Abstract
Background
Obesity is associated with impaired primary and secondary immune responses to influenza infection, with T cells playing a critical role. T cell function is highly influenced by the cellular metabolic state; however, it remains unknown how altered systemic metabolism in obesity alters T cell metabolism and function to influence immune response. Our objective was to identify the altered cellular metabolic state of T cells from obese mice so that we may target T cell metabolism to improve immune response to infection.
Methods
Mice were fed normal chow or high-fat diet for 18–19 weeks. Changes in T cell populations were analyzed in both adipose tissue and spleens using flow cytometry. Splenic T cells were further analyzed for nutrient uptake and extracellular metabolic flux. As changes in T cell mitochondrial oxidation were observed in obesity, obese mice were treated with metformin for six weeks and compared to lean control mice or obese mice undergoing weight loss through diet switch; immunity was measured by survival to influenza infection.
Results
We found changes in T cell populations in adipose tissue of high-fat diet-induced obese mice, characterized by decreased proportions of Treg cells and increased proportions of CD8+ T cells. Activated CD4+ T cells from obese mice had increased glucose uptake and oxygen consumption rate (OCR), compared to T cells from lean controls, indicating increased mitochondrial oxidation of glucose. Treatment of isolated CD4+ T cells with metformin was found to inhibit OCR in vitro and alter the expression of several activation markers. Lastly, treatment of obese mice with metformin, but not weight loss, was able to improve survival to influenza in obesity.
Conclusions
T cells from obese mice have an altered metabolic profile characterized by increased glucose oxidation, which can be targeted to improve survival against influenza infection.
Introduction
Obesity is a metabolic disorder that has reached epidemic proportions in developed countries throughout the world (1). Obesity is characterized by an excess accumulation of fat, resulting in an increase in adipose tissue mass, and associated with an increased risk of cardiovascular disease and diabetes, along with numerous other disorders of health (2). One such disorder of health associated with obesity is the dysregulation of the immune system characterized by adipose and systemic inflammation leading to insulin resistance, a predisposition to developing autoimmune disease, and an impairment of protective immunity (3–5). Specifically, over the last decade, obesity has been identified as an independent risk factor for increased morbidity and mortality from influenza infection (6–8). Moreover, both human and murine studies have implicated T cells as a critical regulator of obesity-associated impairment in response to influenza, and we have found that obese mice and humans have decreased primary and memory T cell responses to influenza infection and vaccination (9–12).
Although obesity is well understood to be a disorder of systemic metabolism, the mechanistic link between the systemic metabolic changes of obesity and altered immune response have not yet been elucidated. This gap in knowledge precludes the development of strategies to prevent and treat influenza in the hundreds of millions of obese individuals worldwide at increased risk of dying from influenza virus infection (13). For that reason, we sought to determine the effect of systemic metabolic dysfunction in obesity on T cell metabolic reprogramming. It is now well-established that T cell function and metabolism are linked, and that metabolic reprograming of T cells can alter T cell differentiation, survival, and function (14, 15). In general, resting T cells utilize a mix of glucose, amino acids, and fatty acids and burn these fuels in the mitochondria in a metabolic program characterized by oxidative metabolism. Upon activation, effector T cells generally increase both glucose and glutamine metabolism in order to generate biomass to support T cell growth and proliferation, whereas memory T cells and regulatory T cells rely more heavily on fatty acid oxidation to fuel immune surveillance and suppressive function (16). There are additional nuances in the metabolism of T cell subsets that have also been described in previous studies (17–19). We recently reported that splenic T cells from influenza-infected obese mice have increased oxidative metabolism compared to splenic T cells from influenza-infected lean mice. Surprisingly, weight loss did not restore these T cell metabolic defects, nor did it improve the memory T cell response to influenza infection in our studies (10). This result suggests that altered T cell metabolism in obesity may contribute to T cell dysfunction and impaired influenza memory response, and changing T cell metabolism directly by specifically inhibiting T cell oxidative metabolism may restore T cell function.
Herein, we examine the changes in T cell metabolism in a mouse model of high fat diet-induced obesity (DIO), and we show that activated CD4+ T cells from DIO mice have increased glucose uptake and elevated oxidative metabolism, consistent with increased glucose oxidation, and not accompanied by any change in the expression of mitochondrial protein complexes. The commonly prescribed anti-diabetic biguanide drug metformin has been previously reported to inhibit T cell oxidative metabolism and prevent allograft rejection (20). We therefore tested the direct effect of metformin on wildtype CD4+ T cells in vitro and found that metformin reduced T cell oxidative metabolism while simultaneously altering T cell activation marker expression. We then compared influenza survival in lean mice, DIO mice, DIO mice treated with dietary weight loss, and DIO mice treated with metformin orally, and we found that metformin treatment improved the survival of influenza-infected obese mice, suggesting that methods that reverse obesity-associated T cell metabolic dysfunction in vivo may also improve immune function in obesity.
