Abstract
Preeclampsia (PE) is a complication of pregnancy characterized by hypertension (HTN-Preg), and often proteinuria. If not managed promptly, PE could lead to eclampsia and seizures. PE could also lead to intrauterine growth restriction (IUGR) and prematurity at birth. Although PE is a major cause of maternal and fetal morbidity and mortality, the underlying mechanisms are unclear. Also, there is a wide variability in the incidence of PE, ranging between 2 to 8% of pregnancies in the Eastern, Western and Developing world, suggesting regional differences in the risk factors and predictors of the pregnancy-related disorder. Several demographic, genetic, dietary and environmental factors, as well as maternal circulating biomarkers have been associated with PE. Demographic factors such as maternal race and ethnicity could play a role in PE. Specific genetic polymorphisms have been identified in PE. Maternal age, parity, education and socioeconomic status could be involved in PE. Dietary fat, protein, calcium and vitamins, body weight, and environmental factors including climate changes and air pollutants could also play a role in PE. Several circulating cytoactive factors including anti-angiogenic factors and cytokines have also been associated with PE. Traditional midwifery care is a common practice in local maternity care units, while advanced perinatal care and new diagnostic tools such as uterine artery Doppler velocimetry have been useful in predicting early PE in major medical centers. These PE risk factors, early predictors and diagnostic tools vary vastly in different regions of the Eastern, Western and Developing world. Further understanding of the differences in the demographic, genetic, dietary and environmental factors among pregnant women in different world regions should help in designing a region-specific cluster of risk factors and predictors of PE, and in turn provide better guidance for region-specific tools for early detection and management of PE.
Keywords: endothelium, hypertension, placenta, preeclampsia, pregnancy
Introduction
Preeclampsia (PE) is a pregnancy-related disorder characterized by hypertension (HTN-Preg) and often proteinuria. If untreated preeclampsia could lead to eclampsia and seizures. PE could also be associated with intrauterine growth restriction (IUGR), premature labor, and low newborn birth weight for gestational age. Although PE is one of the leading causes of maternal and perinatal morbidity and mortality [1], the underlying pathophysiological mechanisms are not clearly understood [2, 3]. Also, the incidence of PE shows a wide range between 2 and 8% of pregnancies worldwide [4, 5], and shows further variability in different countries and world regions [5]. The wide variability in the incidence of PE could be partly related to differences in the risk factors and predictors of PE in different parts of the world. Studies have suggested several demographic, genetic, dietary, environmental, and maternal circulating factors as potential risk factors and predictors of PE [6, 7]. Maternal demographic factors such as race and ethnicity could affect the incidence of PE. Certain maternal genes have been proposed to increase the risk of PE. Maternal age, parity, education and socioeconomic status could contribute to the risk of PE [5, 8]. For instance, pregnant women at very young/older age have higher risk of PE. Dietary fat, protein, calcium and vitamins, body weight, and environmental factors such as climate change and air contaminants could also affect the incidence of PE. For example, pregnant women with higher body mass index (BMI) may be more prone to PE. Importantly, while traditional midwifery care is the main health resource in local maternity units, cutting-edge perinatal care, new diagnostic tools, advanced laboratory tests, and ultrasonography are available in major medical centers. Advanced diagnostic tools have been useful in detecting increased levels of circulating bioactive factors such as anti-angiogenic factors, cytokines, angiotensin receptor antibodies and reactive oxygen species (ROS) in women with PE [9, 10]. Also, Doppler velocimetry often shows uterine artery notching in late PE women. The reliance on these PE risk factors and predictors, and the availability of advanced PE diagnostic tools vary greatly in the Eastern, Western and Developing world.
In this review, we used reports from Pubmed, Medline and the World Health Organization to discuss the incidence and different risk factors and predictors of PE in the Eastern, Western and Developing world. We will first compare the pregnancy outcome data from representative countries in the Eastern world including China and Japan, the Western world including USA and Europe, and the Developing world including the Middle East, Africa and Latin America. We will discuss data gathered from the representative countries by studies conducted between the years 2000 and 2017 and included data from as early as 1967 until 2017, and use these data to compare the average incidence of PE in different regions of the world. We will describe how demographic, genetic, dietary and environmental factors vary among pregnant women in the Eastern, Western and Developing world. We will also describe the various PE biomarkers and diagnostic tools used in clinical practice and in experimental animal models of hypertension in pregnancy (HTN-Preg). We will finally discuss how further understanding of the differences in the demographic, genetic, dietary and environmental factors, and in the availability of advanced diagnostic tools could help in the design of a region-specific cluster of risk factors and predictors of PE, and in turn provide guidance for region-specific tools for early detection and management of PE.
1. Regional Differences in the Incidence of PE
Clinical and epidemiological studies have assessed the incidence of PE among pregnant women in different countries and regions of the world (Fig. 1). Studies from the years 2000 to 2017 that included data from as early as 1967 until 2017, were conducted in different cohorts of pregnant women, and determined the number of PE cases in these cohorts. The incidence of PE was then calculated as the number of PE cases divided by the number of pregnant women in the respective cohort (Fig. 1). In 4 different studies in Eastern countries, the incidence of PE appeared to be lowest in Vietnam (0.2 to 1.19%) and highest in the Philippines (3.6 to 6.3%). In the Western world, 4 studies showed the lowest PE incidence in Spain (0.7 to 1.6%) and the highest incidence in Finland (3.6 to 8.1%). In the Middle East, 4 studies showed that the lowest incidence of PE was in Saudi Arabia (0.9 to 1.3%), the highest incidence was in Iran (3.3 to 7.8%), and the greatest variability in the incidence of PE in Jordan (0.9 to 6.8%). In Africa, 4 studies showed almost similar incidence of PE in most countries (2 to 4%), with the most dramatic variability in DR-Congo (0.8 to 8.5%), and the highest incidence in Africa and among all countries of the world in Ghana (4.4 to 11.8%). In Latin America, the incidence of PE appeared to be high in most of the countries studied, with the most apparent variability in Brazil (1.5 to 8.2%) and the highest incidence in Ecuador (3.5 to 9.6%). Thus while the incidence of PE appears to be somewhat consistent among different studies in Eastern and Western countries, the highest incidences and greatest variability are observed in Developing countries. The large variability in the incidence of PE among different studies makes it important to not rely on only one study, but to study the trends and average incidence of PE from multiple studies.
Fig. 1.
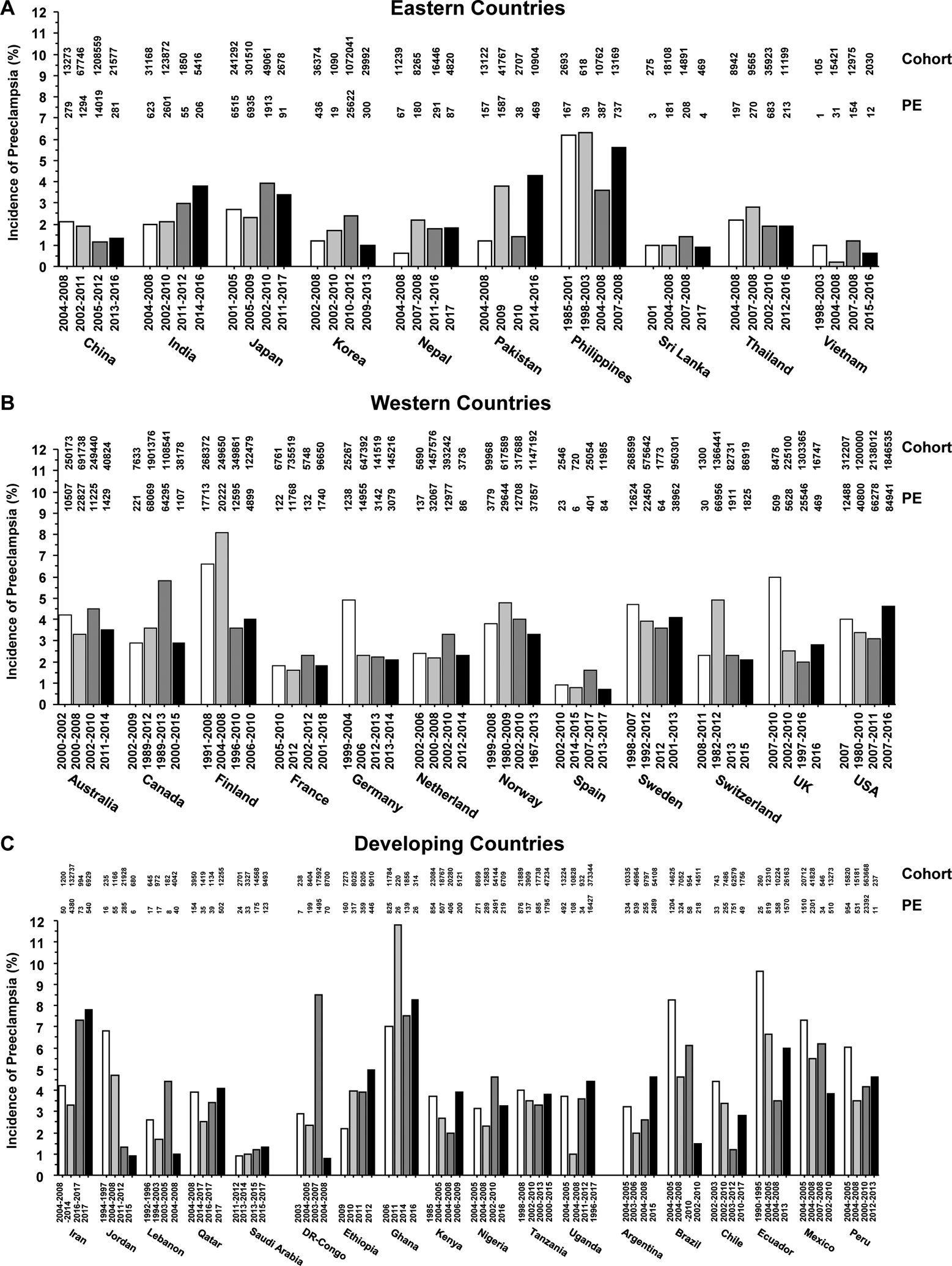
Incidence of PE in representative countries of the Eastern Western and Developing world. The incidence of PE, number of cases of PE, and the number of pregnant women in the cohort from 4 different studies performed between the years 1985 to 2017 in representative countries from the Eastern, Western and Developing world are presented.
We calculated the average incidence of PE from the aforementioned 4 different studies using two approaches. In the first approach, we divided the sum of the number of PE cases from the 4 different studies by the sum number of pregnant women in the 4 respective cohorts (Fig. 2A, 2C, 2E and 2G). In the second approach, we averaged the 4 reported incidences of PE from the 4 different studies to calculate the means±SEM, which allowed us to statistically compare the average incidence of PE in different countries and regions (Fig. 2B, 2D, 2F and 2H). The cumulative data from these 2 different approaches supported the notion that among the Eastern countries the average incidence of PE was lowest in Vietnam and highest in the Philippines. Among Western countries, the average incidence of PE was lowest in Spain (1%) and highest in Finland (5.58%). The calculated incidence of PE in Europe and USA appeared to conform with the general estimates that PE complicates 2 to 5% of pregnancies in the Western world, but showed some differences in specific countries. For example, a previous study has shown that the overall incidence of PE in Norway is 3.5% [11], while the calculated average from 4 different studies suggests that the incidence of PE in this Northern European country is higher (3.97%). In Middle Eastern countries, the average incidence of PE was lowest in Saudi Arabia and highest in Iran. In Africa, the average incidence of PE was almost similar in different countries with an apparent spike in Ghana. Among Latin American countries, the average incidence of PE appeared to be low in Chile, and was largely not statistically different among different countries, except between Mexico and Argentina.
Fig. 2.
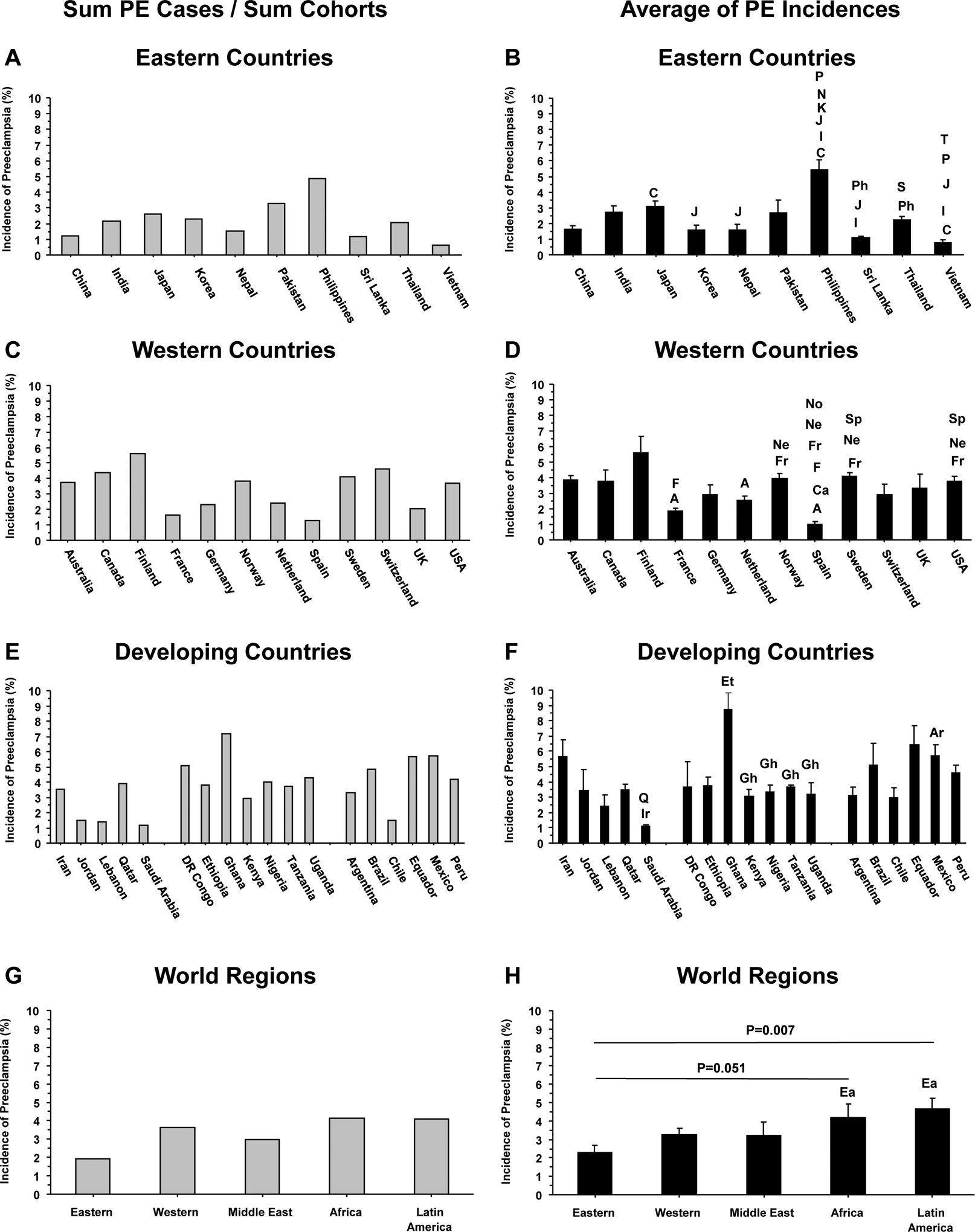
Average incidence of PE in representative countries and in the Eastern Western and Developing world. Data from the 4 studies presented in Figure 1 were used to calculate the average incidence of PE in representative countries and world regions by dividing the Sum number of PE cases by the Sum number of pregnant women in the different cohorts (A, C, E and F), or by calculating the means±SEM of the incidences of PE from the 4 different studies, which allowed statistical comparison of different countries and regions (B, D, F and H). Letters on columns indicate significantly different (p<0.05) from C (China), I (India), J (Japan), K (Korea), N (Nepal), P (Pakistan), Ph (Philippines), S (Sri Lanka), and (T) Thailand in Eastern Countries (B); significantly different (p<0.05) from A (Australia), Ca (Canada), F (Finland), Fr (France), Ne (Netherland), No (Norway), Sp (Spain) in Western Countries (D); significantly different (p<0.05) from Ir (Iran), and Q (Datar) in the Middle East, from Et (Ethiopia), and Gh (Ghana) in Africa, and from Ar (Argentina) in Latin America (F); and borderline (p=0.051) or significantly different (p=0.007) from Ea (Eastern world) in different world regions (H).
When the average incidence of PE was compared by region, the Eastern world appeared to show the lowest incidence (2.27%), with a stepwise increase in the Western world (3.3%) and Middle East (3.22%), and further increase in Africa (4.18%) and Latin America (4.65%) (Fig. 2G, 2H). This is consistent with studies demonstrating that Asian-Chinese, New Zealand-Chinese and Asian-American women have a lower incidence of PE compared with their European, Native American and African-American counterparts [12–14].
A cursory look at the incidence of PE suggests that it is higher in countries with low and middle income populations [15, 16]. Although some studies have suggested that the incidence of PE is higher in countries with low and middle income, the variability observed in these studies and presented in this review does not support such a correlation. For instance, the per capita income in Philippines is higher than that in Vietnam, while PE incidence shows the opposite, with the average incidence of PE lowest in Vietnam and highest in the Philippines. Likewise, in Africa, Ghana shows the highest average incidence of PE at 7.2% and the DR-Congo shows an average PE incidence of 5.1%, but the per capita income is higher in Ghana ($4,650) compared with DR-Congo ($900). Also, Finland has a much higher per capita income ($45,580) and is ranked the 12th country in the Human Development Index (HDI), a summary measure of average achievement in key dimensions of human development including a long and healthy life, being knowledgeable, and having a decent standard of living. Yet, Finland has an average 5.6% incidence of PE, which is much higher than the 0.65% PE incidence in Vietnam with 118th HDI ranking. Additionally, all developing countries in Latin America have a much higher per capita income than any developing country in Africa, yet the PE incidence is almost the same in the two continents. Moreover, the reported incidences of PE do not take into consideration the different regions in each country. For example, in large countries, like Brazil, variation of PE incidence ranging from 1.5% to 8.2% can be related to different levels of development in different regions of the country that are dependent not only on family income, but also healthcare accessibility. These observations suggest the contribution of other factors beyond socioeconomics.
