Abstract
Purpose:
Neutrophils play a critical role in defending against threats such as microbial infection, yet their activation during innate immune response incites collateral damage to healthy tissues. We have previously shown that corneal injury induces mast cells to express the neutrophil chemoattractant CXCL2. Here we delineate the mechanism of injury-induced, non-IgE-mediated mast cell activation at the ocular surface.
Methods:
Corneal injury was induced by mechanical removal of the epithelium and anterior stroma in mast cell deficient (cKitW-sh) and C57BL/6 mice using Algerbrush II. Corneas were analyzed for frequencies of total CD45+ inflammatory cells, CD11b+Ly6G+ neutrophils, and cKit+FcεR1+ mast cells using flow cytometry. Mast cells were stimulated with different inflammatory factors known to increase during corneal injury (IL-33, IL-1β, IL-36γ, IL-6, SDF1α and Substance P) and assessed for the secretion of β-hexosaminidase, tryptase and CXCL2 using ELISA. IL-33 neutralizing antibody (1 mg/ml) was administered locally for mast cell inhibition in vivo.
Results:
Mast cell deficient mice failed to recruit early neutrophils to the injured corneas. IL-33 stimulation upregulated CXCL2 secretion by mast cells. Corneal injury resulted in amplified expression of IL-33 at the cornea and epithelium was identified as its primary source. Topical neutralization of IL-33 at the ocular surface inhibited mast cell activation, limited neutrophil infiltration and reduced corneal inflammatory haze, normalizing tissue architecture following ocular injury.
Conclusions:
These data implicate IL-33 in mast cell activation and early neutrophil recruitment in non-allergic inflammation, suggesting IL-33 as a potential therapeutic target in inflammatory disorders of the ocular surface.
Introduction
Mast cells are long-lived tissue-resident immune cells that are distributed throughout vascularized tissues in the body, and are particularly abundant at mucosal surfaces that interface with the outside environment (e.g. skin, airways and gastrointestinal tract).[1,2] Mast cell activation, for example in the setting of allergy or host defense to parasitic infection, results in the rapid release of preformed and de novo synthesized inflammatory mediators into the extracellular environment.[3,4] These mediators include various cytokines, growth factors, amines, and enzymes such as tryptase and β-hexosaminidase.[3,5] Yet beyond their well-established role as effector cells in pathogenic IgE- and IgG-mediated responses, mast cells are now recognized for their critical function in modulating both innate and adaptive immunity, particularly during the early phases of the inflammatory response.[6-9] Work from our own laboratory has shown that following corneal trauma, tissue-resident mast cells initiate neutrophil recruitment to the site of injury via secretion of the CXC chemokine receptor 2-binding chemoattractant CXCL2.[6] However, while the pathway of mast cell activation in allergic diseases is well defined, the underlying mechanism of mast cell activation following tissue injury have not been fully deciphered.
Neutrophils are the first cells recruited to sites of acute inflammation.[10,11] These motile phagocytes, long understood as the ‘foot soldiers’ of innate immunity, serve a critical function in eliminating microbial pathogens.[12,13] More recently, the uncontrolled effector function of neutrophils has been implicated in chronic inflammatory diseases.[14-16] During the inflammatory response, excessive release of reactive mediators by neutrophils results in collateral damage to adjacent healthy tissues.[14,17,18] Accordingly, there is significant interest in the mechanisms that govern neutrophil infiltration, particularly the contribution of tissue-resident mast cells to this process, which act as sentinels, triggering neutrophil recruitment.
We have previously reported that mast cell activation initiates neutrophil infiltration of the cornea following injury.[6] In the current study, we observed that mast cell deficient mice [19] failed to recruit neutrophils to the injured corneas, and performed a series of in vitro and in vivo experiments to delineate the mechanism of mast cell activation that induces the early recruitment of neutrophils. Our in vivo experiments employed a mouse model of corneal injury, which due to the relative avascularity and scarcity of resident immune cells, provides a highly informative system to evaluate mast cell activation and neutrophil recruitment.[6]
In this study, we report the novel observation that corneal epithelium-derived IL-33 activates mast cell to promote early neutrophil recruitment to the ocular surface following injury. Specifically, we identify epithelium as the primary source of IL-33 following ocular surface injury, and demonstrate that IL-33 promotes mast cell activation. Moreover, we show that in vivo neutralization of IL-33 inhibits mast cell activation and abrogates early neutrophil infiltration reducing corneal inflammatory haze, restoring the tissue architecture following ocular injury.
Materials and Methods
Animals
Six- to eight-week-old male and female C57BL/6 wild-type and mast cell deficient cKitw-sh mice (Stock No: 012861; Jackson Laboratory, Bar Harbor, ME) were purchased for use in the described experiments. The mice were housed in the Schepens Eye Research Institute animal vivarium. All experiments were reviewed and approved by the Schepens Eye Research Institute Animal Care and Use Committee. The mice were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Corneal Injury Model
Corneal injury was performed on deeply anesthetized mice as previously described.[6,20] Briefly, the central cornea of the right eye was demarcated using a 2 mm trephine. The tip of a handheld motor brush (Algerbrush II; Alger Company, Inc., Lago Vista, TX, USA) was used to remove the corneal epithelium and anterior stroma. Following completion of this procedure, a triple antibiotic ointment was applied topically. Mice were administered a subcutaneous injection of buprenorphine in order to mitigate injury-induced pain. In order to evaluate the in vivo effects of mast cells, 2.5 μL of either IL33 blocking antibody (1 mg/ml in PBS; Biolegend) or 2% cromolyn sodium in PBS (Sigma-Aldrich Corp., St. Louis, MO, USA) were administered topically at four time points – one hour prior to injury, at the time of injury, one hour post-injury and 3 hours post-injury. The PBS treated injured mice and uninjured normal mice served as controls. Ocular surface tear wash was collected at 6 hours post-injury. Subsequently, mice were euthanized and their corneas (including the corneal limbus) were harvested for further analyses.
Pharmaceutical intervention
Topical administration of 2% cromolyn sodium (Sigma-Aldrich, St. Louis, MO) eye drops or αIL-33 (1 mg/ml; R&D systems, Minneapolis, MN) were applied to the injured cornea prior to injury, at the time of injury as well as at 1 and 3 hours after injury. A group of control mice were administered phosphate-buffered saline (PBS) eye drops.
