Abstract
Rationale
The non-medical use of new psychoactive substances (NPS) is a worldwide public health concern. The so-called “benzofury” compounds, 5-(2-aminopropyl)benzofuran (5-APB) and 6-(2-aminopropyl)benzofuran (6-APB), are NPS with stimulant-like properties in human users. These substances are known to interact with monoamine transporters and 5-HT receptors in transfected cells, but less is known about their effects in animal models.
Methods
Here, we used in vitro monoamine transporter assays in rat brain synaptosomes to characterize the effects of 5-APB and 6-APB, together with their N-methyl derivatives 5-MAPB and 6-MAPB, in comparison to 3,4-methylenedioxyamphetamine (MDA) and 3,4-methylenedioxymethamphetamine (MDMA). In vivo neurochemical and behavioral effects of 5-APB (0.3 and 1.0 mg/kg, i.v.) and 6-APB (0.3 and 1.0 mg/kg, i.v.) were assessed in comparison to MDA (1.0 and 3.0 mg/kg, i.v.) using microdialysis sampling in the nucleus accumbens of conscious male rats.
Results
All four benzofuran derivatives were substrate-type releasers at dopamine transporters (DAT), norepinephrine transporters (NET) and serotonin transporters (SERT) with nanomolar potencies, similar to the profile of effects produced by MDA and MDMA. However, the benzofurans were at least 3-fold more potent than MDA and MDMA at evoking transporter-mediated release. Like MDA, both benzofurans induced dose-related elevations in extracellular dopamine and serotonin in the brain, but benzofurans were more potent than MDA. The benzofuran derivatives also induced profound behavioral activation characterized by forward locomotion which lasted for at least 2 h post-injection.
Conclusions
Overall, benzofurans are more potent than MDA in vitro and in vivo, producing sustained stimulant-like effects in rats. These data suggest that benzofuran-type compounds may have abuse liability, and could pose risks for adverse effects, especially if used in conjunction with abused drugs or medications which enhance monoamine transmission in the brain.
Keywords: benzofury, designer drugs, monoamine transporter, release, microdialysis, locomotor activity, synthetic stimulants, MDMA, MDA
Introduction
The non-medical (i.e., recreational) use of psychoactive substances that evade legislative control has become an issue of concern for law enforcement, clinicians, and policy makers (Baumann and Volkow 2016; Madras 2017). The structural and pharmacological diversity of these new psychoactive substances (NPS) gives rise to a rich array of drugs available in recreational drug markets worldwide (Hondebrink et al. 2018; Luethi and Liechti 2020), and this is reflected by intensive global monitoring measures of hundreds of such substances that emerged on the market in recent years (Evans-Brown and Sedefov 2018; Tettey et al. 2018). The evolution of novel compounds comes from various sources, such as structural analogs of illicit drugs of abuse and legitimate medication development efforts, but also newly-invented chemical entities without any history of scientific exploration (Brandt et al. 2014b). 1-(1-Benzofuran-5-yl)propan-2-amine (5-(2-aminopropyl)benzofuran or 5-APB) and 1-(1-benzofuran-6-yl)propan-2-amine (6-(2-aminopropyl)benzofuran, 6-APB), and their N-methyl derivatives 5-MAPB and 6-MAPB (Figure 1), first appeared on the recreational drug market almost a decade ago (King 2014). In Europe, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) reported the detection of 5-APB in 2010 (EMCDDA-Europol 2011) followed by 6-APB in 2011 (EMCDDA Europol 2012), whereas both 5-MAPB and 6-MAPB were reported in 2013 (EMCDDA-Europol 2014). Anecdotal evidence from people who use such substances suggests that both 5-APB and 6-APB produce psychostimulant and entactogen-like effects similar those produced by 3,4-methylenedioxymethamphetamine (MDMA) (Greene 2013; Nugteren-van Lonkhuyzen et al. 2015). A number of intoxication cases with adverse effects, including fatalities, have been described where 5-APB and 6-APB were forensically confirmed (Adamowicz et al. 2014; Chan et al. 2013; Daveluy et al. 2016; Elliott and Evans 2014; McIntyre et al. 2015; Nugteren-van Lonkhuyzen et al. 2015), and recent data indicate these compounds are still used (Krpo et al. 2018). The Trans European Drug Information (TEDI) project, designed to carry out chemical analysis of drug samples submitted by recreational users as a harm reduction strategy, identified the appearance of 4-APB, 5-APB and 6-APB in samples and tablets circulating on the “ecstasy” market (Brunt et al. 2017). A case of acute toxicity following consumption of 5-MAPB has also been reported (Hofer et al. 2017). The synthetic preparation of both 5-APB and 6-APB was first published in 2000 as part of a research program designed for the development of selective 5-HT2C receptor agonists (Briner et al. 2000; Briner et al. 2006) and the preparation of other isomers was reported for forensic purposes a decade later (Casale and Hays 2012; Stanczuk et al. 2013). When appearing on the NPS market, one commonly used term associated with 5-APB and 6-APB, which was originally related to the first branded products offered for sale, was “benzofury” (Nugteren-van Lonkhuyzen et al. 2015).
Figure 1.
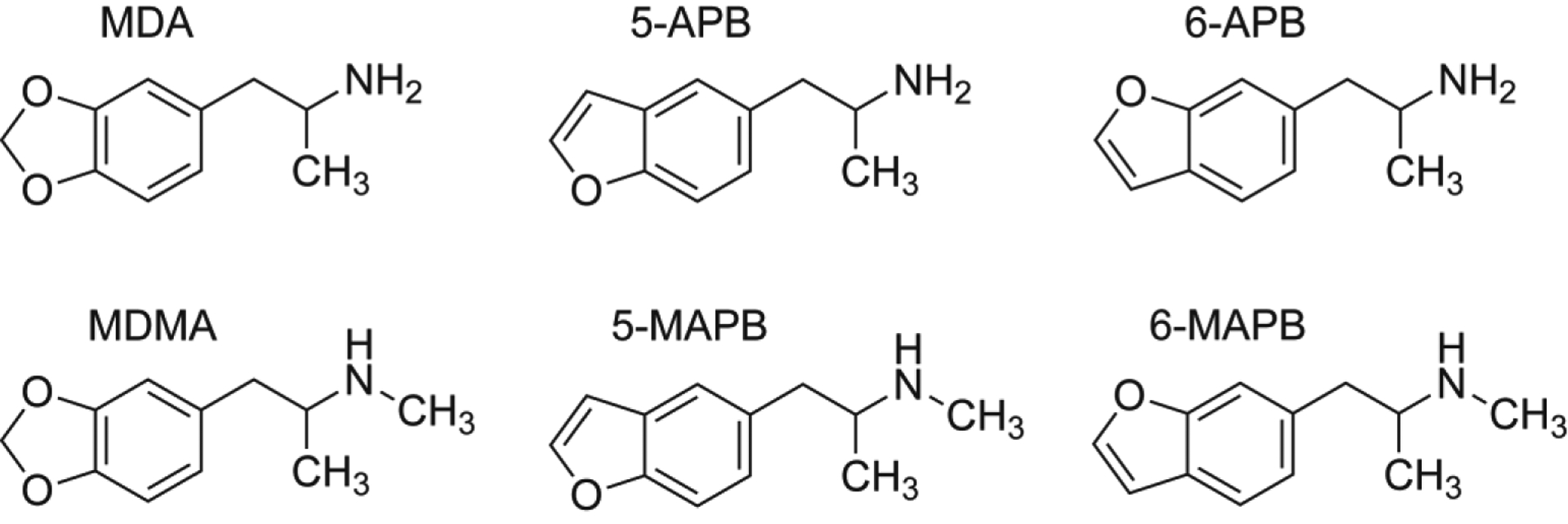
Chemical structures of 5-APB, 6-APB, and their N-methylated counterparts, as compared to MDA and MDMA.
