1. Introduction
Prenatal care is an outstanding prevention strategy widely used in the United States with several studies demonstrating that it improves health outcomes in some women. However, it has not significantly reduced health disparities in minority women.1,2–6. Although nearly two-thirds of Black and Hispanic women receive prenatal care services in the first trimester of pregnancy, those who are emotionally distressed experience multiple adverse outcomes, including pre-eclampsia, preterm birth, operative deliveries, and low birth weight.7–10,1,11,12.
Forty to 50 percent of poor women are symptomatic for prenatal depression with poor minority women meeting the diagnostic criteria for major and minor depression at twice the rate of middle class women.12,13 For those Hispanic and Black depressed women, pregnancy and birth outcomes tend to be poor.14,15 Although the United States Preventive Services Task Force now recommends screening for depression in pregnant women,4 many practices do not conduct depression screening because efficacious interventions and systems are not in place to treat them.16
Pregnant Black woman also are affected by anxiety more than women in the general population, which can lead to poor maternal health and well-being outcomes and adverse consequences on health and development for their children.20–23 Findings from a meta-analysis of anxiety and birth outcomes indicated that increased state anxiety was related to preterm birth and low birthweight.24 Prevalence rates of prenatal anxiety are estimated to be between 16 and 54 percent and are related to poor health outcomes, including pre-eclampsia and excess weight gain.17,18,19, Race could compound the effect of anxiety on birth outcomes as increased trait anxiety in Black women is related to preterm birth.21 These findings are not apparent in White women or studied in Hispanic women.
Studies have found that anxiety, stress, and depressive symptoms are each associated with poor healthy lifestyle behaviors in the general population25 as well as in pregnant women.13,26 These behaviors, such as unhealthy eating and lack of physical activity, have been found to be more prevalent in depressed and stressed Blacks.27 Unhealthy eating, like smoking, is often used to cope or regulate distressing emotions.33–35 In pregnancy, excessive gestational weight gain28, smoking29 and poor nutrition30 are all related to poor maternal health and birth outcomes.31,32 Healthy lifestyle behaviors may be key to mediate the adverse effects of anxiety, stress and depression on maternal and child outcomes.
Poor birth outcomes also may be related to the direct biologic adverse effects of anxiety, stress, or depression. Biological mediators of increased anxiety, stress, and depressive symptoms trigger neuroinflammatory, neuroendocrine, and immune pathways. These pathways can ultimately lead to poor birth outcomes, such as preterm birth.36 Increased anxiety, stress and depressive symptoms also are related to higher admissions to the Neonatal Intensive Care Unit (NICU), operative deliveries and poor infant health.37, 38,39
2. Methods
The purpose of this randomized controlled trial (RCT) is to test a manualized cognitive-behavioral skills building (CBSB) intervention entitled the Creating Opportunities for Personal Empowerment during Pregnancy (COPE-P) program versus an attention control program (i.e., PregnancyPlus) on the mental health, birth and post-partum outcomes of minority pregnant women experiencing depressive, anxiety and stress symptoms. This study, funded by the National Institute of Minority Health and Health Disparities (R01- R01MD012770), is innovative because it is the first known full-scale test of a widely scalable CBSB healthy lifestyle intervention that is embedded in prenatal care with a very high-risk population of emotionally distressed minority pregnant women. The COPE-P Program is a manualized six-session intervention that was adapted from other efficacious COPE interventions,40–42 designed specifically to be a culturally responsive prenatal coping intervention to improve psychosocial and physical health, healthy lifestyle behaviors, birth outcomes and selected post-natal outcomes in a particularly vulnerable group of pregnant minority women (i.e., those experiencing emotional distress).
The specific aims of this study are to: 1) Use a RCT to evaluate the short- and more long-term efficacy of the COPE-P program to improve healthy lifestyle behaviors (i.e., nutrition and physical activity), psychosocial health, and birth and post-natal outcomes in pregnant emotionally distressed minority women as compared to an attention control group; and 2) Examine the role of cognitive beliefs and perceived difficulty in leading a healthy lifestyle in mediating the effects of the COPE-P program on healthy lifestyle behaviors and psychological symptoms in minority pregnant women. An exploratory aim in this study is to determine characteristics that may moderate healthy lifestyle behaviors, psychosocial health, and birth and post-natal outcomes (e.g., race/ethnicity, income, age, parity, language, level of education, marital status).
Hypotheses 1a (primary outcomes). Immediately after the COPE-P program, at 4–6 weeks postpartum (3 months post-intervention), and at 6 months postpartum (8 months post-intervention), COPE-P participants will report higher healthy lifestyle behaviors and less anxiety, stress and depressive symptoms than will the attention control participants.
Hypothesis 1b (secondary outcomes). COPE-P participants will demonstrate more appropriate pregnancy weight gain, better birth outcomes (mode of delivery, birth weight, gestational age), more appropriate postpartum weight loss, and better breastfeeding outcomes than attention control participants.
Hypothesis 2 (theory building exploratory). The effects of the COPE-P program on participants’ healthy lifestyle behaviors and levels of anxiety, stress and depressive symptoms will be mediated by beliefs about their ability to make healthy lifestyles choices and their perceived difficulty in leading a healthy lifestyle.
2.1. Study design overview.
The design of this study is a longitudinal randomized block RCT with repeated measures (beginning with screening prior to 18 weeks, group prenatal care in both groups from 16 ± 1 to 31 ± 1 weeks and ending at 6 months postpartum) at two study sites (New York city and Columbus, Ohio). Race/ethnicity is being blocked to ensure equal numbers of Hispanic and Black women in the COPE-P and PregnancyPlus groups. At each site, there will be a total of at least 30 groups with an average of three to seven women over the course of the study. Groups run in each 18-week time segment (the COPE-P intervention is six sessions each three weeks apart). The PregnancyPlus group also will have six group prenatal care sessions each three weeks apart. Each group within the 18-week time segment will be randomly assigned (using a sealed envelope technique) as either a COPE-P or PregnancyPlus group. These groups will be conducted sequentially to decrease the chance of cross-contamination.
Using random assignment at each site (New York and Ohio) will allow for assessment of an interaction effect as part of the data analytic strategy. Different care providers will run the COPE-P and PregnancyPlus groups, assuring that there is no carryover effect of the intervention into the control group. Three months after the intervention (approximately 4–6 weeks postpartum), all women will be contacted and will once again complete all measures at the routine postpartum visit. At eight months post-intervention (six months postpartum at the six-month well baby visit), all women will once again be contacted and complete all measures (including weights taken on available pediatric clinic adult scales–same model as prenatal scales). Outcome data from charts will be collected by team members blind to group assignment.
2.2. Study population and setting
In New York and Columbus, women will be recruited from antenatal clinics if they are: between 18 and 40 years old, in an uncomplicated singleton pregnancy of less than 18 weeks, and self-identify as either Black or Hispanic. Participants in New York will need to read and speak English or Spanish. At The Ohio State University Total Health and Wellness Clinic and the The Ohio State Wexner Medical Center OB/GYN Clinic, participants will need to speak and read English as there are not enough Spanish-speaking women to warrant conducting groups in Spanish. Women will be excluded if they have chronic medical conditions (e.g., hypertension, or diabetes), are currently receiving treatment or therapy for a psychiatric diagnosis, or have participated in this study with a prior pregnancy. Women with obstetrical complications, such as preeclampsia, gestational diabetes, or fetal abnormalities will be excluded. These exclusion criteria are designed to ensure the likelihood that women in the study are physically able to participate. In Ohio, women who do not speak and read English will be excluded. In New York, women who do not speak and read English or Spanish will be excluded.
