Abstract
Background:
Low red blood cell (RBC) levels are associated with worse intracerebral hemorrhage (ICH) outcomes. However, relationships of RBC transfusions on ICH outcomes are unclear given the overlap of RBC transfusion, comorbidities, and disease severity. We investigated RBC transfusion relationships on ICH outcomes while accounting for comorbidities and disease severity.
Methods:
ICH hospitalizations between 2002–2011 and RBC transfusion exposure were identified from the Nationwide Inpatient Sample using ICD-9-CM codes. Logistic regression was used to study the relationship between RBC transfusion on outcomes after adjusting for demographics, baseline comorbidities, and markers of disease severity. Additional sensitivity analyses stratified by comorbidity burden and disease severity were performed.
Results:
Of 597,046 ICH hospitalizations, RBC transfusions were administered in 22,904 (4%). RBC transfusion was associated with higher odds of in-hospital mortality (adjusted OR: 1.22 [95%CI: 1.10–1.35]). In sensitivity analyses, RBC transfusions resulted in poor outcomes regardless of the comorbidity burden, but attenuation in this relationship was notable with lower comorbidities (adjusted OR 1.43 [95%CI: 1.34–1.51] vs 1.18 [95%CI: 1.10–1.29]). There were no associations of RBC transfusions with poor outcomes in hospitalizations without mechanical ventilation (adjusted OR 0.88 [95%CI: 0.83–1.13]) and in cases requiring ventriculostomy drains (adjusted OR 1.05 [95%CI: 0.97–1.10]).
Conclusions:
In a large, nationally representative sample, RBC transfusion was associated with poor ICH outcomes. However, there were variations in this relationship based on comorbidities and disease severity. Additional prospective studies are required to assess direct risks and benefits from RBC transfusions in ICH.
Keywords: intracerebral hemorrhage, red blood cell transfusions, comorbidities, outcome, administrative data
Introduction
Intracerebral hemorrhage (ICH) is associated with high morbidity and mortality. This is due to initial cerebral tissue injury as well as secondary brain injury that can occur in part due to either direct or indirect impairments of adequate cerebral tissue oxygen delivery. Unfortunately, there are no current treatment targets that have been shown to improve ICH outcomes. However, we and others have shown that low red blood cell (RBC) levels are associated with worse outcomes after ICH1–3. These findings are postulated to be due in part to impaired cerebral oxygenation, extrapolating findings from subarachnoid hemorrhage4,5 and traumatic brain injury6 patients and could suggest a potential role of RBC transfusions as a treatment modality in ICH. Though RBC transfusions are currently being explored in clinical treatment trials for subarachnoid hemorrhage and traumatic brain injury patients7–9, no such trials exist for patients with ICH. While there may be observational evidence that RBC transfusions can improve survival in ICH patients10, it is unclear if these single center findings are widely generalizable. Hence, we sought to explore the relationship between RBC transfusions and outcomes in a large, nationally representative sample of patients hospitalized with ICH while accounting for baseline medical comorbidities and medical and ICH disease severity.
Materials and Methods
Study Design and Sample
We performed a retrospective, cohort study using discharge data from the Nationwide Inpatient Sample (NIS) of the Healthcare Cost and Utilization Project (HCUP) from 2002–2011. The NIS cohort represents a 20% stratified random sample of all inpatient admissions to non-federal US hospitals. The database provides information on patient demographics, hospital characteristics, primary and secondary diagnoses, inpatient procedures, comorbidities, and case-severity measures. All diagnoses and procedures are recorded using International Classification of Diseases version 9 Clinical Modification (ICD-9-CM) codes. Since the NIS database contains de-identified data, this study was exempt from the institutional review as determined by the Weill Cornell Medicine institutional review board.