Initial studies examining the preclinical pharmacology of 5-APB and 6-APB showed they act as agonists at 5-HT2A and 5-HT2B receptors (Dawson et al. 2014; Iversen et al. 2013; Rickli et al. 2015; Shimshoni et al. 2017), whilst agonist or partial agonist properties have been reported for their interactions with trace amine-associated receptor 1 (TAAR1) (rat, mouse, and human) (Simmler et al. 2016). Additionally, both substances inhibit neurotransmitter uptake at dopamine transporters (DAT), norepinephrine transporters (NET) and serotonin (5-HT) transporters (SERT) in a dose-dependent manner (Iversen et al. 2013; Rickli et al. 2015; Zwartsen et al. 2017). Given the structural similarities between benzofuran compounds and 3,4-methylenedioxymethamphetamine (MDMA), it is tempting to speculate that they act as transportable substrates at monoamine transporters. Indeed, Rickli et al. (2015) first showed that 5-APB and 6-APB display transmitter releasing properties in HEK-293 cells transfected with human transporters, and the recent findings of Eshleman et al. (2019) confirm this observation. Application of 10 μM 5-APB to rat brain slices evokes dopamine efflux, consistent with substrate activity at DAT (Dawson et al. 2014). The N-methyl analog 5-MAPB was also reported to show DAT binding and inhibition of reuptake at DAT and SERT (Kim et al. 2019). The evaluation of cytotoxic effects of 5-APB and 5-MAPB in hepatocytes has also been reported (Nakagawa et al. 2018; Nakagawa et al. 2017).
In contrast to the abundance of in vitro data with 5-APB and 6-APB, only a few studies have examined the effects of these compounds in vivo. Microdialysis sampling in mouse striatum revealed that oral administration of 5-APB and 5-MAPB results in significant elevation of extracellular concentrations of dopamine and 5-HT with greater effects on 5-HT (Fuwa et al. 2016). Exposure to 5-MAPB was reported to result in increased levels of 5-HT and decreased concentrations of 3,4-dihydroxyphenylacetic acid (DOPAC) in microdialysate samples obtained from nucleus accumbens in unanesthetized rats (Kim et al. 2019).
In the present study, we sought to extend the findings from rodent models by comparing the pharmacology of the aminoalkylbenzofuran derivatives 5-APB, 5-MAPB, 6-APB and 6-MAPB with the structurally related club drugs, 3,4-methylenedioxyamphetamine (MDA) and MDMA. First, in vitro monoamine transporter assays in rat brain synaptosomes were used to examine the releasing effects of 5-APB, 5-MAPB, 6-APB, and 6-MAPB at DAT, NET and SERT. Next, in vivo microdialysis in rat nucleus accumbens was used to examine changes in extracellular dopamine and 5-HT produced by intravenous injection of 5-APB and 6-APB. Microdialysis sampling was carried out in arenas equipped with photobeam arrays to detect movement, so that neurochemistry and locomotor behaviors could be monitored simultaneously.
Material and methods
Drugs and chemicals
The four aminoalkylbenzofuran derivatives 5-APB, 5-MAPB, 6-APB and 6-MAPB were synthesized as HCl salts according to published procedures (Briner et al. 2000; Stanczuk et al. 2013) and were available from previous studies (Welter et al. 2015a; Welter et al. 2015b). (±)-3,4-Methylenedioxyamphetamine HCl (MDA) and (±)-3,4-methylenedioxymethamphetamine HCl (MDMA) were obtained from the Pharmacy at the National Institute on Drug Abuse (NIDA), Intramural Research Program (IRP), in Baltimore, MD. [3H]1-Methyl-4-phenylpyridinium ([3H]MPP+, specific activity = 85 Ci/mmol) was purchased from American Radiolabeled Chemicals (St Louis, MO, USA) whereas [3H]serotonin ([3H]5-HT, specific activity = 20 Ci/mmol) was purchased from Perkin Elmer (Shelton, CT, USA). All other chemicals and reagents used for the in vitro assays, microdialysis methods, and high-performance liquid chromatography with electrochemical detection (HPLC-ECD) were acquired from Sigma-Aldrich (St Louis, MO, USA) unless otherwise specified.
Animals and housing facilities
Male Sprague-Dawley rats (Envigo, Frederick, MD, USA) weighing 300–400g were housed under conditions of controlled temperature (22 ± 2 °C) and humidity (45 ± 5%), with food and water freely available. Animals were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and procedures were carried out in accordance with the Animal Care and Use Committee of the NIDA IRP. Lights were on from 0700–1900 h and experiments were carried out between 0900–1500 h.
In vitro transporter release assays in synaptosomes
Synaptosomes were prepared from rat striatum for DAT assays, whereas synaptosomes were prepared from whole brain minus striatum and cerebellum for NET and SERT assays, as described previously (Rothman et al. 2001; Rothman et al. 2003). Reserpine (1 μM) was added to the 10% sucrose solution during preparation of synaptosomes to block vesicular uptake of substrates. Radiolabeled substrates used for release assays were 9 nM [3H]1-methyl-4-phenylpyridinium ([3H]MPP+) (DAT and NET) and 5 nM [3H]5-HT (SERT). Unlabeled transporter blockers were employed to prevent uptake of [3H]substrates by competing transporters in some assays. For DAT assays, no unlabeled blockers were required. However, for NET assays, 100 nM GBR12935 and citalopram were added to the sucrose solution to block [3H]MPP+ uptake by DAT and SERT. For SERT assays, 100 nM GBR12935 and nomifensine were added to sucrose to block [3H]5-HT uptake by DAT and NET. Synaptosomes were preloaded with radiolabeled substrate in Krebs-phosphate buffer for 1 h to reach steady state. Release assays were initiated by addition of 850 μL of preloaded synaptosomes to test tubes containing 150 μL buffer solution with or without test drugs. Incubations lasted 30 min for DAT and NET assays, and 5 min for SERT assays. The release reaction was terminated by rapid vacuum filtration, followed by quantitative determination of radioactivity retained on filters by liquid scintillation counting.
In vivo microdialysis in conscious male rats
Microdialysis sampling was carried out in conscious male rats as described previously (Baumann et al. 2012; Baumann et al. 2013). Each rat received a surgically-implanted jugular catheter and an intracranial guide cannula aimed at the nucleus accumbens under pentobarbital anesthesia (sodium pentobarbital, 60 mg/kg, i.p.). Rats were single-housed post-operatively and allowed at least one week to recover from surgery. On the evening before an experiment, rats were moved from their home cages and placed into microdialysis arenas which were equipped with photobeam arrays to detect locomotor activity (TruScan, Harvard Bioscience). For each rat, a microdialysis probe (2×0.5 mm, CMA/12, Harvard Bioscience, Holliston, MA, USA) was inserted into the guide cannula, and an extension tube was attached to the jugular catheter. Rats were connected to a tether system that allowed movement within the arena. Ringers’ salt solution was pumped at a flow rate of 0.6 μL/min to perfuse the probes overnight. On the following morning, dialysate samples were collected at 20 min intervals, and dopamine and 5-HT levels were quantitatively determined using microbore HPLC-ECD as described elsewhere (Baumann et al. 2011). Rats were randomly assigned to groups receiving either drug or saline injections. Once three stable baseline samples were obtained, rats received two sequential intravenous (i.v.) injections of drug: one dose at time zero, followed by a threefold higher dose 60 min later. Control rats received sequential i.v. injections of saline (1 mL/kg) according to the same schedule. Microdialysis samples were collected every 20 min throughout the post-injection period of 120 min. Ambulation within the arena, defined as movement in the horizontal plane, was quantified in 20 min bins that coincided with collection of dialysate samples. At the end of the experiments, rats were euthanized with CO2 and decapitated. The brain sections were examined to verify placement of microdialysis probe tips within the nucleus accumbens. Only those rats with correct placements were included in data analyses.
Data analysis
All statistical analyses were carried out using GraphPad Prism (v. 7.0; GraphPad Scientific, San Diego, CA, USA). For synaptosome assays, EC50 values for stimulation of release were calculated based on non-linear regression analysis. Release efficacy was expressed as a percentage of maximal release, which was determined using saturating concentrations of the non-selective releasing agent tyramine at 10 μM for DAT and NET, and 100 μM for SERT. For in vivo microdialysis experiments, neurotransmitter and behavioral data from individual rats were normalized to percent control values based on three preinjection samples. Normalized group data were expressed as mean ± SEM and were evaluated by a two-factor analysis of variance (ANOVA) (dose × time) where drug effects were compared with saline control. When significant main effects were noted, post hoc comparisons were carried out at each time point using Bonferroni’s post hoc test.