2.3. Power analysis and sample size.
The statistical power estimate is based on our previous research, including a RCT on the efficacy of COPE that demonstrated significant increases in participants’ activity and declines in BMI that were sustained for 12 months post- intervention.43 Our recent pilot study of COPE-P with pregnant minority women also demonstrated small to moderate effect sizes (Cohen’s δ ranging from .22 to 48). Using Optimal Design,44,45 we estimated the power for multilevel models approximating our study design. A sample size of 182 Black and Hispanic women from JMC in the Bronx and 182 Black and Hispanic women from The Ohio State University sites (at 4 data collection points for each, resulting in ~1,450 observations) will have greater than 80% power to detect small to moderate effects (i.e. 25 or greater) between group differences in healthy lifestyle behaviors, psychosocial health, and outcomes testing at alpha = .05 for all proposed models. The sample of 364 will have > .80 to detect a small to medium mediated effect.46 We should have sufficient power to explore moderation processes, given Muthén and Curran’s47 findings that interactions can be detected with high power even for relatively small samples in latent curve models with medium effect sizes.
We also have included a 30% attrition rate that should be adequate based on our longitudinal studies with perinatal minority women.48,49 This allows us to account for women who will need to drop out of the study for either personal (moved) or medical (became high risk) reasons. The proposed study has a longer follow up interval24 so we will recruit up to 473 women, as necessary, to retain an analytic sample of 364. All successful strategies developed in our other studies will be used to reduce attrition. If a data collection point is missed, data will be collected antenatally at the next scheduled visit and postpartally at rescheduled health care visits.
2.4. Screening and sample selection procedure.
Women who meet the inclusion criteria will be approached in the antenatal clinic and told that they may be eligible for the study based on their chronological age and fetal gestational age. Recruiters in New York are fluent in both English and Spanish so recruitment will occur in the language preferred by the potential participant. In Ohio, recruitment will occur in English. Gestational age is based on ultrasound dating or last menstrual period when ultrasound is not available. We will ask questions about race/ethnicity using techniques developed in our prior work.50 If a woman self identifies as Black or Hispanic and provides informed consent, she will be screened for study eligibility.
If a potential participant scores higher than a 10 on the General Anxiety Disorder 7-item scale (GAD-7)51 or a 20 on the Perceived Stress Scale (PSS),52 or a 10 on the Edinburgh Postnatal Depression Scale (EPDS),53 they will be eligible to participate in the study. For the GAD-7, a score of greater than or equal to 10 indicates moderate anxiety and maximizes sensitivity and specificity.51 For the PSS, a score of 20 or greater is the high-stress cut-off. Previous work with pregnant women found that a score of 20 was the 75th percentile.57–59 Cohen, Kamarck & Mermelstein52 assert that PSS scale norms are population specific so using a score as determined by previous work with pregnant women is appropriate. A cut-off score of 10 on the EPDS indicates moderate depressive symptoms in pregnant women.54–56 Total scores of greater than 15 or a response of “hardly ever” to question 10, which relates thoughts of suicide, on the EPDS trigger consultation with the participant’s primary provider who determines if study participation would be appropriate for the participant or if treatment is warranted. Participants responding to question 10 on the EPDS with “sometimes” or “yes, quite often” require immediate psychiatric evaluation per clinic guidelines and are not eligible to participate.
If a participant does not screen into the study, she will be thanked for her time and interest and paid $10.00 for her participation in the screening process. If a woman does screen into the study, she will provide further baseline data at this point. In addition to the screening tools, baseline data will include: demographic and personal information, weight from the clinic’s scale, a 24 hour diet recall, the Healthy Lifestyle Beliefs Scale60 and the Healthy Lifestyle Behaviors Scale.61 A participant will then be assigned to the next available group based on fetal gestational age (groups having been scheduled and previously randomized as intervention or control as described above). Participants will then be given an appointment for their first group prenatal care session and a Fitbit Flex 2 accelerometer, instructions on how to immediately begin using the Fitbit (to provide baseline data that will be uploaded at the first group session) and a Fitbit number (for confidentiality) so that they can compare with other pregnant women. All participants will receive $30.00 for at the baseline visit (Time 0: T0).
3. Theoretical Framework for the Study
The development of the COPE-P Program and selection of study variables is derived from cognitive theories formulated by Ellis62 and Seligman63 as well as behavioral theories by Skinner64 and Lewinsohn.65 A negative view of oneself, the environment, and one’s future, leads to negative mental health outcomes including depression.66,67 The basic premise of cognitive-behavioral therapy (CBT) is that an individual’s emotions and behaviors are, in large part, determined by the way in which he or she cognitively thinks and appraises the world.67 Therefore, a person who has negative or irrational/distorted beliefs tends to have negative emotions (e.g. depression, anxiety) and behaves in negative ways (e.g., overeating, risky behaviors).44,45 Specifically, elevated depressive symptoms are caused by how one perceives situations and events.68 Negative emotions and behaviors are even more profound when there are skill deficits (e.g. poor emotional regulation, poor problem-solving and assertiveness skills, and cognitive distortions that lead to negative perceptions, negative thoughts, negative views of self and future, and failure to attribute positive outcomes to one’s behavior). Based on this theory, we are predicting that pregnant minority women who lack beliefs/confidence in their ability to engage in healthy lifestyle behaviors and perceive those behaviors as difficult to perform will report more stress, anxiety, and depressive symptoms and engage in fewer healthy lifestyle behaviors.
Driven by CBT, the COPE-P program was adapted from other efficacious COPE programs with vulnerable populations (e.g., minority teens, parents of pre-term infants) and is a series of 6 educational and CBSB sessions that are culturally sensitive, readable at the sixth grade reading level and focused on empowering pregnant minority women to engage in healthy lifestyle behaviors (i.e., nutrition, physical activity, positive strategies to cope with stress, problem-solving, regulation of negative mood, and goal setting). The program is designed to be easily integrated into routine prenatal care. Based on CBT, we teach the women how to cognitively restructure their thinking when negative events/interpersonal situations arise that tend to lead them into negative thoughts, and how to turn that thinking into a more positive interpretation of the situation so that they will emotionally feel better and behave in more healthy ways. Emphasis is placed on how patterns of thinking impact behavior and emotions (i.e., the thinking, feeling and behaving triangle). Goal setting to promote engagement in healthy behaviors and problem solving for challenges are part of the CBSB component (e.g., unhealthy behaviors to cope with stress and anxiety; making poor nutritional choices). The program includes content to increase women’s knowledge of how to lead a healthy lifestyle and skills building activities that assists them in putting into practice what they are learning in the group sessions. Prior versions of the program that have been tested have involved large numbers of vulnerable minorities (32% to 100% of the samples),43,69–71 including Blacks and Hispanics, and have consistently produced decreases in anxiety/depressive symptoms and increases in healthy lifestyle behaviors.
Based on CBT, it is hypothesized that the COPE-P program will first strengthen the women’s beliefs/ confidence in their ability to engage in healthy lifestyle behaviors and manage their negative emotions, and lessen their perceived difficulty of performing the behaviors (i.e. the proposed mediating variables), which in turn, will result in more healthy lifestyle behaviors as well as less anxiety, stress and depressive symptoms (our primary outcomes). Ultimately, engaging in healthy lifestyle behaviors (nutrition and exercise) should lead to more appropriate weight and result in better birth and post-natal outcomes.
