Abstract
Purpose:
To compare the clinical characteristics and in vivo confocal microscopy (IVCM) findings of patients with neuropathic corneal pain (NCP) due to refractive surgery (RS-NCP) and herpetic eye disease (H-NCP) to controls.
Methods:
Sixteen patients with RS-NCP and 7 patients with H-NCP, and 37 healthy reference age- and sex-matched healthy controls were included to the study. The medical records were reviewed for demographic features, detailed disease history, ocular surface disease index (OSDI), ocular pain assessment survey (OPAS) scores. IVCM images of patients were analyzed and compared to reference controls by two masked observers.
Results:
The mean pain intensity score for the last 24 h (5.1 ± 2.4 vs. 3.9 ± 1.2; p = 0.27), last 2 weeks (6.1 ± 2.5 vs. 4.8 ± 2.3; p = 0.13) for RS-NCP vs. H-NCP respectively, and quality of life scores (p = 0.23) were similar in both groups. Quality of life, especially mood (p = 0.06) and enjoying life/relations to others (p = 0.10) were affected in both groups, but were not statistically significant between groups. The mean total nerve density was lower in RS-NCP (5,702.4 ± 4,599.0 μm/mm2) compared to their respective controls (26,422.8 ± 4,491.0; p < 0.001) and in the H-NCP group (2,149.5 ± 2,985.9) compared to their respective controls (22,948.8 ± 3,169.0; p < 0.001). Alterations in DC density were similar between all groups (38.3 ± 48.0 cells/mm2 in RS-NCP, 61.0 ± 76.9 in H-NCP, p = 0.95).
Conclusion:
Neuropathic corneal pain patients due to refractive surgery show similar clinical characteristics, pain levels, quality of life impact, and IVCM findings as patients with NCP due to herpetic eye disease.
Keywords: Herpes keratitis, Neuropathic corneal pain, pain, Quality of life, Refractive surgery
1. Introduction
Pain is defined as “unpleasant sensory and emotional experience associated with actual or potential tissue damage” and neuropathic pain (NP) is described as “pain caused by a lesion or disease of the somatosensory nervous system” by International Association for the Study of Pain [1]. Neuropathic corneal pain (NCP) is a new and ill-defined entity, which is characterized by dysfunctional corneal nerves causing non-specific symptoms, such as pain, burning, stinging, photophobia or severe dryness and failure of symptom resolution with conventional dry eye therapy [2–5]. The potential absence of objective slit-lamp findings or overlap with other ocular conditions makes NCP extremely difficult to diagnose.
Our knowledge about NCP, including underlying etiology, pathophysiological mechanism, severity of pain, its effect on quality of life (QoL), treatment and prognosis is very limited [2,6]. Alteration in ocular surface homeostasis, infections, or ocular surgery, among others, may lead to inflammation and peripheral nerve damage, resulting in hypersensitivity and peripheral sensitization (maladaptive nociceptor plasticity) [2–4,7,8]. If peripheral sensitization chronically continues, changes in the central nervous system occur, which lead to persistence of pain (central sensitization) as a result of changes in sensory, emotional, and other brain networks [2–4,7–9].
In addition to many systemic etiologies of NCP [2–5,10–12], underlying ocular etiologies associated with NCP may include dry eye disease (DED), infectious keratitis, herpes simplex keratitis, herpes zoster ophthalmicus, recurrent corneal erosion syndrome, radiation keratopathy, as well as trauma and ocular surgeries, such as cataract and refractive surgery procedures [2,3,7,13]. Refractive surgery is known as an effective method to correct refractive errors with a high predictability and is one of the most commonly performed surgical procedures in the United States [14,15]. However, dry eye symptoms as a consequence of refractive surgery are well described, and some patients may chronically suffer from these symptoms [16,17]. Furthermore, increased number of patients with unexplained ocular pain symptoms [2,3,7] are currently presenting or being identified after refractive surgery procedures [13]. Thus, post-refractive surgery neuralgia or NCP has become of interest to ophthalmologists and vision scientists alike [6,18,19]. While clinical and experimental studies on NCP are gradually increasing, the clinical characteristics, including pain levels and the impact on QoL need to be further elucidated [2–4,20,21]. Thus, we hypothesized that despite the varying etiologies, pain levels and quality of life impact of post-refractive surgery NCP are at least as severe as in post-herpetic neuralgia. Therefore, our aim in this study was to compare the clinical characteristics of NCP due to refractive surgery with the better-known post-herpetic neuralgia (NCP due to herpetic eye disease), in order to aid in understanding features of post-refractive surgery NCP. Elucidation of these aspects of this condition may assist ophthalmologists to better identify diagnosis and management needs, treatment targets and improved outcomes.
2. Methods
This is a cross-sectional, comparative, retrospective, case control study, with two comparison control groups, which was conducted at New England Eye Center, Department of Ophthalmology, Tufts Medical Center, Tufts University School of Medicine, Boston, MA. The study was approved by Institutional Review Board/Ethics Committee of Tufts Medical Center/Tufts University Health Sciences and the study protocol conformed to the Declaration of Helsinki, and adhered to the Health Insurance Portability and Accountability Act (HIPAA).
2.1. Patients
The medical records and in vivo confocal microscopy (IVCM) images of patients who were diagnosed as NCP by the same experienced clinician (PH) between January 2015 and April 2019 were evaluated retrospectively. Diagnosis of NCP was made based on presence of neuropathic ocular symptoms (burning, stinging, photophobia, pain, severe dryness), absent or minimal slit-lamp findings to explain symptoms, corneal nerve abnormalities as detected by IVCM (HRT3/RCM, Heidelberg Engineering GmbH, Heidelberg, Germany). Subjects with refractive surgery related NCP (RS-NCP) and herpetic eye disease-related NCP (H-NCP) were included in the analysis. Patients were excluded if they had any other ocular pathology that might have resulted in pain, such as active corneal infections, abrasions, angle-closure glaucoma, and anterior uveitis, or if they had NCP with a different etiology rather than refractive surgery or herpetic eye disease. Age- and sex-matched reference controls in this study were healthy, asymptomatic individuals, with no ocular pathology, absent ocular surface staining, and tear film break-up time of more than 10 s. All controls were drawn from an IRB-approved prospective normative study database that enrolled healthy subjects after having a complete history and ocular examination. Matching our control group to the other 2 groups in terms of age and sex resulted in the 37 participants from this database. Reference controls were classified as controls for the refractive surgery group (C-1) if their age was ≥ 20 and ≤ 50 and controls for the herpetic group (C-2) if they were > 50 years of age. This subgroup classification was needed for assessment of IVCM images, as the mean age of the population for the refractive surgery patients with NCP was different and less than NCP patients with a history of herpetic eye disease and due to the fact that nerve density may decrease with increasing age.
2.2. Clinical chart review
Demographic features of the patients, time of insult, time between insult and pain onset, duration of pain (time between first consistent pain experience described by patient and the first visit date at our center. Duration of non-specific ocular symptoms and inconsistent pain was not included), Ocular Surface Disease Index (OSDI) [22], Ocular Pain Assessment Survey (OPAS) scores [23], proparacaine challenge test (PCT) results at the initial visits were recorded. For the PCT [2,3], patients were asked to report their pain relief based on visual analogue scale after 1 min of installation of 0.5% proparacaine hydrochloride eye drops (Alcaine; Novartis Ophthalmics, East Hanover, NJ). The PCT was performed in the presence of pain at the day of the visit and results were reported from the initial visit in our clinic. Given that this test had not been routine prior to 2017, not all patients had received the test. Based on complete relief, partial relief, or no relief in symptoms after the PCT, patients were grouped as peripheral, mixed and central NCP, respectively [2,3]. This classification was used in the patients who had PCT at the initial visit, but not in patients for which the PCT was not available.
