Abstract
In a recent clinical trial, the metabolite L-glutamine was shown to reduce painful crises in sickle cell disease (SCD) patients. To support this observation and identify other metabolites implicated in SCD clinical heterogeneity, we profiled 129 metabolites in the plasma of 705 SCD patients. We tested correlations between metabolite levels and six SCD-related complications (painful crises, cholecystectomy, retinopathy, leg ulcer, priapism, aseptic necrosis) or estimated glomerular filtration rate (eGFR), and used Mendelian randomization (MR) to assess causality. We found a potential causal relationship between L-glutamine levels and painful crises (N=1,278, odds ratio (OR) [95% confidence interval] = 0.68 [0.52 – 0.89], P=0.0048). In two smaller SCD cohorts (N=299 and 406), the protective effect of L-glutamine was observed (OR=0.82 [0.50–1.34]), although the MR result was not significant (P=0.44). We identified 66 significant correlations between the levels of other metabolites and SCD-related complications or eGFR. We tested these correlations for causality using MR analyses and found no significant causal relationship. The baseline levels of quinolinic acid was associated with prospectively ascertained survival in SCD patients, and this effect was dependent on eGFR. Metabolomics provide a promising approach to prioritize small molecules that may serve as biomarkers or drug targets in SCD.
Keywords: Sickle cell disease, L-glutamine, Mendelian randomization, 3-ureidopropionate, Biomarkers
INTRODUCTION
Sickle cell disease (SCD) is one of the most common Mendelian diseases in the world, affecting millions of patients living in Sub-Saharan Africa and the Indian sub-continent [1]. In the United States, >100,000 individuals, mostly of African descent, live with SCD, and healthcare costs associated with SCD management and treatment are substantial [2]. Although fundamentally a disease of the blood – caused by mutations in the β-globin gene HBB – SCD is characterized by systemic and debilitating complications, such as painful crises, stroke, pulmonary hypertension and kidney failure. Unfortunately, there are no robust prognostic biomarkers to predict who will develop which complications, and when. SCD treatment still relies primarily on chronic blood transfusions and hydroxyurea (HU), a drug that acts partly by raising the concentration of anti-sickling fetal hemoglobin (HbF) [3].
Progress in gene therapies and genome editing technologies now offer realistic hope of developing a cure for SCD [4]. However, these complex clinical interventions are unlikely to benefit most SCD patients worldwide in the short term. Therefore, we need to continue searching for novel biomarkers and drug targets for SCD. Recently, the US Food and Drug Administration approved three new molecules to treat SCD (L-glutamine, crizanlizumab-tmca and voxelotor). In a double-blind phase 3 clinical trial, L-glutamine was shown to reduce the number of painful crises over a 48-week period [5]. The emergence of L-glutamine as a therapy was based on decades of work investigating the role of oxidative stress in SCD pathophysiology [6]. Red blood cells (RBC) from SCD patients have high oxidative stress and a compromised ability to counteract free radicals due to a low ratio of the reduction-oxidation (redox) co-factor nicotinamide adenine dinucleotide (NAD) and its reduced form ([NADH]:[NAD++NADH]) [7]. L-glutamine is one of the most abundant amino acids in the human body and in addition to its role in protein synthesis, is required to synthesize NAD. Treatment with L-glutamine increases the NAD redox ratio and reduces adhesion of sickle RBC to endothelial cells, a hallmark of vaso-occlusive painful crises [8, 9].
Metabolites, like L-glutamine, are small molecules that result from the activities of endogenous enzymes [10]. The development of high-throughput mass spectrometry-based methodologies makes it possible to profile 100–1000s of metabolites in human biospecimens. Such metabolomic studies have been used to identify metabolite signatures of diseases, but also to pinpoint specific metabolites that may have prognostic and/or therapeutic values [11]. Metabolite levels are variable between individuals (in disease, but also in health) and large genetic studies – termed metabolite genome-wide association studies (mGWAS) – have identified 1000s of genetic variants that control them. Besides providing an opportunity to characterize the biological pathways that control metabolite levels, these genetic discoveries become powerful instruments for Mendelian randomization (MR) studies. MR uses genetic variants to determine the effect of genetically modulated phenotypes on disease outcome [12]. MR mimics randomized clinical trial as it harnesses the random allocation of parental alleles when they are passed on to their offspring. As a consequence, the alleles are independently distributed in the population and free from potential confounders [13, 14]. For instance, it has been possible to show using MR that tobacco smoking, as opposed to other confounders such as socioeconomic status, causes lung cancer by demonstrating that a genetic variant associated with smoking heaviness and located in the nicotinic acetylcholine receptor subunit genes is also associated with lung cancer only through its effect on smoking habits [15]. Other MR studies have validated many drug targets for various human diseases (e.g. statins that lower LDL-cholesterol levels to reduce coronary artery disease (CAD) risk), but have also been useful to rule out many biomarkers as potential causal factors (e.g. HDL-cholesterol or C-reactive protein for CAD) [16–18].
In SCD, only a limited number of studies have used metabolomic approaches to tackle clinical heterogeneity. Zhang et al. discovered increased adenosine levels in blood from SCD patients and transgenic mice: they showed that higher adenosine levels exacerbated sickling, hemolysis and organ damage [19]. Additionally, the same group found that sphingosine-1-phosphate (S1P) and 2,3-bisphosphoglycerate (2,3-BPG) blood concentrations are elevated in SCD patients and mice, which results in the re-programming of the glycolysis program and enhanced disease severity [20, 21]. Finally, Dargouth et al. profiled the metabolome of RBC from healthy individuals and SCD patients and identified several metabolites that highlight differences between the two groups in glycolysis, membrane turnover, and glutathione and nitric oxide metabolism [22]. Although exciting, these pioneering metabolomic studies were performed in a limited number of SCD patients (N=14–30) and did not take advantage of MR methodology to address causality.
To prioritize metabolites that may be important biomarkers or drug targets for SCD, we profiled 129 known metabolites, including L-glutamine, in the plasma of 705 SCD patients. First, we used MR to test the causal relationship between plasma L-glutamine levels and painful crises. Second, we tested the association between all measured metabolites and SCD-related complications and combined these results with previous mGWAS findings to perform MR studies. Finally, we tested if baseline plasma metabolite levels were associated with survival in our SCD cohorts. Our results highlight the value of combining genetic and metabolomic strategies to disentangle the complex pathophysiology of SCD.
SUBJECTS AND METHODS
Study participants
Sample collections and procedures were in accordance with the institutional and national ethical standards of the responsible committees and proper informed written consent was obtained. The Genetic Modifier (GEN-MOD), the Cooperative Study of Sickle Cell Disease (CSSCD), and the Duke University Outcome Modifying Genes (OMG) cohorts have been described elsewhere [23–25]. Plasma samples were collected during steady state in outpatient visit over 3 weeks from treatment for vaso-occlusive crisis in OMG, and over 4 weeks for GEN-MOD. In particular for GEN-MOD, a dedicated research assistant validated all clinical information. Demographic and clinical information for each SCD cohort is available in Table 1. For both GEN-MOD and OMG, patients were not fasting when plasma was collected, and diet information is not available.
Table 1. Demographics and clinical information.
