Abstract
BACKGROUND
We report outcomes for patients with T2N0M0 glottic squamous cell carcinoma (SCC) treated with radiation therapy (RT).
METHODS
Patients who received definitive RT for T2 glottic SCC from 2000 through 2013 were retrospectively reviewed.
RESULTS
113 patients were analyzed (median follow-up time 91 months; 85 patients received 3D-CRT and 28 received IMRT). Fractionation was conventional (58%) or altered (42%); 20 patients (18%) received concurrent chemotherapy. 5-year LC was 83% for the 3D-CRT vs. 81% for the IMRT group (P=0.76). The ultimate locoregional control at 5 years was 100% for IMRT vs. 91% for 3D-CRT (P=0.1). The 5-year OS was 78% for 3D-CRT vs. 81% for IMRT (P=0.83). On multivariate analysis, younger age was the only independent predictor of improved OS (P=0.0002).
CONCLUSIONS
Oncologic and survival outcomes were excellent for patients with T2N0 glottic cancer. Patients treated with IMRT and 3D-CRT had no statistically significant differences in all investigated endpoints.
Keywords: T2, larynx, outcomes, radiotherapy, survival
INTRODUCTION
Radiation therapy (RT) is the treatment of choice for early-stage glottic cancer at many institutions 1–7. Alternatives to external beam RT in the treatment of early-stage glottic cancer include transoral laser excision and open partial laryngectomy, with similar oncological outcomes to external beam RT, although there may be differences in cost and voice outcomes 8–10. T2 glottic cancer is relatively uncommon and should be reported as a separate disease entity as it has different prognosis and treatment outcomes compared with T1 disease. However, the majorty of early-stage glottic cancer studies report the RT outcomes of both T1 and T2 disease combined together without stratification 7,11,12. It is also important to study the outcomes of T2 glottic cancer in the current treatment era, with the advent of more complex radiation therapy techniques.
The three main controversies in treatment of T2 glottic cancer are as follows: First, intensity-modulated radiation therapy (IMRT) is not currently considered the standard of care for early-stage disease, despite its proven dosimetic benefits in normal tissue sparing. In particular for glottic cancer, the use of IMRT has been driven mainly by a desire to reduce the dose to the carotid arteries, which is hoped to reduce the risk of subsequent stroke 13–16. At our institution, IMRT is used to treat T1 glottic cancer for carotid artery dose sparing and has led to excellent oncologic outcomes 17. Although IMRT may be beneficial for T1 disease, it may not be ideal for all patients with T2 disease, especially those with bulky disease, with the corresponding increases in uncertainties in target delineation and risk of missing subclinical disease at the primary site or adjacent lymph nodes 18.
The second controversial topic in stage II glottic cancer are the uses of chemotherapy or altered RT fractionation, especially for patients with impaired vocal cord mobility. Limited series have shown that the addition of concurrent chemotherapy could be beneficial for stage II glottic cancer 19–21, whereas other studies have shown no real benefit 22,23. Similarly, altered RT fractionation may be of benefit, but the the only prospective randomized trial conducted to date showed only a trend to an improvement in local control 24.
The third controversy is whether elective nodal irradiation is required. The risk of nodal disease in T2 glottic cancer is small but higher than that in T1 disease, which has direct bearing on the safety of using IMRT and limited volumes to achieve carotid artery radiation dose sparing for T2 cancers.
The aim of this study was to report oncologic and functional outcomes for patients with stage II (i.e., T2N0M0) squamous cell carcinoma (SCC) of the glottis treated with RT. We investigated the potential correlations of patient- and treatment-related factors with oncologic and survival endpoints. We also focused on outcomes comparison for IMRT versus conventional RT techniques, and for treatment intensification with chemotherapy or altered fractionation versus standard treatment.
PATIENTS AND METHODS
This single-institution retrospective chart review was conducted after approval by the appropriate institutional review board. Patients were identified through an instituitional registry as having T2N0M0 SCC of the glottic larynx and treated with definitive RT from 2000 through 2013. Disease stage was reviewed according to the 8th (2018) edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual. Information on demographics (age at diagnosis, sex, ethnicity), smoking history, Eastern Cooperative Oncology Group (ECOG) performance status (PS) score, clinical factors (tumor grade, disease stage, and pathologic characteristics), imaging findings on contrast-enhanced computed tomography (CT) scans, and treatment modalities (chemotherapy and its sequence with RT, RT total dose, fractionation, and delivery technique) was extracted from the electronic medical records. Biologically effective dose (BED) was calculated by using the simple BED equation 25, and adjusted for overall treatment time with the following formula:
Overall treatment time-adjusted BED = BED - (Ln(2)/0.3) × ((Overall treatment time - 22)/3)
Outcomes were categorized as local recurrence (at the site of primary tumor), locoregional recurrence (at the site of primary tumor and regional lymph nodes), or distant metastasis. Because of the difficulty in distinguishing distant metastasis from a second primary tumor in this retrospective review, all such cases were considered distant metastasis. Death was classified as cancer-related or non-cancer-related depending on the presence of active cancer at the time of death. Functional outcomes including the need for a feeding tube, dysphagia, and hoarseness at last follow-up visit were also recorded. All cerebrovascular events were also recorded. These findings were coded and entered into a database for analysis.
Statistical Analyses
Descriptive statistics were used to analyze distribution of the sample by sex, ethnicity, age, clinical data, treatment modality, fractionation schemes, and functional outcomes. The Kaplan-Meier product limit method was used to calculate survival endpoints, and log-rank tests were used to compare the survival distributions of two samples. All survival endpoints were calculated from the date of diagnosis until the date of the event. Local control (LC) was defined as time without local recurrence, with any local recurrence coded as an event (and all others censored); locoregional control (LRC) as time without locoregional disease, with any local recurrence or neck recurrence coded as an event (and all others censored); ultimate locoregional control (uLRC) as time without a second locoregional disease after salvage surgery; freedom from distant metastasis (FDM) as time without disease outside the therapy fields, with disease outside the treated fields coded as an event (and all others censored); disease-specific survival (DSS) as death from disease as an event (and all others censored); and overall survival (OS) as death from any cause as an event (and all others censored). Both univariate and multivariate Cox proportional hazards analyses were used to investigate potential correlations between patient- and treatment-related factors (age at diagnosis, sex, smoking status, ethnicity, pathologic grade, vocal cord mobility, RT technique, time-adjusted BED, and chemotherapy use) and outcomes (disease control and survival endpoints). Univariate Cox proportional hazards analysis was also used to stratify LC, LRC, uLRC, and OS by RT modality and assess the impact of IMRT as compared with conventional three-dimensional conformal radiotherapy (3D-CRT) or opposed laterals, as well as treatment intensification versus standard treatment. JMP 14 Pro statistical software (SAS Institute, Cary, NC) was used for data analysis, and statistical significance was determined by using a prespecified α of 0.05.