The higher incidence of PE in Africa and Latin America compared with Eastern and Western countries, makes it important to further examine the causes of such disparity. One main reason for the incidence of PE to be as high as 10% of pregnancies in Developing countries could be related to inadequate maternal and prenatal care [4]. This could also be partly related to inadequate keeping of medical records, limited resources and diagnostic tools, and decreased confidence in reported data from clinical and epidemiological studies in Developing countries. Specific demographic, genetic, dietary and environmental factors in the Developing world including maternal age, education, lack/surplus of certain nutrients in diet and BMI could also place pregnant women at higher risk of having PE [6, 9] (Fig. 3). Of note, socioeconomics can influence lifestyle and other demographic factors differently depending on the region and country, and these factors could be superimposed. In the following sections, we will discuss the risk factors and predictors of PE in different countries and world regions, which should help to understand the reasons of the variability in the incidence of PE, and shed further light on the predisposing factors and underlying mechanisms of PE.
Fig. 3.
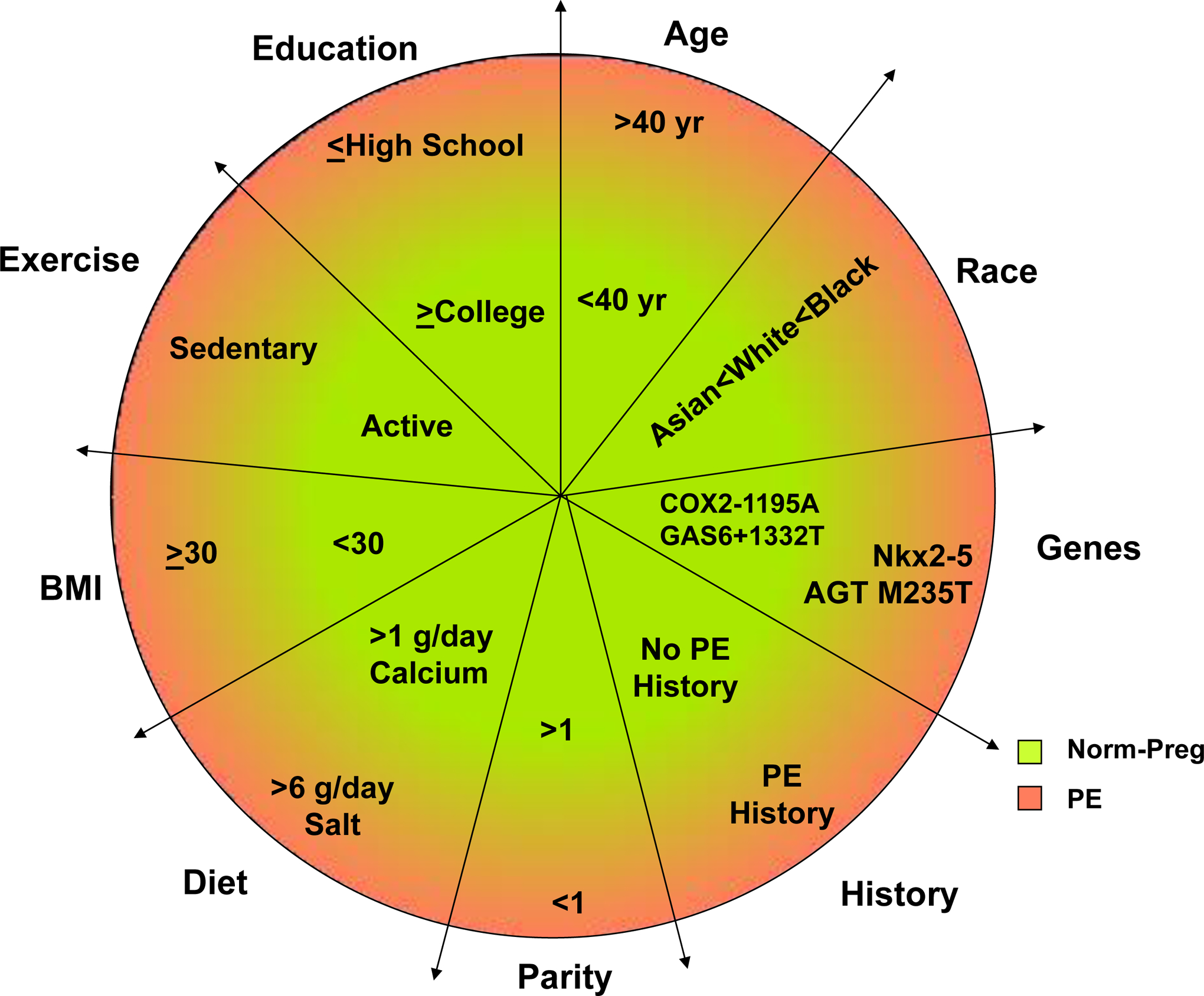
Risk factors of PE. Comparison of demographic, environmental and socioeconomic factors in normal pregnancy (Norm-Preg) and preeclampsia (PE).
2. Demographic and Genetic Factors in PE
2.1. Maternal and Paternal Race
The incidence of PE varies among women of different race, with a higher incidence in African-American (5.2%) than Caucasian, Hispanic (4.0%) and Asian women (3.5%), when controlled for maternal age, parity, education, and gestational age [13] (Fig. 4). Overall, Hispanic and Asian women show a decreased risk while non-Hispanic black women show more severe PE compared with non-Hispanic White women during their first pregnancy [17]. Also, while the overall incidence of HTN-Preg in Asian-American women is 2.72%, Filipino-American women are more likely to experience PE than other Asian ethnic groups [18]. Among Asian-American groups, the incidence of PE is 5.30% in Filipino, 2.80% in Asian-Indian, 2.34% in Korean, 2.21% in Vietnamese, 2.19% in Japanese, 1.41% in Chinese, and 2.98% in other Asian ethnic groups [19]. Also, the rates of PE are higher in Filipinos, Native Hawaiians and other Pacific Islanders, but lower in Chinese and other Asian groups compared with Whites, and this is likely related to maternal age, obesity, and multiple pregnancy [20]. Collectively, while the contribution of race to PE could be better evaluated in more homogenous populations such as in Asia, Africa and the Middle East, the role of race is more difficult to evaluate in a multi-racial and multi-cultural society such as the USA. Therefore, while the average incidence of PE in Asian and African countries could be representative of the whole society, the average incidence of PE in USA should be considered with caution as it may not reflect the racial and ethnic diversity in the society.
Fig. 4.
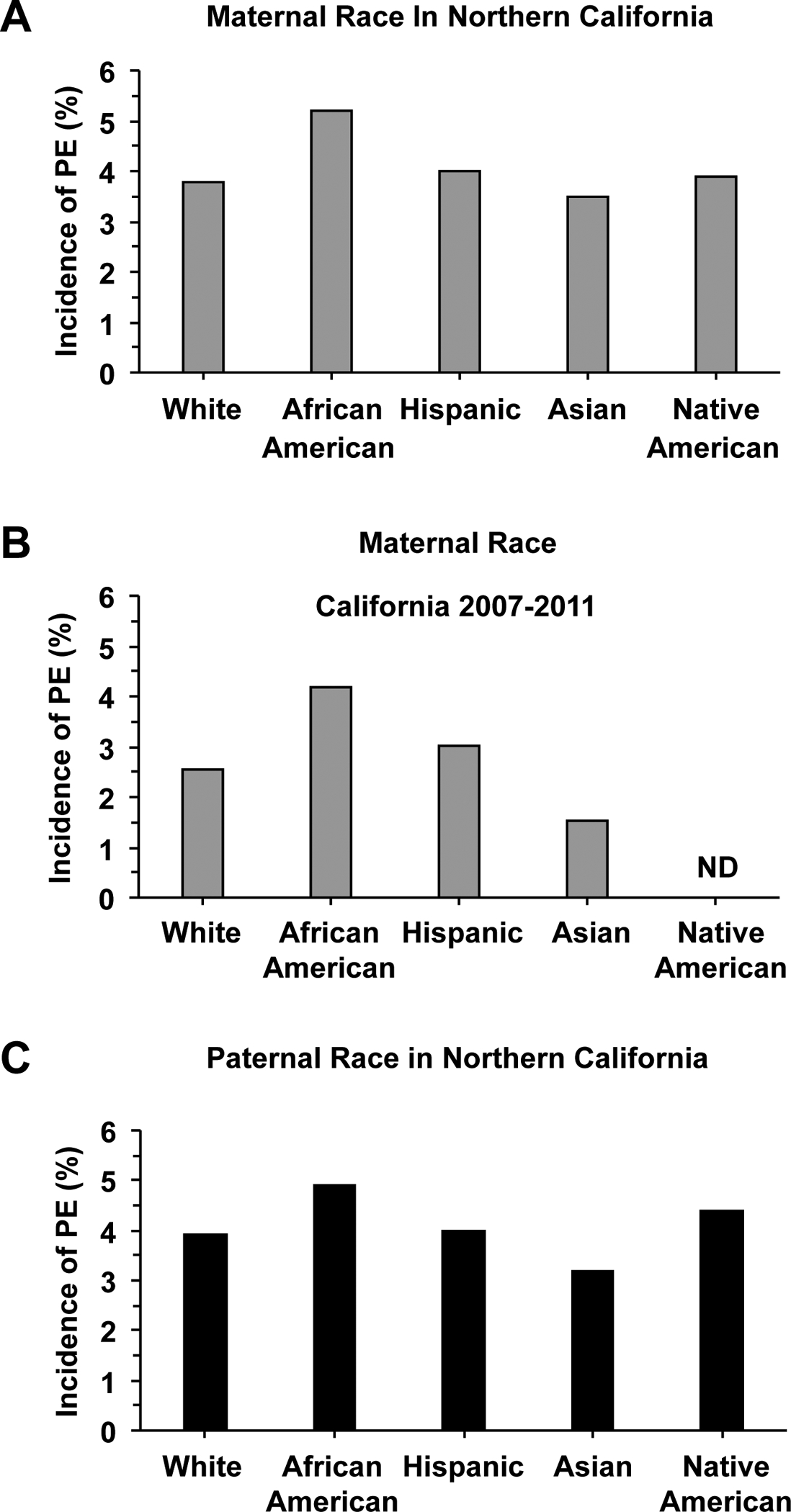
Maternal and paternal ethnicity and the incidence of PE. Comparison of the incidence of PE n different maternal (A, B) and paternal ethnic groups (C) in California, USA. ND, Not determined.
References: [13]
Paternal ethnicity could also play a role in the incidence of PE (Fig. 4). Studies have shown that Asian paternity was associated with the lowest rate of PE (odds ratio [OR] 0.76, 95% confidence interval [CI] 0.68 – 0.85), with an increase in the rate of PE (OR 1.13, 95% CI 1.02–1.26) among parents with different ethnicity compared with those with the same ethnicity [13]. Because of the diverse demographic, cultural, socio-economic characteristics and the different geographic location and environment for different segments of the population in the USA, evaluating the role of paternal ethnicity could be even more complex when compared with a more homogeneous society such as China.
2.2. Hereditary and Genetic factor
Maternal、fetal and paternal genes may play a role in PE [9]. Women and men born from PE pregnancies have higher risk of PE in their own or their partner’s pregnancy, suggesting inheritance of PE susceptibility genes [21]. Differences in the incidence of PE among different races have also supported a role of genes in the racially disparate incidence of PE. Several genes have been implicated in PE including those involved in thrombophilic, vasoactive, immune and metabolic processes as well as cell signaling, and racial differences in genetic variants of PE have been reported [22–24]. Polymorphisms in the methylene tetrahydrofolate reductase 677T, and factor V Leiden mutations among White and Indonesian mothers have been associated with PE [25]. Fetal HLA-C is a major inhibitory ligand for maternal killer cell immunoglobulin-like receptor (KIR) that regulates the cytotoxic activity of natural killer (NK) cells in the placenta. In sub-Saharan Africans, White, and Chinese Han populations, different interactions between maternal KIR gene variants and the genes encoding fetal HLA-C have been associated with PE [26, 27]. The KIR AA genotype is a risk factor for PE in both Europeans and sub-Saharan Africans populations, while different KIR B genotypes were found to protect against PE in both populations [28]. In Caucasian subjects, studies have shown a relation between the frequency of CORIN gene variations and PE [29]. Also, studies in a subset of early onset and severe PE placentae have shown racially disparate expression of the cardiovascular developmental transcription factor Nkx2–5 with Caucasians > African-Americans [30]. Nkx2–5 expression was highly correlated with the mRNA expression of the PE marker sFlt-1, and this correlation was significant in Caucasian, but not African-American women [30]. In Japanese subjects, the TT genotype of angiotensinogen (AGT), heterozygosity of the Glu298Asp variant and homozygosity of the Glu298 genotype of the endothelial nitric oxide synthase (NOS3) gene are independently associated with HTN-Preg [31]. Also, in Dutch women, a M235T polymorphism is associated with a history of elevated blood pressure during pregnancy [32, 33]. In Brazil, the T allele of the rs1319501 of the nicotinamide phosphoribosyl transferase gene is associated with PE [34]. In the Chinese Han population, the G allele of rs-2228570 of the Vitamin D receptor gene、IL-10 −1082A/G and −819T/C、IL-27 rs153109, rs17855750 variants, 11β-HSD2 and KIR genetic variations have been related to PE [35–37]. In contrast, COX2–1195A homozygosity、and variant GAS6+1332 T allele are associated with a decreased risk for PE in the Han Chinese population [38–40].
Although several genes have been linked to high or low incidence of PE, it is not clear how these genes could affect BP and vascular function. Further studies of these genes in different races and in different world regions will further clarify their role in PE.
2.3. Maternal age
PE is more likely to occur at the extremes of reproductive age. The incidence of PE is higher with advanced maternal age [41–43], and is also common in women younger than 20 years of age [44]. Maternal age varies markedly in the Eastern, Western and Developing world (Fig. 5). The maternal age at childbirth has shown substantial increase in many Western countries [45]. The proportion of first births for women aged over 35 years has increased in the United States and Europe (Table 1). The mean age of women at childbirth in Western countries increased from 29.3 years in 2003 to 29.8 years in 2009 [46]. The proportion of women with maternal age ≥35 years is less in Eastern than Western countries. In China, the overall average maternal age is 28.4 years [47]. The relatively young maternal age could partly explain the lower incidence of PE in the Eastern world. However in Developing countries, there is a high teenage pregnancy, which is also associated with higher risks of PE and preterm labor [48]. According to the World Health Organization (WHO) 2010–2011 Multi-country Survey, the percentage of mothers age <20 years old is 14.4% in DR-Congo and 14.6% in the Philippines [49].
Fig. 5.

Maternal age in different world regions. Comparison of maternal age in Eastern world (A), Western world (B), Africa (C), and Latin America (D). ND, Not determined.
References: Eastern World and Latin America [250], Western World [153], Africa [237]
Table 1.
Percentage of pregnant women with maternal age ≥35 years in representative Eastern and Western countries
| Eastern Countries | % Pregnant women age ≥35 years | Western Countries | % Pregnant women age ≥35 years |
|---|---|---|---|
| China (2010–2011) | 9 | Australia (2000–2005) | 20.2 |
| India (2010–2011) | 3 | Canada (2002–2007) | 14.7 |
| Nepal (2010–2011) | 3 | Norway (1999–2006) | 15.9 |
| Philippines (2010–2011) | 15 | Sweden (1997–2006) | 18.1 |
| Thailand (2010–2011) | 15 | UK (1997–2006) | 17.1 |
| Vietnam (2010–2011) | 10 | USA (1998–2007) | 22.0 |
| Average | 9.2±2.2 | Average | 18.0±1.1* |
In recent years, pregnancy has been postponed in Asian cultures and maternal age over 35 years for the first child is not uncommon in Japan [50], Korea [51], and even China [52]. This is attributable to the recent increase in women’s participation in the work force in Eastern countries. On the other extreme, teen pregnancies are common in some Western cultures particularly USA, thus increasing the risk of PE in this young segment of the population.
2.4. Parity
Nulliparity is a risk factor for PE [53], and the incidence of PE is higher in primiparous than multiparous women [19, 54, 55]. Parity varies markedly in the Eastern, Western and Developing world (Fig. 6). In China, due to the one child policy, most pregnant women were nulliparous, which could adversely affect the incidence of PE. In October 2015, China’s one-child policy was changed to a two-child policy, and a large number of women became multiparous, although the maternal age also increased. In comparison, the parity in the Western world has been consistent in the range of 1 to 3. This is because most Western women pursue a career, and the large expense of child-care limits the number of pregnancies. In marked contrast, in some cultures such as the Middle East and Africa, maternal fertility and child-bearing is valued and even rewarded by the society.
Fig. 6.
