Abstract
Background
Socioeconomic and demographic categories such as income, race, insurance status and treatment center type are associated with outcomes in acute leukemia. We aimed to determine if distance to treatment center impacts overall survival in children and young adults with acute leukemia.
Methods
We queried the National Cancer Database for patients ≤39 years of age diagnosed with Acute Myeloid Leukemia (AML) or Acute Lymphoblastic Leukemia (ALL). A backwards elimination procedure was used to select final multivariate Cox models.
Results
In total, 12,301 patients with AML and 22,683 patients with ALL were analyzed. The ALL model included distance to treatment center, Charlson-Deyo score, age, race, insurance status, and community income level. US census definitions of urban vs. rural were not statistically significant, and no interaction was significant for included variables. Compared to distances >50 miles, all other distance groups were associated with improved survival: ≤10 miles (HR 0.91, p=0.04), >10 to ≤20 miles (HR 0.86, p=0.004) and >20 to ≤50 miles (HR 0.87, p=0.005). The final model for AML included the same variables as the ALL model, except distance to treatment center, which was not statistically significant.
Conclusion
For children and young adults with ALL, distances >50 miles are associated with inferior overall survival, however no difference was seen for AML. Although it is unknown if differences in survival for ALL based on distance are driven by relapse or treatment related mortality, increased attention to adherence, supportive care and logistics for patients traveling long distances are warranted.
Keywords: Distance, Treatment Center, ALL, Acute Lymphoblastic Leukemia, Survival, Children, Pediatrics, AYA, Adolescent, Young Adult
Precis:
For children and young adults with ALL, distances >50 miles to treatment center are associated with inferior overall survival. Increased attention to adherence, supportive care and logistics for patients traveling long distances are warranted.
Lay Summary:
For children and young adults with Acute Lymphoblastic Leukemia, living more than 50 miles away from the treatment center was associated with worse outcomes.
Introduction
Outcomes for pediatric patients with Acute Lymphoblastic Leukemia (ALL) and Acute Myeloid Leukemia (AML) have improved with successive generations of clinical trials.1, 2 Improvements in disease directed therapy as well as supportive care have improved relapse rates and decreased treatment related mortality.3 For adolescent and young adults (AYA, ages 15–39 years),4 outcomes for acute leukemia have historically lagged behind those of younger children, though newer approaches directed towards AYAs may lead to improved outcomes.5, 6 Despite improvements in treatment for children and young adults with acute leukemia, certain socioeconomic or health services issues may impact outcomes. Children with both ALL and AML have a higher risk of death if they are of a lower socioeconomic status.7 Treatment facility type may also effect outcomes. AYAs with acute leukemia treated at a National Cancer Institute designated comprehensive cancer center or a Children’s Oncology Group site had improve outcomes compared to those who were not treated at these institutions.8 In adults with AML early mortality is increased in adults treated at non-academic medical centers.9 Additionally, insurance status has demonstrated variable effects on outcomes depending on patient disease and age.10–14 Race also appears to play a role in outcomes as non-white children have inferior outcomes to white children for both ALL and AML,15, 16 although the reasons for this are not entirely clear and have not been demonstrated in all studies.17, 18
The National Cancer Database (NCDB) is a joint quality improvement initiative of the American College of Surgeons Commission on Cancer and the American Cancer Society, and contains registry data from approximately 70% of newly diagnosed malignancies in the United States.19 In addition to data on area income level, race, insurance status, as well as patient and disease specific factors, the NCDB also collects data on distance to treatment center. Among adults with certain solid tumors, distance to treatment center is associated with outcomes.20,21 We hypothesized that distance to treatment center impacts outcomes of children and young adults with acute leukemia, as further distance might strain families and caregivers to a greater degree and may lead to delays in seeking care at both diagnosis and complications during treatment. To test our hypothesis, we queried the NCDB and developed models to predict the contribution of distance to treatment center on overall survival in children and AYAs with AML and ALL.
Methods
Data source
The NCDB population is comprised of patients who were treated or diagnosed at a Commission on Cancer accredited cancer program, which encompasses more than 1,500 facilities.19 Trained abstractors enter clinical and demographic data comparable to those reported in the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry.22 Socio-demographic data are collected on a patient level basis or inferred from patient zip codes at the time of diagnosis (rural vs. urban status, median income). Although de-identified facility level characteristics (e.g. academic vs. community) are collected for adult cases, these data are suppressed for pediatric and AYA cases due to the potential for re-identification due to smaller patient volumes.19 Charlson-Deyo score (0,1,2,3) is used to account for overall comorbidities at the time of diagnosis.19, 23, 24
Case Selection
Patients ≤39 years of age, diagnosed between 2004 and 2015 with AML or ALL were selected from the NCDB (figure 1). The following International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes were used to identify appropriate histological cases: ALL (9811–9818, 9835–9837) and AML: (9840, 9861, 9867, 9871–9874, 9891, 9895–9897, 9910, 9911).
Figure 1.

Included patients based on histology.