Materials and Methods
Animals
Eighteen-week old diet-induced obese (DIO) C57BL/6J and age-matched wildtype (control) C57BL/6J male mice were obtained from Jackson Laboratory (Bar Harbor, ME) and allowed 2 −3 weeks of acclimation during which they were maintained on high fat diet (HFD, 60 kcal% fat, Research Diets, New Brunswick, NJ) or normal chow diet (LabDiet, St. Louis, MO). Mice were group housed (5 per cage), maintained at ambient temperature, and given ad libitum access to food and water. DIO mice and age-matched controls were utilized at approximately 21–22 weeks of age, which was equivalent to 18–19 weeks on HFD. All animal protocols were approved by the Institutional Animal Care and Use Committees (IACUC) at Duke University.
Stromal vascular fraction preparation and T cell isolation
DIO and control mice were euthanized by CO2 inhalation. Mice were perfused with ice cold PBS by injecting 20 ml of buffer into the left ventricle and cutting the right atrium to prevent recirculation. Spleens and epididymal visceral adipose tissue (VAT) were collected. Stromal vesicular fraction (SVF) from VAT was prepared as previously described (10). For the isolation of CD4+ or CD8+ T cells, splenocytes were prepared as previously described (10), and CD4+ or CD8+ cells were isolated by magnetic negative selection (Miltenyi Biotec, Auburn, CA) following manufacturer protocol. Cells were activated by plate bound 1 μg/ml anti-CD3 and 5 μg/ml anti-CD28 antibodies (eBioscience, Fisher Scientific, Hampton, NH) for 48 hours then collected for further analysis.
Flow cytometry and nutrient uptake assays
For the identification of regulatory T cells (Treg cells) the following antibodies were used: APC/Fire 750 Rat anti-mouse CD45 (Biolegend, San Diego, CA), BV605 Armenian Hamster anti-mouse CD3e (BD BioSciences, Franklin Lakes, NJ), Pacific Blue rat anti-mouse CD4 (Biolegend), APC rat anti-moue CD25 (Biolegend), Alexa Fluor 488 rat anti-mouse Foxp3 (Biolegend). Between 500,000 and one million splenocytes or SVF cells were fixed and permeabilized using the Foxp3 Transcription Factor Staining Buffer kit (eBioscience) and stained for Foxp3 following the manufacturer instructions. For the identification of effector T cells (Th1 and Th17) and evaluation of their function, the following antibodies were used: APC/Fire 750 Rat anti-mouse CD45 (Biolegend), BV605 Armenian Hamster anti-mouse CD3e (BD BioSciences), Pacific Blue rat anti-mouse CD4 (Biolegend), PE/Cy7 rat anti-mouse CD8a (Biolegend), APC rat anti-mouse IL-17A (eBioscience) and FITC rat anti-mouse IFNγ (Biolegend). Cells were stimulated for 4.5 hours in complete media containing Golgi Plug (2 μg/ml) (BD Biosciences), PMA (50 ng/ml) (Sigma-Aldrich, St. Louis, MO), and ionomycin (1 μg/ml) (Sigma-Aldrich), then permeabilized and fixed with Cytofix/Cytoperm kit (BD Biosciences) and stained for IFNγ, IL-17A following the manufacturer’s protocol. All samples were acquired on a BD FACSCanto II flow cytometer, and data was analyzed using FlowJo (Treestar, Ashland, OR).
To determine glucose uptake by flow cytometry, cells were incubated for 30min in RPMI 1640 glucose-free media containing 0.5% heat inactivated FBS and 100 μM 2NBDG (Thermo Fisher, Waltham, MA), after which the cells were washed with FACS buffer and stained for surface markers, as previously described. To determine fatty acid uptake, cells were incubated in PBS containing 0.1% fatty acid free BSA and 5 μM BODIPY™ 500/510 C1, C12 (Thermo Fisher) for 30 min, then washed and stained for surface markers.