RESULTS
Patients
The study group consisted of 113 consecutive patients (85% men and 15% women). Mean age at the time of histologic diagnosis was 63 years (range 18–88). Most patients (90%) were fully active and able to carry on all pre-disease activities (ECOG PS score 0). Forty-seven patients (42%) had impaired VC mobility. Patient and disease characteristics are listed in Table 1.
Table 1.
Patient and disease characteristics
| Characteristic | No. of Patients (%) |
|---|---|
| Sex | |
| Male | 96 (85) |
| Female | 17 (15) |
| Ethnicity | |
| White | 79 (70) |
| Black/ African American | 11 (10) |
| Hispanic/Latino | 22 (19) |
| Other/Unspecified | 1 (1) |
| Smoking history at time of diagnosis | |
| None | 16 (14) |
| Positive | 96 (85) |
| Unknown or unspecified | 1 (1) |
| Vocal cord mobility at presentation | |
| Impaired | 47 (42) |
| Not impaired | 66 (58) |
| Eastern Cooperative Oncology Group (ECOG) performance status | |
| 0 | 102 (90) |
| 1 | 8 (7) |
| 2 | 2 (2) |
| Unknown or unspecified | 1 (1) |
| Pathologic grade | |
| Well differentiated | 19 (17) |
| Moderately differentiated | 56 (50) |
| Poorly differentiated | 4 (4) |
| Unknown or unspecified | 34 (29) |
Treatment
All patients were treated with curative intent; 69 (62%) were treated with 4–6 MV x-rays, and 44 (39%) were treated with Cobalt-60. Most patients (85 [75%]) received 3D-CRT with lateral opposed/oblique fields, with both fields treated at each session; the other 28 patients (25%) received IMRT for carotid sparing. Treatment characteristics are summarized in Table 2.
Table 2.
Treatment characteristics
| Characteristic | No. of Patients (%) |
|---|---|
| Radiotherapy technique | |
| 3D-CRT | 85 (75) |
| IMRT | 28 (25) |
| Radiation beam energy | |
| 6 MV | 69 (61) |
| 60Co | 44 (39) |
| Mean total radiation dose, Gy ± SD | 71.1 ± 4.4 |
| Mean no. of fractions received ± SD | 42 ± 13 |
| Fractionation schedule | |
| Conventional | 66 (58) |
| Altered | |
| Twice daily (hyperfractionation) | 30 (27) |
| Six weekly fractions (moderate acceleration) | 2 (2) |
| Concomitant boost | 15 (13) |
| Mean overall treatment time, days, ± SD | 43.3 ± 5.5 |
| Overall treatment time-adjusted BED, Gy, ± SD | 67.1 ± 5.3 |
| Chemotherapy | |
| Concurrent | 20 (18) |
| None | 93 (82) |
Abbreviations: 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; MV, megavolts; 60Co, cobalt-60; SD, standard deviation; BED, biologically effective dose
The 3D-CRT treatments were planned with two- or three-field techniques. No elective nodal irradiation was used, though with parallel opposed fields, the majority of level III was in-field. The IMRT treatment included the entire glottis, with no elective nodal irradiation. Various fractionation patterns and different chemotherapeutic radiosensitizers were used during the study period; 66 patients (58%) received standard fractionation, and 47 received altered fractionation, which included twice-daily treatment (29 patients), concomitant boost (15 patients), and 6 weekly fractions (2 patients 26). The total RT dose ranged from 58 Gy to 79.2 Gy (median 70 Gy, mean total dose 70.8 ± 5.1 Gy). Most patients (93 [82%]) received RT without chemotherapy, and 20 (18%) received concurrent chemoradiation. The most commonly used chemotherapy regimen was concurrent cisplatin. 48 patients (42%) received some form of treatment intensification, 20 with chemotherapy and 32 with overall treatment time-adjusted BED ≥70 Gy (4 patients received both). Of the 47 patients with impaired vocal cord mobility, 23 (49%) received treatment intensification, 12 with chemotherapy and 14 with overall treatment time-adjusted BED ≥70 Gy (3 patients received both). Salvage surgery (total laryngectomy) was used for 26 patients who had residual tumor or developed recurrence.
Oncologic Endpoints
Median follow-up time was 91.2 months (interquartile range [IQR] 50.1−134.4) for all patients and 102.5 months (IQR 66.3–142.5) for patients alive at the time of analysis. By treatment modality, median follow-up time for patients treated with 3D-CRT was 130.5 months (IQR 78.0−159.0 months) and that for patients treated with IMRT was 66.3 months (IQR 53.3−103.2).