Corneal Tissue Isolation and Digestion
Single cell suspensions were prepared from corneas as previously described.[7,21,22] Briefly, corneas were digested in RPMI media (Lonza, Walkersville, MD, USA) containing 4 mg/mL collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 2 mg/mL DNase I (Roche, Basel, Switzerland) for 45 minutes at 37°C. Following this, cells were filtered through a 70-mμ cell strainer.
Cell Culture Assays
Mast cells were generated by culturing bone marrow cells in the presence of stem cell factor (SCF; 50 ng/mL) and IL-3 (10 ng/mL) for 3 to 4 weeks.[6] Media was changed every 3 to 4 days. This method of cell culture generates a mast cell population of >95% purity. For the mast cell stimulation assays, mast cells were cultured either in medium alone, or were stimulated with 1 μM concentration of inflammatory factors IL-1β, IL-6, IL-33, IL-36γ, SDF1α or Substance P for 6 hours at 37°C. The cells and respective supernatants were harvested, and levels of CXCL2, β-hexosaminidase and tryptase were evaluated using the methods described below.
Flow Cytometry
Single cells suspensions were prepared by enzymatic digestion of 3-4 corneas as described above. The cells were stained with fluorochrome-conjugated anti-CD45, anti-CD11b, anti-Ly6G, anti-c-Kit and anti-FcεR1 antibodies for flow cytometry analysis. Bone marrow mast cells were stained with anti-ST2 antibodies. Appropriate isotypes were utilized as antibody controls. Antibodies and isotype controls were acquired from Biolegend (San Diego, CA, USA). LSR II flow cytometer (BD Biosciences, San Jose, CA, USA) and Summit software (Dako Colorado, Inc., Fort Collins, CO, USA) were used to analyze stained cells.
Real-Time PCR
RNeasy Micro Kits (Qiagen, Valencia, CA, USA) were used to isolate total RNA, which was reverse transcribed into cDNA using Superscript III (Invitrogen, Carlsbad, CA, USA) [23]. Quantitative real-time PCR was conducted using Taqman Universal PCR Mastermix and preformulated primers for murine IL-33 (Mm00505403_m1) and glyceraldehype-3-phosphate dehydrogenase (GAPDH, Mm99999915_gl) in a Mastercycler Realplex 2 (Eppendorf, Hamburg, Germany). The comparative threshold cycle method was used to analyze the results, which were normalized to GAPDH as an internal control.
β-hexosaminidase Assays
β-n-acetylglucosaminidase assay kits (Sigma-Aldrich) were used to quantify levels of β-hexosaminidase enzyme. These kits are based on the hydrolysis of 4-Nitrophenyl N-acetyl-β-d-glucosaminide (NP-GlcNAc).[25] In brief, ocular surface tears and culture supernatants were incubated with 0.1 mg/mL NP-GlcNAc (substrate) for 1 hour at 37°C. Subsequently, the enzyme-substrate reaction was stopped with 5 mg/mL sodium carbonate. A SpectraMax Plus 384 Microplate Reader (Molecular Devices, San Jose, CA, USA) was used to measure absorbance at 405 nm. β-hexosaminidase levels were estimated using the formula: U/mL = (A405sample - A405blank) × 0.05 × 0.3 × DF/A405standard × time × volume of sample in milliliters.
Tryptase Assays
Mast Cell Degranulation Assay Kits (Sigma-Aldrich) were used to quantify levels of tryptase enzyme. These kits are based on the spectophotometric detection of the chromophore p-nitroaniline (pNA) after cleavage from the labeled substrate tosyl-gly-pro-lys-pNA.[26] In brief, ocular surface tear and culture supernatants were incubated with 0.1 mg/mL tosyl-gly-pro-lys-pNA (substrate) for 2 hours at 37°C. A SpectraMax Plus 384 Microplate Reader (Molecular Devices, San Jose, CA, USA) was used to quantify free pNA at 405 nm.
ELISA
Commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to quantify levels of CXCL2 or IL-33 in the ocular surface tear wash and supernatants harvested from mast cell cultures, as per the manufacturer's instructions. Exposure to ethylenediamine-tetraacetic acid (EDTA) at 37°C for 30 minutes was used to separate epithelial and stromal layers from corneas harvested from naïve C57BL/6 mice. Subsequently, isolated epithelium and stromal tissues were lysed to evaluate IL-33 levels by ELISA.
Immunohistochemistry and Histology
For immunohistochemistry analysis, corneas with limbus and bulbar conjunctivae were harvested and fixed with 4% paraformaldehyde to stain with Texas red avidin (Company). Stained corneas were mounted on slides and visualized using a confocal microscope (Leica TCS-SP5; Buffalo Grove, IL, USA) at 5X magnification for the detection of avidin-labeled mast cells. For H&E staining, cross sections were prepared from formalin-fixed whole eye ball harvested at 48 hours post-injury as previously described.[27] Corneal tissue structure was analyzed under bright field microscope (Nikon Eclipse E800; Nikon Instruments, Melville, NY, USA) at 20X magnification.
Slit-lamp microscopy
Slit lamp biomicroscopy (with photographs) was used to evaluate inflammation-induced corneal haze following injury, as previously described.[22,28] PBS-treated injured eyes were used as controls. Corneas were visualized in bright field to examine development of haze at 6 hours following injury (Day 0) as well as on Days 1 and 2 post-injury.
Statistical Analysis
Unpaired two-tailed Student t-tests were used to compare means between two groups. The significance level was set at p < 0.05. Data are presented as the mean ± standard deviation. Results shown are representative of at least three independent experiments. Samples sizes were estimated on the basis of previous studies of corneal injury and inflammation.[21,22,29,30]
Results
Mast cell activation is associated with early neutrophil infiltration following corneal injury
To evaluate the distribution of mast cells, corneas were harvested from C57BL/6 mice and stained with a fluorescent conjugate of avidin.[31] Mast cells were observed predominantly in the peripheral cornea and limbus (Fig. 1A). Next, we investigated the effect of corneal injury on mast cell activation at the ocular surface. Corneal injury was performed using an Algerbrush, with 2 mm diameter trephination of the central corneal epithelium and anterior stroma (Fig. 1B). Tear wash was collected at 3-hours following injury. Naïve mice were used as controls. Mast cell activation was assessed by evaluating two markers of mast cell degranulation; β-hexosaminidase and tryptase.[5] Our data demonstrate that both β-hexosaminidase and tryptase levels in the ocular surface tear wash were significantly elevated in the injured mice relative to naïve (2 fold increase [p = 0.003] and 4.5 fold increase [p = 0.018], respectively) (Fig. 1C & 1D). These data indicate that ocular surface injury triggers mast cell activation.