Sickle cell disease patients from three cohorts were included in this study. For the CSSCD, all data are prospective and we only considered patients with genome-wide genotyping data available. For GEN-MOD and OMG, all data were collected at baseline and are retrospective, except survival which is prospective. 1Painful crises in GEN-MOD and OMG are defined as crises requiring hospitalization which was dichotomized (individuals with ≥1 painful crises in the last 12 months are assigned as cases, while individuals with no painful crisis are assigned as controls). In the CSSCD, painful crises are defined as painful episodes requiring emergency room visits, and we dichotomized the data as no crisis (control) or at least one crisis (case) during the follow-up period. For all quantitative variable, we provide mean ± standard deviation. LDH, lactate dehydrogenase; RBC, red blood cell; MCH: mean corpuscular hemoglobin; MCV: mean corpuscular volume; eGFR, estimated glomerular filtration rate; NA, not available.
| Characteristic | GEN-MOD | OMG | CSSCD |
|---|---|---|---|
| Sex (male/female) | 222/184 | 163/136 | 616/662 |
| Age (year) | 31 ± 9 | 35 ± 14 | 14 ± 12 |
| β-globin genotypes (HbSS/ HbS β0-thal/ HbSS α-thal/ HbSC/ HbS β+) | 406/0/0/0/0 | 255/12/0/23/9 | 883/0/395/0/0 |
| Painful crises (cases/controls)1 | 150/180 | 161/128 | 194/907 |
| Leg ulcer (cases/controls) | 30/300 | 79/214 | 185/970 |
| Cholecystectomy (cases/controls) | 200/206 | 173/72 | 152/932 |
| Aseptic necrosis (cases/controls) | 94/236 | 93/194 | 164/991 |
| Priapism (cases/controls) | 41/116 | 55/236 | 96/460 |
| SCD retinopathy (cases/controls) | 182/67 | 65/210 | 274/292 |
| SCD survival (cases/controls) | 19/384 | 35/91 | 44/1235 |
| Bilirubin (mg/dL) | 3.5± 2.1 | 2.9 ± 2.4 | 3.3 ± 2.2 |
| eGFR (mL/min per 1.172 m2) | 143.6± 22.8 | 126.0 ± 40.3 | 165.3 ± 47.0 |
| Hemoglobin (g/dL) | 8.8 ± 1.3 | 8.2 ± 1.8 | 8.4 ± 1.3 |
| Hematocrit (%) | 25.8 ± 4.5 | 25.3 ± 5.7 | 24.8 ± 4.03 |
| Lactate dehydrogenase (units/L) | 400.4 ± 144.7 | 326.8 ± 240.2 | 451.6 ± 244.7 |
| MCH (pg) | 29.3 ± 4.1 | 32.0 ± 4.8 | 29.8 ± 3.1 |
| MCV (fL) | 87.0 ± 10.2 | 92.2 ± 12.7 | 89.2 ± 8.5 |
| RBC count (×106 cells/μL) | 3.0 ± 0.80 | 2.9 ± 0.80 | 2.8 ± 0.57 |
Metabolomics Profiling
Plasma metabolites were profiled using two complimentary liquid chromatography tandem mass spectrometry (LC-MS) methods. Amino acids, amino acid metabolites, acylcarnitines, and other cationic polar metabolites were measured using a Nexera X2 U-HPLC (Shimadzu Corp.) coupled to a Q Exactive Hybrid Quadrupole Orbitrap Mass Spectrometer (Thermo Fisher Scientific). Plasma samples (10 μl) were prepared via protein precipitation, with the addition of 9 volumes of acetonitrile/methanol/formic acid (74.9:24.9:0.2; v/v/v) containing stable isotope-labeled, quality control internal standards (valine-d8, Sigma-Aldrich; St. Louis, MO; and phenylalanine-d8, Cambridge Isotope Laboratories; Andover, MA). The samples were centrifuged (10 min, 9,000 x g, 4°C), and the supernatants were injected directly onto a 150 × 2 mm, 3 μm Atlantis HILIC column (Waters). The column was eluted isocratically at a flow rate of 250 μL/min with 5% mobile phase A (10 mM ammonium formate and 0.1% formic acid in water) for 0.5 minute followed by a linear gradient to 40% mobile phase B (acetonitrile with 0.1% formic acid) over 10 minutes. MS analyses were carried out using electrospray ionization in the positive ion mode using full scan analysis over 70–800 m/z at 70,000 resolution and 3 Hz data acquisition rate. Other MS settings were: sheath gas 40, sweep gas 2, spray voltage 3.5 kV, capillary temperature 350°C, S-lens RF 40, heater temperature 300°C, microscans 1, automatic gain control target 1e6, and maximum ion time 250 ms. Raw data were processed using TraceFinder software (Thermo Fisher Scientific; Waltham, MA) for supervised, targeted extraction of data from a subset of lipids and Progenesis QI (Nonlinear Dynamics; Newcastle upon Tyne, UK). Organic acids, sugars, purines, pyrimidines, and other anionic polar metabolites were measured using an ACQUITY UPLC (Waters Corp, Milford MA) coupled to a 5500 QTRAP triple quadrupole mass spectrometer (AB SCIEX, Framingham MA). Plasma samples (30 μL) were extracted using 120 μL of 80% methanol containing 0.05 ng/μL inosine-15N4, 0.05 ng/μL thymine-d4, and 0.1 ng/μL glycocholate-d4 as quality control internal standards (Cambridge Isotope Laboratories, Inc., Tewksbury MA). The samples were centrifuged (10 min, 9,000 x g, 4°C) and the supernatants (10 μL) were injected directly onto a 150 × 2.0 mm Luna NH2 column (Phenomenex, Torrance CA). The column was eluted at a flow rate of 400 μL/min with initial conditions of 10% mobile phase A (20 mM ammonium acetate and 20 mM ammonium hydroxide (Sigma-Aldrich) in water (VWR)) and 90% mobile phase B (10 mM ammonium hydroxide in 75:25 v/v acetonitrile/methanol (VWR)) followed by a 10 min linear gradient to 100% mobile phase A. The ion spray voltage was −4.5 kV, the source temperature was 500°C, and multiple reaction monitoring (MRM) settings for each metabolite were determined using authentic reference standards. Raw data were processed and visually reviewed using MultiQuant software (AB SCIEX, Framingham MA).
Metabolomics pre-processing
We removed metabolites with >20% missing values. We imputed missing metabolite values using the k-nearest neighbors algorithm [26] as implemented in the R package impute. We log10-transformed metabolite values, and applied batch effect correction based on metabolites’ dates of extraction, the types of ionization, and whether they were obtained from targeted or untargeted approaches. Finally, we applied batch effect correction based on the year of profiling, since sample collection occurred within a 3 years span (2015– 2017). We conducted all batch effect correction using combat [27]. Using a linear model, we then derived residuals correcting for age, sex, SCD genotypes, HU usage, and cohort affiliation. Supplementary Figure 1 summarizes the design of our metabolomic experiment. Although we captured many unknown metabolites, which we used as part of the quality-control steps, this study focuses on the 129 known metabolites that were available in both GEN-MOD and OMG. All metabolite levels that we measured in this study are in arbitrary units as we did not perform absolute quantification.
