Abstract
Fanconi anemia (FA) is associated with bone marrow failure. Bone marrow (BM) from patients with FA and fanca−/− and fancc−/− mice are deficient in hematopoietic stem (HSCs) and progenitor cells (HPCs). Decreased HSCs/HPCs compromise their use in human and mouse hematopoietic cell transplantation (HCT) and gene therapy to correct genetic defects causing FA. We reported increased collection of HSCs from mouse bone marrow and mobilized peripheral blood, and human cord blood of normal donors after collection/processing in low (3%) oxygen (physioxia). We assessed comparative contents of long-term (LT)-HSCs from BM of fanca−/− and fancc−/− when collected/processed at 3% O2, in order to negate effects of extra physiological shock stress (EPHOSS) induced by collection/processing in ambient air. Collection/processing of BM from fanca−/− and fancc−/− mice in physioxia demonstrated a ≥3-fold increase in LT-HSCs compared to that in ambient air. This was associated with decreased phenotypic multipotential progenitor cells and functional granulocyte macrophage, erythroid, and multi-potential progenitors, results similar to that for BM from normal donor mice. Increased collection of HSCs could have clinical applicability for gene therapy and HCT.
Keywords: fanca−/− mice, fancc−/− mice, Hematopoietic stem and progenitor cells, Physioxia (Lowered O2 tension)
1. Introduction
Fanconi anemia (FA) is an autosomal recessive heterogeneous disorder with many defects, the most severe being failure of bone marrow (BM), with associated clonal disorders of the hematopoietic system including evolution of patients to myelodysplastic syndrome disorders (MDS) and acute myeloid leukemia [1, 2]. The first FANC gene, FANCC was identified 65 years after the first case of FA was published, by a complementation-cloning method [3]. FANCA (estimated 60% frequency) and FANCC (estimated 12% frequency) are amongst the most common FANC genes [2, 3]. Currently, more than 20 FA genes have been discovered [1, 3]. Successful treatment includes allogeneic hematopoietic cell transplantation (HCT) [1–5] and also gene therapy to correct the genetic abnormalities in the patients [1, 2, 6]. There are a large number of cDNAs that correspond to distinct FA complementation types. Various functional biological pathways have been implicated in abnormalities associated with FA and fanc genes. This includes DNA repair deficits, the cell cycle, and metabolism [1, 2, 7–12]. Mouse models of fanca−/− and fancc−/− have been used to better understand the hematological abnormalities associated with deletion of these specific fanc genes [7, 8, 11–13]. Being able to collect increased numbers of BM HSCs from fanca−/− and fancc−/− mice could help efforts to get enough long-term (LT)-HSCs for more effective gene transfer/therapy efforts. We used new information from our laboratories that demonstrated that 3–5 times as many highly engrafting LT-HSCs with self-renewal activity could be collected in physioxia conditions (3% O2) from BM [14] and mobilized peripheral blood (mPB) [15] of normal mice and from normal human cord blood cells [14] by collecting and processing cells at 3% O2 so that they are never subjected to ambient air (~21% O2). Using rigorous physioxia collection and processing procedures, we found that we could detect more than 3-fold the numbers of BM LT-HSCs from fanca−/− and fancc−/− mice than if these cells were collected/processed in ambient air, information of potential use for future gene transfer/therapy approaches with these cells, and possibly for human HSCs from patients with FA.
2. Methods and Materials
2.1. Mice
Aged (8–12 weeks old) and sex matched control wild type (WT), fanca−/−, and fancc−/− mice on a C57BL/6 strain background were used [7–12]. Mice were maintained under temperature- and light-controlled conditions (21–24°C, 12-hr light/12-hr dark cycle), and were fed ad libitium. All mouse studies were improved by the Institutional Animal Care and Use Committee members at the Indiana University School of Medicine.
