Abstract
Purpose:
Neuropathic corneal pain (NCP) is a recently acknowledged disease entity. However, there is no consensus in potential treatment strategies, particularly in patients with a centralized component of pain. This study aims to assess the efficacy and tolerability of the tricyclic antidepressant, nortriptyline, among NCP patients.
Methods:
Patients with clinically diagnosed NCP and a centralized component of pain, treated with oral nortriptyline, who had recorded pain scores as assessed by the ocular pain assessment survey at the first and last visit were included. Patients were excluded if they had any other ocular pathology that might result in pain or had less than 4 weeks of nortriptyline use. Demographics, time between visits, concomitant medications, systemic and ocular comorbidities, duration of NCP, side effects, ocular pain scores, and quality of life (QoL) assessment were recorded.
Results:
Thirty patients with a mean age of 53.1±18.5 were included. Male to female ratio was 8:22. Mean ocular pain in the past 24 hours improved from 5.7±2.1 to 3.6±2.1 after 10.5±9.1 months (p<0.0001). Twelve patients (40.0%) had equal to or more than 50% improvement, 6 patients (20.0%) had 30–49% improvement, 6 patients (20.0%) had 1–29% improvement, 4 patients (13.3%) did not improve, while 2 patients (6.7%) reported increase in pain levels. Mean QoL improved from 6.0±2.5 to 4.3±2.4 (p=0.019). Eight patients (26.6%) discontinued treatment due to persistent side effects, despite improvement by 22.4%.
Conclusion:
Nortriptyline was effective in relieving NCP symptoms in patients with centralized component and insufficient response to other systemic and topical therapies who tolerated the drug for at least 4 weeks. Nortriptyline may be used in the management of patients with NCP.
INTRODUCTION
Noxious stimuli generate acute pain and physiological responses, which in the eye, include tearing and motor responses to protect the ocular surface from injury.1,2 These noxious stimuli can be caused by a number of ocular insults, such as trauma, surgery, infection, and inflammation. However, some patients experience neuropathic pain even after resolution of the initial ocular insult in the presence of sub-threshold noxious stimuli (hyperalgesia),3–5 or in the presence of non-noxious stimuli (allodynia, photoallodynia).5–7 This process, which can start in the cornea and can affect the peripheral and/or the central nervous system has been named neuropathic corneal pain (NCP).2,4,8–10 The abnormal responses of nociceptors are caused by sensitization, resulting due to damage to corneal nociceptive nerves that causes the release of pro-inflammatory mediators.5 When this state is long-standing, corneal nociceptors experience an up-regulation in receptors and/or a lowering of excitability thresholds, which initially causes peripheral sensitization.2,11 Peripheral neuronal alterations can result in changes in higher order neurons, including reduced activation thresholds and reduced inhibitory signals, triggering central sensitization.1,2,4,5,8,11–14
Symptoms of NCP include pain,15 discomfort,16 aching,3 irritation,16 dryness,3 grittiness,3 burning,16 and light sensitivity (photoallodynia).6 These symptoms are also often reported in dry eye disease (DED), which makes clinical and differential diagnosis challenging, and causes NCP to be largely under-treated.4,8 Patients with NCP can exhibit peripheral and central sensitization.10 Treatments of peripheral sensitization include neuro-regenerative therapy (e.g. autologous serum tears),6,10 anti-inflammatory therapies, ocular surface rehabilitation (e.g. lubrication, treatment of meibomian gland dysfunction, self-retained cryopreserved amniotic membrane),9 and protective contact lenses.4,8 Treatment for NCP patients with central sensitization is based on that used for non-ocular chronic neuropathic pain.4 These treatments include tricyclic antidepressants (TCA), anticonvulsants (e.g. carbamazepine, gabapentinoids), opioid antagonists (e.g. low-dose naltrexone), opioid agonists (e.g. tramadol), sodium channel blockers (e.g. mexiletine), as well as serotoninnoradrenaline reuptake inhibitors (SNRIs, e.g. duloxetine, venlafaxine).4,17
TCAs are divided into two major groups; secondary amines, such as nortriptyline and desipramine, and tertiary amines, such as imipramine and amitriptyline.18 The pharmacologic analgesic effects of antidepressants involve binding to noradrenaline (NA) and serotonin (5HT) transporters. Reuptake of these neurotransmitters is inhibited, leading to an increased level of NA and 5HT in the synaptic cleft. The secondary amines are relatively selective for NA reuptake, whereas the tertiary amines inhibit the reuptake of NA as well as 5HT.18,19
TCAs have a number of other actions in addition to NA reuptake inhibition that may contribute to the treatment of neuropathic pain, such as sodium channel blockade, sympathetic blockade antagonism of N-methyl-D-aspartate (NMDA) glutamate receptors, and anticholinergic effects.18,20 Nortriptyline, a secondary amine, is sometimes preferred to tertiary amines, such as amitriptyline, for neuropathic pain treatment, because it reportedly has a lower incidence of associated adverse effects,21 which can increase patient compliance and be especially useful in older patients who are likely to experience adverse effects such as confusion, agitation, and postural hypotension.18–23
Since, nortriptyline has been used successfully for the treatment of non-ocular neuropathic pain, we hypothesized that it may also be effective in NCP. Thus, the purpose of this study was to assess the efficacy and tolerability of nortriptyline hydrochloride among NCP patients with a centralized component of pain. Herein, we show the efficacy and safety of nortriptyline among centralized NCP patients.
