) is an effective treatment for many hematological malignancies, and its utilization continues to rise. However, due to the difficult logistics and high cost of HCT, there are significant barriers to accessing the procedure; these barriers are likely greater for older patients. Although numerous factors may influence HCT access, no formal analysis has detailed the cumulative barriers that have been studied thus far.
Methods:
We conducted a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to better categorize the barriers to access and referral to HCT, with a focus on the subgroup of older patients. We searched for articles published in English from PubMed, Embase, Cumulative Index for Nursing and Allied Health (CINAHL), and Cochrane Central Register of Controlled Trials (CENTRAL) between the database inception and January 31st, 2020. We selected articles that met the following inclusion criteria: 1) Study design: qualitative, cross-sectional, observational cohort, or mixed-method study designs; 2) Outcomes: barriers related to patient and physician access to HCT; 3) Population: adults aged ≥18 years with hematological malignancies within the US. Abstracts without full text were excluded. QUALSYST methodology was used to determine article quality. Data on the barriers to access and referral for HCT were extracted, along with other study characteristics. We summarized the findings using descriptive statistics.
Results:
We included twenty-six of 3,859 studies screened for inclusion criteria. Twenty studies were retrospective cohorts and four were cross-sectional. There was one prospective cohort study and one mixed method study. Only one study was rated as high-quality and 16 were rated as fair. Seventeen studies analyzed age as a potential barrier to HCT referral and access with sixteen finding older age to be a barrier. Other consistent barriers to HCT referral and access included non-white race (n=16/20 studies), insurance status (n = 13/14 studies), comorbidities (n=10/11 studies), and lower socioeconomic status (n=7/8 studies).
Conclusions:
High-quality studies are lacking related to HCT barriers. Older age and non-white race were consistently linked to reduced access to HCT. To produce a more just healthcare system, strategies to overcome these barriers for vulnerable populations should be prioritized. Examples include patient and physician education, as well as geriatric-assessment guided care models that can be readily incorporated into clinical practice.
Keywords: Barriers, hematopoietic cell transplantation, age, race
Introduction
In 2018, it was estimated that around 9,000 patients received allogeneic hematopoietic cell transplantation (HCT) and 14,000 patients received autologous HCTs in the US.1 These numbers are expected to gradually increase at the rate of approximately 5% each year due to advances in HCT strategies, increased donor availability, improved pre- and post-transplantation care, and improvement in transplantation outcomes.2, 3 In response, the American Society for Transplantation and Cellular Therapy and the National Marrow Donor Program/Be The Match Registry have developed and sponsored a System Capacity Initiative (SCI).4, 5 The SCI is a series of multifaceted efforts that uses a thoughtful process model to engage multiple large organizations, transplant centers, and medical experts to identify complex problems affecting the care delivery of HCT and to resolve such issues.4, 5
Institutions vary with respect to patient selection, transplant indications, transplantation regimens, and supportive care practices.6, 7 Difficult logistics, complex regulatory requirements, and the high cost of HCT require expensive and robust clinical infrastructures and result in access barriers to these procedures;8 these barriers are likely greater for older patients. Barriers exist at the patient (e.g., age, race, financial burden),9, 10 physician (e.g., physician perceptions and bias),11 and healthcare level (e.g., transplant infrastructure).12 With an increasing attention and focus on population health and socioeconomic factors, it is important to understand the barriers to HCT access so these barriers can be addressed at all levels.13 Although numerous factors may influence HCT access, no formal analysis has detailed these factors.
In this systematic review, we identify the barriers to access and referral to HCT. In addition, we focused on the subgroup of older patients as age is one of the most established factors hindering HCT access. This is postulated to be due to a lack of clinical trial evidence in this population and exclusion by frailty and comorbidity.14, 15
Methods
Data sources
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16 We searched for articles published in English in four databases including PubMed, Embase, Cumulative Index for Nursing and Allied Health (CINAHL), and Cochrane Central Register of Controlled Trials (CENTRAL) between the database inception and January 31st, 2020. The search strategies were developed with the assistance of a librarian (Supplement Table 1).
Inclusion Criteria
We included articles that met the following inclusion criteria: 1) Study design: qualitative, cross-sectional, observational cohort, mixed-method study designs, or intervention trials; 2) Outcomes: barriers related to patient and physician access and referral to HCT; 3) Population: adults aged ≥18 years with hematologic malignancies within the US (since barriers to HCT are likely healthcare system-specific). Abstracts without full text were excluded.
Study Selection
Articles from initial search results were exported into Endnote x9 (Clarivate Analytics), and duplicate articles were removed. The remaining articles were imported into Covidence (Veritas Health Innovation), a systematic review software package. Two authors independently reviewed all titles and abstracts. Disagreements were discussed and resolved by consensus. All eligible full texts were reviewed once again by two authors based on the aforementioned inclusion and exclusion criteria. The references of selected full texts were reviewed for additional articles.
Data Extraction and Analysis
Data from each article were extracted into a template with predetermined variables including: first author, journal name, article title, year of publication, study design, United States geographical location, the study population, the size (n) of the study, type of transplant (allogeneic versus autologous), registry used if applicable, barriers assessed, mean or median age of study participant if applicable, the age definition used in the model (e.g., categorical versus continuous), reasoning for the way age was defined in the model, and barriers found. In an iterative process, each paper was scanned for 28 possible barriers (the total of all barriers extracted from the included studies), and the barriers were categorized as present, no association found, or barrier not assessed. If a barrier was identified in a specific population but not others, it was considered as present. We considered the following as barriers: 1) Positive associations found on multivariable analyses, 2) Positive associations found on univariate analyses (if multivariate analyses were not performed), 3) If no modelling was performed, descriptive statistics were presented; and 4) Elicited via surveys.
Quality Assessment
We used the QUALSYST “Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields” to assess the quality of the studies.17 The checklist for the quantitative studies consisted of fourteen items, each scored on a 3-point scale. The summary score was calculated for each paper and was used to make an overall assessment of the quality of the paper as poor, fair, or good. Details of how this was done are shown in Supplemental Tables 2 and 3. Two authors independently assessed the articles. Disagreements were resolved by a third reviewer.
