Abstract
Background
Individuals with normal weight obesity (NWO) have increased cardiometabolic disease and mortality risk, but factors contributing to NWO development are unknown.
Objective
The objective of this study was to determine if diet quality scores and physical fitness levels differed between adults classified as lean, NWO, and overweight-obese. Secondary objectives of the study were to compare clinical biomarkers and food groups and macronutrient intakes between the three groups, and test for associations between body composition components with diet quality scores and physical fitness levels.
Design
This is a secondary data analysis from a cross-sectional study that included metropolitan university and healthcare system employees. Body composition was measured by dual energy x-ray absorptiometry. Individuals with a body mass index (BMI) below 25 and body fat >23% for men and >30% for women were classified as having NWO. Alternate Healthy Eating Index (AHEI), Dietary Approach to Stop Hypertension (DASH) score, and Mediterranean Diet Score (MDS) were calculated from Block food frequency questionnaires. Physical fitness was assessed by measuring maximum oxygen consumption (VO2 maximum) during treadmill testing.
Participants/setting
This study included 693 adults (65% female, mean age 48.9 ± 11.5 years) enrolled between 2007 and 2013 in Atlanta, Georgia.
Main outcome measures
The main outcome measures were AHEI, DASH, and MDS diet quality scores and VO2 maximum.
Statistical analyses
Multiple linear regression analyses with post-hoc comparisons were used to investigate group differences in fitness, diet quality, and biomarkers. Regression analyses were also used to examine relationships between diet quality scores and fitness with body composition.
Results
VO2 maximum was significantly lower in the NWO compared to the lean group (36.2 ± 0.8 vs. 40.2 ± 1.0 mL/min/kg, p<0.05). Individuals with NWO reported similar diet quality to lean individuals and more favorable AHEI and DASH scores than overweight-obese individuals (p<0.05). Diet quality and VO2 maximum were inversely associated with percent body fat and visceral adipose tissue (p<0.05) regardless of weight status. Individuals with NWO exhibited higher fasting blood insulin concentrations, insulin resistance, LDL cholesterol, and triglyceride levels, and significantly lower HDL cholesterol levels than lean individuals (p<0.05).
Conclusions
Physical fitness was significantly decreased in individuals with NWO compared to lean individuals. Higher diet quality was associated with decreased total and visceral fat but did not distinguish individuals with NWO from lean individuals.
Keywords: Exercise, fat distribution, nutrition, overweight
INTRODUCTION
Obesity is a principal, preventable risk factor for numerous well-characterized diseases such as cardiovascular disease, type 2 diabetes, and some cancers, which represent leading causes of death globally1,2. When examining associations with disease, obesity is typically assessed by body mass index (BMI) to identify at-risk individuals. While BMI is an important measure used for epidemiology surveillance, clinical observations reveal that individuals classified as normal weight or obese using BMI may present with an unhealthy or healthy metabolic profile, respectively3, 4 Thus, BMI does not capture the large heterogeneity in cardiometabolic risk observed across individuals. This is partly due to BMI lacking the sensitivity needed to distinguish the proportions of fat and fat free mass that contribute to total body weight, and BMI provides no indication of fat mass distribution, which are important drivers of metabolic disease development5, 6. Further, BMI does not account for age, race, sex, or fitness level, which influence body composition7. Therefore, assessment of obesity and disease risk using BMI categories may misclassify individuals at risk for chronic disease.
Normal weight obesity (NWO) has emerged as a term to denote individuals who have a normal weight according to BMI guidelines but a disproportionately high body fat mass8. Studies have suggested the NWO body composition phenotype is associated with increased risk of chronic diseases and cardiometabolic abnormalities, including dyslipidemia, hypertension, glucose intolerance, and increased levels of inflammation and oxidative stress markers8–15. Further, individuals with NWO exhibit a higher mortality risk compared to their lean counterparts and metabolically healthy obese individuals 8,13,16,17. Despite this increase in disease risk, individuals with NWO may be overlooked by health professionals and/or misclassified as “healthy” when solely utilizing BMI as a screening tool18.
Specific factors driving the prevalence of NWO are unknown. Lifestyle factors, such as diet quality and physical activity are important factors influencing health and disease. A higher quality diet, characterized by a greater consumption of vegetables, fruits, whole grains, healthy fats, and lean proteins, is associated with lower chronic disease and mortality risk19–23. A higher diet quality, assessed by indexes such as the Dietary Approach to Stop Hypertension (DASH) diet score, Alternate Healthy Eating Index (AHEI), and Alternate Mediterranean Diet Score (MDS), is associated with improvements in blood pressure, lower biomarkers of inflammation and oxidative stress, and reduced risk for type 2 diabetes and cardiovascular disease19–28. Additionally, physical inactivity is a major risk factor for chronic diseases and accounts for an estimated 9% of global premature mortality from leading cardiometabolic diseases29, 30. Physical fitness is a measurable attribute that reflects an individual’s ability to perform physical activity31. Knowledge is limited on the role of diet quality and physical fitness in individuals with NWO. The objective of this study was to examine diet quality scores and physical fitness levels between adults categorized into three body composition subtypes (lean, NWO, and overweight-obese). It was hypothesized that individuals with NWO would have similar diet quality scores and physical fitness levels as individuals with overweight-obesity and lower diet quality scores and physical fitness levels than lean participants. Given that lifestyle behaviors are key drivers of metabolic health and disease32, secondary aims of this study were to provide extensive dietary and metabolic phenotyping of individuals with NWO by comparing clinical biomarkers, markers of oxidative stress and inflammation, and food group and macronutrient intakes between the three groups. A final objective of the study was to test for associations between body composition components with diet quality scores and physical fitness levels.
MATERIALS AND METHODS
Participants and Study Design
This cross-sectional study utilized the Emory-Georgia Tech Center for Health Discovery and Well Being Predictive Health Institute cohort (http://predictivehealth.emory.edu) based in Atlanta, Georgia, USA, and is a secondary analysis of existing data. This cohort is comprised of Emory University and Emory Healthcare employees who had been employed within the Emory system for at least two years. General exclusion criteria were having a poorly controlled chronic disease, acute illness, hospitalization within the previous year, and women who were pregnant or breastfeeding. From an alphabetized list of all eligible employees of Emory University and Emory HealthCare (approximately 30,000), every 10th employee was invited by email to participate in the study. Of the approximately 3,000 invited employees that responded to the email and underwent initial screening in the electronic medical record and then by telephone interview (if available), a total of 739 were ultimately deemed eligible and enrolled in the study. This final number was also influenced by the availability of funds at the time of cohort recruitment. The complete study protocol was previously described33, 34 The study was approved by the Emory Institutional Review Board and all participants provided informed consent. Participants underwent extensive metabolic testing, including clinical laboratory analysis, dietary assessment, and exercise testing. Demographic information, educational attainment, and annual household income were self-reported. Participants were categorized as having a history of chronic disease (hypertension, hyperlipidemia, or diabetes mellitus) if they reported a past or current diagnosis or were currently taking medications to treat hypertension, hyperlipidemia, or diabetes mellitus. Only participants with available body composition and anthropometric data were included in this analysis (n=693).