Metabolite levels association with SCD complications, eGFR, or survival in GEN-MOD and OMG
All statistical tests performed in this study, including models and covariates, are thoroughly described in Supplementary Table 1. We implemented a permutation procedure that considers the correlation between metabolite levels to test the association between metabolite levels and SCD complications (painful crises, aseptic necrosis, cholecystectomy (gall bladder removal), retinopathy, priapism, leg ulcer, survival), estimated glomerular filtration rate (eGFR, calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) equation [28]) or to predict the risk of prospectively ascertained death (survival). For eGFR, we chose the CKD-EPI rather than MDRD equation in order to properly assess high GFR values; this approach allows for the ascertainment of hyperfiltration, which is often observed in SCD patients [24, 29]. We did not measure cystatin C levels, and are not aware of any data on SCD cohorts with concomitant measures of GFR and cystatin C. We randomly permuted the phenotype of interest and computed 100,000 P-values (for each metabolite) in a linear or a logistic model. We then stored the smallest P-value out of the 100,000, and obtained the adjusted/permutated P-value (Pperm) by comparing the number of times the permutated P-values are smaller than the observed P-values:
where b is the number of times Pperm is greater or equal than Pobs, and m the number of permutations. The procedure was implemented in the R statistical package.
Genetic association study in the CSSCD
DNA genotyping and genotype imputation in the CSSCD have been described in detail elsewhere [25]. We restricted our analysis to markers with imputation quality r2 >0.3 and minor allele frequency (MAF) >1%. We removed the effect of sex and age on batch effect-corrected metabolites levels, and used inverse normal transformation to normalize the residuals. We used RvTests (v20171009)[30] to test the association between genotype dosage and the various phenotypes: we used logistic regression models for painful crises or cholecystectomy, and linear regression models for bilirubin and eGFR. All statistical models are defined in Supplementary Table 1. For eGFR, we did not correct for age and sex because the eGFR-EPI equation takes them both into account.
Mendelian Randomization
Instrument identification
Because of our reduced sample size, we selected instruments for MR analyses from large published mGWAS carried out in healthy individual of European ancestry. We identified metabolite-associated variants from the published meta-analysis of KORA and TwinsUK (N=6,056+1,768, 529 metabolites), as well as the whole-genome sequence metabolite association study in TwinsUK (N=1,960, 644 metabolites) [31, 32]. We focused on these publications because they are the two largest published mGWAS to date. We selected sub-genome-wide significant mGWAS SNPs (P<1×10−5) in order to maximize the phenotypic variance explained, and tested two MR models. The first MR model included all sub-genome-wide significant SNPs as valid instruments. For the second MR model, we removed pleiotropic SNPs from the first model if they were associated with other metabolites at a Bonferroni-corrected P<0.05 threshold when considering the number of SNPs in model 1. Pleiotropic SNPs were identified by querying Phenoscanner [33].
Instrument pruning
We employed PLINK1.9v5.2 [34] to identify independent SNP within 5-Mb window and linkage disequilibrium (LD) r2 <0.01 in the CSSCD. This provided us with a list of pseudo-independent variants.
Analysis
We used a two-sample MR approach to test the causal link between metabolites and SCD-related phenotypes. We retrieved association results (effect sizes, standard errors) between instruments and SCD-related phenotypes from the large and clinically well-characterized CSSCD. All MR analyses were performed in R version 3.5.1 with the TwoSampleMR package (v0.4.22)[35]. We used a multiplicative random-effect inverse variance-weighted (IVW) method in each MR analysis. For the analysis of plasma L-glutamine levels and painful crises, we tested 2 models and defined statistical significance using a Bonferroni-corrected threshold of α≤0.025. All other analyses were exploratory and statistical significance was set at nominal α≤0.05. Additionally, we computed the weighted median [36], which selects the median MR estimate as the causal estimate, and MR-Egger [37], which allows the intercept to vary freely and therefore estimates the amount of horizontal pleiotropy, for all the analyses. Moreover, we utilized MR-PRESSO (Pleiotropy Residual Sum and Outlier)[38] to estimate the presence of horizontal pleiotropic bias and to calculate causal estimate adjusted for outliers for all reported results. Finally, we assess the validity of our significant results by conducting additional tests for horizontal pleiotropy, including Cochran’s Q statistic, MR-Egger intercept test of deviation from the null, and I2 heterogeneity statistic [12].
Genetic risk scores (GRS)
Using PLINK1.9v5.2 [34], we calculated the genetic risk scores for L-glutamine and 3-ureidopropionate in CSSCD, GEN-MOD and OMG. Effect size estimates from the two large mGWAS referenced in the MR analysis served as weights. Detailed description of the logistic and linear models employed for CSSCD, GEN-MOD, and OMG for inverse-normal transformed GRS association with painful crisis, eGFR, L-glutamine, and 3-ureidopropionate is available in Supplementary Table 1. We performed principal component analysis in PLINK using 1000 Genomes Project populations as reference.
Data sharing statement
The CSSCD genetic dataset is available on the database of Genotypes and Phenotypes (dbGaP: https://www.ncbi.nlm.nih.gov/gap/). The GEN-MOD and OMG data are available upon requests to the authors.
RESULTS
Plasma metabolites in SCD patients
To identify metabolites that may be useful to predict or treat SCD complications, we measured plasma values of 129 known metabolites in 705 patients from the GEN-MOD and OMG cohorts (Supplementary Figure 1 and Table 1). Although our metabolomic experiment was performed at the same center, it was run in three batches so we applied stringent quality-control and batch-effect correction filters to avoid confounding (Methods and Supplementary Figure 2). The two main classes of metabolites that we measured were amino acids (33%) and lipids (30%) (Supplementary Figure 3 and Supplementary Table 2).
Mendelian randomization supports a potential causal link between L-glutamine and SCD painful crises
L-glutamine therapy in SCD was previously shown to improve the NAD redox ratio, although this effect was not detected in a recent clinical trial [5, 39]. Because we measured plasma L-glutamine levels as part of our metabolomic experiment, we were interested to test association between its plasma levels and SCD-related complications or other clinically-relevant parameters. In GEN-MOD and OMG, we found no evidence of association between plasma L-glutamine levels and SCD complications, including painful crises (Table 2). However, plasma L-glutamine levels were nominally associated with several hematological traits measured at baseline, including reduced hemoglobin concentration and RBC count (Table 2). For SCD complications, interpretation of these results is challenging because clinical events occurred before plasma L-glutamine was measured, and this one-time metabolomic measure may not reflect life-long endogenous exposure to L-glutamine. For these reasons, we sought to further test the relationship between L-glutamine and SCD painful crises using MR.
Table 2. Associations between L-glutamine plasma levels and sickle cell disease (SCD)-related complications and other clinically relevant phenotypes.
