Abstract
Rationale & Objective:
Frailty and poor physical function are associated with adverse kidney transplant outcomes, but how to incorporate this knowledge into clinical practice is uncertain. We studied the association between measured physical performance and clinical outcomes among patients on kidney transplant waitlists.
Study Design:
Prospective observational cohort study.
Setting & Participants:
We studied consecutive patients evaluated in our Transplant Readiness Assessment Clinic, a top-of-the-waitlist management program, from May 2017 through December 2018 (N=305). We incorporated physical performance testing, including the 6-minute walk test (6MWT) and the sit-to-stand (STS) test, into routine clinical assessments.
Exposures:
6MWT and STS test results.
Outcomes:
Primary – Time to adverse waitlist outcomes (removal from waitlist or death). Secondary – Time to transplantation, time to death.
Analytical Approach:
We used linear regression to examine the relationship between clinical characteristics and physical performance test results. We used subdistribution hazards models to examine the association between physical performance test results and outcomes.
Results:
Median 6MWT and STS results were 393 meters (25th-75th percentile range 305-455) and 17 repetitions (25th-75th percentile range 12-21), respectively. Clinical characteristics and Estimated Post-Transplant Survival scores only accounted for 14-21% of the variance in 6MWT/STS results. 6MWT/STS results were associated with adverse waitlist outcomes (adjusted subdistribution hazard ratio [sHR] of 1.42 [95% confidence interval 1.30-1.56 per 50-meter lower in 6MWT and 1.53 [95% confidence interval 1.33-1.75] per 5-repetition lower in STS), and with transplantation (adjusted sHR of 0.80 [95% confidence interval 0.72-0.88] per 50-meter lower in 6MWT and 0.80 [95% confidence interval 0.71-0.89] per 5-repetition lower in STS). Addition of either STS or 6MWT to survival models containing clinical characteristics enhanced fit (likelihood ratio test p<0.001).
Limitations:
Single-center observational study. Other measures of global health status (e.g., Fried frailty index or short physical performance battery) were not examined.
Conclusions:
Among waitlisted kidney transplant candidates with high Kidney Allocation Scores, standardized and easily performed physical performance test results are associated with waitlist outcomes and contain information beyond what is currently routinely collected in clinical practice.
Plain Language Summary
Less physically robust patients on the kidney transplant waitlist have worse outcomes than their more physically robust counterparts. In this study, we applied two tools commonly used by physical therapists, the 6-minute walk and the sit-to-stand test, to assess patients on the kidney transplant waitlist. Among 305 patients followed for at least one year, patients who did better on the two tests were less likely to die or be removed from the waitlist and more likely to receive a transplant. These findings suggest the importance of future studies of physical therapy or other interventions aimed at improving physical performance to improve outcomes for patients awaiting kidney transplantation.
Introduction
Frailty in kidney transplant candidates is a common and consequential risk factor for adverse waitlist and transplant outcomes1. Prior research has associated frailty with lower waitlist access and higher risk of waitlist removal or death2. However, incorporation of frailty assessment into routine pretransplant practice is limited.
A recent survey of transplant programs3 in the United States highlighted knowledge gaps in the area: 1) despite a widespread belief in its importance, 75% of programs do not routinely assess frailty prior to transplant; 2) although frailty metrics such as the Fried frailty index2,4 and Short Physical Performance Battery (SPPB)5,6 are well-established in research, fewer than 8% of programs use them in practice. Regarding the first point, we speculate that programs appreciate the dynamic nature of physical function in transplant candidates7 and may not find assessments at the initial evaluation to be relevant near the actual transplant. Regarding the second point, we speculate that available metrics do not provide actionable benchmarks to help physicians tailor pretransplant management. In other words, we can assess global health by a number of metrics, but we do not know how to best use this information in the transplant setting.
Our program has begun measuring physical performance, rather than frailty per se. We posit that physical performance assessments are better-established in rehabilitation and prehabilitation as an intermediate measure for monitoring response to interventions8. We chose the 60-second sit-to-stand test (STS, also known as chair-stands) and the 6-minute walk test (6MWT). The STS is a sub-component of the SPPB, which measures lower extremity strength6. The 6MWT incorporates elements of lower extremity strength and gait speed, a frailty metric favored by 22% of transplant programs3. The 6MWT also assesses cardiorespiratory fitness9,10, a strong correlate of mortality in persons with end-stage kidney disease11,12 and non-kidney solid organ transplantation13.
We report our real-world experience of implementing physical performance testing into routine pretransplant waitlist management. Our donor service area has one of the longest wait-times in the nation14. We therefore assessed patients near the top of the waitlist, when nutrition and exercise therapies may exert proximal effects on transplant readiness.
The primary objective of this manuscript is to establish that physical performance metrics are associated with waitlist outcomes, even in kidney transplant candidates who have survived to reach the top of the waitlist. We tested whether STS and 6MWT add to the overall assessment of global health status beyond what is currently available, i.e. demographics, clinical comorbidities, and the Estimated Post-Transplant Survival (EPTS) score.
Methods
Cohort Assembly:
We included all patients evaluated through our program’s Transplant Readiness Assessment Clinic (TRAC) from May 2017, when we implemented physical performance testing, through December 2018. The operational details of TRAC, our deceased donor transplant candidate waitlist management strategy, have been published previously14. Briefly, after deceased donor transplant candidates are added to our program’s waitlist (~1800 people in size), those with sufficiently high allocation priority (as determined by Kidney Allocation Scores [KAS]) to be near the top of the waitlist are evaluated in-person by two dedicated physicians, along with two dedicated transplant nurse coordinators (TRAC coordinators). We selected KAS thresholds at which patients qualified for TRAC based on the median KAS at time of offer by blood group. For the study period, the KAS thresholds were 4 for AB, 6 for A, and 8 for B and O. We included status active and inactive patients in follow-up (see Figure S1 for details on TRAC operations).