This meditational model is illustrated in Figure 1. Mediational analyses of data are important to better understand the mechanisms through which treatments are effective.65 However, prior studies have not tended to propose or test mediators of the effects of healthy lifestyle interventions. Thus, there remain large gaps in this body of literature about how interventions impact outcomes. Valid and reliable instruments will be used to measure each of the constructs in Figure 1. Based on our literature review, there are likely to be key influences outside of cognition that may impact a pregnant woman’s healthy lifestyle behaviors and mood. Therefore, we have added some key variables to our conceptual model as potential moderators of the effects of the COPE-P intervention. For example, we may find that our COPE-P program works better for certain sub-groups of women (e.g., Hispanic versus Black women; marital status; income, level of education, or primary language spoken). Analysis of both moderating and mediating variables will allow us to extend the science of healthy lifestyle interventions with pregnant women.
Figure 1.
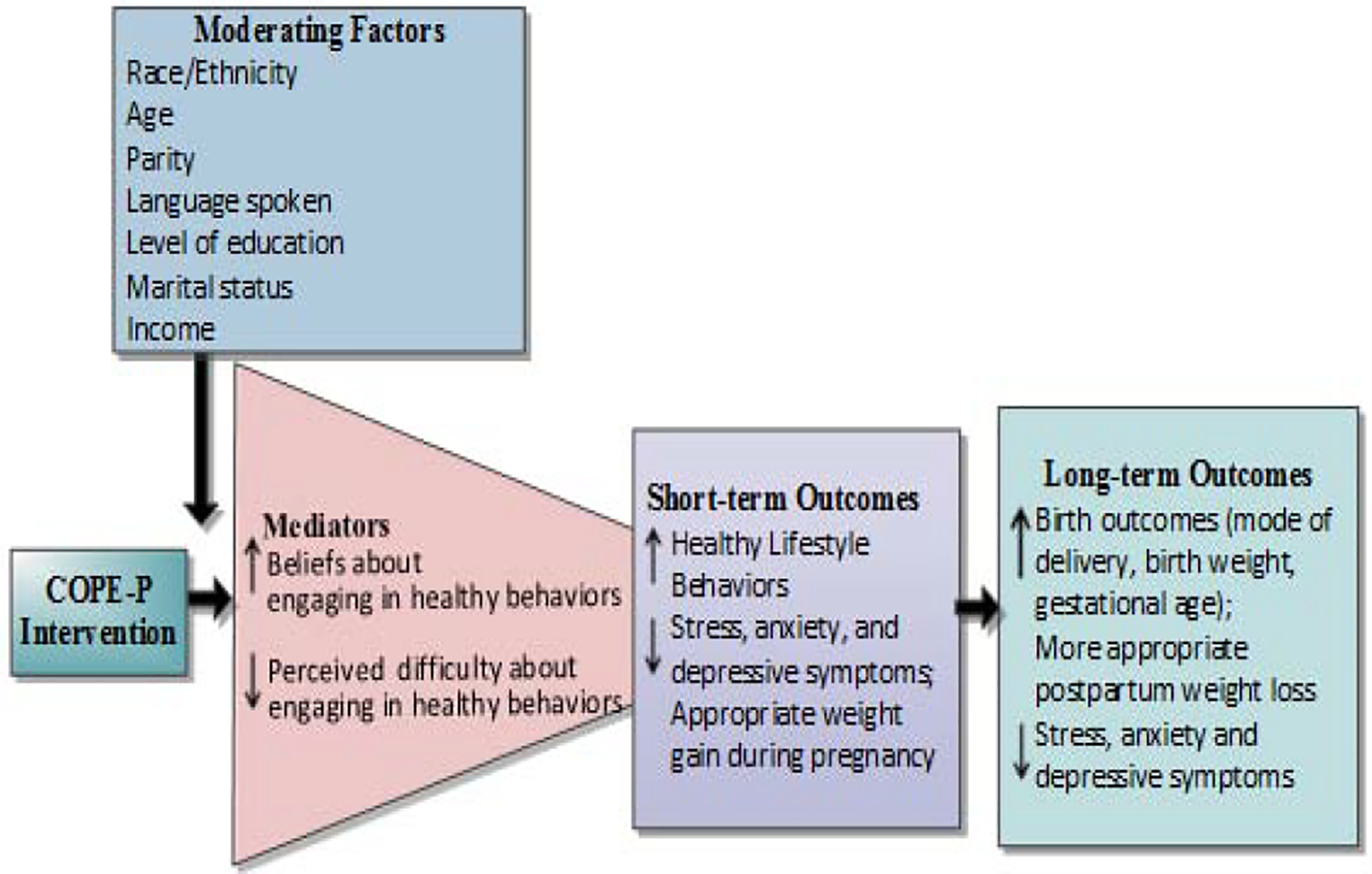
Hypothesized Effects of the COPE-P Intervention
4. Interventions
4.1. Experimental Intervention: COPE-P.
The intervention group is receiving the COPE-P Intervention over the 6 sessions of group prenatal care. The content of the COPE program is driven by the literature review, the theoretical framework, previous studies of COPE interventions with mothers of preterm infants and prior work with pregnant minority women by our team. Advanced practice nurses will be trained in the COPE-P intervention by a primary investigator who has advanced knowledge in the delivery of the program. At each group prenatal care session, appropriate weight gain, nutrition, and exercise will be discussed as they relate to the particular topic of the week.
Session 1 of COPE-P focuses on the thinking, feeling and behaving triangle, and the ABCs (A=Antecedent or Activator event, B=Belief that follows the event, C=Consequence: how you feel and how you behave). The thinking, feeling and behaving triangle and the ABCs are reinforced throughout the program. In Session 1, the women provide examples of a negative belief that is triggered by an antecedent event can be turned into a positive belief, resulting in feeling better and engaging in healthy behaviors. Mindfulness techniques also are presented. The stop light nutrition meal plan and “my pregnancy plate” are described and appropriate weight and physical activity introduced. The skills building activities for Session 1 and for all other sessions take approximately 20–30 minutes and reinforce content from each session. Goal setting and self-monitoring logs are introduced in Session 1 and included in all subsequent sessions. The women will see uploaded Fitbit information and are encouraged to increase their steps by 10% each week. Early uptake of changes in eating and other healthy behaviors will be evaluated by review of skills building activities, logs, and peer discussion.
Session 2 of COPE-P focuses on ways to build self-esteem, positive self-talk and how to change unhealthy habits into healthy ones. Signs of poor and healthy self-esteem are discussed. Discussion of healthy eating and active living is integrated throughout Session 2. Nutrition content includes information on reading labels. Skills building activities for Session 2 include changing an unhealthy habit into a positive one.
Session 3 of COPE-P focuses on stress and coping during pregnancy. Physical and emotional responses to stress are discussed along with healthy snacking. Examples of healthy ways to cope with typical stresses are reviewed. Signs of depression and anxiety are discussed and the group problem solves positive strategies to deal with depression, anxiety, and stress. This skills building session focuses on recognizing unhealthy ways of coping and examples of how to unhealthy coping into healthy coping strategies.
Session 4 of COPE-P focuses on planning, goal setting and the 4-step problem solving process, including overcoming barriers related to eating. The skills building activity for Session 4 focuses on developing strategies for overcoming barriers and problem solving a current challenge.