Body Composition Analysis and Body Composition Subgroups
Body composition, including visceral adipose tissue (VAT), was assessed by dual energy x-ray absorptiometry (DXA) using a Lunar iDXA densitometer and CoreScan software (GE Healthcare, Madison, WI, USA). A single measure of height and weight were taken in light clothing without shoes using a digital scale and stadiometer (Tanita TBF-25, Tanita Health Management, Arlington Heights, IL). Height was recorded to the nearest eighth of an inch and weight was recorded to the nearest tenth of a pound, and both measures were converted to metric units. BMI was calculated as body weight in kilograms divided by height in meters squared (kg/m2). Participants were categorized as either lean, NWO, or overweight-obese based on BMI and sex-specific body fat percent cut points. A body fat percent above 23 was considered elevated for males, and a body fat percent above 30 was considered elevated for females based on previously published literature35. Lean participants had a BMI value between 18.5 and 24.9 kg/m2 and a body fat percent below the sex-specific cut-off values. NWO was characterized as a BMI between 18.5 and 24.9 kg/m2 and a body fat percent above the sex-specific cut-off values. Overweight-obese participants had a BMI ≥25 kg/m2 and a body fat percent above the sex-specific cut-off values. A health professional trained in anthropometry assessed waist circumference (WC) to the closest millimeter using a tape measure. Three WC measurements were taken at the umbilicus, and the average value is reported.
Diet Quality Scores, Dietary Food Groups, and Macronutrients
Diet quality scores were calculated from dietary intake data assessed using 2005 Block food frequency questionnaires (FFQ, NutritionQuest, Berkeley, CA, USA), which reflected dietary intake over the past year36–38. Reported intakes that were less than 500 kcal per day or greater than 5,000 kcal per day were excluded for implausible values. All dietary data were energy adjusted per 1000 kcal. Three diet quality scores were calculated as previously described within this cohort24: AHEI28, DASH39 with adapted scoring of the sweets component25, 40, and MDS41. The AHEI ranges from 0–87.5, DASH score ranges from 0–11, and MDS ranges from 0–9. For all diet quality scores, a higher score is indicative of a higher quality, more healthful diet. Independent of the diet quality scores, reported dietary intake of food group and macronutrients (i.e., grains, fiber, sugar, fruit, vegetables, and proteins) were also compared between lean, NWO, and overweight-obesity groups.
Physical Fitness
Physical fitness was objectively measured by assessing maximal oxygen consumption (VO2 maximum, mL/min/kg) following a modified Balke protocol performed with a trained technician42. All VO2 maximum tests were conducted on a GE T2100 Treadmill (GE Healthcare, Waukesha, WI). VO2 maximum is a measure of cardiorespiratory fitness and captures the ability of the entire cardiovascular system to uptake and utilize oxygen during exercise31.
Clinical, Oxidative Stress, and Inflammatory Markers
All blood samples were taken following an overnight fast, processed, and stored for analysis. Fasting lipid profile, metabolic panel, and inflammatory markers were analyzed commercially by Quest Diagnostics (Valencia, CA). Fasting insulin levels below the level of detection (<2 μIU/mL) were replaced with a value of 1.9 for analyses. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as fasting insulin (μU/mL) x fasting glucose (mg/dL) divided by 40543. An automated machine was used to measure systolic and diastolic blood pressure (Omron, Kyoto, Japan). Plasma aminothiol concentrations, including glutathione (GSH), glutathione disulfide (GSSG), cysteine (Cys), and cystine (CySS), were measured using high performance liquid chromatography following published protocols44 at Emory University. The reduction-oxidation (redox) potentials (Eh) for the thiol/disulfide couples were calculated using the Nernst equation44. Eh provides a measure of the propensity of the redox couples to accept or donate electrons, and a higher value denotes increased oxidative stress. The inflammatory cytokines interleukin-6 (IL-6), IL-8, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) were measured in fasting serum using Fluorokine® MultiAnalyte Profiling multiplex kits (R&D Systems, Minneapolis, MN) with a Bioplex analyzer (Bio-Rad, Hercules, CA).
Statistical Analyses
Data are summarized as mean ± standard error for continuous variables or as counts and proportions for categorical variables. Continuous variables that did not appear to have a bell-shaped distribution were natural log transformed for analyses and back transformed to obtain geometric means. To account for zero values in cytokine analyses, a constant of one was added to all values prior to log transformation. Because the standard error could not be back transformed, 95% confidence intervals along with the geometric mean are presented for log-transformed variables. For categorical variables, χ2 tests were used to test for differences between the body composition subtypes. ANOVA tests were used to examine differences between groups for demographic characteristics that were continuous variables. Overall group differences in the diet quality scores, dietary food groups and macronutrients, and VO2 maximum between the body composition subtypes were tested using multiple regression analyses, controlling for age (continuous), race (White=0, other=1), sex (male=0, female=1), and education (college degree, no=0, yes=1). Post-hoc comparisons between the body composition subtypes were conducted using Tukey’s honestly significant different tests. Regression analyses also provided estimates of the group differences in dietary food groups, macronutrients, and clinical and biochemical variables controlling for age (continuous), race (White=0, other=1), and sex (male=0, female=1) with post-hoc comparisons conducted using Tukey adjustment for multiple testing. Multiple linear regression models also were constructed to test for associations between components of body composition and VO2 maximum and the diet quality scores, controlling for age (continuous), race (White=0, other=1), sex (men=0, women=1), and education (college degree, no=0, yes=1). Presence of a chronic disease and reported annual household income were also considered as potential confounders but were not included in final models because they were not significantly associated with the body composition subtypes, diet quality scores, or physical fitness level. Finally, interactions of body composition subtypes with race, sex, age and education were tested within the fitted linear models for the corresponding product terms (e.g, body composition subtype × race, body composition subtype × sex, etc). All analyses were conducted in JMP® Pro software version 13.0.0 (SAS Institute, Cary, NC)45, using two-sided tests with a significance level of 0.05.
RESULTS
Demographic and clinical characteristics for all participants are shown in Table 1. BMI and DXA body composition data were available for 693 of the study participants of which 14% were classified as lean, 24% as having NWO, and 62% as having overweight-obesity. There were 14 participants (10 males) with BMI levels in the overweight category and percent body fat values below the sex-specific cut points; these individuals were categorized as lean. There were 9 participants (8 females) with BMI levels below 18.5. Seven of these individuals had percent body fat values below the sex-specific cut points and were categorized as lean. One female had a body fat percent above the sex-specific cut point and was categorized as NWO. The lean group was younger than the NWO and overweight-obesity groups (p<0.05). There was a significant difference in sex distribution between the body composition subtypes (p=0.02). Among all female participants, 14% were categorized as lean, 27% as NWO, 59% as overweight-obesity. Among all male participants, 16% were categorized as lean, 17% as NWO, and 67% as overweight-obesity. The proportion of individuals with a history of chronic disease was comparable between the three groups. The lean group had the highest proportion of participants report attaining a college degree or higher (90%) followed by participants with NWO (84%) and participants with overweight-obesity (80%, p=0.047). There were no differences in reported annual household income between the three groups (p>0.05).
Table 1.
Demographic and clinical characteristics of 693 adults participating in the Emory-Georgia Tech Predictive Health Initiative cohort from 2007–2013 according to body composition subtype.
| Characteristic | Lean (n=100) | Normal Weight Obesity (n=164) | Overweight-Obesity (n=429) |
|---|---|---|---|
| Age, y (mean ± SD) | 43.3 ± 12.8y | 49.1 ± 11.4z | 50.0 ± 10.9z |
| Sex [n (%)] | |||
| Women | 62 (62) | 122 (75) | 267 (62)* |
| Men | 38 (38) | 42 (26) | 162 (38)* |
| Race [n (%)] | |||
| White | 81 (83) | 120 (74) | 287 (68) |
| African-American, Asian, American Indian | 19 (17) | 44 (26) | 142 (32) |
| Presence of chronic disease [n (%)] (hypertension, diabetes, hyperlipidemia) | 14 (14) | 34 (21) | 94 (22) |
| College degree or higher [n (%)] | 90 (90) | 137 (84) | 342 (80)* |
| Annual household income [n (%)] a | |||
| ≤ $50,000/year | 7 (8) | 17 (11) | 51 (12) |
| > $50,000-$100,000/year | 23 (26) | 37 (24) | 126 (31) |
| > $100,000-$200,000/year | 31 (34) | 60 (39) | 145 (35) |
| > $200,000/year | 29 (32) | 39 (25) | 89 (22) |
n= 90, 153, and 411 for the lean, NWO, and overweight-obesity groups, respectively
Results of χ2 test showed a significant difference between the three groups, p<0.05.