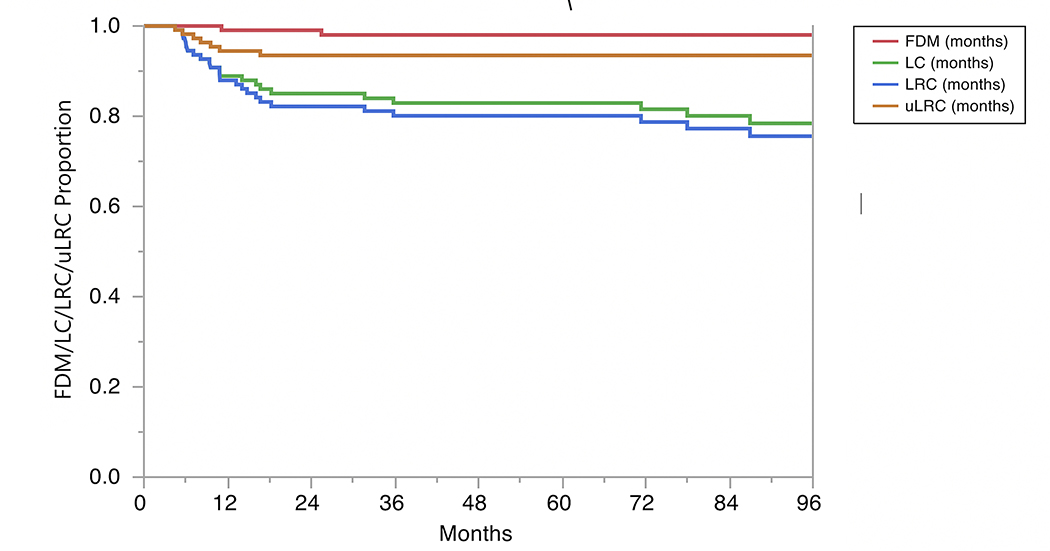
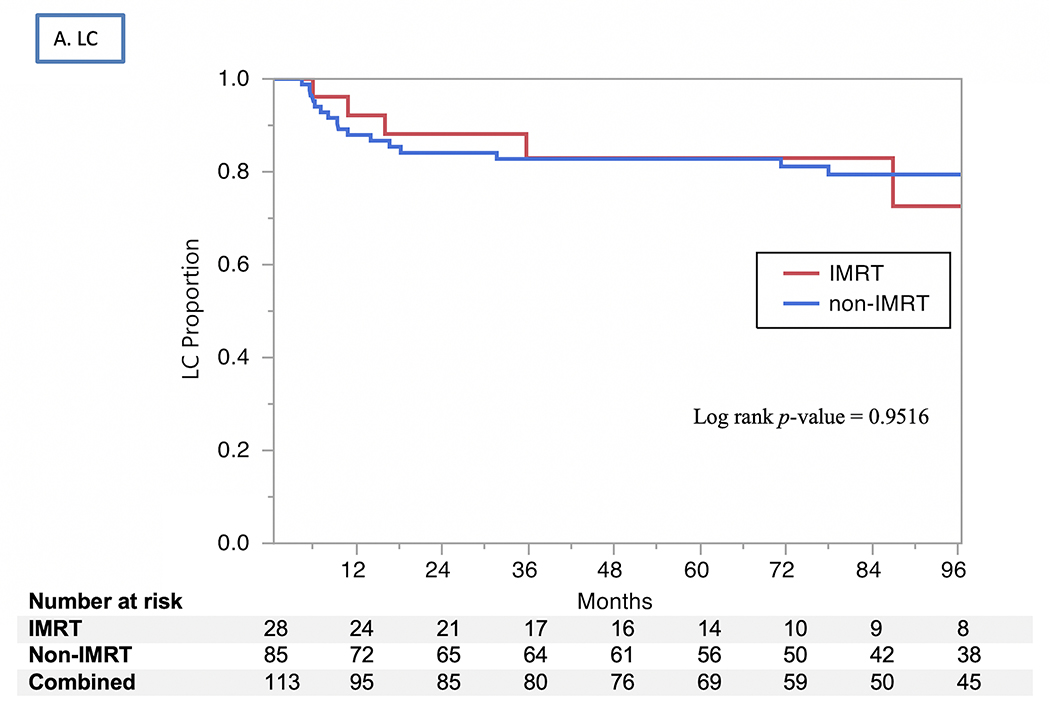
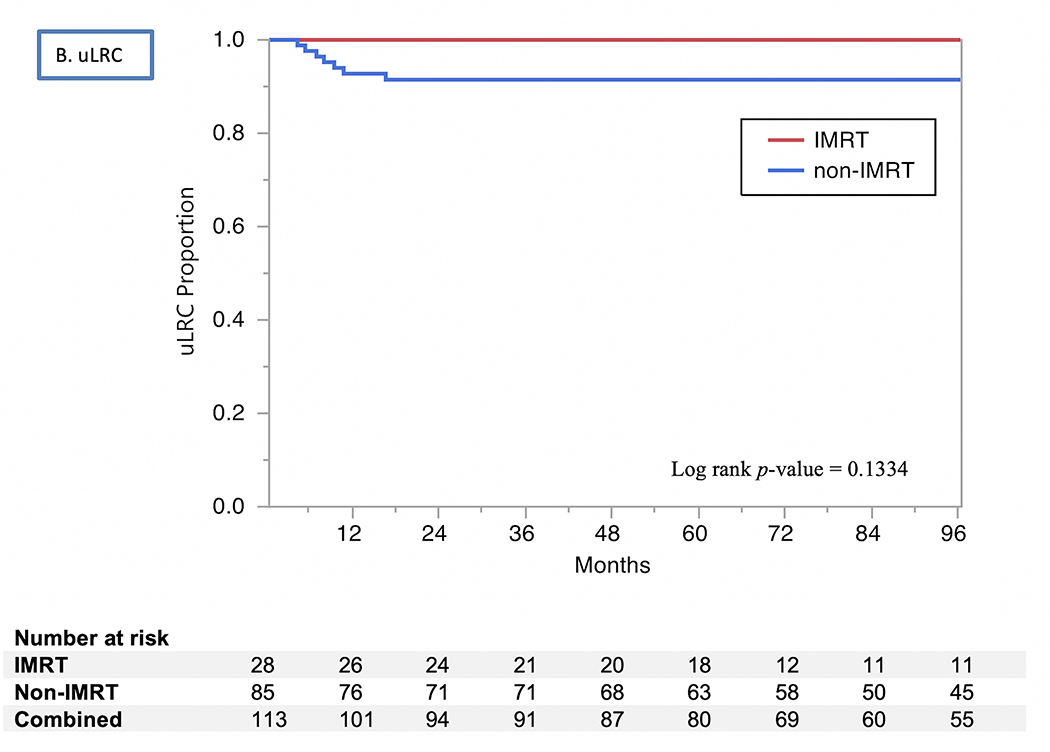
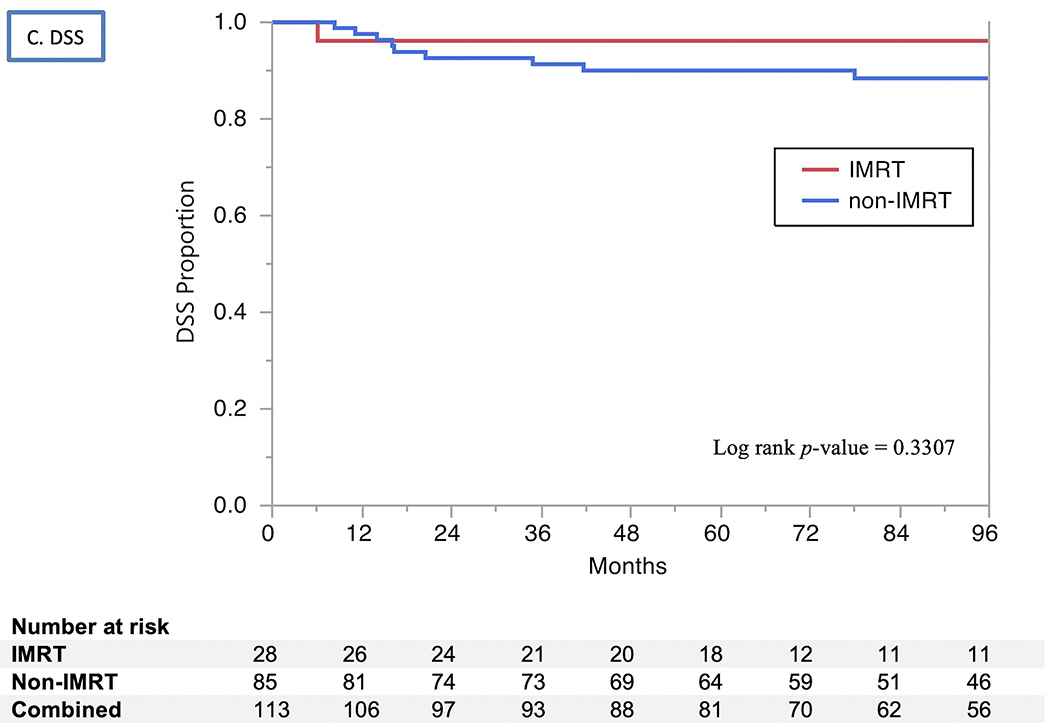
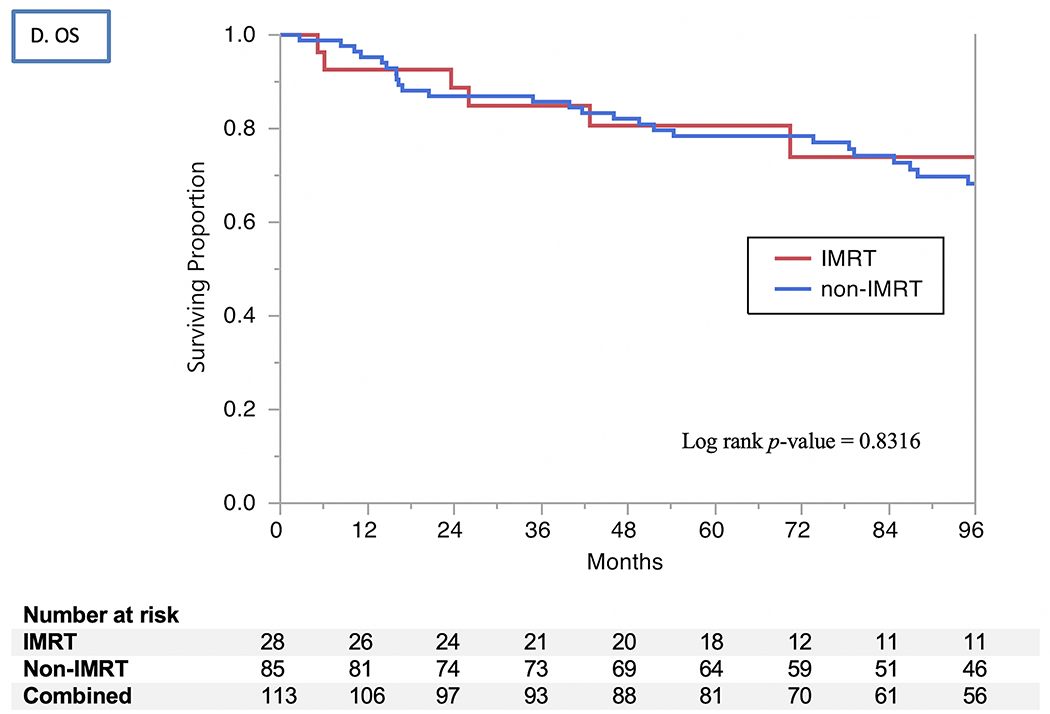
The 2- and 5-year actuarial LC rates 85% and 83%, respectively (Fig. 1). Isolated regional recurrences were encountered only in three patients (two from the 3D-CRT arm and one from the IMRT arm). LRC rates were 82% at 2 years and 80% at 5 years, with no differences by RT modality used (80% vs. 79% at 5 years for 3D-CRT vs. IMRT, respectively, P=0.9) or treatment intensification group (73% vs. 86% at 5 years for treatment intensification vs. none, respectively, P=0.07). The uLRC the rates were 93% at both 2 and 5 years, with all 7 failures after salvage treatment occurring in patients treated with non-IMRT techniques while all patients in the IMRT arm were salvaged successfully with total laryngectomy (91% vs. 100%, P=0.1). The FDM rates were 99% at 2 years and 98% at 5 years (Fig. 1), with no patients having isolated distant failure. The DSS rates for all patients were 93% at 2 years and 91% at 5 years (Fig. 2) and did not differ by vocal cord mobility (normal 94% at 2 and 5 years vs. impaired 93% at 2 years and 88% at 5 years; log rank p =0.33), nor by the use of treatment intensification (87% vs. 73% at 5 years for treatment instensification vs. none, respectively, P=0.5). The OS rates for all patients were 87% at 2 years and 79% at 5 years (Fig. 2) and did not differ by vocal cord mobility (normal 89% at 2 years and 80% at 5 years vs. impaired 85% and 78%; log rank P=0.99) nor by RT modality (3D-CRT 87% at 2 years and 78% at 5 years vs. IMRT group 89% and 81%; log rank P=0.83) nor by the use of treatment intensification. (80% vs. 79% at 5 years for treatment instensification vs. none, respectively, P=0.6).
Fig. 1.
Kaplan Meier curves for freedom from distant metastasis (FDM), local control (LC), locoregional control (LRC), and ultimate locoregional control (uLRC).