2.3. In vivo confocal microscopy and image analysis
Laser IVCM images were conducted on central corneas of all patients and controls, bilaterally, as previously described [24]. Equipped with a 63 × objective immersion lens with a numerical aperture of 0.9 (Olympus, Tokyo, Japan), this microscope uses a 670-nm red wavelength diode laser source to produce an image representing a coronal section of the cornea of 400 × 400 μm (horizontal x vertical). Digital images are recorded at of 30 frames/s. Adjacent images are separated by 1 μm, with a lateral resolution of 1 μm/pixel. To perform this procedure, both eyes were topically anesthetized using 0.5% proparacaine hydrochloride (Alcaine; Novartis Ophthalmics). This was followed by administration of a drop of hydroxypropyl methylcellulose 2.5% (GenTeal gel, Alcon, Fort Worth, TX) to improve the optical coupling with the cornea module of the microscope. The cornea module was mounted with a disposable, sterile polymethylmethacrylate cap (Tomo-Cap; Heidelberg Engineering GmbH), filled with a layer of hydroxypropyl methylcellulose 2.5% (GenTeal gel; Alcon), gel was also applied to the surface of the cap. The equipment is manually advanced until the gel on the cap comes in contact with the surface of the central cornea.
Out of a total of six to eight sequence scans performed on the full thickness of the central cornea, resulting in a total of 50–100 images of the corneal subbasal layer, a masked observer (B.N.B.) selected the three most representative images (best focused, single layer, minimum folds and good contrast) of the subbasal nerve plexus. Two masked observers (B.N.B.; N.M.) analyzed IVCM images for morphology and density of dendritiform cells (DCs) and subbasal nerve plexus (SNP). In case of any discrepancy, the images were analyzed and adjudicated by a third observer. The DC density was measured using Image J (https://imagej.nih.gov/ij/) as previously described [24], and total, trunk, branch nerve density were measured using Neuron J (a semi-automated tracing plugin for Image J) [24]. The IVCM image analyses results were then compared to age- and sex-matched healthy reference controls.
2.4. Questionnaires
The OSDI is a validated 12-item questionnaire with 3 dimensions for rapid assessment of the ocular irritation related to dry eye disease and its impact on vision related functioning [22]. Each question is graded between 0 and 4 (0 = none of the time, 1 = some of the time, 2 = half of the time, 3 = most of the time, 4 = all of the time). The overall OSDI is scored on a scale of 0–100 in which higher scores show more disability.
The OPAS is a validated multidimensional questionnaire for ocular pain [23], which includes 6 dimensions, including eye pain intensity for the last 24 h, eye pain intensity for the last 2 weeks, non-eye pain intensity, quality of life, aggravating factors, associated factors and symptom relief. Patients were asked to respond all questions according pain scale between 0 and 10 (0 = no pain, 10 = worst pain ever) [23].
2.5. Statistical analyses
Statistical analyses were performed with SPSS software version 22.0 (SPSS Inc., IBM, Chicago, IL, USA). Distribution of data was analyzed by Kolmogorov-Smirnov test. Mann Whitney U test and Chi-squared were used to assess the differences in demographic and clinical parameters of RS-NCP and H-NCP groups. The differences in IVCM parameters in patients and healthy groups were compared by Kruskal Wallis-test with pairwise comparison test if needed. Confounding factors (age and sex) were controlled by using a generalized linear model. P values lower than 0.05 were considered statistically significant.
3. Results
Sixty patients were included in the study. Sixteen patients (7 females and 9 males) with post-refractive surgery neuropathic corneal pain (RS-NCP), 7 patients (4 females and 3 males) with post-herpetic neuropathic corneal pain (H-NCP), and thirty-seven healthy (15 young controls; 4 females and 11 males and 22 old controls; 10 females and 12 males) controls were enrolled to the study. The mean age of the patients was 39.7 ± 13.4 years (25.0–66.0 years), 70.7 ± 12.8 (56.0–89.0 years), 33.6 ± 9.5 (22.0–49.0 years) and 61.0 ± 6.8 (51.0–74.0 years) in RS-NCP, H-NCP, C-1 and C-2 controls, respectively (p = 0.61 for RS-NCP vs. C-1, p = 0.21 for H-NCP vs. C-2, and p = 0.001 for RS-NCP vs. H-NCP). Demographic features of patients are summarized in Table 1.
Table 1.
Demographic features of patients and control groups.
| Parameter | RS-NCP | H-NCP | C-1 | C-2 | P value |
|---|---|---|---|---|---|
| N | 16 | 7 | 15 | 22 | |
| Age (years) | 39.7 ± 13.4 (25.0–66.0) | 70.7 ± 12.8 (56.0–89.0) | 33.6 ± 9.5 (22.0–49.0) | 61.0 ± 6.8 (51.0–74.0) | RS-NCP vs. C-1, p = 0.61; H-NCP vs. C-2, p = 0.21; RS-NCP vs. H-NCP, p = 0.001 |
| Gender (female/male) | 7/9 | 4/3 | 4/11 | 10/12 | 0.21 |
| Time between insult and pain onset (weeks) | 9.4 ± 17.3 (0.1–68.0) | 45.0 ± 32.5 (2.0–96.0) | – | – | 0.006 |
| Duration of pain (weeks) | 89.3 ± 75.3 (1.0–252.0) | 172.0 ± 183.3 (6.0–416.0) | – | – | 0.59 |
RS-NCP: Neuropathic corneal pain due to refractive surgery; H-NCP: Neuropathic corneal pain due to herpetic eye disease; C-1: Control group 1; C-2: Control group 2.
3.1. Clinical characteristics
In the RS-NCP group, 1 patient had laser epithelial keratomileusis (LASER), 2 patients had photorefractive keratectomy (PRK) and 13 patients had laser assisted in-situ keratomileusis (LASIK) surgery prior to NCP. In the H-NCP group, 5 patients developed NCP after herpes zoster ophthalmicus and 2 patients developed NCP after herpes simplex keratitis.
A history of DED prior to surgery was recorded in 3 (18.7%) of RS-NCP patients (2 of them developed DED after surgery and 1 had surgery with history of DED) and in 2 (28.5%) of H-NCP patients. In the RS-NCP group, there was no predominance for any systemic condition, but depression in 2 patients (12.5%), trigeminal neuralgia in 1 patient (6.2%), chronic inflammatory demyelinating polyneuropathy in 1 patient (6.2%), and small fiber neuropathy in 1 patient (6.2%) were recorded. The most common systemic disorder recorded in H-NCP group was hyperlipidemia (4 patients, 57.1%) and in 1 patient (14.2%) depression was recorded. Detailed demographic and clinical features of patients were shown in Tables 2 and 3.
Table 2.