Results
Study Characteristics
The search strategy employed (Supplemental Table 1) yielded 3,859 studies which were then refined based on our inclusion criteria to 26 total studies (Figure 1). The studies included had their content extracted for the variables outlined in Table 1. The articles were published in thirteen journals from 1992 to 2019; twenty articles were published after 2009.10, 12, 18–35 Six of the articles published findings from a single center 18,20,24,30,36,37, and twenty articles used patient information from twelve different databases.10–12, 19, 21–23, 25–29, 31, 32, 34–36, 38–40 The most commonly used databases were Center for International Blood and Marrow Transplant Research registry (CIBMTR) (n=4 studies)26, 29, 35, 36 and the Surveillance, Epidemiology and End Results registry (SEER) (n=6 studies).21, 23, 26, 29, 34, 36 Twenty studies were retrospective cohort 10,12,18,20,21,23–30,34–40 four were cross-sectional studies,11, 22, 31, 32 one was a prospective cohort study,19 and one was mixed method study.33 The population size varied from 8818 to over 300 million;22 the latter study evaluated population-level access to HCT.22 The studies covered a variety of hematological malignancies with the most commonly examined population being patients with acute myeloid leukemia (n=20 studies).10, 11, 19, 20, 22, 25–34, 36–40 Twelve studies examined allogeneic HCT only,11, 18, 19, 24–26, 29–32, 36, 37 three studies examined only autologous HCT,12, 21, 35 and eleven studies examined both types of HCT.10, 20, 22, 23, 27, 28, 33, 34, 38–40
Figure 1: PRISMA diagram.
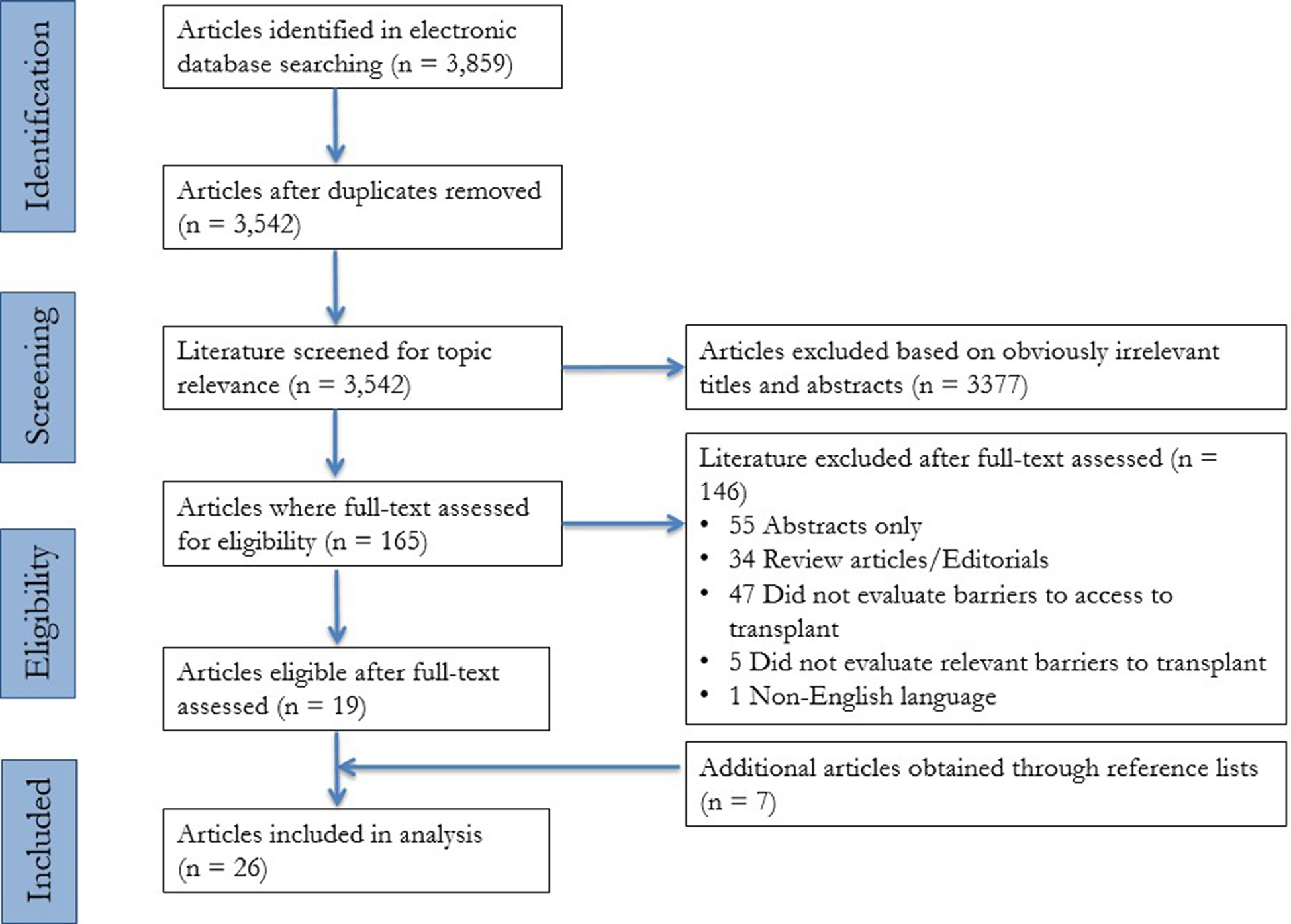
Table 1:
Barriers to hematopoietic cell transplantation
| Article | Study design | Study population | Sample size | Age data reported | Age definitionsa (continuous or categorical, cut-off) | Types of transplant | Data source | Barriers | No associations found |
|---|---|---|---|---|---|---|---|---|---|
| Adrianzen Herrera 201918 | Retrospective | Adult T-cell leukemia-lymphoma | 88 | Median: 56 years (range 28–87) | Categorical (<40, 41–60, or >61) | Allogeneic | EMR of Montefiore Bronx | Socioeconomic status, donor/human leukocyte antigen-subtype availability, insurance, patient preference, Poor understanding or medical non-compliance, cancer type, disease status | Age, performance status |
| Al-Hamadani 201412 | Retrospective | Multiple myeloma | 137,409 | N/A | Categorical (<65, 65–75, or >75) | Autologous | NCDB | Race, socioeconomic status, age, insurance, distance to transplant center/residence, year diagnosed, academic vs. community, bed size | Gender/Sex, comorbidity |
| Barker 201019 | Prospective | Acute leukemia, MDS, myeloproliferative disease, NHL, CLL, prolymphocytic leukemia, HL, MM, or severe AA | 553 | Median: 47 years (range 0.9–73) | - | Allogeneic | - NMDP for URD - Bone Marrow Donors Worldwide, NMDP and New York Blood Center for Cord Blood |
Donor/human leukocyte antigen-subtype availability, race | None |
| Berman 199237 | Retrospective | AML | 350 | 35–46% of patients were aged ≤40 years | - | Allogeneic | MSKCC | Comorbidity, patient preference, disease status | None |
| Bhatt 201810 | Retrospective | AML | 17,555 | Median: 68 years (range 61–75) | Categorical (60–65, 66–70, or 71–75) | Autologous and allogeneic | NCDB | Race, socioeconomic status, age, insurance, comorbidity, distance to transplant center/residence, year diagnosed, academic vs community | Gender/Sex, time from diagnosis to initial therapy initiation |
| Bierenbaum 201220 | Retrospective | AML | 504 | White: median 60.8 years; Black: median 54.4 years | Categorical (<60 or ≥60) | Autologous and allogeneic | UMGCC | Race, age, donor/human leukocyte antigen-subtype availability, substanceuse disorder, comorbidity, performance status, patient preference, disease status | Time from diagnosis to referral |
| Cho 200638 | Retrospective | ALL, CLL, AML, CML, Hodgkin’s, NHL | Leukemia: 2,899; Lymphoma: 3,536 | Leukemia: median 37 years; Lymphoma: median 46 years | Continuous | Autologous and allogeneic | Arizona Hospital Inpatient Discharge Data Public Release File | Age, insurance, comorbidity, cancer type, academic vs community, bed size | Gender/Sex, race |
| Costa 201521 | Retrospective | MM | 22,462 | Median: 71 years (range 61–81) | Continuous | Autologous | SEER | Gender/Sex, race, age | None |
| Delamater 201622 | Cross-sectional | All HCT indications | 306,675,006 | 76% aged ≥18 years | Categorical(0–9, 10–17, 18–29, 30–44, 45–59, 60–74, or ≥75) | Autologous and allogeneic | 2010 US Census Block Group Data | Race, age, distance to transplant center/residence | Gender/Sex |
| Fiala 201723 | Retrospective | MM | 20,916 | Median: 77.1 years | Continuous | Autologous and allogeneic | SEER-Medicare linked database | Gender/Sex, race, socioeconomic status, age, insurance, comorbidity, performance status, year diagnosed | Distance to transplant center/residence |
| Getta 201724 | Retrospective | MDS | 362 | Median: 65 years (range 20–75) | Categorical (<40, 40–64, or ≥65)and (<65 or ≥65) | Allogeneic | MSKCC | Age, comorbidity, physician perception of risk, performance status, patient preference, disease risk, disease status, year diagnosed | Gender/Sex, marital status, race, insurance, socioeconomic status, distance to transplant center/residence |