Figure 1. Mast cell activation is associated with early neutrophil infiltration of the injured cornea.
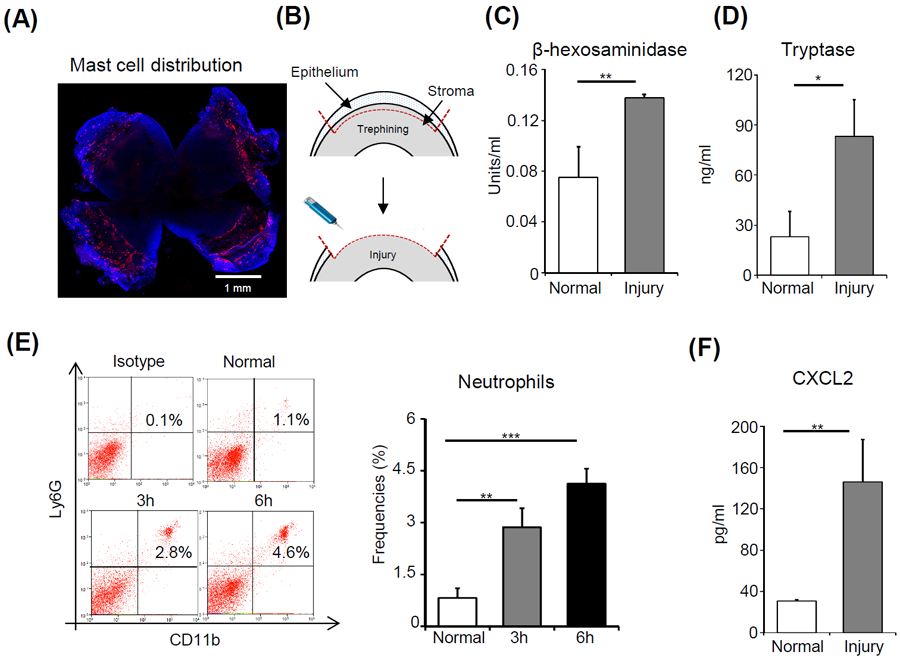
(A) Representative image showing a C57BL/6 murine cornea stained with a fluorescent conjugate of avidin (Texas Red; scale bar: 1 mm). (B) Schematic diagram depicting mouse model of corneal injury. Ocular surface tear wash was collected at 6 hours after injury and mast cell activation markers (C) β-hexosaminidase and (D) Tryptase were estimated relative to naïve mice. (E) Representative flow cytometric dot plots (left) and cumulative bar chart (right) showing the frequencies of CD11b+Ly6G+ neutrophils in the cornea at 3 and 6 hours after injury, compared to normal control animals. (F) Bar chart depicting the secretion levels of CXCL2 in the ocular surface tear wash at 6 hours following injury as estimated by ELISA, relative to normal mice. Representative data from three independent experiments are shown, and each experiment consisted of four to six animals. Data presented are mean ± SD (error bar). *p < 0.05, **p < 0.01, ***p < 0.001.
Next, we investigated neutrophil infiltration into the cornea following ocular injury. Mice were sacrificed at 3- and 6-hours following injury. Single cell suspensions of harvested corneas were prepared and immunostained with CD11b and Ly6G antibodies. Consistent with the results from our previous study,[6] flow cytometry data demonstrated a progressive increase in CD11b+Ly6G+ neutrophil frequencies following corneal injury (3.4 fold increase at 3 hours [p = 0.005] and 5 fold increase at 6 hours [p < 0.001]) (Fig. 1E). The CXC chemokine receptor 2-binding chemokine CXCL2 is recognized as a potent chemoattractant that induces neutrophil recruitment.[17] Accordingly, we evaluated levels of CXCL2 in tear wash at 6 hours following injury relative to naïve controls. Our data show a significant increase in CXCL2 levels following injury (5 fold increase [p = 0.001]). Taken together, our data indicate that corneal injury results in early mast cell activation and neutrophil infiltration to the cornea.
Mast cell deficiency abrogates early neutrophil infiltration
Having demonstrated that corneal injury triggers the activation of mast cells and infiltration of neutrophils, we sought to determine whether activation of mast cells is the cause of the increased neutrophil frequencies observed in the cornea following injury. To investigate this, we employed mast cell-deficient mice (cKitw-sh). The absence of mast cells was confirmed by evaluating frequencies of cKit+FCεR1+ cells in the ocular surface and peritoneum of cKitw-sh mice, with wild type C57BL/6 mice as controls (Fig. 2A). Following the confirmation of mast cell deficiency, corneal injury was performed on cKitw-sh mice. Mice were sacrificed at 6 hours-post injury, single cell suspensions were prepared from harvested corneas, and frequencies of total CD45+ inflammatory cells as well as CD11b+Ly6G+ neutrophils were analyzed by flow cytometry. The frequencies of CD45+ cells observed in the injured corneas harvested from cKitw-sh mice were significantly lower than those detected in injured corneas from C57BL/6 mice (p < 0.03; Fig. 2B). Similarly, the frequencies of CD11b+Ly6G+ neutrophils in corneas derived from cKitw-sh mice were significantly lower (3-fold) than those from wild type controls (p < 0.05; Fig. 2C). However, no significant differences were observed in numbers of total leukocytes (CD45+ cells) and neutrophils (CD11b+Ly6G+) in in the bone marrows of C57BL/6 and cKitw-sh mice (Fig. S1). These data suggest that mast cells play a critical role in promoting early neutrophil infiltration of the injured cornea.
Figure 2. Mast cell deficiency abrogates early neutrophil recruitment.