Clinical characteristics of post-refractive surgery neuropathic corneal pain patients.
| Patient No | Age | Gender (female/male) | Ethnicity | Lateralization (right/left) | Insult | Complication Related to Insult | Time between insult and pain onset (weeks) | Duration of pain (weeks) | Ocular Surgery (except insult) | Accompanying Ocular Disease | Accompanying Systemic Disease | Proparacaine Challenge Test (% relief) | Topical Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | F | Caucasian | R | LASEK | NA | 8 | 44 | NA | NA | NA | 0% | AST |
| 2 | 25 | M | Caucasian | R | PRK | NA | 12 | 172 | Strabismus Ptosis | Oculocutaneous Albinism | Oculocutaneous Albinism | 100% | NA |
| 3 | 27 | M | Caucasian | R | PRK | Corneal abrasion | 1 | 4 | NA | NA | NA | NA | PFAT |
| 4 | 48 | M | Caucasian | L | LASIK | NA | Immediately | 252 | NA | NA | NA | NA | AST, PFAT |
| 5 | 27 | M | Hispanic | L | LASIK | NA | Immediately | 124 | NA | NA | Depression | NA | CsA |
| 6 | 60 | F | Caucasian | L | LASIK | NA | Immediately | 104 | NA | NA | Trigeminal Neuralgia | 33.3% | CsA, PFAT |
| 7 | 33 | M | Caucasian | R | LASIK | NA | 12 | 11 | NA | NA | Chronic Inflammatory Demyelinating Polyneuropathy | 75.0% | AST, PFAT, 0.5% loteprednol etabonate |
| 8 | 66 | M | Caucasian | L | LASIK | NA | 1 | 36 | Cataract Extraction | Fuchs Endothelial Dystrophy | NA | 100% | AST |
| 9 | 33 | F | Caucasian | L | LASIK | NA | 1 | 64 | NA | NA | Depression | NA | AST, 0.5% loteprednol etabonate |
| 10 | 29 | M | Caucasian | R | LASIK | NA | Immediately | 72 | NA | NA | NA | 0% | AST,PFAT |
| 11 | 45 | F | Caucasian | R | LASIK | Flap stria | 26 | 1 | NA | NA | Reynaud phenomenon | 100% | PFAT, 1% prednisolone acetate |
| 12 | 60 | F | Caucasian | L | LASIK | NA | Immediately | 96 | Blepharoplasty | DED | Small fiber neuropathy, Hypothryroidism, Postural Orthostatic Tachycardia, Gastroesophageal Reflux | 50% | PFAT |
| 13 | 42 | M | Asian | R | LASIK | Epithelial ingrowth removal, DED | 16 | 224 | NA | DED | NA | NA | PFAT |
| 14 | 36 | F | Caucasian | R | LASIK | NA | Immediately | 36 | NA | NA | Thyroid Disorder | 10.0% | NA |
| 15 | 32 | F | Caucasian | R | LASIK | Flap stria | 6 | 128 | NA | NA | NA | 100% | AST, PFAT |
| 16 | 48 | M | Caucasian | R | LASIK | Flap dislocation (replacement performed), DED | 68 | 61 | NA | DED | Hyperlipidemia | NA | AST, 0.5% loteprednol etabonate |
AST: Autologous serum tears, CsA: 0.05% cyclosporine A, DED: Dry eye disease, LASEK: Laser epithelial keratomileusis, LASIK: Laser assisted in situ keratomileusis, NA: Not applicable, PFAT: Preservative free artificial tears.
Table 3.
Clinical characteristics of post-herpetic neuropathic corneal pain patients.
| No | Age | Gender (female/male) | Ethnicity | Lateralization (right/left) | Insult | Time between insult and pain onset (weeks) | Duration of pain (weeks) | Ocular Surgery | Accompanying Ocular Disease | Accompanying Systemic Disease | Proparacaine Challenge Test (% relief) | Topical Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | M | Caucasian | L | Herpes zoster | 23 | 20 | NA | NA | NA | NA | PFAT, 0.5% loteprednol etabonate |
| 2 | 56 | F | Caucasian | L | Herpes Zoster | 40 | 6 | NA | NA | Depression Anxiety and Crohn’s Disease | NA | AST, PFAT, 0.5% loteprednol etabonate |
| 3 | 88 | M | Caucasian | R | Herpes Zoster | 63 | NA | Cataract Extraction | NA | Osteoarthritis Hyperlipidemia Hypertension Gastroesophageal Reflux | NA | AST, 1% prednisolone acetate |
| 4 | 66 | M | Caucasian | L | Herpes Zoster | 2 | 158 | Cataract Extraction and Pars Plana Vitrectomy, Ahmed Glaucoma Valve Implantation | DED Glaucoma (steroid responder) | NA | 95.0% | AST, PFAT, 0.5% loteprednol etabonate |
| 5 | 62 | F | Hispanic | R | Herpes Simplex | NA | 380 | Penetrating Keratoplasty, Ahmed Glaucoma Valve | Glaucoma (steroid responder) | Hyperlipidemia | 22.2% | 0.5% loteprednol etabonate |
| 6 | 69 | F | Caucasian | L | Herpes Simplex | 96 | 416 | Cataract Extraction | DED LSCD |
Scleroderma Sjögren’s Syndrome Hyperlipidemia | NA | PFAT |
| 7 | 89 | M | Caucasian | L | Herpes Zoster | 46 | 52 | Cataract Extraction | NA | Hyperlipidemia Hypertension Chronic renal failure | 0% | AST, 0.5% loteprednol etabonate |
AST: Autologous serum tears, DED: Dry eye disease, LSCD: Limbal stem cell deficiency, NA: Not applicable, PFAT: Preservative free artificial tears.
The mean time between insult and pain was 9.4 ± 17.3 weeks (range: 0.1–68.0 weeks) in the RS-NCP and 45.0 ± 32.5 weeks (range: 2.0–96.0 weeks) in H-NCP group (p = 0.006). The mean duration of pain at the visit was 89.3 ± 75.3 weeks (range: 1.0–252.0 weeks) in RS-NCP and 172.0 ± 183.3 weeks (range: 6.0–416.0 weeks) in H-NCP and (p = 0.59) (Tables 1–3).
Proparacaine challenge test results were recorded in 10 RS-NCP patients (55.5%) and in 3 H-NCP patients (42.8%). In the RS-NCP group, 4 patients (40.0%) had peripheral NCP, 4 patients (40.0%) had mixed NCP and 2 patients (20.0%) had central NCP, whereas, in the H-NCP group, 2 patients had mixed NCP (66.6%) and 1 patient had central NCP (33.3%) (p = 0.42).
3.2. Corneal nerve alterations by in vivo corneal confocal microscopy
The mean total, trunk and branch subepithelial nerve plexus density are shown in Table 4 and Fig. 1. The mean total nerve density was 5,702.4 ± 4,599.0 μm/mm2 (range: 234.2–15,257.8) and 2,149.5 ± 2,985.9 (range: 0.0–7,866.5) in RS-NCP and H-NCP respectively, compared to 26,422.8 ± 4,491.0 (range: 37,063.9) in C-1 and 22,948.8 ± 3,169.0 (range: 18,228.4–28,991.9), in C-2 groups.
Table 4.