Measurements
We identified ICH hospitalizations from 2002–2011 using the validated ICD-9-CM code 431 as previously described11,12. Hospitalizations of cases <18 years old were excluded. Cases transferred to another hospital were also excluded to avoid duplicate ICH case inclusions and to best account for entire hospitalization course12. Additionally, we did not include ICH hospitalizations with diagnostic codes suggesting an ICH from traumatic brain injury or other secondary etiologies of ICH including vascular malformation, aneurysm, or malignancy given the known heterogeneity of outcomes in these types of ICH. A cumulative score was calculated for each case using the Charlson comorbidity index13,14 in order to capture baseline comorbidity status. This index is a weighted score of 17 different comorbidities using ICD-9-CM codes with higher scores indicating higher comorbidities. This score has been validated for outcome adjustment for administrative datasets on ischemic stroke and ICH15,16.
Our exposure was RBC transfusions received during ICH hospitalization, identified using the ICD-9-CM code V58.2 and procedure code 99.04, which have both been used previously and validated with high levels of sensitivity and specificity17–19. Our primary outcomes were in-hospital mortality and home discharge. Secondary outcomes studied were a composite favorable outcome of discharge to home or rehabilitation, and a composite unfavorable outcome of discharge to skilled nursing facility, hospice or death. Discharge disposition has been shown to correlate with 90-day and 1-year modified Rankin Scale with discharge to home or rehabilitation indicating higher functional potential than discharge to skilled nursing facility20.
Statistical analysis
Intergroup differences (RBC transfused vs non-RBC transfused ICH cases) were determined by applying Mann-Whitney-U or t-tests for continuous variables and χ2 or Fisher-exact tests for categorical variables. Binary logistic regression was used to evaluate the relationship between RBC transfusion and outcomes. Covariates in the model included baseline demographics (age, sex, race, insurance status), hospital-level characteristics (geographical location, teaching status, and bed size), comorbidities (diabetes, hypertension, hyperlipidemia, atrial fibrillation, valvular heart disease, congestive heart failure, and chronic kidney disease), Charlson comorbidity index, surrogate markers of medical disease and ICH severity: infection (sepsis, urinary tract infections, and pneumonia), withdrawal of care, and procedures (ventriculostomy and mechanical ventilation).
Pre-specified sensitivity analyses were performed to investigate the differential relationship of RBC transfusion on outcome in patients with: a) varying levels of baseline medical comorbidity burden (stratified hospitalizations by Charlson comorbidity index ≥3 vs <3); b) different levels of active medical disease (mechanical ventilation vs no mechanical ventilation); c) and presumptive severity of neurological injury of the ICH (stratified hospitalization by external ventriculostomy drain placement21,22). Neurosurgical ventriculostomy placement is required for cerebrospinal fluid diversion for patients that have obstructive hydrocephalus and/or intraventricular hemorrhage (IVH), the presence of which is a marker of ICH severity23. These clinical parameters have been used in prior studies utilizing administrative data as surrogates of disease severity12,24. These sensitivity analyses were performed in attempts to best investigate the relationship of RBC transfusion, rather than underlying complication/indication, on outcome.
As recommended by HCUP, population estimates were obtained by complex sample analyses that consider weights, clustering, and stratification used for NIS sampling25. All analyses were performed by using IBM SPSS version 20 (IBM Corporation, NY, USA) with statistical significance set at P<0.05.
Results
Baseline Characteristics
Of the 619,166 ICH hospitalizations identified using ICD codes, 597,046 were ultimately included for analysis (figure 1). A total of 22,904 (4%) cases received an RBC transfusion during their ICH hospitalization. Cases receiving RBC transfusions were younger and more likely to be Black or Hispanic, compared to those who were not administered RBC transfusions. There also appeared to be geographic and hospital characteristic variations between cases receiving and not receiving RBC transfusions. Additionally, cases receiving RBC transfusions had more baseline risk factors including chronic kidney disease and anemia, a higher comorbidity burden as noted on the Charlson comorbidity scores, infections during hospitalization, as well as longer hospital length of stay (Table 1).
Figure 1: Patient Selection and Screening from NIS.