Metabolic flux assays
Activated or resting CD4+ or CD8+ T cells were washed with Seahorse XF RPMI 1640 media (Agilent, Santa Clara, CA) and plated at a density of 250,000 cells /well (50 μL) in a Seahorse XFe96 plate (Agilent) pre-coated with Cell-Tak (Corning, Corning, NY). After spinning down the plate at 200 rpm for 1 min, the plate was incubated for 30 min in a humidified 37oC incubator in the absence of CO2. Seahorse XF RPMI 1640 media (130 μL) was added and the plate was incubated for an additional 20 min. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured using a Seahorse XFe96 Analyzer (Agilent). Data were collected under basal conditions and after the addition of the following drugs: 1 μM oligomycin (Sigma-Aldrich), 0.5 μM flurorcarbonyl cynade phenylhydrazone (FCCP) (Sigma-Aldrich), and 0.75 μM rotenone (Sigma-Aldrich) with 1.5 μM antimycin A (Sigma-Aldrich).
Metformin treatment of CD4+ T cells ex vivo
Isolated CD4+ cells from C57BL/6J mice were activated by plate bound 1 μg/ml anti-CD3 and 5 μg/ml anti-CD28 antibodies for 24 hours, then treated with 2 mM metformin (Sigma-Aldrich) prepared in PBS for an additional 24 hours, after which they were collected for further analysis.
Metformin treatment and influenza infection in vivo
Wildtype control and DIO male mice were purchased and acclimated for 2–3 weeks in our animal facility. Following 12 weeks on HFD, obese mice either remained untreated, were switched to normal chow to induce weight loss, or were treated with 250 mg/kg of metformin (Sigma-Aldrich) in drinking water (mouse weight and water consumption were monitored 3 times per week and metformin dose was adjusted accordingly). Following 6 weeks of either weight loss or metformin, mice were lightly anesthetized with isoflurane and infected intranasally with 0.005 HAU per 50μL (TCID50 2.39 ×107) PBS of the H1N1 influenza virus A/Puerto Rico/8/34 (PR8, American Type Culture Collection, Manassas, VA). Body weight was monitored daily following infection. Mice reaching more than 30% body weight loss or other terminal experimental endpoints, such as impaired ambulation or respiratory status, were euthanized by CO2 inhalation.
Histopathology
Lungs were collected and fixed in 10% formaldehyde for 24 hours then washed and stored in 70% ethanol. The tissues were embedded in paraffin and stained for H&E by the Duke University Histopathology Core Facility. Lung inflammation was scored as previously described (21).
Viral titer quantification
Lung tissue was homogenized in 0.2 mL of minimal essential medium (MEM) and centrifuged at 9000 g for 20 minutes. Supernatant was serially diluted starting at 1:10 dilution in MEM containing 20 mg/L trypsin. 100 μL of the supernatant dilutions were added to the 80% confluent MDCK after media removal in replicates of six in a 96 well plate and cultured for 5 days at 37°C at 5% CO2. A suspension of 0.5% of Turkey red blood cells was added to each well and incubated at room temperature. TCID50 was determined by the Reed and Muench calculation (22).
Western blot
Activated CD4+ T cells from control and DIO mice were washed with ice cold PBS and lysed with RIPA buffer. After protein determination using Bio-Rad DC protein determination kit (Bio-Rad, Hercules, CA), 40 μg of protein was run on a Bio-Rad TGX 8–16% gel (Bio-Rad), and transferred to PVDF membrane. Membranes were then blocked and probed following the manufacturer instructions with the following antibodies: Total OXPHOS Rodent WB Antibody Cocktail (Abcam, Cambridge, United Kingdom), rabbit anti-mouse GLUT1 (Boster, Pleasanton, CA), rabbit anti-mouse GLUT3 (Boster), rabbit anti-mouse GLUT6 (Boster) and rabbit anti-mouse β-actin (Cell Signaling Technology, Danvers, MA).
Statistical analysis
T-test with Welch’s correction was used to compare groups assuming Gaussian distribution. To analyze differences in survival, log-rank (Mantel-Cox) test was used. All statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA). All data was determined as significant by p<0.05.