In vivo confocal microscopy parameters in post-herpetic neuropathic corneal pain, post-refractive neuropathic corneal pain and control groups.
| IVCM parameters | RS-NCP (n = 16) | H-NCP (n = 7) | C-1(n = 15) | C-2 (n = 22) | p value | pairwise comparison p values |
|---|---|---|---|---|---|---|
| Total nerve density (μm/mm2) | 5,702.4 ± 4,599.0 (234.2–15,275.8) | 2,149.5 ± 2,985.9 (0.0–7,866.5) | 26,422.8 ± 4,491.0 (21,565.0–37,063.9) | 22,948.8 ± 3,169.0 (18,228.4–28,991.9) | < 0.001 | RS-NCP vs. C-1, p < 0.001; H-NCP vs. C-2, p < 0.001; RS-NCP vs. H-NCP, p = 0.30 |
| Trunk nerve density (μm/mm2) | 3,243.1 ± 2,372.1 (0.0–6,781.6 | 1,399.6 ± 1,847.6 (0.0–5,007.5 | 12,353.2 ± 2,516.5 (8,024.4–17,772.1) | 10,511.7 ± 1,468.3 (8,664.4–15,382.5) | < 0.001 | RS-NCP vs. C-1, p < 0.001; H-NCP vs. C-2, p < 0.001; RS-NCP vs. H-NCP, p = 0.32 |
| Branch nerve density (μm/mm2) | 2,459.2 ± 2,360.8 (0.0–8,476.2) | 749.8 ± 1,159.8 (0.0–2,858.9) | 14,069.6 ± 3,414.2 (8,149.7–19,291.7) | 12,437.0 ± 2,618.9 (8,632.9–18,417.1) | < 0.001 | RS-NCP vs. C-1, p < 0.001; H-NCP vs. C-2, p < 0.001; RS-NCP vs. H-NCP, p = 0.97 |
| DC density (cell/mm2) | 38.3 ± 48.0 (0.0–141.6) | 61.0 ± 76.9 (2.0–189.5 | 28.9 ± 29.8 (2.0–95.8) | 24.6 ± 19.9 (2.0–70.8) | 0.95 | – |
RS-NCP: Neuropathic corneal pain due to refractive surgery, H-NCP: Neuropathic corneal pain due to herpetic eye disease, C-1: Control group 1, C-2: Control group 2.
Fig. 1.
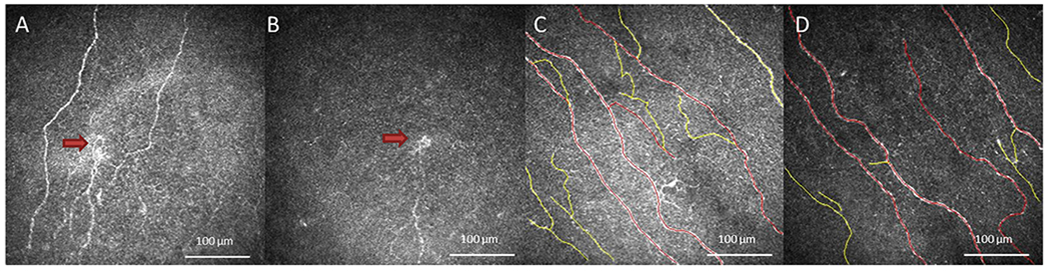
Laser in Vivo Confocal Microscopy (IVCM) Images of Corneal Nerves in Patients and Controls. IVCM images obtained at the level of corneal subepithelial nerve plexus demonstrate changes in corneal nerves in patients and healthy controls. Decreased corneal nerve density, decreased corneal nerve branching, and presence of microneuroma (red arrow) were observed in RS-NCP (A). Decreased corneal nerve density and microneuroma (red arrow) were observed in H-NCP patients (B). Subbasal nerves traced by Neuron J in healthy reference control groups C-1 (C) and C-2 (D) (red tracing; trunk nerve fibers, yellow tracing; branch nerve fibers).
The mean total (p < 0.001), trunk (p < 0.001) and branch (p < 0.001) nerve densities were lower in RS-NCP compared to C-1. The mean total (p < 0.001), trunk (p < 0.001) and branch (p < 0.001) nerve densities were lower in H-NCP compared to C-2. However, total (p = 0.30), trunk (p = 0.32) and branch (p = 0.97) nerve densities were similar in RS-NCP and H-NCP (Figs. 1 and 2). The mean total (p = 0.75), trunk (p = 0.66) and branch (p = 0.43) nerve density were not different in age based control groups.
Fig. 2.
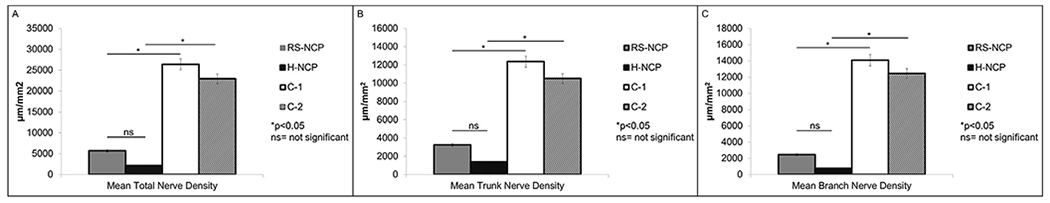
Comparison of total (A), trunk (B) and branch (C) nerve densities detected by in vivo confocal microscopy in RS-NCP, H-NCP and healthy reference controls.
3.3. Dendritiform cell density by in vivo corneal confocal microscopy
The mean DCs densities are shown in Table 4. No statistically significant difference was found between groups regarding DC density (p = 0.95) (Figs. 3 and 4).
Fig. 3.
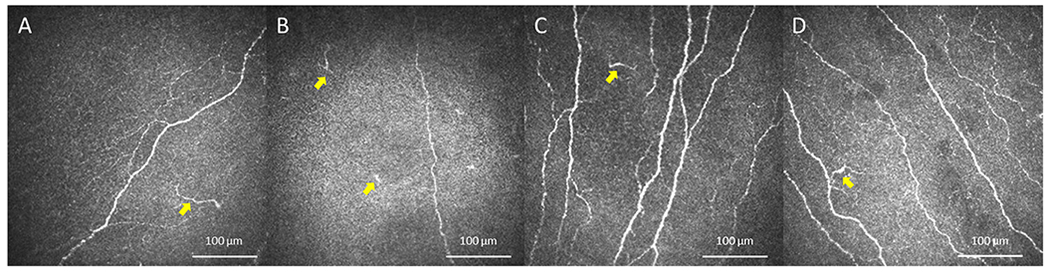
In Vivo Confocal Microscopy (IVCM) images of Dendritiform Cells in Patients and Controls. IVCM images of dendritiform cells (yellow arrows) showed no difference between patients with RS-NCP (A), H-NCP (B) and healthy reference control groups C-1 (C) and C-2 (D).
Fig. 4.
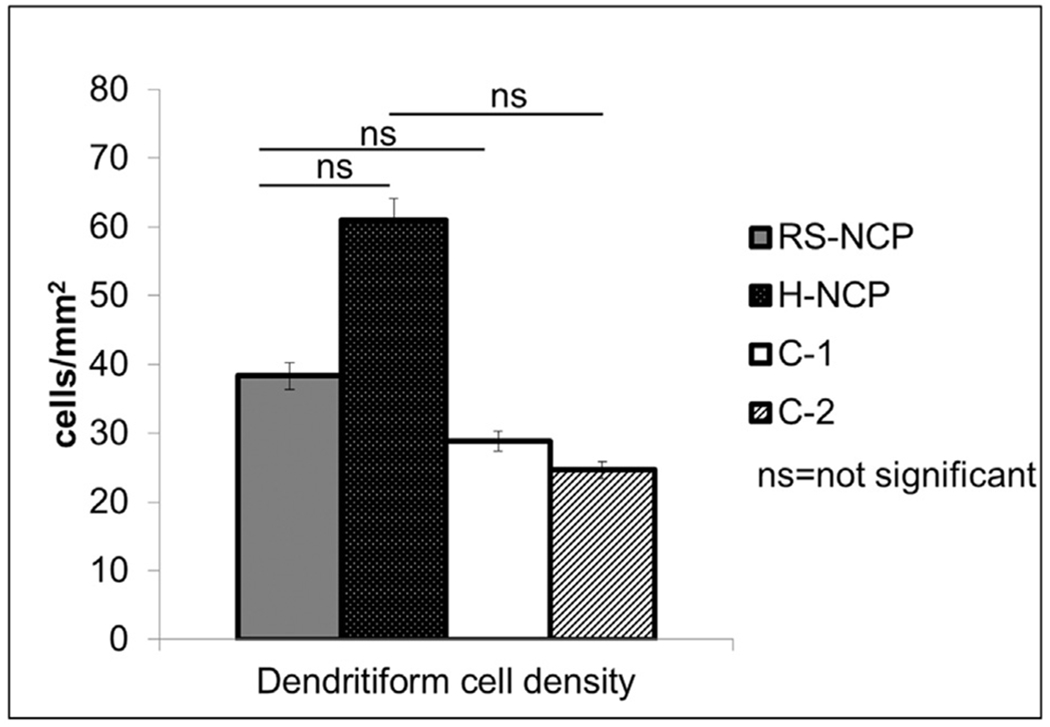
Comparison of dendritiform cell densities detected by in vivo confocal microscopy in RS-NCP, H-NCP and healthy reference controls.