Fig. 2.
Kaplan Meier curves showing (A) local control (LC), (B) utimate locoregional control (uLRC), (C) disease-specific survival (DSS), and (D) overall survival (OS) by RT technique (IMRT vs. non-IMRT) through 96 months.
Correlates with Survival and Other Endpoints
None of the examined clinical and treatment variables were associated with better LRC. Younger age (P=0.0068) was the sole factor associated with better DSS on univariate analysis, and remained significant on multivariate analysis (P=0.0049). Younger age (P<0.0001) and being a non-smoker (HR=0.34, P=0.0334) were associated with improved OS in univariate analysis, but ethnicity, pathologic grade, overall treatment time-adjusted BED, use of chemotherapy and RT modality were not. On multivariate analysis, younger age at diagnosis remained the only independent predictor of improved OS (P=0.0002).
Adverse Events and Functional Outcomes
Eighty-two patients (75%) developed laryngeal mucositis, which in most cases was a patchy pseudomembranous reaction. Grade 1 radiation dermatitis developed in 46 patients (41%), grade 2 in 48 patients (42%), and grade 3 radiation dermatitis in only 4 patients (4%). As for functional outcomes, 48 patients (43%) reported no subjective hoarseness at last follow-up, 83 patients (73%) reported no aspiration, and most patients (98 patients [88%]) did not require a feeding tube (6 patients needed a tube at 6 months, 5 patients at 12 months, and 11 at last follow-up). In patients who received treatment intensification, there was significantly more reported hoarseness (58% versus 33%, P=0.02). There were no significant differences in any of these adverse events and functional outcomes based on RT modality. Two patients had a carotid event at 7 and 8 years respectively after definitive RT (one was treated with 2D/3D-CRT, and one was treated with IMRT but had re-irradiation 3 years after his/her first course of RT).
