Abstract
Exposure to lead (Pb) is linked to a host of adverse health effects. Recent revelations of unmonitored exposures to hazardous levels of lead as seen in the Flint Water Crisis have manifested a need for evaluating biological samples, other than regularly prepared whole blood, for biomonitoring of lead exposure. Here, we present a method utilizing clotted erythrocyte fraction samples, which are commonly archived along with serum (or plasma) in biorepositories, to predict whole blood lead levels to allow for retrospective assessments of environmental exposure to lead. Whole blood and clotted erythrocyte fraction samples were collected from 91 participants in the Airborne Exposure to Semivolatile Organic Pollutants (AESOP) cohort study of mother-child dyads. Clotted erythrocyte fraction samples were prepared either using microwave assisted acid digestion or alkaline dilution and subsequently analyzed for lead using Inductively-couple plasma-mass spectrometry (ICP-MS). Simultaneously withdrawn whole blood samples were also analyzed. A strong linear relationship was observed between lead in whole blood and clotted erythrocyte fraction with Pearson correlation coefficients (r) of 0.90, and 0.89 (p<0.001) for acid digestion and alkaline dilution, respectively. Slopes and intercepts from simple linear regression models of the two clotted erythrocyte fraction methods were not found to be significantly different (p>0.05) when compared to the standard blood lead assay method. Modeled blood lead predicted from clotted erythrocyte fraction was evaluated at a test threshold of 3 μg/dL was found to have diagnostic sensitivity of 88% and specificity of 100%. Results from this study demonstrate clotted erythrocyte fraction samples are a viable alternative biological sample for retrospective public health surveillance of environmental exposure to lead.
Keywords: Biomonitoring, Blood lead levels, Clotted erythrocyte fraction, Exposure assessment, Red blood cell, Whole blood
Graphical Abstract
1. Introduction
Lead (Pb) is a ubiquitous heavy metal that has been widely used since the ancient Romans. In modern time, it’s unique physical and chemical properties have made it a commonly used heavy metal which was extensively mined, smelted, and found in food, water, medicines, cosmetics, paint, used for bullets, and also as fuel additive to reduce engine knocking. These uses combined with long persistence have increased environmental and occupational exposure and biological uptake of this nonessential metal. Exposure to lead is well documented to be linked to a wide range of adverse neurological1, renal2, hematological3, endocrine4, gastrointestinal5, reproductive6,7, and developmental8,9 effects with children being a sensitive population at an elevated risk10.
Efforts to curtail lead exposure were made in the U.S. during the 1970s and 80s with the ban of lead in paint, gasoline, and can solder. This led to a nationwide reduction, but not elimination, of toxic blood lead levels11,12,13. This is important to note since there is no known safe level of Pb exposure. Lead levels as low as 5 μg/dL, the current Center for Disease Control and Prevention (CDC) reference value, were once thought to be “safe” but have been shown to be associated with decreased intelligence and learning and behavioral difficulties in children14,15. Thus, it is imperative from a public health perspective to expand programs for the surveillance of environmental exposure to lead.
The most common method utilized for biomonitoring lead exposure in both research and clinical settings is by measuring the concentration of lead in a whole blood sample. Studies have reported that up to 98% of the lead in a whole blood sample can be found in the erythrocyte fraction due to the high binding affinity of lead for hemoglobin16,17 while only a small fraction is present in serum. For retrospective assessment of lead exposure, the question arises whether the lead analysis in erythrocyte fraction or in inadequately prepared or stored blood samples may correspond adequately with the levels of regular whole blood analysis.
This knowledge gap is important to address due to recent revelations of unmonitored exposures to hazardous levels of lead globally as best exemplified in the Flint Water Crisis18, and more recently in Paris at Notre Dame19. These unfortunate calamities not only highlight that lead exposure remains a public health issue, but also demonstrates a need for expanded lead surveillance including from sample biorepositories to retrospectively assess environmental exposure to lead using archived clotted erythrocyte fraction samples. Archived samples may provide valuable longitudinal information regarding lead exposures in populations, especially if previously unrecognized lead exposure is discovered within a community. Retrospective analyses could provide insight into public health challenges communities may face years after exposures, allowing for proactive testing, interventions and where appropriate, compensation.
Here, we present a simple and robust method for measuring concentration of lead in archived clotted erythrocyte fraction samples using inductively coupled plasma mass spectrometry (ICP-MS). Two methods for preparing clotted erythrocyte fraction samples; acid digestion and alkaline dilution, were evaluated. The sample preparation time and effort are significantly lower for alkaline dilution compared to acid digestion. In addition, exposure of laboratory staff to hazardous substances such as concentrated acids, and acid fumes is reduced. Results from a comparison of whole blood and clotted erythrocyte fraction, as well as clotted erythrocyte fraction preparation methods is presented.