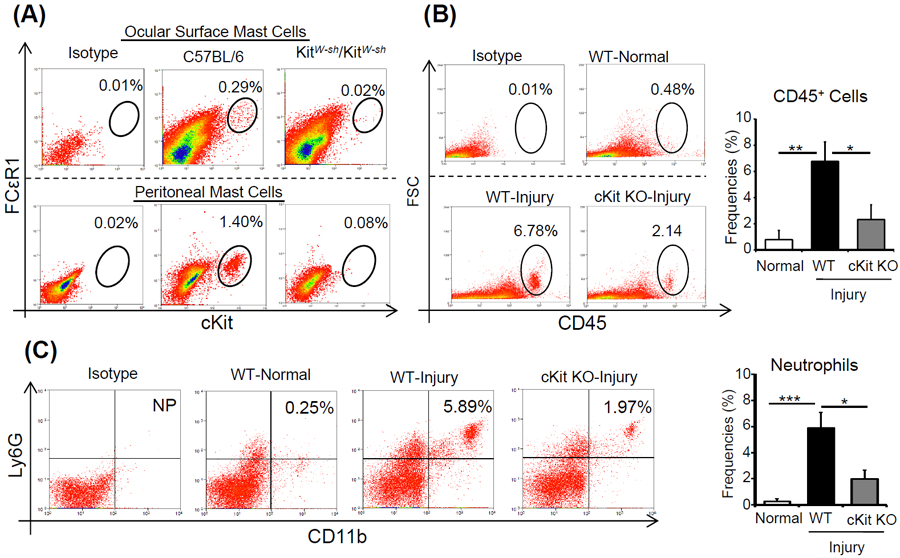
(A) Representative flow cytometry dot plots showing the frequencies of ckit+FcεR1+ mast cells in the ocular surface tissues (upper panels) and peritoneum (lower panels) of cKitW-sh mice, relative to wild type mice. (B) Representative flow cytometry dot plots (left) and cumulative bar chart (right) showing the frequencies of CD45+ inflammatory cells in the corneas of cKitW-sh mice and wild type mice at 6 hours after injury, relative to normal control mice. (C) Representative flow cytometry dot plots (left) and cumulative bar chart (right) showing the frequencies of CD11b+Ly6G+ neutrophils in the corneas of cKitW-sh mice and wild type mice at 6 hours after injury, relative to normal mice. Representative data from two independent experiments are shown, and each experiment consisted of three to four animals. Data presented are mean ± SD (error bar). *p < 0.05, **p < 0.01, ***p < 0.001.
IL-33 activates mast cells to secrete high levels of the neutrophil chemoattractant CXCL2
Having determined that mast cells are critical in the promotion of early neutrophil infiltration following corneal injury, we next investigated the mechanism of mast cell activation at the ocular surface. Due to the cornea harboring very low frequencies of mast cells, we employed bone marrow-derived mast cells that were generated by culturing bone marrow cells with mast cell lineage cytokines, stem cell factor and IL-3 (purity > 95%).[6] We performed in vitro mast cell activation assays in which bone marrow-derived mast cells were stimulated for 6 hours with a variety of pro-inflammatory factors previously reported to be upregulated following ocular surface inflammation (IL-1 β, IL-6, IL-33, IL-36γ, SDF-1α and Substance P).[22,32-37] ELISA analyses demonstrated highest levels of CXCL2 in supernatants of IL-33-stimulated cultures, with negligible expression of CXCL2 observed in unstimulated controls (Fig. 3A). Stimulation of mast cells with other pro-inflammatory factors including IL-1β, IL-6, IL-36γ, SDF-1α and Substance P failed to upregulate CXCL2 expression (Fig. S2). Next, we evaluated whether IL-33 activates mast cells using β-hexosaminidase and tryptase assays. IL-33 stimulation of mast cells resulted in a significant increase in both β-hexosaminidase (p = 0.036) and tryptase levels (p = 0.028), compared to unstimulated controls (Fig. 3B and 3C, respectively). Furthermore, expression of the IL-33 receptor ST2 on mast cells was analyzed using flow cytometry. Our data demonstrate that mast cells constitutively express IL-33R (>99%; Fig. 3D). Collectively these results indicate that IL-33 stimulates mast cells to secrete the neutrophil chemoattractant CXCL2, as well as the markers of mast cell activation β-hexosaminidase and tryptase.
Figure 3. IL-33 activates mast cells and promotes mast cell expression of CXCL2.
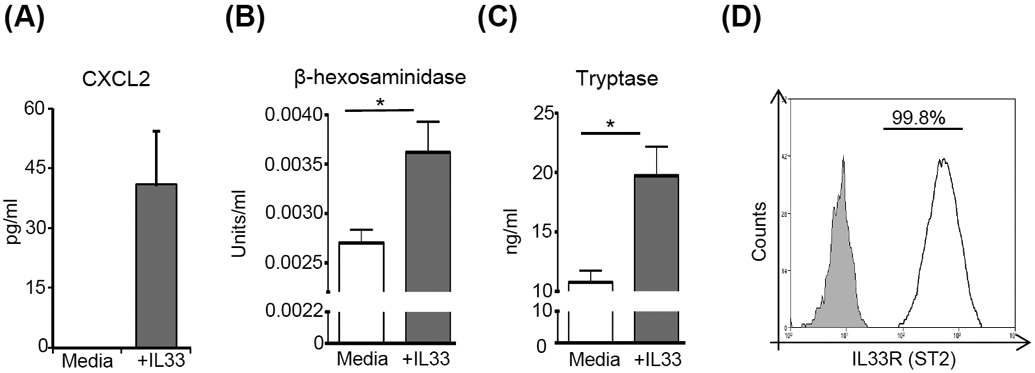
(A) ELISA analysis of CXCL2 levels in the supernatants of mast cells stimulated with IL-33 compared to unstimulated cells. Supernatants from cultures of mast cells stimulated with IL-33 were analyzed for mast cell activation using (B) β-hexosaminidase (C) tryptase. Supernatant from unstimulated mast cells were used as controls. (D) Representative flow cytometry histogram showing the surface expression of IL33R (ST2; White) on mast cells as compared to isotype control (Grey). Representative data from three independent experiments are shown, and each experiment was repeated three times. Data presented are mean ± SD (error bar). *p < 0.05.
Corneal epithelium releases prestored IL-33 during injury
Our next investigation sought to determine whether corneal injury promotes expression of IL-33 at the ocular surface. Whole corneas were harvested at 6 hours post-injury to isolate total mRNA, and expression of IL-33 was evaluated by real-time PCR. Naïve, non-injured corneas were used as controls. Our data demonstrate a substantial increase in IL-33 expression following corneal injury relative to naïve controls (p = 0.033) (Fig. 4A). The upregulated expression of IL-33 following corneal injury was confirmed by analyzing tear washes using ELISA, with a 3-fold increase in IL-33 levels relative to the naïve (p = 0.022) (Fig. 4B). Finally, in order to identify the cellular source of IL-33, corneal epithelial and stromal layers were separated and lysed to quantify IL-33 levels using ELISA. Corneal epithelium was observed to express 4-fold higher levels of IL-33 compared to the stroma (p < 0.001, Fig. 4C), suggesting epithelium to be the primary source of prestored IL-33 released following corneal injury (Fig. 4C).