Physical Performance Testing:
Experienced personnel from an affiliated Veterans Affairs Medical Center’s Exercise Training Unit trained two nurse coordinators to perform the 6MWT and the STS test for all patients evaluated through TRAC, at both initial and follow-up visits. The training personel performed within-subject measurements periodically against the coordinators’ measurements for quality assurance purposes. For the 6MWT, the coordinators instructed patients to walk as fast as they could, but at a safe pace, with their usual walking aid/prosthesis and/or with family assistance (in the case of sensory impairment) in a quiet hallway between two marked objects delineating a distance of 100 feet. Breaks and early stops were permitted, and included in the total 6 minutes. Non-ambulatory patients received a 6MWT result of 0. For the 60-second STS test, the coordinators asked subjects to rise with arms folded on the chest from a standard seat as fast as they could, but at a safe pace. We recorded the number of repetitions completed in 60 seconds. An in-person language interpreter was available to accommodate patients whose preferred language was not English. For implementation tracking purposes, nurse coordinators recorded the time required to administer the tests to a convenience sample of 42 consecutive patients.
Outcomes:
From the time of first physical performance assessment (baseline and time zero of cohort follow-up), we ascertained waitlist outcomes including transplant (at Stanford or another program), removal from our waitlist, and death. To reduce reporting bias (i.e., adverse events are more likely to be reported outside the follow-up schedule), we censored data at the time of last scheduled follow-up.
For every patient, we employed a shared decision-making process, in which we solicited goals and values from the patients and offered counselling on the potential effects that their comorbidities, including diminished physical performance, posed. Patients with poor physical performance were given the option for repeat evaluation after physical therapy, exercise or other lifestyle modifications. Measurements on subsequent visits were taken into consideration for activation and delisting. Programmatically, we did not deny transplant based on any physical performance threshold. Members of the transplant evaluation committee were not privy to the 6MWT and STS results, but were provided information on the patient’s comorbidity burden and general functional status such as independence with activities of daily living and use of assistive devices, as per our standard practice. Decisions for delisting were made on the global assessment of the patient’s candidacy. Reasons for delisting, where applicable, were captured from the meeting minutes. In keeping with our practice of shared decision-making, patients were delisted after verbal communication and agreement with the referring nephrologist. In cases where the referring physician disagreed with delisting, the patient remained on the waitlist with plans for future reassessment.
Data Recording:
Two nurse coordinators recorded the results of the physical performance assessments in the electronic medical record system. We obtained patient characteristics and waitlist outcomes by an automated medical record query augmented with manual chart review.
Baseline Characteristics:
We compared baseline characteristics, stratified by 6MWT and STS tertiles and EPTS categories (0-19, 20-39, 40-59, 60-79, 80-100), where EPTS was calculated at the time of evaluation. We expressed continuous variables as median (25th-75th percentile range), given skewed distributions, and categorical variables as frequencies (percentages). For continuous variables, we tested for trend across 6MWT and STS tertiles and EPTS categories using generalized linear models, and for categorical variables, we tested for trend using the Mantel-Haenzel Chi-Square Test.
Association of physical performance test results with waitlist outcomes:
We applied Fine and Gray’s proportional subdistribution hazards model15 to examine the association between each physical performance test result and adverse waitlist outcomes (removal from waitlist or death, whichever occured first) and transplant in a competing risk framework. We examined Schoenfeld residuals to examine the validity of the proportional hazards assumption. We adjusted for the following clinical characteristics: age, sex, dialysis vintage, diabetes mellitus, and known atherosclerotic disease. Because waitlist removal is possibly confounded by exposure (physical performance), we performed a sensitivity analysis examining death as the primary outcome and all other events as competing risk events.
Relation between the physical performance test results and clinical characteristics:
We used general linear models to assess associations among the physical performance test (6MWT, STS) results (model outcomes) and clinical characteristics (model covariates). We defined clinical characteristics in two ways, by demographics and comorbidities (age, sex, dialysis vintage, diabetes mellitus, and known atherosclerotic disease) and by EPTS. We used the R2 measure to approximate the proportion of variance in physical performance test results explained by clinical characteristics16.
How physical performance testing improves model fit:
We built a series of nested survival models (using the same proportional subdistribution hazards model as above): clinical characteristics alone; and clinical characteristics plus physical performance test results, added on sequentially. As above, we defined clinical characteristics in two ways, by demographics and comorbidities and by EPTS. We assessed whether a more complex model outperformed a simpler model by two methods: 1) comparing the Akaike information criterion (AIC) among the different models, where lower AIC indicates better model fit; and 2) computing the Likelihood Ratio Test among nested models17.
Statistical analyses were conducted using SAS Enterprise version 7.1 (Cary, NC). The Stanford Institutional Review Board approved this project (protocol #43639) in adherence with the Delcaration of Helsinki. The clinical and research activities being reported are consistent with the Principles of the Delcaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficing and Transplant Tourism”. Because the study used data being collected for routine clinical purposes and was defined as “minimal risk”, the Instutitional Review Board waived the need to obtain individual-level consent.
Results
Physical performance test results:
Our cohort consisted of 305 patients. Of them, 305 had 6MWT and 304 had STS results. For ease of interpretation and graphical display, we divided each physical performance test into tertiles. Table 1 summarize their characteristics by 6MWT and STS result tertile and EPTS category. Measures of physical function, including Karnofsky score at listing, were positively correlated with 6MWT and STS results and negatively correlated with EPTS scores.
Table 1.