2.2. Bone Marrow Cell Harvest
Once mice were euthanized, animals were immediately passed through an air lock chamber then into a temperature, humidity, O2 and CO2 controlled, custom built glove box (Hypoxic Chamber, Coy Lab Products) [14]. The glove box was maintained at 3% O2 by N2 balance and 5% CO2. Femurs were collected and flushed in sterile PBS (HyClone, GE Healthcare Life Sciences, Logan, Utah) within the glove box. Collected bone marrow (BM) cells were then split in half, with half kept in the hypoxic glove box and half moved to a biosafety hood exposed to ambient air conditions (~21% O2) where they remained for an hour prior to further processing so that the BM cells can acclimate to ambient air conditions, as we described [14]. Subsequent procedures such as staining cells for flow or setting up colony assays were done simultaneously in the hypoxic glove box or in the biosafety cabinet under ambient air conditions. When culturing cells under hypoxic conditions, cells were equilibrated in the hypoxic glove box such that at no time were the cells exposed to ambient air conditions, and then transferred from the glove box to an incubator maintained at 5% O2 by N2 balance and 5% CO2 via airtight containers. All solutions (e.g., fixatives), media, reagents (e.g., antibodies), plasticware, pipet tips, sterile instruments and gauze as well as anything else that could come into contact with the femur and cells (including the 70% ethanol used to sterilize the mouse prior to removal of the femur) for the hypoxia collected/processed cells were pre-equilibrated to hypoxic conditions in the 3% O2 glove box for at least 18 hours prior to use. All liquids (especially the methyl cellulose used for colony assays due to its viscosity) were vortexed vigorously to displace as much oxygenated air as possible. We emphasize the absolute requirement for extensive equilibration of all materials to the hypoxic conditions of the glove box prior to use and rigorous attention to details to obtain stable and reproducible results for the hypoxia collected/processed cells. The reagents used for ambient air-acclimated cells were kept under ambient air conditions.
2.3. Phenotypic analysis of HSCs/HPCs
These procedures were done exactly as reported [14, 15]. For analyzing hematopoietic stem (HSC) and progenitor (HPC) cell phenotypes in mouse BM, cells were collected at a concentration of ~2–3 × 106 cells per tube, washed in PBS, incubated in fluorescently-conjugated anti-mouse antibody cocktail for 20 minutes at room temperature, washed in PBS, and then fixed in 1% formaldehyde. Samples were analyzed on an LSRII flow cytometer (BD Biosciences). Single color compensation and isotype controls were included for each experiment. Data analysis was performed using FlowJo 7.6.3 software (TreeStar, WA, USA). Gates were determined using fluorescence-minus-one controls. Percent of each population was used to calculate absolute numbers of each population per femur. Phenotyping markers used were FITC-mouse lineage cocktail (CD3, Gr-1, CD11b, CD45R, Ter119; BioLegend; cat. # 133302), PE-CF594-anti-Ly6A/E (a.k.a. Sca-1; clone D7; BD Biosciences), APC-H7-anti-CD117 (a.k.a. c-Kit; clone 2B8; BD Biosciences), APC- or PE-anti-CD135 (a.k.a. Flt3; clone A2F10.1; BD Biosciences), and PE- or BV421-anti-CD34 (clone RAM34; BD Biosciences). HSC and HPC populations for mice are defined as follows- LSK cells: Lin− Sca-1+ c-Kit+, long-term (LT)-HSC: LSK Flt3− CD34−, short-term (ST)-HSC: LSK Flt3− CD34+, and multipotent progenitor (MPP): LSK Flt3+CD34+. For all antibodies used in these studies, the validation for the relevant species and applications can be found on the indicated manufacturer’s website.