| Hwang 200439 | Retrospective | ALL, CLL, AML, CML, and other leukemias | 6,574 | N/A | Categorical (0–17, 18–64, or ≥65) | Autologous and allogeneic | Texas Hospital Inpatient Discharge Public Use Data File | Gender/Sex, age, comorbidity, cancer type | Race, insurance |
| Jabo 201725 | Retrospective | ALL, AML | ALL: 367; AML: 3,657 | ALL: 68% aged ≥60 years; AML: 89% aged ≥60 years | Categorical (15–39, 40–59, or ≥60) | Allogeneic | California Cancer Registry | marital status, race, socioeconomic status, age, year diagnosed | Gender/Sex, distance to transplant center/residence |
| Joshua 201026 | Retrospective | AML, ALL, CML, NHL, MM | 27,725 | 16% aged 6069 years; 29% aged 5059 years; 24% aged 4049 years 13% aged 3039 years; 8% aged 20–29 years; 10% aged 019 years | - | Allogeneic | SEER and CIBMTR | Gender/Sex, race | None |
| Kollman 200111 | Cross-sectional | CML, AML, ALL, other leukemia, MDS, AA, and other malignant and non-malignant diseases | 544 (Physician s, patients, and transplant center coordinators) | Median 29 years (range 0–65) | - | Allogeneic | NMDP | Donor/human leukocyte antigen-subtype availability, insurance, physician perception of risk, patient preference, Disease status, other treatment options | None |
| Mehta 200336 | Retrospective | ALL, AML, CML | Not provided | N/A | - | Allogeneic | CIBMTR and SEER | Gender/Sex | Cancer type |
| Mitchell 199740 | Retrospective | All types of leukemia and lymphoma | Leukemia: 15,116; Lymphoma: 23,304 | Median: 30.7 years | Continuous | Autologous and allogeneic | Inpatient Hospital Discharge Database (California, Maryland, New York, Massachusetts), Bureau of the Census | Insurance, year diagnosed, cancer type, age, race | Gender/Sex |
| Mitchell 201527 | Retrospective | All types of leukemia and lymphoma | Leukemia: 5,721; Lymphoma: 9,137 | Leukemia: 36.7 years (SD 20.9), lymphoma: 47.5 years (SD 13.6) | Continuous | Autologous and allogeneic | California Office of Statewide Planning and Development | Race, socioeconomic status, age, insurance, distance to transplant center/residence, cancer type | Gender/Sex |
| Patel 201528 | Retrospective | AML | 11,084 | 43% 6180 years, 18% >80 years | Categorical (<21, 21–40, 41–60, 61–80, or >80) | Autologous and allogeneic | California Cancer Registry research database, Office of Statewide Health Planning and Development | Race, age, comorbidity, disease risk | Gender/Sex |
| Paulson 201929 | Retrospective | AML, ALL, MDS | 3,147 | Not provided | - | Allogeneic | SEER and CIBMTR | Socioeconomic status | Race, social support, distance to transplant center/residence |
| Pidala 201330 | Retrospective | ALL, AML, MDS, myeloproliferative disorder, MM, Non-Hodgkin lymphoma, Hodgkin lymphoma, CLL, severe AA, PNH | 531 | 50–51 years (range 17–72) | Continuous | Allogeneic | Blood and Marrow Transplantation Program at Moffitt Cancer Center | Race, age, donor/human leukocyte
antigen-subtype availability, insurance, comorbidity, social support,
patient preference, disease status, other treatment options, psychiatric disability |
Gender/Sex, performance status, CMV serologic status |
| Pidala 201331 | Cross-sectional | CML, ALL, AML, MDS | 113 (Physicians) | N/A | Categorical (35–40, 45–50, 50–55, 55–60, 60–65, 65–70, 70–75, or >75) | Allogeneic | AMA Master file | Gender/Sex, race, age, insurance, psychiatric disability, substance use disorder, comorbidity, physician perception of risk, social support, distance to transplant center/residence, other treatment options, language, poor understanding or medical non-compliance, academic vs. community, physician experience with transplant | Donor/human leukocyte antigen- subtype availability |
| Preussler 201432 | Cross-sectional | All HCT Indications | 47 states | N/A | - | Allogeneic | State Medicaid website | Insurance | None |
| Preussler 201633 | Mixed-method | All HCT Indications | Focus group: 15, Survey: 133 (Social workers) | N/A | - | Autologous and allogeneic | N/A | Insurance, social support | None |
| Pulte 201334 | Retrospective | AML, ALL | ALL: 3148, AML:11,735 | ALL: 38–39 years, AML:68–69 years | - | Autologous and allogeneic | SEER | Race | None |
| Schriber 201735 | Retrospective | MM | 24,102 | N/A | Categorical (<45, 45–60, or 61–75) | Autologous | CIBMTR | Gender/Sex, race, age | None |
Abbreviations: AA, aplastic anemia; ALL, acute lymphoblastic leukemia; AMA, American Medical Association; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CMV, cytomegalovirus; HCT, hematopoietic cell transplantation’ HL, Hodgkin’s lymphoma; EMR, electronic medical records; IBMTR, International Bone Marrow Transplant Registry; MDS, myelodysplastic syndrome; MM, multiple myeloma; NCDB, National Cancer Database; NHL, non-Hodgkin’s lymphoma; NMDP, National Marrow Donor Program; MSKCC, Memorial Sloan Kettering Cancer Center; PNH, paroxysmal nocturnal hemoglobinuria; SCT, stem cell transplantation; SEER, Surveillance, Epidemiology, and End Results Program; UMGCC, University of Maryland Greenebaum Cancer Center; URD, unrelated donor