In participants from the GEN-MOD and OMG cohorts, we tested the association between L-glutamine levels measured in plasma and SCD-related complications or clinically relevant blood-based biomarkers. Dichotomous phenotypes were analyzed using logistic regression while correcting for age, sex, hydroxyurea (HU) usage, SCD genotypes and cohort affiliation. Quantitative phenotypes were corrected for age, sex, HU usage, SCD genotypes and cohort affiliation. They were inverse normal-transformed before being tested for association using linear regression. Odds ratio and effect sizes (Beta) are given per standard deviation change in plasma L-glutamine levels. LDH, lactate dehydrogenase; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; CI, confidence interval; SE, standard error.
| Complications | N | Odds ratio | 95% CI | P-value |
| Painful crises | 619 | 1.06 | (0.90–1.24) | 0.52 |
| Survival | 529 | 1.01 | (0.75–1.35) | 0.79 |
| Aseptic necrosis | 617 | 0.97 | (0.97–1.16) | 0.76 |
| Cholecystectomy | 651 | 1.06 | (0.90–1.25) | 0.45 |
| Leg ulcer | 623 | 1.09 | (0.88–1.35) | 0.44 |
| Priapism | 448 | 1.11 | (0.88–1.4) | 0.39 |
| Retinopathy | 524 | 0.99 | (0.82–1.18) | 0.88 |
| Renal Parameter | N | Beta | SE | P-value |
| eGFR | 702 | −0.067 | 0.036 | 0.067 |
| Blood Parameter | N | Beta | SE | P-value |
| Bilirubin | 585 | 0.10 | 0.041 | 0.010 |
| Hematocrit | 697 | −0.08 | 0.035 | 0.019 |
| Hemoglobin | 685 | −0.098 | 0.035 | 0.0048 |
| LDH | 579 | 0.078 | 0.039 | 0.044 |
| MCH | 626 | 0.067 | 0.035 | 0.053 |
| MCV | 697 | 0.07 | 0.032 | 0.03 |
| RBC | 698 | −0.11 | 0.033 | 7.1×10−4 |
Instrument strength plays a critical role in the validity of MR analyses. Although we measured plasma L-glutamine levels in 705 SCD patients, we wanted to take advantage of existing and well-powered mGWAS for the selection of the best metabolite-associated SNPs to use as MR instruments [31, 32]. However, these mGWAS were carried out in Europeans, whereas SCD patients in our cohorts are of African-descent (Supplementary Figure 4), raising the question whether we could use SNPs found in Europeans as MR instruments for phenotypes observed in African-ancestry SCD patients. To validate this strategy, we tested the well-known causal link between bilirubin levels in serum and gallstones leading to surgical removal of the gallbladder (cholecystectomy), a complication often observed in SCD patients [40, 41]. From a GWAS of serum bilirubin levels in 9,464 individuals of European-ancestry, we selected 10 SNPs as MR instruments [42]. In the large and well-characterized CSSCD (Table 1), we tested the association between these SNPs and bilirubin levels or cholecystectomy, and replicated the strong association between these phenotypes and the UGT1A1 locus (Supplementary Table 3). The two-sample inverse variance-weighted (IVW) MR analysis confirmed that high bilirubin levels causes gallbladder disease in SCD: a one standard deviation increase in genetically-controlled bilirubin levels was associated with a 6-fold increase in the risk of cholecystectomy in the CSSCD (odds ratio (OR) [95% confidence interval] = 6.0 [2.8–17.0], PIVW=1.9×10−6)(Supplementary Table 4).
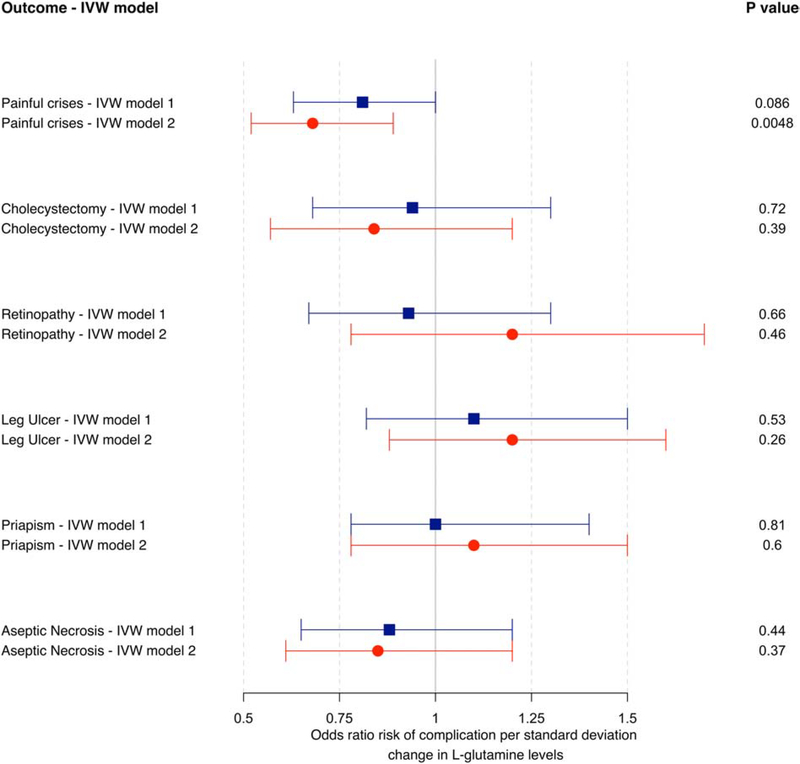
From the available mGWAS results [31, 32], we identified 51 SNPs associated with plasma L-glutamine levels at P<5×10−5 that were available in the CSSCD genetic dataset. Single variant and polygenic trait score association results are available in Supplementary Table 5 [25]. Using these 51 SNPs as instruments in a two-sample IVW MR analysis, we did not detect a causal association between L-glutamine and painful crises (Model 1: OR = 0.81 [0.63–1.00], PIVW=0.086) (Figure 1). When we excluded 24 pleiotropic SNPs (Methods) and repeated the analysis with the remaining 27 SNPs, the MR association with painful crises was significant: a one standard deviation increase in genetically-controlled L-glutamine levels was associated with a 32% reduction in the risk of painful crises in the CSSCD (Model 2: OR=0.68 [0.52–0.89], PIVW=0.0048) (Figure 1). MR analyses using the sensitivity tests MR-Egger and weighted-median did not yield significant associations for Model 2, suggesting insufficient statistical power for these tests [12] or potential residual horizontal pleiotropy (Supplementary Table 6). We repeated the MR analysis in the GEN-MOD and OMG cohorts: although the direction of the effect of the GEN-MOD+OMG meta-analysis indicated a protective effect of L-glutamine on painful crises (OR=0.82 [0.54–1.34]), the result was not significant (PIVW=0.54), presumably due to limited power given the smaller sample size (Supplementary Table 6). In secondary MR analyses, we found no evidence of causal associations between L-glutamine SNPs and several other SCD complications (Supplementary Table 6).
Figure 1. Mendelian randomization (MR) analysis of plasma L-glutamine with sickle cell disease (SCD) painful crises.
Forest plot of MR evaluating the causal relationship between plasma L-glutamine levels and painful crises in SCD patients. Effect sizes and standard errors of 51 variants associated with plasma L-glutamine were retrieved from large European mGWAS. Associations statistics between these 51 variants and SCD complications were calculated in the large prospective and well-characterized CSSCD. In model 1, we considered all 51 SNPs as instruments, whereas model 2 only included 27 variants not associated with other metabolites (Methods). The MR effect size estimates and 95% confidence intervals were calculated using the inverse variance-weighted (IVW) random effect method.
To determine if the 27 SNPs in model 2 capture the known L-glutamine biology, we annotated the nearby genes using ProGeM [43] (Supplementary Table 7). Although genes at many loci remain to be characterized, ProGeM highlighted strong candidate genes: (1) DBT (rs524219) encodes an enzyme involved in the synthesis of glutamate, a precursor to L-glutamine [44], (2) NADSYN1 (rs10431159), which encodes glutamine-dependent NAD(+) synthetase-1, has been implicated in L-glutamine synthesis through a reaction with nitrogen [45, 46], (3) SLC38A8 (rs12447776) is a sodium-coupled neutral amino acid transporter with a preference for glutamine [47, 48], (4) PPA2 (rs4699183) plays a role in the detection of glutamine levels [49], and (5) AADAT (rs138354882) is involved in the conversion of glutamine to glutamate through the production of α-ketoglutarate [50].