METHODS
Patient selection
This study is a retrospective cohort study, which was conducted at the Cornea Service, New England Eye Center, Department of Ophthalmology, Tufts Medical Center, Tufts University Medical School, Boston, Massachusetts. The Institutional Review Board/ Ethics Committee approved the protocol. We ensured compliance with the Health Insurance Portability and Accountability Act and adherence to the tenets of the Declaration of Helsinki.
Electronic records between July 1, 2015 and March 31, 2019 were reviewed. Patients with a diagnosis of ocular pain (ICD-10: H57.1 or ICD-9: 379.91) and whose electronic records included the keyword “nortriptyline” were identified through an electronic search. All patients were clinically diagnosed by a single cornea specialist (PH), who used the ocular pain ICD codes for diagnosis of NCP. Diagnosis was based on medical history, discordance in clinical signs and symptoms, and confirmed by corneal in vivo confocal microscopic findings. Patients with a persistent component of central sensitization, despite topical and/or systemic treatments, were included. Central sensitization was assessed by any level of persistent ocular discomfort/pain after 90 seconds of instillation of 0.5% proparacaine hydrochloride (Alcaine; Alcon, Fort Worth, TX), which anesthetizes the ocular surface nerves.4,10,24 Patients with pure peripheral NCP, only DED, and/or other ocular surface disease would be expected to have complete resolution of symptoms. Patients were excluded if they had any ocular pathology that might cause pain (e.g. active corneal infections, abrasions, recurrent erosion syndrome, angle-closure glaucoma, and anterior uveitis) or had less than 4 weeks of nortriptyline use.
All patients were prescribed nortriptyline in a similar manner. They started at an oral dose of 10 mg and tapered up to 100mg based on treatment response and tolerability. We utilized the Ocular Pain Assessment Survey (OPAS) in this study.25 The OPAS is a multidimensional survey, which includes a dimension for ocular pain intensity and a dimension for quality of life (QoL), among other features. Ocular pain intensity, on a 0–10 scale, is assessed in 6 questions (questions 4–9) using visual analogue scale. These questions are ratings of the patient’s eye pain intensity level (most, least, and average) at 24 hours and 2 weeks. QoL dimension (questions 13–19, range 0–10) describes the interference of pain to certain QoL parameters, such as reading/computer use, driving/TV watching, general activity, mood, sleep, enjoying life/relations with other people, and time spent thinking about eye pain (higher score indicates a higher impact of pain on these QoL parameters).25
Patients were included in the efficacy analysis if there was an electronic documentation of 1) nortriptyline use for at least four weeks; 2) a completed OPAS question 6, which asks the patient to rate the average level of eye pain in the past 24 hours on a scale of 0 to 10 at the visit where nortriptyline was prescribed (termed “first visit” for the purposes of this study); 3) the average level of eye pain in the past 24 hours at the most recent visit to our clinic or at the visit when nortriptyline was discontinued (termed “last visit”).
Chart Reviews
A detailed chart review was conducted. Demographics, time between visits (i.e. duration of nortriptyline use), concomitant medications, systemic and ocular co-morbidities, ocular surgeries, duration of NCP, side effects, reason for discontinuation, and response to OPAS questions 4–9 and 13–19 were recorded.
Statistical Analysis
Statistical analysis was performed with SPSS 20 (IBM SPSS Statistics, Chicago, IL, USA). Distribution of the data was analyzed by Shapiro-Wilk test. Differences between visits were analyzed using paired t-test for normally distributed data and Wilcoxon signed-rank test for non-normally distributed data. Bi-variate correlation analysis was performed where necessary. Mixed model analysis was used to test for the change in question 6 of OPAS between visits with nortriptyline use, age, sex, concomitant medications, and systemic co-morbidities included as covariates. P values less than 0.05 were considered statistically significant.
RESULTS
Demographics
Fifty-four patients were identified as having been prescribed nortriptyline and 30 patients were included in the final dataset for the efficacy analysis, based on inclusion/exclusion criteria. Eleven patients were unable to use nortriptyline more than 4 weeks because of side effects and 13 patients did not have necessary chart data either in the first visit, the last visit, or both. Mean age was 53.1±18.5 years and 22 patients (73.3%) were female. Demographic data of the patients and pertinent concomitant treatments are presented in Tables 1 and 2. All patients were using other topical and/or oral medications during nortriptyline use. A comprehensive list of concomitant treatments can be found in Supplementary Table 1. Systemic co-morbidities included neuro-psychiatric, cardiovascular, autoimmune and miscellaneous diseases. A history of dry eye disease was the most common ocular co-morbidity in this cohort (17 patients). A summary of systemic and ocular comorbidities are provided in Tables 3 and 4 respectively.