Studies that assessed age as a barrier
Quality Assessment
The quality assessment determined that, of the 26 studies, nine were poor, sixteen were fair, and one was of good quality. Based on the QUALSYST criteria,17 the most commonly neglected metrics by articles were estimating and reporting variance within models (n=15) and controlling for confounding variables (n=9). Disagreements between reviewers (as defined in Supplemental Table 2) occurred on four of the studies and a third reviewer examined the papers to make the final determination of quality. The overall low quality of the studies included in this paper is largely due to the lack of prospective assessments of barriers to HCT. Our process of applying the QUALSYST method is shown in Supplemental Table 3. The only study to receive a good quality score was Barker et al. due to the prospective nature of this study.19 The nine papers that received a poor quality assessment failed to meet an average of 65% of the quality metrics as determined by two raters (Supplemental Tables 2 and 3).
Barriers to HCT
The barriers to HCT assessed varied widely as shown in Table 2. Twenty-one studies were done to examine barriers at the patient level,10, 12, 18–21, 23–30, 34–40 two studies at the healthcare professional level,31, 33 two at the state level,22, 32 and one at both the patient and health professional levels.11 Nineteen studies provided information about the age of patients included,10, 18–30, 34, 35, 37, 38, 40 and seventeen studies analyzed age as a potential barrier to access with sixteen of them finding it to be a significant barrier.10, 12, 20–25, 27, 28, 30, 31, 35, 38, 39 Age barrier was found for both autologous and allogeneic HCT. The single study that did not find age to be a barrier was conducted in a single center, had a small sample size (n=88 patients), and analyzed age as a categorical variable (<40, 41–60, and >61 years).18
Table 2:
Potential barriers/factors associated with either referral for and/or receipt of hematopoietic cell transplantation.
| Levels | Barriers/factor s associated with either referral for or receipt of HCT | Total no. of studies | No. of studies indicating the barrier was present* | No. of studies in both autologous and allogeneic HCT | No. of studies indicating the barrier was present* | No. of studies in autologous HCT only | No. of studies indicating the barrier was present* | No. of studies in allogeneic HCT only | No. of studies indicating the barrier was present* |
|---|---|---|---|---|---|---|---|---|---|
| Patient | Age10, 12, 18, 20–25, 27, 28, 30, 31, 35, 38–40 | 17 | 16 | 9 | 9 | 3 | 3 | 5 | 4 |
| Gender/sex10, 12, 21–28, 30, 31, 36, 38–40,35 | 17 | 7 | 8 | 2 | 3 | 2 | 6 | 3 | |
| Race10, 12, 19–31, 34, 35,38–40 | 20 | 16 | 10 | 8 | 3 | 3 | 7 | 5 | |
| Insurance status and coverage10–12, 18, 23, 24, 27, 30–33, 38–40 | 14 | 12 | 7 | 6 | 1 | 1 | 6 | 5 | |
| Comorbidity or medically unstable/ineligible (total number of or specific comorbidity e.g. diabetes)10,12, 20, 23, 24, 27, 30, 31, 37–39 | 11 | 10 | 6 | 6 | 1 | 0 | 4 | 4 | |
| Patient preference11, 18,20, 24, 30, 37 | 6 | 6 | 1 | 1 | - | - | 5 | 5 | |
| Socioeconomic status (education, income, employment)10,12, 18, 23–25, 27, 29 | 8 | 7 | 3 | 3 | 1 | 1 | 4 | 3 | |
| Donor/human leukocyte antigen-subtype availabilitya, 11,18–20, 30, 31 | 6 | 5 | - | - | - | - | 6 | 5 | |
| Performance status18, 20, 23, 24, 30 | 5 | 3 | 1 | 1 | - | - | 4 | 2 | |
| Social support29–31, 33 | 4 | 3 | 1 | 1 | - | - | 3 | 2 | |
| Psychiatric disabilitya, 30, 31 | 2 | 2 | - | - | - | - | 2 | 2 | |
| Substance use disorder20, 31 | 2 | 2 | 1 | 1 | - | - | 1 | 1 | |
| Marital status24, 25 | 2 | 1 | - | - | - | - | 2 | 1 | |
| Poor understanding or medical non-compliancea, 18, 31 | 2 | 2 | - | - | - | - | 2 | 2 | |
| Languagea, 31 | 1 | 1 | - | - | - | - | 1 | 1 | |
| Cytomegalovirus serologic statusa, 30 | 1 | 0 | - | - | - | - | 1 | 0 | |
| Disease | Cancer type18, 27, 36, 38–40 | 6 | 5 | 4 | 4 | - | - | 2 | 1 |
| Disease status (e.g. stable, progression)11, 18, 20, 24, 30, 37 | 6 | 6 | 1 | 1 | - | - | 5 | 5 | |
| Other treatment optionsa, 11, 30, 31 | 3 | 3 | - | - | - | - | 3 | 3 | |
| Disease risk24, 28, 30 | 3 | 3 | 1 | 1 | - | - | 2 | 2 | |
| Physician | Perception of riska, 11, 24, 31 | 3 | 3 | - | - | - | - | 3 | 3 |
| Experience with transplanta, 31 | 1 | 1 | - | - | - | - | 1 | 1 | |
| Organization | Distance to transplant center/residence10, 12, 22–25, 27, 29, 31 | 9 | 5 | 4 | 3 | 1 | 1 | 4 | 1 |
| Year diagnosed10, 12, 23–25, 40 | 6 | 6 | 3 | 3 | 1 | 1 | 2 | 2 | |
| Academic vs. community (including teaching status)10, 12, 31, 38 | 4 | 4 | 2 | 2 | 1 | 1 | 1 | 1 | |
| Bed sizeb, 38 | 1 | 1 | - | - | 1 | 1 | - | - | |
| Time between diagnosis and initial therapy initiation10 | 1 | 0 | 1 | 0 | - | - | - | - | |
| Time between diagnosis and referral20 | 1 | 0 | 1 | 0 | - | - | - | - |
Studied in the setting of allogeneic HCT only
Studied in the setting of autologous HCT only
Barriers to HCT or factors associated with receipt of or referred to HCT were considered present based on the following: 1) Positive associations on multivariable analyses (or univariate analyses if multivariate analyses were not performed), 2) Descriptive statistics (if no modeling was provided), and 3) Elicited via surveys