Results
In vitro transporter release assays
Rat brain synaptosomes were preloaded with [3H]MPP+ or [3H]5-HT followed by incubation with various concentrations of test drug to assess the potential for transporter-mediated release. The dose-response effects for 5-APB, 6-APB and MDA to release [3H]MPP+ via NET and DAT and [3H]5-HT via SERT are depicted in Figure 2. In order to compare the potency of test drugs to induce transporter-mediated release, non-linear regression analysis was employed to calculate potency in terms of EC50 values. As shown in Table 1, all tested substances were potent substrate-type releasers at DAT, NET and SERT. Compared to MDA, the benzofuran counterparts 5-APB and 6-APB were at least 3-fold more potent in their ability to induce release at DAT and SERT, whereas release evoked via NET was 2–3 times more potent than MDA. At DAT, for example, 5-APB (EC50 = 31 nM) was 3.4 times more potent than MDA (EC50 = 106 nM) while 6-APB (EC50 = 10 nM) was 10-fold more potent than MDA. Potency values determined for SERT-mediated release of [3H]5-HT indicated that 5-APB (EC50 = 19 nM) and 6-APB (EC50 = 36 nM) were 8.5 and 4.5 times more potent than MDA. The findings for the N-methyl derivatives are illustrated in Figure 3, and the comparison with MDMA showed that both 5-MAPB and 6-MAPB were at least 3 times more potent at DAT, 3.75 times more potent at NET, and up to 2.5 times more potent at SERT. The calculated DAT/SERT and DAT/NET ratios suggested that all six tested drugs were non-selective substrates across the three transporters. With regard to N-methylation of 5-APB and 6-APB, a difference in potency at NET was not observed whereas a slight reduction in potency was noticeable at DAT. At SERT, 5-APB remained 3.4 times more potent than 5-MAPB (EC50 = 64 nM) although potency of 6-MAPB (EC50 = 33 nM) remained similar compared to 6-APB (EC50 = 36 nM).
Figure 2.
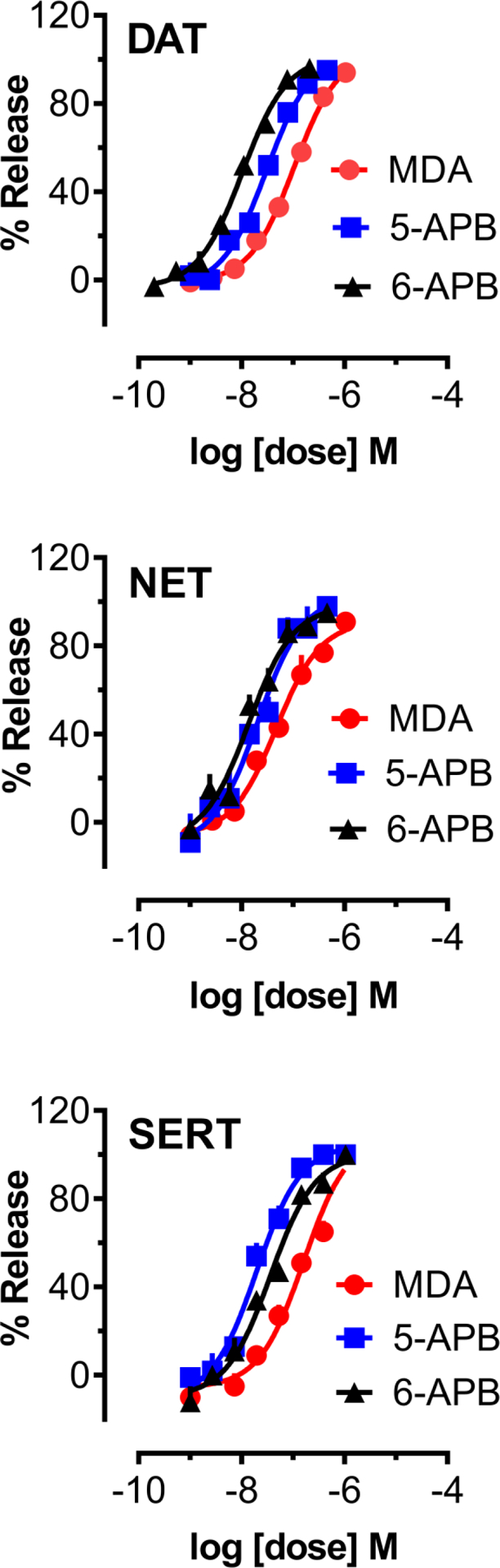
Dose-response effects of MDA, 5-APB, and 6-APB to induce release of [3H]MPP+ via DAT and NET, or [3H]5-HT via SERT, in rat brain synaptosomes. Data are mean +/− SD for N=3 experiments, expressed as a percent of maximal release.
Table 1.
Potency (EC50) and efficacy (%Emax) of psychoactive benzofuran compounds to release [3H]MPP+ via DAT and NET, or [3H]5-HT via SERT, in rat brain synaptosomes.
| Drug | DAT release EC50 (nM) [%Emax] | NET release EC50 (nM) [%Emax] | SERT release EC50 (nM) [%Emax] | DAT/SERT ratio | DAT/NET ratio |
|---|---|---|---|---|---|
| MDA | 106 ±7 [104%] | 47 ±7 [91%] | 162 ±28 [109%] | 1.53 | 0.43 |
| 5-APB | 31 ±3 [103%] | 21 ±4 [103%] | 19 ±2 [104%] | 0.61 | 0.67 |
| 6-APB | 10 ± 1 [101%] | 14 ±2 [98%] | 36 ±5 [100%] | 3.60 | 1.40 |
| MDMA | 120 ±8 [101%] | 90 ±13 [99%] | 85 ±16 [104%] | 0.71 | 0.75 |
| 5-MAPB | 41 ±3 [103%] | 24 ±3 [99%] | 64 ±7 [103%] | 1.56 | 0.58 |
| 6-MAPB | 20 ±2 [106%] | 14 ±2 [100%] | 33 ±4 [106%] | 1.65 | 0.70 |
Data are mean +/− SD for N=3 experiments performed in triplicate. Values in brackets are % of maximal release, as defined in Materials and Methods. DAT/SERT ratios are calculated as (DAT EC50)−1 /(SERT EC50)−1, where larger number indicates higher DAT selectivity. DAT/NET ratios are calculated as (DAT EC50)−1/(NET EC50)−1, where larger number indicates higher DAT selectivity.
Figure 3.
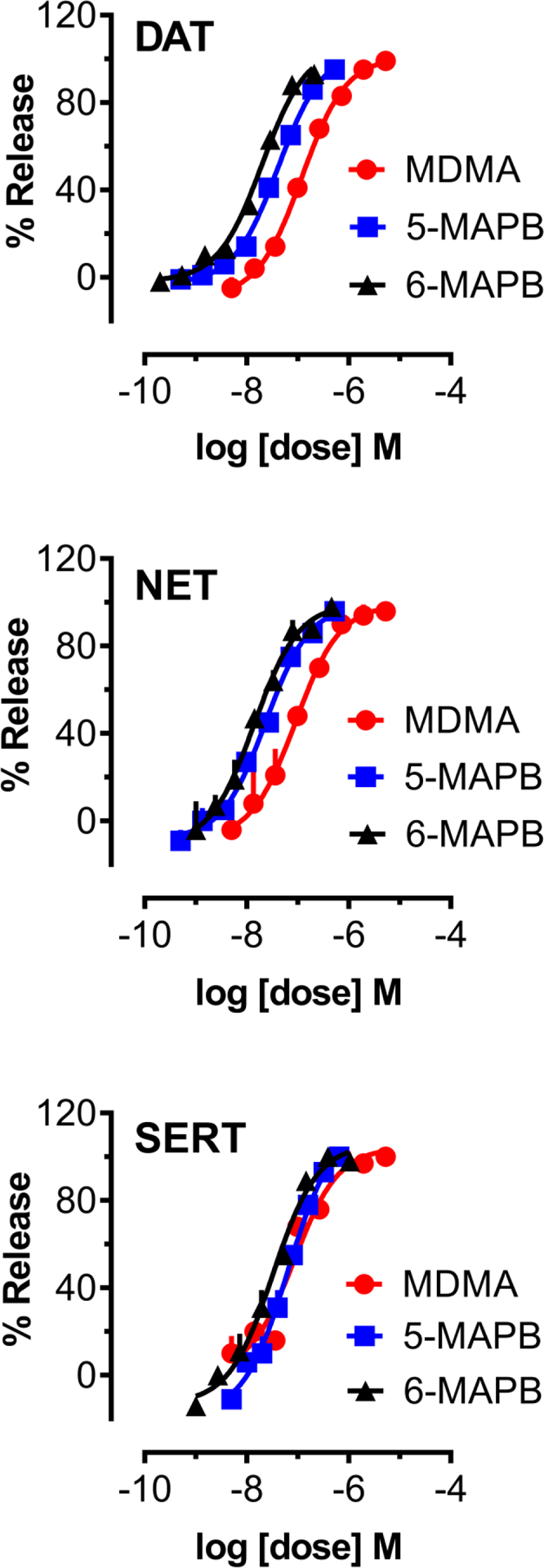
Dose-response effects of MDMA, 5-MAPB, and 6-MAPB to induce release of [3H]MPP+ via DAT and NET, or [3H]5-HT via SERT, in rat brain synaptosomes. Data are mean +/− SD for N=3 experiments, expressed as a percent of maximal release.