DISCUSSION
In this series, definitive RT led to excellent oncologic outcomes for patients with T2 glottic cancer, with 5-year rates of LC (83%) and uLRC (93%) similar to those in the literature (Table 3). Notably, both LC and LRC rates for patients treated with IMRT were as good as, and certainly not inferior to, those for patients treated with 3D-CRT, and have the additional advantage of sparing the carotid artery. In this series, the lack of any elective nodal irradiation in the IMRT cohort did not increase rates of regional relapse, and patients with impairment in vocal cord mobility fared as well as those with intact mobility.
Table 3.
Summary of studies reporting outcomes of patients with T2 laryngeal cancer
| Author, Year | Follow-up Time | Total No. of Pts. | All stages Included | No. of patients with T2 larynx cancer | Treatment modality | LC rates, % | LC rates with laryngeal preservation, % | uLC rates, % | OS rates, % | Cause-specific survival rates, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Fletcher et al., 1980 27 | NR | 507 | T2 | 175 | RT | 74 | ||||
| Harwood et al., 1981 28 | NR | 244 | T2 | 244 | RT | 69 (5-y) | ||||
| Wang, 1997 29 | NR | 902 | T1-T2 | T2a 145 T2b 92 |
RT | 77 (5-y) 71 (5-y) |
92 (5-y) 84 (5-y) |
|||
| Le et al., 1997 30 | Median 9.7 y | 398 | T1-T2 | 83 | RT | 70 (5-y) | uLRC 91 (5-y) | 63 (10-y) | 91 (10-y) | |
| Warde et al 1998 31 | Median 6.8 y | 735 | T1-T2 | 286 | RT | 69 (5-y) | ||||
| Garden et al., 2003 32 | Median 6.8 y | 230 | T2 | 230 | RT | 72 (5-y) | 73 (5-y) | 92 (5-y) | ||
| Short et al., 2006 33 | Median 4.9 y | 145 | T1-T2 | 43 | RT | 80 (5-y LRC) | ||||
| Taguchi et al., 2006 34 | Median 32 mo | 20 | T2 | 20 | CCRT | 95 (3-y) | 100 (3-y) | 100 (3-y) | ||
| Hafidh et al., 2009 35 | Mean 37 mo | 373 | T1-T4 | 38 | RT | 63.3 (5-y) | ||||
| Chera et al., 2010 7 | Median 12 y | 585 | T1-T2 | T2a 165** T2b 95 |
RT | T2a: 80 (5-y) T2b: 70 (5-y) |
T2a 81 (5-y) T2b 74 (5-y) |
T2a 76 (5-y) T2b 78 (5-y) |
T2a 94 (5-y) T2b 90 (5-y) |
|
| Kim et al., 2012 12 | Median 7.1 y | 157 | T1-T2 | 32 | RT | 62 (5-y) | ||||
| Furusaka et al., 2012 36 | Median 9.4 y | 57 | T2 | 57 | RT | 60.4 (5-yr) 50.1 (10-yr) |
88.5 (5-y) 73.5 (10-y) |
|||
| Ermis et al., 2015 37 | Median 72 mo | 132 | T1-T2 | 64 | RT | 80.9 (5-y) | 95.8 (5-y) |
Abbreviations: NR, not reported; y, year(s); mo, month(s); RT, radiation therapy; CCRT, concurrent chemoradiation; LC, local control; LRC: loco-regional control; uLC, ultimate local control; uLRC, ultimate loco-regional control
T2a and T2b classifications were based on the 2nd edition of the American Joint Committee on Cancer (AJCC) staging system. T2 is not subdivided into T2a and T2b in the 8th edition of the AJCC staging system.
Several reports emphasize the dosimetric advantage of IMRT for carotid artery sparing, as carotid irradiation is well known to increase the relative risk of stroke and may limit the use of re-irradiation if needed 13,15,38. For example, a Surveillance, Epidemiology, and End Results (SEER)-Medicare study showed that the 10-year risk of cerebrovascular events for patients with head and neck cancer was 34% after conventionally delivered definitive RT versus 26% after surgery alone (P<0.01) 39. A recent series from our group on T1 glottic cancer showed no cerebrovascular events among patients treated with carotid-sparing IMRT versus 3% among patients treated with conventional RT 17. In the current T2 series, only 2 patients developed a carotid event several years after RT.
The impact of IMRT on outcomes in T2 larynx remains controversial (9,16). Similar to our findings, a recent single-institution retrospective study of 139 patients who received moderately accelerated IMRT with daily laryngeal soft tissue matching reported a high 3-year LC rate of 89% 40. On the other hand, a population-based analysis of 1,929 patients with early stage T1-T2N0 laryngeal cancer from the National Cancer Data Base revealed a statistically significant decline in OS from the use of IMRT relative to 3D-CRT, which was attributed to possible marginal miss or less dose to subclinically involved neck lymph nodes 41. In the current series, no association was found between radiation therapy modality (3D-CRT/opposed laterals versus IMRT) and outcomes, a finding we attribute to the stringent IMRT quality assurance process used for patients with head and neck cancer 42 as well as our routine use of image-guided RT.
Patients given IMRT in this study were treated only to the primary site: the carotid arteries were spared, and so were the adjacent level III lymph nodes. In contrast, patients treated with opposed lateral fields receive level III nodal irradiation. Our isolated regional nodal recurrence rate, 2.7%, was no different than in conventionally treated patients and was comparable to published regional failure rates of about 5% 43. The benefit of elective nodal irradiation in T2 glottic cancer is minimal at best, and its likely overshadowed by long-term carotid artery and other morbidity.
Patients with impaired vocal cord mobility are generally thought to do worse than those with intact vocal cord mobility 7,11,24,29,44–46. A meta-analysis of 21 retrospective studies of patients with T2 glottic cancer showed impaired vocal cord mobility to be associated with a statistically significant decrease in LC 45. In the prospective randomized RTOG 9512 study, LC, LRC, DFS, and OS were significantly worse among patients with impaired vocal cord mobility 24. Contrarily, impaired vocal cord mobility was not associated with worse outcomes in our study. We attribute this finding to selection bias, as approximately half of such patients received intensified treatment, with either altered RT dose fractionation or concurrent chemotherapy. Another potential explanation is inadequacies in the current American Joint Committee on Cancer staging system, as patients with T2 glottic cancer and impaired vocal cord mobility seem to have outcomes similar to patients with stage T3 cancer. The 5-year LC rate for patients with stage III and IV glottic cancer treated with concurrent chemoradiation in RTOG 9111 was 71.1% 47, comparable to the 5-year LC rate for patients with T2 glottic cancer with impaired vocal cord mobility in RTOG 9512, which was 70% 24. The older (2nd edition) staging system was useful for making this distinction, classifying tumors with intact vocal cord mobility as T2a, and tumors with impaired vocal cord mobility as T2b.