Table 1.
Demographics of study population.
| Patients with NCP | N=30 |
|---|---|
| Age, year (SD, Range) | 53.1 (18.5, 18–87) |
| Gender, | |
| Male, n (%) | 8 (26.7) |
| Female, n (%) | 22 (73.3) |
| Ethnicity, n (%) | |
| Caucasian | 23 (73.3) |
| Asian | 2 (6.7) |
| Others/Declined | 5 (16.7) |
Table 2.
Pertinent topical and systemic concomitant treatments.
| N (%) | |
|---|---|
| Topical | |
| Steroids | 20 (66.6) |
| Autologous serum tears | 19 (63.0) |
| Artificial Tears | 6 (20) |
| Cyclosporine-A | 4 (13.3) |
| Lifitegrast | 2 (6.6) |
| PROKERA® | 1 (3.3) |
| TrueTear® | 1 (3.3) |
| Systemic | |
| Gabapentin | 5 (16.7) |
| Carbamazepine | 5 (16.7) |
| SSRIs | 5 (16.7) |
| Bupropion | 4 (13.3) |
| Low dose naltrexone | 2 (6.7) |
| SNRIs | 2 (6.7) |
| Trazodone | 2 (6.7) |
| Atypical anti-psychotic | 2 (6.7) |
| Tramadol | 1 (3.3) |
| Oxycodone | 1 (3.3) |
| Benzodiazepine | 1 (3.3) |
(NSAIDs): non-steroidal anti-inflammatory drugs, SNRIs: selective nor-adrenaline reuptake inhibitors, SSRIs: selective serotonin reuptake inhibitors).
Table 3.
Systemic co-morbidities of patients with NCP.
| Co-morbidities | Patients |
|---|---|
| N (%) | |
| Neuro-psychiatric | |
| Depression | 5 (16.7) |
| Anxiety | 3 (10) |
| Fibromyalgia | 2 (6.7) |
| Stroke | 2 (6.7) |
| Migraine | 1 (3.3) |
| Non-migraine Headaches | 1 (3.3) |
| Multiple Sclerosis | 1 (3.3) |
| Seizures | 1 (3.3) |
| Vertigo | 1 (3.3) |
| Cardiovascular | |
| Hyperlipidemia | 7 (23.3) |
| Hypertension | 5 (16.7) |
| Tachycardia | 1 (3.3) |
| Autoimmune | |
| Rheumatoid Arthritis | 4 (13.3) |
| Myasthenia Gravis | 2 (6.7) |
| Miscellaneous | |
| Diabetes mellitus | 2 (6.7) |
| Hypothyroidism | 5 (16.7) |
| Asthma | 3 (10) |
| Osteoarthritis | 3 (10) |
| History of Cancer | 3 (10) |
| Anemia | 1 (3.3) |
Table 4.
Ocular co-morbidities of patients.
| Patients, N (%) | |
|---|---|
| Dry eye disease | 17 (56.7) |
| Glaucoma | 4 (13.3) |
| Post-herpetic keratitis | 3 (10) |
| Previous Ocular Surgeries | |
| Refractive Surgery (LASIK, PRK) | 4 (13.3) |
| Other ocular surgeries (cataract surgery, pterygium surgery, strabismus surgery) | 4 (13.3) |
| Selective laser trabeculoplasty | 3 (10) |
(LASIK: laser in-situ keratomileusis, PRK: photo refractive keratectomy). All surgeries have been done on both eyes.
Efficacy
According to the OPAS, the patients’ average ocular pain level in the past 24 hours was 5.7±2.1 (range 3–10) at the first visit and 3.6±2.1 (range 0–7) at the last visit (p<0.0001) as seen in Fig. 1. The most severe pain perceived by patients in the past 24 hours had also improved significantly from 7.3±2.2 (range 3–10) to 5.5±2.7 (range 0–10) (p=0.028). For the average pain in the past 24 hours, percent improvement was 37.4%. Twelve patients (40.0%) had equal to or more than 50% improvement, 6 patients (20.0%) had 30–49% improvement, 6 patients (20.0%) had 1–29% improvement, 4 patients (13.3%) did not improve, and 2 patients (6.7%) reported increase in pain levels (Table 5). Pain levels in the past 2 weeks were also significantly improved. Further, average pain scores in the past 2 weeks improved significantly (from 6.3±2.0 to 4.2±2.1, p=0.001), as did the most pain in the past 2 weeks (from 7.7±1.7 to 5.9±2.9, p=0.003). Table 6 shows all other questions in pain level dimension showed a significant improvement at the last visit compared to the first (all p<0.028).
Figure 1.