For continuous variables, one-way ANOVA was performed and results of Tukey post hoc analyses are denoted by superscript letters y and z and indicate significant differences between groups for each row. Values that are not connected by the same letter are significantly different at P < 0.05.
Body Composition and Fat Distribution
Body composition measurements are shown in Table 2. In line with the applied definition of NWO, BMI was similar between the lean and NWO groups and was significantly lower than the overweight-obesity group (p<0.05). Total body fat percent, fat mass, VAT, and WC increased significantly across the three groups from individuals classified as lean to having NWO to having overweight-obesity (p<0.05). Individuals with NWO had the lowest lean body mass (LBM), which differed significantly between the three groups (p<0.05).
Table 2.
Body composition and fat distribution measures among adult participants in the Emory University-Georgia Tech Predictive Health Initiative cohort from 2007–2013 classified by body composition subtype (n=693)a
| Body composition measure | Lean (n=100) | Normal Weight Obesity (n=164) | Overweight- Obesity (n=429) |
|---|---|---|---|
| Body mass index (kg/m2) | 22.4 ± 0.5x | 23.0 ± 0.4x | 31.3 ± 0.2y |
| Body fat (%) | 22.6 ± 0.5x | 31.1 ± 0.4y | 38.1 ± 0.2z |
| Lean mass (kg) | 48.5 ± 0.8x | 44.1 ± 0.6y | 52.3 ± 0.4z |
| Fat mass (kg) | 14.4 ± 1.0x | 20.3 ± 0.8y | 35.0 ± 0.5z |
| Visceral adipose tissue (kg)b | 0.17 (0.15, 0.2)x | 0.44 (0.39, 0.49)y | 1.21 (1.13, 1.3)z |
| Waist circumference (cm) | 79.7 ± 1.9x | 85.7 ± 1.6y | 101.0 ± 0.9z |
Values are presented as mean ± SE or geometric mean (95% confidence interval), adjusting for age, race, and sex.
Variable was natural log transformed for analyses and back transformed for data presentation. Because the standard error cannot be back transformed, 95% confidence intervals are shown.
Results of multiple linear regression and Tukey post hoc analyses are denoted by superscript letters x, y, and z and indicate significant differences between groups for each variable. Within rows, values that are not connected by the same letter are significantly different at P < 0.05.
Diet Quality Scores, Dietary Food Groups, and Macronutrients
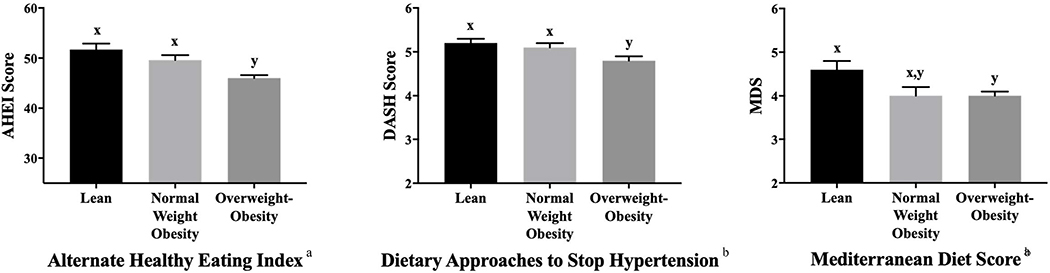
On average, all diet quality scores reflected similar trends: the lean group reported the highest diet quality but was not significantly different from the NWO group (Figure 1). For AHEI and DASH scores, lean and NWO groups had similar diet quality scores (p>0.05), each with significantly higher scores compared to the overweight-obese group (p<0.05). For the MDS, only the lean and overweight-obesity groups significantly differed (p<0.05). The association between body composition and AHEI differed according to education level (p=0.03); the same can be said about the association between body composition and MDS (p=0.04). For individuals without college degrees, there were no differences in AHEI or MDS scores across the body composition subtypes, and for individuals with college degrees the pattern was the same as the overall findings. The association between the DASH diet quality score and body composition groups differed according to race (p=0.008). Among individuals who reported White race, DASH diet quality scores differed only between the lean and overweight-obesity groups with the NWO group reporting similar scores to both. Among other race categories, only the NWO group reported higher DASH diet quality scores than the overweight-obesity group. These results should be interpreted with caution due to the post-hoc nature of these analyses. Stratified analyses with sex as a biological variable are reported in Table 3 (online only). In multiple linear regression analyses, all diet quality scores were significantly, inversely associated with measures of body fat and VAT (p<0.001 for all) but were not associated with measures of WC (Table 4).
Figure 1.
Average (Mean ± SE, adjusting for age, race, sex, and education) reported diet quality scores for participants classified as lean (n=98), having normal weight obesity (n=162), or as having overweight-obesity (n=427) participating in the Emory-Georgia Tech Predictive Health Initiative cohort classified according to body composition subtype. AHEI scores (adjusted mean ± SE) were 51.7 ± 1.2 for the lean, 49.6 ± 1.0 for the NWO, and 46.0 ± 0.6 for the overweight-obesity groups. DASH scores (adjusted mean ± SE) were 5.2 ± 0.1 for the lean, 5.1 ± 0.1 for the NWO, and 4.8 ± 0.1 for the overweight-obesity groups. MDS (adjusted mean ± SE) were 4.6 ± 0.2 for the lean, 4.3 ± 0.2 for the NWO, and 4.0 ± 0.1 for the overweight-obesity groups.
a There was significant effect modification by education status for alternate healthy eating index (AHEI) and Mediterranean diet score (MDS) variables. Among individuals without a college degree, there was no difference in AHEI or MDS between the lean, normal weight obesity, or overweight-obesity groups (p>0.05).
b There was significant effect modification by race for dietary approaches to stop hypertension (DASH) score. Among individuals who reported White race, the lean group reported significantly higher DASH diet quality scores compared to the overweight-obesity group (p<0.05). Values in the normal weight obesity group were similar to both the lean and overweight/obesity group (p>0.05).
x y Results of Tukey’s post hoc analyses are denoted by superscript letters x and y and indicate significant differences between groups for each row. Values that are not connected by the same letter are significantly different at P < 0.05.
Table 3 (online only).
Comparisons of diet quality scores and physical fitness among 687 adults within the Emory University-Georgia Tech Predictive Health Initiative cohort from 2007–2013 classified into three body composition subtypes and stratified by sex a
| Diet Quality Score or Fitness Measure | Lean | Normal Weight Obesity | Overweight-Obesity |
|---|---|---|---|
| Female participants | |||
| Alternative Healthy Eating Indexb | 55.0 ± 1.5x | 51.4 ± 1.1x | 47.3 ± 0.7y |
| DASH Score b,c | 5.2 ± 0.1x | 5.0 ± 0.1x | 4.7 ± 0.1y |
| Mediterranean Diet Scoreb | 5.0 ± 0.2x | 4.4 ± 0.2x,y | 4.0 ± 0.1y |
| VO2 Maximum (mL/min/kg)d | 37.4 ± 1.2x | 32.6 ± 0.8y | 28.9 ± 0.5z |
| Male participants | |||
| Alternative Healthy Eating Indexb | 50.1 ± 1.9 x | 48.1 ± 1.7 x | 47.0 ± 1.1 x |
| DASH Score b,c | 5.3 ± 0.2 x | 5.3 ± 0.2 x | 5.0 ± 0.1 x |
| Mediterranean Diet Scoreb | 4.6 ± 0.3 x | 4.6 ± 0.3 x | 4.4 ± 0.2 x |
| VO2 Maximum (mL/min/kg)d | 44.4 ± 1.7x | 41.4 ± 1.5y | 38.1 ± 1.0y |
Values are presented as mean ± SE adjusting for age and race.
n=62, 121, and 267 in the lean, NWO, and overweight-obesity groups, respectively, in females and n=36, 41, and 160 in the lean, NWO, and overweight-obesity groups, respectively, in males
DASH, Dietary Approaches to Stop Hypertension
VO2, volume of oxygen. n=57, 116, and 232 in the lean, NWO, and overweight-obesity groups, respectively in females and n=34, 38, and 151 in the lean, NWO, and overweight-obesity groups, respectively in males
Results of multiple linear regression and Tukey post hoc analyses are denoted by superscript letters x, y, and z and indicate significant differences between groups for each row. Within rows, values that are not connected by the same letter are significantly different at P< 0.05.