Figure 4. IL-33 released from the cornea following injury is prestored in epithelium.
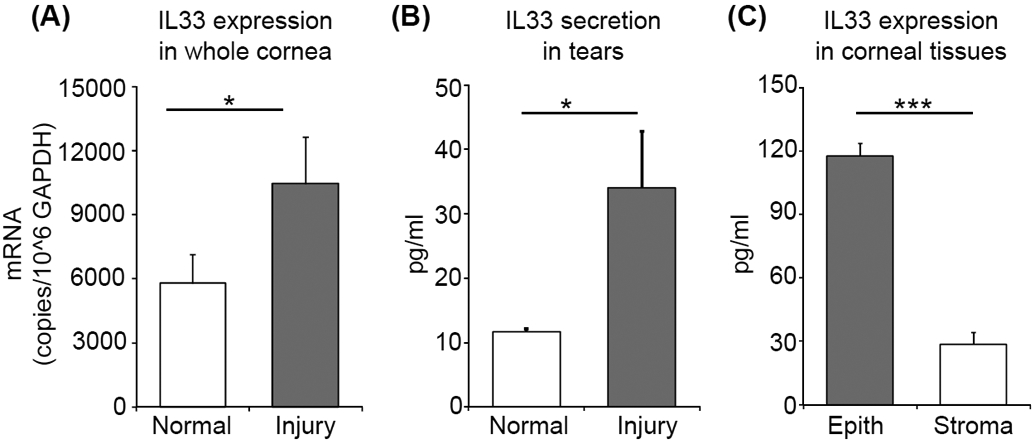
(A) Bar chart depicting mRNA expression of IL-33 in lysate of whole corneas harvested at 6 hours post-injury (normalized to GAPDH) relative to naïive cornea lysate, as quantified by real-time PCR. (B) Bar chart depicting ELISA analysis of IL-33 levels in the ocular surface tear wash at 6 hours post-injury relative to naïive mice. (C) Bar chart depicting ELISA analysis of IL-33 levels in the lysates derived from separated corneal epithelium and stromal tissue lysates. Representative data from three independent experiments are shown, and each experiment consisted of four to six animals. Data presented are mean ± SD (error bar). *p < 0.05, ***p < 0.001.
In vivo blockade of IL-33 suppresses mast cell activation and neutrophil infiltration to the injured cornea
Finally, we investigated whether blockade of IL-33-mediated mast cell activation prevents the early infiltration of neutrophils following corneal injury. Corneal injury was created as shown in Fig. 1B. Anti-IL-33 antibody (1mg/ml) was applied to the cornea at four time points: one hour prior to injury, at the time of injury, as well as at one- and three-hours following injury. Mice treated at the same time points with cromoglycate (a known mast cell inhibitor[38]) or PBS were used as controls. Both topical blockade of IL-33 and administration of cromoglycate inhibited mast cell activation, as demonstrated by a significant reduction in levels of tryptase in the tear wash collected 6 hours following injury (p = 0.002 and p = 0.003 respectively, Fig. 5A). To evaluate early neutrophil infiltration, single cell suspensions were prepared from corneas harvested at 6 hours post-injury, and flow cytometry was performed. Similar to the observed inhibitory effect on mast cell activation, topical application of both anti-IL-33 antibody and cromoglycate limited the infiltration of CD11b+Ly6G+ neutrophils to the injured cornea (p = 0.027 and p = 0.012 respectively, Fig. 5B). To further confirm the inhibitory effect of IL-33 neutralization on corneal inflammation, bright-field images were captured on day 0 (day of injury) as well as on day 1 and 2 post-injury. Topical blockade of IL-33 reduced injury-induced inflammatory haze compared to PBS treated controls (Fig. 5C). Moreover, analysis from corneal cross-sections stained with H&E show that treatment with anti-IL-33 antibody normalize corneal architecture by reducing stromal thickness, edema and disorganization of stromal lamellae, which are associated with injury (Fig. 5D).[39] Taken together, our data indicate that blockade of IL-33 prevents injury-induced mast cell activation, inhibits neutrophil infiltration, and protects against both inflammatory corneal haze and disorganization of tissue architecture following injury.
Figure 5. In vivo blockade of IL-33 suppresses mast cell activation and post-injury corneal inflammation.