Definitions
Urban vs. rural designation is based on zip code of patient address at the time of diagnosis as defined by the year 2000 United States census data. Likewise, community median income reflects that of the patient’s zip code, not patient specific information. Sphere distance to treatment center is the distance on earth between two points, measured from cancer center address to the center of the zip code of patient address. Sphere distance was then categorized by quartiles, as the large sample size allowed for a more granular assessment of distance than if fewer groups were used. In the United States employer‐sponsored private insurance covers a majority of the population. Government insurance programs include Medicaid and Medicare, which provide insurance for persons earning below a designated percentage of the federal poverty level or persons 65 years of age and older, respectively. Additionally, those without insurance do not receive financial assistance for the costs of medical care.25 In the NCDB, insurance is categorized based on the type of primary payer at the time of diagnosis (notably one’s insurance status could change during the course of treatment, though this is not accounted for in the NCDB database). Regarding overall survival, to maintain commission on cancer designation, institutions most maintain an annual follow up of ≥90% within the last five years and ≥80% from the cancer registration reference date. However, no cause specific mortality nor date of relapse is available.
Statistical Methods
Patient characteristics were summarized. The Kaplan-Meier method was used to estimate overall survival (OS) by patient group. Cox proportional hazard models were used to associate patient and other characteristics (age, gender, etc.) with OS. A backwards elimination procedure was used to identify the final multivariate Cox models, starting with all factors in the univariate analysis, including diagnosis year (no screen from the univariate analysis was performed). At each step of backwards elimination, the factor with the largest p-value was excluded. The procedure continued until all factors in the model had p-values of 0.05 or less. Akaike information criterion (AIC) statistics were used as well. Interactions between distance and age, race, comorbidity score, community income, and insurance status which were defined a priori, were tested. Diagnosis year was included as a stratification factor in the final model to account for time effect on overall survival. All tests were two-sided and a p-value of 0.05 or less was considered statistically significant. Statistical analysis was carried out using SAS Studio version 3.7 (SAS Institute, Cary, NC). Plots were produced by R version 3.6 (R Foundation, Vienna, Austria).
Results
Patients
In total, 12,301 patients with AML and 22,683 patients with ALL were available for analysis (Table 1). For both patients with AML and ALL, slightly more than 20% of patients had a travel distance of more than 50 miles. For all patients with AML, the five and ten year overall survival was 51% (95% CI: 50–52%) and 48% (95% CI: 47–49%) respectively, whereas, for ALL, the five and ten year overall survival was 79% (95% CI: 79–80%) and 76% (95% CI: 75–77%).
Table 1.
Characteristics of patients analyzed with ALL and AML based on distance to treatment center
| Acute Lymphoblastic Leukemia | Acute Myeloid Leukemia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Distance to Center by Zip Code (Miles) | Distance to Center by Zip Code (Miles) | ||||||||||
| ≤10 | >10– ≤20 | >20– ≤50 | >50 | Total | ≤10 | >10– ≤20 | >20– ≤50 | >50 | Total | ||
| Total N (%) | 7774 (34) | 4686 (21) | 5378 (24) | 4845 (21) | 22683 | Total N (%) | 4522 (37) | 2282 (19) | 2607 (22) | 2890 (23) | 12301 |
| Charlson-Deyo Score | Charlson-Deyo Score | ||||||||||
| 0 | 7249 (34) | 4389 (21) | 5016 (24) | 4508 (21) | 21162 | 0 | 4049 (36) | 2062 (19) | 2374 (21) | 2641 (24) | 11126 |
| 1 | 462 (34) | 263 (20) | 318 (24) | 301 (22) | 1344 | 1 | 391 (40) | 191 (19) | 195 (20) | 210 (21) | 987 |
| 2 | 41 (34) | 24 (20) | 28 (24) | 26 (22) | 119 | 2 | 48 (40) | 21 (18) | 24 (20) | 26 (22) | 119 |
| 3 | 22 (38) | 10 (17) | 16 (28) | 10 (17) | 58 | 3 | 34 (49) | 8 (12) | 14 (20) | 13 (19) | 69 |
| Gender | Gender | ||||||||||
| Female | 3181 (34) | 1913 (21) | 2212 (24) | 1936 (21) | 9242 | Female | 2234 (37) | 1125 (19) | 1263 (21) | 1394 (23) | 6016 |
| Male | 4593 (34) | 2773 (21) | 3166 (24) | 2909 (22) | 13441 | Male | 2288 (36) | 1157 (18) | 1344 (21) | 1496 (24) | 6285 |
| Race/Ethnicity | Race/Ethnicity | ||||||||||
| African American | 860 (47) | 349 (19) | 322 (17) | 314 (17) | 1845 | African American | 821 (52) | 271 (17) | 228 (14) | 273 (17) | 1593 |
| Asian | 455 (47) | 269 (28) | 140 (14) | 106 (11) | 970 | Asian | 324 (49) | 145 (22) | 84 (13) | 108 (16) | 661 |