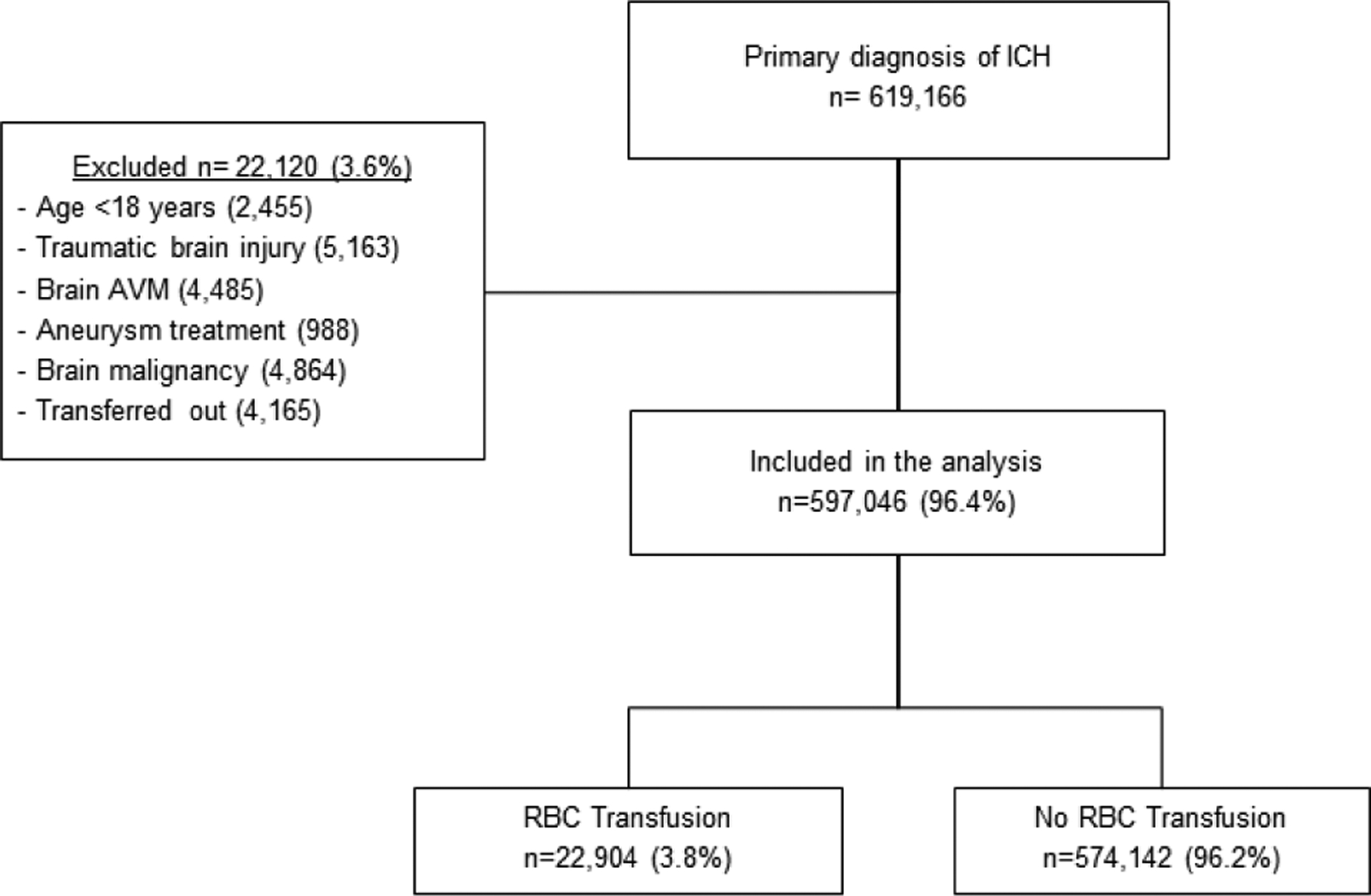
ICH: intracerebral hemorrhage; AVM: arteriovenous malformation; RBC: red blood cells
Table 1.