2. Materials and methods
2.1. Cohort sample collection, preservation, and storage
Samples were collected by bilingual field staff from participants of the Airborne Exposure to Semi-volatile Organic Pollutants (AESOP) Study which has been tracking mother-child dyads in East Chicago, IN and Columbus Junction, IA since 2008 to study exposures to persistent environmental pollutants. The AESOP Study design involved enrollment of children from middle schools and their mothers. Multiple children from the same mother were allowed to be enrolled if they met inclusion criteria20. Most children were 14 years of age at enrollment. All protocols were approved by the University of Iowa’s Institutional Review Board (IRB). Written consent and assent along with administered questionnaires were obtained in English or in Spanish from all participants as previously described20. Blood samples utilized for this study were collected in participants homes between April 2017 and March 2019. The demographics of AESOP Study participants contributing biospecimens for this analysis are shown in Table 1.
Table 1.
Demographics of the AESOP Study participants in East Chicago who provided blood for this study.
| Adult Women (Mothers) | ||||||
|---|---|---|---|---|---|---|
| 2017-2018 | 2018-2019 | |||||
| Characteristics | % | n | % | n | ||
| Total | - | 25 | - | 20 | ||
| Demographic | ||||||
| Mean Age, Years | 30-39 | 20 | 5 | 15 | 3 | |
| 40-49 | 48 | 12 | 50 | 10 | ||
| 50-59 | 32 | 8 | 35 | 7 | ||
| Race/Ethnicity | Hispanic | 72 | 18 | 75 | 15 | |
| Black | 16 | 4 | 15 | 3 | ||
| Non-Hispanic White | 8 | 2 | 10 | 2 | ||
| Education Level | 8th Grade or Less | 36 | 9 | 40 | 8 | |
| Some High School | 8 | 2 | 10 | 2 | ||
| High School Diploma/GED | 36 | 9 | 30 | 6 | ||
| Some College, No Degree | 16 | 4 | 15 | 3 | ||
| Two-Year Degree (AA/AS) | 4 | 1 | 5 | 1 | ||
| Average Yearly | Less than $19,999 | 44 | 11 | 50 | 10 | |
| Income | Greater Than $19,999 | 56 | 14 | 50 | 10 | |
| Children | ||||||
| 2017-2018 | 2018-2019 | |||||
| Characteristics (male/female) | % | n | % | n | ||
| Total | - | 26 (11/15) | - | 20 (6/14) | ||
| Demographic | ||||||
| Mean Age, Years | Under 18 | 31 | 8 (8/0) | 25 | 5 (5/0) | |
| 18-20 | 12 | 3 (2/1) | 10 | 2 (0/2) | ||
| 21+ | 57 | 15 (1/14) | 65 | 13 (12/1) | ||
| Race/Ethnicity | Hispanic | 73 | 19 (8/11) | 75 | 15 (4/11) | |
| Black | 19 | 5 (2/3) | 15 | 3 (2/1) | ||
| Non-Hispanic White | 8 | 2 (1/1) | 10 | 2 (1/1) | ||
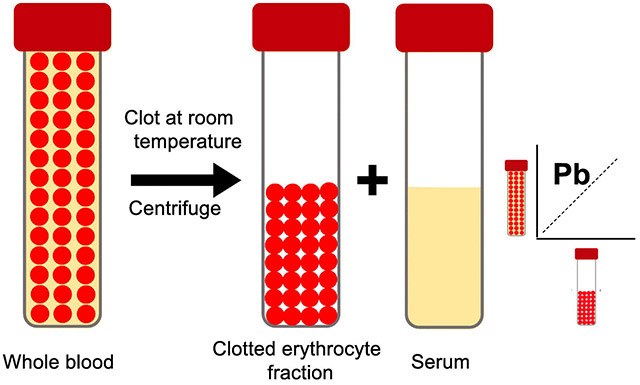
Blood samples were collected using venipuncture into evacuated red top tubes with no preservatives (BD Vacutainer, Model No. 366430), and a tan top tube coated with K2EDTA (BD Vacutainer, Model No. 367855) for whole blood. The tubes were placed in a cooler at 0°C and transported to our nearby field office. There, red top tubes were allowed to clot at room temperature for 30 min, then centrifuged at 3000 g for 10 min, followed by aliquoting of the serum, leaving behind the clotted erythrocyte fraction. Samples were then shipped, frozen on dry ice, to the Human Toxicology and Exposomics Laboratory (HTEL) at the University of Iowa for storage in our biorepository prior to analysis.