| Hispanic/Latinos | 2408 (43) | 1118 (20) | 1083 (20) | 928 (17) | 5537 | Hispanic/Latinos | 878 (46) | 376 (20) | 324 (17) | 321 (17) | 1899 |
| Native American | 24 (15) | 17 (11) | 29 (18) | 90 (56) | 160 | Native American | 17 (18) | 9 (10) | 18 (19) | 50 (53) | 94 |
| Others | 118 (37) | 87 (27) | 62 (20) | 50 (16) | 317 | Others | 66 (45) | 31 (21) | 20 (14) | 29 (20) | 146 |
| White | 3789 (28) | 2786 (21) | 3668 (27) | 3281 (24) | 13524 | White | 2365 (30) | 1429 (18) | 1899 (24) | 2073 (27) | 7766 |
| Unknown | 120 (36) | 60 (18) | 74 (22) | 76 (23) | 330 | Unknown | 51 (36) | 21 (15) | 34 (24) | 36 (25) | 142 |
| Insurance | Insurance | ||||||||||
| Government | 2,812 (34) | 1460 (18) | 1898 (23) | 1995 (24) | 8165 | Government | 1467 (37) | 620 (16) | 776 (20) | 1068 (27) | 3931 |
| Not Insured | 476 (42) | 210 (18) | 245 (21) | 212 (19) | 1143 | Not Insured | 427 (46) | 164 (18) | 176 (19) | 155 (17) | 922 |
| Private | 4226 (34) | 2860 (23) | 3087 (25) | 2400 (19) | 12573 | Private | 2489 (36) | 1424 (20) | 1552 (22) | 1511 (22) | 6976 |
| Unknown | 260 (32) | 156 (19) | 148 (18) | 238 (30) | 802 | Unknown | 139 (29) | 74 (16) | 103 (22) | 156 (33) | 472 |
| Community Median Income | Community Median Income | ||||||||||
| <38 K | 1654 (41) | 354 (9) | 680 (17) | 1334 (33) | 4022 | <38 K | 1050 (44) | 170 (7) | 329 (14) | 860 (36) | 2409 |
| 38–63 K | 3522 (31) | 2011 (17) | 3061 (27) | 2952 (26) | 11546 | 38–63 K | 2013 (33) | 1050 (17) | 1442 (23) | 1642 (27) | 6147 |
| ≥63 K | 2308 (34) | 2318 (34) | 1633 (24) | 552 (8) | 6811 | ≥63 K | 1238 (35) | 1062 (30) | 834 (24) | 384 (11) | 3518 |
| Unknown | 290 (95) | 3 (1) | 4 (1) | 7 (2) | 304 | Unknown | 221 (97) | 0 (0) | 2 (1) | 4 (2) | 227 |
| Year of Diagnosis | Year of Diagnosis | ||||||||||
| 2004 | 631 (35) | 360 (20) | 402 (22) | 403 (22) | 1796 | 2004 | 373 (39) | 178 (18) | 189 (20) | 228 (24) | 968 |
| 2005 | 652 (37) | 320 (18) | 415 (23) | 394 (22) | 1781 | 2005 | 384 (40) | 174 (18) | 187 (19) | 219 (23) | 964 |
| 2006 | 664 (35) | 387 (20) | 444 (23) | 396 (21) | 1891 | 2006 | 364 (37) | 202 (21) | 187 (19) | 230 (23) | 983 |
| 2007 | 681 (35) | 402 (20) | 456 (23) | 426 (22) | 1965 | 2007 | 405 (40) | 186 (18) | 211 (21) | 218 (21) | 1020 |
| 2008 | 707 (35) | 436 (22) | 469 (23) | 404 (20) | 2016 | 2008 | 429 (40) | 189 (18) | 214 (20) | 230 (22) | 1062 |
| 2009 | 697 (35) | 429 (22) | 463 (23) | 402 (20) | 1991 | 2009 | 356 (37) | 186 (19) | 191 (20) | 241 (25) | 974 |
| 2010 | 616 (31) | 417 (21) | 487 (25) | 440 (22) | 1960 | 2010 | 363 (34) | 197 (19) | 245 (23) | 255 (24) | 1060 |
| 2011 | 718 (36) | 411 (21) | 476 (24) | 398 (20) | 2003 | 2011 | 400 (39) | 180 (17) | 217 (21) | 233 (23) | 1030 |
| 2012 | 568 (32) | 376 (21) | 437 (25) | 396 (22) | 1777 | 2012 | 363 (35) | 202 (20) | 218 (21) | 243 (24) | 1026 |
| 2013 | 593 (34) | 351 (20) | 424 (24) | 400 (23) | 1768 | 2013 | 351 (33) | 195 (18) | 250 (24) | 259 (25) | 1055 |
| 2014 | 635 (34) | 410 (22) | 443 (24) | 363 (20) | 1851 | 2014 | 383 (34) | 200 (18) | 275 (24) | 268 (24) | 1126 |
| 2015 | 612 (32) | 387 (21) | 462 (25) | 423 (22) | 1884 | 2015 | 351 (34) | 193 (19) | 223 (22) | 266 (26) | 1033 |
| Age | Age | ||||||||||
| <18 | 5152 (32) | 3444 (22) | 4030 (25) | 3375 (21) | 16001 | <18 | 1065 (33) | 659 (21) | 707 (22) | 753 (24) | 3184 |
| ≥18 | 2622 (39) | 1242 (19) | 1348 (20) | 1470 (22) | 6682 | ≥18 | 3457 (38) | 1623 (18) | 1900 (21) | 2137 (23) | 9117 |
| Rural | Rural | ||||||||||
| No | 7349 (34) | 4555 (21) | 5160 (24) | 4529 (21) | 21593 | No | 4229 (36) | 2218 (19) | 2511 (22) | 2670 (23) | 11628 |
| Yes | 8 (3) | 4 (1) | 54 (19) | 221 (77) | 287 | Yes | 2 (1) | 3 (2) | 37 (20) | 147 (78) | 189 |
| Unknown | 417 (52) | 127 (16) | 164 (20) | 95 (12) | 803 | Unknown | 291 (60) | 61 (13) | 59 (12) | 73 (15) | 484 |
| Vital Status | Vital Status | ||||||||||
| Alive | 5547 (33) | 3554 (21) | 4061 (24) | 3457 (21) | 16619 | Alive | 2215 (36) | 1162 (19) | 1326 (22) | 1410 (23) | 6113 |
| Dead | 1615 (39) | 745 (18) | 855 (20) | 965 (23) | 4180 | Dead | 1956 (38) | 927 (18) | 1058 (21) | 1214 (24) | 5155 |
| Unknown | 612 (32) | 387 (21) | 462 (25) | 423 (22) | 1884 | Unknown | 351 (34) | 193 (19) | 223 (22) | 266 (26) | 1033 |
Association between Covariates and Overall Survival
The association between covariates and overall survival was carried out for ALL and AML separately (Table 2). For patients with ALL, distance to treatment center, Charlson-Deyo score, gender, race, insurance status, community median income, and age were all associated with overall survival. (Figure 2). However, there was no difference in survival based on a rural v. urban zip code. No statistically significant interactions were detected for any of the variables. Similar findings were seen for patients with AML, although no difference was detected based on gender. Again, no statistically significant interactions were detected for any of the variables predicting overall survival in AML.