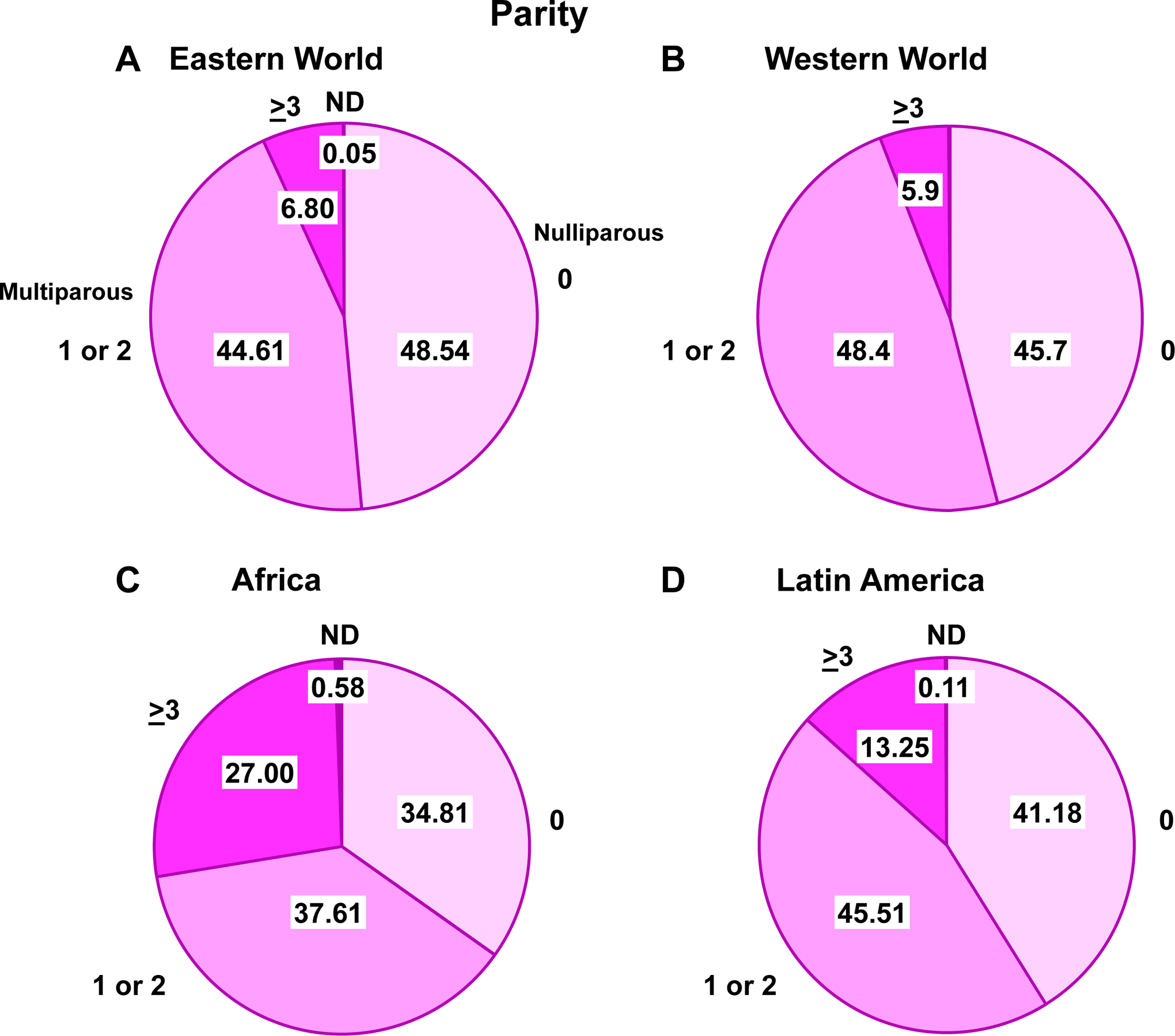
Parity in different world regions. Comparison of the number of pregnancies among women in Eastern World (A), Western World (B), Africa (C) and Latin America (D). ND, Not determined.
References: Eastern World, Africa and Latin America [250], Western World [251]
2.5. Singleton vs multiples pregnancy
Women with multiples pregnancy, e.g. twins, are more likely to develop PE than women with singleton pregnancy [56, 57] (Fig. 7).The relative risk of PE is ~2.8 fold in multifetal over singleton pregnancies [58], and the risk is directly correlated with the number of babies [59]. This could be related, in part, to placental efficiency (i.e. the ratio of fetal weight to placental weight). Also, women who conceive multiples pregnancy through Assisted Reproductive Technologies have a 2.1-fold higher risk of PE than those who conceive naturally [60].
Fig. 7.
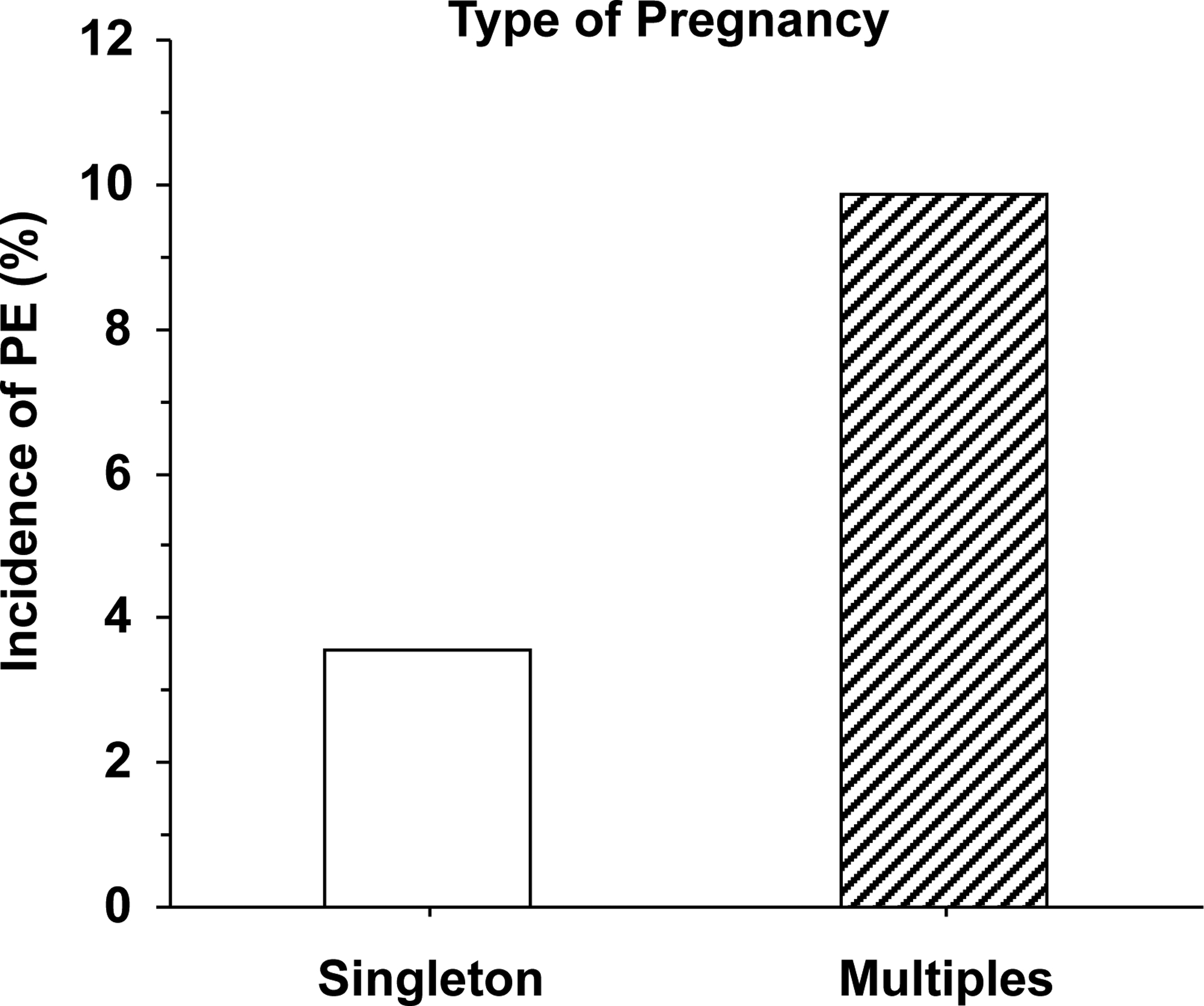
Incidence of PE in singleton versus multiples pregnancy.
Reference: [58]
The reliance of women on Assisted Reproductive Technologies to improve fertility could be related to socio-demographic factors. For instance, Caucasian and older women are more likely to seek Assisted Reproductive Technology. A study in California found that Caucasian and Asian women comprised most (90%), whereas Hispanic women comprised only 8% of all Assisted Reproductive Technology pregnancies [61].
2.6. Maternal Education
The relationship between maternal education and pregnancy outcome has shown marked variability in different regions [62–64]. Post-secondary education is more common in the Western world compared to the Eastern and Developing world (Fig. 8). This is largely because of the marked difference in the standard of living, and the lower income and decreased affordability of higher education in the Developing world. Although the relation between the level of maternal education and the incidence of PE has not been directly studied, maternal higher education may be associated with a lower risk of HTN-Preg [65].
Fig. 8.
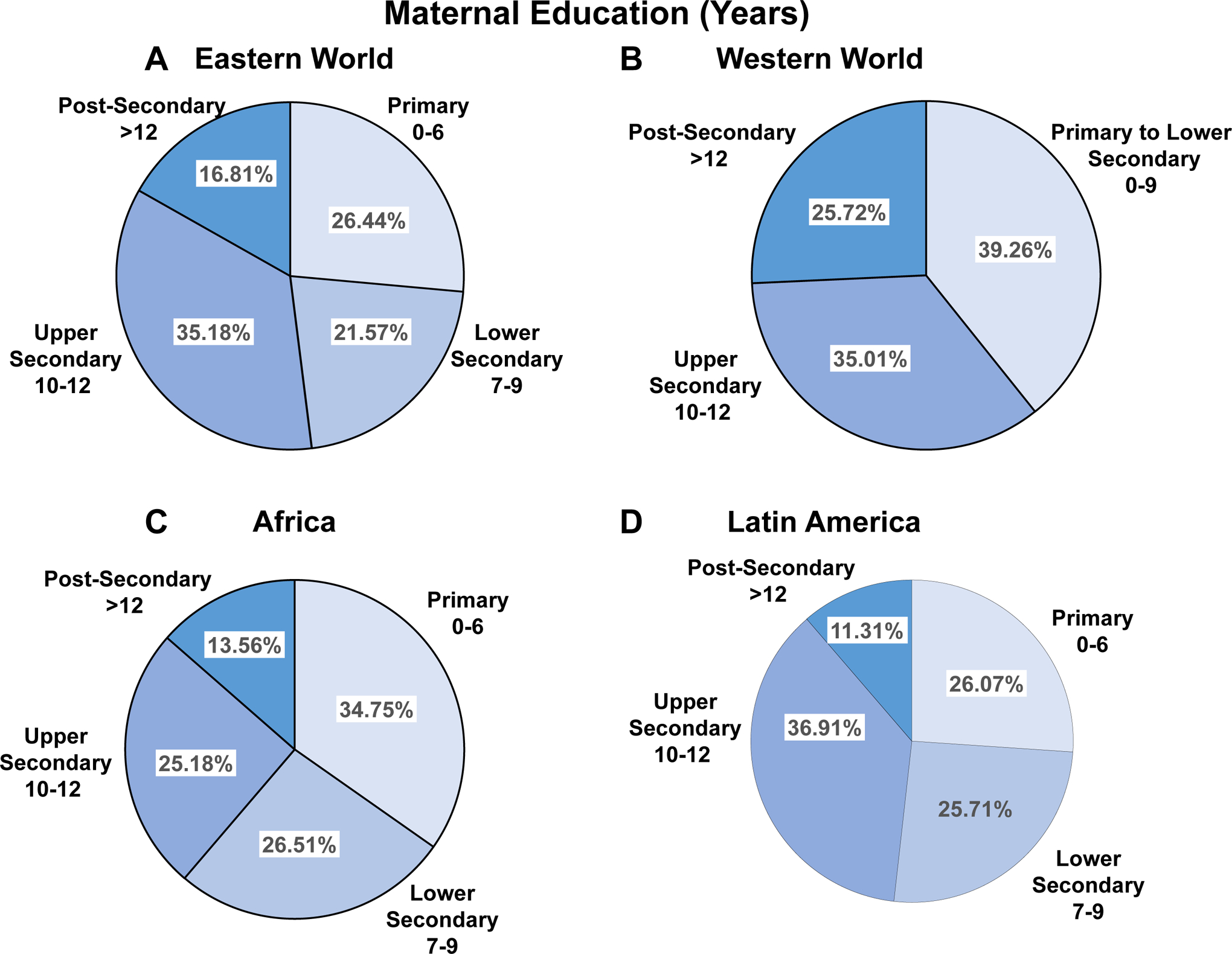
Education level of pregnant women in different world regions. Comparison of the number of years of education among pregnant women in Eastern World (A), Western World (B), Africa (C) and Latin America (D).
References: Eastern World, Africa and Latin America [252], Western World [253]
2.7. Number of Partners
In women without a history of PE, a change of partner is thought to increase the risk of PE [66, 67]. Because of cultural differences, Eastern women may have fewer partners than Western women. For instance, the majority of Chinese women maintain a stable sexual relationship for more than a year before becoming pregnant, and they usually have a long-lasting sexual relationship with the same partner.
3. Dietary and Environmental Risk Factors in PE
3.1. Diet
The incidence of PE may be affected by dietary patterns and nutritional factors. High total energy, high salt, and low dietary magnesium and calcium intake during pregnancy have been related to HTN-Preg [68, 69]. In support, pregnant rats on a high fat diet demonstrate aberrant lipid metabolism and PE-like manifestations. In comparison, diets high in fiber and potassium and plant-derived food seem to reduce the risk of PE [70, 71]. Studies in China and Norway suggest that vegetarian diet is associated with decreased risk of PE [72, 73]. A study among Australian women has shown that implementation of a Mediterranean diet with large vegetable components before pregnancy could help in the prevention of HTN-Preg [74].
Dietary patterns vary markedly in different world regions. The Western diet is higher in salt and sugar compared with that in Eastern countries. In USA, the “meat” dietary pattern is common among Hawaiians, while the “bean” pattern is more common among women with Chinese and Japanese background [75]. Differences in dietary pattern have been found between Chinese and European communities living in the same country [76]. Compared to Caucasians, Chinese migrants have lower energy and fat dietary intakes and consume higher amounts of soy, grains, vegetables and fish, and lower amounts of meat, dairy products, sweets and alcohol than Caucasians. One study has shown that the energy-adjusted total fat intake is at least 10% lower in Chinese and Japanese women than African, Caucasian and Hispanic women [77]. The differences in caloric, salt and meat intake between different ethnic groups may contribute to the differences in the incidence of PE.
Other nutrition factors have shown effects in animal models of HTN-Preg and could play a role in PE. In an animal model of HTN-Preg, a maternal diet containing high levels of folate, vitamin B12 and docosahexaenoic acid (DHA) reduced the risk for cognitive disorders in the adult offspring [78]. Also, a combined supplementation of folic acid, vitamin B12, and omega-3 fatty acid improved placental levels of the anti-inflammatory cytokine IL-10 in a rat model of HTN-Preg, and decreased the levels of the inflammatory cytokine TNF-α in livers of 3 month old offspring [79].
Low dietary calcium less than 700 mg increases the risk of PE, and calcium supplementation for women with low dietary calcium could reduce the risk of PE by 30% to 50% [80]. The potential role of dietary calcium and vitamin D in reducing the incidence of PE has been highlighted in WHO reports (Table 2). Following nutrition data, the WHO issued a recommendation that pregnant women should have calcium supplements to prevent PE. The guidelines recommend daily administration of supplemental calcium and vitamin D from 20 week of gestation. However, these recommendations should be adopted with caution, as the incidence of PE is higher in Western than Eastern countries although diets rich in calcium are more common in Western countries. Also, the recommended dietary intake of vitamin D for pregnant and lactating women may need to be adjusted in different countries. For instance, deficiency in the intake of calcium and antioxidant vitamins is more common and may be responsible for the higher incidence of PE in the Developing world compared to the Western and more developed countries [81]. Pregnant women in Asia, the Middle East, Africa and Latin America are at risk of vitamin D deficiency [82]. A study in Ecuador showed that pregnant women in Ecuador’s Andean area consumed only 60% of the calcium intake recommended by WHO [83]. Thus, if used adequately in different world regions, calcium and vitamin D could be a relatively affordable supplement to reduce the incidence of PE.
Table 2.
Recommended dietary intake of vitamin D (IU) for pregnant and lactating women by different agencies in different countries and conglomerates of countries
| Country/Region | Recommending Agency | Pregnancy | Lactation |
|---|---|---|---|
| Australia and New Zealand | NHMRC 2006 | 200 | 200 |
| China | Chinese Nutrition Society 2013 | 400 | 400 |
| Austria-Germany- Switzerland | DACH 2012 | 800 | 800 |
| Nordic Countries | Nordic nutrition recommendations, Nordic Council of Ministers 2012 | 200 | 200 |
| Central Europe | Polish Scientific Committee on Vitamin D 2013 | 1500–2000 | 1500–2000 |
| Europe | EC 1993 | 400 | 400 |
| USA | Endocrine Society 2010 | 600–2000 | 600–2000 |
| USA and Canada | IOM 2010 | 600 | 600 |
| Worldwide | WHO/FAO 2012/2014 | 200 | 200 |
DACH, Joint Nutritional Society of Germany, Austria and Switzerland; EC, European Commission; FAO: Food and Agriculture Organization of the United Nations; IOM, Institute of Medicine; NHMRC: National Health and Medical Research Council of Australia; Nordic countries, Denmark, Finland, Iceland, Norway and Sweden; WHO, World Health Organization.
Reference: [154]
3.2. Obesity
Obesity is a major risk factor for cardiovascular and metabolic disorders. Increased body mass index (BMI) have been associated with late-onset PE, and studies have suggested that obesity increases the risk of PE [84–87]. The prevalence of overweight and obesity has been increasing in the population of many developed countries particularly USA (Fig. 9). Of note, in USA, obesity varies in different ethnic groups with the largest proportion of obesity among African-American, followed by Latinas and White women [88]. In comparison, overweight and obesity are less common and amount to only 21.2% of pregnant women in mainland China [89].
Fig. 9.