Baseline Characteristics of ICH Hospitalizations
| Characteristic | RBC transfusion N=22,904 | No transfusion N=574,142 | P value |
|---|---|---|---|
| Age, years | |||
| 18–64 | 9,625 (42.0) | 207,261 (36.1) | <0.001 |
| 65–79 | 7,874 (34.4) | 201,340 (35.1) | |
| 80 or more | 5,406 (23.6) | 165,541 (28.8) | |
| Female | 12,068 (52.7) | 301,643 (52.5) | <0.001 |
| Race | |||
| White | 10,676 (46.5) | 312,002 (54.3) | <0.001 |
| Black | 4,735 (20.7) | 75,697 (13.2) | |
| Hispanic | 2,406 (10.5) | 42,945 (7.5) | |
| Asian | 1,053 (4.6) | 21,049 (3.6) | |
| Other | 888 (3.9) | 16,466 (2.9) | |
| Missing | 3,147 (13.7) | 105,983 (18.5) | |
| Hospital geographic region | |||
| Northeast | 5,335 (23.3) | 105,392 (18.4) | <0.0001 |
| Midwest | 3,452 (15.1) | 122,424 (21.3) | |
| South | 9,086 (41.1) | 228,200 (39.7) | |
| West | 5,032 (21.9) | 118,126 (20.6) | |
| Urban hospital | 21,881 (95.5) | 546,638 (95.2) | 0.045 |
| Academic hospital | 14,214 (62.1) | 341,083 (59.4) | <0.001 |
| Hospital size | |||
| Small | 1,210 (5.3) | 44,029 (7.7) | <0.001 |
| Medium | 5,079 (22.2) | 120,443 (20.9) | |
| Large | 16,524 (72.1) | 409,670 (71.4) | |
| Health insurance | |||
| Medicare | 13,473 (58.8) | 365,204 (63.6) | <0.001 |
| Medicaid | 2,985 (13.0) | 42,930 (7.5) | |
| Private insurance | 4,439 (19.4) | 115,778 (20.2) | |
| Other | 1,984 (8.7) | 58,230 (10.1) | |
| Comorbidities | |||
| Anemia | 10,758 (46.9) | 61,881 (10.8) | <0.001 |
| Dyslipidemia | 3,932 (17.2) | 140,269 (24.4) | <0.001 |
| Coronary Artery Disease | 4,845 (21.2) | 115,852 (20.1) | <0.001 |
| Atrial Fibrillation | 5,221 (22.8) | 103,278 (17.9) | <0.001 |
| Hypertension | 15,975 (69.7) | 449,786 (78.3) | <0.001 |
| Diabetes | 5,226 (22.8) | 123,371 (21.5) | <0.001 |
| Chronic kidney disease | 3,117 (13.6) | 38,417 (6.7) | <0.001 |
| Charlson Comorbidity Index | <0.001 | ||
| Mechanical Ventilation | 12,554 (54.8) | 163,322 (28.4) | <0.001 |
| External Ventriculostomy | 3,682 (16.1) | 35,782 (6.2) | <0.001 |
| Sepsis | 2,906 (12.7) | 16,957 (2.9) | <0.001 |
| Pneumonia | 5,036 (21.9) | 78,235 (13.6) | <0.001 |
| Urinary tract infection | 4,740 (20.7) | 38,940 (6.8) | <0.001 |
| Gastrointestinal hemorrhage | 1,265 (5.5) | 5,694 (1.0) | <0.001 |
| ESRD requiring dialysis | 1,674 (7.3) | 10,039 (1.7) | <0.001 |
| Length of stay, mean [SD] | 17.0 (20.1) | 8.0 (10.5) | <0.001 |
Abbreviations: ICH: intracerebral hemorrhage; RBC, red blood cell; ESRD: end stage renal disease; SD: standard deviation
Primary Analyses
The overall in-hospital mortality was 30% in our study and there was a higher mortality in cases exposed to RBC transfusion (35% vs 29%). In the unadjusted analysis, RBC transfusion was associated with an increased odds of in-hospital mortality (odds ratio [OR] 1.29; 95%CI: 1.25–1.33). We noted a continued association of RBC transfusion on hospital mortality after adjusting for relevant covariates (adjusted OR 1.22; 95%CI 1.10–1.35). Similarly, logistic regression models identified an association of RBC exposure and increased odds of unfavorable discharge disposition (adjusted OR 1.44; 95%CI: 1.36–1.51). Conversely, RBC transfusion was associated with a lower odds of home discharge (adjusted OR 0.45; 95%CI: 0.42–0.51) and favorable discharge disposition (adjusted OR 0.56; 95%CI: 0.54–0.60) (Table 2). In post-hoc analysis, we additionally included gastrointestinal hemorrhage, end stage renal disease on hemodialysis, and hospital length of stay as covariates in regression models and did not note a change in the relationship of RBC transfusion increased odds of mortality (adjusted OR 1.24; 95%CI: 1.16–1.35) or lower odds of home discharge (adjusted OR 0.73; 95%CI: 0.68–0.77).