Table 2.
Univariate model of patient characteristics and overall survival. Note, a lower hazard ratio implies a decreased risk of death.
| Acute Lymphoblastic Leukemia | Acute Myeloid Leukemia | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR (95% CI) | Pairwise P | Overall P | HR (95% CI) | Pairwise P | Overall P |
| Sphere Distance to Center | ||||||
| ≤10 Miles v. >50 Miles | 1.04 (0.96–1.13) | 0.29 | <.0001 | 1.05 (0.98–1.13) | 0.20 | 0.0066 |
| 10–20 Miles v. >50 Miles | 0.76 (0.69–0.84) | <.0001 | 0.94 (0.87–1.03) | 0.18 | ||
| 20–50 Miles v. >50 Miles | 0.76 (0.70–0.84) | <.0001 | 0.93 (0.86–1.01) | 0.10 | ||
| Charlson-Deyo Score | ||||||
| 0 v. 3 | 0.30 (0.20–0.43) | <.0001 | <.0001 | 0.41 (0.31–0.56) | <.0001 | <.0001 |
| 1 v. 3 | 0.53 (0.35–0.79) | 0.0018 | 0.61 (0.44–0.84) | 0.0023 | ||
| 2 v. 3 | 0.91 (0.56–1.50) | 0.71 | 0.92 (0.62–1.35) | 0.66 | ||
| Gender | ||||||
| Male v. Female | 1.16 (1.09–1.24) | <.0001 | <.0001 | 1.02 (0.97–1.08) | 0.4276 | 0.4276 |
| Race | ||||||
| African American v. Caucasian | 1.57 (1.41–1.74) | <.0001 | <.0001 | 1.33 (1.23–1.44) | <.0001 | <.0001 |
| Asian v. Caucasian | 0.95 (0.80–1.12) | 0.54 | 0.97 (0.85–1.10) | 0.62 | ||
| Hispanic/Latinos v. Caucasian | 1.48 (1.38–1.59) | <.0001 | 0.94 (0.86–1.01) | 0.11 | ||
| Native American v. Caucasian | 1.72 (1.25–2.37) | 0.0009 | 1.03 (0.76–1.40) | 0.84 | ||
| Others v. Caucasian | 0.91 (0.68–1.23) | 0.54 | 0.74 (0.55–1.00) | 0.0477 | ||
| Insurance | ||||||
| Government v. Private | 1.27 (1.19–1.36) | <.0001 | <.0001 | 1.20 (1.13–1.27) | <.0001 | <.0001 |
| Not Insured v. Private | 2.59 (2.32–2.89) | <.0001 | 1.39 (1.25–1.54) | <.0001 | ||
| Community Median Income | ||||||
| < $38,000 v. ≥ $63,000 | 1.72 (1.57–1.88) | <.0001 | <.0001 | 1.32 (1.21–1.42) | <.0001 | <.0001 |
| $38,000– $63,000 v. ≥ $63,000 | 1.35 (1.26–1.46) | <.0001 | 1.17 (1.10–1.26) | <.0001 | ||
| Age | ||||||
| ≥18 v. <18 | 5.56 (5.22–5.92) | <.0001 | <.0001 | 1.56 (1.46–1.67) | <.0001 | <.0001 |
| Rural | ||||||
| Yes v. No | 0.89 (0.67–1.19) | 0.43 | 0.4325 | 0.97 (0.77–1.21) | 0.76 | 0.76 |
Figure 2:
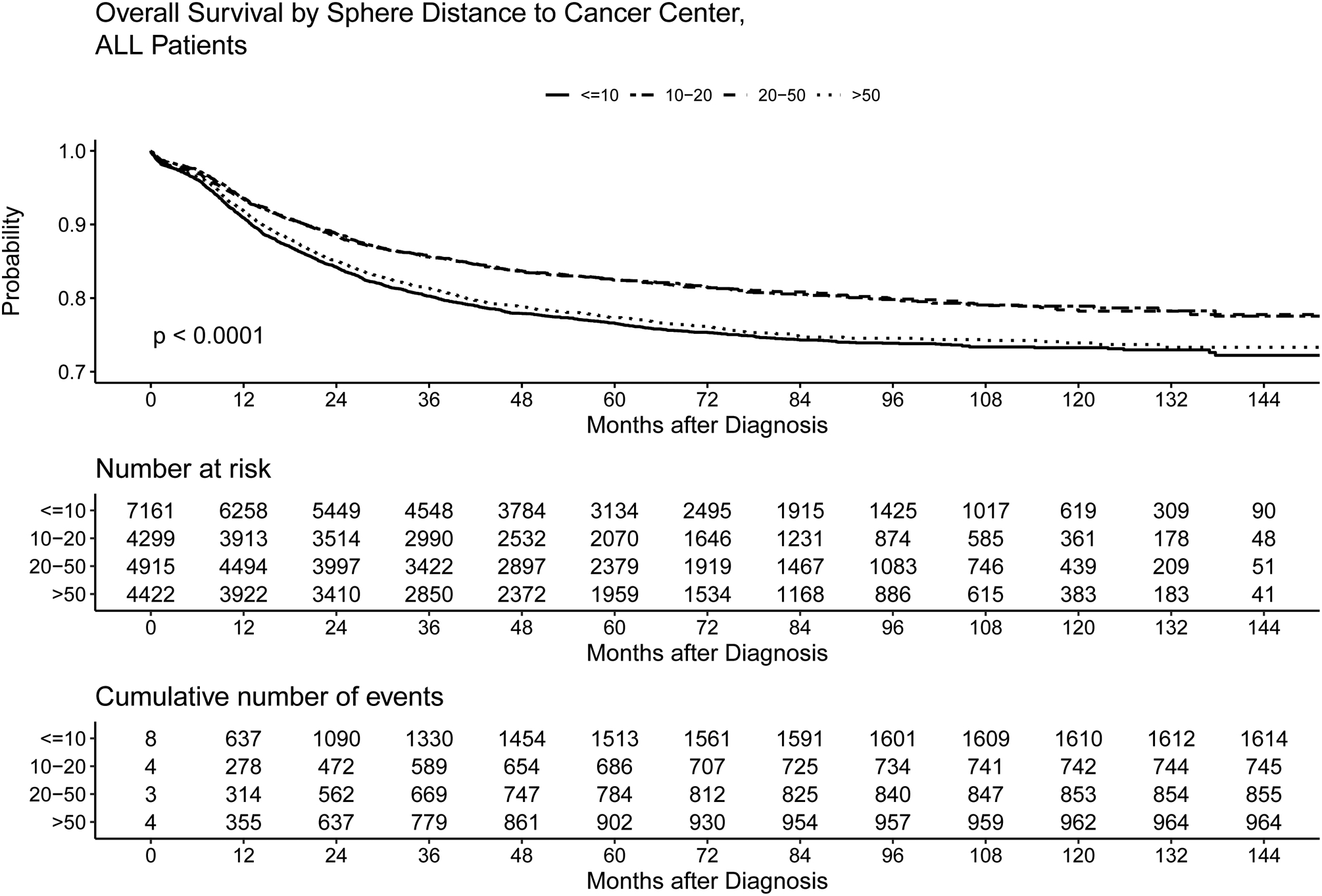
Kaplan-Meier plot of overall survival for patients with ALL based on distance to treatment center. At 60 months, the overall survival rate was 77%, 82%, 83% and 77% for patients ≤10 miles, >10 to ≤20 miles, >20 to ≤50, and >50 miles to treatment center, respectively. In this analysis, which does not incorporate covariates, overall survival was not significantly different for those ≤10 miles and those >50 miles. However, inferior survival was seen for those >50 miles from treatment center versus those who were>10 to ≤20 miles or >20 to ≤50 miles.
ALL Multivariate Model