Table 4.
Cross-sectional associations between body composition measures, diet quality scores, and physical fitness in 693 adult participating in the Emory Georgia-Tech Predictive Health Initiative cohort from 2007–2013 [β ± SE (p-value)]a
| Body Composition Measure | AHEIb | DASHc | MDSd | VO2 maximum (mL/min/kg)e |
|---|---|---|---|---|
| BMI | −0.09 ± 0.02 (<0.001) | −1.0 ± 0.23(<0.001) | −0.27 ± 0.13 (0.04) | −0.22 ± 0.06 (<0.001) |
| Body fat % | −0.16 ± 0.02 (<0.001) | −1.54 ± 0.26 (<0.001) | −0.73 ± 0.15 (<0.001) | −0.35 ± 0.03 (<0.001) |
| Lean mass (kg) | −0.02 ± 0.03 (0.45) | −0.42 ± 0.29 (0.15) | 0.17 ± 0.16 (0.29) | −0.11 ± 0.04 (0.003) |
| Fat m ass (kg) | −0.21 ± 0.04 (<0.001) | −2.05 ± 0.47 (<0.001) | −0.71 ± 0.26 (0.008) | −0.52 ± 0.05 (<0.001) |
| Visceral adipose tissue (kg)f | −0.02 ± 0.003 (<0.001) | −0.18 ± 0.04 (<0.001) | −0.06 ± 0.02 (0.003) | −0.04 ± 0.004 (<0.001) |
| Waist circumference (cm) | −0.15 ± 0.08 (0.05) | −1.00 ± 0.80 (0.21) | −0.37 ± 0.47 (0.43) | −0.63 ± 0.11 (<0.001) |
All coefficient estimates are from multiple linear regression analyses with body composition measures as a continuous variable. Analyses were conducted individually for each measure of body composition. All estimates are adjusted for age, race, sex, and education.
AHEI, Alternate Healthy Eating Index
DASH, Dietary Approaches to Stop Hypertension Score
MDS, Mediterranean Diet Score
VO2, volume of oxygen consumption
Variable was log transformed for analyses.
In comparisons of reported daily food group and macronutrient consumption, participants with overweight-obesity reported significantly higher consumption of saturated fats, trans-fats, and meats and significantly lower consumption of carbohydrates, fiber, fruits and legumes, nuts, and soy compared to lean and NWO participants (Table 5 [online only], p<0.05 for all). The NWO group reported lower consumption of total fat and protein, refined grains and significantly higher consumption of yellow/orange vegetables than the overweight-obese group (p<0.05 for all). Only reported potato consumption differed significantly between each group (p<0.05).
Table 5 (online only).
Comparison of reported daily intake of food groups and macronutrients between 687 adults within the Emory- Georgia Tech Predictive Health Initiative cohort from 2007–2013 categorized into body composition subtypes a
| Food Group/Macronutrient Variable | Lean (n=98) | Normal Weight Obesity (n=162) | Overweight-Obesity (n=427) |
|---|---|---|---|
| Total fat (grams) | 38.6 ± 0.7y,z | 38.4 ± 0.6y | 40.4 ± 0.4z |
| Saturated fat (grams)b | 10.4 (9.8, 10.9)y | 10.3 (9.9, 10.7)y | 11.2 (11.0, 11.5)z |
| Monounsaturated fat (grams) | 15.4 ± 0.3x | 15.4 ± 0.3x | 16.1 ± 0.2x |
| Polyunsaturated fat (grams) b | 9.3 (8.9, 9.8)x | 9.3 (8.9, 9.6)x | 9.5 (9.3, 9.7)x |
| Trans-fat (gram) b | 0.9 (0.8, 1)y | 0.9 (0.8, 0.9)y | 1.1 (1.1, 1.2)z |
| Total carbohydrates (grams) | 123.6 ± 2.0y | 125.6 ± 1.6y | 118.3 ± 1.0z |
| Total grains (ounce equivalents) | 2.9 ± 0.1x | 2.6 ± 0.1x | 2.8 ± 0.04x |
| Whole grains (ounce equivalents) | 0.9 ± 0.1y | 0.8 ± 0.04y,z | 0.7 ± 0.03z |
| Refined grains (ounce equivalents) | 2.0 ± 0.1y,z | 1.9 ± 0.1y | 2.1 ± 0.04z |
| Total dietary fiber (grams) b | 12.1 (11.3, 12.9)y | 11.7 (11.1, 12.4)y | 10.7 (10.4, 11.1)z |
| Added sugars (teaspoon equivalents) b | 5.6 (5.1, 6.1)x | 5.5 (5.1, 6.0)x | 5.4 (5.2, 5.7)x |
| Total fruits (cups) b | 0.8 (0.7, 0.9)y | 0.8 (0.8, 0.9)y | 0.6 (0.6, 0.7)z |
| Whole fruit (cups) b | 0.6 (0.5, 0.6)y | 0.6 (0.5, 0.7)y | 0.4 (0.4, 0.5)z |
| Total Vegetables (cups) b | 1.2 (1.1, 1.3)x | 1.3 (1.2, 1.4)x | 1.2 (1.1, 1.2)x |
| Yellow/orange (cups) b | 0.08 (0.07, 0.1)y,z | 0.1 (0.08, 0.11)y | 0.07 (0.07, 0.08)z |
| Dark leafy greens (cups) b | 0.03 (0.02, 0.03)x | 0.03 (0.02, 0.03)x | 0.03 (0.02, 0.03)x |
| Potatoes (cups) b | 0.08 (0.07, 0.09)x | 0.10 (0.09, 0.11)y | 0.12 (0.11, 0.12)z |
| Total protein (grams) b | 38.2 (36.8, 39.7)y,z | 37.2 (36.1, 38.3)y | 39.5 (38.8, 40.2)z |
| Milk and dairy products (milk equivalents) b,c | 0.5 (0.5, 0.6)x | 0.5 (0.5, 0.6)x | 0.6 (0.5, 0.6)x |
| Eggs (count) b | 0.1 (9.6, 0.2)x | 0.1 (0.1, 0.1)x | 0.1 (0.1, 0.2)x |
| Beef, pork, and lamb (ounces) b | 0.3 (0.3, 0.4)y | 0.4 (0.3, 0.5)y | 0.5 (0.5, 0.6)z |
| Poultry (ounces) b | 0.3 (0.2, 0.3)y | 0.3 (0.2, 0.3)y | 0.4 (0.3, 0.4)z |
| Fish/seafood (ounces) b | 0.3 (0.3, 0.4)x | 0.3 (0.2, 0.4)x | 0.4 (0.3, 0.4)x |
| Beans/legumes/nuts/soy (servings) b | 1.7 (1.4, 2.0)y | 1.6 (1.4, 1.8)y | 1.2 (1.1, 1.3)z |
Values are presented as units per 1000 kcal and as mean ± SE for normally distributed data or geometric mean (95% confidence interval) for variables requiring natural log-transformation. All analyses were adjusted for age, race, and sex.