Session 5 of COPE-P focuses on dealing with emotions in healthy ways through positive thinking and effective communication. Women practice regulating their emotions along with mental and guided imagery. Positive self-control strategies for emotions and unhealthy eating are explored. The skills building activities in this session focus on practicing mental imagery and monitoring for emotional triggers with a response plan.
Session 6 of COPE-P focuses on coping with stressful situations commonly encountered during pregnancy while continuing to reinforce the thinking, feeling, and behaving triangle. Influences on healthy eating are explored. In skills building activities, women practice positively dealing with challenging situations.
4.2. The Attention Control Intervention.
At each group session, the PregnancyPlus attention control group will receive the usual prenatal care. Additionally, for each of the six group sessions, they will discuss a pamphlet designed for patient education by American College of Obstetricians and Gynecologists (ACOG). These six pamphlets are: 1) Routine tests during pregnancy; 2) Nutrition during pregnancy; 3) Exercise during pregnancy; 4) Obesity and pregnancy;; 5) Breastfeeding your baby; 6) Exercise after pregnancy. Group sessions are designed to last the same amount of time (1½ hours) as the COPE-P group.
5. Measures
5.1. General Anxiety Scale (GAD-7).
The GAD-7 is a 7-item, 4-point Likert-type scale ranging from (0) Not at all to (3) Everyday, that assesses the participants’ level of anxiety over the last 2 weeks. Scores range from 0 to 21, with higher scores indicating greater functional impairment related to the patient’s experience of anxiety. The scale’s reliability and validity have been tested across clinical care settings. It has been found useful in monitoring symptom severity and changes across time. In our COPE-P pilot work, the Cronbach’s alpha was .89.
5.2. Perceived Stress Scale (PSS).
The PSS is a widely used, standardized measure of global stress designed to elicit the degree to which respondents find their lives unpredictable, uncontrollable, and overloading. Concurrent and predictive validity were supported by comparing the PSS with depression scores and life-event scales in college samples. The PSS has demonstrated internal consistency reliability of .84 to .86 with young adult populations52 and .80 with pregnant minority women57 and takes about 5 minutes to complete. Cronbach’s alpha in the COPE-P pilot was .80.
5.3. The Edinburgh Postnatal Depression Scale (EPDS).
The EPDS is a 10 item self-report perinatal depression questionnaire.72 The scale asks participants to describe how they have felt in the previous week. Unlike other depression screening tools, the EPDS excludes questions regarding somatic symptoms of pregnancy and has been found to be equivalent to a structured interview in determining prevalence of depression.73,74 Scores range from 0–30 with higher scores signifying higher severity of depressive symptoms.75 ACOG supports the EPDS as a depression screening tool in pregnant women because of its brevity, readability and scoring ease.76 As will be indicated in participant informed consent forms, if a woman answers item 10 (self-harm) with a 1,2, or 3, she will be accompanied to a mental health provider for immediate care. Women with high scores will be referred to their primary care provider and we will monitor to assure follow up.
5.4. The 24 Hour Adult Nutrition Log.
Nutrition will be examined by conducting a 24-hour diet recall for each participant at each data point. Food models and pictures will be used to accurately identify portion sizes and participants will be asked brand names of food. The target day for the diet recall will be 24 hours in advance of the data visit. Information from the diet histories will be analyzed using the Food Processor database that contains over 2400 foods.77 Nutrient values are in accord with information provided by the United States Department of Agriculture and over 550 other research sources. Fast foods are included as are over 1182 convenience food items. The database is updated annually.
5.5. Healthy Lifestyle Beliefs Scale (HLBS)
The HLBS is a 16-item scale adapted from other valid and reliable belief scales.60 This scale taps beliefs about various facets of maintaining a healthy lifestyle (e.g., “I believe that I can be more active” and “I am sure that I will do what is best to lead a healthy life”). Subjects respond to each item on a five point Likert-type scale that ranging from 1 strongly disagree to 5 strongly agree. Construct validity of the scale has been supported through factor analysis with over 1,000 young adults. Cronbach’s alpha from the COPE-P pilot was .91.
5.6. Healthy Lifestyles Behaviors Scale (HLBES)
Healthy lifestyle behaviors will be measured with the HLBES used in our prior studies.61 Subjects respond to each of the 15 items (e.g., I exercised regularly; I talked about my worries) on a 5-point Likert-type scale that ranges from 1 strongly disagree to 5 strongly agree. Construct validity has been supported through factor analysis from data obtained in our prior work.61 Cronbach’s alpha in the COPE-P pilot was .86 and took 5 minutes to complete.
5.7. Physical Activity.
During pregnancy, spring loaded pedometers have been found to be inaccurate78 due, to the pedometer being tilted away from the vertical plane such that the spring suspended lever arm does not register all steps. This is particularly true for overweight or obese pregnant women so an accelerometer measure of step counts is much more accurate.78 However, accelerometer type measures of physical activity may over count steps especially when participants are involved in motor vehicle traffic and most especially on bumpy roads.79 Devices, such as the Fitbit Flex 2, combine accelerometer and pedometer features to improve accuracy80 and have the advantage of being worn on the wrist like a bracelet so women are less likely to forget to use it, a common problem with devices that necessitate being affixed to clothing.81
5.8. Birth and Post-Natal Outcome Data.
These data will be abstracted from clinic charts by team members blinded to treatment group and from the Demographic and Personal Information Form (DPI) completed by the women.
5.9. Assessment of Intervention Fidelity and Intervention Dose.
Monitoring fidelity of the intervention program and dose response is essential to having greater confidence in the findings, being able to explain the results obtained, and in helping to ensure internal validity of the study. In order to assess and ensure fidelity, we will: 1) implement a manualized protocol; 2) determine the dosage (number of sessions attended and number of skills building activities completed) and duration of the intervention in order to determine whether the intervention has a greater impact on those participants who complete more of the intervention and skills building assignments; 3) account for variations in intervention dosage statistically; 4) measure participant’s adherence to the intervention; 5) train the interveners to deliver the intervention consistently as intended; 6) monitor the intervention with an observation fidelity check list used in our prior studies; 7) utilize checklists to ensure that all intervention components are included in each session; and 8) evaluate participant receipt of the intervention by building into the protocol quantitative evaluative measures, such as nutrition and activity knowledge. Trained research staff on the use of the previously developed fidelity tool (Melnyk, 2003) will be used to complete fidelity observations on randomly assigned intervention and control groups for fidelity adherence. The observer will not be a part of the group and their presence will be explained prior to the start of the session. Meticulous record keeping will be maintained in order to evaluate the content of each session and completion of skills building activities. We also will have the midwives delivering the intervention record the tasks accomplished in each intervention session, the methods used to complete the tasks, as well as time spent on each task and impressions of the flow, content and acceptance of the sessions in an intervention diary.
6. Data Management and Analysis
6.1. Data Management.
Survey data will be obtained using paper and pencil surveys. At each site, all surveys will be scanned using Remark software to ensure accuracy and avoid the necessity of double data entry. Fitbit data will be uploaded using Bluetooth technology at each data point (with T0 baseline data being collected between screening and the first group prenatal care session) and entered into an Excel database. Data from the 24-hour diet recalls will be manually entered at each site into Food Processor and then exported into an Excel file for data analyses. At each visit, weights will be recorded. Obstetrical chart data will be extracted at each site. Dr. Szalacha will merge the Ohio and New York data files, perform data cleaning, construct analytic variables, assess psychometrics and conduct both interim and final analyses.