In vivo microdialysis data
Neurochemical and behavioral effects of acute i.v. administration of MDA to male rats (n = 7 rats per group) are shown in Figure 4. MDA treatment had a significant main effect on dialysate dopamine (F[1,108]=132.2, p<0.0001) and 5-HT (F[1,108]=154.0, p<0.0001) concentrations. Post hoc tests revealed that dopamine levels were significantly greater than saline control at all time points after the 3 mg/kg dose, whereas 5-HT levels were elevated at the 20 min time point after the 1 mg/kg dose, and at all time points after the 3 mg/kg dose (Figure 4). At the 80 min mark, i.e. 20 min following injection of the 3 mg/kg dose of MDA, dialysate dopamine levels increased 7.6-fold above baseline, which represented the maximum increase. The peak effect on 5-HT was 4.6-fold above baseline after 1 mg/kg and 11.2-fold after 3 mg/kg. MDA had a main effect on ambulation (F[1,108]=102.2, p<0.0001) when compared with saline controls. Post hoc analysis showed that MDA did not significantly increase locomotion after 1 mg/kg but did significantly stimulate activity (p<0.0001) at all times following administration of the 3 mg/kg dose. The peak effect of MDA on ambulation was 30-fold above baseline, which declined to about 18-fold when measured at the 120 min point.
Figure 4.
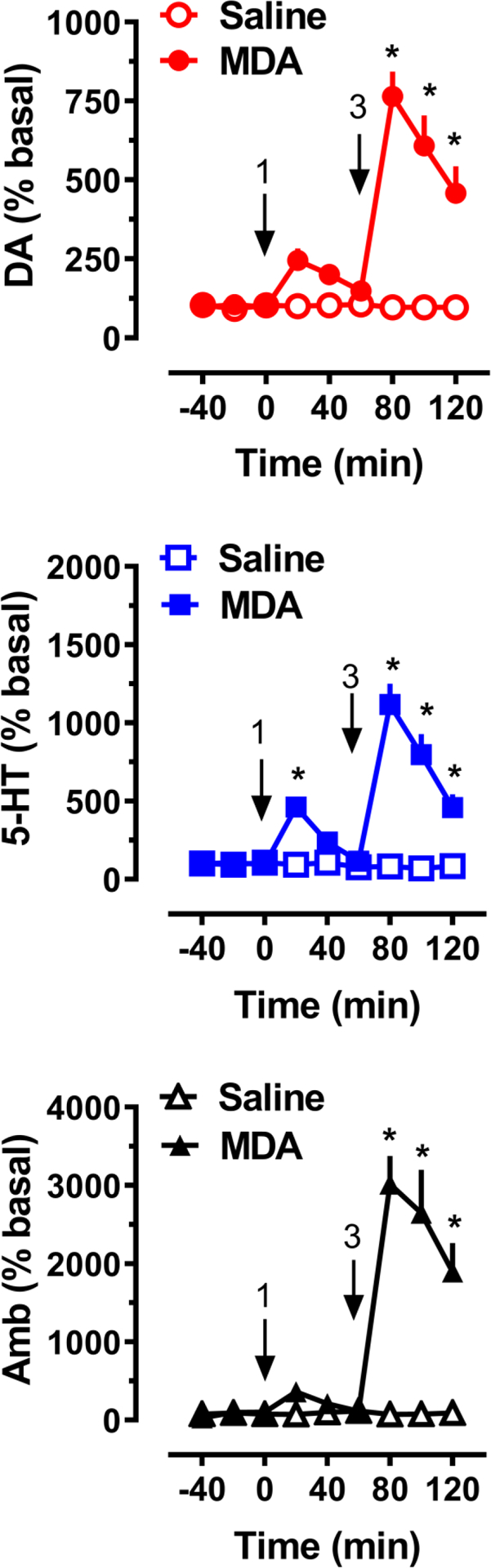
Effects of MDA administration on extracellular dopamine (DA) and 5-HT concentrations, and horizontal ambulation (Amb), in rats undergoing microdialysis in nucleus accumbens. Rats received i.v. drug injections at t=0 and 60 min, at the doses indicated, as shown by arrows. Data are mean +/− SEM for N=7 rats/group, expressed as percent of pre-injection basal values for each endpoint. * = significant effect compared to saline at corresponding time point.
The effects of 5-APB on neurochemistry and motor behavior (n = 7 rats per group) are displayed in Figure 5. 5-APB treatment had a significant main effect on dialysate levels of dopamine (F[1,108]=92.1, p<0.0001) and 5-HT (F[1,108]=107.4, p<0.0001). Post hoc tests showed that 5-APB failed to significantly increase dopamine levels at the 0.3 mg/kg dose, but a significant neurotransmitter elevation (p<0.0001) was detected at all time points after the 1.0 mg/kg dose. The peak increase of dopamine after the 1.0 mg/kg dose was 6.7-fold above baseline. 5-APB elevated dialysate 5-HT at 20 min after 0.3 mg/kg (5.7-fold, p<0.05), and at all time points after 1.0 mg/kg (p<0.0001). The peak effect on 5-HT was 17.7-fold above baseline after administration of 1.0 mg/kg. At the 120 min point, 5-HT dialysate levels remained elevated 11.3-fold above baseline. 5-APB administration at 1.0 mg/kg caused a main effect on ambulation (F[1,108]=73.5, p<0.0001) when compared with saline controls. Post hoc analysis also indicated that 5-APB significantly stimulated activity at all times following administration of the 1.0 mg/kg dose. The peak effect of 5-APB on ambulation was 21-fold above baseline, and when measured at the 120 min point, a 14.0-fold increase above baseline was still noticeable.
Figure 5.
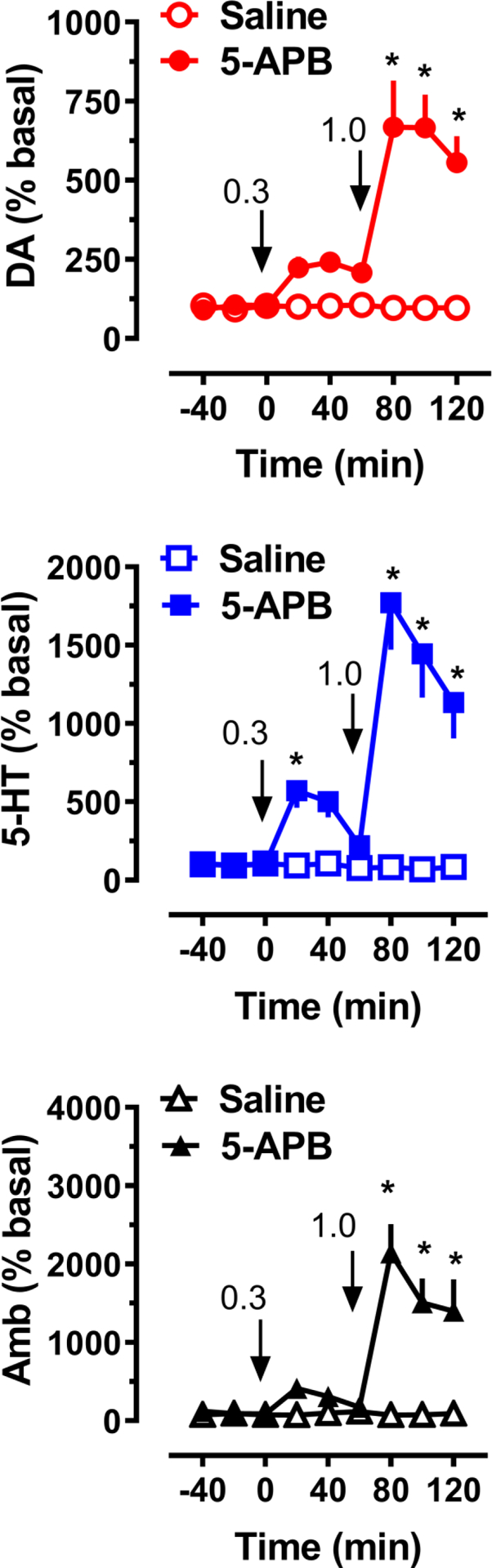
Effects of 5-APB administration on extracellular dopamine (DA) and 5-HT concentrations, and horizontal ambulation (Amb), in rats undergoing microdialysis in nucleus accumbens. Rats received i.v. drug injections at t=0 and 60 min, at the doses indicated, as shown by arrows. Data are mean +/− SEM for N=7 rats/group, expressed as percent of pre-injection basal values for each endpoint. * = significant effect compared to saline at corresponding time point.