After backwards stepwise elimination, the final ALL model included distance to treatment center, Charlson-Deyo score, age, race, insurance status, and community median income (Table 3). Compared to distances >50 miles, all other distance groups were associated with improved survival: ≤10 miles (HR 0.91, p=0.04), >10 to ≤20 miles (HR 0.86, p=0.004) and >20 to ≤50 miles (HR 0.87, p=0.005). Compared to Caucasian race, those of African-American, Hispanic/Latino, and Native American race had inferior overall survival, whereas no difference was detected for Asian race or other race. Compared to private insurance, those with government insurance, or no insurance at diagnosis had inferior survival, and compared to community median income of ≥ $63,000, those with lower median incomes had inferior survival. No statistically significant interactions were seen with any of the variables (i.e. the effect of distance on overall survival did not depend on other factors examined). Therefore, subgroup analyses were not performed.
Table 3.
Multivariate model of ALL patient characteristics and overall survival. Note, a lower hazard ratio implies a decreased risk of death. After adjusting for covariates, a distance of >50 miles to treatment center is associated with the lowest overall survival.
| Characteristic | HR (95% CI) | P |
|---|---|---|
| Sphere Distance to Center | ||
| ≤10 Miles v. >50 Miles | 0.91 (0.84–0.995) | 0.04 |
| 10–20 Miles v. >50 Miles | 0.86 (0.78–0.95) | 0.0043 |
| 20–50 Miles v. >50 Miles | 0.87 (0.79–0.96) | 0.0048 |
| Charlson-Deyo Score | ||
| 0 v. 3 | 0.41 (0.28–0.61) | <.0001 |
| 1 v. 3 | 0.60 (0.40–0.90) | 0.01 |
| 2 v. 3 | 0.68 (0.41–1.13) | 0.13 |
| Age | ||
| ≥18 v. <18 | 5.59 (5.24–5.99) | <.0001 |
| Race | ||
| African American v. Caucasian | 1.30 (1.16–1.45) | <.0001 |
| Asian v. Caucasian | 0.95 (0.80–1.13) | 0.53 |
| Hispanic/Latinos v. Caucasian | 1.19 (1.10–1.28) | <.0001 |
| Native American v. Caucasian | 1.54 (1.10–2.14) | 0.01 |
| Others v. Caucasian | 0.93 (0.67–1.29) | 0.68 |
| Insurance | ||
| Government v. Private | 1.26 (1.17–1.35) | <.0001 |
| Not Insured v. Private | 1.27 (1.13–1.43) | <.0001 |
| Community Median Income | ||
| < $38,000 v. ≥ $63,000 | 1.27 (1.15–1.40) | <.0001 |
| $38,000– $63,000 v. ≥ $63,000 | 1.16 (1.07–1.26) | 0.0003 |
AML Multivariate Model
The final AML model included Charlson-Deyo score, age, race, insurance status, and community median income (Table 4). As compared to ALL, distance to treatment center was not significantly associated with OS after adjusting for other factors in the final model. Like the ALL model, compared to private insurance, those with government insurance, or no insurance at diagnosis had inferior survival. Additionally, compared to community median income of ≥ $63,000, those with lower median incomes had inferior survival. Those of African American race had inferior overall survival compared to Caucasian race, but no difference was seen for Asian, Hispanic/Latino, Native American, or other race compared to Caucasian race. No statistically significant interactions were seen with any of the variables.