Results
Mice on high fat diet display limited changes in VAT T cells, but not lymphoid T cells
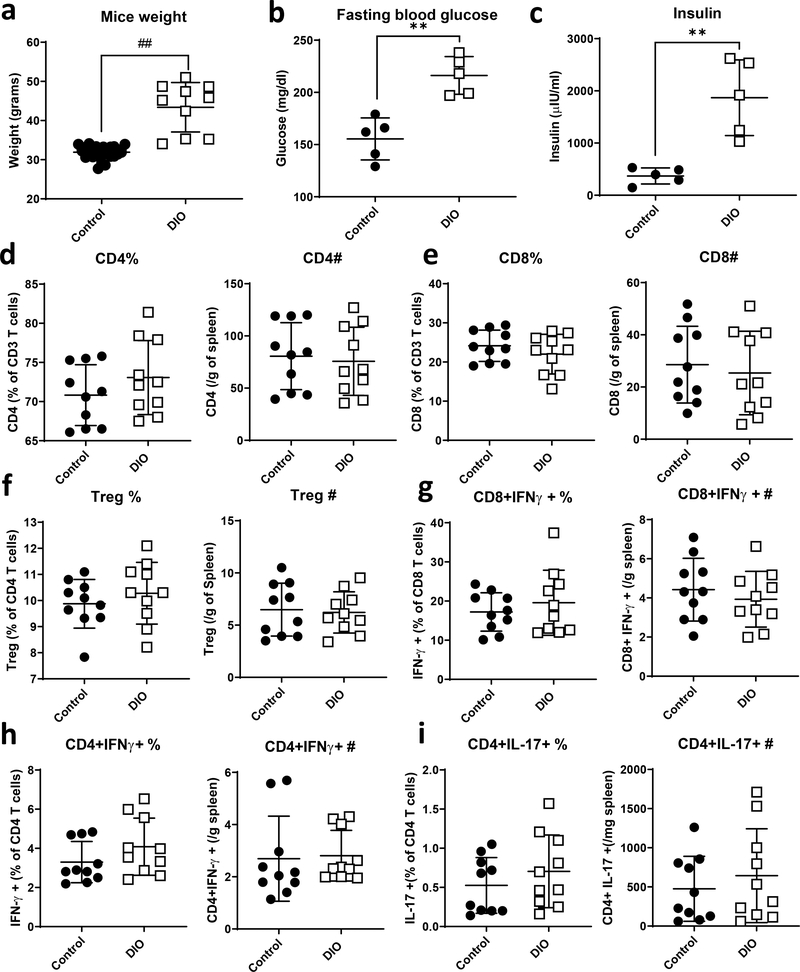
To study the effect of obesity on T cells, we used the high-fat diet induced obese (DIO) mouse model. Mice were placed on chow or high fat diet (HFD; 60 kcal% fat) at weaning and remained on their corresponding diet for 18–19 weeks. DIO mice displayed a phenotype similar to human obesity with an increase in body weight, fasting blood glucose and serum insulin, as well as increased visceral adipose tissue (VAT) mass, as compared to lean controls (Fig. 1a–c and S1A). DIO mice also had an increase in VAT stromal vascular fraction (SVF) cell number compared to lean control mice, indicating an increase in adipose tissue inflammation (Fig S1B). We then isolated splenocytes and SVF cells from VAT in lean versus DIO mice and examined the proportions and numbers of T cell populations by flow cytometry. In the spleen, we observed a trend increase in CD4+ T cell proportion, but no change in CD4+ T cell number, and no change in either CD8+ T cell proportion or number in DIO mice compared to lean control mice (Fig. 1d, e). VAT T cells, on the other hand, showed a significant decrease in CD4+ T cell proportion, but not number, as well as a significant increase in CD8+ T cell proportion and number in DIO mice compared to lean controls (Fig. S1C, D). Upon examining CD4+ T cell subpopulations, we found a sharp decrease in the Treg cell population in VAT from DIO mice compared to lean controls, as well as a decrease in IL-17 secreting CD4+ T cells in VAT from DIO mice compared to lean controls (Fig. S1E, H). There was no change in the proportion or number of IFNγ expressing CD4+ or CD8+ T cells in VAT in DIO mice compared to normal chow fed controls (Fig. S1F, G), nor in the number or proportion of Treg cells, IFNγ expressing CD4+ or CD8+ T cells, or IL-17 expressing CD4+ T cells in splenocytes from DIO mice compared to lean controls (Fig. 1f–i).
Figure 1. High fat diet induced obese (DIO) mice have human obesity characteristics with no significant change in the proportions of peripheral T cells.
(a) C57BL/6J mice were placed on 60% high fat diet (DIO) or normal chow (control) at 3 weeks of age following weaning. Body weights were measured following 18–19 weeks on diet. (b) Fasting blood glucose was measured from peripheral blood prior to euthanization. (c) Insulin was measured from serum. (d-i) Splenocytes were isolated, stained and analyzed by flow cytometry for CD4 (d), CD8 (e), CD4+CD25+Foxp3+ (f), CD8+IFNγ+ cells (g), CD4+IFNγ+ (h), and CD4+IL-17+ (i). Each dot represents a single mouse. Data were pooled from two independent experiments (n=5). T-test with Welch’s correction was used to compare groups. **p<0.01, ## p<0.000001.