Variable was natural log-transformed for analyses and back transformed for data presentation. Because the standard error cannot be back transformed, 95% confidence intervals are shown.
A milk equivalent is equal to consumption of one cup of low-fat milk.
Results of Tukey post hoc analyses are denoted by superscript letters x, y, and z and indicate significant differences between groups for each row. Within each row, values that are not connected by the same letter are significantly different at P< 0.05.
Physical Fitness
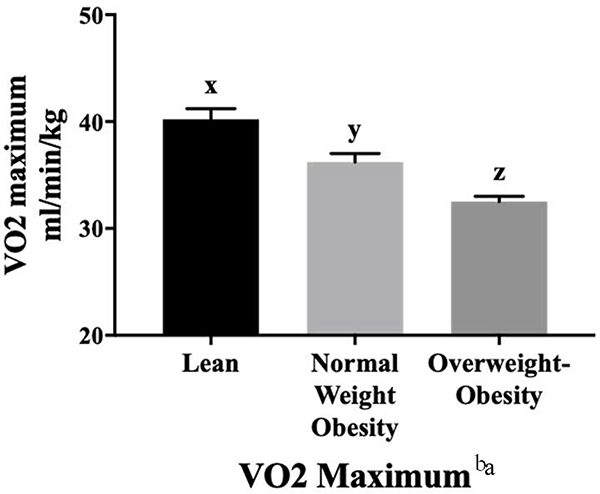
Fitness levels were incrementally lower across the three groups (p<0.05), with the NWO and overweight-obesity groups having significantly lower fitness levels compared to the lean group (Figure 2). There was significant effect modification between VO2 maximum and age (p=0.046). Among individuals with NWO, there was a significant decline in VO2 maximum with aging. Analyses of VO2 maximum stratified by sex are shown in Table 3 (online only). In multiple linear regression analyses, VO2 maximum was inversely associated with all measures of total and abdominal adiposity (Table 4, p<0.001 for all).
Figure 2.
Average (Mean ± SE, adjusting for age, race, sex, and education) VO2 maximum values for participants classified as lean (n=91), having normal weight obesity (n=154), or as having overweight-obesity (n=383) participating in the Emory-Georgia Tech Predictive Health Initiative cohort classified according to body composition subtype. VO2 maximum values in ml/min/kg (adjusted mean ± SE) were 40.2 ± 1.0 for the lean, 36.2 ± 0.8 for the NWO, and 32.5 ± 0.5 for the overweight-obesity groups.
a There was significant effect modification between age and VO2 maximum. Among individuals with NWO, there was a significant decline in VO2 maximum with aging.
xyz Results of Tukey’s post hoc analyses are denoted by superscript letters x, y, and z and indicate significant differences between groups for each row. Values that are not connected by the same letter are significantly different at P < 0.05.
Clinical, Oxidative Stress, and Inflammatory Markers
Fasting glucose concentrations were similar in the lean and NWO groups and significantly higher in the overweight-obesity group (Table 6, p<0.05). Fasting insulin concentrations and HOMA-IR were significantly different between each group, with the NWO group exhibiting values between the other two groups (p<0.05). Total cholesterol was higher in the NWO group compared to the lean group (p<0.05). LDL cholesterol levels were similar in the NWO and overweight-obesity groups and significantly elevated compared to the lean group (p<0.05). HDL cholesterol and triglycerides differed significantly between each group (p<0.05 for both). Systolic and diastolic blood pressure were significantly higher in the overweight-obese group compared to the lean and NWO groups (p<0.05). All plasma aminothiol concentrations were similar between lean participants and individuals with NWO (p>0.05) and reflected a less oxidized redox state compared to the overweight-obesity group who exhibited higher CySS, lower GSH, higher GSH redox potential, and higher CySS/GSH ratio. Inflammatory cytokines did not differ between the three groups (p>0.05 for all).
Table 6.
Clinical, oxidative stress, and inflammatory markers in 693 adults participating in the Emory-Georgia Tech Predictive Health Initiative cohort from 2007–2013 classified according to body composition subtype a
| Lean (n=100) | Normal Weight Obesity (n=164) | Overweight- Obesity (n=429) | |
|---|---|---|---|
| Blood glucose (mg/dL) b,c,d | 86.0 (83.6, 88.5)x | 86.0 (84.1, 88.0)x | 90.2 (89.0, 91.5)y |
| Insulin (μIU /mL) b,e,f | 2.41 (2.07, 2.8)x | 3.09 (2.74, 3.48)y | 5.41 (5.03, 5.82)z |
| HOMA-IR b,e,g | 0.51 (0.44, 0.60)x | 0.66 (0.58, 0.75)y | 1.21 (1.11, 1.3)z |
| Total cholesterol (mg/dL) h | 185.0 ± 3.8x | 196.9 ± 3.0y | 191.7 ± 1.9x,y |
| LDL-C (mg/dL) h | 98.4 ± 3.4x | 113.0 ± 2.7y | 113.0 ± 1.6y |
| HDL-C (mg/dL) h | 72.1 ± 1.7x | 65.2 ± 1.3y | 56.5 ± 0.8z |
| Triglycerides (mg/dL) i,j | 72.2 ± 6.0x | 93.8 ± 4.8y | 112.4 ± 3.0z |
| Systolic blood pressure (mmHg) k | 114.9 ± 1.5x | 116.9 ± 1.2x | 125.9 ± 0.7y |
| Diastolic blood pressure (mmHg) k | 72.3 ± 1.1x | 75.6 ± 0.9x | 79.2 ± 0.5y |
| Cysteine, μM l | 8.9 ± 0.2x | 9.2 ± 0.2x | 9.4 ± 0.1x |
| Cystine, μM l | 78.7 ± 1.8x | 79.7 ± 1.4x | 87.9 ± 0.9y |
| Glutathione, μM b,l | 1.77 (1.64, 1.91)x | 1.76 (1.66, 1.87)x | 1.5 (1.45, 1.5 6)y |
| Glutathione disulfide, μM b,l | 0.052 (0.045, 0.06)x | 0.053 (0.048, 0.06)x | 0.049 (0.045, 0.052)x |
| Eh Cysteine, mV l,m | −69.7 ± 0.6x | −70.3 ± 0.5x | −69.5 ± 0.3x |
| Eh Glutathione, mV l,m | −137.3 ± 1.1x | −136.9 ± 0.8x | −134.0 ± 0.5y |
| Cystine/Glutathione ratio b,l | 43.5 (39.8, 47.7)x | 44.6 (41.5, 47.8)x | 57.3 (54.8, 59.8)y |
| Interleukin-6 (pg/mL) b,n,o | 2.51 (1.99, 3.18) x | 2.54 (2.11, 3.06) x | 3.1 (2.76, 3.48) x |
| Tumor necrosis factor-α (pg/mL) b,n,l | 3.25 (2.88, 3.66) x | 3.47 (3.16, 3.81) x | 3.64 (3.43, 3.85) x |
| Interleukin-8 (pg/mL) b,n,q | 7.87 (6.9, 8.98) x | 7.96 (7.18, 8.82) x | 7.91 (7.42, 8.43) x |
| Interferon gamma (pg/mL) b,n,r | 0.35 (0.25, 0.5)x | 0.32 (0.25, 0.42) x | 0.42 (0.35, 0.49) x |
Values are presented as mean ± SE or as geometric mean (95% confidence interval), adjusting for age, race, and sex.
Variable was natural log transformed for analyses and back transformed for data presentation. Because the standard error cannot be back transformed, 95% confidence intervals for the geometric mean are shown.
To convert mg/dL glucose to mmol/L, multiple mg/dL by 0.0555. To convert mmol/L glucose to mg/dL, multiple mmol/L by 18.0182.
n=162 and 427 for the normal weight obesity (NWO) and overweight-obesity groups, respectively
n=162 and 426 for the NWO overweight-obesity groups, respectively
To convert μIU/mL insulin to pmol/L, multiply μIU/mL by 6.0. To convert pmol/L insulin to μIU/mL, multiply pmol/L by 0.167.