Table 4.
Multivariate model of ALL patient characteristics and overall survival. Note, a lower hazard ratio implies a decreased risk of death. After adjusting for covariates, distance to treatment center is no longer significant.
| Characteristic | HR (95% CI) | P |
|---|---|---|
| Charlson-Deyo Score | ||
| 0 v. 3 | 0.45 (0.33–0.62) | <.0001 |
| 1 v. 3 | 0.64 (0.46–0.89) | 0.01 |
| 2 v. 3 | 0.91 (0.61–1.35) | 0.63 |
| Age | ||
| ≥18 v. <18 | 1.55 (1.45–1.67) | <.0001 |
| Race | ||
| African American v. Caucasian | 1.24 (1.14–1.34) | <.0001 |
| Asian v. Caucasian | 1.02 (0.89–1.16) | 0.80 |
| Hispanic/Latinos v. Caucasian | 0.92 (0.85–1.01) | 0.07 |
| Native American v. Caucasian | 1.02 (0.74–1.40) | 0.90 |
| Others v. Caucasian | 0.75 (0.55–1.04) | 0.09 |
| Insurance | ||
| Government v. Private | 1.19 (1.12–1.27) | <.0001 |
| Not Insured v. Private | 1.26 (1.13–1.41) | <.0001 |
| Community median income | ||
| < $38,000 v. ≥ $63,000 | 1.21 (1.11–1.32) | <.0001 |
| $38,000– $63,000 v. ≥ $63,000 | 1.14 (1.07–1.23) | 0.0002 |
Discussion
The objective of this study was to determine if distance to treatment center affects outcomes of children and AYAs with acute leukemia. Our findings demonstrate that distances greater than 50 miles to treatment center are associated with inferior survival for patients with ALL but not AML. For patients with ALL, this association holds for both children less than 18 years old and young adults age 18–39. Distance to treatment center remained significant after adjusting for other socioeconomic factors such as community income and insurance status. Further, rural vs. urban zip codes were not statistically significantly different in this study, as has been seen in other work.7 Taken together, these data suggest that the physical distance to treatment center for ALL patients, as opposed to only the intrinsic community factors, influence overall survival. This study adds to the existing literature using a larger population showing multiple socioeconomic factors such as race, insurance status, and income are all associated with survival for children and young adults with acute leukemia.
The association between distance to treatment center of more than 50 miles with poorer overall survival in ALL for both children and young adults but not AML is intriguing. Data from the NCDB does not discern between treatment related and disease related mortality. However, it is plausible that the difference in general treatment approaches for ALL and AML may be responsible. Typical treatment for ALL is delivered mostly on an outpatient basis and extends for multiple years requiring patients to take many trips to the treatment center for therapy as well as for complications such as febrile neutropenia. Although some young adult regimens utilized during the study timeframe include greater amounts of inpatient chemotherapy administration (e.g. hyper-fractionated cyclophosphamide, vincristine, Adriamycin, and dexamethasone)26 they still include a maintenance period where adherence and travel to the treatment center is needed.27, 28 Conversely, AML treatment is typically given almost exclusively inpatient (although some centers deliver outpatient therapy) and lasts for months as opposed to years. The lack of an association with treatment center distance for AML is consistent with a smaller previous study of adults.29 Potential challenges relating to patients with ALL traveling to appointments leading to delays in therapy or difficulty getting to the treatment center for emergent complications might be responsible for the differences observed in this study, although data from the NCDB is not available to specifically test that hypothesis. Perhaps greater emphasis on partnering with regional centers for emergency management or pre-emergent planning/training with patients and families might benefit this population. Additionally, our results did not show that for each distance category there was a proportionally worse hazard ratio as patients became more distant from their treatment center, but rather a threshold effect at 50 miles. As the NCDB dataset only measures from the center of a patient’s zip code to the treatment center, we were unable to measure other aspects of travel to treatment center, such as traffic patterns or type of transportation utilized. Therefore, this distance may not fully account for the total transportation time for a given patient.