CD4+ T cells from high fat diet-induced obese mice display altered cellular metabolism and nutrient uptake characteristics
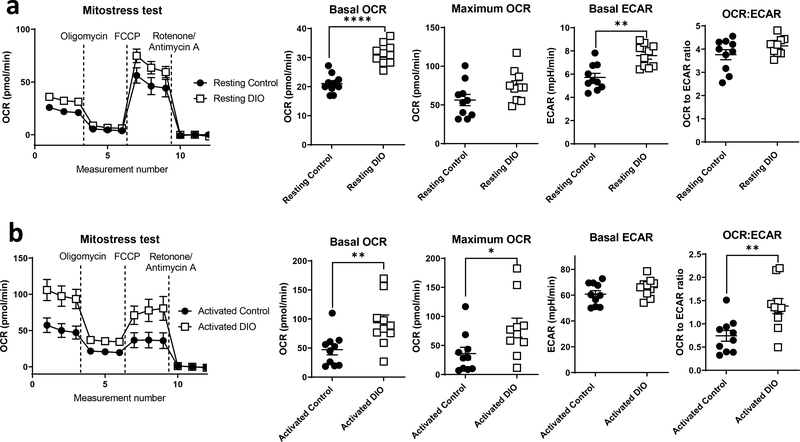
To better understand T cell metabolism functionally under obese conditions, we utilized extracellular flux analysis to measure both extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) as surrogate markers for glycolytic and oxidative metabolism, respectively. First, we isolated splenic CD4+ and CD8+ T cells from lean control versus DIO mice and examined these cells at rest or following activation with plate bound anti-CD3 and anti-CD28 antibodies for 48 hours. We then performed a Seahorse Mito Stress Test using the Seahorse XFe96 extracellular flux analyzer. Resting CD4+ T cells from DIO mice were generally more metabolically active than CD4+ T cells from lean control mice, with an increase in both basal OCR and ECAR, but without any significant change in the OCR: ECAR ratio (Fig. 2a). Upon activation, however, there was a specific and striking increase in both basal and max OCR in CD4+ T cells from DIO mice compared to lean controls, but no additional increase in ECAR in obesity beyond the already large increase in ECAR observed following T cell activation, resulting in an increased OCR:ECAR ratio consistent with increased CD4+ T cell oxidative metabolism (Fig. 2b). On the other hand, CD8+ T cells did not show any significant changes in metabolism under obese conditions, although there was a trend increase observed in basal OCR in activated CD8+ T cells from DIO mice compared to lean controls (Fig. S2A, B).
Figure 2. Obesity is associated with changes in CD4+ T cell metabolism.
CD4+ T cells were isolated from the spleens of lean (control) versus diet induced obese (DIO) mice. Cells were rested or activated by plate-bound anti-CD3 and anti-CD28 for 48hr. Cells were then plated at 250,000 cells per well, and extracellular flux analysis was performed using the Seahorse XFe96 Flux analyzer in response to the mitochondrial stress test with injections of 1 μM oligomycin, 0.5 μM flurorcarbonyl cynade phenylhydrazone (FCCP) and 0.75 μM rotenone with 1.5μM antimycin A. OCR and ECAR were measured for rested (a) and activated (b) CD4 cells. Data were pooled from two independent experiments (n=5). T-test with Welch’s correction was used to compare groups. *p<0.05, **p<0.01, ****p<0.0001.