Table 2.
Logistic Regression Analysis Evaluating the Relationship between RBC Transfusion and ICH Outcomes
| RBC Transfusion* | No RBC Transfusion* | Adjusted OR (95% Cl) | P value | |
|---|---|---|---|---|
| In-hospital mortality | 7,927 (34.6) | 173,738 (29.1) | 1.22 (1.10–1.35) | <0.001 |
| Home discharge/Self-care | 2,135 (9.3) | 121,166 (20.3) | 0.45 (0.42–0.51) | <0.001 |
| Unfavorable discharge (SNF/hospice/inpatient death) | 11,312 (61.9) | 264,020 (52.2) | 1.44 (1.36–1.51) | <0.001 |
| Favorable discharge* (Home/Rehabilitation) | 3,891 (21.3) | 172,591 (34.1) | 0.56 (0.54–0.60) | <0.001 |
Abbreviations: CI, confidence interval; ICH, intracerebral hemorrhage; OR, odds ratio; RBC, red blood cell; SNF, skilled nursing facility.
Logistic regression models adjusted for age, sex, modified Charlson Comorbidity Index, hypertension, diabetes, dyslipidemia, atrial fibrillation, mechanical ventilation, insurance status, teaching status of hospital, chronic kidney disease, ventriculostomy procedure, infections, and withdrawal of care
Data represented as N (%) unless otherwise specified
Sensitivity Analyses
We additionally performed pre-specified sensitivity analyses. In the first analysis, RBC transfusion remained associated with poor ICH outcomes regardless of baseline comorbidity burden as stratified by Charlson comorbidity index ≥3 vs <3 (adjusted OR 1.43 [95%CI: 1.34–1.51] vs adjusted OR 1.18 [95%CI: 1.10–1.29] respectively). However, it was notable that there was an attenuation of the association of RBC transfusion exposure on poor outcomes in cases with less baseline comorbidities. In our second analysis, we similarly identified a variation in the relationship of RBC transfusion on ICH outcome when stratifying the cohort based on need for mechanical ventilation, a surrogate marker of medical disease severity. While RBC transfusion was associated with poor outcomes in cases requiring mechanical ventilation, there was no association in cases not requiring mechanical ventilation (adjusted OR 1.33 [95%CI: 1.27–1.39] vs adjusted OR 0.88 [95%CI: 0.83–1.13] respectively). In our third analyses, we stratified cohorts by external ventriculostomy drain use as a surrogate measure of ICH specific disease severity. RBC transfusion was associated with poor ICH outcomes (adjusted OR 1.51; 95%CI: 1.46–1.57) among hospitalizations not requiring ventriculostomy. However, there was no relationship between RBC transfusion and poor outcomes in cases receiving ventriculostomy (adjusted OR 1.05; 95%CI: 0.97–1.10). Additional analyses were performed stratifying for both anemia and gastrointestinal hemorrhage complication diagnostic codes and there continued to be associations of RBC transfusions on poor outcome regardless of the presence/absence of diagnosis (table 3). There were no interactions between RBC transfusion and the stratification variable for each outcome.
Table 3.