HOMA-IR, homeostasis model assessment of insulin resistance.
To convert mg/dL cholesterol to mmol/L, multiply mg/dL by 0.0259. To convert mmol/L cholesterol to mg/dL, multiply by 38.7.
n=161 and 426 for the NWO and overweight-obesity groups, respectively
To convert mg/dL triglycerides to mmol/L, multiply mg/dL by 0.0113. To convert mmol/L triglycerides to mg/dL, multiply mmol/L by 88.6.
n=163 and 429 in the NWO and overweight-obesity groups, respectively
Aminothiol redox measures: n=93, 155, and 412 for the lean, NWO, and overweight-obesity groups, respectively
Eh millivolts, redox potential
Cytokine measure
n=93, 155, and 415 for the lean, NWO, and overweight-obesity groups, respectively
n=95, 159, and 418 for the lean, NWO, and overweight-obesity groups, respectively
n=94, 157, and 415 for the lean, NWO, and overweight-obesity groups, respectively
n=93, 156, and 416 for the lean, NWO, and overweight-obesity groups, respectively
Results of multiple linear regression and Tukey post hoc analyses are denoted by superscript letters x, y, and z and indicate significant differences between groups for each value. Within each row, values that are not connected by the same letter are significantly different at P < 0.05.
DISCUSSION
NWO is associated with cardiometabolic derangements that place seemingly lean individuals at risk for metabolic disease8–15. Notably, more adults in this cohort were classified as NWO than were classified as lean. Individuals with NWO had significantly lower physical fitness levels compared to their lean counterparts. Individuals with NWO reported higher diet quality than individuals classified as having overweight-obesity, although higher diet quality was inversely associated with measures of adiposity in all participants regardless of weight status. Furthermore, the metabolic panel of individuals with NWO indicated higher levels of risk factors for cardiometabolic disease, particularly in markers of insulin resistance and lipid concentrations.
Individuals with NWO have a higher chronic disease and mortality risk compared to normal-weight, lean individuals or metabolically healthy, obese individuals8, 15–17, and the definition used to classify individuals with NWO plays an important role in establishing these risks. Oliveros and colleagues describe the history of investigating subtypes of obesity and summarize the metabolic dysfunction noted in individuals with NWO9. The prevalence of NWO has been reported as high as 30% and differs by race and sex8, 10. Importantly, there are no established percent body fat cut-points to define obesity46, which contributes to the variability in reported prevalence46, 47 One study found the prevalence of NWO in women ranged from 1.4 to 27.8% when applying various thresholds47. Using age- and sex-specific cut points may decrease the variability in prevalence47. In this cohort, there was a 24% prevalence of NWO among all participants, a 27% prevalence of NWO among women, and a 17% prevalence of NWO among men. Additional reports are generally consistent that women have a higher prevalence of NWO than men47–50. However, a recent nationwide study of Chinese adults noted a higher prevalence of NWO in males (9.5% vs 6.1%)14, showing the importance of screening for NWO in both males and females. Additionally, obesity misclassification by BMI may differ by sex51. One study showed males were more likely to be misclassified at a BMI between 25–27 kg/m2, but women were more likely to be misclassified at a BMI between 24–26 kg/m2 51. Altogether, while the prevalence and definition of NWO is variable, there is strong evidence from the current study and other studies that supports increased cardiometabolic and mortality risk factors in individuals with normal weight but high body fat and a need to effectively screen and identify these individuals8–15, 17, 52.
The NWO group in this study had significantly lower levels of objectively measured physical fitness compared to the lean group. Similarly, a Chinese study using objective assessments reported impaired physical fitness and muscular strength in college-aged individuals with NWO53. Measures of self-reported physical activity participation data have shown mixed results54, 55. Circuit training has been successfully applied as a 10-week physical activity intervention in women with NWO56, showing significant improvements in clinical measures and reductions in total and trunk body fat which resulted in participants no longer being classified as NWO56. Low physical fitness is a leading risk factor for chronic diseases57, and increasing participation in aerobic physical activity is an effective primary and secondary prevention strategy to reduce chronic disease risk58. The health benefits from aerobic physical activity participation may exceed the effects of prescription medications59. Thus, there is a need to address the low levels of physical fitness in this population to reduce disease risk.
Much attention in NWO research is paid to increased adiposity; however, another important characteristic of NWO indicated by this study is decreased lean mass. Physical activity, especially strength training, is integral for stimulating skeletal muscle protein synthesis and maintaining lean body mass, particularly as one ages60. Further, physical activity improves glucose metabolism and insulin sensitivity, and individuals with higher fitness levels have better insulin sensitivity61. Skeletal muscle secretes a variety of myokines, especially during exercise, such as follistatin-like 1, fibroblast growth factor-21 (FGF-21), brain-derived neurotrophic factor (BDNF), myonectin, and interleukin-6 (IL-6), that have both local and systemic health-promoting effects59. These chemicals increase glucose uptake, promote uptake and lipolysis of free fatty acids in skeletal muscle and the liver, have neurocognitive benefits, promote angiogenesis, improve endothelial function, and protect against ectopic fat deposition59. Thus, strategies designed to increase lean body mass in individuals with NWO may be an important factor to target for health improvements through a variety of mechanisms.
Higher diet quality is associated with decreased chronic disease risk19–23, 25–28. In this study, individuals with NWO reported similar diet quality as lean individuals and higher diet quality than individuals classified as overweight-obese, with group differences primarily driven by females. There were few similarities in reported food group and macronutrient intakes between NWO and overweight-obesity groups. Diet quality scores reflected suboptimal diet quality for all groups, although average AHEI score for all groups was above a previously reported U.S. average62. Few studies have investigated dietary intake or diet quality of individuals with NWO54, 55, 63. Mannisto et al. found components of dietary intake related to diet quality were associated with NWO, including lower intakes of cereals, fish, and root vegetables, and higher intakes of sugar54. Amani et al. found that individuals with NWO consumed lower amounts of antioxidant compounds compared to lean individuals and had similar total antioxidant capacity as individuals categorized as having overweight-obesity63. Further, the NWO group consumed higher total energy, less fiber, and fewer servings of fruit, legumes, and nuts and seeds compared to the lean group63. Notably, in the entire cohort, higher diet quality was associated with lower total body fat and VAT. In longitudinal studies, poor diet quality has been shown to predict higher visceral adiposity, and interventions that increase physical activity and/or improve diet quality have been effective in reducing VAT and liver fat while improving cardiometabolic risk factors64–66. While diet quality may not differentiate individuals with NWO from lean individuals in this cohort, maintaining a higher diet quality may help prevent additional weight gain.
In the current study, individuals with NWO and overweight-obesity exhibited adverse metabolic biomarkers compared to the lean group, including fasting insulin concentrations, HOMA-IR, total cholesterol, and LDL cholesterol. There is substantial evidence of cardiometabolic dysregulation in NWO cohorts, including dyslipidemia9, 10, 12, 50, 67, 68, increased inflammation12,13, increased oxidative stress11,13, altered adipokine levels8,11, and the presence of metabolic syndrome components including hypertension, insulin resistance, and hyperglycemia10, 11, 14, 16, 17, 48, 69–71. In this cohort, although individuals with NWO showed dysregulated insulin function and altered lipid levels compared to lean individuals, there was no evidence of significant oxidative stress or inflammation in the NWO group compared to the lean group. We previously reported that higher diet quality is associated with lower levels of oxidative stress24. It is possible that individuals with NWO in the current cohort maintain a diet quality high enough to sustain aminothiol redox balance. While there is heterogeneity in reported metabolic profiles of individuals with NWO, there is consistent evidence of adverse metabolic health in these individuals, highlighting the need to screen for and prevent NWO9, 10, 12, 15, 50, 67, 68.