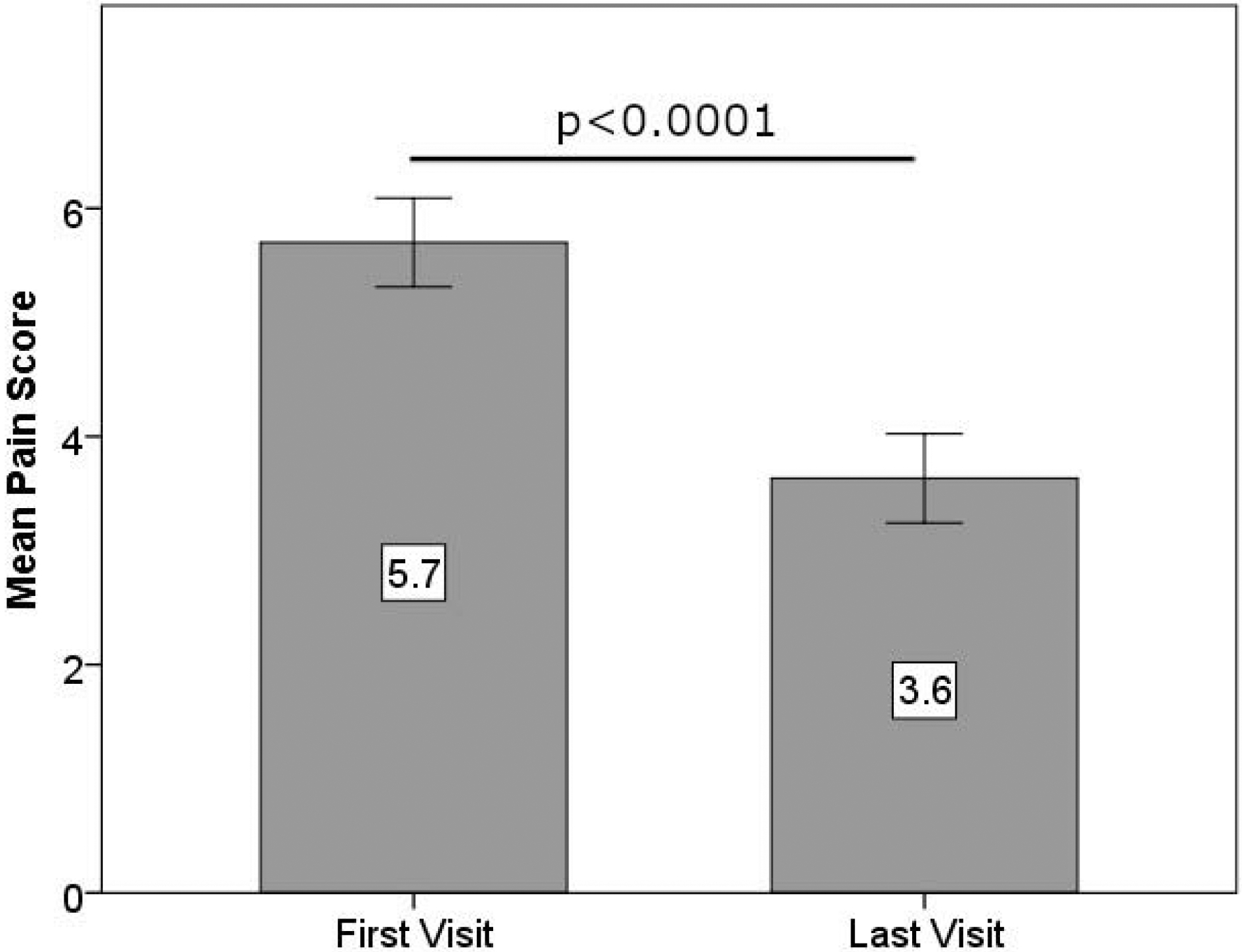
Mean pain scores in first and last visits (error bars represent standard error of means).
Table 5.
Percent change in pain scores.
| Percent Change in Pain Score(%) | Number of Patients (N) | Percent of Patients (%) |
|---|---|---|
| <0 | 2 | 6.7 |
| 0 | 4 | 13.3 |
| 1–29 | 6 | 20.0 |
| 30–49 | 6 | 20.0 |
| ≥50 | 12 | 40.0 |
Table 6.
Results from the questions in pain level dimension of the Ocular Pain Assessment Survey.
| OPAS | Percent Change in Pain Score | ||||
|---|---|---|---|---|---|
| # | Eye Pain Question | First Visit Score | Last Visit Score | p | |
| 4 | Most in 24h | 7.3±2.2 | 5.5±2.7 | −9.3±83.3 | 0.028 |
| 5 | Least in 24h | 3.8±2.6 | 2.4±2.0 | −16.7±76.5 | 0.013 |
| 6 | Average in 24h | 5.7±2.1 | 3.6±2.1 | −37.4±32.6 | <0.0001 |
| 7 | Most in 2w | 7.7±1.7 | 5.9±2.9 | −25.0±35.0 | 0.003 |
| 8 | Least in 2w | 4.8±2.8 | 2.8±2.2 | −34.1±46.6 | 0.002 |
| 9 | Average in 2w | 6.3±2.0 | 4.2±2.1 | −30.5±39.9 | 0.001 |
(h= hours, w= weeks, data in mean±SD).