Potential causal link between 3-ureidopropionate and kidney function in SCD
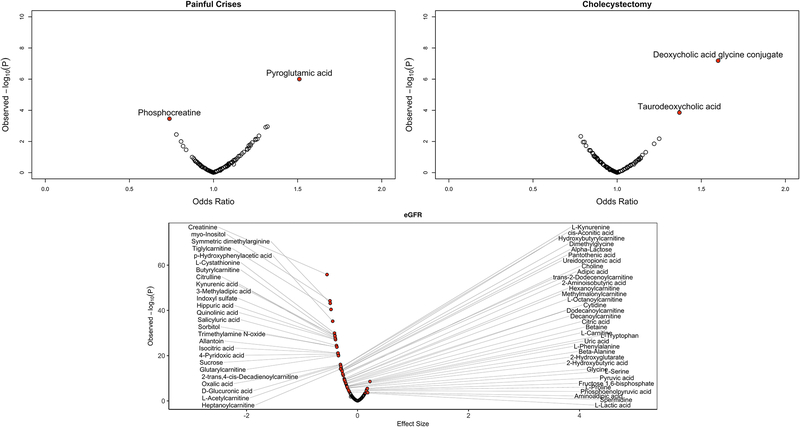
We tested 6 SCD-related complications as well as eGFR against the levels of the 129 known metabolites measured in GEN-MOD and OMG. In total, we found 66 metabolites with Pperm ≤0.05, including 62 metabolites associated with eGFR, two metabolites associated with painful crises and two metabolites associated with cholecystectomy (Figure 2 and Supplementary Table 8). There was a strong association between eGFR and creatinine levels, which serves as an internal control given that we use this metabolite to calculate eGFR. Most of these metabolites have never been linked to SCD and future work could therefore test if they represent potential novel biomarkers of disease severity. Previous metabolomic studies had found high levels of adenosine, S1P and 2,3-BPG in the blood of SCD patients [19–21]. Unfortunately, we did not measure S1P and 2,3-BPG in our experiment. We could retrieve adenosine levels in a subset of patients (N=404), but we are cautious with interpretation since plasma adenosine has a short half-life and can be generated during blood extraction. Within the limitations of our experimental design, we observed higher adenosine levels in SCD patients with painful crises and cholecystectomy, although the results were not significant (Supplementary Table 9).
Figure 2. Known metabolites associated with SCD complications and estimated glomerular filtration rate (eGFR) in GEN-MOD and OMG.
We tested 129 metabolites against clinical complications by logistic regression (linear regression for quantitative eGFR). On the x-axis, we report odd ratios (effect sizes for eGFR) in metabolite standard deviation units, whereas the y-axis presents the observed analytical P-values. Red circles highlight metabolites with Pperm <0.05 calculated using 100,000 permutations. In total, we found 2 metabolites for painful crises, 2 metabolites with cholecystectomy, and 62 metabolites for eGFR.
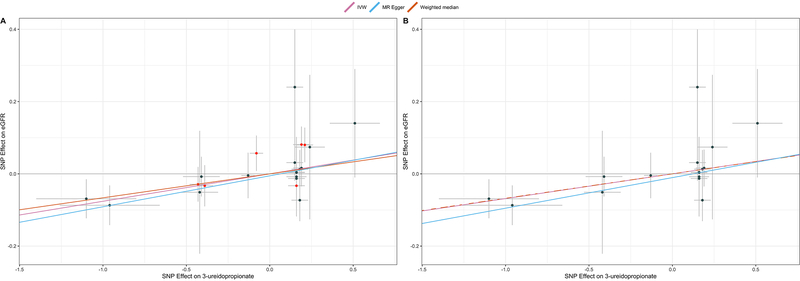
Using the same strategy as for L-glutamine, we derived MR instruments for 48 of the 66 metabolites identified in the pairwise analyses with SCD phenotypes; there were no significant mGWAS variants for the remaining 18 metabolites. Across these 48 tests, we identified a single nominally significant association in our two-sample MR analyses involving eGFR and 3-ureidoproprionate levels (see URL for all available MR results, including sensitivity tests). In a European mGWAS [32], we retrieved 22 SNPs associated with 3-ureidoproprionate levels, including 16 that were not pleiotropic (Supplementary Table 10). Our results indicate that a one standard deviation increase in genetically-controlled 3-ureidopropionate levels was associated with improved eGFR of 0.07 mL/min per 1.172 m2 (PIVW-model1=8.7×10−4; PIVW-model2=9.7×10−4) (Figure 3 and Supplementary Table 11). The sensitivity analyses did not allow us to exclude the possibility of confounding due to horizontal pleiotropy (Figure 3 and Supplementary Table 11). Furthermore, we could not replicate the MR result in GEN-MOD and OMG, indicating that larger SCD cohorts are needed to confirm the potential causal link between 3-ureidoproprionate and eGFR (Supplementary Table 11).
Figure 3. 3-ureidopropionate causally influences estimated glomerular filtration rate (eGFR) in sickle cell disease (SCD) patients.
(A) Mendelian randomization (MR) plot comparing the effects of SNPs on 3-ureidopropionate in Europeans (retrieved from large European mGWAS) (x-axis) and eGFR in SCD patients (CSSCD) (y-axis). The slope of each line corresponds to the MR effect for each method (inverse variance-weighted (IVW), MR-Egger or weighted median). Data are expressed as effect sizes with 95% confidence intervals. SNPs in red are pleiotropic. (B) Same as A, except that we removed pleiotropic variants.
Again, we used ProGeM to annotate the genes located near the 16 non-pleiotropic SNPs used in the 3-ureidoproprionate MR analyses [43] (Supplementary Table 12). This metabolite has been less studied, but we found that one of the variants, rs11704820, maps to an intron of the beta-ureidoproprionase (UPB1) gene. Otherwise, we retrieved few genes that have been implicated in kidney functions and renal disease: (1) WDR72 (rs555045773) has been associated with eGFR variation[51] and (2) LPIN1 (rs78734409 and rs71394795) is linked to myoglobinuria, which leads to renal failure due to the accumulation of creatine kinase in the kidneys [52–55].
Predicting survival status using baseline metabolite levels
Given the clinical heterogeneity that characterizes this disease, being able to predict which SCD patients will follow a severe clinical course could be extremely useful. This is particularly true for more invasive therapeutic options (e.g. gene therapy) or in settings where resources to treat SCD are limited, such as Sub-Saharan Africa. Thus, we decided to explore the prognostic value of plasma metabolites in SCD. As discussed above, the data currently available in GEN-MOD and OMG are largely retrospective. However, we could prospectively ascertain SCD severity using a simple definition based on survival status during the follow-up period (Table 1 and Methods). We identified 10 metabolites that were nominally associated with survival status, but only quinolinic acid remained significant after permutations to account for the number of tests performed (Table 3). For all 10 metabolites, increased levels were associated with increased risk of death, and for all but cytosine levels, the effect on survival was mediated by an association with eGFR. Quinolinic acid is a product of the kynurenine pathway, which also metabolizes the amino acid tryptophan.