3.4. Questionnaires
The mean OSDI score and the mean pain intensity scores calculated from OPAS are presented in Table 5. The mean OSDI score (p = 0.10), the mean pain intensity score for the past 24 h (p = 0.27) (when it was most; p = 0.24 or least painful; p = 0.37), the mean pain intensity score in the past 2 weeks (p = 0.13) (when it was most; p = 0.08 or least painful; p = 0.57), and non-eye pain in the past 24 h (p = 0.79) and in the past 2 weeks (p = 0.62) were not statistically different between RS-NCP and H-NCP (Tables 5 and 6).
Table 5.
Symptom questionnaire results for post-herpetic and post-refractive surgery neuropathic corneal pain patients.
| RS-NCP (n = 16) | H-NCP Group (n = 7) | p value | |
|---|---|---|---|
| OSDI | |||
| Mean ± SD (range) | 59.1 ± 19.8 (14.5–90.9) | 60.0 ± 23.8 (22.9–100.0) | 0.10 |
| Pain Intensity for the last 24 h | |||
| Mean ± SD (range) | 5.1 ± 2.4 (1.0–9.6) | 3.9 ± 1.2 (2.0–5.6) | 0.27 |
| Pain Intensity for the last 2 weeks | |||
| Mean ± SD (range) | 6.1 ± 2.5 (1.6–9.6) | 4.8 ± 2.2 (2.0–9.0) | 0.13 |
| QoL score | |||
| Mean ± SD (range) | 6.1 ± 2.4 (2.0–9.5) | 4.7 ± 2.5 (1.8–9.0) | 0.23 |
| Aggravating Factors | |||
| Mean ± SD (range) | 4.3 ± 3.2 (0.0–10.0) | 5.5 ± 3.4 (2.5–10.0) | 0.58 |
| Associated Factors | |||
| Mean ± SD (range) | 3.8 ± 2.5 (0.0–10.0) | 4.4 ± 1.8 (2.0–7.0) | 0.36 |
RS-NCP: Neuropathic corneal pain due to refractive surgery, H-NCP: Neuropathic corneal pain due to herpetic eye disease, OSDI: Ocular surface disease index, QoL: Quality of life, SD: standard deviation.
Table 6.
Question by question analysis of Ocular Pain Assessment Survey.
| Ocular Pain Assessment Survey Questions (0–10) (0 = no pain, 10 = worst pain) |
RS-NCP (n = 16) | H-NCP Group (n = 7) | p value |
|---|---|---|---|
| Q1 - The overall severity of your pain today | |||
| Mean ± SD (range) | 5.4 ± 3.3 (0.0–10.0) | 5.1 ± 2.8 (2.0–10.0) | 0.76 |
| Q4 - Level of pain when it is most painful in the past 24 h | |||
| Mean ± SD (range) | 6.7 ± 2.3 (2.0–10.0) | 5.5 ± 2.5 (2.0–10.0) | 0.24 |
| Q5 - Level of pain when it is least painful in the past 24 h | |||
| Mean ± SD (range) | 3.6 ± 2.9 (0.0–9.0) | 2.2 ± 1.2 (1.0–4.0) | 0.37 |
| Q6 - Level of pain on average in the past 24 h | |||
| Mean ± SD (range) | 5.0 ± 2.4 (1.0–10.0) | 4.0 ± 1.5 (2.0–6.0) | 0.37 |
| Q7 - Level of pain when it is most painful in the past 2 weeks | |||
| Mean ± SD (range) | 7.9 ± 2.6 (2.0–10.0) | 6.2 ± 2.6 (2.0–10.0) | 0.08 |
| Q8 - Level of pain when it is least painful in the past 2 weeks | |||
| Mean ± SD (range) | 3.7 ± 3.2 (0.0–9.0) | 3.3 ± 2.3 (1.0–7.0) | 0.57 |
| Q9 - Level of pain on average in the past 2 weeks | |||
| Mean ± SD (range) | 5.8 ± 2.4 (2.0–10.0) | 5.0 ± 2.4 (2.0–10.0) | 0.24 |
| Q10 - Level of worst non-eye pain in the past 24 h | |||
| Mean ± SD (range) | 3.4 ± 3.8 (0.0–10.0) | 2.8 ± 2.7 (0.0–7.0) | 0.79 |
| Q11 - Level of worst non-eye pain in the past 2 weeks | |||
| Mean ± SD (range) | 4.3 ± 4.3 (0.0–10.0) | 3.0 ± 3.0 (0.0–8.0) | 0.62 |
| Q12 - Percentage of time you spend thinking about your non-eye pain (%) | |||
| Mean ± SD (range) | 44.3 ± 40.6 (0–100.0) | 32.0 ± 40.8 (0–100.0) | 0.61 |
| Q13 - How much your pain has interfered with/affected reading and/or computer use | |||
| Mean ± SD (range) | 6.6 ± 2.5 (0.0–10.0) | 6.0 ± 3.0 (2.0–10.0) | 0.53 |
| Q14 - How much your pain has interfered with/affected driving and/or watching TV | |||
| Mean ± SD (range) | 5.1 ± 3.0 (0.0–9.0) | 5.1 ± 2.6 (2.0–10.0) | 0.63 |
| Q15 - How much your pain has interfered with/affected general activity (walking, doing house chores) | |||
| Mean ± SD (range) | 4.9 ± 3.2 (0.0–10.0) | 4.4 ± 3.6 (0.0–10.0) | 0.78 |
| Q16 - How much your pain has interfered with/affected mood | |||
| Mean ± SD (range) | 7.5 ± 3.1 (0.0–10.0) | 4.5 ± 3.1 (0.0–10.0) | 0.06 |
| Q17 - How much your pain has interfered with/affected sleep | |||
| Mean ± SD (range) | 4.3 ± 3.9 (0.0–10.0) | 1.5 ± 1.7 (0.0–4.0) | 0.18 |
| Q18 - How much your pain has interfered with/affected enjoying life/relations with other people | |||
| Mean ± SD (range) | 6.9 ± 3.4 (0.0–10.0) | 4.1 ± 3.7 (0.0–10.0) | 0.10 |
| Q19 - Percentage of time you spend thinking about your eye pain (%) | |||
| Mean ± SD (range) | 72.3 ± 30.5 (10.0–100.0) | 77.1 ± 22.8 (50.0–100.0) | 0.83 |
| Q20 - How much your pain increased when exposed to wind, dry air, heat, air conditioning (%) | |||
| Mean ± SD (range) | 53.9 ± 37.8 (0.0–100.0) | 68.5 ± 24.1 (50.0–100.0) | 0.40 |
| Q21 - How much your pain increased when exposed to volatile chemicals (cleaning agents, fumes, cosmetic fragrances) (%) | |||
| Mean ± SD (range) | 28.8 ± 33.4 (0.0–100.0) | 41.4 ± 51.7 (0.0–100.0) | 0.75 |
| Q22 - How often your eye pain accompanied by redness (%) | |||
| Mean ± SD (range) | 33.0 ± 37.1 (0.0–100.0) | 54.2 ± 37.3 (0.0–100.0) | 0.29 |
| Q23 - How often your eye pain accompanied by burning (%) | |||
| Mean ± SD (range) | 54.3 ± 34.2 (0.0–100.0) | 80.0 ± 18.2 (50.0–100.0) | 0.10 |
| Q24 - How often your eye pain accompanied by sensitivity to light (%) | |||
| Mean ± SD (range) | 43.6 ± 39.0 (0.0–100.0) | 60.7 ± 44.5 (0.0–100.0) | 0.36 |
| Q25 - How often your eye pain accompanied by tearing (%) | |||
| Mean ± SD (range) | 21.0 ± 25.6 (0.0–100.0) | 22.8 ± 30.9 (0.0–80.0) | 0.73 |
| Q26 - How much eye pain relief you have experienced since the last visit (%) | |||
| Mean ± SD (range) | 16.2 ± 23.8 (0.0–70.0) | 17.5 ± 28.7 (0.0–60.0) | 0.80 |
| Q27 - How much non-eye pain relief you have experienced since the last visit (%) | |||
| Mean ± SD (range) | 16.6 ± 23.8 (0.0–70.0) | 20.0 ± 40.0 (0.0–80.0) | 0.89 |
RS-NCP: Neuropathic corneal pain due to refractive surgery, H-NCP: Neuropathic corneal pain due to herpetic eye disease.