2.2. Reagents and standards
All vials and containers used in this study were acid washed in 10% HCl solution overnight and left to dry in a Class II biosafety cabinet equipped with positive pressure. All reagents were of trace metal grade or better. Concentrated HNO3 and HCl were purchased from Fisher Scientific. Deionized water (resistance ≥ 18.2 MΩ*cm) was dispensed from a water purification system (Model. GenPure Pro UV-TOC/UF, Thermo Scientific). Alkaline diluent solution was comprised of 0.4% (v/v) tetramethyl ammonium hydroxide (TMAH, Alfa Aesar), 1% (v/v) ethanol (Sigma-Aldrich), 0.01%(w/v) ammonium pyrrolidine dithiocarbamate (APDC, Alfa Aesar), 0.05% (v/v) Triton X-100™ (Sigma-Aldrich). National Institute of Standards and Technology (NIST) traceable single and multielement stock solutions were used for preparing internal standards, and calibration standard solutions were purchased from Inorganic Ventures (Christiansburg, VA).
2.3. Acid digestion
To date, there is little information in the literature on procedures for acid digestion of clotted erythrocyte fraction samples. Massanyi et al. investigated levels of lead and other trace metals in whole blood, serum, and blood clots within horses, however lacked crucial details on the procedure and method validation21. Thus, we modified EPA Method 3051 as follows. A tube (~4 mL) of clotted erythrocyte fraction was weighed and transferred to a pre-weighed Teflon digestion vessel containing 9 mL of concentrated HNO3 and 1 mL of 30% H2O2. Samples were then acid digested using a Milestone ETHOS UP (Sorisole, Italy) microwave digestion system with a ramp set to 210°C for 20 min, and a hold at 210 °C for 15 min. Samples were then diluted with 40 mL of deionized water. The digestion vessel mass was recorded after digestion to obtain a final solution mass. Samples were then diluted 10-fold in 2% HNO3 containing 10 μg/L of 89yitrium, and 209bismuth as internal standards. The concentrations of trace metals in clotted erythrocyte fraction samples were expressed as volume by converting the final recorded mass using a previously reported22 density of 1.08×103 kg/m3.
2.4. Alkaline dilution
Whole blood and clotted erythrocyte fraction samples were prepared by alkaline dilution using a method23 developed by the Division of Laboratory Sciences at the Centers for Disease Control and Prevention (Method No. DLS-3040.1-01). Briefly, 100 μL aliquot of either whole blood or clotted erythrocyte fraction was diluted 50-fold in a solution of 0.4% (v/v) tetramethyl ammonium hydroxide (TMAH), 1% (v/v) ethanol, 0.01% (w/v) ammonium pyrrolidine dithiocarbamate (APDC), 0.05% (v/v) Triton X-100™, with 5 μg/L of 103rhodium, 130tellerium, and 193iridium as internal standards. A lab-pooled whole blood sample was utilized for creating matrix matched calibration standards. The homogeneity of the variance among aliquots of the lab-pooled sample was assessed using Levene's test (P-value > 0.05 indicated no statistically significant difference among aliquots).
2.5. ICP-MS Quality Assurance and Quality Control
Samples were analyzed with ICP-MS (Agilent 7900) using helium gas in collision cell mode to remove isobaric interferences and increase sensitivity. Our laboratory is enrolled in the Lead and Multielement Proficiency Program (LAMP), a lab standardization program run by the Centers for Disease Control and Prevention (CDC). Each quarter, our lab blindly analyzes a provided set of bovine blood samples and reports the results. The CDC then provides an assessment of the precision of participating laboratories’ measurements of lead, cadmium, mercury, selenium, and manganese benchmarked against their gold standard value. Performance data on LAMP analyses is provided in the supplemental materials (Figure S1).
2.6. Statistical analysis
R-studio equipped with the ggplot2 package was utilized for statistical analyses. The Shapiro-Wilk (p≥0.05) test demonstrated that elemental concentrations were log-normally distributed. Thus, linear regression modeling was used to assess relationships between logarithmized levels of whole blood measurements and clotted erythrocyte fraction samples prepared using either acid digestion or alkaline dilution. Pearson correlation coefficients of log-transformed data were calculated to assess goodness of fit of the linear model. Bland-Altman plots served to assess method differences. Diagnostic sensitivity and specificity were tested as a function of various blood lead level thresholds.