Table 3. Nominally significant associations between survival and plasma levels of 129 metabolites in sickle cell disease (SCD).
In participants from the GEN-MOD and OMG cohorts, we tested the association between metabolite levels measured in plasma and prospectively ascertained survival (529 SCD patients: 54 deaths). We analyzed the data using logistic regression while correcting for age, sex, hydroxyurea (HU) usage, SCD genotypes and cohort affiliation. Metabolite levels were inverse normal-transformed so that odds ratios are given per standard deviation change in plasma metabolite levels. Only quinolinic acid remains significant after permutations to account for the number of tests performed. OR, odds ratio; CI, confidence interval.
| Metabolite | OR | 95% CI | P-value | Permuted P-value | Permuted P-value after adjusting for eGFR |
|---|---|---|---|---|---|
| Quinolinic acid | 1.76 | (1.29 – 2.41) | 3.8 × 10−4 | 0.03 | 0.89 |
| Cytosine | 1.60 | (1.19 – 2.15) | 1.8 × 10−3 | 0.15 | 0.16 |
| Indoxyl sulfate | 1.59 | (1.17 – 2.17) | 3.1 × 10−3 | 0.24 | 1.0 |
| 2-trans,4-cis-Decadienoylcarnitine | 1.53 | (1.14 – 2.05) | 4.2 Ù 10−3 | 0.3 | 1.0 |
| Cytidine | 1.52 | (1.14 – 2.04) | 5.0 × 10−3 | 0.34 | 0.97 |
| Asymmetric dimethylarginine | 1.53 | (1.13 – 2.07) | 5.6 × 10−3 | 0.38 | 0.78 |
| L-Cystathionine | 1.56 | (1.13 – 2.14) | 6.6 × 10−3 | 0.42 | 1.0 |
| Alpha-Lactose | 1.47 | (1.1 – 1.97) | 9.2 × 10−3 | 0.53 | 1.0 |
| D-Glucuronic acid | 1.5 | (1.11 – 2.05) | 9.3 × 10−3 | 0.54 | 0.97 |
| Ureidopropionic acid | 1.47 | (1.1 – 1.97) | 1.0 × 10−2 | 0.56 | 1.0 |
DISCUSSION
While the cause of SCD has been known for over a century, treatment options are limited and it is extremely difficult to predict which patients will have a more severe presentation of the disease. To continue to address these challenges, we performed the largest metabolomic study in SCD patients, measuring 129 known metabolites using a targeted approach in 705 participants. We also measured 1,985 unknown metabolites as part of our experiment, but they were not considered in our analyses except during the pre-processing quality-control steps. Our effort was motivated by recent successes using this metabolomic approach to find new prognostic biomarkers and potential drug targets for human diseases [56]. In fact, a recent study showed using MR the causal impact of L-glutamine on RBC, mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) in healthy Europeans [57].
Among the 62 eGFR-associated metabolites, we noted 11 acylcarnitines that were inversely correlated with eGFR. It is known that acylcarnitines accumulate in the plasma of patients with renal disease due to reduced clearance of esterified carnitine moieties, probably mediated by the renal tubular carnitine transporter OCTN2 [58]. Our eGFR analyses also identified metabolites from the tryptophan metabolism (kynurenic acid, kynurenine) and the choline derivatives (choline, trimethylamine-N-oxide) pathways which are indicative of tubulointerstitial dysfunction and have been associated with incident chronic kidney disease [59]. We found two metabolites from secondary bile acid metabolism – deoxycholic acid glycine conjugate and taurodeoxycholic acid – that were associated with cholecystectomy. This observation is consistent with the fact that bile salts contribute to gallstones and gallbladder disease. Finally, we identified phosphocreatine and pyroglutamic acid as two metabolites associated with SCD painful crises. Little is known about a role for phosphocreatine in pain biology, although its levels are increased in skeletal muscles of fibromyalgia patients [60]. Pyroglutamic acid belongs to the glutathione metabolism pathway and its levels were elevated in SCD patients with painful crises. Because high pyroglutamic acid levels are sometimes observed in individuals who chronically use acetaminophen [61], this association might therefore reflect pain management as opposed to a causal link between pyroglutamic acid and pain in SCD patients. This conclusion is supported by the fact that our MR analyses did not causally implicate pyroglutamic acid in painful crises.
By combining metabolite profiles with mGWAS results, we could use MR methods to test causality between metabolites and SCD-related complications. Using this strategy, we identified in the large CSSCD a promising causal relationship between plasma L-glutamine levels and painful crises, which provides independent evidence consistent with recent results from a phase 3 clinical trial [5]. Given that the CSSCD was initiated in the ~1980s and that L-glutamine was only recently approved, it supports the idea that new drugs targeting known pathophysiological mechanisms (e.g. increased oxidative stress) could yield effective SCD therapeutic options. Our analyses also highlighted 3-ureidoproprionate, an intermediate in the metabolism of uracil, as a potential positive modulator of eGFR. Interpretation of this result is difficult because little is known about this metabolite and the result was not replicated in additional SCD patients. Mutations in the gene UPB1, which encodes the enzyme that transforms 3-ureidopropionate into beta-alanine, cause beta-ureidopropionase deficiency, a rare monogenic disease characterized by high plasma levels of 3-ureidoproprionate [62]. Only a few patients with this disease have so far been described and they presented mostly with neurologic development issues. However, there is no report of abnormal glomerular filtration rate or other kidney defects in these patients. We propose that future MR replication in independent SCD cohorts and animal studies could be extremely useful to investigate the possible role of 3-ureidoproprionate in regulating kidney functions, and in particular whether raising 3-ureidopropionate levels could improve glomerular filtration rate in SCD patients.
Our study presents with a few limitations, especially as it relates to some of the MR assumptions and the ability to detect a causal effect between L-glutamine and SCD painful crises. First, our statistical power to detect heterogeneity due to confounding in our MR analyses and to replicate our main findings was limited because there are few large, well-characterized and genotyped SCD cohorts available. Second, some of the SNPs used as MR instruments may be pleiotropic beyond the filtering that we applied (i.e. have an effect on multiple unknown metabolites and other phenotypes) so that it is not possible to rule out an effect on SCD complications that is independent from the tested metabolites. For these two reasons, it will be important to replicate the L-glutamine-painful crises MR analyses in independent large SCD cohorts when they become available. Third, we used MR instruments derived from mGWAS performed in Europeans to test for causality in African-ancestry SCD patients. There have been many reports on the transferability (or lack thereof) of GWAS findings across ancestries [63]. We used the well-known relationship between bilirubin levels and gallbladder disease to show that our approach can work. However, it is likely that having access to large mGWAS results in African-ancestry populations would provide better instruments, and may lead to the identification of additional causal links between metabolites and SCD phenotypes by MR. Finally, we measured metabolite levels in plasma, but their levels in RBC could have provided complementary information (in particular for L-glutamine).
One characteristic of our study is that we measured metabolites in SCD cohorts that have mostly collected retrospective clinical data. One exception is information on the survival status of the participants. Using a simple linear model, we found a significant association between prospectively-ascertained survival status and baseline quinolinic acid levels. This association was mediated by eGFR, consistent with our previous observation that quinolinic acid levels correlate with rapid renal function decline in SCD patients [24]. In the future, it will be interesting and important to test whether metabolites predict other complications in prospective SCD cohorts. In conclusion, our results motivate future experiments to integrate metabolite profiles and other orthogonal omics datasets (e.g. genetics) to build better predictors of SCD-related complications and overall severity.