Interestingly, in some studies of adults with solid tumors, longer distance to treatment centers seems to predict better outcomes.20, 21, For instance, in adults with head and neck cancer, those in the highest quartile for travel distance were more likely to present with early stage disease and those with oral cavity cancer were more likely to receive appropriate surgical therapy.22 For men with prostate cancer, longer travel distances are associated with decreased overall mortality, except in those with Medicaid insurance.21 This general improvement seen with longer treatment distances is likely confounded by center type (i.e. patients traveling to a tertiary care facility) or socioeconomic status (i.e. those able to travel further may be of higher status).20, 21 Improved outcomes in lung transplant recipients who travel further for transplantation suggests this phenomena is not unique to cancer therapy.30 Our study did not show improved survival for patients with longer distance to treatment centers for either AML or ALL. One possible explanation for this is that most children and young adults with acute leukemia are treated at tertiary centers (whereas many other cancer types in adults may be treated in the community setting), perhaps negating the effect of center type.
To our knowledge this study is also the largest performed in children and young adults to demonstrate the impact of income, and insurance status on outcomes in acute leukemia. Our study found that lower community median income was associated with lower overall survival for both ALL and AML. These findings confirm prior studies in a more robust sample. A previous meta-analysis of children with acute leukemia found that, among those with ALL, lower individual level socioeconomic status is associated an almost twofold increased risk of death, and in AML, low area-wide socioeconomic status is associated with an approximately 25% higher risk of death.7 Additionally, our study showed patients with government or no insurance had inferior outcomes compared to private insurance for both ALL and AML. Previously, among young adults with AML, insurance status has been shown to impact survival,10 and in a NCDB study of adults with AML, non-private insurance has been associated with worse survival.12 Alternatively, studies of acute leukemia induction mortality in children using the Pediatric Health Information System (PHIS) database did not detect a difference based on insurance type (notably most patient undergoing induction therapy are treated inpatient or are followed very closely as an outpatient).11, 13
Our study also demonstrated inferior outcomes for African Americans vs. Caucasians in both AML and ALL, as well as inferior outcomes for Hispanic/ Latino and Native American’s with ALL. Previous studies in children with acute leukemia have shown variable results. A study from the Children’s Oncology group demonstrated that Hispanic and black children with AML have worse survival than white children,15 though a study from St. Jude Children’s Research Hospital did not detect a difference between white and black children with AML.18 A study using the PHIS database found that black and white patients with AML have comparable on-therapy mortality17 A study of children utilizing the National Cancer Institute’s Surveillance, Epidemiology, and End Results database from 1973–1999 showed that black, Hispanic, and Native American patient’s with ALL have decreased survival compared to white and Asian/Pacific Islander patients.16 The frequency of Native American’s living in more remote areas might play a role in their inferior overall survival. In a large NCDB study of adults with AML, black race has been associated with worse survival.12 Hematopoietic Cell Transplantation (HCT) may be required for cure of acute leukemia. Having Hispanic or black race/ethnicity has been associated with lower utilization of HCT in adults,31 and in adults with AML, post-remission therapy is more often delayed in blacks compared to whites.32 In our study of over 32,000 patients with acute leukemia, race clearly impacted overall survival for both children and young adults after adjusting for additional socioeconomic risk factors.
Our study has notable limitations. First, the NCDB is not able to discern between relapse and treatment related mortality as a cause of death. As this is a population level study, the differences seen between ALL and AML may generate hypothesis, but cannot definitively explain the reason why treatment distance impacts outcome. Additionally, distance to treatment center data provided by the NCDB is based on the distance from the center of the patient’s zip code and does not specifically address how far or how long the patient traveled to their treatment center, or if they obtained new lodging closer to the treatment center during therapy. In this dataset, only 1–2% of patients were designated as being from a rural zip code, which may have limited the power to detect a difference based on urban v. rural zip code. Our study found an association with Charlson-Deyo score and overall mortality, however previous studies have shown that different comorbidity scales may be more optimal to predict risk of inpatient mortality in children.24 Unfortunately, Charlson-Deyo score is the only available comorbidity score in the data-set. The NCDB censors treatment center type for patients younger than 40 years so we were unable to correct for potential confounding of this variable. Although the NCDB contains registry data from approximately 65% of newly diagnosed malignancies in the United States, approximately 8% of all patients in this study did not have outcome data, and it is unclear if this may bias our findings.19 Additionally, the NCDB is limited to centers with accreditation from the Commission on Cancer, and it is unknown if the results of our study remain valid for non-accredited facilities, or if patients living further from their treatment facility are more or less likely to received care at an accredited facility. AYAs with acute leukemia appear to have improved outcomes when treated at specialized centers.8 We also were not able to assess treatment center volume, but a previous study of children with ALL showed that induction mortality in ALL does not vary by center volume.33
Overall our study demonstrated that for patients with ALL, a distance to treatment center of >50 miles, is associated with inferior survival. This group represents more than 20% of children and young adults with ALL and an increase in attention to adherence, supportive care and logistics for patients living >50 miles from their treatment center is warranted. Further research to determine if differences in survival are driven by relapse or treatment related mortality can help inform which factors (e.g. compliance with medication and appointments, caregiver emotional stress, financial toxicity, etc.) should be targeted for intervention.