6.2. Data Analysis.
Statistical analyses of these data must not only demonstrate the efficacy of the COPE-P intervention, but also map the trajectories of group differences over time, and identify subgroup differences. Analysis will begin with characterization of the sample with descriptive statistics that identify differences between the COPE-P and PregnancyPlus groups 1) evident at baseline, despite randomization and 2) between groups due to differential attrition. Data will be screened for normality, outliers, and homogeneity. Appropriate nonparametric analyses will be considered in situations of irresolvable heterogeneity. We will evaluate the psychometric qualities (reliability, convergent and discriminant validity) of the scales, employing both exploratory factor analyses and SEM measurement models. Descriptive statistics will be used to summarize the sample characteristics and distribution of each variable. Missing Data. All data analyses will be completed as intention to treat analyses (i.e., individuals analyzed by group according to original random assignment, without regard to adherence to the intervention). Missing data will be imputed, when appropriate, using multiple imputation in SAS v9.4 PROC MI.
Hypothesis-driven analyses.
Various bivariate tests (e.g., independent and dependent t-tests, correlations, ANCOVA and repeated measures analyses of covariance) will be used to evaluate differences and longitudinal patterns of change across time and between relevant subgroups (e.g., intervention groups, language groups) for each significant outcome in the preliminary bivariate analyses, (primary outcomes are healthy lifestyle behaviors, anxiety, stress, and depressive symptoms). For secondary outcomes (appropriate weight gain during pregnancy, birth and post-natal outcomes (e.g., mode of delivery, birth weight/length, gestational age, postpartum weight loss and breastfeeding practices) we will fit a series of longitudinal multilevel growth models to the data.
For all continuous outcomes, such as anxiety, the basic model, to which we can add our controls and predictors, can be written as: The level 1 submodel estimates how each participant’s anxiety changes over the four data collection periods. The level 2 submodel will relate the inter-individual differences to intervention group and other time-invariant predictors and controls, such as medication (i.e., antidepressants) and have begun therapy (i.e., in usual care for mental health diagnoses), and will estimate a participant’s initial anxiety level (π0i) and rate of change in anxiety (π1i). Anxiety will be modeled in terms of random subject effects (intercept and time trends) to account for individual differences in how participants change over time. We will begin with a linear model for time but will investigate nonlinear effects as suggested by the data. Subsequent models will contain time-varying covariates (i.e., dose of intervention, weight, exercise) and thus will focus on within-participants effects, that is, whether within-participant differences in the covariates are associated with within-participant differences in anxiety. We will examine the potential of cognitive beliefs and perceived difficulty in leading a healthy lifestyle in mediating the effects of the COPE-P program on healthy lifestyle behaviors and psychological symptoms. Rather than detecting mediated effects with the causal steps approach of Baron and Kenny,82,83 we will use the Joint Significance Test established by MacKinnon, which assesses the statistical significance of the X to M relation, α path, and then the M to Y relation, β path. If both are statistically significant, there is evidence of mediation.84 Models with more than one mediator are simply extensions of the single-mediator case.85 In our multilevel models, we will investigate mediation effects within the different levels of analysis and across levels.86 Finally, we will explore characteristics that may moderate healthy lifestyle behaviors, psychosocial health, and birth outcomes (e.g., race/ethnicity, age, parity, language spoken, level of education, income, marital status) in the above models. The proposed examination of mediators and moderators should allow for increased understanding of mechanisms underlying any intervention effect and different responses among the participants. The most appropriate covariance structure for the residuals εij will be determined after data collection and the correlation of responses over time is estimated. Several covariance structures will be examined and the resulting models compared for fit using the quasi-likelihood independence model information criterion (QIC) fit using SAS 9.4 (Proc MIXED).
Should any of the outcomes need to be dichotomized (i.e., present vs. absent), we will fit a multi-level random effects logistic regression models with time-varying covariates. The basic model, to which we will add our controls and predictors, can be written: where i indexes participants, t indexes time, COPE-P is an indicator of the intervention group, COPE-P x Time is the intervention by time interaction, Xk is a set of time-varying covariates measured for each participant I at time t and ui is participant-specific error term. The interaction term COPE-P x Time is the test of the effectiveness of the COPE-P program. If successful, the regression coefficient will be negative, indicating that the modeled probability of psychological symptoms declined more rapidly with time in the COPE-P group than they did in the PregnancyPlus group. We will fit these models using SAS 9.4 (Proc NLMIXED).
7. Discussion
8a. Strengths
Strengths of this study include that it is a randomized controlled trial that targets a very high-risk population of pregnant minority women with longitudinal follow-up to determine both short and more long-term effects of the intervention. Although the United States Preventive Services Task Force recommends routine screening of pregnant women,87 it is typically not conducted as practices do not have systems in place to manage those women who screen positive for depression. If found to be efficacious with this high-risk group of pregnant women, COPE-P could be a key solution to managing those with emotional distress and improving their outcomes. Because the intervention is manualized, it could be easily scaled across the United States as other versions of COPE, which are currently being used across the nation and globe.88
8b. Potential Difficulties and Solutions
The intervention for this study is provided within a group prenatal care context to ensure feasibility, ease of administration and to decrease participant burden. Group prenatal care, to be successful, requires flexibility on the part of the providers to accommodate participation.89 While retention is always a concern in longitudinal studies, we are employing strategies that we have used in the past to maintain contact with minority women. We have planned for over enrollment to ensure our needed sample size. Further, our choice of multilevel models allows for variably spaced measurement occasions (allowing for differing data collection schedules for participants) and for varying numbers of waves of data per participant; thus, missing data (unbalanced designs) are more easily managed. Women at both sites have historically attended postpartum and well-baby care, which will also help in minimizing attrition.
Obtaining actual data (not self-reported) about health behaviors is a challenge. We are relying on self-report for nutrition data but maximize accuracy using food models and FoodProcessor. Although weekend eating is often different than weekday eating, our overall interest in healthy food choices will be minimally impacted by the choice of day. The numerous data points help to ensure that we are obtaining an accurate picture of healthy eating both during pregnancy and in the postpartum. We will be obtaining weight at each visit and although differences in clothing or time of day may allow for some error, there are enough time points that trends in weight gain and loss will be accurately recorded. Finally, using an accelerometer that can be worn on the wrist enhances use of these devices and accuracy. Pregnant women cannot clip pedometers to their waist and wearing pedometers on the trunk leads to inaccuracy in counting steps as pregnancy progresses and trunk axes change. Wearing a pedometer on the foot is difficult as feet swell and reaching the feet is difficult as pregnancy progresses. A wrist accelerometer is ideal in ensuring accuracy and wearability.
Having more than one site also can create challenges in ensuring that the intervention is being uniformly provided, however, this intervention is completely manualized to enhance fidelity and facilitate wide-scale scalability if found to be efficacious and fidelity will be addressed continually throughout the study. Moreover, our multisite design contributes to greater generalizability.
Table 1.