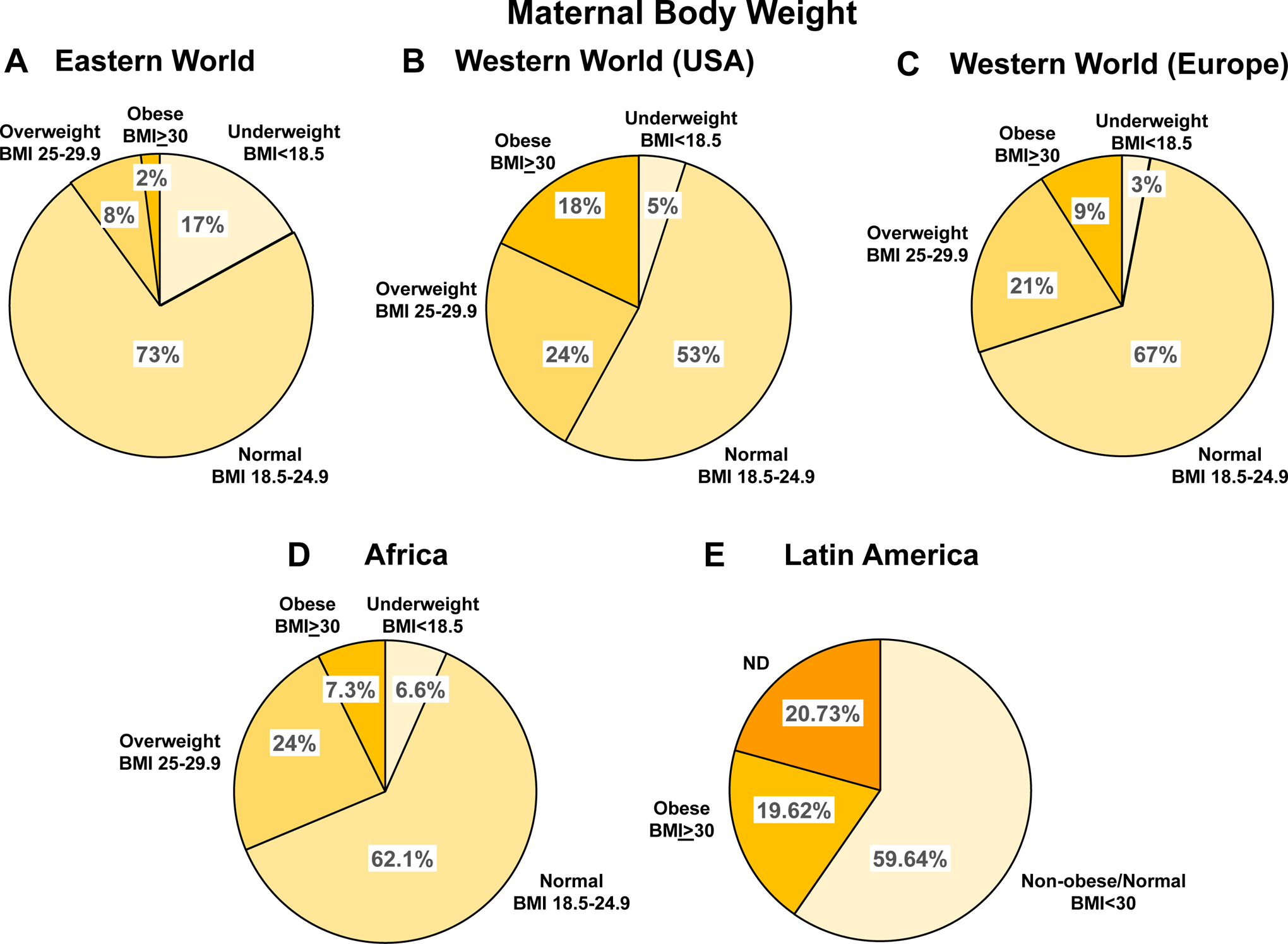
Maternal body weight in different world regions. Comparison of maternal body weight among pregnant women in Eastern World (A), USA (B), Europe (C), Africa (D) and Latin America (E).
References: Eastern and Western World [92], Africa [237], Latin America [250]
Weight gain during the course of pregnancy also varies with race. A study found that weight gain during pregnancy was less in Asians and Blacks than Caucasians [90]. Another study found that white women experienced excessive gestational weight gain (54%), which was more than that in Asian (43%) and Hispanic women (46%) [91]. Women in USA and Western Europe have higher pre-pregnancy BMI and higher rates of gestational weight gain, often surpassing healthy guidelines when compared with women in East Asia [92]. These observations highlight the importance of further studying the interrelationship between obesity, ethnicity, and pregnancy outcomes.
3.3. Exercise
Because overweight and obesity increase the risk of PE, prenatal exercise could reduce gestational weight gain and in turn the incidence of PE. Studies have shown that regular exercise during pregnancy decreases the risk of developing PE [93–96]. Exercise during pregnancy has been suggested to improve placental angiogenesis and endothelial function, and reduce the risk of vascular dysfunction during pregnancy and PE [97].
The benefit of physical activity before and during pregnancy has also been reported in animal models of HTN-Preg [98–100]. Physical activity has been shown to decrease BP and improve the angiogenic profile and placental efficiency in both normal pregnancy and in a rat model of placental ischemia and reduced uterine perfusion pressure [99].
The practice of exercise is different among races. Some studies suggest that black adolescents have less physical activity compared with their white counterparts [101]. Physical activity was lower in non-Hispanic Black or women of other race/ethnic groups than non-Hispanic white women [102, 103]. Some studies in Brazil suggest that pregnant women have low level of physical activity [104]. In China, physical activity is low among pregnant women, likely due to psychological factors such as fear of miscarriage and fatigue. Also, threatened abortion and preterm labor are common in China, which has led many pregnant women to prefer bed rest. Thus in general, Chinese women are more sedentary during pregnancy compared with women in Western countries
3.4. Climate
Seasonal variation in the incidence of PE has been reported in many countries [105–108]. Most studies suggest that women who give birth in Winter are at higher risk of developing PE than women who give birth in warmer seasons. A study from Tygerberg Hospital in South Africa showed that PE occurred in 11.5% of all admissions (1,329/11,585), with the highest incidence in Winter (13.6%) [107]. Women admitted in Winter had a higher risk of developing PE than those admitted in Summer. The risk of developing PE in June (Winter in South Africa) was higher than in February (Summer in South Africa). There was also a correlation between the number of PE cases and the minimum daily temperature [107]. In Europe, countries with colder climate such as Finland and Norway have higher incidence of PE than countries with warmer climate. There could also be an interrelationship between climate and race. A study found that while the incidence of PE decreased in white women during the Summer months, that pattern was not observed in black women [109].
3.5. Air pollution
Exposure to air pollution has been associated with systemic inflammation and oxidative stress, which could affect vascular remodeling and function during pregnancy [110]. Studies found that exposure to certain environmental contaminants may adversely affect cytotrophoblasts and contribute to PE [111]. Exposure to fine particulate matter <2.5 microns, particulate matter <10 microns, and nitrogen dioxide was associated with increased risk of gestational hypertension and PE [112, 113]. A study in China has suggested that prenatal exposure to particulate matter <10 microns and sulfur dioxide increases the risk of PE, and that these effects could be exacerbated by humidity [114]. However, in a large Norwegian pregnant women cohort, no statistically significant associations were found between moderate to low levels of nitrogen dioxide exposure during pregnancy and HTN-Preg or PE [115]. Environmental noise pollution may also be another risk factor for PE [116].
4. Prenatal care and minimizing complications of pregnancy
Prenatal care could minimize complications of pregnancy including PE, gestational diabetes and preterm birth. Guidelines for prenatal care have developed over the years and are now highly recommended for health pregnancy. Advocates for prenatal care recommend integrated maternal and fetal care, screening for diabetes, blood factors, iron deficiency anemia, vaccination history, tobacco or drug use, bacterial infection and sexually-transmitted disease, medical care, vitamins and folic acid supplementation, and counseling and psychosocial support. The number of women receiving prenatal care has steadily increased in the United States and other Western countries. On average, women in Western countries have between 7 to 12 prenatal visits to their family physician or obstetrician [117]. To minimize the incidence of PE, BP is monitored at each prenatal visit, and women are counseled on warning signs of PE. Women with history of chronic hypertension or PE in a previous pregnancy have additional measurements of baseline urine protein, uterine artery Doppler velocimetry, and other laboratory tests for biomarkers of PE [80].
Prenatal care is not well-adopted in developing countries partly because prenatal facilities are either absent or inadequate. Despite the dire economic conditions in many developing countries, pregnant women should be encouraged to use the existing facilities to their best advantage, and governments should provide resources and access to maternal care facilities. Unfortunately, even when prenatal facilities are adequate they are often underutilized because they are very distant or expensive, or due to widespread illiteracy or ignorance. Even literate women may not use prenatal services due to traditional and cultural beliefs and prejudices. Health Departments in developing countries should educate the population about the importance and availability of prenatal programs. With further education, access to information, and collaboration with the developed world, these prejudices should gradually disappear. Pregnant women attitude towards prenatal care could also be improved by further training of already existing and entrusted local traditional birth attendants or midwives, and their integration in the healthcare system [118].
5. Predictors and markers of PE
Apart from delivery of the baby and placenta, there is no effective treatment for PE, making primary and secondary prevention of PE a major public health objective. Screening for women at high risk of PE and providing them with adequate care, could substantially improve the maternal and perinatal outcomes. The International Federation of Gynecology and Obstetrics (FIGO) encourages all countries to adopt and promote PE prediction strategies. The predictive methods range from basic diagnostic tools to advanced biomarkers. Clinical history of PE is an important risk factor of PE. There is also a large number of bioactive factors in the circulation and amniotic fluid that can be used as biomarkers of PE (Fig. 10). Some of these biomarkers have also shown similar pattern in animal models of HTN-Preg (Fig. 10). Uterine artery Doppler can also be used to predict PE. The reliance on these different methods in predicting PE varies in Western countries compared to Eastern and Developing countries.
Fig. 10.
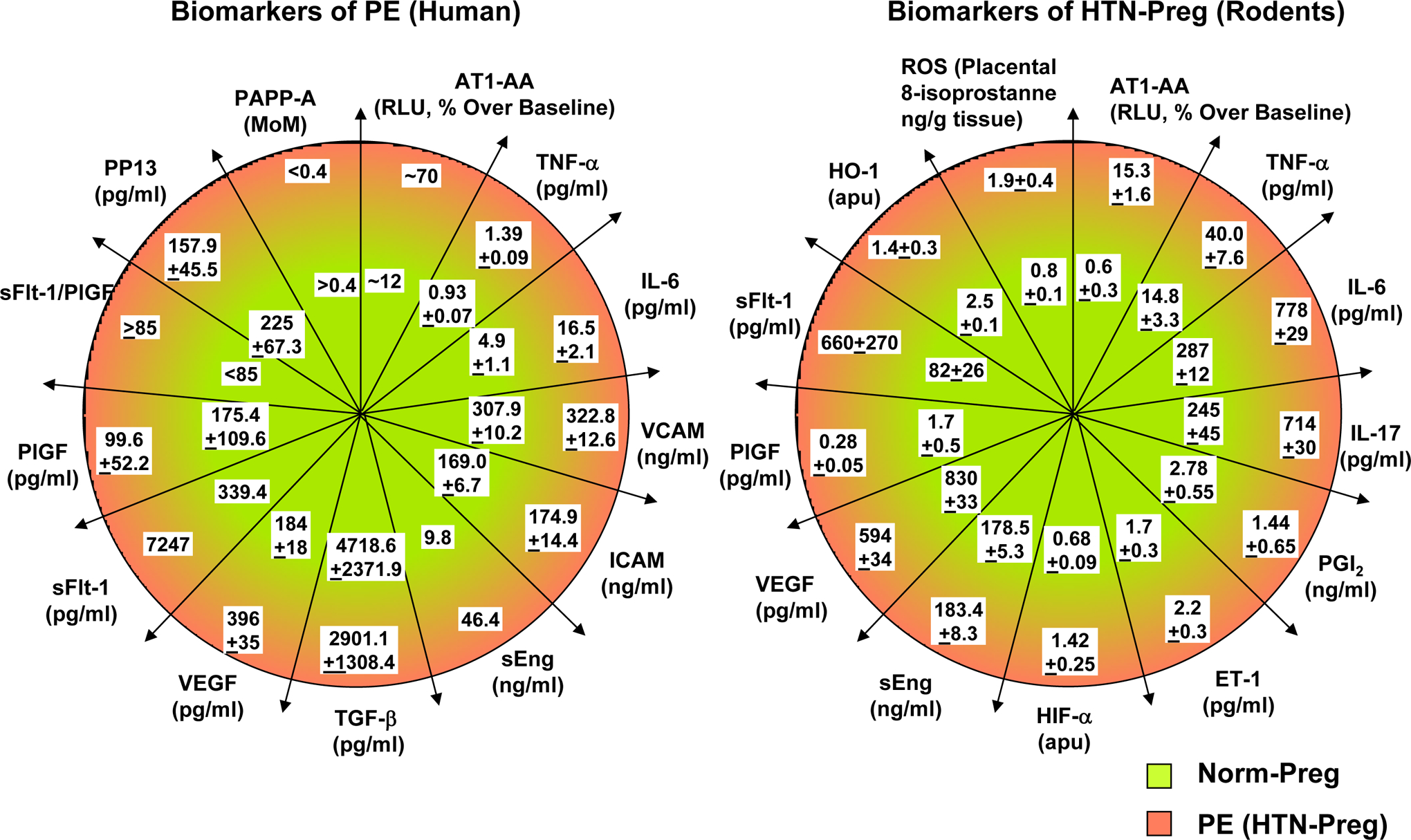
Biomarkers of PE and HTN_Preg. Comparison of biomarkers in Norm-Preg and PE women (A), and in Norm-Preg and HTN-Preg rodents (B).
AT1-AA, angiotensin type 1 receptor agonistic autoantibody; ET-1, endothelin-1; HIF-α, hypoxia inducible factor-α; HO-1, hemeoxygenase 1; ICAM, intercellular adhesion molecule; IL-6, interleukin-6; MoM, multiple of the median; PAPP-A, pregnancy-associated plasma protein-A; PGI2, prostacyclin; PlGF, placental growth factor; PP13, placental protein 13; sEng, soluble endoglin; ROS, reactive oxygen species; sFlt-1, soluble fms-like tyrosine kinase-1; TGFβ, transforming growth factor-β; TNF-α, tumor necrosis factor-α; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor,
5.1. Clinical history and early diagnosis of PE
History of PE in a previous pregnancy is a risk factor of PE. A study found that the risk of recurrent PE is 14.7% in the second pregnancy and 31.9% in the third pregnancy among women with a history of PE [119]. Family history of PE or a cardiovascular disease is also a risk for PE. History of chronic hypertension, renal disease and diabetes mellitus is also considered in determining the risk of PE. As described above, other genetic, demographic and lifestyle factors such as maternal age and weight, different partners, and high salt diet could also be considered in PE risk prediction. If a pregnant woman is positive for these predictors, additional signs and symptoms are needed for early diagnosis of PE.
According to the guidelines of the American College of Obstetric and Gynecology (ACOG), the following signs and symptoms could be used for early diagnosis of PE:
Systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg on 2 occasions at least 4 hours apart after 20 weeks of gestation.
Proteinuria (≥300 mg per 24 hour urine collection, protein/creatinine ratio ≥0.3, or dipstick reading of 1+).
With high BP alone and even in the absence of proteinuria, PE can still be diagnosed if any of the following conditions is present. Evidence of other maternal organ dysfunction, including new development of renal insufficiency, liver involvement with or without right upper quadrant or epigastric abdominal pain; neurological complications (e.g. eclampsia, altered mental status, blindness, stroke, clonus, severe headaches, and persistent visual scotomata); hematological complications; or uteroplacental dysfunction (such as fetal growth restriction, abnormal umbilical artery Doppler waveform analysis, or stillbirth).
These guidelines for PE have evolved over the years and could vary among different countries. In 2010, The National Institute for Health and Care Excellence in the United Kingdom introduced evidence-based guidelines on the diagnosis and management of HTN-Preg, birth, and the postnatal period (www.nice.org.uk/guidance/cg107). In USA, the classification scheme of HTN-Preg adopted by ACOG in 2013 comprised four categories, i.e. chronic HTN that predates pregnancy, PE-eclampsia, chronic HTN with superimposed PE, and nonproteinuric gestational HTN [120]. In 2014, the Society of Obstetricians and Gynecologists of Canada released revised recommendations for HTN-Preg on the basis of literature reviews and criteria from the Canadian Task Force on Preventative Health Care [121]. The Society of Obstetric Medicine of Australia and New Zealand has expanded its definition of chronic HTN [122].
While these guidelines are readily available from various health agencies, they have not been fully implemented in different countries and world regions. As described in WHO’s 2014 consultation on quality of maternal and newborn care: “… there has been limited progress in improving maternal and pediatric outcomes because of a major gap between coverage and the quality of care provided in health facilities”. For instance, despite the issuance of these PE diagnosis criteria, the adoption of these guidelines is low in some African countries. In sub-Saharan African countries, a survey found less than a quarter (24%) of women admitted to labor and delivery services were asked about signs of PE or eclampsia, ranging from 11% in Mozambique to 34% in Kenya and Ethiopia. Only 77% had their BP checked upon admission, and less than half (46%) underwent testing for proteinuria [123].
5.2. Biomarkers and biochemical profile of PE
Studies have suggested multiple screening biomarkers for PE including angiogenic and antiangiogenic factors involved in placental development, markers of endothelial dysfunction, inflammatory cytokines, and angiotensin receptor antibodies [124, 125] (Fig. 10). Some of the biochemical indexes closely related to the onset of PE include increased sFlt-1/PlGF ratio, soluble fms-like tyrosine kinase-1 (sFlt-1) levels, free vascular endpothelial groth factor (VEGF), soluble endoglin (sEng), and cell-free fetal DNA (cffDNA), and decreased placental growth factor (PlGF), placental protein 13 (PP13) and pregnancy-associated plasma protein A (PAPP-A) [126–128]. Maternal circulating sFlt-1 and sEng are increased and free PlGF is decreased several weeks prior to the signs and symptoms of PE [129–131]. Among these biochemical factors, the sFlt-1/PlGF ratio shows certain accuracy in predicting PE [132, 133]. Some of the biomarkers of PE in human show a similar pattern in HTN-Preg rodents [6] (Fig. 10).
The levels of circulating biomarkers could show some racial/ethnic differences. A study found that the associations between low levels of PlGF and high levels of sEng and early-onset PE were stronger in Whites and Hispanics than in Blacks. Higher sFlt-1 (or sVEGFR-1) levels were associated with increased risk of early-onset PE only in Hispanic and White women [134]. Racial/ethnic differences have also been reported in tumor necrosis factor-α (TNF-α). A pro-inflammatory pattern with increased TNF-α biological activity was more common among African-American compared to Caucasian women [135].