Baseline characteristics on the date of 1st TRAC evaluation, stratified by 6MWT result tertile or EPTS category. Continuous variables are represented as median (25-75th percentile range). Categorical variables are represented as count (percentage). P-values refer to the test for trend across categories. Data is 100% complete unless stated otherwise.
| Baseline Characteristic | 6MWT Result in Meters | p | ||
|---|---|---|---|---|
| 0-329 | 334-425 | 426-681 | ||
| N=101 | N=102 | N=102 | ||
| Demographics and Follow-Up | ||||
| Age (year) | 61 (55-66) | 59 (51-64) | 50 (43-58) | <0.0001 |
| Sex (% female) | 59 (58%) | 53 (52%) | 30 (29%) | <0.0001 |
| Race/ethnicity (%) | ||||
| White | 16 (16%) | 20 (20%) | 12 (12%) | 0.6 |
| Black | 6 (6%) | 10 (10%) | 6 (6%) | |
| Hispanic, non-black | 52 (51%) | 35 (34%) | 56 (55%) | |
| Asian | 23 (23%) | 31 (30%) | 24 (24%) | |
| Other/Mixed | 4 (4%) | 6 (6%) | 4 (4%) | |
| Time from listing (years) | 6.2(4.5-8.7) | 5.7(2.8-7.6) | 6.4(4.1-7.7) | 0.2 |
| Follow-up timea (days) | 194 (35-449) | 411 (281-585) | 373 (175-476) | 0.007 |
| Comorbidities | ||||
| Dialysis vintage (year) | 8.1(5.1-9.6) | 6.8(4.6-8.4) | 6.8(4.5-8.3) | 0.05 |
| Diabetes mellitus (%) | 68 (67%) | 49 (48%) | 32 (31%) | <0.0001 |
| Hypertension (%) | 95 (94%) | 93 (92%) | 94 (92%) | 0.6 |
| Atherosclerotic diseaseb (%) | 53 (52%) | 34 (33%) | 22 (22%) | <0.0001 |
| Coronary artery disease*,c (%) | 22 (22%) | 21 (21%) | 16 (16%) | 0.2 |
| Peripheral artery diseased(%) | 35 (35%) | 11 (11%) | 5 (5%) | <0.0001 |
| Lower extremity amputation (%) | 13 (13%) | 2 (2%) | 4 (4%) | 0.01 |
| Ever smoked tobacco (%) | 35 (35%) | 33 (32%) | 44 (43%) | 0.2 |
| Measures of Physical Function | ||||
| Karnofsky score (at listing) | 80 (70-90) | 80 (80-90) | 80 (80-90) | 0.0007 |
| STS result (repetitions)* | 11 (0-14) | 17 (14-21) | 21 (19-26) | <0.0001 |
| Assistive walking device (%) | <0.0001 | |||
| None | 51 (50%) | 95 (93%) | 101 (99%) | |
| Cane/Walker | 45 (45%) | 6 (6%) | 1 (1%) | |
| Prosthesis | 1 (1%) | 1 (1%) | 0 (0%) | |
| Wheelchair | 4 (4%) | 0 (0%) | 0 (0%) | |
| Baseline Characteristic | STS Result in Number of Repetitions | p | ||
| 0-13 | 14-20 | 21-54 | ||
| N=103 | N=111 | N=90 | ||
| Demographics and Follow-Up | ||||
| Age (year) | 62 (55-67) | 54 (47-61) | 55 (44-63) | <0.001 |
| Sex (% female) | 58 (56%) | 49 (44%) | 35 (39%) | 0.01 |
| Race/ethnicity (%) | ||||
| White | 20 (19%) | 13 (12%) | 15 (17%) | 0.3 |
| Black | 13 (13%) | 4 (4%) | 5 (6%) | |
| Hispanic, non-black | 46 (45%) | 53 (48%) | 43 (48%) | |
| Asian | 18 (17%) | 36 (32%) | 24 (27%) | |
| Other/Mixed | 6 (6%) | 5 (5%) | 3 (3%) | |
| Time from listing (years) | 6.0(4.1-8.2) | 6.1(3.7-8.4) | 6.1(4.1-7.5) | 0.29 |
| Follow-up timea (days) | 281 (35-534) | 352 (148-492) | 499 (294-494) | 0.02 |
| Comorbidities | ||||
| Dialysis vintage (year) | 7.1(4.9-9.0) | 7.7(5.5-9.4) | 6.6(4.1-8.3) | 0.1 |
| Diabetes mellitus (%) | 62 (60%) | 50 (45%) | 37 (41%) | 0.007 |
| Hypertension (%) | 97 (94%) | 98 (88%) | 86 (96%) | 0.8 |
| Atherosclerotic diseaseb (%) | 54 (52%) | 36 (32%) | 19 (21%) | <0.0001 |
| Coronary artery disease*,c (%) | 25 (24%) | 22 (20%) | 12 (13%) | 0.06 |
| Peripheral artery diseased(%) | 26 (25%) | 17 (15%) | 8 (9%) | 0.002 |
| Lower extremity amputation (%) | 11 (11%) | 5 (5%) | 3 (3%) | 0.04 |
| Ever smoked tobacco (%) | 34 (33%) | 41 (37%) | 36 (40%) | 0.3 |
| Measures of Physical Function | ||||
| Karnofsky score (at listing) | 80 (70-90) | 80 (70-90) | 90 (80-90) | 0.006 |
| 6MWT result (meters) | 274 (137-362) | 396 (340-447) | 457 (413-499) | <0.0001 |
| Assistive walking device (%) | <0.0001 | |||
| None | 53 (51%) | 102 (92%) | 88 (98%) | |
| Cane/Walker | 45 (44%) | 9 (8%) | 2 (2%) | |
| Prosthesis | 1 (1%) | 0 (0%) | 0 (0%) | |