2.4. Functional analysis of HPCs
For HPC colony assays, BM cells flushed from femurs of indicated mice were plated at 5 × 104 cells/mL in 1% methylcellulose culture medium with 0.1 mM hemin (Sigma-Aldrich; St. Louis, MO, USA), 30% FBS, 1 U/mL recombinant human erythropoietin (rhEPO; Amgen; Thousand Oaks, CA, USA), 50 ng/mL recombinant mouse stem cell factor (rmSCF; R&D Systems; cat. # 455-MC), and 5% vol/vol pokeweed mitogen mouse spleen cell conditioned medium. Colonies were scored after 6 days of incubation at 5% CO2 and lowered 5% O2 in a humidified chamber, and granulocyte-macrophage colony-forming units (CFU-GM), erythrocyte burst-forming units (BFU-E), and granulocyte, erythrocyte, macrophage, megakaryocyte colony-forming units (CFU-GEMM) were distinguished by morphology of colonies. Total numbers of colonies per femur were calculated. For high specific activity tritiated thymidine kill assays, BM cells were treated with 50 μCi of high specific activity [3H]Tdr (20 Ci/mmol; DuPont NEN) at room temperature for 40 minutes then washed twice prior to plating in HPC colony assays. These assays were performed exactly as reported [14–16].
2.5. Statistics
Results are expressed as average mean values ± standard error mean (SEM). Statistics were performed using one-way ANOVA with post-hoc Tukey’s Multiple Comparison test.
3. Results and Discussion
3.1. Phenotype analysis of LT-HSCs, ST-HSCs, and MPPs
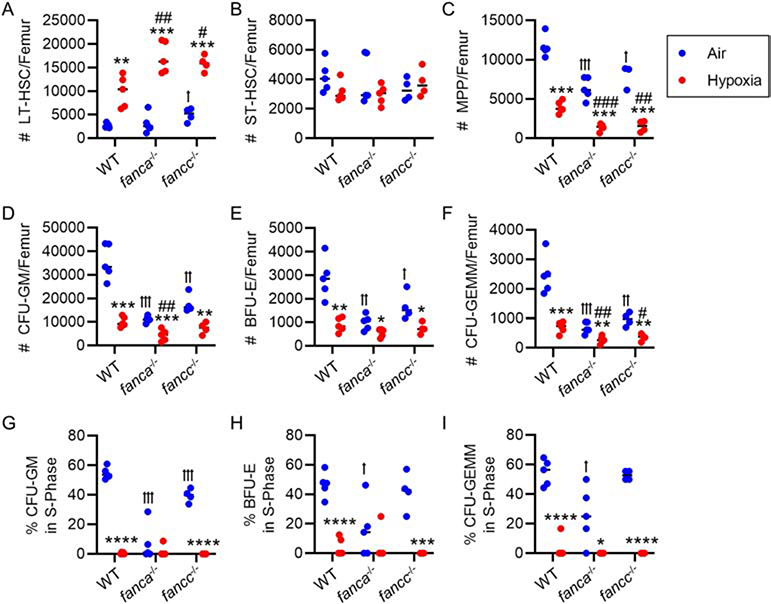
As previously reported for normal BM from C57BL/6 mice [14], we noted significantly increased numbers of LT-HSCs and decreased numbers of MPPs, with no changes in ST-HSCs when cells were collected/processed at 3% O2 (physioxia), compared to cells collected in hypoxia and then placed into ambient air O2 conditions for one hour (Fig. 1A–C). We previously published that mouse BM cells collected in ambient air manifested the same changes/phenotypes as those cells collected at 3% O2 and then transferred to ambient air for at least one hour [14]. While we didn’t detect significant differences in absolute numbers of LT-HSCs and ST-HSCs per femur between WT, fanca−/− and fancc−/− mice collected in ambient air (Fig. 1A&B), there were highly significant decreases in numbers of MPPs from the BM of fanca−/− and fancc−/− mice compared to that of normal control wildtype (WT) mouse BM (Fig. 1C). What was very clear was that fanca−/− and fancc−/− LT-HSCs, as well as WT-HSCs, were significantly increased at least 3-fold when cells were collected/processed at 3% O2 compared to cells collected at 3% O2 and placed at ambient air O2 tension for one hour before analysis (Fig. 1A). Coincident with the increased numbers of LT-HSCs from the WT, fanca−/− and fancc−/− mice collected/processed at 3% O2, there was an associated significant decrease in absolute numbers of phenotypically-identified MPPs (Fig. 1C). This suggests that, as previously reported [14], collection/processing of these BM cells under physioxia conditions decreases differentiation of HSCs to HPCs induced by ambient air induced extra physiological shock/stress (EPHOSS) effects. Consistent with decreases in phenotypically-defined MPPs, when BM from WT, fanca−/− and fancc−/− are assessed for numbers/femur and cycling status of functional colony forming cells (CFU-GM, BFU-E, and CFU-GEMM) after collection/processing cells at 3% O2, these HPCs are decreased (Fig. 1D–F) and are in a slow or non-cycling state (Fig. 1G–I).