5.3. Uterine artery Doppler velocimetry (notching)
Uterine artery Doppler waveform analysis has been proposed as a predictive marker for late PE and fetal growth restriction. PE is predicted from either the presence of bilateral uterine artery early diastolic notches or a mean pulsatility index above 95% for gestational age (Fig. 11). Although uterine artery Doppler is a convenient and noninvasive tool, its use as the sole predictive test for PE has shown poor accuracy [136]. On the other hand, predictive models combining first-trimester uterine artery Doppler waveform analysis with maternal characteristics and biochemical markers, can achieve a detection rate of over 90% for early-onset PE [137].
Fig. 11.
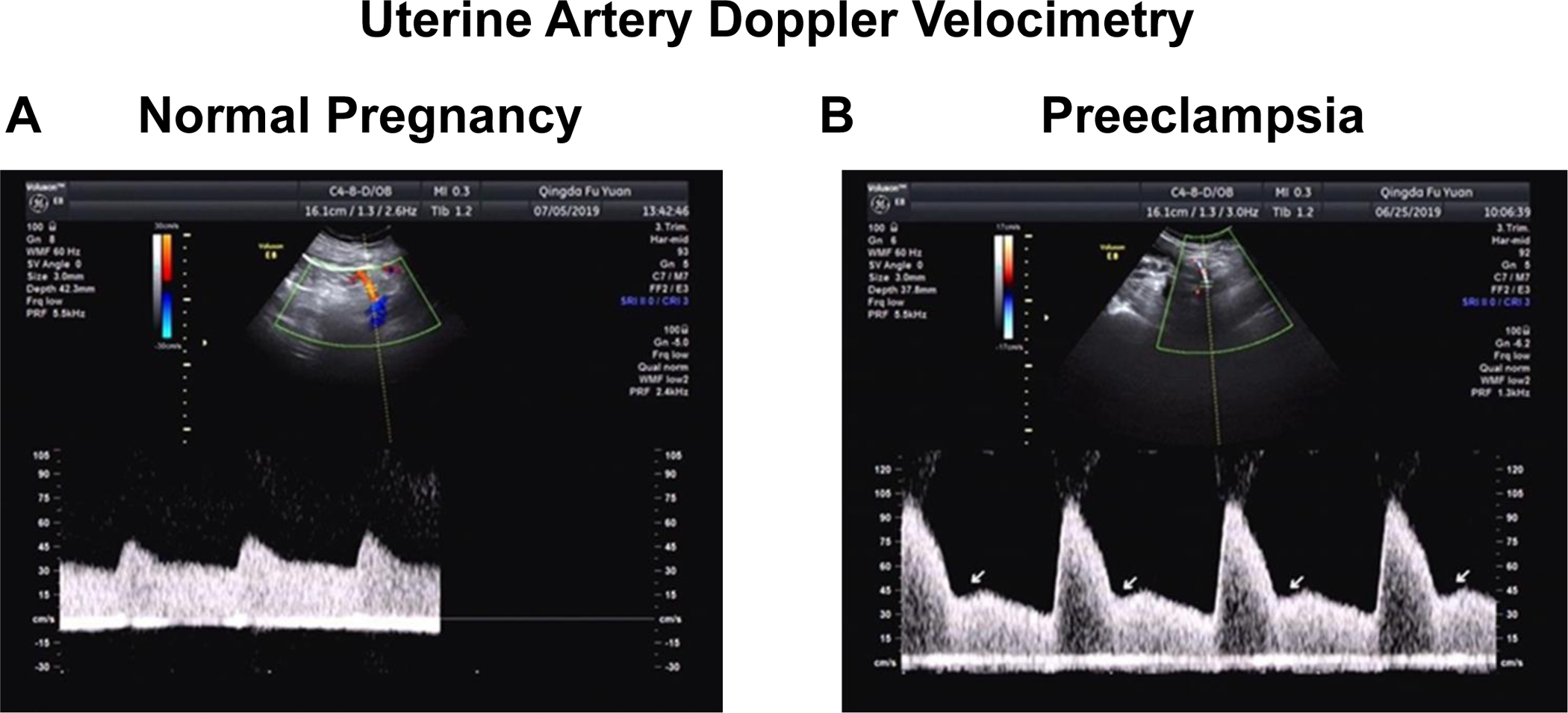
Uterine artery Doppler velocimetry in normal versus preeclamptic pregnancy. Representative uterine artery Doppler velocimetry obtained from normal pregnant (A) and preeclamptic women (B) in the second trimester. PE is suspected by either the presence of bilateral uterine artery early diastolic notches (arrows) or a mean pulsatility index above the 95th percentile for gestational age.
5.4. Brachial artery flow-mediated dilation (FMD)
Brachial artery FMD has been used for evaluation of endothelial cell function in healthy subjects and individuals with cardiovascular disease. Because systemic endothelial dysfunction has been identified as one of the main pathophysiological events in PE, FMD could be used to predict PE [138]. FMD is a cost-effective, convenient and non-invasive method to measure endothelial function in PE women. A study has reported that the use of FMD to assess endothelial dysfunction in patients with PE and as a tool to predict the clinical onset of the disorder have shown promising results [139].
5.5. Cell-free fetal RNA/DNA
Amniotic fluid cell-free RNA (cff-RNA) has been used widely in diagnosing chromosome abnormalities. Recent research suggests potential predictive value of analyzing the amniotic fluid transcriptome in PE. Studies have demonstrated that RNAs associated with the ribosome pathway, insulin-like growth factor B, and ubiquitin C are upregulated more than 10-fold in PE compared with control pregnant women [140], suggesting that these genes could be used as predictive biomarkers for PE in early pregnancy.
Cell-free fetal DNA (cffDNA), a product of normal placental apoptosis, has also been evaluated as a screening tool for PE. Increased levels of cffDNA have been observed in patients before the onset of symptoms of PE, purportedly from increased placental hypoxia and apoptosis [141]. A disadvantage of cffDNA analysis is that it may be reliable only from the beginning of the second trimester. Also, the procedure may not gain popularity among patients and healthcare practitioners due to its invasive nature.
Considering the complexity of the pathogenesis of PE, it is not possible to use a single predictor or biomarker to predict the disorder, and a combination of these factors could provide more accurate prediction. The use of clinical risk factors combined with biochemical profile and biomarkers can improve their predictive value in PE [142]. Among the different biomarkers, the sFlt-1/PlGF ratio is considered a clinically useful predictor of the risk of PE. Studies have also recommended a combined and feasible test that includes evaluation of maternal history and risk factors, measurement of mean arterial pressure, measurement of biomarkers such as serum PlGF, and assessment of uterine artery pulsatility index [143].
6. Complications and management of PE
If not managed properly, PE could lead to eclampsia with severe hypertension, headache, visual disturbances and seizures. Because the pathophysiology of PE is not clearly understood, prevention and management of PE have eluded healthcare practitioners for decades.. Interestingly, the rate of recurrent PE among women with a history of PE decreased by 30% after the release of the US Preventive Services Task Force recommendation of aspirin for PE prevention [144]. However, the use of aspirin as preventive measure of PE is not universally accepted. Instead, management of PE is usually focused on control of acute HTN, prevention of seizures, and timely delivery of the fetus. In patients with late-onset PE, delivery is an effective way to treat the disorder [145]. Patients with early-onset PE need further evaluation and weighing of available options. The risk of wait-and-see approach versus rapid delivery of the baby must be carefully balanced. These options may have different weights and may be considered differently in various regions and countries.
Most of the PE patients in China deliver by Caesarean section compared to induced labor in Western countries. This is likely because in China and the Eastern world especially in rural areas, pregnant women do not have adequate education or financial resources with limited access to prenatal care. In many instances, Eastern women do not have prenatal care and plan to give birth at home with the help of the local midwife. Eastern women usually do not go to the hospital unless they feel sick, with symptoms such as headache, upper abdominal pain, or visual disturbances, suggesting advanced stages and complications of PE. In comparison, pregnant women in the Western world have a better healthcare system and better access to perinatal care. Thus while the overall incidence of PE is higher in the Western than the Eastern world, due to advanced perinatal care the complications of PE are less common in Western countries with the PE maternal fatality rate at 1% or less.
Magnesium sulfate (MgSO4) has been recommended internationally as the first‐line drug for treatment of severe PE and eclampsia. WHO and other international organizations recommend two MgSO4 regimens for eclampsia prophylaxis, intramuscularly and intravenously [146]. However, the use of MgSO4 in clinical practice for prevention and treatment of eclampsia varies widely. One survey including 147 health facilities in 15 countries across Africa, Latin America and Asia has shown that intramuscular maintenance regimens were more commonly used in the African region (45.7%) than in Latin American (3.0%) and Asian regions (22.9%), whereas intravenous maintenance regimens were used more often in Latin American (94.0%) and Asian regions (60.0%) than in the African region (21.7%) [147].
The availability of MgSO4 in health facilities also varied in different countries [123]. In a study of health facilities, the labor and delivery wards that carried MgSO4 were 16% in Ethiopia and 55% in Madagascar. Also, the availability of MgSO4 in health centers varied, from 4% in Rwanda to 96% in Mozambique. The causes of these variabilities could be related to the specific Ministry of Health policy which did not allow the use of MgSO4 at lower level facilities in Ethiopia and Rwanda. Also, a study in Pakistan found that health care workers, in particular older generation physicians, felt that using MgSO4 outside facilities with intensive care units was unsafe [148].
7. Other Considerations
Despite the variability in the incidence, risk factors and predictors of PE highlighted in this review, certain limitations should be considered. First, the review evaluated the incidence of PE in representative countries in the Eastern, Western and Developing world. Inclusion of a larger number of countries could be more representative of the incidence of PE in a specific world region. Second, the incidence of PE in different countries was evaluated in different years and time periods. This is particularly limiting because the incidence of PE is expected to change with the economic development and advances in medicine and technology over time. Ideally, evaluation of the incidence of PE in different countries and world regions should be compared over the same period of time. Third, although we have attempted to provide a complete picture of the incidence of PE in different countries, data from some countries have been incomplete, inadequate or not reliable. While the incidence and predictors of PE have been extensively studied and well-documented in the Western world, access to adequate maternal data is somewhat limited in developing countries and in rural areas. The PE data could also be limited in non-English speaking countries, where scientific data are published mainly in local journals with limited access to world-wide readers.
8. Perspective
The incidence of PE varies in different world regions and in different countries of the same region, and the causes of this variability involve multiple demographic, genetic, dietary and environmental factors. Generally, the incidence of PE is less in the Eastern than the Western and Developing world. In the Eastern World, the incidence of PE is highest in the Philippines likely due to the advanced maternal age and variants of the VEGF-A and VEGFR1 gene [149]. In the Western World, the incidence of PE is higher in Norway and Finland possibly due to the seasonal extremes in both temperature and hours of daylight as these countries are stretched north-south over a vast span of latitudes, and far from the equator [150]. The incidence of hypertensive disorders of pregnancy and PE is higher in Africa and Latin America likely due to early pregnancies, maternal anemia and infections, low socioeconomic status, limited access to healthcare services, and lack of advanced tools and capabilities to predict and diagnose PE in pregnant women at high risk and at early stages of the disorder [151, 152].
Several approaches are needed to reduce the incidence of PE. Because of the high incidence of PE in low income countries, greater efforts should be made in these countries to raise awareness of the benefits of early prenatal visits, improve maternal education, enhance the hospitals perinatal care, and provide advanced technologies to collect and analyze PE data and risk factors. Also, significant efforts should be made to enhance the perinatal life style and promote low-salt diet, calcium supplementation, exercise, and appropriate weight gain in pregnancy, and treat any underlying health conditions such as cardiovascular, metabolic, and kidney diseases. Additionally, diagnostic guidelines and criteria and advanced diagnostic tools should be made available in different regions particularly in rural areas. Baseline screening measures should include evaluation of various maternal risk factors, and measurements of mean arterial pressure, and should be confirmed with serum PlGF, and uterine artery pulsatility index. Future research should further examine the relationship between gene polymorphisms, maternal lifestyle and demographic and environmental factors risk factors of PE. These efforts should help design region-specific clusters of risk factors, diagnostic tools and approaches for prevention and management of PE
Acknowledgements
This work was supported by BRI Fund to Sustain Research Excellence from Brigham Research Institute, and grants from National Heart, Lung, and Blood Institute (HL65998, HL111775, R56HL147889, R01HL147889-A1). Dr. Ning Zhang was a visiting scholar from Department of Obstetrics and Gynecology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong Province, P. R. China. Dr. Jing Tan was a visiting scholar from School of Acupuncture, Moxibustion & Tuina, Hunan University of Chinese Medicine, Changsha, China. Dr. HaiFeng Yang was a visiting scholar from Department of Neurosurgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China. We thank Elizabeth Hiebert, Bonnie Liu, Tejas Shah, Mayuri Venkatesh, and Jacob Zinner for critically reviewing, proof-reading, and editing the manuscript.