| Wheelchair | 4 (4%) | 0 (0%) | 0 (0%) | |
| Baseline Characteristic | EPTS Category | p | ||||
|---|---|---|---|---|---|---|
| 0-19 | 20-39 | 40-59 | 60-79 | 80-100 | ||
| N=32 | N=52 | N=39 | N=61 | N=121 | ||
| Demographics and Follow-Up | ||||||
| Age (year) | 35 (26-40) | 47 (44-52) | 56 (47-59) | 54 (51-64) | 64 (60-69) | <0.0001 |
| Sex (% female) | 15 (47%) | 26 (50%) | 17 (44%) | 28 (46%) | 56 (46%) | 0.8 |
| Race/ethnicity (%) | ||||||
| White | 3 (9%) | 9 (17%) | 8 (21%) | 8 (13%) | 20 (17%) | 0.5 |
| Black | 1 (3%) | 4 (8%) | 4 (10%) | 6 (10%) | 7 (6%) | |
| Hispanic, non-black | 20 (63%) | 31 (60%) | 15 (38%) | 27 (44%) | 50 (41%) | |
| Asian | 7 (22%) | 8 (15%) | 10 (26%) | 18 (30%) | 35 (29%) | |
| Other/Mixed | 1 (3%) | 0 | 2 (5%) | 2 (3%) | 9 (7%) | |
| Time from listing (years) | 5.3 (2.8-8.0) | 6.8 (3.9-9.0) | 6.1 (4.3-6.9) | 5.8 (3.3-7.8) | 6.2 (4.4-8.4) | 0.1 |
| Follow-up timea (days) | 310(163-451) | 400(146-568) | 470(392-612) | 339(209-472) | 273(76-486) | 0.06 |
| Comorbidities | ||||||
| Dialysis vintage (year) | 6.2 (2.6-8.1) | 8.2 (4.5-10.0) | 6.7 (5.8-8.3) | 6.7 (4.6-8.3) | 7.8 (6-9.6) | 0.009 |
| Diabetes mellitus (%) | 0 (0%) | 3 (6%) | 10 (26%) | 37 (61%) | 99 (82%) | <0.0001 |
| Hypertension (%) | 30 (94%) | 42 (81%) | 39 (100%) | 57 (93%) | 114 (94%) | 0.1 |
| Atherosclerotic diseaseb (%) | 2 (6%) | 10 (19%) | 10 (26%) | 21 (34%) | 66 (55%) | <0.0001 |
| Coronary artery disease*,c (%) | 0 (0%) | 5 (10%) | 8 (21%) | 11 (18%) | 35 (29%) | <0.0001 |
| Peripheral artery diseased(%) | 0 (0%) | 5 (10%) | 2 (5%) | 14 (23%) | 30 (25%) | <0.0001 |
| Lower extremity amputation (%) | 0 (0%) | 2 (4%) | 1 (3%) | 2 (3%) | 14 (12%) | 0.008 |
| Ever smoked tobacco (%) | 8 (25%) | 17 (33%) | 18 (46%) | 22 (36%) | 47 (39%) | 0.2 |
| Measures of Physical Function | ||||||
| Karnofsky score (at listing) | 90 (80-100) | 90 (80-90) | 90 (80-90) | 80 (70-90) | 80 (70-90) | 0.0002 |
| 6MWT result (meters) | 454 (390-457) | 444 (399-488) | 425 (388-473) | 367 (305-445) | 324 (238-400) | <0.0001 |
| STS result (repetitions)* | 21 (16-25) | 20 (15-25) | 20 (15-23) | 17 (12-20) | 14 (9-20) | <0.0001 |
| Assistive walking device (%) | 0.02 | |||||
| None | 30 (94%) | 45 (87%) | 35 (90%) | 45 (74%) | 92 (76%) | |
| Cane/Walker | 2 (6%) | 5 (10%) | 4 (10%) | 15 (25%) | 26 (21%) | |
| Prosthesis | 0 (0%) | 0 (0%) | 0 (%) | 0 (%) | 2 (2%) | |
| Wheelchair | 0 (0%) | 2 (4%) | 0 (0%) | 1 (2%) | 1 (1%) | |
Missing fewer than 1% of the data.
Follow-up time: Time between 1st TRAC visit (and ascertainment of all baseline characteristics) and date of censoring.
Atherosclerotic disease: A history of clinically significant coronary artery disease (see below), peripheral artery disease (see below), or cerebrovascular disease (ischemic cerebrovascular accident or revascularization).
Coronary artery disease: A history of acute coronary syndrome or coronary revascularization.
Peripheral artery disease: A history of revascularization, amputation, or clinical claudication with confirmed diagnosis.
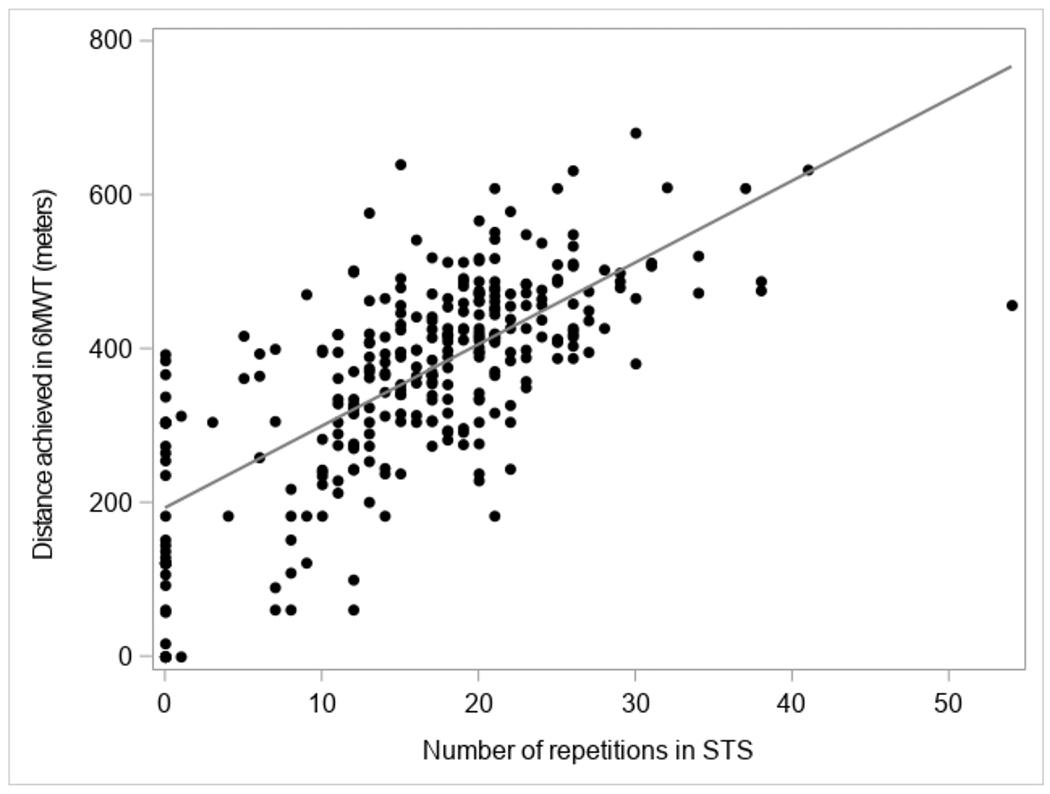
Figure 1 depicts the distribution of the 6MWT results and its relation to the STS results. We plotted it alongside the corresponding metabolic equivalence of tasks (METs), calculated by the formula MET = (6MWT/210) + 1, and the equivalent activities of daily living21. The median 6MWT result was 393 meters (25th-75th percentile range 305-455), corresponding to 2.9 METs, and a physical activity level between the “physical care for children” and a “brisk walk”. The median STS result was 17 repetitions (25th-75th percentile range 12-21). We observed a direct correlation between the two physical performance test results, with R2 values of 0.49 for the STS and the 6MWT. Figure S2 depicts the distribution of STS results.