Figure 1. Effect of hypoxic (3% O2) isolation and processing of bone marrow (BM) from fanca−/− and fancc−/− mice.
In a hypoxic glove box (acclimated for 18 hours to 3% O2) femurs of wildtype (WT), fanca−/− and fancc−/− mice were flushed in sterile PBS, counted, and then split in half. One half remained under hypoxic conditions (indicated as ‘Hypoxia’) and the other was removed from the hypoxic glove box and acclimated for 1 hour to ambient air (indicated as ‘Air’). (A-C) Long-term (LT)-HSC defined as Lin− Sca-1+ c-Kit+ Flt3− CD34− (A), short-term (ST)-HSC defined as Lin− Sca-1+ c-Kit+ Flt3− CD34+ (B) and multipotent progenitors (MPP) defined as Lin− Sca-1+ c-Kit+ Flt3+ CD34+ (C) number per femur were determined by flow cytometry. (D-I) Nucleated BM cells from each group were utilized in a hematopoietic progenitor cell (HPC) colony forming assay stimulated in vitro with EPO, SCF, PWMSCM, and hemin and cultured at 5% O2 (D-F) with the percent of HPC in S-phase defined by high specific activity tritiated thymidine kill assay (G-I). The number of colony-forming units granulocyte-macrophage (CFU-GM; D&G), burst-forming unit erythroid (BFU-E; E&H) and CFU granulocyte-erythrocyte-macrophage-megakaryocyte (CFU-GEMM; F&I) was calculated per femur (with each mouse plated in triplicate). (A-I) n=4–5 mice/group. Data are the mean ± SEM. Statistics were performed using one-way ANOVA with post-hoc Tukey’s Multiple Comparison test. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 when comparing air to hypoxia in the same mouse type. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 when comparing air to hypoxia in the same mouse type. ꝉ p < 0.05, ꝉꝉ p < 0.01, ꝉꝉꝉ p < 0.001 when comparing to WT in air. # p < 0.05, ## p < 0.01, ### p < 0.001 when comparing to WT in hypoxia.
The main take away message of this present study is that it is possible to obtain significantly increased numbers of LT-HSCs from fanca −/− and fancc −/− BM, if the cells are collected/processed in physioxia conditions of 3% O2, and not exposed to EPHOSS induced ambient air conditions which cause loss of LT-HSCs with increased numbers of MPPs and cycling functional populations CFU-GM, BFU-E, and CFU-GEMM. It should be noted that while physioxia collection/processing of BM cells results in decreased numbers of HPCs, there are still plenty of HPCs left for engraftment [14, 15, 18]. We have recently demonstrated that collecting/processing HSCs at cold (4°C) temperature results in a similar preservation of HSC numbers/function as hypoxia collection/processing procedures [19]. However, it is not yet clear if some of the intracellular mechanisms associated with physioxia vs. ambient air collections (p53-mitochondrial permeability transition pore opening-cyclophilin D, and hypoxia inducing factor-1 alpha and the hypoxamir, miR210) [14, 16] are similar to that of cold collection/processing of the cells [19].