Sensitivity Analysis of the Association between RBC Transfusion and ICH Outcomes1
| Characteristic | In-hospital Mortality | Home Discharge/Self-Care |
|---|---|---|
| OR (95% Cl) | OR (95% Cl) | |
| Modified Charlson’s index ≥ 3 | 1.43 (1.34–1.51)* | 0.45 (0.39–0.52)* |
| Modified Charlson’s index <3 | 1.18 (1.10–1.29)* | 0.33 (0.30–0.40)* |
| Cases with mechanical Ventilation | 1.33 (1.27–1.39)* | 0.98 (0.94–1.04) |
| Cases without mechanical Ventilation | 0.88 (0.83–1.13) | 0.64 (0.61–0.68)* |
| Cases with EVD | 1.05 (0.97–1.10) | 0.91 (0.78–1.05) |
| Cases without EVD | 1.51 (1.46–1.57)* | 0.69 (0.66–0.73)* |
| Excluding cases with Gl hemorrhage | 1.23 (1.18–1.29)* | 0.66 (0.61–0.69)* |
| Cases with anemia only | 1.48 (1.41–1.55)* | 0.58 (0.54–0.62)* |
| Cases without anemia | 1.55 (1.48–1.62)* | 0.31 (0.29–0.33)* |
| Patients with ESRD on dialysis | 1.23 (1.02–1.34)* | 0.54 (0.49–0.61)* |
| Patients without ESRD | 1.18 (1.13–1.24)* | 0.65 (0.60–0.72)* |
Abbreviations: CI, confidence interval; EVD, external ventriculostomy drain; GI: gastrointestinal; ESRD: end stage renal disease; OR, odds ratio; RBC, red blood cell.
Logisitc regression models adjusted for age, sex, modified Charlson Comorbidity Index, hypertension, diabetes, dyslipidemia, atrial fibrillation, mechanical ventilation, insurance status, teaching status of hospital, chronic kidney disease, ventriculostomy procedure, infections, and withdrawal of care
Indicates p value < 0.05
Discussion
In a large, nationally representative cohort of hospitalizations with non-traumatic ICH, approximately 4% of cases received an RBC transfusion during their ICH hospitalization. Intergroup differences in hospital characteristics, geographic location of the hospital, and baseline demographics were seen in ICH hospitalizations requiring RBC transfusions compared to those that did not. Expectedly, we also identified that cases receiving RBC transfusions had higher medical comorbidities and surrogate markers of severity of illness during hospitalization (infections, procedures) compared to cases not requiring RBC transfusions. While RBC transfusions were associated with poor ICH outcomes even after adjusting for these comorbidities, the relationship of RBC transfusion on outcome appeared to vary based on baseline comorbidities as well as presumed medical and neurological injury severity.
There is increasing recognition that anemia or low RBC levels correlate with poor ICH outcomes due in part to impaired cerebral oxygenation1–3. Subsequently, RBC levels have been considered a potential therapeutic target to improve outcomes in these patients. While the logical next step would be to examine if RBC transfusion has any therapeutic benefit in ICH as has been initiated in other diseases of acute brain injury7,9, there is a paucity of RBC transfusion data on this topic in ICH. A single center cohort study from a tertiary care center identified better survival with RBC transfusions after ICH10. In this context, our findings from a much larger cohort of ICH cases across the United States suggesting poor outcomes after RBC transfusion did not confirm these single center observational study results. However, rather than directly contradicting these observational ICH study findings, it is possible that our results reinforce observations seen in other studies of primarily non-brain injured patients which similarly identified that RBC transfusions were associated with poor outcomes26. It is worth noting that these studies in non-brain injured patients cautioned that the relationships of RBC transfusions with increased morbidity/mortality did not imply causality and could reflect the significant overlap of baseline comorbidities, severity of illness during hospitalization with low RBC levels, RBC transfusion requirements, and subsequent outcomes. Perhaps in line with this concept is that we noted an attenuation of the relationship of RBC transfusion exposure on poor outcome when looking at ICH cases with lower baseline comorbidities. Similarly, there was no association of RBC transfusions with poor outcomes in ICH cases that did not require mechanical ventilation.