Figure 1.
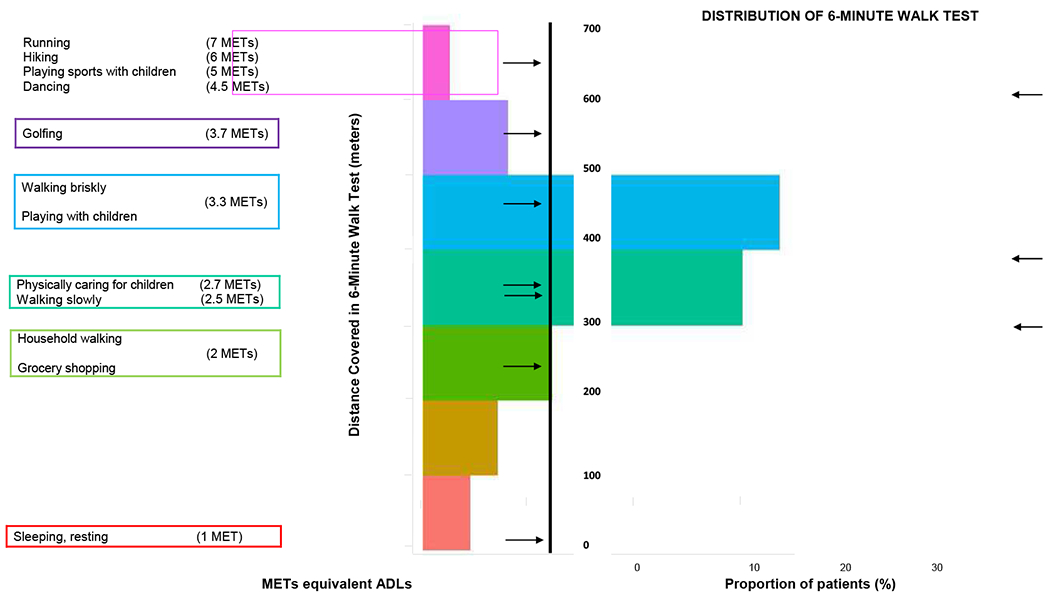
Distribution of the 6MWT results from patients’ 1st TRAC evaluation (right) relative to everyday activities (left), and STS results (bottom). 6MWT: 6-minute walk test. STS: Sit-to-stand test. *Reference mean 6MWT results in healthy adults22, adults with chronic obstructive pulmonary disease23 (mean age 63 years), and frail elderly individuals24 (mean age 70 years).
Of the 305 patients, 103 (34%) had at least one instance of repeat testing. On repeat testing, 6MWT remained unchanged (within 50 meters of initial result) in 60% of patients, while 22% experienced an increase of >50 meters and 18% experience an decrease of >50 meters. On repeat testing, STS remained unchanged (within 5 repetitions of initial result) in 63% of patients, while 28% experienced an increase of >5 repetitions and 9% experience an decrease of >5 repetitions.
Seven (2%) patients had 6MWT results of 0: four were wheelchair-bound, one had a recent below-the-knee amputation, and two ambulated with cane and walkers and declined testing due to fatigue. One patient with the recent amputation was able to perform STS (12 repetitions); the other six patients were unable to perform STS. Within this subgroup, one patient (initially wheelchair-bound) improved with aggressive physical therapy and underwent a successful transplant, while the rest either died (n=1) or were removed from the waitlist (n=5).
Association of physical performance test results with waitlist outcomes:
Over a median follow-up period of 362 days, 58 patients were removed from the waitlist for comorbidities, 15 died on the waitlist, 104 transplanted, and 128 were alive on the waitlist with survival time censored. Reasons for waitlist removal were cardiopulmonary disease (59%), non-cardiovascular disease (52%), frailty and poor physical function (48%), and vascular disease (41%) (Table S1).
The median 6MWT results were 305 meters (25th-75th percentile 183-367) for patients who died, 244 meters (25th-75th percentile 122-328) for patients removed from the waitlist, 419 meters (25th-75th percentile 370-472) for transplanted patients, and 403 meters (25th-75th percentile 335-469) for patients alive on the waitlist with survival time censored. The median STS results were 14 repetitions (25th-75th percentile 9-19) for patients who died, 10 repetitions (25th-75th percentile 0-15) for patients removed from the waitlist, 20 repetitions (25th-75th percentile 16-23) for transplanted patients, and 18 (25th-75th percentile 13-21) for patients alive on the waitlist with survival time censored.
Results of the 6MWT and STS tests were negatively associated with waitlist removal or death and positively associated with kidney transplant. Relative risks of death or waitlist removal for patients in the lower and middle tertiles of physical performance were significantly higher than those in the top tertile, and likelihood of transplant significantly lower (Figure 2). Inspection of Schoenfeld residuals demonstrated no evidence that the proportionality assumption was violated. Adjustment for covariates did not attenuate the effect size (Table 2). Each 50-meter lower 6MWT result was associated with an increased risk of death or waitlist removal (adjusted subdistribution hazard ratio (sHR) 1.42 [95% C.I. 1.30-1.56]) and decreased likelihood of transplant (adjusted sHR 0.80 [95% C.I. 0.72-0.88]). Each 5-repetition lower in the STS result was associated with an increased risk of death or waitlist removal (adjusted sHR 1.53 [95% C.I. 1.33-1.75]) and decreased likelihood of transplant (adjusted sHR 0.80 [0.71-0.89]). In the sensitivity analysis examining the association between 6MWT and STS results and death, each 50-meter lower 6MWT was associated with an increased risk of death (adjusted sHR 1.29 [1.06-1.58]. The association of death with STS approached but did not reach statistical significance (adjusted sHR 1.23 [0.92-1.66] for every 5-repetition decrease).