List of Abbreviations:
- AT1-AA
angiotensin type 1 receptor agonistic autoantibody
- BP
blood pressure
- COX
cyclooxygenase
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- HO
hemeoxygenase
- HIF
hypoxia-inducible factor
- HTN-Preg
hypertension in pregnancy
- IL
interleukin
- IUGR
intrauterine growth restriction
- NO
nitric oxide
- NOS
nitric oxide synthase
- PE
preeclampsia
- PGI2
prostacyclin
- PlGF
placental growth factor
- ROS
reactive oxygen species
- sEng
soluble endoglin
- sFlt-1
soluble fms-like tyrosine kinase-1
- TGF-β
transforming growth factor-β
- TNFα
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
Footnotes
Conflict of Interest
None
References
- [1].Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global health 2014;2:e323–33. [DOI] [PubMed] [Google Scholar]
- [2].Gathiram P, Moodley J. Pre-eclampsia: its pathogenesis and pathophysiolgy. Cardiovasc J Afr 2016;27:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol (Oxf) 2013;208:224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Duley L The global impact of pre-eclampsia and eclampsia. Seminars in perinatology 2009;33:130–7. [DOI] [PubMed] [Google Scholar]
- [5].Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. European journal of obstetrics, gynecology, and reproductive biology 2013;170:1–7. [DOI] [PubMed] [Google Scholar]
- [6].Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochemical pharmacology 2015;95:211–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yu W, Gao W, Rong D, Wu Z, Khalil RA. Molecular determinants of microvascular dysfunction in hypertensive pregnancy and preeclampsia. Microcirculation 2018:e12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control 2015;8:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ali SM, Khalil RA. Genetic, immune and vasoactive factors in the vascular dysfunction associated with hypertension in pregnancy. Expert opinion on therapeutic targets 2015;19:1495–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Possomato-Vieira JS, Khalil RA. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv Pharmacol 2016;77:361–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sole KB, Staff AC, Laine K. The association of maternal country of birth and education with hypertensive disorders of pregnancy: A population-based study of 960 516 deliveries in Norway. Acta Obstet Gynecol Scand 2018;97:1237–47. [DOI] [PubMed] [Google Scholar]
- [12].Anderson NH, Sadler LC, Stewart AW, Fyfe EM, McCowan LM. Ethnicity, body mass index and risk of pre-eclampsia in a multiethnic New Zealand population. The Australian & New Zealand journal of obstetrics & gynaecology 2012;52:552–8. [DOI] [PubMed] [Google Scholar]
- [13].Caughey AB, Stotland NE, Washington AE, Escobar GJ. Maternal ethnicity, paternal ethnicity, and parental ethnic discordance: predictors of preeclampsia. Obstet Gynecol 2005;106:156–61. [DOI] [PubMed] [Google Scholar]
- [14].Gong J, Savitz DA, Stein CR, Engel SM. Maternal ethnicity and pre-eclampsia in New York City, 1995–2003. Paediatr Perinat Epidemiol 2012;26:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Souza JP, Gulmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet 2013;381:1747–55. [DOI] [PubMed] [Google Scholar]
- [16].Zanette E, Parpinelli MA, Surita FG, Costa ML, Haddad SM, Sousa MH, et al. Maternal near miss and death among women with severe hypertensive disorders: a Brazilian multicenter surveillance study. Reprod Health 2014;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ghosh G, Grewal J, Mannisto T, Mendola P, Chen Z, Xie Y, et al. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn Dis 2014;24:283–9. [PMC free article] [PubMed] [Google Scholar]
- [18].Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups. Am J Public Health 2005;95:1545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li C, Binongo JN, Kancherla V. Effect of Parity on Pregnancy-Associated Hypertension Among Asian American Women in the United States. Matern Child Health J 2019;23:1098–107. [DOI] [PubMed] [Google Scholar]
- [20].Nakagawa K, Lim E, Harvey S, Miyamura J, Juarez DT. Racial/Ethnic Disparities in the Association Between Preeclampsia Risk Factors and Preeclampsia Among Women Residing in Hawaii. Matern Child Health J 2016;20:1814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Valenzuela FJ, Perez-Sepulveda A, Torres MJ, Correa P, Repetto GM, Illanes SE. Pathogenesis of preeclampsia: the genetic component. Journal of pregnancy 2012;2012:632732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rana S, Karumanchi SA, Lindheimer MD. Angiogenic factors in diagnosis, management, and research in preeclampsia. Hypertension 2014;63:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jebbink J, Wolters A, Fernando F, Afink G, van der Post J, Ris-Stalpers C. Molecular genetics of preeclampsia and HELLP syndrome - a review. Biochim Biophys Acta 2012;1822:1960–9. [DOI] [PubMed] [Google Scholar]
- [24].Fong FM, Sahemey MK, Hamedi G, Eyitayo R, Yates D, Kuan V, et al. Maternal genotype and severe preeclampsia: a HuGE review. Am J Epidemiol 2014;180:335–45. [DOI] [PubMed] [Google Scholar]
- [25].Prasmusinto D, Skrablin S, Fimmers R, van der Ven K. Ethnic differences in the association of factor V Leiden mutation and the C677T methylenetetrahydrofolate reductase gene polymorphism with preeclampsia. European journal of obstetrics, gynecology, and reproductive biology 2004;112:162–9. [DOI] [PubMed] [Google Scholar]
- [26].Hiby SE, Walker JJ, O’Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 2004;200:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Long W, Shi Z, Fan S, Liu L, Lu Y, Guo X, et al. Association of maternal KIR and fetal HLA-C genes with the risk of preeclampsia in the Chinese Han population. Placenta 2015;36:433–7. [DOI] [PubMed] [Google Scholar]
- [28].Nakimuli A, Chazara O, Hiby SE, Farrell L, Tukwasibwe S, Jayaraman J, et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc Natl Acad Sci U S A 2015;112:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stepanian A, Alcais A, de Prost D, Tsatsaris V, Dreyfus M, Treluyer JM, et al. Highly significant association between two common single nucleotide polymorphisms in CORIN gene and preeclampsia in Caucasian women. PloS one 2014;9:e113176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rivers ER, Horton AJ, Hawk AF, Favre EG, Senf KM, Nietert PJ, et al. Placental Nkx2–5 and target gene expression in early-onset and severe preeclampsia. Hypertension in pregnancy 2014;33:412–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kobashi G, Yamada H, Ohta K, Kato E, Ebina Y, Fujimoto S. Endothelial nitric oxide synthase gene (NOS3) variant and hypertension in pregnancy. Am J Med Genet 2001;103:241–4. [PubMed] [Google Scholar]
- [32].Zafarmand MH, Franx A, Sabour S, van der Schouw YT, Grobbee DE, de Leeuw PW, et al. The M235T variant of the angiotensinogen gene is related to development of self-reported hypertension during pregnancy: the Prospect-EPIC cohort study. Hypertension research : official journal of the Japanese Society of Hypertension 2008;31:1299–305. [DOI] [PubMed] [Google Scholar]
- [33].Kobashi G, Hata A, Shido K, Ohta K, Yamada H, Fujimoto S, et al. The M235T variant of the angiotensinogen gene and the body mass index are useful markers for prevention of hypertension in pregnancy: a tree-based analysis of gene-environment interaction. Semin Thromb Hemost 2002;28:501–6. [DOI] [PubMed] [Google Scholar]
- [34].Luizon MR, Belo VA, Palei AC, Amaral LM, Lacchini R, Sandrim VC, et al. Effects of NAMPT polymorphisms and haplotypes on circulating visfatin/NAMPT levels in hypertensive disorders of pregnancy. Hypertension research : official journal of the Japanese Society of Hypertension 2015;38:361–6. [DOI] [PubMed] [Google Scholar]
- [35].Zhan Y, Liu M, You Y, Zhang Y, Wang J, Wang X, et al. Genetic variations in the vitamin-D receptor (VDR) gene in preeclampsia patients in the Chinese Han population. Hypertension research : official journal of the Japanese Society of Hypertension 2015;38:513–7. [DOI] [PubMed] [Google Scholar]
- [36].Liu QY, Gao FY, Liu XR, Li J, Ji M, Dong J, et al. Investigations into the association between polymorphisms in the interleukin-10 gene and risk of early-onset preeclampsia. Genet Mol Res 2015;14:19323–8. [DOI] [PubMed] [Google Scholar]
- [37].Liu B, Li Y, Yao Y, Li H, Liang H, Xin M, et al. Polymorphisms of the IL27 gene in a Chinese Han population complicated with pre-eclampsia. Sci Rep 2016;6:23029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ren R, Gao M, Fan P, Liu X, Liu R, Ma L, et al. [Association study between −765G > C and −1195G > A functional polymorphisms in the cyclooxygenase 2 gene and risk of preeclampsia]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2015;32:245–9. [DOI] [PubMed] [Google Scholar]
- [39].Ye L, Guan L, Fan P, Liu X, Liu R, Chen J, et al. [Association study between 834+7G/A and +1332C/T polymorphisms in the growth arrest specific 6 gene and risk of severe preeclampsia in Chinese population]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2017;34:45–9. [DOI] [PubMed] [Google Scholar]
- [40].Ye L, Guan L, Fan P, Liu X, Liu R, Chen J, et al. Association study between GAS6 gene polymorphisms and risk of preeclampsia in Chinese population. European journal of obstetrics, gynecology, and reproductive biology 2017;211:122–6. [DOI] [PubMed] [Google Scholar]
- [41].Kenny LC, Lavender T, McNamee R, O’Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PloS one 2013;8:e56583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol 2013;42:634–43. [DOI] [PubMed] [Google Scholar]
- [43].Schimmel MS, Bromiker R, Hammerman C, Chertman L, Ioscovich A, Granovsky-Grisaru S, et al. The effects of maternal age and parity on maternal and neonatal outcome. Archives of gynecology and obstetrics 2015;291:793–8. [DOI] [PubMed] [Google Scholar]
- [44].Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, Bommarito K, Madden T, Olsen MA, et al. Maternal age and risk of labor and delivery complications. Matern Child Health J 2015;19:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Huang L, Sauve R, Birkett N, Fergusson D, van Walraven C. Maternal age and risk of stillbirth: a systematic review. CMAJ 2008;178:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Breart G, Barros H, Wagener Y, Prati S. Characteristics of the childbearing population in Europe. European journal of obstetrics, gynecology, and reproductive biology 2003;111 Suppl 1:S45–52. [DOI] [PubMed] [Google Scholar]
- [47].Xiao J, Shen F, Xue Q, Chen G, Zeng K, Stone P, et al. Is ethnicity a risk factor for developing preeclampsia? An analysis of the prevalence of preeclampsia in China. Journal of human hypertension 2014;28:694–8. [DOI] [PubMed] [Google Scholar]
- [48].Blum LS, Khan R, Sultana M, Soltana N, Siddiqua Y, Khondker R, et al. Using a gender lens to understand eating behaviours of adolescent females living in low-income households in Bangladesh. Matern Child Nutr 2019;15:e12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ganchimeg T, Ota E, Morisaki N, Laopaiboon M, Lumbiganon P, Zhang J, et al. Pregnancy and childbirth outcomes among adolescent mothers: a World Health Organization multicountry study. BJOG 2014;121 Suppl 1:40–8. [DOI] [PubMed] [Google Scholar]
- [50].Ogawa K, Urayama KY, Tanigaki S, Sago H, Sato S, Saito S, et al. Association between very advanced maternal age and adverse pregnancy outcomes: a cross sectional Japanese study. BMC Pregnancy Childbirth 2017;17:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lim JW. The changing trends in live birth statistics in Korea, 1970 to 2010. Korean J Pediatr 2011;54:429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Li YH, Wang YP, Dai L, Zhou GX, Liang J, Li Q, et al. [The trend of national advanced maternal age woman proportion in hospital-based surveillance]. Zhonghua Yu Fang Yi Xue Za Zhi 2009;43:1073–6. [PubMed] [Google Scholar]
- [53].Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Risk factors and clinical manifestations of pre-eclampsia. BJOG 2000;107:1410–6. [DOI] [PubMed] [Google Scholar]
- [54].Gold RA, Gold KR, Schilling MF, Modilevsky T. Effect of age, parity, and race on the incidence of pregnancy associated hypertension and eclampsia in the United States. Pregnancy hypertension 2014;4:46–53. [DOI] [PubMed] [Google Scholar]
- [55].Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. American journal of obstetrics and gynecology 2013;209:544 e1–e12. [DOI] [PubMed] [Google Scholar]
- [56].Young BC, Wylie BJ. Effects of twin gestation on maternal morbidity. Seminars in perinatology 2012;36:162–8. [DOI] [PubMed] [Google Scholar]
- [57].Day MC, Barton JR, O’Brien JM, Istwan NB, Sibai BM. The effect of fetal number on the development of hypertensive conditions of pregnancy. Obstet Gynecol 2005;106:927–31. [DOI] [PubMed] [Google Scholar]
- [58].Walker MC, Murphy KE, Pan S, Yang Q, Wen SW. Adverse maternal outcomes in multifetal pregnancies. BJOG 2004;111:1294–6. [DOI] [PubMed] [Google Scholar]
- [59].Luke B, Brown MB. Maternal morbidity and infant death in twin vs triplet and quadruplet pregnancies. American journal of obstetrics and gynecology 2008;198:401 e1–10. [DOI] [PubMed] [Google Scholar]
- [60].Lynch A, McDuffie R Jr., Murphy J, Faber K, Orleans M. Preeclampsia in multiple gestation: the role of assisted reproductive technologies. Obstet Gynecol 2002;99:445–51. [DOI] [PubMed] [Google Scholar]
- [61].Johnston R, Fong A, Lovell S, Sobolewski PS, Rad S, Turner A. Demographic and Obstetric Outcomes of Pregnancies conceived by Assisted Reproductive Technology (ART) compared to Non-ART Pregnancies. JBRA Assist Reprod 2015;19:16–20. [DOI] [PubMed] [Google Scholar]
- [62].Acevedo-Garcia D, Soobader MJ, Berkman LF. Low birthweight among US Hispanic/Latino subgroups: the effect of maternal foreign-born status and education. Soc Sci Med 2007;65:2503–16. [DOI] [PubMed] [Google Scholar]
- [63].Acevedo-Garcia D, Soobader MJ, Berkman LF. The differential effect of foreign-born status on low birth weight by race/ethnicity and education. Pediatrics 2005;115:e20–30. [DOI] [PubMed] [Google Scholar]
- [64].Ross KM, Dunkel Schetter C, McLemore MR, Chambers BD, Paynter RA, Baer R, et al. Socioeconomic Status, Preeclampsia Risk and Gestational Length in Black and White Women. J Racial Ethn Health Disparities 2019;6:1182–91. [DOI] [PubMed] [Google Scholar]
- [65].Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med 2010;39:263–72. [DOI] [PubMed] [Google Scholar]
- [66].Li DK, Wi S. Changing paternity and the risk of preeclampsia/eclampsia in the subsequent pregnancy. Am J Epidemiol 2000;151:57–62. [DOI] [PubMed] [Google Scholar]
- [67].Broughton Pipkin F Risk factors for preeclampsia. The New England journal of medicine 2001;344:925–6. [DOI] [PubMed] [Google Scholar]
- [68].Birukov A, Andersen LB, Herse F, Rakova N, Kitlen G, Kyhl HB, et al. Aldosterone, Salt, and Potassium Intakes as Predictors of Pregnancy Outcome, Including Preeclampsia. Hypertension 2019;74:391–8. [DOI] [PubMed] [Google Scholar]
- [69].Schoenaker DA, Soedamah-Muthu SS, Mishra GD. The association between dietary factors and gestational hypertension and pre-eclampsia: a systematic review and meta-analysis of observational studies. BMC Med 2014;12:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Longo-Mbenza B, Kadima-Tshimanga B, Buassa-bu-Tsumbu B, M’Buyamba K Jr. Diets rich in vegetables and physical activity are associated with a decreased risk of pregnancy induced hypertension among rural women from Kimpese, DR Congo. Niger J Med 2008;17:45–9. [PubMed] [Google Scholar]
- [71].Frederick IO, Williams MA, Dashow E, Kestin M, Zhang C, Leisenring WM. Dietary fiber, potassium, magnesium and calcium in relation to the risk of preeclampsia. J Reprod Med 2005;50:332–44. [PubMed] [Google Scholar]
- [72].Brantsaeter AL, Haugen M, Samuelsen SO, Torjusen H, Trogstad L, Alexander J, et al. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr 2009;139:1162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mi B, Wen X, Li S, Liu D, Lei F, Liu R, et al. Vegetable dietary pattern associated with low risk of preeclampsia possibly through reducing proteinuria. Pregnancy hypertension 2019;16:131–8. [DOI] [PubMed] [Google Scholar]
- [74].Schoenaker DA, Soedamah-Muthu SS, Callaway LK, Mishra GD. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: results from the Australian Longitudinal Study on Women’s Health. Am J Clin Nutr 2015;102:94–101. [DOI] [PubMed] [Google Scholar]
- [75].Maskarinec G, Novotny R, Tasaki K. Dietary patterns are associated with body mass index in multiethnic women. J Nutr 2000;130:3068–72. [DOI] [PubMed] [Google Scholar]
- [76].Tam CY, Hislop G, Hanley AJ, Minkin S, Boyd NF, Martin LJ. Food, beverage, and macronutrient intakes in postmenopausal Caucasian and Chinese-Canadian women. Nutr Cancer 2011;63:687–98. [DOI] [PubMed] [Google Scholar]
- [77].Huang MH, Schocken M, Block G, Sowers M, Gold E, Sternfeld B, et al. Variation in nutrient intakes by ethnicity: results from the Study of Women’s Health Across the Nation (SWAN). Menopause 2002;9:309–19. [DOI] [PubMed] [Google Scholar]
- [78].Kemse N, Kale A, Chavan-Gautam P, Joshi S. Increased intake of vitamin B12, folate, and omega-3 fatty acids to improve cognitive performance in offspring born to rats with induced hypertension during pregnancy. Food Funct 2018;9:3872–83. [DOI] [PubMed] [Google Scholar]
- [79].Kemse N, Sundrani D, Kale A, Joshi S. Maternal Micronutrients, Omega-3 Fatty Acids and Gene Expression of Angiogenic and Inflammatory Markers in Pregnancy Induced Hypertension Rats. Archives of medical research 2017;48:414–22. [DOI] [PubMed] [Google Scholar]
- [80].Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev 2010:CD001059. [DOI] [PubMed] [Google Scholar]
- [81].Lopez-Jaramillo P, Garcia RG, Lopez M. Preventing pregnancy-induced hypertension: are there regional differences for this global problem? Journal of hypertension 2005;23:1121–9. [DOI] [PubMed] [Google Scholar]
- [82].van der Pligt P, Willcox J, Szymlek-Gay EA, Murray E, Worsley A, Daly RM. Associations of Maternal Vitamin D Deficiency with Pregnancy and Neonatal Complications in Developing Countries: A Systematic Review. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Weigel MM, Narvaez WM, Lopez A, Felix C, Lopez P. Prenatal diet, nutrient intake and pregnancy outcome in urban Ecuadorian primiparas. Arch Latinoam Nutr 1991;41:21–37. [PubMed] [Google Scholar]
- [84].Rosenberg TJ, Garbers S, Chavkin W, Chiasson MA. Prepregnancy weight and adverse perinatal outcomes in an ethnically diverse population. Obstet Gynecol 2003;102:1022–7. [DOI] [PubMed] [Google Scholar]
- [85].Spradley FT. Metabolic abnormalities and obesity’s impact on the risk for developing preeclampsia. American journal of physiology Regulatory, integrative and comparative physiology 2016;312:R5–R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Melchor I, Burgos J, Del Campo A, Aiartzaguena A, Gutierrez J, Melchor JC. Effect of maternal obesity on pregnancy outcomes in women delivering singleton babies: a historical cohort study. J Perinat Med 2019;47:625–30. [DOI] [PubMed] [Google Scholar]