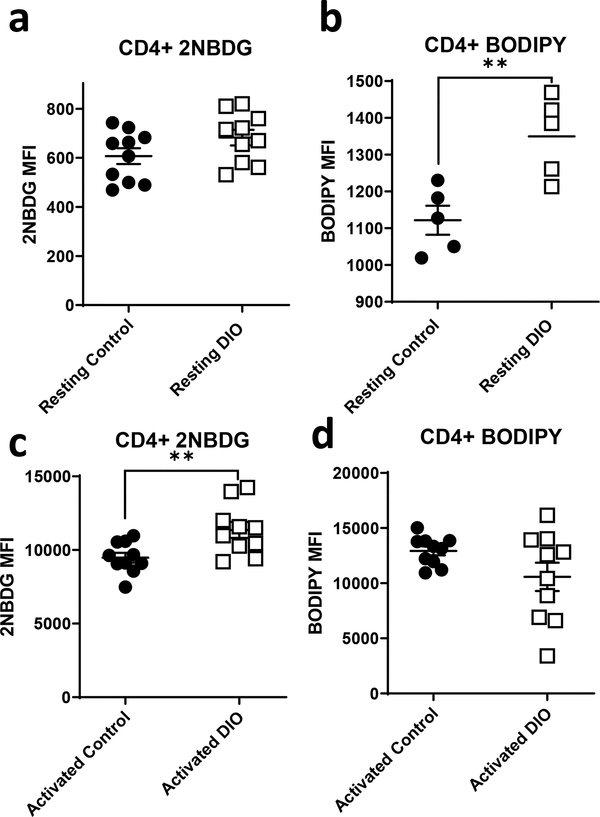
The observed changes in oxidative metabolism in CD4+ T cells from obese mice did not correlate with any change in expression of mitochondrial complexes, as demonstrated by Western blot using an OXPHOS antibody cocktail (Fig. S3A), but were accompanied by altered nutrient uptake (Fig 3). Specifically, resting CD4+ T cells from DIO mice showed equivalent glucose uptake but increased fatty acid uptake (Fig. 3a, b), whereas activated CD4+ T cells from DIO mice showing increased glucose uptake but no change in fatty acid uptake (Fig. 3c, d), as compared to CD4+ T cells from lean controls. However, this increase in glucose uptake in activated CD4+ T cells from DIO mice was not associated with changes in the expression of the glucose transporters GLUT1, GLUT3 and GLUT6, which are known to be induced in T cells following activation (Fig. S3B).
Figure 3. Obesity is associated with changes in CD4+ T cell nutrient uptake.
CD4+ T cells were isolated from lean (control) and diet induced obese (DIO) mice. Cells were rested or activated by plate-bound anti-CD3 and anti-CD28 for 48hr after which were stained for 2NBDG (a, c) and BODIPY (b, d) and analyzed by flow cytometry. Data were pooled from two independent experiments except for BODIPY uptake were the results represent data from one of the experiments both showed same trend and statistical significance (n=5). T-test with Welch’s correction was used to compare groups. **p<0.01.
Metformin inhibits mitochondrial oxidation in vitro and changes the expression of several T cell activation markers
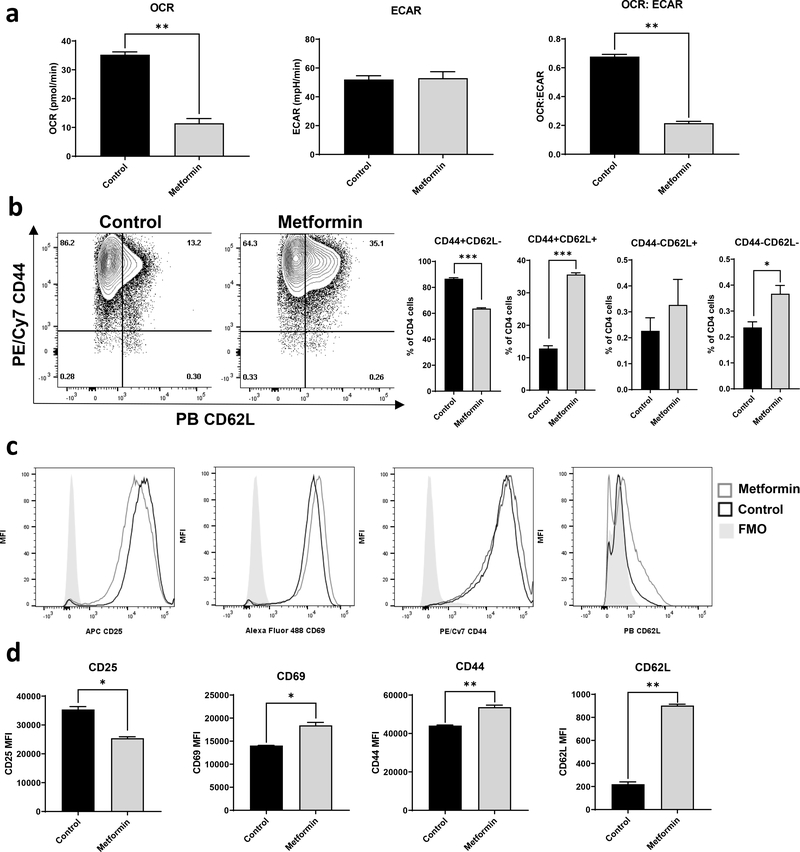
As shown above, CD4+ T cells display increased oxidative metabolism in both the resting state and following activation, and have an increase in the OCR:ECAR ratio following activation. This is consistent with our previously published data that T cells from influenza-primed obese mice challenged with a second influenza infection displayed increased OCR and OCR:ECAR ratios following infection, which did not reverse with weight loss (10). As weight loss was also insufficient in those studies to reverse T cell dysfunction and normalize response to influenza, we asked if normalization of T cell metabolism, specifically targeting increased T cell oxidative metabolism, could restore immunity to influenza in obese mice. To target increased T cell oxidative metabolism in obesity, we chose to utilize the commonly prescribed diabetic drug metformin, which works by disrupting electron transport in the mitochondria; the prevailing view is that it does so by inhibiting complex I of the electron transport chain, thereby decreasing mitochondrial oxidation (23). To test the ability of metformin to alter T cell oxidative metabolism, CD4+ T cells from lean control C57BL/6J mice were activated with