Figure 2.
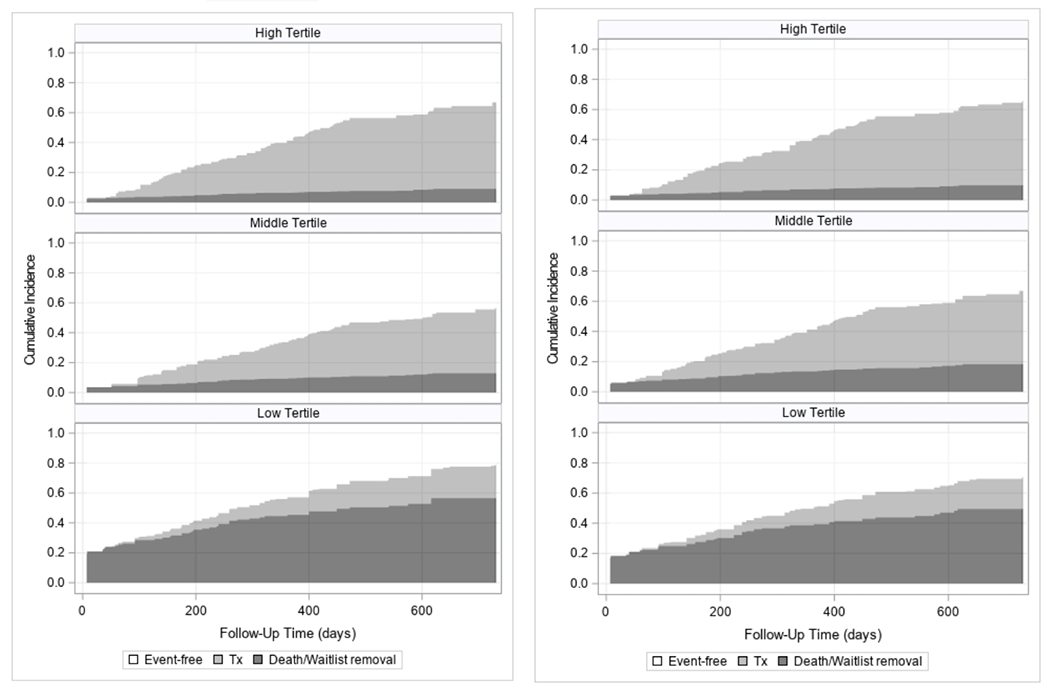
Stacked cumulative incidence plots of time (in days) to 1) waitlist removal or death (dark grey) and 2) transplant (light grey), stratified by 6MWT (left) and STS (right) results at the patients’ 1st TRAC evaluation. 6MWT: high tertile 426-681; middle tertile 334-425; low tertile 0-329. STS: high tertile 21-54; middle tertile 14-20; low tertile 0-13.
Table 2.
Association between physical performance measures and time to outcome in a competing risk framework (subdistribution hazard ratios). 6MWT: 6 minute walk test. STS: Sit to stand test.
| Physical Performance Measure | N | Waitlist Removal or Death | Transplant | ||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| 6MWT per 50m lower | 305 | 1.43 (1.31-1.55) | 1.42 (1.30-1.56) | 0.82 (0.76-0.89) | 0.80 (0.72-0.88) |
| STS per 5-rep lower | 304 | 1.63 (1.43-1.86) | 1.53 (1.33-1.75) | 0.80 (0.72-0.88) | 0.80 (0.71-0.89) |
Relation between clinical characteristics and physical performance test results (Table S2):
When clinical characteristics were approximated by EPTS categories, results of the 6MWT and STS test were inversely related to EPTS. A second model, approximating clinical characteristics by demographic and comorbidities (age, sex, dialysis vintage, diabetes mellitus, and known atherosclerotic disease), performed better than the model containing only EPTS. The proportions of variance in 6MWT and STS scores explained by the comorbidities noted above were 0.21 and 0.14, respectively.
How physical performance testing enhances risk assessment:
Figure 3 depicts the AICs of different models examining time to death or removal from waitlist. The family of models using demographics and comorbidities to approximate clinical characteristics (circle) outperformed the family of models using EPTS to approximate clinical characteristics (triangles). The addition of either STS or 6MWT to clinical characteristics enhanced model fit (likelihood ratio test p<0.001) compared to models without physical performance test results. While there was not complete concordance in the STS and 6MWT results, the addition of STS to a model already containing clinical characteristics + 6MWT did not significantly enhance model fit.
Figure 3.