Treatment intensification for T2 glottic cancer via use of concurrent chemotherapy or altered RT fractionation did not improve outcomes in this study, but the introduction of chemotherapy was relatively recent and was given to only 18% of patients. The addition of chemotherapy for T2 glottic cancer has been shown to be beneficial in several retrospective studies 19,20. In contrast, a recent SEER cohort analysis reported increased disease-specific mortality in patients with T1–2N0M0 glottic cancer treated with chemoradiation relative to patients treated with RT alone, presumably from the acute and late toxicity of concurrent chemotherapy 23. In view of this limited evidence on the concurrent addition of chemotherapy to RT for patients with T2 laryngeal cancer, further studies should focus on selecting appropriate patients for concurrent therapy versus altered fractionation according to criteria such as tumor volume and impaired vocal cord mobility.
Altered RT fractionation also did not affect outcomes in this series. This is in contrast to results from other series, for which hypofractionation and hyperfractionation were found to be superior (or at least trending to superiority) to standard fractionation 32,48,49. Moreover, in the prospective RTOG 9512 trial, while hyperfractionated RT was associated with modestly but not significantly better LC rates relative to standard fractionation, it did have higher rates of acute toxicity 24.
We believe that selection is the reason that treatment intensification with either chemotherapy or altered fractionation did not improve outcomes in this study. Patients were triaged to these modalities when once-daily RT alone was thought to be insufficient based on tumor volume, unfavorable growth pattern, or impaired vocal cord mobility; in other words, had these patients not received intensified treatment, they might have fared worse than those who received once-daily RT alone. We currently tend to favor concurrent chemotherapy for patients with impaired vocal cord mobility, and altered fractionation for bulky but unimpaired vocal cord mobility.
We acknowledge that this study had inherent limitations related to its retrospective nature and the small numbers of patients with (a) impaired vocal cord mobility, (b) IMRT, and (c) chemotherapy. Also, the follow-up duration for patients treated with non-IMRT was significiantly longer than that for patients treated with IMRT. Longer follow-up is likely to be required to detect potential cerebrovascular events, but carotid events might have been underreported if they occurred in an outside hospital, or in more remotely treated patients potentially lost to follow-up. So far, no events have been reported in our IMRT cohort. Another limitation of our study is the lack of formal voice quality assessment, which should be included in future prospective trials. Future studies should also incorporate human papillomavirus (HPV) testing, HPV has been implicated in laryngeal cancer oncogenesis, although its influence on prognosis remains unclear 50–52. Despite these limitations, this study represents one of the largest series reporting outcomes for patients with T2 glottic cancer in the modern era.
CONCLUSION
Excellent 5-year oncologic and functional outcomes were achieved in this series of 113 patients with T2 glottic cancer. The use of altered fractionation, concurrent chemotherapy, or IMRT (vs. 3D-CRT) was not associated with differences in outcomes. Additional experience with larger groups of patients and longer follow-up will be necessary to determine outcomes associated with carotid artery irradiation to better explore cerebrovascular events, and studies with formal assessment of voice quality and swallowing are also needed to understand functional outcomes in patients with T2 glottic cancer. IMRT without elective nodal irradiation is now our standard approach, with treatment intensification for patients with bulky cancers and/or those with impaired vocal cord mobility.
Funding
Dr. Fuller received funding and salary support related to this project from the National Institutes of Health (NIH), including: the National Institute for Dental and Craniofacial Research (NIDCR) Academic Industrial Partnership Grant (R01DE028290); National Cancer Institute (NCI) Early Phase Clinical Trials in Imaging and Image-Guided Interventions Program (1R01CA218148); an NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672) and an NIH/NCI Head and Neck Specialized Programs of Research Excellence (SPORE) Developmental Research Program Award (P50CA097007).
Dr. Fuller received funding and salary support unrelated to this project from: the NIDCR Establishing Outcome Measures Award (1R01DE025248/R56DE025248) and National Science Foundation (NSF), Division of Mathematical Sciences, Joint NIH/NSF Initiative on Quantitative Approaches to Biomedical Big Data (QuBBD) Grant (NSF 1557679); NSF Division of Civil, Mechanical, and Manufacturing Innovation (CMMI) standard grant (NSF 1933369) a National Institute of Biomedical Imaging and Bioengineering (NIBIB) Research Education Programs for Residents and Clinical Fellows Grant (R25EB025787–01); the NIH Big Data to Knowledge (BD2K) Program of the NCI Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Award (1R01CA214825), and the MD Anderson Sister Institution Network Fund.
Direct infrastructure support was provided by the multidisciplinary Stiefel Oropharyngeal Research Fund of the University of Texas MD Anderson Cancer Center Charles and Daneen Stiefel Center for Head and Neck Cancer and the Cancer Center Support Grant (P30CA016672) and the MD Anderson Program in Image-guided Cancer Therapy.
Dr. Fuller has received direct industry grant support, honoraria, and travel funding from Elekta AB. None of these fundings constitute conflicts of interest with this project.
Footnotes
COI Disclosure: Authors declare no COI.