Major strengths of this study were the use of sensitive body composition and fat distribution assessment methods in a large cohort of adults to classify body composition subtypes. This study also provides extensive clinical and metabolic phenotyping of individuals with NWO to add to the existing literature of the adverse clinical profiles presented in individuals with NWO. There are also some limitations to this study. FFQs are subject to recall bias, have a high participant burden, and varying reliability72, 73. This is a cross-sectional analysis, and causality cannot be inferred in the reported relationships. Participants in this cohort reported high education and income levels, which may limit the generalizability of this population. Future research should examine the most appropriate cut points for defining obesity considering age, sex, and race. Of note, additional classifications exist for individuals with a normal weight but increased disease risk such as “metabolically obese, normal weight3 ”, “lean, insulin resistant74,” “lean, type 2 diabetics75,” and “non-alcoholic steatohepatitis in lean individuals76.” Many of these classifications are based on BMI, whereas NWO is classified by body fat percent and BMI. Indeed, many of these noted classifications are a subset of individuals with NWO with underlying obesity, abdominal adiposity, and inflammation as a driver of cardiometabolic disease77, 78. Finally, in addition to diet and physical fitness, numerous factors influence body weight and composition and metabolic health, including genetics, epigenetics, and environmental exposures, which are not addressed here79, 80
Conclusions
In conclusion, while diet quality was similar between individuals with NWO and lean individuals, physical fitness was significantly lower in the NWO group. Focus on increasing physical activity and physical fitness may be an important lifestyle factor to target for risk reduction in individuals with NWO. Future research should determine if achieving an adequately high diet quality with concomitant increase in physical activity is an effective strategy for individuals with NWO to reduce fat mass and increase muscle synthesis.
RESEARCH SNAPSHOT.
Research Question
Do diet quality scores and physical fitness levels differ between adults categorized as lean, as having normal weight obesity (NWO), or as having overweight-obesity? Also, do clinical biomarkers and food groups and macronutrient intakes differ between the three groups, and are body composition components associated with diet quality scores and physical fitness levels?
Key Findings
In a large cohort of working adults, participants with normal weight obesity had lower physical fitness levels than lean individuals but reported similar diet quality to lean participants and higher diet quality scores than overweight-obese participants. For clinical biomarkers, individuals with NWO and overweight-obesity exhibited overall worse metabolic panels compared to lean participants. Regardless of body composition group, higher physical fitness and diet quality was associated with lower total and visceral adiposity.
Acknowledgments
Funding Support:
This work is based on information from the Emory Predictive Health Institute and Center for Health Discovery and Well Being Database supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR002378. Additional grant support includes NIH K01 DK102851 (JAA), K24 DK096574 (TRZ), and P30 ES019776 (JAA, TRZ, DPJ).
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global recommendations on physical activity for health. Geneva, Switzerland: https://www.who.int/dietphysicalactivitv/publications/9789241599979/en/. Published 2010. Accessed June 27, 2020. [PubMed] [Google Scholar]
- 3.Conus F, Rabasa-Lhoret R, Peronnet F. Characteristics of metabolically obese normal-weight (MONW) subjects. App PhysiolNutrMe. 2007;32:4–12. [DOI] [PubMed] [Google Scholar]
- 4.Primeau V, Coderre L, Karelis AD, et al. Characterizing the profile of obese patients who are metabolically healthy. Int JObes. 2011;35:971–981. [DOI] [PubMed] [Google Scholar]
- 5.Gruzdeva O, Borodkina D, Uchasova E, Dyleva Y, Barbarash O. Localization of fat depots and cardiovascular risk. Lipids Health Dis. 2018;17:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 7.Heymsfield SB, Cefalu WT. Does body mass index adequately convey a patient’s mortality risk? JAMA. 2013;309:87–88. [DOI] [PubMed] [Google Scholar]
- 8.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56:426–433. [DOI] [PubMed] [Google Scholar]
- 10.De Lorenzo A, Martinoli R, Vaia F, Di Renzo L. Normal weight obese (NWO) women: an evaluation of a candidate new syndrome. NutrMetab Cardiovas. 2006;16:513–523. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, Paik JK, Kang R, Kim SY, Lee SH, Lee JH. Increased oxidative stress in normal-weight postmenopausal women with metabolic syndrome compared with metabolically healthy overweight/obese individuals. Metabolism. 2013;62:554–560. [DOI] [PubMed] [Google Scholar]
- 12.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Renzo L, Galvano F, Orlandi C, et al. Oxidative stress in normal-weight obese syndrome. Obesity. 2010;18:2125–2130. [DOI] [PubMed] [Google Scholar]
- 14.Jia A, Xu S, Xing Y, et al. Prevalence and cardiometabolic risks of normal weight obesity in Chinese population: A nationwide study. Nutr Metab Cardiovas. 2018;28:1045–1053. [DOI] [PubMed] [Google Scholar]
- 15.Jean N, Somers VK, Sochor O, Medina-Inojosa J, Llano EM, Lopez-Jimenez F. Normal-weight obesity: implications for cardiovascular health. Curr Atheroscler Rep. 2014;16:464. [DOI] [PubMed] [Google Scholar]
- 16.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Somers VK, Lopez- Jimenez F. Normal weight obesity and mortality in United States subjects >/=60 years of age (from the Third National Health and Nutrition Examination Survey). Am J Cardiol. 2013;112:1592–1598. [DOI] [PubMed] [Google Scholar]
- 17.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med. 2015;163:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. 2010;34:791–799. [DOI] [PubMed] [Google Scholar]
- 19.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J AcadNutrDiet. 2018;118:74–100.e111. [DOI] [PubMed] [Google Scholar]
- 20.Fung TT, McCullough M, van Dam RM, Hu FB. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care. 2007;30:1753–1757. [DOI] [PubMed] [Google Scholar]
- 21.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bo S, Ponzo V, Goitre I, et al. Predictive role of the Mediterranean diet on mortality in individuals at low cardiovascular risk: a 12-year follow-up population-based cohort study. JTranslMed.. 2016;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fargnoli JL, Fung TT, Olenczuk DM, Chamberland JP, Hu FB, Mantzoros CS. Adherence to healthy eating patterns is associated with higher circulating total and high-molecular-weight adiponectin and lower resistin concentrations in women from the Nurses’ Health Study. Am J Clin Nutr. 2008;88:1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettermann EL, Hartman TJ, Easley KA, et al. Higher mediterranean diet quality scores and lower body mass index are associated with a less oxidized plasma glutathione and cysteine redox status in adults. J Nutr. 2018;148:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferranti EP, Dunbar SB, Higgins M, et al. Psychosocial factors associated with diet quality in a working adult population. Res Nurse Health. 2013;36:242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitan EB, Wolk A, Mittleman MA. Relation of consistency with the dietary approaches to stop hypertension diet and incidence of heart failure in men aged 45 to 79 years. Am J Cardiol. 2009;104:1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liese AD, Nichols M, Sun X, D’Agostino RB Jr., Haffner SM. Adherence to the DASH Diet is inversely associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care. 2009;32:1434–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–1271. [DOI] [PubMed] [Google Scholar]
- 29.Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390:2643–2654. [DOI] [PubMed] [Google Scholar]
- 30.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu F Assessment of Physical Activity in Nutritional Epidemiology In: Willett W, ed. Nutritional Epidemiology. 3rd ed. New York: Oxford University Press; 2013:241–259. [Google Scholar]
- 32.Mokdad AH, Ballestros K, Echko M, et al. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319:1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rask KJ, Brigham KL, Johns MM. Integrating comparative effectiveness research programs into predictive health: a unique role for academic health centers. Acad Med. 2011;86:718–723. [DOI] [PubMed] [Google Scholar]
- 34.Brigham KL. Predictive health: the imminent revolution in health care. JAm Geriatr Soc. 2010;58 Suppl 2:S298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madeira FB, Silva AA, Veloso HF, et al. Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle-income country. PLoS One. 2013;8:e60673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 37.Mares-Perlman JA, Klein BE, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr. 1993;123:489–501. [DOI] [PubMed] [Google Scholar]
- 38.NutritionQuest. Our Research: Questionnaires. https://nutritionquest.com/company/our-research-questionnaires/. Published 2019. Accessed June 16, 2020.