Mean QoL score improved from 6.0±2.5 (range 0.29–10) at the first visit to 4.3±2.4 (range 0.29–8.43) at the last visit (p=0.019). The effect of pain on reading/computer use, mood, sleep, and time spent thinking about pain improved significantly (all p <0.041). Table 7 summarizes the results of individual QoL questions. Time between visits (duration of nortriptyline use) was 10.5±9.1 months (range 1–35 months) and duration of NCP before the treatment was 16.9±23.0 months (range 1–83 months) both of which were not correlated with percent change in average ocular pain in the past 24 hours (Spearman’s rho=−0.118, p=0.534 and Spearman’s rho=−0.383, p=0.177 respectively). The mean nortriptyline dose was 66.88±25.90 mg and the dose was not correlated to the level of pain reduction (Spearman’s rho=−0.567, p=0.143). The addition of co-variates (age, sex, NCP duration, nortriptyline use duration, concomitant medications, and systemic and ocular co-morbidities) via mixed model showed that nortriptyline had a significant effect on the pain scores (F=12.08, p<0.0001).
Table 7.
Results from the questions in quality of life dimension of the Ocular Pain Assessment Survey.
| OPAS | QoL dimension: pain effecting, | Percent Change in QoL Score | |||
|---|---|---|---|---|---|
| # | First Visit Score | Last Visit Score | p | ||
| 13 | Reading/ computer use | 6.8±3.3 | 4.3±2.4 | −29.2±55.5 | 0.007 |
| 14 | Driving/ watching tv | 5.3±3.5 | 3.9±3.5 | −11.5±107.7 | 0.247 |
| 15 | General activity | 5.3±2.8 | 3.9±3.1 | −26.8±62.9 | 0.053 |
| 16 | Mood | 6.7±2.9 | 4.4±3.1 | −16.0±89.8 | 0.029 |
| 17 | Sleep | 3.8±4.0 | 1.5±2.7 | −41.1±52.7 | 0.011 |
| 18 | Enjoying life | 6.5±3.2 | 4.6±3.5 | −20.4±63.5 | 0.068 |
| 19 | Time spent thinking about pain | 7.6±3.3 | 5.2±3.5 | −23.9±57.6 | 0.041 |
(QoL data in mean±SD).
Side Effects
A total of nineteen out of fifty-four (35.0%) patients who were prescribed nortriptyline, discontinued due to side effects. Eight of these patients (14.8%) who used nortriptyline more than 4 weeks and were included in the efficacy analysis, discontinued due to persistent mild side effects despite the improvement of pain score by 22.4% (from 7.0±2.1 (range 4–10) to 5.4±2.2 (range 2–8), p=0.041). Their mean duration of use prior to discontinuation was 7.6±5.7 months (range 2–16 months). Patient-reported side effects for the 19 patients included lethargy (n=4), dry mouth (n=4), constipation (n=2), nausea (n=2), headache (n=2), tachycardia (n=1), and unspecified (n=4).
DISCUSSION
Herein, we demonstrate that nortriptyline use reduces pain score and improves QoL scores in patients with NCP with a centralized component, and that therapeutic approaches for non-ocular neuropathic pain might be useful in NCP as well. Currently NCP does not have clearly outlined diagnostic criteria, but limited response to topical proparacaine, nerve abnormalities shown by corneal confocal microscopy described by previous studies,6,10 along with medical history, discordance between signs and symptoms, failed DED treatment, and photoallodynia have been used to diagnose NCP.4,6 Residual pain and discomfort after the instillation of proparacaine eye drops points to centralized neuropathic component of the pain in our cohort.24
Many of the proposed therapies discussed for NCP are derived from evidence-based literature for systemic neuropathic pain and ocular post-herpetic neuralgia (PHN).4 Neuropathic pain has complex pathophysiology, which generally requires the use of more than one systemic neuromodulatory drugs as well as interventional therapies (e.g. nerve blocks) as treatment options. Neuropathic pain typically does not respond to analgesics (e.g. acetaminophen, NSAIDs) or weak opioids (e.g. codeine), although they are frequently used.17,26 TCAs along with gabapentinoids, and SNRIs are recommended as the first line treatment options in peripheral and central neuropathic pain.23,27
Although not approved by the Food and Drug Administration for the treatment of neuropathic conditions, many trials have shown that TCAs are beneficial for neuropathic pain.23,27,28,29 TCAs are known to be very effective in the treatment of neuropathic pain compared to other potential treatments as assessed by the commonly used quantitative measure of number needed to treat (NNT). NNT is defined as the number of patients needed to treat to have a 50% pain reduction in one patient. The literature related to PHN has shown that combined NNT for TCAs was 3.6 (15 studies), gabapentin was 6.3 (14 studies), pregabalin was 7.7 (25 studies), and SNRIs was 6.4 (14 studies).17,23
The effect of TCAs in neuropathic pain is independent of their antidepressant effects. Studies have shown that they produce analgesic effect in patients without depression and in doses smaller than those usually effective in depression.28 Their analgesic efficacy seems to be mediated by their action on descending modulatory inhibitory controls, as well as locus coeruleus and spinal cord.17,19 Because of its efficacy in neuropathic pain, and lower reported side effects among TCAs, we chose to investigate nortriptyline for this study of NCP.26 To our knowledge, this is the first study reporting the effect of nortriptyline use in trigeminal neuropathic pain.