Because cerebral oxygen delivery is paramount in settings of severe neurological injury, we attempted to address the differential impact of RBC transfusions amongst cases with different degrees of neurologic injury utilizing ventriculostomy placement as a surrogate marker of increased neurologic injury. An intriguing observation from our data was that while RBC transfusions had a deleterious relationship with outcomes among hospitalizations not requiring ventriculostomy placement, there was no relationship with outcomes when ventriculostomies were placed. Because patients receiving ventriculostomies are more likely to have intraventricular hemorrhage, larger hematoma volumes, deep ICH location, and poor mental status, all of which independently portend poor prognosis, it could be speculated that brain injury severity can mask any potential underlying outcome effect (either deleterious or beneficial) of RBC transfusion. It is unclear whether our study further suggests that patients with less severe neurological injury may be more susceptible to the risks rather than benefits associated with RBC transfusions. However, at the very least our findings appear to indicate that future studies will need to account for measures of both acute medical and neurological critical illness, such as physiologic scores (APACHE), ICH location, ICH score23, hematoma expansion1, in addition to baseline comorbidities when addressing the relationship of RBC transfusions on outcome. It could be possible that RBC transfusions indicated for cerebral oxygenation delivery may have different, and potentially beneficial relationships with outcomes compared to those transfusions given for blood loss or conditions related to medical illness severity.
Though our study strengths included a very large sample of nationally represented ICH hospitalizations and attempts to adjust for potential confounders, the results need to be interpreted with caution due to inherent limitations of administrative databases. The absence of granular data, specifically hemoglobin laboratory levels and indication for transfusions in such databases prohibit a closer look at the relationship of RBC transfusion themselves (rather than underlying disease/indication) on outcome. While we attempted to adjust for medical comorbidities, active disease severity, and also performed analyses while excluding those encountering hemorrhagic complications in order to estimate the relationship of RBC transfusion itself on outcome, further work using prospective datasets will be required to elucidate this complex relationship. Coding errors and misclassification are additional inherent limitations to administrative datasets, however in order to address this issue, we utilized well-validated codes to identify ICH cases and RBC transfusion exposure18,19. Similarly, while random ICD-9-CM coding errors are possible, they would bias the results toward the null, and are hence unlikely to account for the measured differences in mortality rates found in this study. Further limitations included the absence of well-validated ICH severity measures, such as ICH volume, ICH location, and Glasgow Coma Scale in our data. The use of ventriculostomy procedure codes as a surrogate marker of ICH severity may be limited in certain scenarios and a closer look at specific ICH characteristics will be important to elucidate in future studies. It is also possible that our analyses could be limited by treatment bias, where patients not receiving RBC transfusions could simply be patients who did not receive aggressive treatment due to withdrawal of care practices. However, we attempted to account for this by incorporating withdrawal of care as a covariate in all models. Additional limitations included the inability to assess the number of transfusions and temporality of the transfusion in relation to the day of hospitalization in this dataset. This prevented us from looking at the known relationship between RBC transfusions and incident complications (infections and thrombosis) to identify whether our relationships of RBC transfusions and poor outcomes were driven by inherent risks of RBC transfusions themselves.
Finally, our study lacked functional outcome data at discharge as well as long-term follow-up which is a critical measure of outcome in neurological disease. While we used discharge disposition, a validated, accurate surrogate for functional outcomes20 in these settings at discharge, further work is required to establish the relationship of RBC transfusions on long term functional and cognitive outcomes.
Conclusions
In this nationally representative study, RBC transfusions were associated with poor outcomes after ICH. However there appeared to be variations in the relationship of RBC transfusion on poor outcomes based on comorbidities and hospitalization related medical and ICH severity. In order to disentangle the complex relationship of RBC transfusions, underlying medical indication/disease, and their confounders on clinical ICH outcome, further prospective work is required to elucidate the specific risks and benefits of RBC transfusion for patients with ICH. And ultimately, further work is required to clarify whether ICH patients necessitate different hemoglobin threshold triggers for RBC transfusions.
Funding/Support
Dr. Roh is supported by the National Blood Foundation. Dr. Murthy is supported by the National Institutes of Health (K23NS105948). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NBF or NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
Authors do not report any conflict of interest disclosures.