Study Variables, Measures and Timing
| Variables | Measures | Timing |
|---|---|---|
|
Demographic Characteristics Age, Height Education Level, Relationship Status, Race/Ethnicity, Language Spoken, Income, Parity, Medications, Mental Health Referral/Rx post screening |
Demographic and Personal Information (DPI) Form |
T0 =Baseline (screening visit) T1 = 31 weeks T2= 6–8 weeks postpartum visit T3=6 months well baby visit |
| Healthy Lifestyle Behaviors | Healthy Lifestyle Behaviors | T0, T1, T2, T3 |
| Healthy Lifestyle Beliefs | Healthy Lifestyle Beliefs Scale60 | T0, T1, T2, T3 |
| Nutrition | 24 Hour Nutrition Log/Food Processor Database | T0, T1, T2, T3 |
| Weight Gain/Loss, BMI | Scale | T0, T1, T2, T3 |
| Exercise | Steps Fitbit Flex 2 Accelerometer | T0 (given at screening T1, T2, T3) |
| Psychosocial Health | ||
| Anxiety | GAD751 | T0, T1, T2, T3 |
| Depressive Symptoms | EPDS53 | T0, T1, T2, T3 |
| Perceived Stress | PSS52 | T0, T1, T2, T3 |
| Birth and Post-Natal Outcomes | ||
| Gestational Age at birth, Gender | Chart review | Post delivery |
| Birth Weight, Birth Length, Overall Health | Chart review | Post delivery |
| Mode of Delivery (C/S, Vaginal, | Chart review | Post delivery |
| Breastfeeding (initiation and duration) | DPI Form | T2, T3 |
| COPE-P Acceptability | Index (COPE-P group) | T1 and T2 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest and Source of Funding:
Bernadette Melnyk has a company, COPE2Thrive, which disseminates other versions of the COPE program. For the remaining authors, there are no disclosures to declare.
This paper was supported by National Institute of Minority Health and Health Disparities of the National Institutes of Health under award number RO1MD012770 to Drs. Gennaro and Melnyk. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Bernadette Mazurek Melnyk, Vice President for Health Promotion, University Chief Wellness Officer, Dean and Professor, College of Nursing, Professor of Pediatrics & Psychiatry, College of Medicine, Executive Director, the Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, The Ohio State University, 1585 Neil Ave, Columbus, Ohio 43210.
Susan Gennaro, Dean and Professor, William F. Connell School of Nursing, Boston College, Chestnut Hill, MA.
Laura A. Szalacha., Professor Research Methodology and Biostatistics Core, USF Health Morsani College of Medicine and College of Nursing, University of South Florida.
Jacqueline Hoying, Assistant Professor of Clinical Practice Director, MINDSTRONG© Director, Consumer Core, the Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, The Ohio State University College of Nursing, Columbus, Ohio.
Caitlin O’Connor, Research Associate, William F. Connell School of Nursing, Boston College, Chestnut Hill, MA.
Andrea Cooper, Research Nurse Coordinator, The Ohio State University College of Nursing, Columbus, Ohio.
Anne Gibeau, Director of Midwifery, Jacobi Medical Center, Bronx, NY.
REFERENCES
- 1.Alexander G, Kotelchuck M. (2001). Assessing the role and effectiveness of prenatal care: History, challenges and directions for future research. Public Health Rep. 2001;116(4):306–316. doi: 10.1016/S0033-3549(04)50052-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya S. How effective is antenatal care to promote maternal and neonatal health? Int J Gynaecol Obstet.1995;50 Suppl 2:S35–S42. doi: 10.1016/0020-7292(95)02483-S. [DOI] [PubMed] [Google Scholar]
- 3.Fiscella K Does prenatal care improve birth outcomes? A critical review. Obstet Gynecol.1995;85(3):468–79. DOI: 10.1016/0029-7844(94)00408-6 [DOI] [PubMed] [Google Scholar]
- 4.Krans EE, Davis MM. Preventing low birthweight: 25 years, prenatal risk, and the failure to reinvent prenatal care. Am J Obstet Gynecol. 2012;206(5):398–403. doi: 10.1016/j.ajog.2011.06.082. [DOI] [PubMed] [Google Scholar]
- 5.Van Dijk JW, Anderko L, Stetzer F. The impact of prenatal care coordination on birth outcomes. J Obstet Gynecol Neonatal Nurs. 2011;40(1):98–108. doi: 10.1111/j.1552-6909.2010.01206.x. [DOI] [PubMed] [Google Scholar]
- 6.Conway KS, Kutinova A. Maternal health: does prenatal care make a difference? Health Econ. 2006;15(5):461–88. DOI: 10.1002/hec.1097 [DOI] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics, final natality data.2014. Retrieved March 2, 2020 from www.marchofdimes.com/peristats.
- 8.Healy AJ, Malone FD, Sullivan LM, et al. Early access to prenatal care: implications for racial disparity in perinatal mortality. Obstet Gynecol. 2006;107(3):625–31. DOI: 10.1097/01.AOG.0000201978.83607.96 [DOI] [PubMed] [Google Scholar]
- 9.Lu MC, Kotelchuck M, Hogan V, Jones L, Wright K, Halfon N. Closing the black-white gap in birth outcomes: A life-course approach. Ethn Dis. 2010;20(1 Suppl 2):S2–62–76. [PMC free article] [PubMed] [Google Scholar]
- 10.Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research and practice. Curr Opin Psychiatry. 2012;25(2):141–8. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominguez TP, Schetter CD, Mancuso R, Rini CM, Hobel C. Stress in African American pregnancies: testing the roles of various stress concepts in prediction of birth outcomes. Ann Behav Med. 2005;29(1):12–21. doi: 10.1207/s15324796abm2901_3 [DOI] [PubMed] [Google Scholar]
- 12.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mckee MD, Cunningham M, Jankowski KR, Zayas L. Health-related functional status in pregnancy: relationship to depression and social support in a multi-ethnic population. Obstet Gynecol. 2001;97(6):988–993. doi: 10.1016/s0029-7844(01)01377-1 [DOI] [PubMed] [Google Scholar]
- 14.Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797–802. doi: 10.1093/aje/kwf131 [DOI] [PubMed] [Google Scholar]
- 15.Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95(4):487–490. doi: 10.1016/s0029-7844(99)00602-x [DOI] [PubMed] [Google Scholar]
- 16.Thombs BD, Arthurs E, Coronado-Montoya S, et al. Depression screening and patient outcomes in pregnancy or postpartum: a systematic review. J Psychosom Res. 2014;76(6):433–446. doi: 10.1016/j.jpsychores.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 17.Class QA, Lichtenstein P, Långström N, D’Onofrio BM. Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: a population study of 2.6 million pregnancies. Psychosom Med. 2011;73(3):234–241. doi: 10.1097/PSY.0b013e31820a62ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priest SR, Austin MP, Barnett BB, Buist A. A psychosocial risk assessment model (PRAM) for use with pregnant and postpartum women in primary care settings. Arch Womens Ment Health. 2008;11(5–6):307–317. doi: 10.1007/s00737-008-0028-3 [DOI] [PubMed] [Google Scholar]
- 19.Lee AM, Lam SK, Sze Mun Lau SM, Chong CS, Chui HW, Fong DY. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet Gynecol. 2007;110(5):1102–1112. doi: 10.1097/01.AOG.0000287065.59491.70 [DOI] [PubMed] [Google Scholar]
- 20.Zelkowitz P, Papageorgiou A. Easing maternal anxiety: an update. Womens Health (Lond). 2012;8(2):205–213. doi: 10.2217/whe.11.96 [DOI] [PubMed] [Google Scholar]