REFERENCES
- 1.Harwood AR, Hawkins NV, Rider WD, Bryce DP. Radiotherapy of early glottic cancer--I. Int J Radiat Oncol Biol Phys. 1979;5(4):473–476. [DOI] [PubMed] [Google Scholar]
- 2.Harwood AR, Tierie A. Radiotherapy of early glottic cancer--II. Int J Radiat Oncol Biol Phys. 1979;5(4):477–482. [DOI] [PubMed] [Google Scholar]
- 3.Horiot JC, Fletcher GH, Ballantyne AJ, Lindberg RD. Analysis of failures in early vocal-cord cancer. Radiology. 1972;103(3):663–665. doi: 10.1148/103.3.663 [DOI] [PubMed] [Google Scholar]
- 4.Johansen LV, Overgaard J, Hjelm-Hansen M, Gadeberg CC. Primary radiotherapy of T1 squamous cell carcinoma of the larynx: analysis of 478 patients treated from 1963 to 1985. Int J Radiat Oncol Biol Phys. 1990;18(6):1307–1313. [DOI] [PubMed] [Google Scholar]
- 5.Lindelov B, Lauritzen AF, Hansen HS. Stage I glottic carcinoma: an analysis of tumour recurrence after primary radiotherapy. Clin Oncol (R Coll Radiol). 1990;2(2):94–96. [DOI] [PubMed] [Google Scholar]
- 6.Lusinchi A, Dube P, Wibault P, Kunkler I, Luboinski B, Eschwege F. Radiation therapy in the treatment of early glottic carcinoma: the experience of Villejuif. Radiother Oncol. 1989;15(4):313–319. [DOI] [PubMed] [Google Scholar]
- 7.Chera BS, Amdur RJ, Morris CG, Kirwan JM, Mendenhall WM. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(2):461–466. doi: 10.1016/j.ijrobp.2009.08.066 [DOI] [PubMed] [Google Scholar]
- 8.Mendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1-T2 glottic carcinomas. Cancer. 2004;100(9):1786–1792. doi: 10.1002/cncr.20181 [DOI] [PubMed] [Google Scholar]
- 9.Agrawal N, Ha PK. Management of early-stage laryngeal cancer. Otolaryngol Clin North Am. 2008;41(4):757–769, vi–vii. doi: 10.1016/j.otc.2008.01.014 [DOI] [PubMed] [Google Scholar]
- 10.Aaltonen L-M, Rautiainen N, Sellman J, et al. Voice quality after treatment of early vocal cord cancer: a randomized trial comparing laser surgery with radiation therapy. Int J Radiat Oncol Biol Phys. 2014;90(2):255–260. doi: 10.1016/j.ijrobp.2014.06.032 [DOI] [PubMed] [Google Scholar]
- 11.Mendenhall WM, Amdur RJ, Morris CG, Hinerman RW. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol. 2001;19(20):4029–4036. doi: 10.1200/JCO.2001.19.20.4029 [DOI] [PubMed] [Google Scholar]
- 12.Kim TG, Ahn YC, Nam HR, et al. Definitive radiation therapy for early glottic cancer: Experience of two fractionation schedules. Clin Exp Otorhinolaryngol. 2012;5(2):94–100. doi: 10.3342/ceo.2012.5.2.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenthal DI, Fuller CD, Barker JLJ, et al. Simple carotid-sparing intensity-modulated radiotherapy technique and preliminary experience for T1–2 glottic cancer. Int J Radiat Oncol Biol Phys. 2010;77(2):455–461. doi: 10.1016/j.ijrobp.2009.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chera BS, Amdur RJ, Morris CG, Mendenhall WM. Carotid-sparing intensity-modulated radiotherapy for early-stage squamous cell carcinoma of the true vocal cord. Int J Radiat Oncol Biol Phys. 2010;77(5):1380–1385. doi: 10.1016/j.ijrobp.2009.07.1687 [DOI] [PubMed] [Google Scholar]
- 15.Gomez D, Cahlon O, Mechalakos J, Lee N. An investigation of intensity-modulated radiation therapy versus conventional two-dimensional and 3D-conformal radiation therapy for early stage larynx cancer. Radiat Oncol. 2010;5:74. doi: 10.1186/1748-717X-5-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi HS, Jeong BK, Jeong H, et al. Carotid sparing intensity modulated radiotherapy on early glottic cancer: preliminary study. Radiat Oncol J. 2016;34(1):26–33. doi: 10.3857/roj.2016.34.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed ASR, Smith BD, Smith JB, et al. Outcomes of carotid-sparing IMRT for T1 glottic cancer: Comparison with conventional radiation. Laryngoscope. February 2019. doi: 10.1002/lary.27873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feigenberg SJ, Lango M, Nicolaou N, Ridge JA. Intensity-modulated radiotherapy for early larynx cancer: is there a role? Int J Radiat Oncol Biol Phys. 2007;68(1):2–3. doi: 10.1016/j.ijrobp.2007.01.006 [DOI] [PubMed] [Google Scholar]
- 19.Nishimura G, Tsukuda M, Mikami Y, et al. Efficacy of concurrent chemoradiotherapy for T1 and T2 laryngeal squamous cell carcinoma regarding organ preservation. Anticancer Res. 2009;29(2):661–666. [PubMed] [Google Scholar]
- 20.Okami K, Hamano T, Takeo T, et al. Concurrent chemoradiotherapy with docetaxel for T2 laryngeal carcinoma. Tokai J Exp Clin Med. 2008;33(3):130–134. [PubMed] [Google Scholar]
- 21.Itoh Y, Fuwa N. Retrospective analysis: concurrent chemoradiotherapy using protracted continuous infusion of low-dose cisplatin and 5-fluorouracil for T2N0 glottic cancer. Radiat Med. 2006;24(4):277–281. doi: 10.1007/s11604-005-1517-1 [DOI] [PubMed] [Google Scholar]
- 22.Hirasawa N, Itoh Y, Ishihara S, et al. Radiotherapy with or without chemotherapy for patients with T1-T2 glottic carcinoma: retrospective analysis. Head Neck Oncol. 2010;2:20. doi: 10.1186/1758-3284-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Kishan AU, Raldow A, et al. Addition of Chemotherapy Is Associated With Decreased Survival in Early-Stage (T1–2N0M0) Glottic Squamous Cell Carcinoma Treated With Definitive Radiotherapy. JCO Precis Oncol. 2019;(3):1–14. doi: 10.1200/PO.18.00099 [DOI] [PubMed] [Google Scholar]
- 24.Trotti A 3rd, Zhang Q, Bentzen SM, et al. Randomized trial of hyperfractionation versus conventional fractionation in T2 squamous cell carcinoma of the vocal cord (RTOG 9512). Int J Radiat Oncol Biol Phys. 2014;89(5):958–963. doi: 10.1016/j.ijrobp.2014.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83(991):554–568. doi: 10.1259/bjr/31372149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyhne NM, Primdahl H, Kristensen CA, et al. The DAHANCA 6 randomized trial: Effect of 6 vs 5 weekly fractions of radiotherapy in patients with glottic squamous cell carcinoma. Radiother Oncol. 2015;117(1):91–98. doi: 10.1016/j.radonc.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 27.Fletcher GH, Goepfert H. Larynx and pyriform sinus In: Fletcher GH, ed. Textbook of Radiotherapy. Ed 3 Philadelphia, PA: Lea & Febiger; 1980:331–363. [Google Scholar]