Funding Statement:
career development support was provided by NIH NCATS 2KL2TR002457 (PI Dweik; SJR)
Footnotes
COI Statement: The authors report no conflicts of interest
References
- 1.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. 2015;33: 2949–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33: 2938–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander TB, Wang L, Inaba H, et al. Decreased relapsed rate and treatment-related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials. Cancer. 2017;123: 3791–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68: 7–30. [DOI] [PubMed] [Google Scholar]
- 5.DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29: 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132: 362–368. [DOI] [PubMed] [Google Scholar]
- 7.Petridou ET, Sergentanis TN, Perlepe C, et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Ann Oncol. 2015;26: 589–597. [DOI] [PubMed] [Google Scholar]
- 8.Wolfson J, Sun CL, Wyatt L, Stock W, Bhatia S. Adolescents and young adults with acute lymphoblastic leukemia and acute myeloid leukemia: impact of care at specialized cancer centers on survival outcome. Cancer Epidemiol Biomarkers Prev. 2017;26: 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt VR, Shostrom V, Giri S, et al. Early mortality and overall survival of acute myeloid leukemia based on facility type. Am J Hematol. 2017;92: 764–771. [DOI] [PubMed] [Google Scholar]
- 10.Borate UM, Mineishi S, Costa LJ. Nonbiological factors affecting survival in younger patients with acute myeloid leukemia. Cancer. 2015;121: 3877–3884. [DOI] [PubMed] [Google Scholar]
- 11.Kavcic M, Fisher BT, Li Y, et al. Induction mortality and resource utilization in children treated for acute myeloid leukemia at free-standing pediatric hospitals in the United States. Cancer. 2013;119: 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Master S, Mansour R, Devarakonda SS, Shi Z, Mills G, Shi R. Predictors of survival in acute myeloid leukemia by treatment modality. Anticancer Res. 2016;36: 1719–1727. [PubMed] [Google Scholar]
- 13.Seif AE, Fisher BT, Li Y, et al. Patient and hospital factors associated with induction mortality in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61: 846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smits-Seemann RR, Kaul S, Hersh AO, et al. ReCAP: Gaps in insurance coverage for pediatric patients with acute lymphoblastic leukemia. J Oncol Pract. 2016;12: 175–176; e207–114. [DOI] [PubMed] [Google Scholar]
- 15.Aplenc R, Alonzo TA, Gerbing RB, et al. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2006;108: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290: 2008–2014. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Newton JG, Getz KD, et al. Comparable on-therapy mortality and supportive care requirements in Black and White patients following initial induction for pediatric acute myeloid leukemia. Pediatr Blood Cancer. 2019;66: e27583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubnitz JE, Lensing S, Razzouk BI, Pounds S, Pui CH, Ribeiro RC. Effect of race on outcome of white and black children with acute myeloid leukemia: the St. Jude experience. Pediatr Blood Cancer. 2007;48: 10–15. [DOI] [PubMed] [Google Scholar]
- 19.Boffa DJ, Rosen JE, Mallin K, et al. Using the national cancer database for outcomes research: a review. JAMA Oncol. 2017;3: 1722–1728. [DOI] [PubMed] [Google Scholar]
- 20.Ringstrom MJ, Christian J, Bush ML, Levy JE, Huang B, Gal TJ. Travel distance: Impact on stage of presentation and treatment choices in head and neck cancer. Am J Otolaryngol. 2018;39: 575–581. [DOI] [PubMed] [Google Scholar]
- 21.Vetterlein MW, Loppenberg B, Karabon P, et al. Impact of travel distance to the treatment facility on overall mortality in US patients with prostate cancer. Cancer. 2017;123: 3241–3252. [DOI] [PubMed] [Google Scholar]
- 22.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutacker N, Bloor K, Cookson R. Comparing the performance of the Charlson/Deyo and Elixhauser comorbidity measures across five European countries and three conditions. Eur J Public Health. 2015;25 Suppl 1: 15–20. [DOI] [PubMed] [Google Scholar]
- 24.Hessels AJ, Liu J, Cohen B, Shang J, Larson EL. Severity of illness measures for pediatric inpatients. J Healthc Qual. 2018;40: e77–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medford-Davis LN, Fonarow GC, Bhatt DL, et al. Impact of insurance status on outcomes and use of rehabilitation services in acute ischemic stroke: findings from get with the guidelines-stroke. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rytting ME, Jabbour EJ, Jorgensen JL, et al. Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Munster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol. 2016;91: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGrady ME, Pai ALH. A systematic review of rates, outcomes, and predictors of medication non-adherence among adolescents and young adults with cancer. J Adolesc Young Adult Oncol. 2019;8: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124: 2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez CP, Baz R, Jawde RA, et al. Impact of socioeconomic status and distance from treatment center on survival in patients receiving remission induction therapy for newly diagnosed acute myeloid leukemia. Leuk Res. 2008;32: 413–420. [DOI] [PubMed] [Google Scholar]
- 30.Tsuang WM, Lin S, Valapour M, Udeh BL, Budev M, Schold JD. The association between lung recipient travel distance and posttransplant survival. Progress in Transplantation. 2018;28: 231–235. [DOI] [PubMed] [Google Scholar]
- 31.Jabo B, Morgan JW, Martinez ME, Ghamsary M, Wieduwilt MJ. Sociodemographic disparities in chemotherapy and hematopoietic cell transplantation utilization among adult acute lymphoblastic and acute myeloid leukemia patients. PLoS One. 2017;12: e0174760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brady AK, Fu AZ, Earl M, et al. Race and intensity of post-remission therapy in acute myeloid leukemia. Leuk Res. 2011;35: 346–350. [DOI] [PubMed] [Google Scholar]
- 33.Wilkes JJ, Hennessy S, Xiao R, et al. Volume-outcome relationships in pediatric acute lymphoblastic leukemia: association between hospital pediatric and pediatric oncology volume with mortality and intensive care resources during initial therapy. Clin Lymphoma Myeloma Leuk. 2016;16: 404–410 e401. [DOI] [PMC free article] [PubMed] [Google Scholar]