Being able to collect more LT-HSCs could allow for more efficacious efforts for gene transfer and gene therapy for gene correction [2, 6, 8, 20] and for studies on fanca−/− and fancc−/− BM effects of eventual transition of these cells to a pre-malignant (e.g., MDS) or malignant state (e.g., leukemia). Of course, there are a number of considerations that must still be addressed. First, it is not clear how efficiently the physioxia collected and processed LT-HSCs can be gene transduced, studies still need to be done. It is possible that these physioxia collected cells may be in a state less susceptible to efficient gene transduction efforts (e.g., they may be in a more slowly cycling state and may need a more effective pre-stimulus to make them efficient for gene transduction protocol). Also, we have not yet analyzed how efficiently the physioxia collected/processed HSCs can engraft in a mouse transplant setting as defined by chimerism in a competitive repopulating assay using congenic donor, competitor, and recipients, and using limitng dilution analysis of donor cells to calculate competitive repopulating units (a quantitative measure of functional HSCs) [17] in order to determine if the increase in LT-HSCs noted for fanca−/− and fancc−/− BM cells collected/processed in physioxia is as good as, better than, or less effective than our previous experience with WT HSCs [14, 15]. A final question is how relevant the mouse studies will be for eventual translation to a clinical situation to improve HSC numbers and fitness from patients with FA for autologous HCT. We consider the present study a start to a rigorous understanding of eventually improving the treatment of patients with FA.
Acknowledgements
These studies were supported by United States of America Public Health Service Grants from the National Institutes of Health: R35 HL139599 to HEB, R01 DK 109188 to HEB and MLC, U54 DK106846 to HEB, R01 HL 132921 to DWC and supplement to R01 HL 132921 to ESP.
Footnotes
Conflict of Interest Statement
None of the co-authors have any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dror Y (2018). Inherited Bone Marrow Failure Syndromes In: Hematology: Basic Principles and Practice. 7th Edition (Hoffman R, Benz E, Silberstein L, Heslop H, Weitz JI, and Anastasi J, Salama ME, and Abutalib SA, Editors). Chapter 29. Pages 350–393. [Google Scholar]
- 2.Nalepa G, & Clapp DW (2018). Fanconi anaemia and cancer: an intricate relationship. Nature reviews. Cancer, 18(3), 168–185. 10.1038/nrc.2017.116 [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Nebert DW, Bruford EA, Thompson DC, Joenje H, & Vasiliou V (2015). Update of the human and mouse Fanconi anemia genes. Human genomics, 9, 32 10.1186/s40246-015-0054-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, & Lehn P (1989). Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. The New England journal of medicine, 321(17), 1174–1178. 10.1056/NEJM198910263211707 [DOI] [PubMed] [Google Scholar]
- 5.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, & Boyse EA (1989). Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 86(10), 3828–3832. 10.1073/pnas.86.10.3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Río P, Navarro S, Wang W, Sánchez-Domínguez R, Pujol RM, Segovia JC, Bogliolo M, Merino E, Wu N, Salgado R, Lamana ML, Yañez RM, Casado JA, Giménez Y, Román-Rodríguez FJ, Álvarez L, Alberquilla O, Raimbault A, Guenechea G, Lozano ML, Cerrato L, Hernando M, Gálvez E, Hladun R, Giralt I, Barquinero J, Galy A, García de Andoín N, López R, Catalá A, Schwartz JD, Surrallés J, Soulier J, Schmidt M, Díaz de Heredia C, Sevilla J, & Bueren JA (2019). Successful engraftment of gene-corrected hematopoietic stem cells in non-conditioned patients with Fanconi anemia. Nature medicine, 25(9), 1396–1401. 10.1038/s41591-019-0550-z [DOI] [PubMed] [Google Scholar]