- 21.Catov JM, Abatemarco DJ, Markovic N, Roberts JM. Anxiety and optimism associated with gestational age at birth and fetal growth. Matern Child Health J. 2010;14(5):758–764. doi: 10.1007/s10995-009-0513-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker ED, Jaffee SR, Uher R, Maughan B. The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depress Anxiety. 2011;28(8):696–702. doi: 10.1002/da.20856 [DOI] [PubMed] [Google Scholar]
- 23.Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126(2):e401–e409. doi: 10.1542/peds.2009-3226 [DOI] [PubMed] [Google Scholar]
- 24.Ding XX, Wu YL, Xu SJ, et al. Maternal anxiety during pregnancy and adverse birth outcomes: a systematic review and meta-analysis of prospective cohort studies. J Affect Disord. 2014;159:103–110. doi: 10.1016/j.jad.2014.02.027 [DOI] [PubMed] [Google Scholar]
- 25.Walsh JL, Senn TE, Carey MP. Longitudinal associations between health behaviors and mental health in low-income adults. Transl Behav Med. 2013;3(1):104–113. doi: 10.1007/s13142-012-0189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27(5):604–615. doi: 10.1037/a0013242 [DOI] [PubMed] [Google Scholar]
- 27.Mezuk B, Rafferty JA, Kershaw KN, et al. Reconsidering the role of social disadvantage in physical and mental health: stressful life events, health behaviors, race, and depression. Am J Epidemiol. 2010;172(11):1238–1249. doi: 10.1093/aje/kwq283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald SD, Han Z, Mulla S, Beyene J; Knowledge Synthesis Group. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ. 2010;341:c3428 Published 2010 Jul 20. doi: 10.1136/bmj.c3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28(2):152–160. doi: 10.1016/j.reprotox.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 30.Barger MK. Maternal nutrition and perinatal outcomes. J Midwifery Womens Health. 2010;55(6):502–511. doi: 10.1016/j.jmwh.2010.02.017 [DOI] [PubMed] [Google Scholar]
- 31.Nucci LB, Schmidt MI, Duncan BB, Fuchs SC, Fleck ET, Santos Britto MM. Nutritional status of pregnant women: prevalence and associated pregnancy outcomes. Rev Saude Publica. 2001;35(6):502–507. doi: 10.1590/s0034-89102001000600002 [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Solomon CG, Manson JE, Hu FB. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Arch Intern Med. 2006;166(5):543–548. doi: 10.1001/archinte.166.5.543 [DOI] [PubMed] [Google Scholar]
- 33.George GC, Hanss-Nuss H, Milani TJ, Freeland-Graves JH. Food choices of low-income women during pregnancy and postpartum. J Am Diet Assoc. 2005;105(6):899–907. doi: 10.1016/j.jada.2005.03.028 [DOI] [PubMed] [Google Scholar]
- 34.Hurley KM, Caulfield LE, Sacco LM, Costigan KA, Dipietro JA. Psychosocial influences in dietary patterns during pregnancy. J Am Diet Assoc. 2005;105(6):963–966. doi: 10.1016/j.jada.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 35.Paul KH, Graham ML, Olson CM. The web of risk factors for excessive gestational weight gain in low-income women. Matern Child Health J. 2013;17(2):344–351. doi: 10.1007/s10995-012-0979-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro GD, Fraser WD, Frasch MG, Séguin JR. Psychosocial stress in pregnancy and preterm birth: associations and mechanisms. J Perinat Med. 2013;41(6):631–645. doi: 10.1515/jpm-2012-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apter G, Devouche E, Gratier M. Perinatal mental health. J Nerv Ment Dis. 2011;199(8):575–577. doi: 10.1097/NMD.0b013e318225f2f4 [DOI] [PubMed] [Google Scholar]
- 38.Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735. doi: 10.1177/070674370404901103 [DOI] [PubMed] [Google Scholar]
- 39.Chung TK, Lau TK, Yip AS, Chiu HF, Lee DT. Antepartum depressive symptomatology is associated with adverse obstetric and neonatal outcomes. Psychosom Med. 2001;63(5):830–834. doi: 10.1097/00006842-200109000-00017 [DOI] [PubMed] [Google Scholar]
- 40.Melnyk BM, Feinstein NF. Reducing Hospital Expenditures With the COPE (Creating Opportunities for Parent Empowerment) Program for Parents and Premature Infants. An Analysis of Direct Healthcare Neonatal Intensive Care Unit Costs and Savings. Nurs Admin Q. 2009;33(1):32–37. doi: 10.1097/01.NAQ.0000343346.47795.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melnyk BM, Feinstein NF, Alpert-Gillis L, et al. Reducing premature infants’ length of stay and improving parents’ mental health outcomes with the Creating Opportunities for Parent Empowerment (COPE) neonatal intensive care unit program: a randomized, controlled trial. Pediatrics. 2006;118(5):e1414–e1427. doi: 10.1542/peds.2005-2580 [DOI] [PubMed] [Google Scholar]
- 42.Melnyk BM, Jacobson D, Kelly S, et al. Promoting healthy lifestyles in high school adolescents: a randomized controlled trial. Am J Prev Med. 2013;45(4):407–415. doi: 10.1016/j.amepre.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melnyk BM, Jacobson D, Kelly SA, et al. Twelve-Month Effects of the COPE Healthy Lifestyles TEEN Program on Overweight and Depressive Symptoms in High School Adolescents. J Sch Health. 2015;85(12):861–870. doi: 10.1111/josh.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melnyk BM, Moldenhauer Z, eds. The KySS Guide to Child and Adolescent Mental Health Screening, Early Intervention and Health Promotion. Cherry Hill, NJ: National Association of Pediatric Nurse Practitioners; 2006. [Google Scholar]
- 45.Lam D A brief overview of CBT techniques In: Cognitive Behavior Therapy in Nursing Practice. Freeman S, Freeman A, eds. New York, NY: Springer Publishing Company; 2005; 29–47. [Google Scholar]
- 46.Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muthén BO, Curran PJ. General longitudinal modeling of individual differences in Experimental designs: A latent variable framework for analysis and power estimation. Psychol Methods. 1997;2(4):371–402. 10.1037/1082-989X.2.4.371 [DOI] [Google Scholar]
- 48.Gennaro S, Biesecker B, Fantasia HC, Nguyen M, Garry D. Nutrition profiles of African [corrected] American women in the third trimester [published correction appears in MCN Am J Matern Child Nurs. 2011 May-Jun;36(3):168]. MCN Am J Matern Child Nurs. 2011;36(2):120–126. doi: 10.1097/NMC.0b013e3182057a13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gennaro S, Bloch JR. Postpartum health in mothers of term and preterm infants. Women Health. 2005;41(3):99–112. doi: 10.1300/J013v41n03_06 [DOI] [PubMed] [Google Scholar]
- 50.Gennaro S, Fantasia HC, Keshinover T, Garry D, Wilcox W, Uppal E. Racial and ethnic identity in nursing research. Nurs Outlook. 2013;61(3):174–180. doi: 10.1016/j.outlook.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 52.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 53.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- 54.Hanusa BH, Scholle SH, Haskett RF, Spadaro K, Wisner KL. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585–596. doi: 10.1089/jwh.2006.0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157:288–290. doi: 10.1192/bjp.157.2.288 [DOI] [PubMed] [Google Scholar]