Abbreviation: HCT, hematopoietic cell transplantation
Of the seventeen studies10, 12, 18, 20–25, 27, 28, 30, 31, 35, 38–40 that evaluated age as a barrier, definitions of age varied (Table 1). Age was treated as a categorical distribution in eleven studies and a continuous variable in six studies. The analytic models often controlled for available confounding variables (e.g. comorbidities). When age was treated as a categorical variable, the definitions often differed. The cut-offs were either 60 or 65 years. Thirteen of 17 studies did not provide a rationale for the age cut-off.10, 12, 18, 20–23, 27, 28, 30, 38–40 The remaining studies primarily defined it based on the clinical likelihood that anyone above that age would receive an HCT.24, 25, 31
An analysis of race showed that sixteen10, 12, 19–23, 25–28, 30, 31, 34, 35, 40 out of twenty studies10, 12, 19–31, 34, 35, 38–40 found it was a significant barrier to HCT access. Race was a barrier in both autologous and allogeneic HCT. In a large retrospective study (N=137,409) Al-Hamadani et al. found that, despite a steady rise in the autologous HCT rate among all populations, Black and Hispanic patients had a significantly lower autologous HCT growth rate.12 Barker et al. found that for allogeneic HCT, racial barrier may be due to the decreased representation seen in the bone marrow transplant registry for southern European, Asian, African, White Hispanic, and mixed non-European patients.19 Another single-center study showed that Black patients with acute myeloid leukemia were significantly less likely to receive an HCT referral; however, overall survival did not differ for those who were referred.20 The differences in referral pattern persisted even controlling for the more complex karyotypes often found in Black patients.20 Other factors that have been suggested to explain the racial discrepancy in HCT referral and treatment include socioeconomic status, increased comorbidities, and inadequate health insurance. In 20,916 patients with multiple myeloma, Fiala et al. demonstrated that racial disparities in autologous HCT persisted despite adjusting for the aforementioned factors. Black patients were 37% less likely to utilize autologous HCT and also less likely to receive bortezomib treatment.23
The next most analyzed barrier was gender/sex and seven of the seventeen studies10, 12, 21–28, 30, 31, 35, 36, 38–40 found it to be a significant barrier to access. Of the seven studies, female gender/sex was a barrier in four studies,21, 23, 36, 39 male gender/sex was a barrier in one study,31one study in allogeneic HCT26 found that it depended on the cancer and transplant types, and one study in autologous HCT found it depended on race/ethnicity.35 Joshua et al. found that overall the results for gender/sex were much more inconsistent than for race or age with some significant findings only existing in certain subgroups of both cancer and donor type.26 Hwang et al. found gender/sex only to be a significant barrier in older adults with leukemia.39
Other assessed barriers include insurance status, with 13 of 14 studies finding this to be a barrier (e.g., lack of insurance status, non-private insurance, or non-managed care insurance) 10, 11, 12, 18, 23, 24, 27, 30, 31, 32, 33, 38, 39, 40, followed by comorbidities (n = 10/11) 10, 12, 20, 23, 24, 27, 30, 31, 37, 38, 39, socioeconomic status (n=7/8),10, 12, 18, 23–25, 27, 29 cancer type (n=5/6),18, 27, 36, 38–40 year diagnosed (n=6/6),10, 12, 23–25, 40 and disease status (n=6/6).11, 18, 20, 24, 30, 37 The aforementioned barriers occurred in both autologous and allogeneic HCT. Barriers that were specific to allogeneic HCT included: donor/human leukocyte antigen-subtype availability, psychiatric disability, marital status, poor understanding or medical non-compliance, language, availability of other treatment options, perception of risk by physicians, and experience with HCT.
Discussion
HCT can be a curative or life-extending treatment for many leukemias, lymphomas, and myelodysplastic syndromes, as well as a plethora of other malignant and non-malignant conditions. To the authors’ knowledge, this is the first large-scale systematic review of factors that may impede access to HCT in various populations. The studies included in this systematic review generally limited themselves to specific diseases (e.g., acute myeloid leukemia, multiple myeloma, myelodysplastic syndrome), specific types of HCT (e.g. autologous, matched-related allogeneic, or non-related allogeneic), and the types of barriers that patients encounter (e.g. race, age, sex, household income, insurance, education level, etc.). Only one study was prospective and considered high-quality, with the remaining being low to fair quality. Among the 28 variables that were assessed as possible barriers, most were patient-level with age, race, and insurance status being the most consistent. Studies evaluating barriers at the levels of physician, organization, and policy are severely lacking. Our results highlight inequalities in healthcare provision between groups.