The mechanism of action of nortriptyline is largely based on NA reuptake inhibition. The NA reuptake inhibition enhances analgesic effects, mainly through two mechanisms. First, it acts on the α2-adrenergic receptor in the dorsal horn of the spinal cord.18 The α2-adrenergic receptors are coupled to the inhibitory G protein, which in turns inhibit the presynaptic voltage-gated Ca2+ channels that inhibit the release of excitatory neurotransmitters from primary afferent fibers. Second, increased NA acts on the locus coeruleus and improves the function of an impaired noradrenergic inhibitory system.18,20 Activation of the α2-adrenergic receptor is exceptionally effective against allodynia and hyperalgesia associated with neuropathic pain.30,31 In addition, sodium blockade might contribute to its analgesic effects along with NMDA blockade.32
Pain scores in the past 24 hours and in the past 2 weeks improved significantly in our cohort. In a double-blind, randomized, controlled trial on patients with neuropathic pain, Gilron et al. reported that nortriptyline reduced mean daily pain assessed by visual analogue scale from 5.4±1.3 to 2.9±1.6 (p=0.02), which is similar to the improvement in our study.22 Another randomized placebo controlled study showed that pain improvement with nortriptyline is better than placebo (32% and 11% respectively), which supports our findings.33 In a randomized, double-blind, parallel-group trial in PHN patients, nortriptyline has reduced pain scores by 47.6%.34 Watson et al. reported in a randomized, double-blind, crossover study comparing nortriptyline to amitriptyline that both drugs were similar in efficacy and 21 out of 31 patients (67.7%) had a good response.21 Panerai et al. showed a better analgesic effect for nortriptyline over placebo (p<0.0001).35 Another prospective, randomized, crossover study on chronic leg pain showed a 14% reduction of pain below placebo; however, this difference was not significant.36 Although our study did not have a placebo group, the placebo effect is an important aspect of studies in patients with neuropathic pain, which shows the patients’ perception and experience of receiving a pain-reducing treatment. The placebo effect size in neuropathic pain studies are generally above a Cohen’s D of 0.8.37
Previously our group has demonstrated treatment efficacy of self-retained cryopreserved amniotic membrane and autologous serum tears for peripheral NCP.6,9,10 We have shown that autologous serum tears reduced pain scores from 9.1±0.8 to 3.1±1.2 at the time point when patients reported improvement in symptoms.10 Self-retained cryopreserved amniotic membrane showed a similar outcome with improvement of pain scores from 6.4±2.7 to 1.9±1.8.9 The greater reduction in pain scores observed in these studies could be due to the fact that the patients in these studies had peripheral NCP as opposed to our centralized NCP cohort. This may indicate that peripheral NCP is more responsive to treatment, and therefore that NCP should be treated before central sensitization. Furthermore, the time between reported pain scores was longer in our study.
The current study, in patients with a high female:male ratio as observed in other ocular surface diseases, demonstrates that 60% of the patients had more than 30% improvement in pain scores. In a study with data from 2,724 subjects, it has been shown that a 30% reduction in pain level, as assessed via visual analogue scale, was reflective of a clinically important change in the standard seven-point patient global impression of change scale.38 When all patients in this cohort who were prescribed nortriptyline were considered, 33.3% (18/54) of patients had more than 30% improvement in pain and 22.2% (12/54) had more than 50% improvement in pain. Although it has been shown in PHN that duration of disease and duration of TCA use effects the outcome,39 we did not find any of these parameters to affect the outcome.
NCP seems to have a significant impact on QoL in our cohort, which improved after nortriptyline treatment. Similarly, in a double-blind, randomized, controlled trial with 40 patients who have diabetic polyneuropathy and PHN, the authors reported that nortriptyline was able to reduce pain interference with QoL items from 4.0±0.8 to 2.1±0.4. The effect seems to be similar to that in DED as shown in a previous study, which included 5,884 DED patients with a 5.5 baseline impact of symptoms on daily activities, which improved to 3.5 after treatment.40 A phase III study evaluating nortriptyline in neuropathic pain showed that the hours of sleep increased significantly with the use of nortriptyline.41 We found that the effect of pain on reading/computer use, sleep, and mood were significantly improved. Specifically, improved reading and computer use may suggest a significant decrease in hyperalgesia.
Common side effects for nortriptyline include dry mouth, dry eye, constipation, weight gain, postural hypotension, and sedation.4,17,26,42 Similar to the current study, nortriptyline has been shown to have a withdrawal rate of up to 35% due to side effects.21,22,41,43 In the efficacy analysis, we excluded the patients who discontinued before 4 weeks. The start of the effect of nortriptyline differs in patients and it may take between 2 to 8 weeks.44 It has been shown that nortriptyline has fewer side effects, which necessitates discontinuation, than amitriptyline (5/31 and 10/31 respectively), although both have similar efficacy on neuropathic pain (based on pain, disability, sleep, depression, and satisfaction ratings, both medications were similar in 21/31 patients).21 Regardless, physicians prescribing nortriptyline should be aware of the side effects and drug interactions.4,19,20 One especially concerning vision threatening side effect is angle closure glaucoma, which can occur due to the anticholinergic effect of nortriptyline, and it should be used with caution in patients with angle-closure glaucoma risk.4,17,45 However, the risk is very low, and none of our patients experienced this side effect. Interestingly, there was no report of worsening of dry eye symptoms in our cohort. Moreover, life-threatening side effects were not observed, and all side effects were transient and resolved after discontinuation.