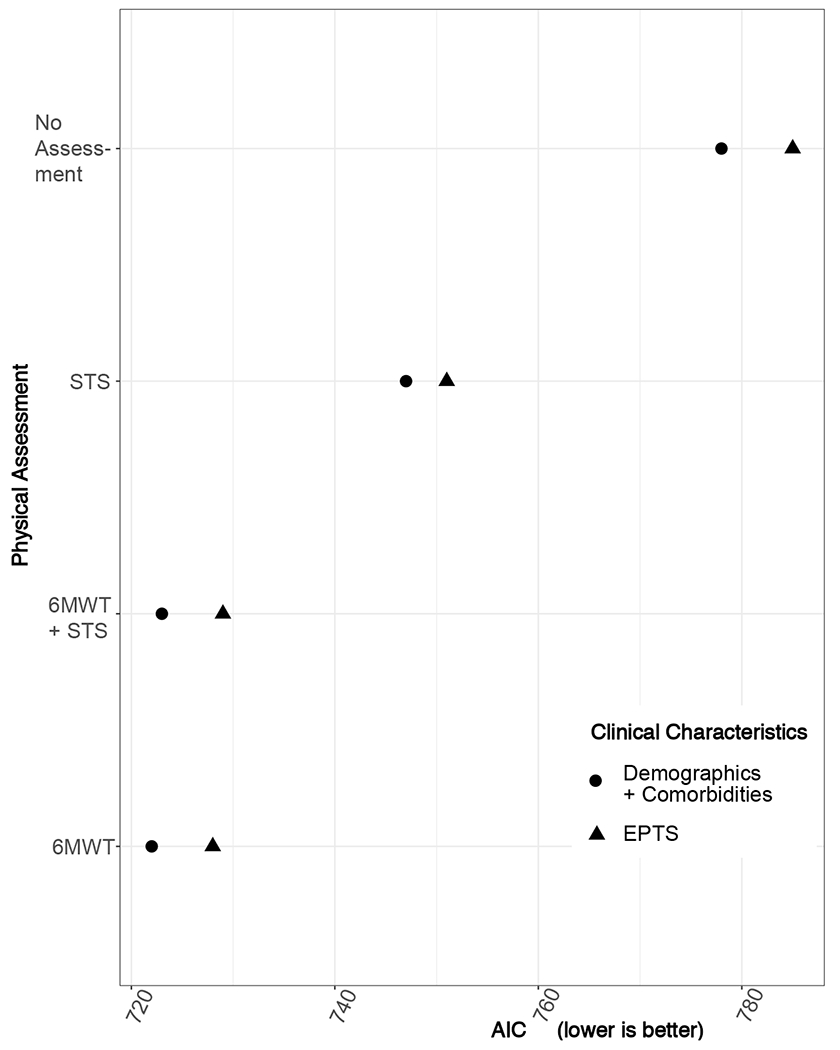
Comparison of AICs among different survival models. For each set of models, we tested clinical characteristics only and clinical characteristics plus physical performance metrics. Clinical characteristics are modelled as either demographics (age, sex) + comorbidities (dialysis vintage, diabetes mellitus, and known atherosclerotic disease) or EPTS. Lower AIC (x-axis) indicates better model fit.
Implementation of physical performance testing:
In a convenience sample of 42 consecutive patients, the time for administering the 6MWT and STS test in the clinic were 9.9±1.6 and 2.7±1.1 minutes, respectively.
Discussion
In this cohort of kidney transplant candidates, we demonstrated how to incorporate two physical performance measures, the 6MWT and the STS, into the comprehensive medical assessment of the deceased donor kidney transplant candidate near the top of the waitlist. Our study adds to existing literature in several ways. First, we focused on physical performance metrics commonly accepted in the physical medicine and rehabilitation literature. Showing that they are closely associated with waitlist outcomes begs the question: will prehabilitation and physical therapy alter the trajectory for at-risk transplant candidates? Second, rather than assessing physical performance at the time of transplantation or initial transplant evaluation, we tested physical performance at a transplant readiness visit close to the anticipated time of transplant, a timepoint which likely represents the best pretransplant point to intervene to improve posttransplant outcomes. Our main findings show that physical performance tests, when performed in patients with high KAS at the top of the waitlist, are 1) portable and implementable; 2) associated with waitlist outcomes; and 3) contain information beyond what is currently collected. Our overarching goal is to offer new possibilities in pretransplant clinic which may be objective and more adaptable to prehabilitation.
This study encompasses our real-life experiences with implementation and resource utilization. We trained two nurse coordinators to perform the testing outlined herein. Both the 6MWT and STS evaluations were completed within 15 minutes, even in patients who did not speak English, and results were available in real-time for patient counselling. These findings suggest that the 6MWT and STS can be reliably incorporated into the pretransplant evaluation at a modest cost.
Both measures of physical performance yield information relevant to risk stratification beyond what is routinely collected. Age, sex, comorbidities, and EPTS score explain a relatively low proportion (<20%) of variance in physical performance measures. Adding physical performance test results to the survival model significantly improves the prediction of adverse waitlist outcomes. Taken together, these observations suggest that physical performance measures contain information beyond traditional clinical characteristics, and may be used to refine risk stratification in the clinical setting, enabling more individualized patient counselling and shared decision-making when transplant is imminent.
Assessing physical performance at the top of the waitlist may help identify patients in need of prehabilitation. Prehabilitation has been shown to be efficacious in the setting of cardiothoracic and abdominal surgeries22. A recent single-arm, uncontrolled pilot study reported preliminary results of prehabilitation in kidney transplant candidates and found a modest improvement in physical activity based on accelerometry23. The objective and quantitative nature of the STS and 6MWT measurements, along with their strong association with adverse waitlist outcomes, allow for establishing patient goals and tracking progress. Furthermore, the ease of administering STS and 6MWT may decrease bias in selection of transplant candidates for prehabilitation.
Regarding the choice between 6MWT and STS, our data hints at 6MWT being a more holistic marker. The association between 6MWT and death was slightly more robust than that between STS and death. Adding 6MWT to clinical characteristics also resulted in the best model fit (Figure 3). The 6MWT includes an assessment of cardiorespiratory fitness9,10 and is already widely used in heart and lung transplantation13. Practically, more patients were able to perform the 6MWT than the STS, possibly because ambulation (with aid if needed) is less demanding of joint movements and balance than the STS. For these reasons, of the two metrics, 6MWT may be an overall more useful test.
Our data were derived from a single center, representing an important limitation. While our population was quite diverse in terms of race, ethnicity, comorbidity burden, and primary language, they are not representative of the whole country. Furthermore, we only studied a subset of our waitlist population, those candidates with the highest KAS and, by definition, longer dialysis duration and/or high sensitization status. Nationally, waitlisted candidates are becoming older and sicker, as reflected in rising proportion of diabetes and long dialysis duration24. This snapshot of patients at the top of our waitlist may therefore be a harbinger of what is to come for other transplant programs. That we were able to implement the 6MWT and STS in our population where nearly half of the patients’ preferred language was not English is promising for its portability to other programs.