Figure 6 depicts the neurochemical and behavioral results obtained from acute i.v. administration of 6-APB to a group of male rats (n = 7 rats per group) and significant main effects on dialysate dopamine (F[1,108]=385.8, p<0.0001) and 5-HT (F[1,108]=80.9, p<0.0001) were noted. Post hoc tests revealed that 6-APB elevated dialysate dopamine at 20 min (2.6-fold, p<0.01) and 40 min (3.3-fold, p<0.0001) after 0.3 mg/kg, and at all-time points after 1.0 mg/kg (p<0.0001) when compared to saline. The peak increase in dopamine following 1 mg/kg 6-APB was 8.4-fold above baseline (Figure 6). 6-APB elevated dialysate 5-HT immediately after administration of 1.0 mg/kg (p<0.0001), a 16-fold increase relative to baseline, and this elevation was still present at the last sampling period (p<0.0001). 6-APB had a main effect on ambulation (F[1,108]=75.4, p<0.0001) when compared with saline controls, as shown in Figure 6. Post hoc analysis showed that 6-APB administration induced a 24-fold increase in ambulation after the 1 mg/kg dose, whereas the 0.3 mg/kg dose failed to result in significant increases in locomotion.
Figure 6.
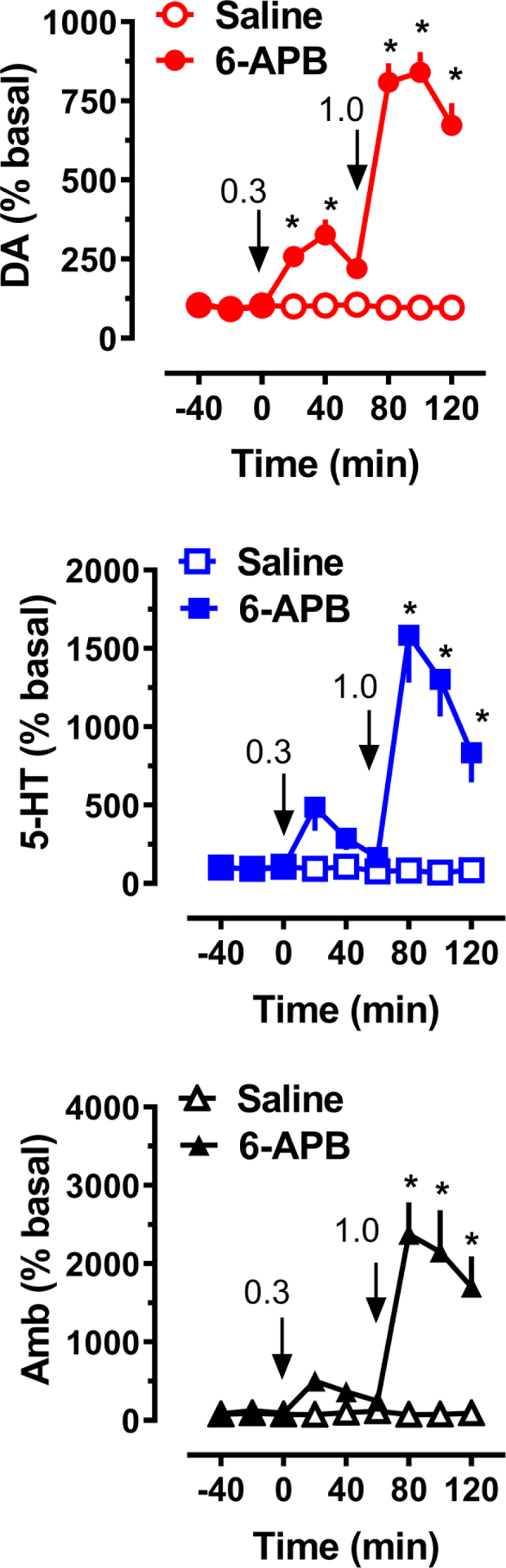
Effects of 6-APB administration on extracellular dopamine (DA) and 5-HT concentrations, and horizontal ambulation (Amb), in rats undergoing microdialysis in nucleus accumbens. Rats received i.v. drug injections at t=0 and 60 min, at the doses indicated, as shown by arrows. Data are mean +/− SEM for N=7 rats/group, expressed as percent of pre-injection basal values for each endpoint. * = significant effect compared to saline at corresponding time point.
Discussion
The present study investigated neurochemical and behavioral actions of the aminoalkylbenzofuran derivatives 5-APB and 6-APB, which together with their N-methylated counterparts 5-MAPB and 6-MAPB, appeared on the NPS market several years ago. As shown in Figures 2 and 3, the four benzofuran compounds are potent, non-selective, substrate-type releasing agents at DAT, NET, and SERT. In contrast to pure reuptake inhibitors such as cocaine, the benzofurans induce non-exocytotic transporter-mediated release of monoamines via reversal of normal transporter flux, consistent with the molecular mechanism of action for ring-substituted amphetamines (Baumann et al. 2013; Glennon 2014; Rothman and Baumann 2003; Sitte and Freissmuth 2015). Similar to what we have reported in previous studies, MDA and MDMA showed nM potencies as releasers at all three transporters in rat brain synaptosomes (Baumann et al. 2007; Sandtner et al. 2016). It is noteworthy that we found 5-APB and 6-APB to be substantially more potent than MDA and MDMA, and N-methylation of the benzofurans did not alter releasing potencies, which suggests this chemical modification is accommodated by the orthosteric binding site of monoamine transporters (Table 1).
The transporter-mediated releasing properties of 5-APB and 6-APB were first reported by Rickli et al. (2015) who examined [3H]monoamine efflux after exposure to a single large dose (100 μM) in human embryonic kidney 293 (HEK) cells transfected with human transporters. Rickli et al. used uptake inhibition potencies from transfected cells to calculate DAT/SERT ratios of 0.05 and 0.29 for 5-APB and 6-APB, respectively; these values indicate substantial SERT selectivity and are much lower than the DAT/SERT ratios of 0.61 and 3.60 that we found using release assays in synaptosomes (see Table 1). The 10-fold lower DAT/SERT ratios reported for 5-APB and 6-APB in transfected cells may seem at odds with our findings in synaptosomes, but the DAT/SERT ratios for MDMA and MDA in transfected cells (i.e., 0.12 and 0.25) are also much lower than our findings in synaptosomes (Luethi et al. 2019). Thus, in both transfected cells and rat brain synaptosomes, the DAT/SERT ratios for benzofurans are similar to those reported for MDMA and MDA. Although 5-MAPB and 6-MAPB were not included in the study of Rickli et al., the N-ethyl derivative of 5-APB, i.e. 5-EAPB, was evaluated and exhibited a reduction in the magnitude of norepinephrine and dopamine release while maintaining 5-HT releasing properties (Rickli et al. 2015). More recently, Eshleman et al. (2019) examined the dose-response relationships for the releasing activity of 6-APB and 6-MAPB, and several other benzofurans in HEK cell transfected with human transporters. These investigators demonstrated that 6-APB and 6-MAPB display nM potencies for releasing [3H]monoamines at human DAT, NET and SERT, in agreement with the data from rat synaptosomes reported here. Other studies show that 5-APB and 5-MAPB enhance electrically-induced dopamine efflux in slices of rat nucleus accumbens (Dawson et al. 2014; Sahai et al. 2017) consistent with DAT-mediated dopamine release. It is noteworthy that 5-(2-aminopropyl)indole (5-IT) and 6-(2-aminopropyl)indole (6-IT), two structurally related NPS which differ from 5-APB and 6-APB by replacement of the heteroatom in the five-membered ring, also are reported to exhibit potent releasing activities at DAT and SERT (Luethi et al. 2018; Marusich et al. 2016).