Supplementary Material
HIGHLIGHTS.
Using Mendelian randomization, we tested the causal relationship between high L-glutamine levels and reduced risk of painful crises in SCD patients.
By targeted metabolomics, we identified 66 metabolites that are associated with SCD complications, such as gall bladder disease or renal dysfunction.
Acknowledgments
We thank all participants for their contribution to this project. We also thank Adil Harroud and Brent Richards for advices on the Mendelian randomization analyses. G.L. is funded by the Canadian Institutes of Health Research (CIHR, PJT #156248), the Doris Duke Charitable Foundation, and the Canada Research Chair program. GEN-MOD sample and data collection were supported by NIH grant HL-68922. A.A-K. M.J.T. and establishment and analysis of the OMG cohort has been funded by NHLBI (R01HL68959, HL79915, HL70769, HL87681).
Footnotes
Disclosure of Conflicts of Interest
The authors have declared that no conflict of interest exists.
URL
All Mendelian randomization results are available at: http://www.mhi-humangenetics.org/dataset/MR_Analysis_SCD_everything.html.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Piel FB, Steinberg MH, Rees DC: Sickle Cell Disease. N Engl J Med 2017, 376(16):1561–1573. [DOI] [PubMed] [Google Scholar]
- 2.Kauf TL, Coates TD, Huazhi L, Mody-Patel N, Hartzema AG: The cost of health care for children and adults with sickle cell disease. Am J Hematol 2009, 84(6):323–327. [DOI] [PubMed] [Google Scholar]
- 3.Telen MJ: Beyond hydroxyurea: new and old drugs in the pipeline for sickle cell disease. Blood 2016, 127(7):810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orkin SH, Bauer DE: Emerging Genetic Therapy for Sickle Cell Disease. Annu Rev Med 2019, 70:257–271. [DOI] [PubMed] [Google Scholar]
- 5.Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, Gordeuk VR, Viswanathan K, Sarnaik S, Osunkwo I et al. : A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. N Engl J Med 2018, 379(3):226–235. [DOI] [PubMed] [Google Scholar]
- 6.Chirico EN, Pialoux V: Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB life 2012, 64(1):72–80. [DOI] [PubMed] [Google Scholar]
- 7.Zerez CR, Lachant NA, Lee SJ, Tanaka KR: Decreased erythrocyte nicotinamide adenine dinucleotide redox potential and abnormal pyridine nucleotide content in sickle cell disease. Blood 1988, 71(2):512–515. [PubMed] [Google Scholar]
- 8.Niihara Y, Matsui NM, Shen YM, Akiyama DA, Johnson CS, Sunga MA, Magpayo J, Embury SH, Kalra VK, Cho SH et al. : L-glutamine therapy reduces endothelial adhesion of sickle red blood cells to human umbilical vein endothelial cells. BMC Blood Disord 2005, 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris CR, Suh JH, Hagar W, Larkin S, Bland DA, Steinberg MH, Vichinsky EP, Shigenaga M, Ames B, Kuypers FA et al. : Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood 2008, 111(1):402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suhre K, Gieger C: Genetic variation in metabolic phenotypes: study designs and applications. Nat Rev Genet 2012, 13(11):759–769. [DOI] [PubMed] [Google Scholar]
- 11.Zampieri M, Sekar K, Zamboni N, Sauer U: Frontiers of high-throughput metabolomics. Curr Opin Chem Biol 2017, 36:15–23. [DOI] [PubMed] [Google Scholar]
- 12.Hemani G, Bowden J, Davey Smith G: Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet 2018, 27(R2):R195–R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith GD, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S: Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med 2007, 4(12):e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith GD, Ebrahim S: ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003, 32(1):1–22. [DOI] [PubMed] [Google Scholar]
- 15.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P et al. : A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 2008, 452(7187):633–637. [DOI] [PubMed] [Google Scholar]
- 16.Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, Kahn J, Afonso L, Williams KA, Flack JM: Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol 2012, 60(25):2631–2639. [DOI] [PubMed] [Google Scholar]
- 17.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T et al. : Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 2012, 380(9841):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collaboration CRPCHDG, Wensley F, Gao P, Burgess S, Kaptoge S, Di Angelantonio E, Shah T, Engert JC, Clarke R, Davey-Smith G et al. : Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ 2011, 342:d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Berka V, Song A, Sun K, Wang W, Zhang W, Ning C, Li C, Zhang Q, Bogdanov M et al. : Elevated sphingosine-1-phosphate promotes sickling and sickle cell disease progression. J Clin Invest 2014, 124(6):2750–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV et al. : Detrimental effects of adenosine signaling in sickle cell disease. Nat Med 2011, 17(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun K, D’Alessandro A, Ahmed MH, Zhang Y, Song A, Ko TP, Nemkov T, Reisz JA, Wu H, Adebiyi M et al. : Structural and Functional Insight of Sphingosine 1-Phosphate-Mediated Pathogenic Metabolic Reprogramming in Sickle Cell Disease. Sci Rep 2017, 7(1):15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darghouth D, Koehl B, Madalinski G, Heilier JF, Bovee P, Xu Y, Olivier MF, Bartolucci P, Benkerrou M, Pissard S et al. : Pathophysiology of sickle cell disease is mirrored by the red blood cell metabolome. Blood 2011, 117(6):e57–66. [DOI] [PubMed] [Google Scholar]
- 23.Solovieff N, Milton JN, Hartley SW, Sherva R, Sebastiani P, Dworkis DA, Klings ES, Farrer LA, Garrett ME, Ashley-Koch A et al. : Fetal hemoglobin in sickle cell anemia: genome-wide association studies suggest a regulatory region in the 5’ olfactory receptor gene cluster. Blood 2010, 115(9):1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu JZ, Garrett ME, Soldano KL, Chen ST, Clish CB, Ashley-Koch AE, Telen MJ: Clinical and metabolomic risk factors associated with rapid renal function decline in sickle cell disease. Am J Hematol 2018, 93(12):1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilboudo Y, Bartolucci P, Rivera A, Sedzro JC, Beaudoin M, Trudel M, Alper SL, Brugnara C, Galacteros F, Lettre G: Genome-wide association study of erythrocyte density in sickle cell disease patients. Blood Cells Mol Dis 2017, 65:60–65. [DOI] [PubMed] [Google Scholar]
- 26.Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB: Missing value estimation methods for DNA microarrays. Bioinformatics 2001, 17(6):520–525. [DOI] [PubMed] [Google Scholar]
- 27.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD: The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28(6):882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asnani M, Serjeant G, Royal-Thomas T, Reid M: Predictors of renal function progression in adults with homozygous sickle cell disease. Br J Haematol 2016, 173(3):461–468. [DOI] [PubMed] [Google Scholar]
- 29.Arlet JB, Ribeil JA, Chatellier G, Eladari D, De Seigneux S, Souberbielle JC, Friedlander G, de Montalembert M, Pouchot J, Prie D et al. : Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol 2012, 13:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan X, Hu Y, Li B, Abecasis GR, Liu DJ: RVTESTS: an efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics 2016, 32(9):1423–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, Arnold M, Erte I, Forgetta V, Yang TP et al. : An atlas of genetic influences on human blood metabolites. Nat Genet 2014, 46(6):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long T, Hicks M, Yu HC, Biggs WH, Kirkness EF, Menni C, Zierer J, Small KS, Mangino M, Messier H et al. : Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet 2017, 49(4):568–578. [DOI] [PubMed] [Google Scholar]