Disclosures
Dr. Roh receives consulting fees from Portola Pharmaceuticals
References:
- 1.Roh DJ et al. Low hemoglobin and hematoma expansion after intracerebral hemorrhage. Neurology (2019) doi: 10.1212/WNL.0000000000007820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuramatsu JB et al. Anemia is an independent prognostic factor in intracerebral hemorrhage: an observational cohort study. Crit. Care 17, R148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diedler J et al. Low hemoglobin is associated with poor functional outcome after non-traumatic, supratentorial intracerebral hemorrhage. Crit. Care Lond. Engl 14, R63 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz P et al. The Effect of Packed Red Blood Cell Transfusion on Cerebral Oxygenation and Metabolism After Subarachnoid Hemorrhage. Neurocrit. Care 24, 118–121 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Dhar R, Zazulia AR, Derdeyn CP & Diringer MN RBC Transfusion Improves Cerebral Oxygen Delivery in Subarachnoid Hemorrhage. Crit. Care Med 45, 653–659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oddo M et al. Anemia and brain oxygen after severe traumatic brain injury. Intensive Care Med. 38, 1497–1504 (2012). [DOI] [PubMed] [Google Scholar]
- 7.English SW et al. Aneurysmal SubArachnoid Hemorrhage-Red Blood Cell Transfusion And Outcome (SAHaRA): a pilot randomised controlled trial protocol. BMJ Open 6, e012623 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okonkwo DO et al. Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II: A Phase II Randomized Trial. Crit. Care Med 45, 1907–1914 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gobatto ALN et al. Transfusion requirements after head trauma: a randomized feasibility controlled trial. Crit. Care Lond. Engl 23, 89 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheth KN et al. Packed red blood cell transfusion and decreased mortality in intracerebral hemorrhage. Neurosurgery 68, 1286–1292 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Kokotailo RA & Hill MD Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke 36, 1776–1781 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Murthy SB et al. Outcomes after intracerebral hemorrhage from arteriovenous malformations. Neurology 88, 1882–1888 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL & MacKenzie CR A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis 40, 373–383 (1987). [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC & Ciol MA Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol 45, 613–619 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Bar B & Hemphill JC Charlson comorbidity index adjustment in intracerebral hemorrhage. Stroke 42, 2944–2946 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiménez Caballero PE et al. Charlson comorbidity index in ischemic stroke and intracerebral hemorrhage as predictor of mortality and functional outcome after 6 months. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc 22, e214–218 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Long B, Xiao Z-N, Shang L-H, Pan B-Y & Chai J Impact of perioperative transfusion in patients undergoing resection of colorectal cancer liver metastases: A population-based study. World J. Clin. Cases 7, 1093–1102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segal JB, Ness PM & Powe NR Validating billing data for RBC transfusions: a brief report. Transfusion (Paris) 41, 530–533 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Howard DH, Karcz A & Roback JD The accuracy of claims data for measuring transfusion rates. Transfus. Med 26, 457–459 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Qureshi AI, Chaudhry SA, Sapkota BL, Rodriguez GJ & Suri MFK Discharge destination as a surrogate for Modified Rankin Scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch. Phys. Med. Rehabil 93, 1408–1413.e1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moradiya Y, Murthy SB, Newman-Toker DE, Hanley DF & Ziai WC Intraventricular thrombolysis in intracerebral hemorrhage requiring ventriculostomy: a decade-long real-world experience. Stroke 45, 2629–2635 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekula RF, Cohen DB, Patek PM, Jannetta PJ & Oh MY Epidemiology of ventriculostomy in the United States from 1997 to 2001. Br. J. Neurosurg 22, 213–218 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Hemphill JC, Bonovich DC, Besmertis L, Manley GT & Johnston SC The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke J. Cereb. Circ 32, 891–897 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Murthy SB et al. Intracerebral Hemorrhage Outcomes in Patients with Systemic Cancer. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc 25, 2918–2924 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houchens R & Elixhauser A Final report on calculating Nationwide Inpatient Sample (NIS) variances, 2001. Rockv. MD Agency Healthc. Res. Qual 2003–2 (2005). [Google Scholar]
- 26.Marik PE & Corwin HL Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit. Care Med 36, 2667–2674 (2008). [DOI] [PubMed] [Google Scholar]