3. Results
3.1. Blood lead levels in AESOP study cohort
In total, whole blood and clotted erythrocyte fraction samples from 91 subjects were analyzed for this study with a combined sample size of 182. Demographic data of the AESOP Study participants who provided blood for this study are presented in Table 1. Whole blood lead concentrations ranged from 0.39 to 7.95 μg/dL with a geometric mean of 1.36 μg/dL. Clotted erythrocyte fraction samples prepared using acid digestion were found to have lead concentrations ranging from 0.43 to 7.64 μg/dL with a geometric mean of 1.25 μg/dL. Concentrations of lead in clotted erythrocyte fraction samples prepared using alkaline dilution ranged from 0.40 to 7.14 μg/dL with a geometric mean of 1.16 μg/dL. A breakdown of whole blood lead levels among AESOP study participants in East Chicago is presented in Table 2.
Table 2.
Breakdown of concentrations of lead in whole blood of AESOP Study participants in East Chicago.
| Adult Women (Mothers) | 2017-2018 | 2018-2019 | |
|---|---|---|---|
| Mean±1SD | 1.88 ± 1.30 | 2.34 ± 1.59 | |
| Median | 1.39 | 1.77 | |
| GM | 1.57 | 2.04 | |
| IQR | 1.31 | 1.06 | |
| Children (male/female) | 2017-2018 | 2018-2019 | |
| Mean±1SD | 1.08 ± 0.62 (1.06 ± 0.70 / 1.10 ± 0.57) | 1.38 ± 0.60 (1.60 ± 0.93 / 1.28 ± 0.40) | |
| Median | 0.92 (0.79 / 0.93) | 1.27 (1.30 / 1.20) | |
| GM | 0.96 (0.91 / 1.00) | 1.29 (1.44 / 1.23) | |
| IQR | 0.57 (0.51 / 0.57) | 0.52 (0.37 / 0.47) |
3.2. Measured lead in whole blood and clotted erythrocyte fraction
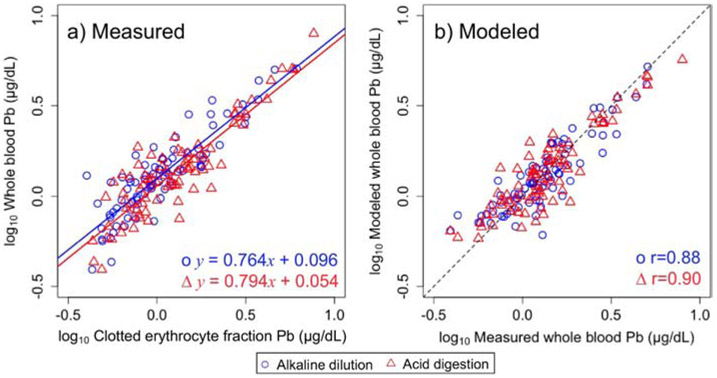
A scatterplot displaying the relationship between the concentration of lead measured in whole blood against clotted erythrocyte fraction is displayed in Figure 1a. In addition, the linear regression of whole blood and clotted erythrocyte fraction prepared using either acid digestion (red line) or alkaline dilution (blue line) is displayed in Figure 1a. A strong linear relationship between lead in whole blood and clotted erythrocyte fraction for both preparation methods was found. The results of the linear regression of log transformed values for the two methods were similar, with slopes of 0.764 and 0.794, and y intercepts of 0.096 and 0.054 for alkaline dilution and acid digestion, respectively.
Figure 1.
a) Plot of concentrations of Pb measured in clotted erythrocyte fraction against whole blood overlaid with the linear regression line colored to corresponding clotted erythrocyte fraction preparation method. b) Plot of concentrations measured in whole blood against modeled whole blood predicted from clotted erythrocyte fraction prepared either using acid digestion or alkaline dilution. Dashed line represents a 1 to 1 relationship.
3.3. Modeled whole blood lead levels
The results from the log-linear regression model was used to predict values of whole blood using clotted erythrocyte fraction values as shown in Figure 1b for lead using Equation 1. Pearson correlation coefficients of measured whole blood lead against clotted erythrocyte fraction predicted whole blood lead were 0.90 and 0.88 for acid digestion and alkaline dilution, respectively.