The major limitations of our study are its retrospective nature along with relatively small sample size and multiple cofactors. However, the severity of pain, and significant impact on QoL, and the constrained availability of effective treatment options, the current study is of significant importance in the management of these patients and provides the largest study, to date, in NCP patients. Patients with neuropathic pain often receive combination therapy with multiple agents.46 The most commonly used concomitant medications in our cohort was topical steroids and autologous serum tears. Gabapentinoids and carbamazepine were the most commonly used concomitant oral neuropathic pain medication. One of the important factors in the treatment of neuropathic pain is the failure to assess or treat comorbidities that make coping with chronic pain difficult.47 Among these comorbidities, depression (16.7%) and anxiety (10.0%) were the most common ones in the current study. Although 56.7% of the patients had a previous history of DED, all patients had none to minimal ocular surface findings at the time of the study. Several variables among the patients were controlled with mixed model analyses. Despite these limitations, we show significant efficacy of nortriptyline. Nevertheless, our study warrants additional prospective trials with the use of TCAs for NCP with a centralized component.
In summary, nortriptyline resulted in a noteworthy pain decrease in patients with a centralized component of NCP. Additional prospective, multi-center, placebo-controlled studies are required to confirm our findings. However, it is important to note that in our experience, there is no single treatment that is effective in all patients with NCP, and that multiple drugs, as also found by others for non-ocular neuropathic pain,22 or a multi-modal approach may be necessary to achieve pain relieve.
Supplementary Material
DISCLOSURE/CONFLICT OF INTEREST
Financial support: NIH R61-NS113341 (PH), Massachusetts Lions Eye Research Fund Inc. (PH), Lions Club International Foundation (PH), Tufts Medical Center Institutional support (PH), American Society for Cataract and Refractive Surgery (MIM), the Scientific and Technological Research Council of Turkey (MCO), Gazi University Institutional Support (MCO). The sponsors or funding organizations had no role in the design or conduct of this research.
NIH R61-NS113341 (PH), Massachusetts Lions Eye Research Fund Inc. (PH), Lions Club International Foundation (PH), Tufts Medical Center Institutional support (PH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work has been previously presented, in part, at the Association for Research in Vision and Ophthalmology Annual Meeting, 2019, Vancouver, Canada and at the American Academy of Ophthalmology Annual Meeting, 2019, San Francisco, CA.
No conflicting relationship exists for any author.
REFERENCES
- 1.Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What Causes Eye Pain? Curr Ophthalmol Rep. 2015;3(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15(3):404–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond). 2015;29(3):301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dieckmann G, Goyal S, Hamrah P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology. 2017;124(11S):S34–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galor A, Moein HR, Lee C, et al. Neuropathic pain and dry eye. Ocul Surf. 2018;16(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul Surf. 2015;13(3):250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamrah P, Qazi Y, Shahatit B, et al. Corneal Nerve and Epithelial Cell Alterations in Corneal Allodynia: An In Vivo Confocal Microscopy Case Series. Ocul Surf. 2017;15(1):139–151. [DOI] [PubMed] [Google Scholar]
- 8.Goyal S, Hamrah P. Understanding Neuropathic Corneal Pain--Gaps and Current Therapeutic Approaches. Semin Ophthalmol. 2016;31(1–2):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morkin MI, Hamrah P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul Surf. 2018;16(1):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal S, Colon C, Kheirkhah A, Hamrah P. Efficacy of autologous serum tears for treatment of neuropathic corneal pain. Ocul Surf. 2019;17(3):532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78(3):513–525. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10(1):2–14. [DOI] [PubMed] [Google Scholar]
- 13.Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, Galor A. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. The British journal of ophthalmology. 2017;101(2):227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen HH, Yosipovitch G, Galor A. Neuropathic symptoms of the ocular surface: dryness, pain, and itch. Curr Opin Allergy Clin Immunol. 2017;17(5):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theophanous C, Jacobs DS, Hamrah P. Corneal Neuralgia after LASIK. Optometry and vision science : official publication of the American Academy of Optometry. 2015;92(9):e233–240. [DOI] [PubMed] [Google Scholar]