- 39.Folsom AR, Parker ED, Harnack LJ. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am JHypertens. 2007;20:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon LB, Subar AF, Peters U, et al. Adherence to the USDA Food Guide, DASH Eating Plan, and Mediterranean dietary pattern reduces risk of colorectal adenoma. J Nutr. 2007;137:2443–2450. [DOI] [PubMed] [Google Scholar]
- 41.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. [DOI] [PubMed] [Google Scholar]
- 42.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U.S. Armed Forces Med J 1959;10:675–688. [PubMed] [Google Scholar]
- 43.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 44.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.JMP Pro (for Windows) [computer program]. Version 13.1. Cary, NC: SAS Institute; 2017. [Google Scholar]
- 46.Franco LP, Morais CC, Cominetti C. Normal-weight obesity syndrome: diagnosis, prevalence, and clinical implications. NutrRev 2016;74:558–570. [DOI] [PubMed] [Google Scholar]
- 47.Marques-Vidal P, Pecoud A, Hayoz D, et al. Prevalence of normal weight obesity in Switzerland: effect of various definitions. Eur JNutr 2008;47:251–257. [DOI] [PubMed] [Google Scholar]
- 48.Kim MK, Han K, Kwon HS, et al. Normal weight obesity in Korean adults. Clin Endocrinol. 2014;80:214–220. [DOI] [PubMed] [Google Scholar]
- 49.Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Normal-weight obesity and disability in older adults: data from the National Health and Nutrition Examination Study 1999–2004. J Am Geriatr Soc. 2016;64:1367–1368. [DOI] [PubMed] [Google Scholar]
- 50.Marques-Vidal P, Pecoud A, Hayoz D, et al. Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovas. 2010;20:669–675. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen SD, Astrup AV, Skovgaard IM. Reduction of misclassification rates of obesity by body mass index using dual-energy X-ray absorptiometry scans to improve subsequent prediction of per cent fat mass in a Caucasian population. Clin Obes 2011;1:69–76. [DOI] [PubMed] [Google Scholar]
- 52.Bellissimo MP, Cai Q, Ziegler TR, et al. Plasma high-resolution metabolomics differentiates adults with normal weight obesity from lean individuals. Obesity. 2019;27:1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang M, Schumann M, Huang T, Tormakangas T, Cheng S. Normal weight obesity and physical fitness in Chinese university students: an overlooked association. BMC Public Health. 2018;18:1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mannisto S, Harald K, Kontto J, et al. Dietary and lifestyle characteristics associated with normal-weight obesity: the National FINRISK 2007 Study. Br JNutr. 2014;111:887–894. [DOI] [PubMed] [Google Scholar]
- 55.Moy FM, Loh DA. Cardiometabolic risks profile of normal weight obese and multiethnic women in a developing country. Maturitas. 2015;81:389–393. [DOI] [PubMed] [Google Scholar]
- 56.Ferreira FC, Bertucci DR, Barbosa MR, et al. Circuit resistance training in women with normal weight obesity syndrome: body composition, cardiometabolic and echocardiographic parameters, and cardiovascular and skeletal muscle fitness. J Sport MedPhys Fit. 2017;57:1033–1044. [DOI] [PubMed] [Google Scholar]
- 57.Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med. 2009;43:1–2. [PubMed] [Google Scholar]
- 58.Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541–556. [DOI] [PubMed] [Google Scholar]
- 59.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology. 2013;28:330–358. [DOI] [PubMed] [Google Scholar]
- 60.McGlory C, Phillips SM. Exercise and the regulation of skeletal muscle hypertrophy. Prog Mol Biol Transl. 2015;135:153–173. [DOI] [PubMed] [Google Scholar]
- 61.Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med. 2000;21:1–12. [DOI] [PubMed] [Google Scholar]
- 62.Wang DD, Leung CW, Li Y, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174:1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amani R, Parohan M, Jomehzadeh N, Haghighizadeh MH. Dietary and biochemical characteristics associated with normal-weight obesity. Int J Vitam Nutr Res. 2019:1–6. [DOI] [PubMed] [Google Scholar]
- 64.Maskarinec G, Lim U, Jacobs S, et al. Diet quality in midadulthood predicts visceral adiposity and liver fatness in older ages: the Multiethnic Cohort Study. Obesity. 2017;25:1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nazare JA, Smith J, Borel AL, et al. Changes in both global diet quality and physical activity level synergistically reduce visceral adiposity in men with features of metabolic syndrome. J Nutr. 2013;143:1074–1083. [DOI] [PubMed] [Google Scholar]
- 66.Ma J, Hennein R, Liu C, et al. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology. 2018;155:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berg C, Strandhagen E, Mehlig K, Subramoney S, Lissner L, Bjorck L. Normal weight adiposity in a Swedish population: how well is cardiovascular risk associated with excess body fat captured by BMI? Obes Sci Pract. 2015;1:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramsaran C, Maharaj RG. Normal weight obesity among young adults in Trinidad and Tobago: prevalence and associated factors. Int J Adolesc Med Health. 2017;29. [DOI] [PubMed] [Google Scholar]
- 69.Karkhaneh M, Qorbani M, Mohajeri-Tehrani MR, Hoseini S. Association of serum complement C3 with metabolic syndrome components in normal weight obese women. J Diabetes Metab Disord 2017;16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circ CardiovascImaging. 2012;5:349–356. [DOI] [PubMed] [Google Scholar]
- 71.Liu PJ, Ma F, Lou HP, Zhu YN. Normal-weight central obesity is associated with metabolic disorders in Chinese postmenopausal women. Asia Pac J Clin Nutr. 2017;26:692–697. [DOI] [PubMed] [Google Scholar]
- 72.Bingham SA. Limitations of the various methods for collecting dietary intake data. Ann Nutr Metab. 1991;35:117–127. [DOI] [PubMed] [Google Scholar]
- 73.Willett W, Lenart E. Reproducibility and validity of food frequency questionnaires In: Willett W, ed. Nutritional Epidemiology. 3rd ed. New York: Oxford University Press; 2013:151–212. [Google Scholar]
- 74.Moscavitch SD, Kang HC, Filho RA, Mesquita ET, Neto HC, Rosa ML. Comparison of adipokines in a cross-sectional study with healthy overweight, insulin-sensitive and healthy lean, insulin-resistant subjects, assisted by a family doctor primary care program. DiebetolMetab Syndr 2016;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.George AM, Jacob AG, Fogelfeld L. Lean diabetes mellitus: An emerging entity in the era of obesity. World JDiabetes. 2015;6:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Younes R, Bugianesi E. NASH in lean individuals. Semin Liver Dis. 2019;39:86–95. [DOI] [PubMed] [Google Scholar]
- 77.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014;15:6184–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luna-Luna M, Medina-ürrutia A, Vargas-Alarcon G, Coss-Rovirosa F, Vargas-Barron J, Perez-Mendez O. Adipose tissue in metabolic syndrome: onset and progression of atherosclerosis. Arch Med Med. 2015;46:392–407. [DOI] [PubMed] [Google Scholar]
- 79.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. [DOI] [PubMed] [Google Scholar]
- 80.Neeland IJ, Ross R, Despres JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endo. 2019;7:715–725. [DOI] [PubMed] [Google Scholar]