Mechanistically, the non-selective transporter releasing activity of 5-APB, 6-APB, and related analogs is comparable to other NPS such as the ring-substituted cathinones mephedrone and methylone (Baumann et al. 2012), (±)-cis-para-methyl-4-methylaminorex (4,4’-DMAR) and (±)-cis-4-methylaminorex (Brandt et al. 2014a), and (±)-cis- and trans- 3,4’methylenedioxy-4-methylaminorex (MDMAR) (Maier et al. 2018; McLaughlin et al. 2015). By contrast, cathinone analogs which contain a pyrrolidine ring, such as α-pyrrolidinovalerophenone (α-PVP) and 3,4-methylenedioxypyrovalerone (MDPV), are pure uptake inhibitors devoid of substrate-type releasing activity (Baumann et al. 2013; Kolanos et al. 2015). It is tempting to speculate that the bulky pyrrolidine ring moiety of α-PVP and MDPV renders these substances too large to fit through the transporter permeation pore, confining their interaction to the extracellular face of the protein.
Once the potent monoamine releasing properties of 5-APB and 6-APB were confirmed using synaptosome assays in vitro, we predicted that an elevation of neurotransmitters would be observed under in vivo conditions. To this end, we examined extracellular levels of dopamine and 5-HT using microdialysis in rat nucleus accumbens, a brain region associated with the reward circuitry underlying drug abuse liability and dependence (Bonci et al. 2003; Willuhn et al. 2010). Here we show that MDA, 5-APB, and 6-APB significantly elevate extracellular dopamine levels in rats undergoing microdialysis. Importantly, MDA was about 3-fold less potent than the benzofuran compounds, in agreement with the in vitro findings from rat brain synaptosomes. It is noteworthy that only 6-APB induced a significant elevation of dopamine after the low dose (0.3 mg/kg), and this observation is consistent with the more potent releasing effects of this drug at DAT (EC50 = 10 nM). 6-APB also caused the highest peak and most sustained dopamine increase, though all drugs induced substantial elevations in dopamine. The in vivo microdialysis data also reveal that administration of MDA, 5-APB and 6-APB give rise to robust increases in dialysate 5-HT levels, but MDA is 3-fold less potent in this respect. It is interesting that the low dose of 5-APB increased dialysate 5-HT while the same dose of 6-APB did not. Again, the differences in potency with regard to 5-HT release were also observed in the in vitro data where 5-APB (EC50 = 19 nM) was slightly more potent than 6-APB (EC50 = 36 nM), and much more potent than MDA (EC50 = 162 nM) to release 5-HT from synaptosomes.
A previous microdialysis study revealed that oral administration of 5-APB (0.08 mmol/kg, equivalent to ~17 mg/kg) led to an 8-fold increase in dialysate dopamine, and a 23-fold increase in dialysate 5-HT, in the corpus striatum of male CD-1 mice (Fuwa et al. 2016). In the Fuwa et al. study, the massive rise in extracellular 5-HT produced by 5-APB was maintained for up to 3 h post-injection. The extended time course of effects for 5-APB observed by Fuwa et al. (2016) might be explained by the larger dose administered or the oral route, which would be predicted to afford slower drug kinetics. Fuwa et al. also reported that oral administration of 5-MAPB (0.08 mmol/kg, equivalent to ~18 mg/kg) resulted in a peak maximum elevation in 5-HT which was much more pronounced than that produced by an equivalent dose of MDMA (0.08 mmol/kg, equivalent to ~18.4 mg/kg) (Fuwa et al. 2016). Interestingly, oral administration of either 1-(1-benzofuran-2-yl)-N-methylpropan-2-amine (2-MAPB) or 5-EAPB also produced large elevations in 5-HT levels, but the effects were smaller when compared to 5-MAPB. Overall, the present microdialysis studies, and those carried out in mice, show that 5-APB and 6-APB produce robust increases in extracellular dopamine and 5-HT, and both benzofuran compounds are more potent than MDA and MDMA. In the study reported by Kim et al. (2019), 5-MAPB induced an increase in 5-HT after administration of 1 mg/kg and a decrease of DOPAC after injection of lower doses (0.1 and 0.3 mg/kg) following analysis of microdialysates collected from rat nucleus accumbens.
In the present study, we found that MDA, 5-APB, and 6-APB induce profound dose-related behavioral activation characterized by forward ambulation, effects that last for at least 2 h post-injection. 6-APB produced a somewhat greater increase in activity (24-fold) than 5-APB (20-fold), perhaps due to higher elevations in extracellular dopamine produced by 6-APB. Our previous studies in rats have shown a significant positive correlation between the extent of motor activation and dialysate dopamine levels detected in the nucleus accumbens in vivo (Baumann et al. 2011; Zolkowska et al. 2009). The present behavioral findings in rats agree with the report of Dolan et al. (2017), who showed that intraperitoneal (i.p.) injection of 5-APB in mice increases forward locomotion with an ED50 of 3.27 mg/kg. The potency of 5-APB to stimulate activity in mice is at least 3-fold greater than that of MDMA (e.g. Gatch et al. 2019) Dolan et al. (2017) also investigated the discriminative stimulus effects of 5-APB in groups of rats trained to recognize either methamphetamine (1 mg/kg, i.p.), MDMA (1.5 mg/kg, i.p.), or cocaine (10 mg/kg, i.p.). The studies revealed that 5-APB fully substitutes for the discriminative stimulus effects of MDMA, but not methamphetamine or cocaine. Moreover, the ED50 value for 5-APB to substitute for the MDMA stimulus cue (i.e., ED50 = 0.27 mg/kg, i.p.) is substantially lower than the training dose of MDMA, indicating greater potency for 5-APB. Taken together, the behavioral results with 5-APB in rodents are fully consistent with neurochemical findings reported here that benzofuran compounds mimic the effects of MDMA but are substantially more potent.
Reported clinical features associated with 5-APB, 6-APB, or 5-MAPB overdose include cardiovascular and neurological effects consistent with a stimulant toxidrome (Chan et al. 2013; Hofer et al. 2017; Kamour et al. 2014; McIntyre et al. 2015; Nugteren-van Lonkhuyzen et al. 2015), which could be mediated by potent monoamine releasing properties identified in vitro and in vivo. Additionally, it has been reported that 5-APB and 6-APB act as agonists at the 5-HT2A and 5-HT2B receptor (Dawson et al. 2014; Iversen et al. 2013; Rickli et al. 2015), which might be clinically relevant for adverse cardiovascular events, especially after prolonged drug use (Setola et al. 2003). Appreciable affinity toward subtypes of α1-adrenoceptors (α1A, α2A) (Rickli et al. 2015) and α2-adrenoceptors (α2A, α2B, α2C) (Iversen et al. 2013) has also been observed, which could be a contributing factor for vasoconstriction and prolonged systolic and diastolic hypertension observed in a 5-MAPB intoxication case (Hofer et al. 2017).
In the present study, 5-APB and 6-APB were more potent than MDA in vitro and in vivo, and produced sustained stimulant-like effects in rats. The extent to which the potent and non-selective releasing properties of benzofurans translate into abuse potential warrants further investigation in animal models. In the only study to examine the abuse liability of benzofuran compounds, Cha et al. (2016) showed that 5-APB induces conditioned place preference but does not support intravenous drug self-administration. These intriguing findings suggest that 5-APB is rewarding yet does not possess substantial reinforcing effects. Nevertheless, the potency of the aminoalkylbenzofurans studied here could pose risks for adverse effects in humans, especially if used in conjunction with abused drugs or medications which enhance overall monoamine transmission in the brain.
In summary, we found that 5-APB, 6-APB, and their N-methylated counterparts act as potent substrate-type releasers at DAT, NET, and SERT, which mimics the molecular mechanism of action of MDA and MDMA. Importantly, the benzofuran compounds are more potent than MDA in vitro and in vivo, and they produce sustained stimulant-like effects in rats. The preclinical data suggest that benzofuran-type compounds may have abuse potential and could pose risks for adverse effects, especially if used in conjunction with abused drugs or medications which enhance overall monoamine transmission in the brain.
Funding
The research reported here was generously supported by the NIDA IRP (grant number DA 000523 to MHB).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
Animal use procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Care and Use Committee of the Intramural Research Program of the National Institute on Drug Abuse (Baltimore, MD, USA).