- [87].Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, et al. Obesity, obstetric complications and cesarean delivery rate--a population-based screening study. American journal of obstetrics and gynecology 2004;190:1091–7. [DOI] [PubMed] [Google Scholar]
- [88].Ramos GA, Caughey AB. The interrelationship between ethnicity and obesity on obstetric outcomes. American journal of obstetrics and gynecology 2005;193:1089–93. [DOI] [PubMed] [Google Scholar]
- [89].Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- [90].Ochsenbein-Kolble N, Roos M, Gasser T, Zimmermann R. Cross-sectional study of weight gain and increase in BMI throughout pregnancy. European journal of obstetrics, gynecology, and reproductive biology 2007;130:180–6. [DOI] [PubMed] [Google Scholar]
- [91].Denize KM, Acharya N, Prince SA, da Silva DF, Harvey ALJ, Ferraro ZM, et al. Addressing cultural, racial and ethnic discrepancies in guideline discordant gestational weight gain: a systematic review and meta-analysis. PeerJ 2018;6:e5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med 2018;16:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Magro-Malosso ER, Saccone G, Di Tommaso M, Roman A, Berghella V. Exercise during pregnancy and risk of gestational hypertensive disorders: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2017;96:921–31. [DOI] [PubMed] [Google Scholar]
- [94].Rudra CB, Sorensen TK, Luthy DA, Williams MA. A prospective analysis of recreational physical activity and preeclampsia risk. Med Sci Sports Exerc 2008;40:1581–8. [DOI] [PubMed] [Google Scholar]
- [95].Rudra CB, Williams MA, Lee IM, Miller RS, Sorensen TK. Perceived exertion during prepregnancy physical activity and preeclampsia risk. Med Sci Sports Exerc 2005;37:1836–41. [DOI] [PubMed] [Google Scholar]
- [96].Kasawara KT, do Nascimento SL, Costa ML, Surita FG, e Silva JL. Exercise and physical activity in the prevention of pre-eclampsia: systematic review. Acta Obstet Gynecol Scand 2012;91:1147–57. [DOI] [PubMed] [Google Scholar]
- [97].Skow RJ, King EC, Steinback CD, Davenport MH. The influence of prenatal exercise and pre-eclampsia on maternal vascular function. Clinical science 2017;131:2223–40. [DOI] [PubMed] [Google Scholar]
- [98].Gilbert JS. From apelin to exercise: emerging therapies for management of hypertension in pregnancy. Hypertension research : official journal of the Japanese Society of Hypertension 2017;40:519–25. [DOI] [PubMed] [Google Scholar]
- [99].Gilbert JS, Banek CT, Bauer AJ, Gingery A, Needham K. Exercise training attenuates placental ischemia-induced hypertension and angiogenic imbalance in the rat. Hypertension 2012;60:1545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gilbert JS, Banek CT, Bauer AJ, Gingery A, Dreyer HC. Placental and vascular adaptations to exercise training before and during pregnancy in the rat. American journal of physiology Regulatory, integrative and comparative physiology 2012;303:R520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Steinl GK, Whisner CM, Pressman EK, Cooper EM, Groth SW, O’Brien KO. Patterns and Correlates of Self-Reported Physical Activity in a Cohort of Racially Diverse Pregnant Adolescents. J Pediatr Adolesc Gynecol 2018;32:51–6. [DOI] [PubMed] [Google Scholar]
- [102].Evenson KR, Wen F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev Med 2011;53:39–43. [DOI] [PubMed] [Google Scholar]
- [103].Evenson KR, Wen F. National trends in self-reported physical activity and sedentary behaviors among pregnant women: NHANES 1999–2006. Prev Med 2010;50:123–8. [DOI] [PubMed] [Google Scholar]
- [104].Domingues MR, Barros AJ. Leisure-time physical activity during pregnancy in the 2004 Pelotas Birth Cohort Study. Rev Saude Publica 2007;41:173–80. [DOI] [PubMed] [Google Scholar]
- [105].Algert CS, Roberts CL, Shand AW, Morris JM, Ford JB. Seasonal variation in pregnancy hypertension is correlated with sunlight intensity. American journal of obstetrics and gynecology 2010;203:215 e1–5. [DOI] [PubMed] [Google Scholar]
- [106].Tam WH, Sahota DS, Lau TK, Li CY, Fung TY. Seasonal variation in pre-eclamptic rate and its association with the ambient temperature and humidity in early pregnancy. Gynecol Obstet Invest 2008;66:22–6. [DOI] [PubMed] [Google Scholar]
- [107].Immink A, Scherjon S, Wolterbeek R, Steyn DW. Seasonal influence on the admittance of pre-eclampsia patients in Tygerberg Hospital. Acta Obstet Gynecol Scand 2008;87:36–42. [DOI] [PubMed] [Google Scholar]
- [108].Rudra CB, Williams MA. Monthly variation in preeclampsia prevalence: Washington State, 1987–2001. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2005;18:319–24. [DOI] [PubMed] [Google Scholar]
- [109].Bodnar LM, Catov JM, Roberts JM. Racial/ethnic differences in the monthly variation of preeclampsia incidence. American journal of obstetrics and gynecology 2007;196:324 e1–5. [DOI] [PubMed] [Google Scholar]
- [110].Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circulation research 2016;119:1204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Fowler PA, Bellingham M, Sinclair KD, Evans NP, Pocar P, Fischer B, et al. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol Cell Endocrinol 2012;355:231–9. [DOI] [PubMed] [Google Scholar]
- [112].Hu H, Ha S, Roth J, Kearney G, Talbott EO, Xu X. Ambient Air Pollution and Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-analysis. Atmos Environ (1994) 2014;97:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Pedersen M, Stayner L, Slama R, Sorensen M, Figueras F, Nieuwenhuijsen MJ, et al. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension 2014;64:494–500. [DOI] [PubMed] [Google Scholar]
- [114].Wang Q, Zhang H, Liang Q, Knibbs LD, Ren M, Li C, et al. Effects of prenatal exposure to air pollution on preeclampsia in Shenzhen, China. Environ Pollut 2018;237:18–27. [DOI] [PubMed] [Google Scholar]
- [115].Madsen C, Haberg SE, Aamodt G, Stigum H, Magnus P, London SJ, et al. Preeclampsia and Hypertension During Pregnancy in Areas with Relatively Low Levels of Traffic Air Pollution. Matern Child Health J 2018;22:512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Auger N, Duplaix M, Bilodeau-Bertrand M, Lo E, Smargiassi A. Environmental noise pollution and risk of preeclampsia. Environ Pollut 2018;239:599–606. [DOI] [PubMed] [Google Scholar]
- [117].Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician 2014;89:199–208. [PubMed] [Google Scholar]
- [118].Nylander PP, Adekunle AO. Antenatal care in developing countries. Baillieres Clin Obstet Gynaecol 1990;4:169–86. [DOI] [PubMed] [Google Scholar]
- [119].Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ 2009;338:b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- [121].Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy hypertension 2014;4:105–45. [DOI] [PubMed] [Google Scholar]
- [122].Lowe SA, Brown MA, Dekker GA, Gatt S, McLintock CK, McMahon LP, et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. The Australian & New Zealand journal of obstetrics & gynaecology 2009;49:242–6. [DOI] [PubMed] [Google Scholar]
- [123].Rawlins B, Plotkin M, Rakotovao JP, Getachew A, Vaz M, Ricca J, et al. Screening and management of pre-eclampsia and eclampsia in antenatal and labor and delivery services: findings from cross-sectional observation studies in six sub-Saharan African countries. BMC Pregnancy Childbirth 2018;18:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 2014;10:466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Forest JC, Charland M, Masse J, Bujold E, Rousseau F, Lafond J, et al. Candidate biochemical markers for screening of pre-eclampsia in early pregnancy. Clin Chem Lab Med 2012;50:973–84. [DOI] [PubMed] [Google Scholar]
- [126].Naljayan MV, Karumanchi SA. New developments in the pathogenesis of preeclampsia. Advances in chronic kidney disease 2013;20:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Scazzocchio E, Figueras F. Contemporary prediction of preeclampsia. Curr Opin Obstet Gynecol 2011;23:65–71. [DOI] [PubMed] [Google Scholar]
- [128].Raghupathy R Cytokines as key players in the pathophysiology of preeclampsia. Med Princ Pract 2013;22 Suppl 1:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Widmer M, Villar J, Benigni A, Conde-Agudelo A, Karumanchi SA, Lindheimer M. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol 2007;109:168–80. [DOI] [PubMed] [Google Scholar]
- [130].Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2008;21:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. The New England journal of medicine 2006;355:992–1005. [DOI] [PubMed] [Google Scholar]
- [132].Liu Y, Zhao Y, Yu A, Zhao B, Gao Y, Niu H. Diagnostic accuracy of the soluble Fms-like tyrosine kinase-1/placental growth factor ratio for preeclampsia: a meta-analysis based on 20 studies. Archives of gynecology and obstetrics 2015;292:507–18. [DOI] [PubMed] [Google Scholar]
- [133].Ohkuchi A, Hirashima C, Takahashi K, Suzuki H, Matsubara S, Suzuki M. Onset threshold of the plasma levels of soluble fms-like tyrosine kinase 1/placental growth factor ratio for predicting the imminent onset of preeclampsia within 4 weeks after blood sampling at 19–31 weeks of gestation. Hypertension research : official journal of the Japanese Society of Hypertension 2013;36:1073–80. [DOI] [PubMed] [Google Scholar]
- [134].Yang J, Pearl M, DeLorenze GN, Romero R, Dong Z, Jelliffe-Pawlowski L, et al. Racial-ethnic differences in midtrimester maternal serum levels of angiogenic and antiangiogenic factors. American journal of obstetrics and gynecology 2016;215:359 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Menon R, Dunlop AL, Kramer MR, Fortunato SJ, Hogue CJ. An overview of racial disparities in preterm birth rates: caused by infection or inflammatory response? Acta Obstet Gynecol Scand 2011;90:1325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Pedroso MA, Palmer KR, Hodges RJ, Costa FDS, Rolnik DL. Uterine Artery Doppler in Screening for Preeclampsia and Fetal Growth Restriction. Rev Bras Ginecol Obstet 2018;40:287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Khong SL, Kane SC, Brennecke SP, da Silva Costa F. First-trimester uterine artery Doppler analysis in the prediction of later pregnancy complications. Dis Markers 2015;2015:679730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Guimaraes MF, Brandao AH, Rezende CA, Cabral AC, Brum AP, Leite HV, et al. Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow-mediated dilation of brachial artery. Archives of gynecology and obstetrics 2014;290:441–7. [DOI] [PubMed] [Google Scholar]
- [139].Brandao AH, Felix LR, Patricio Edo C, Leite HV, Cabral AC. Difference of endothelial function during pregnancies as a method to predict preeclampsia. Archives of gynecology and obstetrics 2014;290:471–7. [DOI] [PubMed] [Google Scholar]
- [140].Jung YW, Shim JI, Shim SH, Shin YJ, Chang SW, Cha DH. Global gene expression analysis of cell-free RNA in amniotic fluid from women destined to develop preeclampsia. Medicine (Baltimore) 2019;98:e13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Hahn S, Rusterholz C, Hosli I, Lapaire O. Cell-free nucleic acids as potential markers for preeclampsia. Placenta 2011;32 Suppl:S17–20. [DOI] [PubMed] [Google Scholar]
- [142].Quan LM, Xu QL, Zhang GQ, Wu LL, Xu H. An analysis of the risk factors of preeclampsia and prediction based on combined biochemical indexes. Kaohsiung J Med Sci 2018;34:109–12. [DOI] [PubMed] [Google Scholar]
- [143].Tan MY, Syngelaki A, Poon LC, Rolnik DL, O’Gorman N, Delgado JL, et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol 2018;52:186–95. [DOI] [PubMed] [Google Scholar]
- [144].Tolcher MC, Chu DM, Hollier LM, Mastrobattista JM, Racusin DA, Ramin SM, et al. Impact of USPSTF recommendations for aspirin for prevention of recurrent preeclampsia. American journal of obstetrics and gynecology 2017;217:365 e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol 2014;10:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Jain V Letter to the Editor: Diagnosis, Evaluation, and Management of the Hypertensive Disorders of Pregnancy: Executive Summary. J Obstet Gynaecol Can 2015;37:774–5. [DOI] [PubMed] [Google Scholar]
- [147].Long Q, Oladapo OT, Leathersich S, Vogel JP, Carroli G, Lumbiganon P, et al. Clinical practice patterns on the use of magnesium sulphate for treatment of pre-eclampsia and eclampsia: a multi-country survey. BJOG 2016;124:1883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bigdeli M, Zafar S, Assad H, Ghaffar A. Health system barriers to access and use of magnesium sulfate for women with severe pre-eclampsia and eclampsia in Pakistan: evidence for policy and practice. PloS one 2013;8:e59158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel JP, et al. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG 2014;121 Suppl 1:49–56. [DOI] [PubMed] [Google Scholar]
- [150].Weinberg CR, Shi M, Basso O, DeRoo LA, Harmon Q, Wilcox AJ, et al. Season of Conception, Smoking, and Preeclampsia in Norway. Environ Health Perspect 2017;125:067022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Fokom-Domgue J, Noubiap JJ. Diagnosis of hypertensive disorders of pregnancy in sub-Saharan Africa: a poorly assessed but increasingly important issue. J Clin Hypertens (Greenwich) 2015;17:70–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Allanson ER, Muller M, Pattinson RC. Causes of perinatal mortality and associated maternal complications in a South African province: challenges in predicting poor outcomes. BMC Pregnancy Childbirth 2015;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open 2011;1:e000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Schoenmakers I, Pettifor JM, Pena-Rosas JP, Lamberg-Allardt C, Shaw N, Jones KS, et al. Prevention and consequences of vitamin D deficiency in pregnant and lactating women and children: A symposium to prioritise vitamin D on the global agenda. J Steroid Biochem Mol Biol 2016;164:156–60. [DOI] [PubMed] [Google Scholar]
- [155].Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertension research : official journal of the Japanese Society of Hypertension 2016;40:213–20. [DOI] [PubMed] [Google Scholar]
- [156].Wang C, Lin L, Su R, Zhu W, Wei Y, Yan J, et al. Hemoglobin levels during the first trimester of pregnancy are associated with the risk of gestational diabetes mellitus, pre-eclampsia and preterm birth in Chinese women: a retrospective study. BMC Pregnancy Childbirth 2018;18:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Sachan R, Patel ML, Sachan P, Gaurav A, Singh M, Bansal B. Outcomes in hypertensive disorders of pregnancy in the North Indian population. Int J Womens Health 2013;5:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Magee LA, Sharma S, Nathan HL, Adetoro OO, Bellad MB, Goudar S, et al. The incidence of pregnancy hypertension in India, Pakistan, Mozambique, and Nigeria: A prospective population-level analysis. PLoS Med 2019;16:e1002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Matsuda Y, Kawamichi Y, Hayashi K, Shiozaki A, Satoh S, Saito S. Impact of maternal age on the incidence of obstetrical complications in Japan. The journal of obstetrics and gynaecology research 2011;37:1409–14. [DOI] [PubMed] [Google Scholar]
- [160].Mayama M, Morikawa M, Umazume T, Nakagawa K, Hosokawa A, Yamaguchi M, et al. Increase in the number of patients diagnosed using the new classification of hypertensive disorders of pregnancy in Japan. The journal of obstetrics and gynaecology research 2019;45:1118–26. [DOI] [PubMed] [Google Scholar]
- [161].Choe SA, Min HS, Cho SI. Decreased Risk of Preeclampsia After the Introduction of Universal Voucher Scheme for Antenatal Care and Birth Services in the Republic of Korea. Matern Child Health J 2017;21:222–7. [DOI] [PubMed] [Google Scholar]
- [162].Park Y, Cho GJ, Kim LY, Lee TS, Oh MJ, Kim YH. Preeclampsia Increases the Incidence of Postpartum Cerebrovascular Disease in Korean Population. J Korean Med Sci 2018;33:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Bilano VL, Ota E, Ganchimeg T, Mori R, Souza JP. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PloS one 2014;9:e91198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Das S, Das R, Bajracharya R, Baral G, Jabegu B, Odland JO, et al. Incidence and Risk Factors of Pre-Eclampsia in the Paropakar Maternity and Women’s Hospital, Nepal: A Retrospective Study. Int J Environ Res Public Health 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Short VL, Geller SE, Moore JL, McClure EM, Goudar SS, Dhaded SM, et al. The Relationship between Body Mass Index in Pregnancy and Adverse Maternal, Perinatal, and Neonatal Outcomes in Rural India and Pakistan. Am J Perinatol 2018;35:844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Perveen S. Frequency and impact of hypertensive disorders of pregnancy. J Ayub Med Coll Abbottabad 2014;26:518–21. [PubMed] [Google Scholar]
- [167].Rao AK, Cheng YW, Caughey AB. Perinatal complications among different Asian-American subgroups. American journal of obstetrics and gynecology 2006;194:e39–41. [DOI] [PubMed] [Google Scholar]
- [168].Rao AK, Daniels K, El-Sayed YY, Moshesh MK, Caughey AB. Perinatal outcomes among Asian American and Pacific Islander women. American journal of obstetrics and gynecology 2006;195:834–8. [DOI] [PubMed] [Google Scholar]
- [169].Goonewardene IM, Deeyagaha Waduge RP. Adverse effects of teenage pregnancy. Ceylon Med J 2005;50:116–20. [DOI] [PubMed] [Google Scholar]
- [170].Herath RP, Siriwardana SR, Ekanayake CD, Abeysekara V, Kodithuwakku SUA, Herath HP. Non-alcoholic fatty liver disease and pregnancy complications among Sri Lankan women: A cross sectional analytical study. PloS one 2019;14:e0215326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Kongwattanakul K, Saksiriwuttho P, Chaiyarach S, Thepsuthammarat K. Incidence, characteristics, maternal complications, and perinatal outcomes associated with preeclampsia with severe features and HELLP syndrome. Int J Womens Health 2018;10:371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Nguyen CL, Nguyen PTH, Chu TK, Ha AVV, Pham NM, Duong DV, et al. Cohort profile: maternal lifestyle and diet in relation to pregnancy, postpartum and infant health outcomes in Vietnam: A multicentre prospective cohort study. BMJ Open 2017;7:e016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Roberts CL, Algert CS, Morris JM, Ford JB, Henderson-Smart DJ. Hypertensive disorders in pregnancy: a population-based study. Med J Aust 2005;182:332–5. [DOI] [PubMed] [Google Scholar]
- [174].Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000–2008. American journal of obstetrics and gynecology 2013;208:476 e1–5. [DOI] [PubMed] [Google Scholar]
- [175].Al-Rubaie ZTA, Malcolm Hudson H, Jenkins G, Mahmoud I, Ray JG, Askie LM, et al. The association between ethnicity and pre-eclampsia in Australia: A multicentre retrospective cohort study. The Australian & New Zealand journal of obstetrics & gynaecology 2019. [DOI] [PubMed] [Google Scholar]
- [176].Shen M, Smith GN, Rodger M, White RR, Walker MC, Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PloS one 2017;12:e0175914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Auger N, Luo ZC, Nuyt AM, Kaufman JS, Naimi AI, Platt RW, et al. Secular Trends in Preeclampsia Incidence and Outcomes in a Large Canada Database: A Longitudinal Study Over 24 Years. Can J Cardiol 2016;32:987 e15–23. [DOI] [PubMed] [Google Scholar]