- 56.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review [published correction appears in Obstet Gynecol. 2004 Jun;103(6):1344]. Obstet Gynecol. 2004;103(4):698–709. doi: 10.1097/01.AOG.0000116689.75396.5f [DOI] [PubMed] [Google Scholar]
- 57.Ruiz RJ, Fullerton J, Brown CE, Schoolfield J. Relationships of cortisol, perceived stress, genitourinary infections, and fetal fibronectin to gestational age at birth. Biol Res Nurs. 2001;3(1):39–48. doi: 10.1177/109980040100300106 [DOI] [PubMed] [Google Scholar]
- 58.Ruiz RJ, Fullerton J, Brown CE, Dudley DJ. Predicting risk of preterm birth: the roles of stress, clinical risk factors, and corticotropin-releasing hormone. Biol Res Nurs. 2002;4(1):54–64. doi: 10.1177/1099800402004001007 [DOI] [PubMed] [Google Scholar]
- 59.Geslani GP, Gaebelein CJ. Perceived stress, stressors, and mental distress among doctor of pharmacy students. Social Behavior and Personality: An International Journal. 2013:41(9);1457–1468. 10.2224/sbp.2013.41.9.1457 [DOI] [Google Scholar]
- 60.Melnyk BM. Healthy Lifestyle Beliefs Scale. Hammondsport, NY: COPE for HOPE, Inc.;2003. [Google Scholar]
- 61.Melnyk BM. Healthy Lifestyle Behaviors Scale. Hammondsport, NY: COPE for HOPE, Inc.;2003. [Google Scholar]
- 62.Ellis A. Reason and emotion in psychotherapy. New York, NY: Lyle Stuart;1962. [Google Scholar]
- 63.Seligman MEP. Helplessness: On depression, development and death. San Francisco, CA: Freeman;1975. [Google Scholar]
- 64.Skinner BF. Science and human behavior. New York, NY: Macmillan; 1953. [Google Scholar]
- 65.Lewinsohn PM. Manual of instructions for the behavioral ratings used for the observation of interpersonal behavior In: Behavior therapy assessment. Mash EJ, Terdal LG, eds. New York, NY: Springer Publishing Company; 335–345. [Google Scholar]
- 66.Beck AT. Depression: Clinical, experimental, and theoretical aspects. New York, NY: Harper & Row; 1967. [Google Scholar]
- 67.Beck AT, Rush AJ, Shaw B, Emery G. Cognitive therapy of depression. New York, NY: Guilford Press; 1979. [Google Scholar]
- 68.Compton SN, March JS, Brent D, Albano AM 5th, Weersing R, Curry J. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43(8):930–959. doi: 10.1097/01.chi.0000127589.57468.bf [DOI] [PubMed] [Google Scholar]
- 69.Hoying J, Melnyk BM. COPE: A Pilot Study With Urban-Dwelling Minority Sixth-Grade Youth to Improve Physical Activity and Mental Health Outcomes. J Sch Nurs. 2016;32(5):347–356. doi: 10.1177/1059840516635713 [DOI] [PubMed] [Google Scholar]
- 70.Melnyk BM, Jacobson D, Kelly S, O’Haver J, Small L, Mays MZ. Improving the mental health, healthy lifestyle choices, and physical health of Hispanic adolescents: a randomized controlled pilot study. J Sch Health. 2009;79(12):575–584. doi: 10.1111/j.1746-1561.2009.00451.x [DOI] [PubMed] [Google Scholar]
- 71.Melnyk BM, Feinstein NF, Alpert-Gillis L, et al. Reducing premature infants’ length of stay and improving parents’ mental health outcomes with the Creating Opportunities for Parent Empowerment (COPE) neonatal intensive care unit program: a randomized, controlled trial. Pediatrics. 2006;118(5):e1414–e1427. doi: 10.1542/peds.2005-2580 [DOI] [PubMed] [Google Scholar]
- 72.Cox JL, Chapman G, Murray D, Jones P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. J Affect Disord. 1996;39(3):185–189. doi: 10.1016/0165-0327(96)00008-0 [DOI] [PubMed] [Google Scholar]
- 73.Tandon SD, Cluxton-Keller F, Leis J, Le HN, Perry DF. A comparison of three screening tools to identify perinatal depression among low-income African American women. J Affect Disord. 2012;136(1–2):155–162. doi: 10.1016/j.jad.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ji S, Long Q, Newport DJ, et al. Validity of depression rating scales during pregnancy and the postpartum period: impact of trimester and parity. J Psychiatr Res. 2011;45(2):213–219. doi: 10.1016/j.jpsychires.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaudron LH, Szilagyi PG, Tang W, et al. Accuracy of depression screening tools for identifying postpartum depression among urban mothers. Pediatrics. 2010;125(3):e609–e617. doi: 10.1542/peds.2008-3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268–1271. doi: 10.1097/01.AOG.0000465192.34779.dc [DOI] [PubMed] [Google Scholar]
- 77.ESHA Research. Food Nutrition Database. Available at http://www.esha.com/nutritional-database/. Accessed March 1, 2020.
- 78.Crouter SE, Schneider PL, Bassett DR Jr. Spring-levered versus piezo-electric pedometer accuracy in overweight and obese adults. Med Sci Sports Exerc. 2005;37(10):1673–1679. doi: 10.1249/01.mss.0000181677.36658.a8 [DOI] [PubMed] [Google Scholar]
- 79.Le Masurier GC, Tudor-Locke C. Comparison of pedometer and accelerometer accuracy under controlled conditions. Med Sci Sports Exerc. 2003;35(5):867–871. doi: 10.1249/01.MSS.0000064996.63632.10 [DOI] [PubMed] [Google Scholar]
- 80.Mancuso PJ, Thompson M, Tietze M, Kelk S, Roux G. Can patient use of daily activity monitors change nurse practitioner practice? The Journal for Nurse Practitioners. 10(10):787–793.e4. 10.1016/j.nurpra.2014.09.002 [DOI] [Google Scholar]
- 81.Garbers S, Nelson JA, Rosenberg T, Chiasson MA. Using pedometers to promote physical activity among working urban women [letter]. Preventing Chronic Disease: Public Health Research, Practice, and Policy [serial online], 3(2), 1–3. Retrieved from: http://www.cdc.gov/pcd/issues/2006/apr/05_0157.htm [PMC free article] [PubMed] [Google Scholar]
- 82.Spillane V, Byrne MC, Byrne M, Leathem CS, O’Malley M, Cupples ME. Monitoring treatment fidelity in a randomized controlled trial of a complex intervention. J Adv Nurs. 2007;60(3):343–352. doi: 10.1111/j.1365-2648.2007.04386.x [DOI] [PubMed] [Google Scholar]
- 83.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 84.Kenny DA, Kashy DA, Bolger N. Data analysis in social psychology In: The Handbook of Social Psychology, Volume 1. Gilbert DT, Fiske ST, Lindzey G, eds. New York, NY: Oxford University Press;1998; 233–265. [Google Scholar]
- 85.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacKinnon DP. Contrasts in multiple mediator models In: Multivariate Applications in Substance Use Research: New Methods for New Questions. Rose JS, Chassin L, Presson CC, Sherman SJ, eds. Mahway, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 87.US Preventive Services Task Force. Interventions to Prevent Perinatal Depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321(6):580–587. doi: 10.1001/jama.2019.0007 [DOI] [PubMed] [Google Scholar]
- 88.Melnyk BM Reducing Healthcare Costs for Mental Health Hospitalizations With the Evidence-based COPE Program for Child and Adolescent Depression and Anxiety: A Cost Analysis. J Pediatr Health Care. (2020) 34, 117–121 [DOI] [PubMed] [Google Scholar]
- 89.Pekkala J, Cross-Barnet C, Kirkegaard M, Silow-Carroll S, Courtot B, Hill I. Key Considerations for Implementing Group Prenatal Care: Lessons from 60 Practices. J Midwifery Womens Health. 2019. October 23. doi: 10.1111/jmwh.13047. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