References
- Adamowicz P, Zuba D, Byrska B (2014) Fatal intoxication with 3-methyl-N-methylcathinone (3-MMC) and 5-(2-aminopropyl)benzofuran (5-APB). Forensic Sci Int 245:126–132. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37:1192–203. 10.1038/npp.2011.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee SM, Blough BE, Rothman RB (2011) In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther 337:218–225. 10.1124/jpet.110.176271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Srihari RT, Cozzi NV, Schindler CW (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology 38:552–562. 10.1038/npp.2012.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Volkow ND (2016) Abuse of new psychoactive substances: threats and solutions. Neuropsychopharmacology 41:663–665. 10.1038/npp.2015.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Wang XY, Rothman RB (2007) 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology 189:407–424. 10.1007/s00213-006-0322-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Bernardi G, Grillner P, Mercuri NB (2003) The dopamine-containing neuron: maestro or simple musician in the orchestra of addiction? Trends Pharmacol Sci 24:172–177. 10.1016/S0165-6147(03)00068-3 [DOI] [PubMed] [Google Scholar]
- Brandt SD, Baumann MH, Partilla JS, Kavanagh PV, Power JD, Talbot B, Twamley B, O’Brien J, Mahony O, Elliott SP, Archer RP, Patrick J, Singh K, Dempster NM, Cosbey SH (2014a) Characterization of a novel and potentially lethal designer drug, (±)-cis-para-methyl-4-methylaminorex (4,4’-DMAR, or ‘Serotoni’). Drug Test Anal 6:684–695. 10.1002/dta.1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, King LA, Evans-Brown M (2014b) The new drug phenomenon. Drug Test Anal 6:587–597. 10.1002/dta.1686 [DOI] [PubMed] [Google Scholar]
- Briner K, Burkhart JP, Burkholder TP, Fisher MJ, Gritton WH, Kohlman DT, Liang SX, Miller SC, Mullaney JT, Xu Y-C, Xu Y (2000) Aminoalkylbenzofurans as serotonin (5-HT(2C)) agonists Patent No. WO2000044737A1, Eli Lilly and Company, Indianapolis, IN, USA, 2000. [Google Scholar]
- Briner K, Burkhart JP, Burkholder TP, Fisher MJ, Gritton WH, Kohlman DT, Liang SX, Miller SC, Mullaney JT, Xu YC (2006) Aminoalkylbenzofurans as serotonin (5-HT(2C)) agonists Patent No. US7045545B1, Eli Lilly and Company, Indianapolis, IN, USA, 2006. [Google Scholar]
- Brunt TM, Nagy C, Bücheli A, Martins D, Ugarte M, Beduwe C, Ventura Vilamala M (2017) Drug testing in Europe: monitoring results of the Trans European Drug Information (TEDI) project. Drug Test Anal 9:188–198. 10.1002/dta.1954 [DOI] [PubMed] [Google Scholar]
- Casale JF, Hays PA (2012) The characterization of 6-(2-aminopropyl)benzofuran and differentiation from its 4-, 5-, and 7-positional analogues. Microgram J 9:61–74. [Google Scholar]
- Cha HJ, Lee KW, Eom JH, Kim YH, Shin J, Yun J, Han K, Kim HS (2016) 5-(2-Aminopropyl)benzofuran and phenazepam demonstrate the possibility of dependence by increasing dopamine levels in the brain. Pharmacol Biochem Behav 149:17–22. 10.1016/j.pbb.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Chan WL, Wood DM, Hudson S, Dargan PI (2013) Acute psychosis associated with recreational use of benzofuran 6-(2-aminopropyl)benzofuran (6-APB) and cannabis. J Med Toxicol 9:278–281. 10.1007/s13181-013-0306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daveluy A, Géniaux H, Eiden C, Boucher A, Chenaf C, Deheul S, Spadari M, Gérardin M, Miremont-Salamé G, Haramburu F, French Network of Addictovigilance Centres (2016) Illicit drugs or medicines taken by parachuting. Fundam Clin Pharmacol 30:185–190. 10.1111/fcp.12172 [DOI] [PubMed] [Google Scholar]
- Dawson P, Opacka-Juffry J, Moffatt JD, Daniju Y, Dutta N, Ramsey J, Davidson C (2014) The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat. Prog Neuro Psychopharmacol Biol Psychiatry 48:57–63. 10.1016/j.pnpbp.2013.08.013 [DOI] [PubMed] [Google Scholar]
- Dolan SB, Forster MJ, Gatch MB (2017) Discriminative stimulus and locomotor effects of para-substituted and benzofuran analogs of amphetamine. Drug Alcohol Depend 180:39–45. 10.1016/j.drugalcdep.2017.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S, Evans J (2014) A 3-year review of new psychoactive substances in casework. Forensic Sci Int 243:55–60. 10.1016/j.forsciint.2014.04.017 [DOI] [PubMed] [Google Scholar]
- EMCDDA-Europol (2011) EMCDDA-Europol 2010 Annual Report on the implementation of Council Decision 2005/387/JHA. Lisbon, Portugal: Available at: http://www.emcdda.europa.eu/system/files/publications/644/EMCDDA-Europol_Annual_Report_2010A_281336.pdf [15 May 2016] [Google Scholar]
- EMCDDA-Europol (2012) EMCDDA-Europol 2011 Annual Report on the implementation of Council Decision 2005/387/JHA. Lisbon, Portugal: Available at: http://www.emcdda.europa.eu/system/files/publications/689/EMCDDA-Europol_Annual_Report_2011_2012_final_335568.pdf [15 May 2016] [Google Scholar]
- EMCDDA-Europol (2014) EMCDDA-Europol 2013 Annual Report on the implementation of Council Decision 2005/387/JHA. Lisbon, Portugal: Available at: http://www.emcdda.europa.eu/system/files/publications/814/TDAN14001ENN_475519.pdf [15 May 2016] [Google Scholar]
- Eshleman AJ, Nagarajan S, Wolfrum KM, Reed JF, Swanson TL, Nilsen A, Janowsky A (2019) Structure-activity relationships of bath salt components: substituted cathinones and benzofurans at biogenic amine transporters. Psychopharmacology (Berl) 236:939–952. 10.1007/s00213-018-5059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Brown M, Sedefov R (2018) Responding to new psychoactive substances in the European Union: early warning, risk assessment, and control measures. Handb Exp Pharmacol 252:3–49. 10.1007/164_2018_160 [DOI] [PubMed] [Google Scholar]
- Fuwa T, Suzuki J, Tanaka T, Inomata A, Honda Y, Kodama T (2016) Novel psychoactive benzofurans strongly increase extracellular serotonin level in mouse corpus striatum. J Toxicol Sci 41:329–37. 10.2131/jts.41.329 [DOI] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2019) Locomotor activity and discriminative stimulus effects of five novel synthetic cathinone analogs in mice and rats. Drug Alcohol Depend 199:50–58. 10.1016/j.drugalcdep.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA (2014) Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention In: Linda PD(ed) Advances in Pharmacology. Academic Press, pp 581–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SL (2013) Benzofurans and benzodifurans In: Dargan PI, Wood DM (eds) Novel Psychoactive Substances Classification, Pharmacology and Toxicology. Academic Press, London, UK, pp 383–392 [Google Scholar]
- Hofer KE, Faber K, Muller DM, Hauffe T, Wenger U, Kupferschmidt H, Rauber-Luthy C (2017) Acute toxicity associated with the recreational use of the novel psychoactive benzofuran N-methyl-5-(2 aminopropyl)benzofuran. Ann Emerg Med 69:79–82. 10.1016/j.annemergmed.2016.03.042 [DOI] [PubMed] [Google Scholar]
- Hondebrink L, Zwartsen A, Westerink RHS (2018) Effect fingerprinting of new psychoactive substances (NPS): what can we learn from in vitro data? Pharmacol Ther 182:193–224. [DOI] [PubMed] [Google Scholar]
- Iversen L, Gibbons S, Treble R, Setola V, Huang X-P, Roth BL (2013) Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol 700:147–151. 10.1016/j.ejphar.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamour A, James D, Lupton DJ, Cooper G, Eddleston M, Vale A, Thompson JP, Thanacoody R, Hill SL, Thomas SH (2014) Patterns of presentation and clinical features of toxicity after reported use of ([2-aminopropyl]-2,3-dihydrobenzofurans), the ‘benzofuran’ compounds. A report from the United Kingdom National Poisons Information Service. Clin Toxicol 52:1025–31. 10.3109/15563650.2014.973115 [DOI] [PubMed] [Google Scholar]