- [178].Auger N, Rheaume MA, Paradis G, Healy-Profitos J, Hsieh A, Fraser WD. Preeclampsia and the risk of cataract extraction in life. American journal of obstetrics and gynecology 2016;216:417 e1–e8. [DOI] [PubMed] [Google Scholar]
- [179].Hanley GE, Hutcheon JA, Kinniburgh BA, Lee L. Interpregnancy Interval and Adverse Pregnancy Outcomes: An Analysis of Successive Pregnancies. Obstet Gynecol 2017;129:408–15. [DOI] [PubMed] [Google Scholar]
- [180].Bernard N, Forest JC, Tarabulsy GM, Bujold E, Bouvier D, Giguere Y. Use of antidepressants and anxiolytics in early pregnancy and the risk of preeclampsia and gestational hypertension: a prospective study. BMC Pregnancy Childbirth 2019;19:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017;60:636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Tran PL, Robillard PY, Dumont C, Schweizer C, Omarjee A, Iacobelli S, et al. Recurrent or first preeclampsia in multiparae: A case-control study of singleton pregnancies in Reunion Island. European journal of obstetrics, gynecology, and reproductive biology 2019;240:80–6. [DOI] [PubMed] [Google Scholar]
- [183].Lamminpaa R, Vehvilainen-Julkunen K, Gissler M, Heinonen S. Preeclampsia complicated by advanced maternal age: a registry-based study on primiparous women in Finland 1997–2008. BMC Pregnancy Childbirth 2012;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Lamminpaa R, Vehvilainen-Julkunen K, Gissler M, Selander T, Heinonen S. Pregnancy outcomes of overweight and obese women aged 35 years or older - A registry-based study in Finland. Obes Res Clin Pract 2015;10:133–42. [DOI] [PubMed] [Google Scholar]
- [185].Parker SE, Gissler M, Ananth CV, Werler MM. Induced Abortions and the Risk of Preeclampsia Among Nulliparous Women. Am J Epidemiol 2015;182:663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Metsala J, Stach-Lempinen B, Gissler M, Eriksson JG, Koivusalo S. Risk of Pregnancy Complications in Relation to Maternal Prepregnancy Body Mass Index: Population-Based Study from Finland 2006–10. Paediatr Perinat Epidemiol 2015;30:28–37. [DOI] [PubMed] [Google Scholar]
- [187].Martins-Costa SH, Vettorazzi J, Valerio E, Maurmman C, Benevides G, Hemessath M, et al. Protein creatinine ratio in random urine sample of hypertensive pregnant women: maternal and perinatal outcomes. Hypertension in pregnancy 2011;30:331–7. [DOI] [PubMed] [Google Scholar]
- [188].Schneider S, Freerksen N, Rohrig S, Hoeft B, Maul H. Gestational diabetes and preeclampsia--similar risk factor profiles? Early Hum Dev 2012;88:179–84. [DOI] [PubMed] [Google Scholar]
- [189].Tamayo T, Tamayo M, Rathmann W, Potthoff P. Prevalence of gestational diabetes and risk of complications before and after initiation of a general systematic two-step screening strategy in Germany (2012–2014). Diabetes Res Clin Pract 2016;115:1–8. [DOI] [PubMed] [Google Scholar]
- [190].Lee Y, Magnus P. Maternal and Paternal Height and the Risk of Preeclampsia. Hypertension 2018;71:666–70. [DOI] [PubMed] [Google Scholar]
- [191].Riise HKR, Sulo G, Tell GS, Igland J, Nygard O, Iversen AC, et al. Association Between Gestational Hypertension and Risk of Cardiovascular Disease Among 617 589 Norwegian Women. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Adank MC, Benschop L, Peterbroers KR, Smak Gregoor AM, Kors AW, Mulder MT, et al. Is maternal lipid profile in early pregnancy associated with pregnancy complications and blood pressure in pregnancy and long term postpartum? American journal of obstetrics and gynecology 2019;221:150 e1–e13. [DOI] [PubMed] [Google Scholar]
- [193].von Schmidt auf Altenstadt JF, Hukkelhoven CW, van Roosmalen J, Bloemenkamp KW. Pre-eclampsia increases the risk of postpartum haemorrhage: a nationwide cohort study in the Netherlands. PloS one 2013;8:e81959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Lamain-de Ruiter M, Kwee A, Naaktgeboren CA, Louhanepessy RD, De Groot I, Evers IM, et al. External validation of prognostic models for preeclampsia in a Dutch multicenter prospective cohort. Hypertension in pregnancy 2019;38:78–88. [DOI] [PubMed] [Google Scholar]
- [195].Rodriguez-Mesa N, Robles-Benayas P, Rodriguez-Lopez Y, Perez-Fernandez EM, Cobo-Cuenca AI. Influence of Body Mass Index on Gestation and Delivery in Nulliparous Women: A Cohort Study. Int J Environ Res Public Health 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Thellin O, Elmoualij B, Zorzi W, Jensen JS, Close R, Deregowski V, et al. Four-color multiplex real-time PCR assay prototype targeting azithromycin resistance mutations in Mycoplasma genitalium. BMC Infect Dis 2019;19:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad PR, et al. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum Reprod 2015;30:1724–31. [DOI] [PubMed] [Google Scholar]
- [198].Cnattingius S, Wikstrom AK, Stephansson O, Johansson K. The Impact of Small for Gestational Age Births in Early and Late Preeclamptic Pregnancies for Preeclampsia Recurrence: a Cohort Study of Successive Pregnancies in Sweden. Paediatr Perinat Epidemiol 2016;30:563–70. [DOI] [PubMed] [Google Scholar]
- [199].Wikstrom S, Lindh CH, Shu H, Bornehag CG. Early pregnancy serum levels of perfluoroalkyl substances and risk of preeclampsia in Swedish women. Sci Rep 2019;9:9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Rejno G, Lundholm C, Oberg S, Lichtenstein P, Larsson H, D’Onofrio B, et al. Maternal anxiety, depression and asthma and adverse pregnancy outcomes - a population based study. Sci Rep 2019;9:13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Purde MT, Baumann M, Wiedemann U, Nydegger UE, Risch L, Surbek D, et al. Incidence of preeclampsia in pregnant Swiss women. Swiss Med Wkly 2015;145:w14175. [DOI] [PubMed] [Google Scholar]
- [202].Khashan AS, Evans M, Kublickas M, McCarthy FP, Kenny LC, Stenvinkel P, et al. Preeclampsia and risk of end stage kidney disease: A Swedish nationwide cohort study. PLoS Med 2019;16:e1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP, et al. Preeclampsia and Cardiovascular Disease in a Large UK Pregnancy Cohort of Linked Electronic Health Records: A CALIBER Study. Circulation 2019;140:1050–60. [DOI] [PubMed] [Google Scholar]
- [204].Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Maric I, Mayo JA, Druzin ML, Wong RJ, Winn VD, Stevenson DK, et al. Maternal Height and Risk of Preeclampsia among Race/Ethnic Groups. Am J Perinatol 2019;36:864–71. [DOI] [PubMed] [Google Scholar]
- [206].Khandwala YS, Baker VL, Shaw GM, Stevenson DK, Lu Y, Eisenberg ML. Association of paternal age with perinatal outcomes between 2007 and 2016 in the United States: population based cohort study. BMJ 2018;363:k4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Hodel M, Blank PR, Marty P, Lapaire O. sFlt-1/PlGF Ratio as a Predictive Marker in Women with Suspected Preeclampsia: An Economic Evaluation from a Swiss Perspective. Dis Markers 2019;2019:4096847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Kharaghani R, Cheraghi Z, Okhovat Esfahani B, Mohammadian Z, Nooreldinc RS. Prevalence of Preeclampsia and Eclampsia in Iran. Arch Iran Med 2016;19:64–71. [PubMed] [Google Scholar]
- [209].Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol 2018;51:743–50. [DOI] [PubMed] [Google Scholar]
- [210].Pakniat H, Bahman A, Ansari I. The Relationship of Pregnancy-Associated Plasma Protein A and Human Chorionic Gonadotropin with Adverse Pregnancy Outcomes: A Prospective Study. J Obstet Gynaecol India 2019;69:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Farzaneh F, Tavakolikia Z, Soleimanzadeh Mousavi SH. Assessment of occurrence of preeclampsia and some clinical and demographic risk factors in Zahedan city in 2017. Clinical and experimental hypertension 2018;41:583–8. [DOI] [PubMed] [Google Scholar]
- [212].Abu-Heija AT, Jallad MF, Abukteish F. Maternal and perinatal outcome of pregnancies after the age of 45. The journal of obstetrics and gynaecology research 2000;26:27–30. [DOI] [PubMed] [Google Scholar]
- [213].Khader YS, Batieha A, Al-Njadat RA, Hijazi SS. Preeclampsia in Jordan: incidence, risk factors, and its associated maternal and neonatal outcomes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2017;31:770–6. [DOI] [PubMed] [Google Scholar]
- [214].Khraise WN, Allouh MZ, El-Radaideh KM, Said RS, Al-Rusan AM. Assessment of risk factors for postdural puncture headache in women undergoing cesarean delivery in Jordan: a retrospective analytical study. Local Reg Anesth 2017;10:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Seoud MA, Nassar AH, Usta IM, Melhem Z, Kazma A, Khalil AM. Impact of advanced maternal age on pregnancy outcome. Am J Perinatol 2002;19:1–8. [DOI] [PubMed] [Google Scholar]
- [216].Usta IM, Zoorob D, Abu-Musa A, Naassan G, Nassar AH. Obstetric outcome of teenage pregnancies compared with adult pregnancies. Acta Obstet Gynecol Scand 2008;87:178–83. [DOI] [PubMed] [Google Scholar]
- [217].Zahed LF, Rayes RF, Mahfouz RA, Taher AT, Maarouf HH, Nassar AH. Prevalence of factor V Leiden, prothrombin and methylene tetrahydrofolate reductase mutations in women with adverse pregnancy outcomes in Lebanon. American journal of obstetrics and gynecology 2006;195:1114–8. [DOI] [PubMed] [Google Scholar]
- [218].Bashir M, Naem E, Taha F, Konje JC, Abou-Samra AB. Outcomes of type 1 diabetes mellitus in pregnancy; effect of excessive gestational weight gain and hyperglycaemia on fetal growth. Diabetes Metab Syndr 2019;13:84–8. [DOI] [PubMed] [Google Scholar]
- [219].Shaukat S, Nur U. Effect of prepregnancy maternal BMI on adverse pregnancy and neonatal outcomes: results from a retrospective cohort study of a multiethnic population in Qatar. BMJ Open 2019;9:e029757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Soliman A, Salama H, Al Rifai H, De Sanctis V, Al-Obaidly S, Al Qubasi M, et al. The effect of different forms of dysglycemia during pregnancy on maternal and fetal outcomes in treated women and comparison with large cohort studies. Acta Biomed 2018;89:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Wahabi HA, Fayed AA, Alzeidan RA, Mandil AA. The independent effects of maternal obesity and gestational diabetes on the pregnancy outcomes. BMC Endocr Disord 2014;14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Al-Shaikh GK, Ibrahim GH, Fayed AA, Al-Mandeel H. Grand multiparity and the possible risk of adverse maternal and neonatal outcomes: a dilemma to be deciphered. BMC Pregnancy Childbirth 2017;17:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Wahabi H, Fayed A, Esmaeil S, Alzeidan R, Elawad M, Tabassum R, et al. Riyadh Mother and Baby Multicenter Cohort Study: The Cohort Profile. PloS one 2016;11:e0150297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Subki AH, Algethami MR, Baabdullah WM, Alnefaie MN, Alzanbagi MA, Alsolami RM, et al. Prevalence, Risk Factors, and Fetal and Maternal Outcomes of Hypertensive Disorders of Pregnancy: A Retrospective Study in Western Saudi Arabia. Oman Med J 2018;33:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Longo-Mbenza B, Tshimanga KB, Buassa-bu-Tsumbu B, Kabangu MJ. Diets rich in vegetables and physical activity are associated with a decreased risk of pregnancy induced hypertension among rural women from Kimpese, DR Congo. Niger J Med 2008;17:265–9. [PubMed] [Google Scholar]
- [226].Elongi JP, Tandu B, Spitz B, Verdonck F. [Influence of the seasonal variation on the prevalence of pre-eclampsia in Kinshasa]. Gynecol Obstet Fertil 2011;39:132–5. [DOI] [PubMed] [Google Scholar]
- [227].Wagnew M, Dessalegn M, Worku A, Nyagero J. Trends of preeclampsia/eclampsia and maternal and neonatal outcomes among women delivering in addis ababa selected government hospitals, Ethiopia: a retrospective cross-sectional study. Pan Afr Med J 2016;25:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Obed S, Patience A. Birth weight and ponderal index in pre-eclampsia: a comparative study. Ghana Med J 2006;40:8–13. [PMC free article] [PubMed] [Google Scholar]
- [229].Owusu JT, Anderson FJ, Coleman J, Oppong S, Seffah JD, Aikins A, et al. Association of maternal sleep practices with pre-eclampsia, low birth weight, and stillbirth among Ghanaian women. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2013;121:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Adu-Bonsaffoh K, Ntumy MY, Obed SA, Seffah JD. Perinatal outcomes of hypertensive disorders in pregnancy at a tertiary hospital in Ghana. BMC Pregnancy Childbirth 2017;17:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Noubiap JJ, Bigna JJ, Nyaga UF, Jingi AM, Kaze AD, Nansseu JR, et al. The burden of hypertensive disorders of pregnancy in Africa: A systematic review and meta-analysis. J Clin Hypertens (Greenwich) 2019;21:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Bansal YP. Pre-eclampsia/eclampsia: a profile from Pumwani Maternity Hospital, Nairobi, Kenya. East Afr Med J 1985;62:691–8. [PubMed] [Google Scholar]
- [233].Widmer M, Cuesta C, Khan KS, Conde-Agudelo A, Carroli G, Fusey S, et al. Accuracy of angiogenic biomarkers at 20weeks’ gestation in predicting the risk of pre-eclampsia: A WHO multicentre study. Pregnancy hypertension 2015;5:330–8. [DOI] [PubMed] [Google Scholar]
- [234].Kiondo P, Wamuyu-Maina G, Wandabwa J, Bimenya GS, Tumwesigye NM, Okong P. The effects of vitamin C supplementation on pre-eclampsia in Mulago Hospital, Kampala, Uganda: a randomized placebo controlled clinical trial. BMC Pregnancy Childbirth 2014;14:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Ogundipe O, Hoyo C, Ostbye T, Oneko O, Manongi R, Lie RT, et al. Factors associated with prenatal folic acid and iron supplementation among 21,889 pregnant women in Northern Tanzania: a cross-sectional hospital-based study. BMC Public Health 2012;12:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Mahande MJ, Daltveit AK, Mmbaga BT, Masenga G, Obure J, Manongi R, et al. Recurrence of preeclampsia in northern Tanzania: a registry-based cohort study. PloS one 2013;8:e79116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Mrema D, Lie RT, Ostbye T, Mahande MJ, Daltveit AK. The association between pre pregnancy body mass index and risk of preeclampsia: a registry based study from Tanzania. BMC Pregnancy Childbirth 2018;18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Chuwa FS, Mwanamsangu AH, Brown BG, Msuya SE, Senkoro EE, Mnali OP, et al. Maternal and fetal risk factors for stillbirth in Northern Tanzania: A registry-based retrospective cohort study. PloS one 2017;12:e0182250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Hauger MS, Gibbons L, Vik T, Belizan JM. Prepregnancy weight status and the risk of adverse pregnancy outcome. Acta Obstet Gynecol Scand 2008;87:953–9. [DOI] [PubMed] [Google Scholar]
- [240].Dowswell T, Carroli G, Duley L, Gates S, Gulmezoglu AM, Khan-Neelofur D, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev 2010:CD000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Marques MR, Grandi C, Nascente LMP, Cavalli RC, Cardoso VC. Placental morphometry in hypertensive disorders of pregnancy and its relationship with birth weight in a Latin American population. Pregnancy hypertension 2018;13:235–41. [DOI] [PubMed] [Google Scholar]
- [242].Parra M, Rodrigo R, Barja P, Bosco C, Fernandez V, Munoz H, et al. Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. American journal of obstetrics and gynecology 2005;193:1486–91. [DOI] [PubMed] [Google Scholar]
- [243].Garmendia ML, Matus O, Mondschein S, Kusanovic JP. Gestational weight gain recommendations for Chilean women: a mathematical optimization approach. Public Health 2018;163:80–6. [DOI] [PubMed] [Google Scholar]
- [244].Sepulveda-Martinez A, Rencoret G, Silva MC, Ahumada P, Pedraza D, Munoz H, et al. First trimester screening for preterm and term pre-eclampsia by maternal characteristics and biophysical markers in a low-risk population. The journal of obstetrics and gynaecology research 2018;45:104–12. [DOI] [PubMed] [Google Scholar]
- [245].Lopez-Jaramillo P, Delgado F, Jacome P, Teran E, Ruano C, Rivera J. Calcium supplementation and the risk of preeclampsia in Ecuadorian pregnant teenagers. Obstet Gynecol 1997;90:162–7. [DOI] [PubMed] [Google Scholar]
- [246].Parra-Pingel PE, Quisiguina-Avellan LA, Hidalgo L, Chedraui P, Perez-Lopez FR. Pregnancy outcomes in younger and older adolescent mothers with severe preeclampsia. Adolesc Health Med Ther 2017;8:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Reyes E, Martinez N, Parra A, Castillo-Mora A, Ortega-Gonzalez C. Early intensive obstetric and medical nutrition care is associated with decreased prepregnancy obesity impact on perinatal outcomes. Gynecol Obstet Invest 2012;73:75–81. [DOI] [PubMed] [Google Scholar]
- [248].Gonzales GF, Tapia VL, Fort AL, Betran AP. Pregnancy outcomes associated with Cesarean deliveries in Peruvian public health facilities. Int J Womens Health 2013;5:637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Wynn A, Cabeza J, Adachi K, Needleman J, Garcia PJ, Klausner JD. Frequency of maternal and newborn birth outcomes, Lima, Peru, 2013. PloS one 2015;10:e0116102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Hirayama F, Koyanagi A, Mori R, Zhang J, Souza JP, Gulmezoglu AM. Prevalence and risk factors for third- and fourth-degree perineal lacerations during vaginal delivery: a multi-country study. BJOG 2012;119:340–7. [DOI] [PubMed] [Google Scholar]
- [251].Ray JG, Wanigaratne S, Park AL, Bartsch E, Dzakpasu S, Urquia ML. Preterm preeclampsia in relation to country of birth. J Perinatol 2016;36:718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Karlsen S, Say L, Souza JP, Hogue CJ, Calles DL, Gulmezoglu AM, et al. The relationship between maternal education and mortality among women giving birth in health care institutions: analysis of the cross sectional WHO Global Survey on Maternal and Perinatal Health. BMC Public Health 2011;11:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Ruiz M, Goldblatt P, Morrison J, Kukla L, Svancara J, Riitta-Jarvelin M, et al. Mother’s education and the risk of preterm and small for gestational age birth: a DRIVERS meta-analysis of 12 European cohorts. J Epidemiol Community Health 2015;69:826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Verlohren S, Herraiz I, Lapaire O, Schlembach D, Zeisler H, Calda P, et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014;63:346–52. [DOI] [PubMed] [Google Scholar]
- [255].El Sherbiny WS, Soliman A, Nasr AS. Placental protein 13 as an early predictor in Egyptian patients with preeclampsia, correlation to risk, and association with outcome. J Investig Med 2012;60:818–22. [DOI] [PubMed] [Google Scholar]
- [256].Angelova M, Todorov I, Kovachev E. Early Prognostic Factors for the Progress of Preeclampsia - Our Experience in the Period 2010–2011. Open Access Maced J Med Sci 2016;4:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Odibo AO, Zhong Y, Goetzinger KR, Odibo L, Bick JL, Bower CR, et al. First-trimester placental protein 13, PAPP-A, uterine artery Doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta 2011;32:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]