- 33.Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, Paul DS, Freitag D, Burgess S, Danesh J et al. : PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics 2016, 32(20):3207–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ: Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015, 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R et al. : The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowden J, Davey Smith G, Haycock PC, Burgess S: Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol 2016, 40(4):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR: Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol 2016, 45(6):1961–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verbanck M, Chen CY, Neale B, Do R: Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018, 50(5):693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niihara Y, Zerez CR, Akiyama DS, Tanaka KR: Oral L-glutamine therapy for sickle cell anemia: I. Subjective clinical improvement and favorable change in red cell NAD redox potential. Am J Hematol 1998, 58(2):117–121. [DOI] [PubMed] [Google Scholar]
- 40.Passon RG, Howard TA, Zimmerman SA, Schultz WH, Ware RE: Influence of bilirubin uridine diphosphate-glucuronosyltransferase 1A promoter polymorphisms on serum bilirubin levels and cholelithiasis in children with sickle cell anemia. J Pediatr Hematol Oncol 2001, 23(7):448–451. [DOI] [PubMed] [Google Scholar]
- 41.Stender S, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A: Extreme bilirubin levels as a causal risk factor for symptomatic gallstone disease. JAMA Intern Med 2013, 173(13):1222–1228. [DOI] [PubMed] [Google Scholar]
- 42.Johnson AD, Kavousi M, Smith AV, Chen MH, Dehghan A, Aspelund T, Lin JP, van Duijn CM, Harris TB, Cupples LA et al. : Genome-wide association meta-analysis for total serum bilirubin levels. Hum Mol Genet 2009, 18(14):2700–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stacey D, Fauman EB, Ziemek D, Sun BB, Harshfield EL, Wood AM, Butterworth AS, Suhre K, Paul DS: ProGeM: a framework for the prioritization of candidate causal genes at molecular quantitative trait loci. Nucleic Acids Res 2019, 47(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng W, Jia J, Guan H, Tian Q: Case report: maple syrup urine disease with a novel DBT gene mutation. BMC Pediatr 2019, 19(1):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hara N, Yamada K, Terashima M, Osago H, Shimoyama M, Tsuchiya M: Molecular identification of human glutamine- and ammonia-dependent NAD synthetases. Carbon-nitrogen hydrolase domain confers glutamine dependency. J Biol Chem 2003, 278(13):10914–10921. [DOI] [PubMed] [Google Scholar]
- 46.Wojcik M, Seidle HF, Bieganowski P, Brenner C: Glutamine-dependent NAD+ synthetase. How a two-domain, three-substrate enzyme avoids waste. J Biol Chem 2006, 281(44):33395–33402. [DOI] [PubMed] [Google Scholar]
- 47.Hagglund MGA, Hellsten SV, Bagchi S, Philippot G, Lofqvist E, Nilsson VCO, Almkvist I, Karlsson E, Sreedharan S, Tafreshiha A et al. : Transport of L-glutamine, L-alanine, L-arginine and L-histidine by the neuron-specific Slc38a8 (SNAT8) in CNS. J Mol Biol 2015, 427(6 Pt B):1495–1512. [DOI] [PubMed] [Google Scholar]
- 48.Toral MA, Velez G, Boudreault K, Schaefer KA, Xu Y, Saffra N, Bassuk AG, Tsang SH, Mahajan VB: Structural modeling of a novel SLC38A8 mutation that causes foveal hypoplasia. Mol Genet Genomic Med 2017, 5(3):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid MA, Wang WI, Rosales KR, Welliver MX, Pan M, Kong M: The B55alpha subunit of PP2A drives a p53-dependent metabolic adaptation to glutamine deprivation. Mol Cell 2013, 50(2):200–211. [DOI] [PubMed] [Google Scholar]
- 50.Sookoian S, Pirola CJ: Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol 2012, 18(29):3775–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osman WM, Jelinek HF, Tay GK, Khandoker AH, Khalaf K, Almahmeed W, Hassan MH, Alsafar HS: Clinical and genetic associations of renal function and diabetic kidney disease in the United Arab Emirates: a cross-sectional study. BMJ Open 2018, 8(12):e020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meijer IA, Sasarman F, Maftei C, Rossignol E, Vanasse M, Major P, Mitchell GA, Brunel-Guitton C: LPIN1 deficiency with severe recurrent rhabdomyolysis and persistent elevation of creatine kinase levels due to chromosome 2 maternal isodisomy. Mol Genet Metab Rep 2015, 5:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nunes D, Nogueira C, Lopes A, Chaves P, Rodrigues E, Cardoso T, Leao Teles E, Vilarinho L: LPIN1 deficiency: A novel mutation associated with different phenotypes in the same family. Mol Genet Metab Rep 2016, 9:29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schweitzer GG, Collier SL, Chen Z, McCommis KS, Pittman SK, Yoshino J, Matkovich SJ, Hsu FF, Chrast R, Eaton JM et al. : Loss of lipin 1-mediated phosphatidic acid phosphohydrolase activity in muscle leads to skeletal myopathy in mice. FASEB J 2019, 33(1):652–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stepien KM, Schmidt WM, Bittner RE, O’Toole O, McNamara B, Treacy EP: Long-term outcomes in a 25-year-old female affected with lipin-1 deficiency. JIMD Rep 2019, 46(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wishart DS: Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov 2016, 15(7):473–484. [DOI] [PubMed] [Google Scholar]
- 57.Adams CD: A Mendelian randomization study of circulating glutamine and red blood cell traits. Pediatr Blood Cancer 2020:e28333. [DOI] [PubMed] [Google Scholar]
- 58.Calvani M, Benatti P, Mancinelli A, D’Iddio S, Giordano V, Koverech A, Amato A, Brass EP: Carnitine replacement in end-stage renal disease and hemodialysis. Ann N Y Acad Sci 2004, 1033:52–66. [DOI] [PubMed] [Google Scholar]
- 59.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik A et al. : A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 2013, 24(8):1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerdle B, Forsgren MF, Bengtsson A, Leinhard OD, Soren B, Karlsson A, Brandejsky V, Lund E, Lundberg P: Decreased muscle concentrations of ATP and PCR in the quadriceps muscle of fibromyalgia patients--a 31P-MRS study. Eur J Pain 2013, 17(8):1205–1215. [DOI] [PubMed] [Google Scholar]
- 61.Alhourani HM, Kumar A, George LK, Sarwar T, Wall BM: Recurrent Pyroglutamic Acidosis Related to Therapeutic Acetaminophen. The American journal of the medical sciences 2018, 355(4):387–389. [DOI] [PubMed] [Google Scholar]
- 62.Moolenaar SH, Gohlich-Ratmann G, Engelke UF, Spraul M, Humpfer E, Dvortsak P, Voit T, Hoffmann GF, Brautigam C, van Kuilenburg AB et al. : beta-Ureidopropionase deficiency: a novel inborn error of metabolism discovered using NMR spectroscopy on urine. Magn Reson Med 2001, 46(5):1014–1017. [DOI] [PubMed] [Google Scholar]
- 63.Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, Daly MJ, Bustamante CD, Kenny EE: Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am J Hum Genet 2017, 100(4):635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.