We found older age to be the one of the most frequent barriers reported. The studies attributed the barriers of older age to a range of issues although evidence for underlying age-related barriers were rarely provided. When provided, studies attributed it to a lack of prospective studies in older adults,10, 21, 24 perceived higher risks vs. benefits,27, 39 current guidelines,23 higher levels of comorbidities,10, 23, 25 and a bias against HCT as a modality in older adults among physicians.24, 25, 31 Other potential explanations include a lack of information on the efficacy of treatment in the older population, patient preferences, healthcare system barriers, insurance, and the rapid changes in transplant practice making it difficult for referring physicians to remain up to date. Studies used a range of ages when analyzing age as a variable. These studies often defined older adults as the age above (60 or 65) which HCT was generally not recommended. The use of various age cut-offs suggests uncertainty surrounding the use of HCT in older adults. Nonetheless, several studies and guidelines have supported the use of HCT in older adults (>60 years), with improvement in survival rates.41–48 In four prospective AML trials of 1,155 patients aged 60 years and over, allogeneic HCT was associated with improvement in 5-year overall survival compared to non-allogeneic post-remission therapies among those with intermediate- or adverse-risk AML.42
Several strategies may improve the challenge of limited HCT use (and more broadly treatment decision-making and selection) among older adults outlined in this systematic review. First, prospective and/or randomized controlled trials investigating HCT in this population, focusing on the efficacy, tolerability, and outcomes important to older adults (e.g., functional status, cognition) should enhance generalizability. One such example is the BMT CTN Protocol 1704 CHARM study (ClinicalTrials.gov Identifier: NCT03992352) that aims to validate pre-HCT factors (patient-reported factors, clinical factors, and biomarkers) and to risk stratify for non-relapse mortality after allogeneic HCT in older adults. Second, we believe education for both referring physicians and patients on the increasing utility and promising outcomes of HCT in older adults will promote shared decision-making and referral to transplant centers. Third, geriatric assessment may help identify older patients who are fit enough for HCT.49, 50 Earlier referral and use of geriatric assessment may not only improve HCT access but can be used to identify subtle issues that may preclude HCT (e.g., lack of social support, comorbidities, functional impairment), as well as to predict morbidity and mortality.49, 51 One recent single center study demonstrated a new model of care incorporating a cancer-specific geriatric assessment and a multi-disciplinary team of providers to create individualized supportive care plans for allogeneic HCT among those ≥60 years.52 Patients had better survival, fewer inpatient deaths, shorter lengths of stay, and fewer discharges to nursing facilities than historical control subjects arguing for prospective studies.52
Like age, our systematic review found systemic barriers of race, socioeconomic status, and insurance that are not unique to HCT. For example, Fiala et al. showed that HCT and receipt of bortezomib differed by race.23 This finding confirms what is already colloquially known i.e., racial disparities are not be unique to HCT, but persist throughout the American healthcare system.23 Race as a barrier to HCT has been well-described by several population-based studies;23, 26, 34 it is country-specific and exists in multiple levels including donor availability, access to HCT, and outcomes of HCT.26, 53 The Agency for Healthcare Research and Quality (AHRQ) reported on national and state healthcare disparities after an Institute of Medicine report called for national attention to the large racial and ethnic disparities in the United States. A multifaceted, interdisciplinary approach like the one outlined by Fiscella and Sanders is needed in addressing these racial barriers that persist throughout the United States health care system. However, there are more immediate actions that can be taken within the field. Barker et al. outline a path forward with increasing donor matching utilizing cord blood, Schriber et al. propose education on early referral to transplantation centers, and Joshua et al. highlight that additional studies are urgently needed in searching for reasoning behind the barriers.19, 26, 35 In addition, advances in and increasing use of haploidentical transplantation may reduce racial barriers associated with lack of donor availability for allogeneic HCT.55
Insurance status was also a significant barrier to HCT referral or treatment. Several studies found that type of insurance mattered (private vs. non-private insurance, managed vs. non-managed care),23, 24 and whether or not patients had insurance played a role in the utilization of HCT.39 These findings suggest economic factors present significant consideration in treatment decisions, specifically the decision to refer and transplant. Barriers based on gender/sex had some of the most mixed results from the studies analyzed, with some further sub-population analysis showing a positive relationship,12, 36 whereas others showed a negative relationship.35 Large prospective studies are therefore needed to understand whether gender/sex is a barrier to HCT.
Twenty studies10–12, 19, 21–23, 25–29, 31, 32, 34–36, 38–40 included in our systematic review utilized population-based databases but only eleven10, 12, 21, 23, 25, 27, 28, 32, 36, 38, 39 included information about the database and its possible limitations. These databases are robust resources for information and allow for powerful analyses, but much of the information overlaps among studies and thus they can replicate some of the innate biases of the databases. The SEER registry, which was used by 6 studies, includes 18 cancer registries and covers about 28% of the Unites States population. When compared with the non-SEER population, SEER populations overrepresented minorities, economically disadvantaged persons, urban geography, and young people.56 Thus, studies that used the SEER registry should attempt to enhance the robustness of their studies by combining different databases (assuming other databases do not have the same characteristics). Many studies that did report limitations of their databases cited missing data for some of their variables. For example, the National Cancer Database (NCDB), used by two of the studies,10, 57 only collects data on the first course of treatment defined as “methods of treatment recorded in the treatment plan and administered to the patient before disease progression or recurrence”.58 Therefore, prospective collaborations across institutions, registries, and countries to validate and address barriers to HCT may yield more valid and actionable findings.59
Our study has some limitations. Important exclusions included non-English language, and studies conducted in non-United States countries to reduce heterogeneity. Nevertheless, comparisons to other healthcare systems may yield insights into the influence of structural factors (e.g., insurance status). Second, most papers included were perceived to be of low quality amd Third, many of the studies did not analyze barriers within various sub-populations thereby limiting our ability to compare barriers across subgroups. Forth, studies analyzing insurance as a barrier did not provide specifics of insurance coverage (e.g., immunosuppressive and antimicrobial medications post-transplantation). Lastly, seven additional articles were identified through review of references in included papers, so it is therefore possible that other relevant papers may have been omitted from our review. It may also indicate that access or barriers may not be well-defined in the literature.