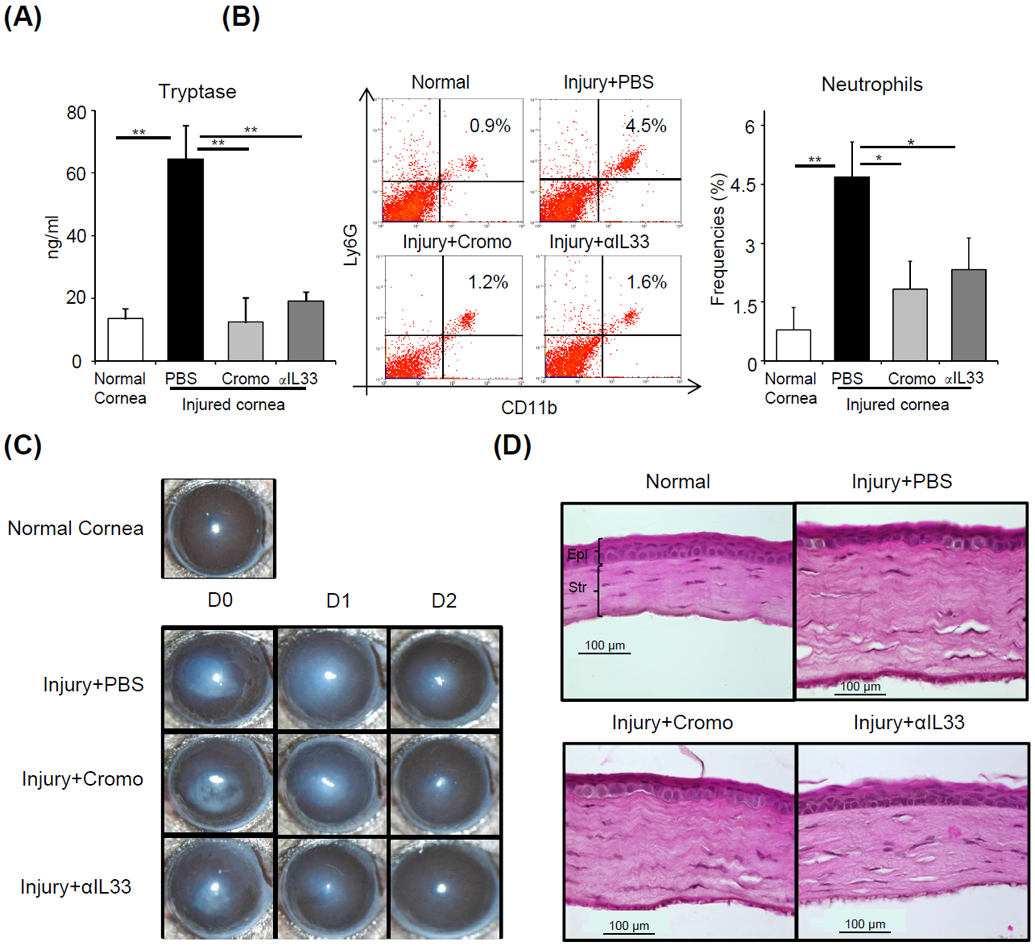
C57BL/6 mice were treated topically with either cromoglycate (2%) or αIL-33 (1 mg/ml) at 1 hour prior to injury, at the time of injury as well as 1 and 3 hours after injury. (A) Bar chart depicting tryptase levels in ocular surface tear wash at 6 hours post-injury in the indicated groups relative to normal mice. (B) Representative flow cytometry dot plots (left) and cumulative bar chart (right) showing the frequencies of CD11b+Ly6G+ neutrophils in the cornea at 6 hours after injury in the indicated groups, relative to normal mice. (C) Representative bright-field microscopic images of corneas of indicated groups at Day 0, 1 and 2 post-injury. (D) Cross-sections stained with hematoxylin and eosin to visualize corneal thickness, edema and stromal lamellae (Epi: epithelium; Str: Stroma; scale bar: 100 μm). Representative data from three independent experiments are shown, and each experiment consisted of four to six animals. Data presented are mean ± SD (error bar). *p < 0.05, **p < 0.01.
Discussion
Neutrophils play vital roles in host defence against noxious pathogens, yet paradoxically, neutrophils are also involved in a range of inflammatory diseases.[40] Indeed, the release of cytotoxic molecules into the extracellular milieu by neutrophils can result in substantial host tissue damage.[41] The functional toll of this collateral tissue damage is clearly evidenced in inflammatory diseases of the ocular surface, where corneal opacification results in loss of vision.[42,43] Previous work by our laboratory[6,24] and others[44] has demonstrated that mast cells are implicated in early neutrophil recruitment, yet the mechanisms that govern mast cell activation and its subsequent neutrophil recruitment following tissue injury have not been defined. Here, using a murine model of corneal injury, we show that: (i) injury-induced mast cell activation is associated with both heightened levels of the neutrophil chemoattractant chemokine CXCL2 and increased neutrophil frequencies; (ii) epithelium-derived IL-33 stimulates mast cells to secrete higher levels of CXCL2 following injury; and (iii) in vivo blockade of IL-33 abrogates mast cell activation and neutrophil infiltration at early time points following injury.
Mast cells are notorious for their role in initiating the allergic immune response by releasing cytokines and chemokines that recruit a variety of leukocytes including eosinophils, basophils and T cells to the site of allergen exposure.[45,46] Recently, using a non-allergic ocular surface inflammation model, we reported that topical application of cromoglycate (a mast cell inhibitor formulated as eye drops for the treatment of allergic conjunctivitis[47]) following corneal injury significantly reduces local infiltration of inflammatory cells.[6] In the current study we developed this work further by employing mast cell-deficient cKitW-sh mice[48] and investigated the mechanisms by which sterile injury of the ocular surface leads to mast cell-mediated neutrophil recruitment. Our data indicate that mast cells secrete CXCL2 at early timepoints following injury, which recruits neutrophils to the injured tissue. Corroborating the importance of mast cell activation in promoting early neutrophil recruitment, our data demonstrate that mast cell deficiency substantially reduces the frequencies of infiltrating neutrophils following corneal injury, relative to wild type controls. We considered the possibility that cKitW-sh mice may have reduced total numbers of neutrophils, and that this might be exerting a confounding effect on our data, but we observed similar frequencies of both total CD45+ immune cells and CD11b+Ly6G+ neutrophils in c-Kit knockout and wild type C57BL/6 mice (Fig. S1).
IL-33, a member of the IL-1 family, is a pleiotropic cytokine that has been shown to modulate type 1, type 2 and regulatory immune responses [49]. In our study, we screened a range of inflammatory mediators known to be released following tissue injury (including IL-1β, IL-6, IL-33, IL-36γ, SDF-1α and Substance P)[22,32-37] for their capacity to stimulate the secretion of CXCL2 by mast cells. We detected substantially higher secretion of CXCL2 following stimulation with IL-33 compared to other candidate factors. IL-33 is known to be released into the extracellular environment during necrosis or tissue injury, where it binds to the receptor complex of ST2 and IL-1 receptor accessory protein.[50] Accordingly, we evaluated the expression of IL-33 at the cornea, and found it to be substantially upregulated following injury relative to naïve controls. This is consistent with reports showing heightened expression of IL-33 following acute kidney injury [51] and acute hepatitis [52]. We traced the source of prestored IL-33 in the cornea, and found it to be primarily stored in epithelial cells. In order to further assess whether the IL-33/ST2 axis drives the mast cell activation following injury, we evaluated the surface expression of ST2 by mast cells, and found near-uniform expression of ST2. Finally to confirm in vivo whether ligation of IL33 with ST2 leads to mast cell activation and subsequent early neutrophil recruitment to the injured site, we topically administered neutralizing anti-IL33 antibody to the injured ocular surface. We observed that IL-33 neutralization substantially reduced both mast cell activation and neutrophil infiltration following injury. We found the scale of these effects to be comparable to topical application of the mast cell inhibitor cromoglycate. Moreover, we observed that blockade of IL-33 resulted in reduced inflammatory corneal haze following injury, and protected against disruption of the corneal tissue architecture.