| Equation 1: |
3.4. Bland-Altman Analysis
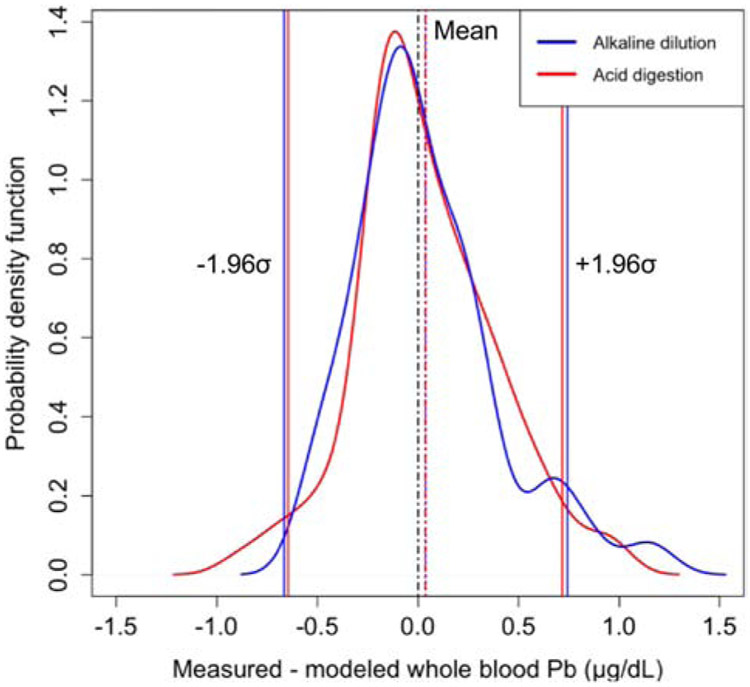
In addition to evaluating the correlations between the methods, we also evaluated the differences by modifying the Bland-Altman approach for assessing agreement between two clinical methods24 (Figure S2). Rather than plotting the individual points which can make it difficult to visualize trends in the data, we plotted the distribution of the method difference between measured and modelled blood lead as a probability density function (Figure 2). The mean method difference between whole blood and clotted erythrocyte fraction is 0.06 ± 0.83 and 0.04 ± 0.70 μg/dL for acid digestion and alkaline dilution, respectively. Dashed lines represent errors calculated as 1,96*σ. Fewer than 5% of samples method differences (n=8) were outside of the ± 1,96*σ range. The method differences were observed to be normally distributed.
Figure 2.
Modified visual representation of the Bland-Altman analysis showing the distribution of the differences between the measured and modeled whole blood lead predicted using clotted erythrocyte fraction prepared using either acid digestion or alkaline dilution. The solid horizontal lines represent the standard deviation multiplied by 1.96, and dashed lines represent the mean method difference. Dashed black line represents a measured modeled difference of 0. The colors of the lines correspond to the clotted erythrocyte fraction sample predation method.
3.5. Sensitivity and specificity
Since blood lead levels are used as a biomarker of exposure and also for classifying subjects with elevated levels compared to the CDC reference blood lead level, the diagnostic sensitivity and specificity of the method was tested and results are shown in Table 3. The whole blood lead measurement was selected as the gold standard for comparison. At a test threshold blood lead level of 3 μg/dL the acid digestion method had 88% diagnostic sensitivity and 100% diagnostic specificity. For the alkaline dilution method, the diagnostic sensitivity was 89% and the diagnostic specificity was 97%.
Table 3.
Diagnostic sensitivity (D-SN) and specificity (D-SP) of blood lead at a cut point of 3 μg/dL as modeled using clotted erythrocyte fraction with whole blood lead measurements designated as the gold standard for comparison.
| Measured | |||||
|---|---|---|---|---|---|
| Modeled | Test Result | ≥3 μg/dL | <3 μg/dL | D-SN | D-SP |
| Acid digestion | ≥3 μg/dL | 7 | 0 | 88% | 100% |
| <3 μg/dL | 1 | 76 | |||
| Alkaline dilution | ≥3 μg/dL | 4 | 2 | 89% | 97% |
| <3 μg/dL | 2 | 74 | |||
4. Discussion
This work provides a method that enables researchers to use archived clotted erythrocyte fraction samples to retrospectively analyze blood lead levels in previously studied populations. The recent environmental calamities seen in Flint, Michigan and Paris have highlighted the fact that lead exposure remains a major public health issue. The method validation presented here allows for retrospective epidemiological studies to be undertaken in varying populations, especially where a prior lead exposure is discovered within a community.