In conclusion, the literature about barriers to access for HCT is growing; although, high-quality prospective studies are lacking. Inequalities of care among different populations have become evident in patients needing HCT. Our review demonstrates that even in those patients receiving specialized care, the United States healthcare system struggles with persistent inequalities. Many systemic factors that may promote inequality of care were identified. Older age and non-white race were consistently associated with constrained access to HCT. Focused interventions and research to equalize HCT access and produce a more just healthcare system are a high priority. These include more foundational work (e.g. qualitative interviews with patients, physicians, and healthcare leaders) to better understand the barriers. Ongoing efforts to reduce barriers for older patients should be encouraged and promoted in other under-served populations.
Supplementary Material
Highlights:
There are significant barriers to accessing hematopoietic cell transplantation (HCT).
High-quality studies are lacking related to HCT barriers.
Older age and non-white race were consistently linked to reduced access to HCT.
Strategies to overcome these barriers for these vulnerable populations should be prioritized.
Acknowledgement:
We would like to thank Mr. Daniel Castillo for performing the systematic search. We would like to thank Dr. Susan Rosenthal for her editorial assistance.
Funding Statement:
Dr. Loh is supported by the National Cancer Institute at the National Institute of Health (K99CA237744) and Wilmot Research Fellowship Award. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01 HL139985-01A1 and R01HL146884-01). Dr. Klepin is supported by National Institute on Aging at the National Institute of Health (R33AG059206). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentation: The study was presented as a poster at the 2018 American Society of Hematology Annual Meeting
Declarations of interest:
Dr. Loh has served as a consultant to Pfizer and Seattle Genetics. Dr. Lagu has served as a consultant for the Yale Center for Outcomes Research and Evaluation, under contract to the Centers for Medicare and Medicaid Services, for which she has provided clinical and methodological expertise and input on the development, reevaluation, and implementation of hospital outcome and efficiency measures. The views expressed in this manuscript do necessarily reflect those of the Yale Center for Outcomes Research and Evaluation or the Centers for Medicare and Medicaid Services. Dr. Klepin is a contributor to UpToDate. Dr. Wildes has served as a consultant for Seattle Genetics and Carevive Systems, and received research funding from Janssen. Dr. Stock has served as a consultant to Agios, Amgen, Kite, Jazz, Servier, Pfizer, Morphosys, and Adpative Biotechnologies. She has received speaking honoraria from Pfizer and Abbvie. Dr. Majhail has served as a consultant to Anthem, Inc., served on the advisory board for Nkarta, and received honoraria from Mallinckrodt. All other authors declare no conflict of interest.
References
- 1.D’Souza A, Lee S, Zhu X, Pasquini M. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant. 2017;23:1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CIBMTR Summary Slides - HCT Trends and Survival Data. [Cited 2019 Dec 7]. Available from: https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx#DownloadSummarySlides.
- 3.Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19:1498–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denzen EM, Majhail NS, Stickney Ferguson S, et al. Hematopoietic cell transplantation in 2020: summary of year 2 recommendations of the National Marrow Donor Program’s System Capacity Initiative. Biol Blood Marrow Transplant. 2013;19:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majhail NS, Murphy EA, Denzen EM, et al. The National Marrow Donor Program’s Symposium on Hematopoietic Cell Transplantation in 2020: a health care resource and infrastructure assessment. Biol Blood Marrow Transplant. 2012;18:172–182. [DOI] [PubMed] [Google Scholar]
- 6.Majhail NS, Mau LW, Chitphakdithai P, et al. Transplant center characteristics and survival after allogeneic hematopoietic cell transplantation in adults. Bone Marrow Transplant. 2020;55:906–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majhail NS, Mau LW, Chitphakdithai P, et al. National Survey of Hematopoietic Cell Transplantation Center Personnel, Infrastructure, and Models of Care Delivery. Biol Blood Marrow Transplant. 2015;21:1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SJ, Joffe S, Artz AS, et al. Individual physician practice variation in hematopoietic cell transplantation. J Clin Oncol. 2008;26:2162–2170. [DOI] [PubMed] [Google Scholar]
- 9.Khera N, Chang YH, Hashmi S, et al. Financial burden in recipients of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:1375–1381. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt VR, Chen B, Gyawali B, Lee SJ. Socioeconomic and health system factors associated with lower utilization of hematopoietic cell transplantation in older patients with acute myeloid leukemia. Bone Marrow Transplant. 2018;53:1288–1294. [DOI] [PubMed] [Google Scholar]
- 11.Kollman C, Weis T, Switzer GE, et al. Non-HLA barriers to unrelated donor stem cell transplantation. Bone Marrow Transplant. 2001;27:581–587. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hamadani M, Hashmi SK, Go RS. Use of autologous hematopoietic cell transplantation as initial therapy in multiple myeloma and the impact of socio-geo-demographic factors in the era of novel agents. Am J Hematol. 2014;89:825–830. [DOI] [PubMed] [Google Scholar]
- 13.Majhail NS, Omondi NA, Denzen E, Murphy EA, Rizzo JD. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2010;16:1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broekhuizen K, Pothof A, de Craen AJ, Mooijaart SP. Characteristics of randomized controlled trials designed for elderly: a systematic review. PLoS One. 2015;10:e0126709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal G, Gundabolu K, Vallabhajosyula S, Silberstein PT, Bhatt VR. Reduced-intensity conditioning allogeneic hematopoietic-cell transplantation for older patients with acute myeloid leukemia. Ther Adv Hematol. 2016;7:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- 17.Kmet L, Lee R, Cook L. Standard Quality Assessment Criteria For Evaluating Primary Research Papers from a variety of fields. 2004. [Cited on 26 Dec 2019]. Available from: http://www.ihe.ca/documents/HTA-FR13.pdf.