- 28.Harwood AR, Beale FA, Cummings BJ, Keane TJ, Rider WD. T2 glottic cancer: an analysis of dose-time-volume factors. Int J Radiat Oncol Biol Phys. 1981;7(11):1501–1505. [DOI] [PubMed] [Google Scholar]
- 29.Wang CC. Carcinoma of the larynx In: Radiation Therapy for Head and Neck Neoplasms. Ed 3 New York, NY: Wiley-Liss; 1997:221–255. [Google Scholar]
- 30.Le QT, Fu KK, Kroll S, et al. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiat Oncol Biol Phys. 1997;39(1):115–126. [DOI] [PubMed] [Google Scholar]
- 31.Warde P, O’Sullivan B, Bristow RG, et al. T1/T2 glottic cancer managed by external beam radiotherapy: the influence of pretreatment hemoglobin on local control. Int J Radiat Oncol Biol Phys. 1998;41(2):347–353. [DOI] [PubMed] [Google Scholar]
- 32.Garden AS, Forster K, Wong P-F, Morrison WH, Schechter NR, Ang KK. Results of radiotherapy for T2N0 glottic carcinoma: does the “2” stand for twice-daily treatment? Int J Radiat Oncol Biol Phys. 2003;55(2):322–328. [DOI] [PubMed] [Google Scholar]
- 33.Short S, Krawitz H, Macann A, et al. TN/TN glottic carcinoma: a comparison of two fractionation schedules. Australas Radiol. 2006;50(2):152–157. doi: 10.1111/j.1440-1673.2006.01559.x [DOI] [PubMed] [Google Scholar]
- 34.Taguchi T, Tsukuda M, Mikami Y, Horiuchi C, Ishitoya J-I, Katori H. Concurrent chemoradiotherapy with carboplatin and uracil-ftegafur in patients with stage two (T2 N0 M0) squamous cell carcinoma of the glottic larynx. J Laryngol Otol. 2006;120(6):478–481. doi: 10.1017/S0022215106000880 [DOI] [PubMed] [Google Scholar]
- 35.Hafidh M, Tibbo J, Trites J, et al. Radiotherapy for T1 and T2 laryngeal cancer: the Dalhousie University experience. J Otolaryngol Head Neck Surg. 2009;38(4):434–439. [PubMed] [Google Scholar]
- 36.Furusaka T, Matuda H, Saito T, Katsura Y, Ikeda M. Long-term observations and salvage operations on patients with T2N0M0 squamous cell carcinoma of the glottic larynx treated with radiation therapy alone. Acta Otolaryngol. 2012;132(5):546–551. doi: 10.3109/00016489.2011.646008 [DOI] [PubMed] [Google Scholar]
- 37.Ermiş E, Teo M, Dyker KE, Fosker C, Sen M, Prestwich RJD. Definitive hypofractionated radiotherapy for early glottic carcinoma: Experience of 55Gy in 20 fractions. Radiat Oncol. 2015;10(1). doi: 10.1186/s13014-015-0505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthiesen C, Herman TDLF, Singh H, et al. Dosimetric and radiobiologic comparison of 3D conformal, IMRT, VMAT and proton therapy for the treatment of early-stage glottic cancer. J Med Imaging Radiat Oncol. 2015;59(2):221–228. doi: 10.1111/1754-9485.12227 [DOI] [PubMed] [Google Scholar]
- 39.Smith GL, Smith BD, Buchholz TA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;26(31):5119–5125. doi: 10.1200/JCO.2008.16.6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rock K, Huang SH, Tiong A, et al. Partial Laryngeal IMRT for T2N0 Glottic Cancer: Impact of Image Guidance and Radiation Therapy Intensification. Int J Radiat Oncol Biol Phys. 2018;102(4):941–949. doi: 10.1016/j.ijrobp.2018.03.034 [DOI] [PubMed] [Google Scholar]
- 41.Jackson MW, Amini A, Jones B, Karam S. Improved Survival With 3DCRT Over IMRT in Early-Stage Larynx Cancer: A National Cancer Data Base Analysis. Int J Radiat Oncol. 2016;94(4):872–873. doi: 10.1016/j.ijrobp.2015.12.039 [DOI] [Google Scholar]
- 42.Cardenas CE, Mohamed ASR, Tao R, et al. Prospective Qualitative and Quantitative Analysis of Real-Time Peer Review Quality Assurance Rounds Incorporating Direct Physical Examination for Head and Neck Cancer Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;98(3):532–540. doi: 10.1016/j.ijrobp.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansen LV, Grau C, Overgaard J. Glottic carcinoma--patterns of failure and salvage treatment after curative radiotherapy in 861 consecutive patients. Radiother Oncol. 2002;63(3):257–267. [DOI] [PubMed] [Google Scholar]
- 44.Mendenhall WM, Parsons JT, Million RR, Fletcher GH. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiation therapy: relationship of dose-fractionation factors to local control and complications. Int J Radiat Oncol Biol Phys. 1988;15(6):1267–1273. [DOI] [PubMed] [Google Scholar]
- 45.McCoul ED, Har-El G. Meta-analysis of impaired vocal cord mobility as a prognostic factor in T2 glottic carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135(5):479–486. doi: 10.1001/archoto.2009.47 [DOI] [PubMed] [Google Scholar]
- 46.Bhateja P, Ward MC, Hunter GH, et al. Impaired vocal cord mobility in T2N0 glottic carcinoma: Suboptimal local control with Radiation alone. Head Neck. 2016;38(12):1832–1836. doi: 10.1002/hed.24520 [DOI] [PubMed] [Google Scholar]
- 47.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91–11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31(7):845–852. doi: 10.1200/JCO.2012.43.6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64(1):77–82. doi: 10.1016/j.ijrobp.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 49.Moon SH, Cho KH, Chung EJ, et al. A prospective randomized trial comparing hypofractionation with conventional fractionation radiotherapy for T1–2 glottic squamous cell carcinomas: results of a Korean Radiation Oncology Group (KROG-0201) study. Radiother Oncol. 2014;110(1):98–103. doi: 10.1016/j.radonc.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 50.Torrente MC, Rodrigo JP, Haigentz MJ, et al. Human papillomavirus infections in laryngeal cancer. Head Neck. 2011;33(4):581–586. doi: 10.1002/hed.21421 [DOI] [PubMed] [Google Scholar]
- 51.Sanchez Barrueco A, Gonzalez Galan F, Lora Pablos D, et al. HPV in Larynx Squamous Cell Carcinoma: New Serotypes and Survival Study within 10-Year Follow-up. Otolaryngol Head Neck Surg. 2017;156(4):677–682. doi: 10.1177/0194599817695545 [DOI] [PubMed] [Google Scholar]
- 52.Erkul E, Yilmaz I, Narli G, Babayigit MA, Gungor A, Demirel D. The presence and prognostic significance of human papillomavirus in squamous cell carcinoma of the larynx. Eur Arch Otorhinolaryngol. 2017;274(7):2921–2926. doi: 10.1007/s00405-017-4573-0 [DOI] [PubMed] [Google Scholar]