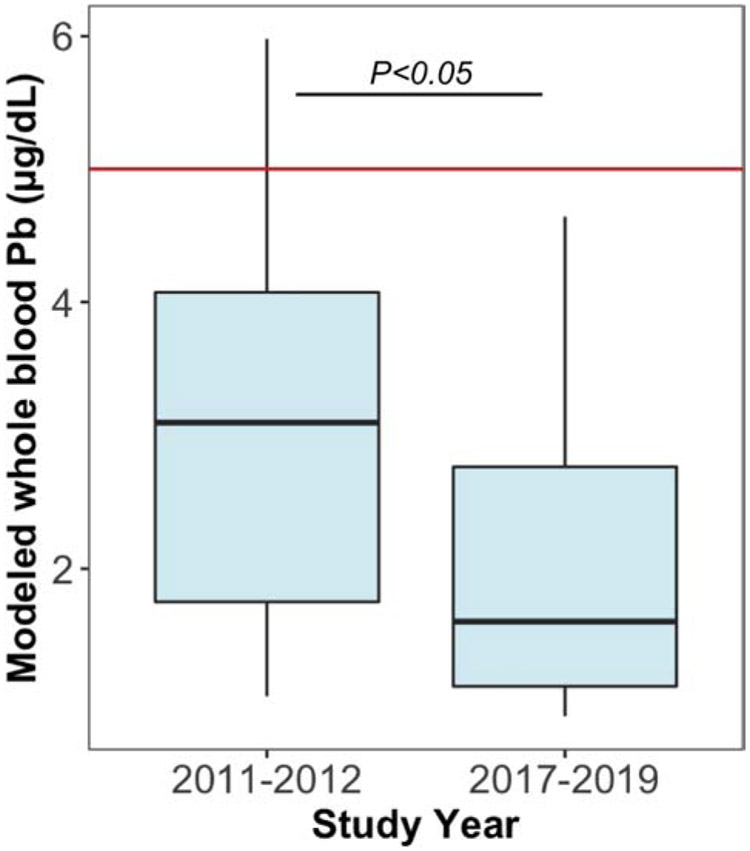
This is the case in East Chicago, IN where in 2016 it was discovered that a public housing development and an elementary school were built on the site of a former lead smelting operation (USS Lead Superfund Site). A subset of archived clotted erythrocyte fraction samples (n=15) from 2011 to 2012 of AESOP study participants in East Chicago was used to apply the method presented here to model whole blood lead levels. A comparison of these 15 subjects modeled whole blood lead levels between 2011 to 2012 and 2017 to 2019, presented in Figure 3, revealed a significantly (paired t-test, p<0.05) elevated level prior to the establishment of the USS Lead EPA Superfund Cleanup Site.
Figure 3.
Application of clotted erythrocyte fraction for retrospective assessment of lead exposure in East Chicago. A subset of clotted erythrocyte fraction samples of 15 subjects from two time points, before and after the establishment of USS Lead Superfund Site in 2016, was used to model whole blood lead levels. A significant decrease in modeled whole blood lead overtime was observed (paired sample t-test, p<0.05). Solid red line represents CDC reference blood lead level of 5 μg/dL.
However, it is difficult with the limited sample size to fully attribute this decrease to remediation efforts. It is interesting to see a trend of decreasing blood lead levels in East Chicago although several reports11,12 have suggested that blood lead levels have plateaued in the U.S. population even before 2011. These reports have also indicated that communities comprised of majority Black and Hispanic populations, low income, and low educational attainment, such those of East Chicago, had disproportionately higher blood lead levels than those on the national scale suggesting these minority communities had yet to reach a plateau.
In addition to acid digestion, alkaline dilution of clotted erythrocyte fraction was evaluated. The sample preparation time and effort are significantly lower for alkaline dilution compared to acid digestion. In addition, exposure of lab staff to hazardous substances such as concentrated acids, and acid fumes is reduced. Taking these factors into consideration suggests that alkaline dilution is the more favorable method for analysis of clotted erythrocyte fraction.
Results from the comparison of these two methods for lead showed comparable degrees of correlation with a highly significant (p<0.001) correlation coefficient (r) of 90 and 0.88 for acid digestion and alkaline dilution, respectively. The slope and intercept of the linear regression model comparing clotted erythrocyte fraction preparation methods to whole blood did not differ significantly (p>0.05).
Blood lead levels are used in clinical settings for identifying children who may be overexposed. Sensitivity and specificity were evaluated at a test threshold value of 3 μg/dL. This value was been identified as the intended update to the CDC blood lead reference value25. In addition, this threshold value is similar to the detection limit of Leadcare II (Meridian Bioscience, Cincinnati OH), the most commonly used test kit for blood lead. Diagnostic sensitivity and specificity were 89% and 97%, respectively, for the alkaline dilution method, comparing favorably to NHANES values26.