The mean effect of pain on reading and/or computer use (p = 0.53), driving and/or watching TV (p = 0.63), general activity (walking, doing house chores) (p = 0.78), mood (p = 0.06), sleep (p = 0.18), enjoying life/relations with other people (p = 0.10), and time spent thinking about eye pain (p = 0.83) did not show statistical difference between RS-NCP and H-NCP patients (Table 6). However, mood, sleep, and enjoying life were more affected by pain in the RS-NCP group compared to the H-NCP group (Table 6).
In both groups, patients reported that they spent most of their time by thinking about their eye pain (72.3% in RS-NCP and 77.1% in H-NCP, p = 0.83). Aggravating factors such as wind, dry air, heat and air conditioning (p=0.40) and volatile chemicals (p=0.75) had a similar effect on pain in both groups (Table 6). Redness (p = 0.29), burning (p = 0.10), sensitivity to light (p = 0.36) and tearing (p = 0.73) accompanied to pain with comparable frequency in RS-NCP and H-NCP (Table 6). Burning was the most associated symptom with pain in both groups (54.3% in RS-NCP and 80.0% in H-NCP, p = 0.10). The mean quality of life score (QoL, questions 13–19) (p = 0.23), aggravating factors (questions 20–21) (p = 0.58) and associated factors (questions 22–25) (p = 0.36) scores calculated from OPAS were all affected in both groups and did show similar findings (Table 5).
4. Discussion
All NP disorders have a common denominator (damage or malfunctioning of somatosensory nervous system) with different underlying etiologies and pathogenesis [25,26]. Pain perception begins with detection of noxious stimuli by nociceptors and induction of action potentials created by nociceptor to somatosensory cortex and paralimbic structures [2]. Peripheral axonal injury may result in release of pro-inflammatory mediators, which may lower the threshold of action potentials of nociceptors, spread the stimuli to adjacent nociceptor and activate silent nociceptor [2,6,12]. Increased peripheral sensitization leads to central sensitization over time, which may result in increased pain levels and awareness [2,6,12]. Trauma, inflammation and iatrogenic damage may trigger this pain signaling pathway [4,6,27].
It is well-known that herpes simplex [28], herpes zoster [29] and refractive surgery [30] can cause corneal nerve damage, which can be detected as decreased nerve density by IVCM. Post-herpetic neuralgia stems from damage to peripheral and central neurons that may be due to either inflammation and/or viral infection, and it is known as one of the most painful conditions [31–35]. Although the classical presentation of post-herpetic neuralgia is pain starting with acute viral disease (acute) and persisting chronically, subacute and chronic neuralgia may also occur, and occasionally pain may develop after a pain-free period [36–38] as presented in our H-NCP group. Moreover, chronic post-herpetic neuralgia does not develop with all acute episodes and can occur after several acute episodes. Interestingly, even long-term follow-up has shown that nerve density may not be restored to healthy levels after herpetic eye disease [28,29]. Similarly, persistent nerve loss over many years has been shown following refractive surgery [17,30,39–41]. Furthermore, stimulated keratocytes during refractive surgeries may produce several cytokines, chemokines, and growth factors that might initiate corneal inflammation after surgical procedures [42,43]. Corneal nerve damage and post-operative inflammation are most likely involved in the development of NCP after refractive surgery, similar to post-herpetic neuralgia.
Herein, we present and compare clinical characteristics of post-refractive surgery NCP and post-herpetic neuralgia patients. Both refractive surgery and herpetic eye disease related NCP present with moderate to severe pain levels, which affect quality of life negatively and significantly. OSDI scores showed that RS-NCP and H-NCP patients suffered from ocular discomfort symptoms with a similar frequency. Further, pain intensity scores showed that pain following refractive surgery was at least as severe as H-NCP patients. Pain levels of patients were strong enough to have moderate impact on daily activities such as reading, computer use, driving, watching TV, walking, and doing house chores. The pain not only affects daily activities, but also mood, enjoying life, and relations with other people, particularly in the post-refractive surgery group.
Although, QoL scores and the sub-questions (reading and/or computer use, driving and/or watching TV, general activities like walking, doing house chores, mood, sleep, enjoying life/relations with other people) are similar in both groups, RS-NCP patients were affected more significantly. However, in contrast to QoL, RS-NCP patients were affected less by aggravating factors (wind, dry air, heat, air conditioning, and volatile chemicals). Furthermore, RS-NCP patients’ symptoms were less likely to be associated with other ocular surface symptoms such as redness, burning, sensitivity to light and tearing compared to H-NCP. When comparisons have been made between other serious chronic conditions including cancer, cardiovascular or neuromuscular disorders and chronic pain, it has been showed that chronic pain conditions had at least as much impact on QoL as those conditions [44,45]. NCP symptoms are generally much more severe and persistent than dry eye symptoms [46–48], which as another chronic ocular condition has shown utility scores similar to moderate angina [49].
Previous studies have shown that negative mood also might influence the experience of neuropathic pain [50,51]. Diaries from patients with reflex sympathetic dystrophy (complex regional pain syndrome) demonstrated that yesterday’s depressed mood contributed today’s increased pain and yesterday’s pain also contributed today’s depression, anxiety and anger [51]. Moreover, it has been shown that better baseline emotional health and physical functioning are likely to respond to treatment better in painful neuropathies [52–54]. The same vicious cycle may be present in NCP patients, which makes the treatment more challenging and also more critical, as NCP patients may be at risk to develop mood disorders.