- 7.Freie B, Li X, Ciccone SL, Nawa K, Cooper S, Vogelweid C, Schantz L, Haneline LS, Orazi A, Broxmeyer HE, Lee SH, & Clapp DW (2003). Fanconi anemia type C and p53 cooperate in apoptosis and tumorigenesis. Blood, 102(12), 4146–4152. 10.1182/blood-2003-03-0971 [DOI] [PubMed] [Google Scholar]
- 8.Haneline LS, Li X, Ciccone SL, Hong P, Yang Y, Broxmeyer HE, Lee SH, Orazi A, Srour EF, & Clapp DW (2003). Retroviral-mediated expression of recombinant Fancc enhances the repopulating ability of Fancc−/− hematopoietic stem cells and decreases the risk of clonal evolution. Blood, 101(4), 1299–1307. 10.1182/blood-2002-08-2404 [DOI] [PubMed] [Google Scholar]
- 9.Nalepa G, & Clapp DW (2014). Fanconi anemia and the cell cycle: new perspectives on aneuploidy. F1000prime reports, 6, 23 10.12703/P6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravera S, Dufour C, Degan P, & Cappelli E (2018). Fanconi anemia: from DNA repair to metabolism. European journal of human genetics: EJHG, 26(4), 475–476. 10.1038/s41431-017-0046-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdul-Sater Z, Cerabona D, Potchanant ES, Sun Z, Enzor R, He Y, Robertson K, Goebel WS, & Nalepa G (2015). FANCA safeguards interphase and mitosis during hematopoiesis in vivo. Experimental hematology, 43(12), 1031–1046.e12. 10.1016/j.exphem.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Wilson AF, Du W, & Pang Q (2018). Cell-Cycle-Specific Function of p53 in Fanconi Anemia Hematopoietic Stem and Progenitor Cell Proliferation. Stem cell reports, 10(2), 339–346. 10.1016/j.stemcr.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulliam AC, Hobson MJ, Ciccone SL, Li Y, Chen S, Srour EF, Yang FC, Broxmeyer HE, & Clapp DW (2008). AMD3100 synergizes with G-CSF to mobilize repopulating stem cells in Fanconi anemia knockout mice. Experimental hematology, 36(9), 1084–1090. 10.1016/j.exphem.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantel CR, O’Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, Brustovetsky N, Srour EF, Lee MR, Messina-Graham S, Haas DM, Falah N, Kapur R, Pelus LM, Bardeesy N, Fitamant J, Ivan M, Kim KS, & Broxmeyer HE (2015). Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell, 161(7), 1553–1565. 10.1016/j.cell.2015.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aljoufi A, Cooper S, & Broxmeyer HE (2020). Collection and Processing of Mobilized Mouse Peripheral Blood at Lowered Oxygen Tension Yields Enhanced Numbers of Hematopoietic Stem Cells. Stem Cell Reviews and Reports. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capitano ML, Griesenauer B, Guo B, Cooper S, Paczesny S, & Broxmeyer HE (2020). The IL-33 Receptor/ST2 Acts as a Positive Regulator of Functional Mouse Bone Marrow Hematopoietic Stem and Progenitor Cells. Blood Cells Molecules and Diseases. 84, 102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper SH, Broxmeyer HE, & Capitano ML (2020). Experimental Mouse Models of Mouse and Human Hematopoietic Stem Cell Transplantation In, Methods in Molecular Biology: Hematopoietic Stem Cells (Eds: Pelus LM and Hoggatt J). Springer Nature, New York, In Press. [DOI] [PubMed] [Google Scholar]
- 18.Broxmeyer HE, O’Leary HA, Huang X, & Mantel C (2015). The importance of hypoxia and extra physiologic oxygen shock/stress for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex vivo. Current opinion in hematology, 22(4), 273–278. 10.1097/MOH.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broxmeyer HE, Cooper S, & Capitano ML (2020). Enhanced Collection of Phenotypic and Engrafting Human Cord Blood Hematopoietic Stem Cells at 4°C. Stem Cells. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zonari E, Desantis G, Petrillo C, Boccalatte FE, Lidonnici MR, Kajaste-Rudnitski A, Aiuti A, Ferrari G, Naldini L, & Gentner B (2017). Efficient Ex Vivo Engineering and Expansion of Highly Purified Human Hematopoietic Stem and Progenitor Cell Populations for Gene Therapy. Stem cell reports, 8(4), 977–990. 10.1016/j.stemcr.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]