Since this method measures total lead and is not aimed at measuring speciation or chemical structures of lead, close attention and tight rigor do not have to be applied to the length of time the samples have been stored. Acid digestion occurs at pH close to 0 which ensures complete solubilization of lead in the sample. Suggesting comparable solubilization of lead between the two clotted erythrocyte fraction sample preparation methods.
There are several limitations to this study which suggest further improvement. We did not measure hematocrit which is often used as an indicator to assess the fraction of a whole blood sample that is comprised of serum and erythrocyte fraction. This is an important factor as studies27 have reported relative variation of up to 15% resulting from seasonal variability and hydration. Furthermore, it is well known that sex differences exist for hematocrit which can also influence the extrapolation to whole blood28. This can be especially important to consider for studies focusing on pregnant women where hemodilution can occur29. However, we cannot comment on the effect of hemodilution on clotted erythrocyte fraction samples as pregnant women were not included in this study. Lastly, the partitioning of lead between serum and erythrocyte fraction is not always constant particularly after very recent high concentration exposures30. Although these acute exposures are not as common, they can lead to underestimations of lead exposure. These variabilities in proportion and partitioning of lead in clotted erythrocyte fraction may help explain the scatter observed between whole blood and clotted erythrocyte fraction measurements.
5. Conclusion
To our knowledge, this is the first study to demonstrate clotted erythrocyte fraction measurement of lead as predictors of exposure comparable to whole blood. The results from this study highlight that clotted erythrocyte fraction samples can be utilized to predict lead exposure in a similar manner to whole blood. We attribute this to the high affinity of lead towards iron, primarily in the form of hemoglobin. Thus, in studies of lead exposure surveillance, clotted erythrocyte fraction lead should be considered as a viable complement to whole blood for retrospective analysis. Efforts are underway for applying this method in order to retrospectively assess unmonitored environmental exposure to lead in East Chicago.
Supplementary Material
Highlights.
Whole blood lead levels were predicted using clotted erythrocyte fraction
Two sample-preparation methods, acid digestion and alkaline dilution, were evaluated
Lead was strongly correlated between whole blood and clotted erythrocyte fraction
Clotted erythrocyte fraction is a viable alternative for retrospective surveillance of lead exposure
Acknowledgements
We would like to acknowledge our study staff, Barbara Mendenhall, Nancy Morales and Jeanne DeWall for home visits and blood collection and retrieval. We would also like to thank Dr. Brian Wels and Dr. Drew Latta for ICP-MS related technical assistance, Dr. Andrea Adamcakova-Dodd for laboratory assistance and Cara Held for non-technical review of this manuscript. Funding for the AESOP Study is from the Iowa Superfund Program, grant NIH P42ES013661. The lead analysis described in this report was funded by the Environmental Health Sciences Research Center, grant NIH P30ES005605.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goyer RA. Results of Lead Research: Prenatal Exposure and Neurological Consequences. October 1996;104(10):10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loghman-Adham M Renal effects of environmental and occupational lead exposure. Environ Health Perspect. 1997;105(9):928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazumdar I, Goswami K, Ali MS. Status of Serum Calcium, Vitamin D and Parathyroid Hormone and Hematological Indices Among Lead Exposed Jewelry Workers in Dhaka, Bangladesh. Indian J Clin Biochem. 2017;32(1):110–116. doi: 10.1007/s12291-016-0582-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tališman S, Cvitković P, Jurasović J, Pizent A, Gavella M, Ročić B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect. 2000;108(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabinowitz MB, Kopple JD, Wetherill GW. Effect of food intake and fasting on gastrointestinal lead absorption in humans. Am J Clin Nutr. 1980;33(8):1784–1788. [DOI] [PubMed] [Google Scholar]
- 6.Selevan SG, Rice DC, Hogan KA, Euling SY, Pfahles-Hutchens A, Bethel J. Blood lead concentration and delayed puberty in girls. N Engl J Med. 2003;348(16):1527–1536. doi: 10.1056/NEJMoa020880 [DOI] [PubMed] [Google Scholar]