plate bound anti-CD3 and anti-CD28 for 24 hours in vitro, followed by treatment with or without metformin at a concentration of 2 mM for an additional 24 hours. Activated CD4+ T cells treated with metformin had a significant and striking decrease in OCR with no accompanying change in ECAR, resulting in a lower OCR:ECAR ratio (Fig. 4a). These metformin-induced changes in activated CD4+ T cell OCR were accompanied by changes in several activation markers, including an increase in the expression of CD44, CD62L, and CD69 along with a decrease in CD25 (Fig. 4b–d). These results suggest that metformin has direct effects on both T cell function and T cell metabolism.
Figure 4. The antidiabetic drug metformin reverses T cell metabolic dysfunction in CD4+ T cells and enhances memory markers.
CD4+ T cells were isolated from C57BL/6J male mice and activated for 24 hours by plate bound anti-CD3 and anti-CD28 antibodies then treated with 2 mM metformin for an additional 24 hours. The cells were assayed for extracellular metabolic flux (a), and stained for activation marker expression (b-d). Splenocytes were pooled from 3 mice and data represents results from two independent experiments. T-test with Welch’s correction was used to compare groups. *p<0.05, **p<0.01, ***p<0.001.
Obese mice treated with metformin are protected against mortality from influenza
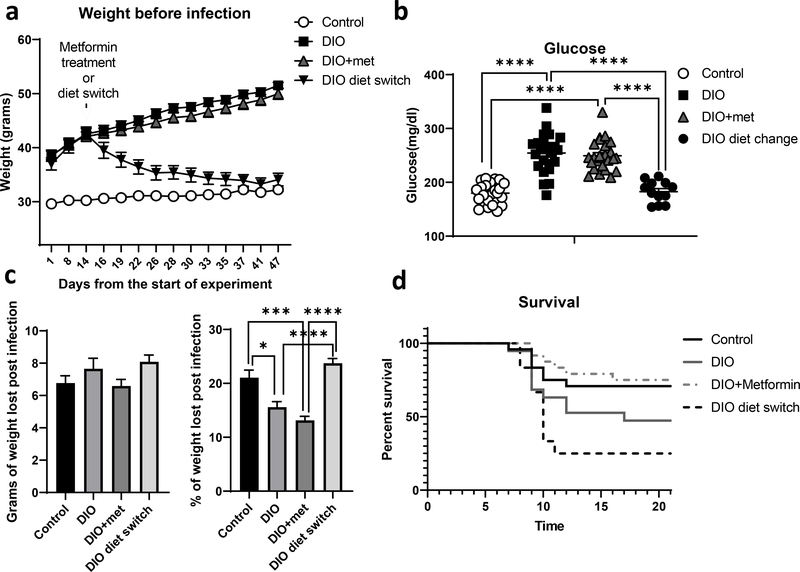
To assess the effect of metformin on immunity in DIO mice in vivo, we challenged mice with influenza infection. Our experimental conditions included four groups: lean mice on normal chow, DIO mice on HFD for 18 weeks, DIO mice on HFD for 12 weeks then switched to normal chow diet for 6 weeks to induce weight loss, and DIO mice on HFD for 18 weeks but treated with metformin in the drinking water (250 mg/kg) for the final 6 weeks of HFD. Body weights were measured weekly (Fig. 5a). The dose of metformin used in this experiment was insufficient to induce weight loss or restore glucose homeostasis, as measured by fasting blood glucose (Fig. 5b). All mice were then infected with the H1N1 influenza virus A/Puerto Rico/8/34 (PR8) intranasally, and infection was confirmed by weight loss (Fig. 5c). Interestingly, influenza-infected DIO mice pre-treated with metformin had improved survival compared to untreated obese mice, and significantly improved survival compared to obese mice that underwent weight loss prior to influenza infection (Fig. 5d). The ability of metformin to improve survival in obese mice infected with influenza was not associated with significant changes in lung pathology (Supplementary Fig. 4a and b), but was associated with a decrease in CD4+ T cell oxidative metabolism (Supplementary Fig. 4c). Surprisingly, weight loss had a negative effect on survival compared to DIO mice maintained on HFD (Fig. 5d).