In summary, these findings provide novel insights on the role of mast cells in ocular mucosal inflammation. Injury to the ocular surface leads to upregulation of epithelium-derived IL-33 that activates mast cells to secrete CXCL2 and initiate neutrophil infiltration. Furthermore, mast cell-deficient mice with injured ocular surface show a significant reduction in neutrophil infiltration. The demonstration of in vivo blockade of IL-33 preventing mast cell activation and neutrophil recruitment to the injured ocular surface, suggest IL-33 could be a potenital thearpeutic target for ocular surface inflammatory disorders.
Supplementary Material
Figure S1. Bone marrows of mast cell deficient and wild type mice shows similar frequency of total immune cells and neutrophils.
Bone marrows were harvested from femurs and tibias of cKitW-sh and wild type mice. Thereafter, single cell suspensions were prepared and analyzed for the frequency of CD45+ immune cells and CD11b+Ly6G+ neutrophils by flow cytometry. (A) Representative flow cytometry dot plots (left) and cumulative bar chart (right) showing comparable frequencies of CD45+ immune cells in the bone marrow of cKitW-sh mice and wild type mice. (B) Representative flow cytometry dot plots (left) and cumulative bar chart (right) showing comparable frequencies of CD11b+Ly6G+ neutrophils in the in the bone marrow of cKitW-sh mice and wild type mice. Representative data from two independent experiments are shown, and each experiment consisted of three to four animals. Data are represented as mean ± SD.
Figure S2. Expression of CXCL2 by mast cells upon stimulation with inflammatory factors released during tissue injury.
Mast cells were stimulated with different inflammatory factors that are known to increase during corneal injury (IL-33, IL-1β, IL-36γ, IL-6, SDF1α and Substance P)[22,31-36]. Representative data from three independent experiments showing increased secretion of CXCL2 with IL33 stimulation, and undetectable expression following stimulation with other factors. Mast cell were derived from the bone marrow of C57BL/6 mice in the presence of IL3 (10 ng/ml) and SCF (50 ng/ml). Representative data from three independent experiments are shown, and each experiment was repeated three times. Data presented are mean ± SD (error bar).
Acknowledgments
Disclosure/conflicts of interest: The authors have no financial conflict of interests. This work was supported by the National Institutes of Health grants R01EY029727 and P30EY003790.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat Immunol 2011;12:1035–44. 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 1997;77:1033–79. 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- [3].Wernersson S, Pejler G. Mast cell secretory granules: Armed for battle. Nat Rev Immunol 2014; 14:478–94. 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- [4].Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CMM, Tsai M. MAST CELLS AS “TUNABLE” EFFECTOR AND IMMUNOREGULATORY CELLS: Recent Advances. Annu Rev Immunol 2005;23:749–86. 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- [5].Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol 2014;5:569 10.3389/fimmu.2014.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sahu SK, Mittal SK, Foulsham W, Li M, Sangwan VS, Chauhan SK. Mast cells initiate the recruitment of neutrophils following ocular surface injury (Under review). Investig Opthalmology Vis Sci 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li M, Mittal SK, Foulsham W, Amouzegar A, Sahu SK, Chauhan SK. Mast cells contribute to the induction of ocular mucosal alloimmunity. Am J Transplant 2019;19:662–73. 10.1111/ajt.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: A multi-functional master cell. Front Immunol 2016;6 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beghdadi W, Madjene LC, Benhamou M, Charles N, Gautier G, Launay P, et al. Mast cells as cellular sensors in inflammation and immunity. Front Immunol 2011. ;2 10.3389/fimmu.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang J Neutrophils in tissue injury and repair. Cell Tissue Res 2018;371:531–9. https://doi.Org/10.1007/s00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosales C Neutrophil: A cell with many roles in inflammation or several cell types? Front Physiol 2018;9 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rungelrath V, Kobayashi SD, DeLeo FR. Neutrophils in innate immunity and systems biology-level approaches: An update. Wiley Interdiscip Rev Syst Biol Med 2019. 10.1002/wsbm.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13:159–75. 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- [14].Mittal SK, Mashaghi A, Amouzegar A, Li M, Foulsham W, Sahu SK, et al. Mesenchymal stromal inhibit neutrophil effector functions in a murine model of ocular inflammation (In press). Investig Opthalmology Vis Sci 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nicolás-Ávila JÁ, Adrover JM, Hidalgo A. Neutrophils in Homeostasis, Immunity, and Cancer. Immunity 2017;46:15–28. 10.1016/j.immuni.2016.12.012. [DOI] [PubMed] [Google Scholar]
- [16].Rock KL, Latz E, Ontiveros F, Kono H. The Sterile Inflammatory Response. Annu Rev Immunol 2010;28:321–42. 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Girbl T, Lenn T, Perez L, Rolas L, Barkaway A, Thiriot A, et al. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity 2018;49:1062–1076.e6. 10.1016/j.immuni.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity 2015;42:1005–19. 10.1016/j.immuni.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant kitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 2005;167:835–48. 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shukla S, Mittal SK, Foulsham W, Elbasiony E, Singhania D, Sahu SK, et al. Therapeutic efficacy of different routes of mesenchymal stem cell administration in corneal injury. Ocul Surf 2019; 17:729–36. 10.1016/j.jtos.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mittal SK, Foulsham W, Shukla S, Elbasiony E, Omoto M, Chauhan SK. Mesenchymal Stromal Cells Modulate Corneal Alloimmunity via Secretion of Hepatocyte Growth Factor. Stem Cells Transl Med 2019:sctm.19-0004. 10.1002/sctm.19-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mittal SK, Omoto M, Amouzegar A, Sahu A, Rezazadeh A, Katikireddy KR, et al. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Reports 2016;7:583–90. 10.1016/j.stemcr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee HS, Chauhan SK, Okanobo A, Nallasamy N, Dana R. Therapeutic efficacy of topical epigallocatechin gallate in murine dry eye. Cornea. 2011;30(12):1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li M, Mittal SK, Foulsham W, Amouzegar A, Sahu SK, Chauhan SK. Mast cells contribute to the induction of ocular mucosal alloimmunity. Am J Transplant 2018. 10.1111/ajt.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML, et al. Hexokinase Is an Innate Immune Receptor for the Detection of Bacterial Peptidoglycan. Cell 2016;166:624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Summers SA, Gan P-Y, Dewage L, Ma FT, Ooi JD, O’Sullivan KM, et al. Mast cell activation and degranulation promotes renal fibrosis in experimental unilateral ureteric obstruction. Kidney Int 2012;82:676–85. 10.1038/ki.2012.211. [DOI] [PubMed] [Google Scholar]