In our NCP patients, peripheral nerve damage was detected in both groups by IVCM. The nerve densities were comparable in RS-NCP and H-NCP patients. However, both groups demonstrated significantly lower nerve densities than their respective age- and sex-matched healthy controls groups. Previous studies have shown that although corneal nerve regeneration occurs in patients with herpetic eye disease, it still remains low as compared to age-matched healthy individuals, even after 3 years of follow-up [28]. Further, it has been reported that reaching normal corneal nerve density levels can be achieved after 2 years of PRK and by 5 years following LASIK surgery [55]. The results warrant future studies to compare corneal nerve alterations between post-herpetic patients with and without NCP, as well as in post-refractive surgery patients with and without NCP to assess if corneal nerve alterations are specific to NCP or the underlying condition. Although, exact mechanisms underlying corneal nerve regeneration are not completely understood, neuropeptides, neurotrophins, and growth factors released by corneal epithelium and keratocytes, including nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), neurotrophin 3, neurotrophin 4/5, epidermal growth factor (EGF), and glial cell derived neurotrophic factor (GDNF) seem to have role in nerve fiber survival, differentiation and maturation [56,57]. However, delayed or abnormal corneal nerve regeneration after refractive surgery or herpetic eye disease may precipitate development of NCP. Furthermore, corneal inflammation induced by surgical trauma during refractive surgery [13,58] and during the course of herpetic infection [59] contribute to disease processes. Interestingly, in our patient population, DC alterations as shown by IVCM did not show a significant difference compared to healthy individuals. Notably, the presentation times between refractive surgical procedure/active herpetic disease and the initial study visit were widely distributed, which may have influenced the level of corneal inflammation. However, inflammation is required for sensitization of peripheral nerves and the development of NCP, but is not necessarily required to be maintained during the course of the disease after peripheral and central sensitization of neuronal pathways have been initiated. Accordingly, DC densities in some of our NCP patients that were on topical steroids and cyclosporine A during the study visits, may have confounded by topical therapies without resolution of pain.
The goal of NCP treatment should be to reduce pain in the short term and to inhibit and/or resolve central sensitization in the long term. Therefore, treatment of NCP requires differentiation of peripheral and central component pain for adequate management [2,3,12]. In peripheral NCP patients, topical ocular surface treatments, such as artificial tears, topical steroids [3], autologous serum tears [60,61], cryopreserved amniotic membrane [62], and bandage contact lenses may be sufficient by down-regulating ectopic, spontaneous, or hypersensitive signaling in peripheral nociceptor pathways [13]. However, central sensitization requires systemic pharmacotherapies, such as tricyclic antidepressants, low dose-naltrexone, anticonvulsants, serotonin-norepinephrine inhibitors, and other analgesics addition to topical ocular surface treatments [3,63]. The proparacaine challenge test is used to assess central component of pain as previously described [3]. Despite our limited data for proparacaine challenge test, available patient results show that the majority of RS-NCP patients had at least a nonocular component of pain that did not resolve with anesthetic drops. Similar to our patients, previous studies have also reported different amount of centralization component in refractive surgery related NCP, which suggests severe pain, longer and complex treatment and more diminished quality of life [9,12,13,64,65]. A high rate of centralization suggest delayed diagnosis and treatment in both groups. Thus, in order to prevent central sensitization and impairment of quality of life and mood, early diagnosis and treatment have a crucial importance in NCP, especially in a younger age group with a more active work and social life like refractive surgery patients.
In conclusion, despite the limitations of our study, including the retrospective design, relatively small sample size, and heterogeneity of the groups, our study suggests that RS-NCP patients may present with a wide spectrum of clinical features similar to post-herpetic neuralgia. However, pain severity, impaired quality of life and mood, severe nerve damage and the presence of central sensitization are dominant findings in these patients. Therefore, NCP should be kept in mind in refractive surgery patients with non-specific persistent symptoms, who are unresponsive to conventional dry eye treatments and additional evaluation, such as IVCM to assess nerve abnormalities, and the proparacaine challenge test to assess central component of symptoms, should be considered for diagnosis and management purposes. Early diagnosis prior to the development of central sensitization is thus of great importance.
Acknowledgement
Authors declare no acknowledgement.
Funding
NIHR61-NS113341 (PH), Massachusetts Lions Eye Research Fund Inc. (PH), Bettingen Foundation (PH), Lions Club International Foundation (PH), Research to Prevent Blindness Challenge Grant, Tufts Medical Center Institutional Support (PH), Turkish Scientific Research Council (MCO), Gazi University Medical School (MCO).
Footnotes
Conference Presentation: Presented, in part, at the Ocular Microbiology and Immunology Group 52nd Annual Meeting, October 2018, Chicago, IL, USA
Declaration of competing interest
Authors declare no financial disclosure.
References
- [1].IASP Terminology. https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698, Accessed date: 7 July 2019.
- [2].Goyal S, Hamrah P. Understanding neuropathic corneal pain-gaps and current therapeutic approaches. Semin Ophthalmol 2016;31 (1–2): 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dieckmann G, Goyal S, Hamrah P. Neuropathic corneal pain: approaches for management. Ophthalmology 2017;124(11S):S34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moshirfar M, Benstead EE, Sorrentino PM, Tripathy K. Ocular neuropathic pain. Treasure Island (FL): StatPearls; 2019. [PubMed] [Google Scholar]
- [5].Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf 2009;7(1):28–40. [DOI] [PubMed] [Google Scholar]
- [6].Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf 2017;15(3):404–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond) 2015;29(3):301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Borsook D, Youssef AM, Simons L, Elman I, Eccleston C. When pain gets stuck: the evolution of pain chronification and treatment resistance. Pain 2018;159(12):2421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Crane AM, Feuer W, Felix ER, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol 2017;101(9):1238–43. [DOI] [PubMed] [Google Scholar]
- [10].Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, Galor A. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol 2017; 101 (2):227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Galor A, Covington D, Levitt AE, et al. Neuropathic ocular pain due to dry eye is associated with multiple comorbid chronic pain syndromes. J Pain 2016; 17(3): 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol 2016; 100(1): 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Theophanous C, Jacobs DS, Hamrah P. Corneal neuralgia after LASIK. Optom Vis Sci 2015;92(9):e233–40. [DOI] [PubMed] [Google Scholar]
- [14].Wen D, McAlinden C, Flitcroft I, et al. Postoperative efficacy, predictability, safety, and visual quality of laser corneal refractive surgery: a network meta-analysis. Am J Ophthalmol 2017;178:65–78. [DOI] [PubMed] [Google Scholar]
- [15].Sambhi RS, Sambhi GDS, Mather R, Malvankar-Mehta MS. Dry eye after refractive surgery: a meta-analysis. Can J Ophthalmol 2020;55(2):99–l06. [DOI] [PubMed] [Google Scholar]
- [16].Toda I Dry eye after LASIK. Invest Ophthalmol Vis Sci 2018;59(14):DES109–15. [DOI] [PubMed] [Google Scholar]
- [17].Levitt AE, Galor A, Weiss JS, et al. Chronic dry eye symptoms after LASIK: parallels and lessons to be learned from other persistent post-operative pain disorders. Mol Pain 2015;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf 2014;12(1):32–45. [DOI] [PubMed] [Google Scholar]