- 18.Adrianzen Herrera D, Kornblum N, Acuna-Villaorduna A, et al. Barriers to Allogeneic Hematopoietic Stem Cell Transplantation for Human T Cell Lymphotropic Virus 1-Associated Adult T Cell Lymphoma-Leukemia in the United States: Experience from a Large Cohort in a Major Tertiary Center. Biol Blood Marrow Transplant. 2019;25:e199–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bierenbaum J, Davidoff AJ, Ning Y, Tidwell ML, Gojo I, Baer MR. Racial differences in presentation, referral and treatment patterns and survival in adult patients with acute myeloid leukemia: a single-institution experience. Leuk Res. 2012;36:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa LJ, Huang JX, Hari PN. Disparities in utilization of autologous hematopoietic cell transplantation for treatment of multiple myeloma. Biol Blood Marrow Transplant. 2015;21:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delamater PL, Uberti JP. Geographic access to hematopoietic cell transplantation services in the United States. Bone Marrow Transplant. 2016;51:241–248. [DOI] [PubMed] [Google Scholar]
- 23.Fiala MA, Wildes TM. Racial disparities in treatment use for multiple myeloma. Cancer. 2017;123:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Getta BM, Kishtagari A, Hilden P, et al. Allogeneic Hematopoietic Stem Cell Transplantation Is Underutilized in Older Patients with Myelodysplastic Syndromes. Biol Blood Marrow Transplant. 2017;23:1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabo B, Morgan JW, Martinez ME, Ghamsary M, Wieduwilt MJ. Sociodemographic disparities in chemotherapy and hematopoietic cell transplantation utilization among adult acute lymphoblastic and acute myeloid leukemia patients. PLoS One. 2017;12:e0174760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshua TV, Rizzo JD, Zhang MJ, et al. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer. 2010;116:3469–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell JM, Conklin EA. Factors affecting receipt of expensive cancer treatments and mortality: evidence from stem cell transplantation for leukemia and lymphoma. Health Serv Res. 2015;50:197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24:344–349. [DOI] [PubMed] [Google Scholar]
- 29.Paulson K, Brazauskas R, Khera N, et al. Inferior Access to Allogeneic Transplant in Disadvantaged Populations: A Center for International Blood and Marrow Transplant Research Analysis. Biol Blood Marrow Transplant. 2019;25:2086–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pidala J, Kim J, Schell M, et al. Race/ethnicity affects the probability of finding an HLA-A, -B, -C and -DRB1 allele-matched unrelated donor and likelihood of subsequent transplant utilization. Bone Marrow Transplant. 2013;48:346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pidala J, Craig BM, Lee SJ, Majhail N, Quinn G, Anasetti C. Practice variation in physician referral for allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preussler JM, Farnia SH, Denzen EM, Majhail NS. Variation in medicaid coverage for hematopoietic cell transplantation. J Oncol Pract. 2014;10:e196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preussler JM, Mau LW, Majhail NS, et al. Patient housing barriers to hematopoietic cell transplantation: results from a mixed-methods study of transplant center social workers. Support Care Cancer. 2016;24:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulte D, Redaniel MT, Jansen L, Brenner H, Jeffreys M. Recent trends in survival of adult patients with acute leukemia: overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica. 2013;98:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schriber JR, Hari PN, Ahn KW, et al. Hispanics have the lowest stem cell transplant utilization rate for autologous hematopoietic cell transplantation for multiple myeloma in the United States: A CIBMTR report. Cancer. 2017;123:3141–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta P, Pollock BH, Nugent M, Horowitz M, Wingard JR. Access to stem cell transplantation: do women fare as well as men? Am J Hematol. 2003;72:99–102. [DOI] [PubMed] [Google Scholar]
- 37.Berman E, Little C, Gee T, O’Reilly R, Clarkson B. Reasons that patients with acute myelogenous leukemia do not undergo allogeneic bone marrow transplantation. N Engl J Med. 1992;326:156–160. [DOI] [PubMed] [Google Scholar]
- 38.Cho C, 2006. Factors affecting stem cell transplanation for leukemia and lymphoma. Thesis, Georgetown University, Washington, D.C.. [Google Scholar]
- 39.Hwang JP, Lam TP, Cohen DS, Donato ML, Geraci JM. Hematopoietic stem cell transplantation among patients with leukemia of all ages in Texas. Cancer. 2004;101:2230–2238. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell JM, Meehan KR, Kong J, Schulman KA. Access to bone marrow transplantation for leukemia and lymphoma: the role of sociodemographic factors. J Clin Oncol. 1997;15:2644–2651. [DOI] [PubMed] [Google Scholar]
- 41.Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of Allogeneic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. Biol Blood Marrow Transplant. 2016;22:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Versluis J, Hazenberg CL, Passweg JR, et al. Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: a time-dependent analysis. Lancet Haematol. 2015;2:e427–436. [DOI] [PubMed] [Google Scholar]
- 43.Muffly L, Pasquini MC, Martens M, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017;130:1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ustun C, Le-Rademacher J, Wang HL, et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60–75 years in first complete remission (CR1): an alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia. 2019;33:2599–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devine SM, Owzar K, Blum W, et al. Phase II Study of Allogeneic Transplantation for Older Patients With Acute Myeloid Leukemia in First Complete Remission Using a Reduced-Intensity Conditioning Regimen: Results From Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. J Clin Oncol. 2015;33:4167–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosko A, Wang HL, de Lima M, et al. Reduced intensity conditioned allograft yields favorable survival for older adults with B-cell acute lymphoblastic leukemia. Am J Hematol. 2017;92:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atallah E, Horowitz MM, Logan B, et al. Outcome of Patients 65 Years and Older with Myelodysplastic Syndrome (MDS) Receiving Allogeneic Hematopoietic Stem Cell Transplantation Compared to Patients 55–64 Years of Age. Blood. 2015;126:193–193. [Google Scholar]
- 49.Olin RL, Fretham C, Pasquini MC, et al. Geriatric assessment in older alloHCT recipients: association of functional and cognitive impairment with outcomes. Blood Adv. 2020;4:2810–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayani R, Rosko A, Olin R, Artz A. Use of geriatric assessment in hematopoietic cell transplant. J Geriatr Oncol. 2020;11:225–236. [DOI] [PubMed] [Google Scholar]
- 51.Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99:1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Derman BA, Kordas K, Ridgeway J, et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv. 2019;3:3488–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Majhail NS, Nayyar S, Santibañez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant. 2012;47:1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiscella K, Sanders MR. Racial and Ethnic Disparities in the Quality of Health Care. Annual review of public health. 2016;37:375–394. [DOI] [PubMed] [Google Scholar]
- 55.Ciurea SO, Bittencourt MCB, Milton DR, et al. Is a matched unrelated donor search needed for all allogeneic transplant candidates? Blood Adv. 2018;2:2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuo TM, Mobley LR. How generalizable are the SEER registries to the cancer populations of the USA? Cancer Causes Control. 2016;27:1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017;3:1722–1728. [DOI] [PubMed] [Google Scholar]
- 59.Horowitz MM. The role of registries in facilitating clinical research in BMT: Examples from the Center for International Blood and Marrow Transplant Research. Vol. 42, Bone Marrow Transplantation. Nature Publishing Group; 2008. p. S1–2. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