- [27].Amouzegar A, Mittal SK, Sahu A, Sahu SK, Chauhan SK. Mesenchymal Stem Cells Modulate Differentiation of Myeloid Progenitor Cells During Inflammation. Stem Cells 2017;35:1532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Omoto M, Katikireddy KR, Rezazadeh A, Dohlman TH, Chauhan SK. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Invest Ophthalmol Vis Sci 2014. 10.1167/iovs.14-15413. [DOI] [PubMed] [Google Scholar]
- [29].Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med 2014;6:266ra172 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Omoto M, Suri K, Amouzegar A, Li M, Katikireddy KR, Mittal SK, et al. Hepatocyte Growth Factor Suppresses Inflammation and Promotes Epithelium Repair in Corneal Injury. Mol Ther 2017;25:1881–8. 10.1016/j.ymthe.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu J, Fu T, Song F, Xue Y, Xia C, Liu P, et al. Mast Cells Participate in Corneal Development in Mice. Sci Rep 2015;5:1–14. 10.1038/srep17569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ji YW, Mittal SK, Hwang HS, Chang E, Lee JH, Seo Y, et al. Lacrimal gland - derived IL-22 regulates IL-17-mediated ocular mucosal inflammation 2017;10:1–9. 10.1038/mi.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg 1996;224:647–64. 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang S, Zhang H. Upregulation of the IL-33/ST2 pathway in dry eye. Mol Vis 2019;25:583–92. [PMC free article] [PubMed] [Google Scholar]
- [35].Gao N, Me R, Dai C, Seyoum B, Yu FX. Opposing Effects of IL-1Ra and IL-36Ra on Innate Immune Response to Pseudomonas aeruginosa Infection in C57BL/6 Mouse Corneas . J Immunol 2018;201:688–99. 10.4049/jimmunol.1800046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bamdad S, Khademi B, Chenari N, Taseh A, Razmkhah M. Stromal cell derived factor-1, CXCR4 and CXCR7 gene transcripts in pterygia. J Curr Ophthalmol 2017;29:28–32. 10.1016/j.joco.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Suvas S Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J Immunol 2017;199:1543–52. 10.4049/jimmunol.1601751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Altounyan RECE. Review of clinical activity and mode of action of sodium cromoglycate. Clin Exp Allergy 1980;10:481–9. [DOI] [PubMed] [Google Scholar]
- [39].Torricelli AAM, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial-Stromal injury. Exp Eye Res 2016;142:110–8. 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol 1994;56:672–86. 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- [41].Bardoel BW, Kenny EF, Sollberger G, Zychlinsky A. The Balancing Act of Neutrophils. Cell Host Microbe 2014;15:526–36. 10.1016/J.CHOM.2014.04.011. [DOI] [PubMed] [Google Scholar]
- [42].Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ 2001;79:214–21. [PMC free article] [PubMed] [Google Scholar]
- [43].Elsayed EM, Saleh SM, Sabry D, Hassan AM. Causes and clinical manifestations of ocular trauma 2019. 10.4103/DJO.DJO_62_18. [DOI] [Google Scholar]
- [44].De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013; 121. [DOI] [PubMed] [Google Scholar]
- [45].Amin K The role of mast cells in allergic inflammation. Respir Med 2012;106:9–14. 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- [46].Hofmann AM, Abraham SN. New roles for mast cells in modulating allergic reactions and immunity against pathogens. Curr Opin Immunol 2009;21:679–86. 10.1016/j.coi.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Owen CG, Shah A, Henshaw K, Smeeth L, Sheikh A. Topical treatments for seasonal allergic conjunctivitis: systematic review and meta-analysis of efficacy and effectiveness. Br J Gen Pract 2004;54:451–6. [PMC free article] [PubMed] [Google Scholar]
- [48].Grimbaldeston MA, Chen C-C, Piliponsky AM, Tsai M, Tam S-Y, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 2005;167:835–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liew FY, Girard J-P, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol 2016;16:676–89. [DOI] [PubMed] [Google Scholar]
- [50].Scott IC, Majithiya JB, Sanden C, Thornton P, Sanders PN, Moore T, et al. Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage. Sci Rep 2018;8:3363 10.1038/s41598-018-21589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Akcay A, Nguyen Q, He Z, Turkmen K, Won Lee D, Hernando AA, et al. IL-33 exacerbates acute kidney injury. J Am Soc Nephrol 2011;22:2057–67. 10.1681/ASN.2010091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Carrière V, Arshad MI, Le Seyec J, Lefevre B, Farooq M, Jan A, et al. Endogenous IL-33 Deficiency Exacerbates Liver Injury and Increases Hepatic Influx of Neutrophils in Acute Murine Viral Hepatitis. Mediators Inflamm 2017;2017:1359064 10.1155/2017/1359064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Bone marrows of mast cell deficient and wild type mice shows similar frequency of total immune cells and neutrophils.
Bone marrows were harvested from femurs and tibias of cKitW-sh and wild type mice. Thereafter, single cell suspensions were prepared and analyzed for the frequency of CD45+ immune cells and CD11b+Ly6G+ neutrophils by flow cytometry. (A) Representative flow cytometry dot plots (left) and cumulative bar chart (right) showing comparable frequencies of CD45+ immune cells in the bone marrow of cKitW-sh mice and wild type mice. (B) Representative flow cytometry dot plots (left) and cumulative bar chart (right) showing comparable frequencies of CD11b+Ly6G+ neutrophils in the in the bone marrow of cKitW-sh mice and wild type mice. Representative data from two independent experiments are shown, and each experiment consisted of three to four animals. Data are represented as mean ± SD.
Figure S2. Expression of CXCL2 by mast cells upon stimulation with inflammatory factors released during tissue injury.
Mast cells were stimulated with different inflammatory factors that are known to increase during corneal injury (IL-33, IL-1β, IL-36γ, IL-6, SDF1α and Substance P)[22,31-36]. Representative data from three independent experiments showing increased secretion of CXCL2 with IL33 stimulation, and undetectable expression following stimulation with other factors. Mast cell were derived from the bone marrow of C57BL/6 mice in the presence of IL3 (10 ng/ml) and SCF (50 ng/ml). Representative data from three independent experiments are shown, and each experiment was repeated three times. Data presented are mean ± SD (error bar).