- [19].Galor A, Patel S, Small LR, et al. Pregabalin failed to prevent dry eye symptoms after laser-assisted in situ keratomileusis (LASIK) in a randomized Pilot study. J Clin Med 2019;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jacobs DS. Diagnosis and treatment of ocular pain: the ophthalmologisťs perspective. Curr Ophthalmol Rep 2017;5(4):271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McKay TB, Seyed-Razavi Y, Ghezzi CE, et al. Corneal pain and experimental model development. Prog Retin Eye Res 2019;71:88–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol 2000; 118(5): 615–21. [DOI] [PubMed] [Google Scholar]
- [23].Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and reliability of a novel ocular pain assessment survey (OPAS) in quantifying and monitoring corneal and ocular surface pain. Ophthalmology 2016;123(7):1458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci 2011;52(8):5136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baron R, Maier C, Attal N, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain 2017;158(2):261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9(8):807–19. [DOI] [PubMed] [Google Scholar]
- [27].Dieckmann GKN, Jamali A, Lopez MJ, Moein H, Hamrah P. Epidemiological factors of neuropathic corneal pain. Paper presented at: IASP2019; Vancouver, BC, Canada. [Google Scholar]
- [28].Moein HR, Kheirkhah A, Muller RT, Cruzat AC, Pavan-Langston D, Hamrah P. Corneal nerve regeneration after herpes simplex keratitis: a longitudinal in vivo confocal microscopy study. Ocul Surf 2018;16(2):218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hamrah P, Cruzat A, Dastjerdi MH, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology 2013;120(1):40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bandeira F, Yusoff NZ, Yam GH, Mehta JS. Corneal re-innervation following refractive surgery treatments. Neural Regen Res 2019;14(4):557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mallick-Searle T, Snodgrass B, Brant JM. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J Multidiscip Healthc 2016;9:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sommer C, Leinders M, Uceyler N. Inflammation in the pathophysiology of neuropathic pain. Pain 2018;159(3):595–602. [DOI] [PubMed] [Google Scholar]
- [33].Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain 2008;9(1 Suppl 1):S10–8. [DOI] [PubMed] [Google Scholar]
- [34].Dworkin RH, Gnann JW Jr., Oaklander AL, Raja SN, Schmader KE, Whitley RJ. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain 2008;9(1 Suppl 1):S37–44. [DOI] [PubMed] [Google Scholar]
- [35].Pavan-Langston D Herpes zoster ophthalmicus. Neurology 1995;45(12 Suppl 8):S50–1. [DOI] [PubMed] [Google Scholar]
- [36].Scholz J, Finnerup NB, Attal N, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 2019;160(1):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schott GD. Triggering of delayed-onset postherpetic neuralgia. Lancet 1998;351(9100):419–20. [DOI] [PubMed] [Google Scholar]
- [38].Dworkin RH, Portenoy RK. Proposed classification of herpes zoster pain. Lancet 1994;343(8913):1648. [DOI] [PubMed] [Google Scholar]
- [39].Lee BH, McLaren JW, Erie JC, Hodge DO, Bourne WM. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci 2002;43(12):3660–4. [PubMed] [Google Scholar]
- [40].Calvillo MP, McLaren JW, Hodge DO, Bourne WM. Corneal reinnervation after LASIK: prospective 3-year longitudinal study. Invest Ophthalmol Vis Sci 2004;45(11): 3991–6. [DOI] [PubMed] [Google Scholar]
- [41].Moilanen JA, Holopainen JM, Vesaluoma MH, Tervo TM. Corneal recovery after lasik for high myopia: a 2-year prospective confocal microscopic study. Br J Ophthalmol 2008;92(10):1397–402. [DOI] [PubMed] [Google Scholar]
- [42].Leonardi A, Tavolato M, Curnow SJ, Fregona IA, Violato D, Alio JL. Cytokine and chemokine levels in tears and in corneal fibroblast cultures before and after excimer laser treatment. J Cataract Refract Surg 2009;35(2):240–7. [DOI] [PubMed] [Google Scholar]
- [43].Alio JL, Javaloy J. Corneal inflammation following corneal photoablative refractive surgery with excimer laser. Surv Ophthalmol 2013;58(1):11–25. [DOI] [PubMed] [Google Scholar]
- [44].Sprangers MA, de Regt EB, Andries F, et al. Which chronic conditions are associated with better or poorer quality of life? J Clin Epidemiol 2000;53(9):895–907. [DOI] [PubMed] [Google Scholar]
- [45].Andrew R, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Pract 2014;14(1):79–94. [DOI] [PubMed] [Google Scholar]
- [46].Galor A, Batawi H, Felix ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol 2016; 100(6): 745–9. [DOI] [PubMed] [Google Scholar]
- [47].Galor A, Zlotcavitch L, Walter SD, et al. Dry eye symptom severity and persistence are associated with symptoms of neuropathic pain. Br J Ophthalmol 2015;99(5): 665–8. [DOI] [PubMed] [Google Scholar]
- [48].Galor A, Small L, Feuer W, Levitt RC, Sarantopoulos KD, Yosipovitch G. The relationship between ocular itch, ocular pain, and dry eye symptoms (an American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 2017;115:T5. [PMC free article] [PubMed] [Google Scholar]
- [49].Wan KH, Chen LJ, Young AL. Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye (Lond) 2016; 30(12): 1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Arena JG, Sherman RA, Bruno GM, Smith JD. The relationship between situational stress and phantom limb pain: cross-lagged correlational data from six month pain logs. J Psychosom Res 1990; 34(1): 71–7. [DOI] [PubMed] [Google Scholar]
- [51].Feldman SI, Downey G, Schaffer-Neitz R. Pain, negative mood, and perceived support in chronic pain patients: a daily diary study of people with reflex sympathetic dystrophy syndrome. J Consult Clin Psychol 1999;67(5):776–85. [DOI] [PubMed] [Google Scholar]
- [52].Girach A, Julian TH, Varrassi G, Paladini A, Vadalouka A, Zis P. Quality of life in painful peripheral neuropathies: a systematic review. Pain Res Manag 2019;2019:2091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Smith EM, Pang H, Ye C, et al. Predictors of duloxetine response in patients with oxaliplatin-induced painful chemotherapy-induced peripheral neuropathy (CIPN): a secondary analysis of randomised controlled trial - CALGB/alliance 170601. Eur J Cancer Care (Engl) 2017;26(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Otto M, Bach FW, Jensen TS, Sindrup SH. Health-related quality of life and its predictive role for analgesic effect in patients with painful polyneuropathy. Eur J Pain 2007; 11 (5): 572–8. [DOI] [PubMed] [Google Scholar]
- [55].Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol 2005; 140(6): 1059–64. [DOI] [PubMed] [Google Scholar]
- [56].Sacchetti M, Lambiase A. Neurotrophic factors and corneal nerve regeneration. Neural Regen Res 2017;12(8):1220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol 2017;232(4):717–24. [DOI] [PubMed] [Google Scholar]
- [58].Solomon R, Donnenfeld ED, Perry HD. The effects of LASIK on the ocular surface. Ocul Surf 2004;2(1):34–44. [DOI] [PubMed] [Google Scholar]
- [59].Hamrah P, Pavan-Langston D, Dana R. Herpes simplex keratitis and dendritic cells at the crossroads: lessons from the past and a view into the future. Int Ophthalmol Clin 2009;49(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Aggarwal S, Colon C, Kheirkhah A, Hamrah P. Efficacy of autologous serum tears for treatment of neuropathic corneal pain. Ocul Surf 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous serum tears for treatment of Photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. Ocul Surf 2015;13(3):250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Morkin MI, Hamrah P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul Surf 2018;16(1):132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Galor A, Moein HR, Lee C, et al. Neuropathic pain and dry eye. Ocul Surf 2018; 16(1): 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Dieckmann G, Koseoglu ND, Akhlaq A, Pondelis N, Hamrah P. Efficacy of intranasal stimulation for peripheral pain among neuropathic corneal pain patients. Paper presented at: ARVO annual meeting abstract. 2018. [Google Scholar]
- [65].Shetty R, Deshpande K, Ghosh A, Sethu S. Management of ocular neuropathic pain with vitamin B12 supplements: a case report. Cornea 2015;34(10): 1324–5. [DOI] [PubMed] [Google Scholar]