- 16.Galor A, Covington D, Levitt AE, et al. Neuropathic Ocular Pain due to Dry Eye is Associated with Multiple Comorbid Chronic Pain Syndromes. The journal of pain : official journal of the American Pain Society. 2016;17(3):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obata H Analgesic Mechanisms of Antidepressants for Neuropathic Pain. International journal of molecular sciences. 2017;18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Max MB. Treatment of post-herpetic neuralgia: antidepressants. Ann Neurol. 1994;35 Suppl:S50–53. [DOI] [PubMed] [Google Scholar]
- 20.Kremer M, Salvat E, Muller A, Yalcin I, Barrot M. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience. 2016;338:183–206. [DOI] [PubMed] [Google Scholar]
- 21.Watson CP, Vernich L, Chipman M, Reed K. Nortriptyline versus amitriptyline in postherpetic neuralgia: a randomized trial. Neurology. 1998;51(4):1166–1171. [DOI] [PubMed] [Google Scholar]
- 22.Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009;374(9697):1252–1261. [DOI] [PubMed] [Google Scholar]
- 23.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crane AM, Feuer W, Felix ER, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. The British journal of ophthalmology. 2017;101(9):1238–1243. [DOI] [PubMed] [Google Scholar]
- 25.Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology. 2016;123(7):1458–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derry S, Wiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finnerup NB, Attal N. Pharmacotherapy of neuropathic pain: time to rewrite the rulebook? Pain Manag. 2016;6(1):1–3. [DOI] [PubMed] [Google Scholar]
- 28.Onghena P, Van Houdenhove B. Antidepressant-induced analgesia in chronic non-malignant pain: a meta-analysis of 39 placebo-controlled studies. Pain. 1992;49(2):205–219. [DOI] [PubMed] [Google Scholar]
- 29.Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399–409. [DOI] [PubMed] [Google Scholar]
- 30.Paqueron X, Conklin D, Eisenach JC. Plasticity in action of intrathecal clonidine to mechanical but not thermal nociception after peripheral nerve injury. Anesthesiology. 2003;99(1):199–204. [DOI] [PubMed] [Google Scholar]
- 31.Kimura M, Saito S, Obata H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neuroscience letters. 2012;529(1):70–74. [DOI] [PubMed] [Google Scholar]
- 32.Dick IE, Brochu RM, Purohit Y, Kaczorowski GJ, Martin WJ, Priest BT. Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. The journal of pain : official journal of the American Pain Society. 2007;8(4):315–324. [DOI] [PubMed] [Google Scholar]
- 33.Raja SN, Haythornthwaite JA, Pappagallo M, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59(7):1015–1021. [DOI] [PubMed] [Google Scholar]
- 34.Chandra K, Shafiq N, Pandhi P, Gupta S, Malhotra S. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial--the GONIP Trial. Int J Clin Pharmacol Ther. 2006;44(8):358–363. [DOI] [PubMed] [Google Scholar]
- 35.Panerai AE, Monza G, Movilia P, Bianchi M, Francucci BM, Tiengo M. A randomized, within-patient, cross-over, placebo-controlled trial on the efficacy and tolerability of the tricyclic antidepressants chlorimipramine and nortriptyline in central pain. Acta Neurol Scand. 1990;82(1):34–38. [DOI] [PubMed] [Google Scholar]
- 36.Khoromi S, Cui L, Nackers L, Max MB. Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain. 2007;130(1–2):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vase L, Skyt I, Hall KT. Placebo, nocebo, and neuropathic pain. Pain. 2016;157 Suppl 1:S98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. [DOI] [PubMed] [Google Scholar]
- 39.Bowsher D Factors influencing the features of postherpetic neuralgia and outcome when treated with tricyclics. Eur J Pain. 2003;7(1):1–7. [DOI] [PubMed] [Google Scholar]
- 40.Stonecipher K, Perry HD, Gross RH, Kerney DL. The impact of topical cyclosporine A emulsion 0.05% on the outcomes of patients with keratoconjunctivitis sicca. Current medical research and opinion. 2005;21(7):1057–1063. [DOI] [PubMed] [Google Scholar]
- 41.Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98(1–2):195–203. [DOI] [PubMed] [Google Scholar]
- 42.Fraunfelder FT, Sciubba JJ, Mathers WD. The role of medications in causing dry eye. Journal of ophthalmology. 2012;2012:285851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson JC, Kennedy JS, Pollock BG, et al. Treatment of major depression with nortriptyline and paroxetine in patients with ischemic heart disease. Am J Psychiatry. 1999;156(7):1024–1028. [DOI] [PubMed] [Google Scholar]
- 44.Society TBP. Information for Adult Patients Prescribed Nortriptyline for Treatment of Pain. britishpainsociety.org/static/uploads/resources/files/FPM-Nortriptyline_0.pdf. Published 2014. Accessed2020.
- 45.Lachkar Y, Bouassida W. Drug-induced acute angle closure glaucoma. Curr Opin Ophthalmol. 2007;18(2):129–133. [DOI] [PubMed] [Google Scholar]
- 46.Schug SA, Parsons B, Almas M, Whalen E. Effect of Concomitant Pain Medications on Response to Pregabalin in Patients with Postherpetic Neuralgia or Spinal Cord Injury-Related Neuropathic Pain. Pain Physician. 2017;20(1):E53–E63. [PubMed] [Google Scholar]
- 47.Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5 Suppl 1:S9–S27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