- Kim M, Yang CH, Lee YS, Jang C-G, Oh S, Lee S (2019) Effects of aromatic ring-substituted phenethylamines on the release of dopamine and serotonin. Forensic Toxicol 37:104–112. 10.1007/s11419-018-0440-y [DOI] [Google Scholar]
- King LA (2014) New phenethylamines in Europe. Drug Test Anal 6:808–818. 10.1002/dta.1570 [DOI] [PubMed] [Google Scholar]
- Kolanos R, Saldoth F, Jain AD, Partilla JS, Baumann MH, Glennon RA (2015) Structural modification of the designer stimulant α-pyrrolidinovalerophenone (α-PVP) influences potency at dopamine transporters. ACS Chem Neurosci 6:1726–1731. 10.1021/acschemneuro.5b00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krpo M, Luytkis HC, Haneborg AM, Hoiseth G (2018) A fatal blood concentration of 5-APB. Forensic Sci Int 291:e1–e3. 10.1016/j.forsciint.2018.08.044 [DOI] [PubMed] [Google Scholar]
- Luethi D, Kolaczynska KE, Docci L, Krahenbuhl S, Hoener MC, Liechti ME (2018) Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology 134:4–12. 10.1016/j.neuropharm.2017.07.026 [DOI] [PubMed] [Google Scholar]
- Luethi D, Kolaczynska KE, Walter M, Suzuki M, Rice KC, Blough BE, Hoener MC, Baumann MH, Liechti ME (2019) Metabolites of the ring-substituted stimulants MDMA, methylone and MDPV differentially affect human monoaminergic systems. J Psychopharmacol 33:831–841. 10.1177/0269881119844185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luethi D, Liechti ME (2020) Designer drugs: mechanism of action and adverse effects. Arch Toxicol 94:1085–1133. 10.1007/s00204-020-02693-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK (2017) The growing problem of new psychoactive substances (NPS). Curr Top Behav Neurosci 32:1–18. 10.1007/7854_2016_34 [DOI] [PubMed] [Google Scholar]
- Maier J, Mayer FP, Luethi D, Holy M, Jäntsch K, Reither H, Hirtler L, Hoener MC, Liechti ME, Pifl C, Brandt SD, Sitte HH (2018) The psychostimulant (±)-cis-4,4’-dimethylaminorex (4,4’-DMAR) interacts with human plasmalemmal and vesicular monoamine transporters. Neuropharmacology 138:282–291. 10.1016/j.neuropharm.2018.06.018 [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Blough BE, Brandt SD, Kavanagh PV, Partilla JS, Baumann MH (2016) The new psychoactive substances 5-(2-aminopropyl)indole (5-IT) and 6-(2-aminopropyl)indole (6-IT) interact with monoamine transporters in brain tissue. Neuropharmacology 101:68–75. 10.1016/j.neuropharm.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre IM, Gary RD, Trochta A, Stolberg S, Stabley R (2015) Acute 5-(2-aminopropyl)benzofuran (5-APB) intoxication and fatality: a case report with postmortem concentrations. J Anal Toxicol 39:156–159. 10.1093/jat/bku131 [DOI] [PubMed] [Google Scholar]
- McLaughlin G, Morris N, Kavanagh PV, Power JD, Twamley B, O’Brien J, Talbot B, Dowling G, Mahony O, Brandt SD, Patrick J, Archer RP, Partilla JS, Baumann MH (2015) Synthesis, characterization, and monoamine transporter activity of the new psychoactive substance 3’,4’-methylenedioxy-4-methylaminorex (MDMAR). Drug Test Anal 7:555–564. 10.1002/dta.1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Suzuki T, Inomata A (2018) Preventive effects of fructose and N-acetyl-L-cysteine against cytotoxicity induced by the psychoactive compounds N-methyl-5-(2-aminopropyl)benzofuran and 3,4-methylenedioxy-N-methamphetamine in isolated rat hepatocytes. J Appl Toxicol 38:284–291. 10.1002/jat.3523 [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Suzuki T, Tada Y, Inomata A (2017) Cytotoxic effects of psychotropic benzofuran derivatives, N-methyl-5-(2-aminopropyl)benzofuran and its N-demethylated derivative, on isolated rat hepatocytes. J Appl Toxicol 37:243–252. 10.1002/jat.3351 [DOI] [PubMed] [Google Scholar]
- Nugteren-van Lonkhuyzen JJ, van Riel AJHP, Brunt TM, Hondebrink (2015) Pharmacokinetics, pharmacodynamics and toxicology of new psychoactive substances (NPS): 2C-B, 4-fluoroamphetamine and benzofurans. Drug Alcohol Depend 157:18–27. 10.1016/j.drugalcdep.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Rickli A, Kopf S, Hoener MC, Liechti ME (2015) Pharmacological profile of novel psychoactive benzofurans. Br J Pharmacol 172:3412–3425. 10.1111/bph.13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH (2003) Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479:23–40. 10.1016/j.ejphar.2003.08.054 [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39:32–41. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA (2003) In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther 307:138–145. 10.1124/jpet.103.053975 [DOI] [PubMed] [Google Scholar]
- Sahai MA, Davidson C, Khelashvili G, Barrese V, Dutta N, Weinstein H, Opacka-Juffry J (2017) Combined in vitro and in silico approaches to the assessment of stimulant properties of novel psychoactive substances - the case of the benzofuran 5-MAPB. Prog Neuropsychopharmacol Biol Psychiatry 75:1–9. 10.1016/j.pnpbp.2016.11.004 [DOI] [PubMed] [Google Scholar]
- Sandtner W, Stockner T, Hasenhuetl PS, Partilla JS, Seddik A, Zhang YW, Cao JJ, Holy M, Steinkellner T, Rudnick G, Baumann MH, Ecker GF, Newman AH, Sitte HH (2016) Binding mode selection determines the action of ecstasy homologs at monoamine transporters. Mol Pharmacol 89:165–175. 10.1124/mol.115.101394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL (2003) 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol 63:1223–1229. [DOI] [PubMed] [Google Scholar]
- Shimshoni JA, Winkler I, Golan E, Nutt D (2017) Neurochemical binding profiles of novel indole and benzofuran MDMA analogues. Naunyn Schmiedebergs Arch Pharmacol 390:15–24. 10.1007/s00210-016-1297-4 [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buchy D, Chaboz S, Hoener MC, Liechti ME (2016) In vitro characterization of psychoactive substances at rat, mouse, and human trace amine-associated receptor 1. J Pharmacol Exp Ther 357:134 10.1124/jpet.115.229765 [DOI] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M (2015) Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol Sci 36:41–50. 10.1016/j.tips.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczuk A, Morris N, Gardner EA, Kavanagh P (2013) Identification of (2-aminopropyl)benzofuran (APB) phenyl ring positional isomers in Internet purchased products. Drug Test Anal 5:270–276. 10.1002/dta.1451 [DOI] [PubMed] [Google Scholar]
- Tettey JNA, Crean C, Ifeagwu SC, Raithelhuber M (2018) Emergence, diversity, and control of new psychoactive substances: a global perspective. Handb Exp Pharmacol 252:51–67. 10.1007/164_2018_127 [DOI] [PubMed] [Google Scholar]
- Welter J, Brandt SD, Kavanagh P, Meyer MR, Maurer HH (2015a) Metabolic fate, mass spectral fragmentation, detectability, and differentiation in urine of the benzofuran designer drugs 6-APB and 6-MAPB in comparison to their 5-isomers using GC-MS and LC-(HR)-MSn techniques. Anal Bioanal Chem 407:3457–3470. 10.1007/s00216-015-8552-2 [DOI] [PubMed] [Google Scholar]
- Welter J, Kavanagh P, Meyer MR, Maurer HH (2015b) Benzofuran analogues of amphetamine and methamphetamine: studies on the metabolism and toxicological analysis of 5-APB and 5-MAPB in urine and plasma using GC-MS and LC-(HR)-MSn techniques. Anal Bioanal Chem 407:1371–1388. 10.1007/s00216-014-8360-0 [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PEM (2010) Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci 3:29–71. 10.1007/7854_2009_27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH (2009) Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther 329:738–746. 10.1124/jpet.108.146142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwartsen A, Verboven AHA, van Kleef R, Wijnolts FMJ, Westerink RHS, Hondebrink L (2017) Measuring inhibition of monoamine reuptake transporters by new psychoactive substances (NPS) in real-time using a high-throughput, fluorescence-based assay. Toxicol In Vitro 45:60–71. 10.1016/j.tiv.2017.05.010 [DOI] [PubMed] [Google Scholar]