Additional limitations exist. Due to the long distance that many of our patients had to travel, we were unable to do a formal pilot study to address operator variability. In our study, we limited physical performance testing to two operators, and multiple studies have validated the high reliability of the 6MWT and STS25,26. A further limitation is that the primary outcome, removal from waitlist or death, is not completely disentangled from the exposure, physical performance assessment. As such, some may argue that physical performance assessments merely confirm the evaluating physician’s suspicion that the patient is ill-suited for transplant. Partly mitigating this concern are more modest, but statistically significant, associations between the exposure and transplantation and death on the waitlist. While frailty, or poor physical function, was cited as a reason in ~50% of patients removed from the waitlist, it was not the sole season in any patient. Indeed, the inability to disentangle adverse outcome from physician’s knowledge of the exposure is a limitation common to most research in this area. Our objective is to establish physical performance assessments that can be adapted to clinical settings, rather than limited to research settings. Our results therefore represent a real-life scenario in which physical assessments were incorporated into routine pretransplant care.
In summary, we report our experience in applying two physical performance tests to risk-stratify patients at the top of the deceased donor kidney transplant waitlist. Our results demonstrate that the physical performance testing yields valuable information beyond clinical characteristics or EPTS. Given their ease of implementation and quantitative nature, we suggest that the 6MWT and/or STS be incorporated for patient counselling and preparation pretransplant and as an intermediate outcome of prehabilitation programs.
Supplementary Material
Figure S1. Workflow in the Transplant Readiness and Assessment Clinic.
Figure S2. Distribution of sit-to-stand results (N=304).
Table S1. Reasons for waitlist removal.
Table S2. Association of clinical characteristics and measured physical performance (6MWT and STS results).
Acknowledgements:
We would like to acknowledge Lynn M. Clinton for her administrative and database assistance.
Support: Research reported here was supported by the John M. Sobrato Gift Fund (J.C.T.) and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K24 DK085446 (G.M.C.). The funders had no role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Prior Presentation: Aspects of this work were presented in poster form the World Congress of Nephrology, Melbourne Australia, April 2019, and as oral presentations at the American Transplant Congress, Boston MA, June 2019 and American Society of Nephrology Kidney Week, Washington DC, November 2019.
References:
- 1.Cheng XS, Lentine KL, Koraishy F, Myers J, Tan JC. Implications of frailty for peritransplant outcomes in kidney transplant recipients. Curr Transpl Rep. 2019;6(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haugen CE, Chu NM, Ying H, et al. Frailty and access to kidney transplantation. Clin J Am Soc Nephrol. 2019;14:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAdams-DeMarco MA, Van Pilsum Rasmussen S, Chu NM, et al. Perceptions and practices regarding frailty in kidney transplantation: Results of a national survey. Transplantation. 2020;104(2):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15(1):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haugen CE, Agoons D, Chu NM, et al. Physical Impairment and Access to Kidney Transplantation. Transplantation. 2019;104(2):367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nastasi AJ, McAdams-DeMarco MA, Schrack J, et al. Pre-Kidney Transplant Lower Extremity Impairment and Post-Kidney Transplant Mortality. Am J Transplant. 2018;18(1):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu NM, Deng A, Haugen CE, Segev DL, McAdams-DeMarco MA. Dynamic Frailty before Kidney Transplantation—Time of Measurement Matters. Transplantation. 2019;103(8):1700–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers JN, Fonda H. The Impact of Fitness on Surgical Outcomes: The Case for Prehabilitation. Curr Sports Med Rep. 2016;15(4):282–289. [DOI] [PubMed] [Google Scholar]
- 9.Ross RM, Murthy JN, Wollak ID, Jackson AS. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross R, Blair SN, Arena R, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134(24):e653–e699. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Zheng D, Zhang L, et al. Six-minute walk test predicts all-cause mortality and technique failure in ambulatory peritoneal dialysis patients. Nephrol. 2017;22(2):118–124. [DOI] [PubMed] [Google Scholar]
- 12.Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int. 2004;65(2):719–724. [DOI] [PubMed] [Google Scholar]
- 13.Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19(4):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng XS, Busque S, Lee J, et al. A New Approach to Kidney Waitlist Management in the Kidney Allocation System Era: Pilot Implementation and Evaluation. Clin Transplant. September 2018:e13406. [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assocation. 94(446):495–509. [Google Scholar]
- 16.Zhang D A coefficient of determination for generalized linear models. Am Stat. 2017;71(4):310–316. [Google Scholar]
- 17.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data (Statistics for Biology and Health). 2nd ed. Springer; 2005. [Google Scholar]
- 18.Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Card Rehabil. 2001;21(2):87–93. [DOI] [PubMed] [Google Scholar]
- 19.Polkey M, Spruce M, Edwards L, et al. Six-minute-walk test in chronic obstructive pulmonary disease: Minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 187(4):382–386. [DOI] [PubMed] [Google Scholar]
- 20.Gremeaux V, Troisgros O, Benaim S, et al. Determining the minimal clinically important difference for the six-minute walk test and the 200-meter fast-walk test during cardiac rehabilitation program in coronary artery disease patients after acute coronary syndrome. Arch Phys Med Rehabil. 2011. ;92(4):611–619. [DOI] [PubMed] [Google Scholar]
- 21.Glass S, Dwyer GB. American College of Sports Medicine’s Metabolic Calculations Handbook. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 22.Cheng XS, Myers JN, Chertow GM, et al. Prehabilitation for kidney transplant candidates: Is it time? Clin Transplant. 2017;31(8):e13020. [DOI] [PubMed] [Google Scholar]
- 23.McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, et al. Prehabilitation prior to kidney transplant: Results from a pilot study. Clin Transplant. 2019;33(1):e13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Kidney. Am J Transplant. 20(S1):20–130. [DOI] [PubMed] [Google Scholar]
- 25.Segura-Orti E, Martinez-Olmos F. Test-retest reliability and minimal detectable change scores for sit-to-stand-to-sit tests, the six-minute walk test, the one-leg heel-rise test, and handgrip strength in people undergoing hemodialysis. Phys Ther.2011. ;91(8):1244–1252. [DOI] [PubMed] [Google Scholar]
- 26.Crook S, Busching G, Schultz K, et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur Respir J. 2017;49:1601871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Workflow in the Transplant Readiness and Assessment Clinic.
Figure S2. Distribution of sit-to-stand results (N=304).
Table S1. Reasons for waitlist removal.
Table S2. Association of clinical characteristics and measured physical performance (6MWT and STS results).