- 7.Meeker JD, Rossano MG, Protas B, et al. Cadmium, lead, and other metals in relation to semen quality: Human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect. 2008;116(11):1473–1479. doi: 10.1289/ehp.11490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasserman GA, Liu X, Lolacono NJ, et al. Lead exposure and intelligence in 7-year-old children: the Yugoslavia Prospective Study. Environ Health Perspect. 1997;105(9):956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich KN, Berger OG, Succop PA, Hammond PB, Bornschein RL. The developmental consequences of low to moderate prenatal and postnatal lead exposure: Intellectual attainment in the Cincinnati lead study cohort following school entry. Neurotoxicol Teratol. 1993;15(1):37–44. doi: 10.1016/0892-0362(93)90043-N [DOI] [PubMed] [Google Scholar]
- 10.Krul IM, Sc M, Lugtenburg PJ, Ph D, Van Leeuwen FE, Ph D. Intellectual Impairment in Children with Blood Lead Concentrations below 10 μg per Deciliter. N Engl J Med. 2003;348(16):1517–1526. doi: 10.1056/NEJMoa1505949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RL, Homa DM, Meyer PA, et al. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988–2004. Pediatrics. 2009;123(3):e376–e385. [DOI] [PubMed] [Google Scholar]
- 12.Brody DJ, Pirkle JL, Kramer RA, et al. Blood lead levels in the US population: phase 1 of the Third National Health and Nutrition Examination Survey (NHANES III, 1988 to 1991). Jama. 1994;272(4):277–283. [DOI] [PubMed] [Google Scholar]
- 13.Ettinger AS, Egan KB, Homa DM, Brown MJ. Blood Lead Levels in US Women of Childbearing Age, 1976–2016. Environ Health Perspect. 2020;128(1):17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jedrychowski W, Perera FP, Jankowski J, et al. Very low prenatal exposure to lead and mental development of children in infancy and early childhood. Neuroepidemiology. 2009;32(4):270–278. doi: 10.1159/000203075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ Health Perspect. 2005;113(7):894–899. doi: 10.1289/ehp.7688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mañay N, Cousillas A, Heller T. Blood Lead Level (BLL, B-Pb) in Human and Animal Populations: B-Pb as a Biological Marker to Environmental Lead Exposure. Cell Eff Heavy Met. 2011:315–330. doi: 10.1007/978-94-007-0428-2 [DOI] [Google Scholar]
- 17.Barbosa F, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: Advantages, limitations, and future needs. Environ Health Perspect. 2005;113(12):1669–1674. doi: 10.1289/ehp.7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna-Attisha M, LaChance J, Sadler RC, Schnepp AC. Elevated blood lead levels in children associated with the Flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283–290. doi: 10.2105/AJPH.2015.303003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Geen A, Yao Y, Ellis T, Gelman A. Fallout of Lead over Paris from the 2019 Notre-Dame Cathedral Fire. GeoHealth. 2020:e2020GH000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ampleman MD, Martinez A, DeWall J, Rawn DFK, Hornbuckle KC, Thorne PS. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ Sci Technol. 2015;49(2):1156–1164. doi: 10.1021/es5048039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massanyi P, Stawarz R, Halo M, et al. Blood concentration of copper, cadmium, zinc and lead in horses and its relation to hematological and biochemical parameters. J Environ Sci Heal Part A. 2014;49(8):973–979. [DOI] [PubMed] [Google Scholar]
- 22.Nahirnyak VM, Yoon SW, Holland CK. Acousto-mechanical and thermal properties of clotted blood. J Acoust Soc Am. 2005;117(4):2413–2413. doi: 10.1121/1.4786299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffery M Jarrett JLP. Blood Multi-element Analysis by a Triple Quadrupole Inductively Coupled Plasma Mass Spectrometer (ICP-QQQ-MS). Vol 13; 2019. [Google Scholar]
- 24.Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. [PubMed] [Google Scholar]
- 25.Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 μg/dL. Neurotoxicology. 2006;27(5):693–701. doi: 10.1016/j.neuro.2006.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vargas CM, Burt VL, Gillum RF, Pamuk ER. Validity of self-reported hypertension in the National Health and Nutrition Examination Survey III, 1988–1991. Prev Med (Baltim). 1997;26(5):678–685. [DOI] [PubMed] [Google Scholar]
- 27.Thirup P Haematocrit: Within-subject and seasonal variation. Sport Med. 2003;33(3):231–243. doi: 10.2165/00007256-200333030-00005 [DOI] [PubMed] [Google Scholar]
- 28.Kelton JG, Powers P, Julian J, et al. Sex-related differences in platelet aggregation: influence of the hematocrit. Blood. 1980;56(1):38–41. [PubMed] [Google Scholar]
- 29.Rothenberg SJ, Karchmer S, Schnaas L, Perroni E, Zea F, Fernandez Alba J. Changes in serial blood lead levels during pregnancy. Environ Health Perspect. 1994;102(10):876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith D, Hernandez-Avila M, Téllez-Rojo MM, Mercado A, Hu H. The relationship between lead in plasma and whole blood in women. Environ Health Perspect. 2002;110(3):263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.