Figure 5. Metformin improves survival to influenza infected obese mice independent of its effects on systemic metabolism.
Diet induced obese (DIO) mice were treated with or without 250 mg/kg metformin for 6 weeks, then infected intransally with PR8 influenza virus. Comaparison groups included lean mice fed normal chow (control) and a weight loss group fed high fat diet for 12 weeks followed by normal chow for 6 weeks (DIO diet switch). Body weights were monitored throughout the experiment (a), and fasting blood glucoses were measured prior to influenza infection (b). Infection-induced weight loss was determined for all groups (c), and survival was monitored (d), showing a significant increase in survival when obese mice were treated with metformin (n=24) compared to untreated obese mice (n=19) (Log-rank test, p<0.05) and a significant decrease in survival of the weight loss DIO mice (n=12) compared to control lean mice (n=24) (Log-rank test, P<0.01). The median survival for the DIO group and the diet switch group was 17 days and 10 days respectively, while it was undefined for the other groups due to the survival probabilty not reaching the 50 percent mark prior to the end of the experiment. Data were pooled from two independent experements. T-test with Welch’s correction was used to compare groups’ weight. *p<0.05, ***p<0.001, ****p<0.0001.
Discussion
Obesity impairs protective immunity and is an independent risk factor for increased morbidity and mortality from all strains of influenza infection. Moreover, despite vaccination, obese individuals are twice as likely to develop influenza or influenza-like illness. (6–8, 24). Both human and animal studies have identified T cell dysfunction as a key player in the dysfunctional immune response to influenza in the setting of obesity. It is now well established that T cell differentiation and function depend highly on the T cell metabolic state (25). In general, naïve T cells utilize a mixture of glucose, lipids, and amino acids and burn these fuels in the mitochondria to fuel immune surveillance. Upon encounter with antigen, activated T cells increase glucose and glutamine metabolism, with a striking increase in aerobic glycolysis, meaning that glucose-derived pyruvate is converted to lactate despite sufficient availability of oxygen to perform mitochondrial metabolism (25). Here, we have identified an alternative metabolic program for activated T cells from obese mice: an increase in glucose uptake along with an increase in mitochondrial oxidation, suggesting increased glucose oxidation.
To study the effect of obesity on T cell metabolism, we used the high fat diet induced obese (DIO) mouse model. This mouse model is more appropriate to study T cells in the context of obesity than ob−/− or db−/− mice, as leptin-deficient or leptin receptor-deficient mice have dysfunctional T cell responses. In fact, we have reported that leptin receptor signaling is required for increased T cell glucose metabolism following activation, and T cells lacking leptin receptor have impaired Th1 and Th17 responses in vivo (26, 27). For these experiments, we used male mice, similar to our previous study examining protective immunity and memory T cell function in obesity (10). Female DIO mice were not used because they are more resistant to weight gain on high fat diet and to the development of obesity-associated inflammation, making them less suitable for the study of human disease (28).
Our results here are consistent with our previous study, in which we saw increased OCR and OCR:ECAR ratios in T cells isolated from obese mice undergoing an influenza memory challenge (10). Interestingly, weight loss prior to influenza reinfection did not rescue abnormal T cell metabolism, nor did it restore T cell response to infection in that study (10). Here, we found that the increase in OCR in T cells from obese mice was not due to changes in the expression levels of mitochondrial protein complexes. This led us to examine levels of nutrient uptake, and we found a significant increase in glucose uptake following T cell activation in obese mice, with a similar trend in resting CD4+ T cells. This increase in glucose uptake was not accompanied by any significant change in the total expression of the glucose transporters that are known to be present on activated T cells (29). This may indicate that the increase in glucose uptake is due to changes in glucose transporter activity or surface expression (30, 31).
Because weight loss in our previous study did not reverse abnormal T cell function or metabolism in obese mice (10), we hypothesized that changes in T cell metabolism under obese conditions are responsible for T cell dysfunction seen in obesity. We therefore set out to determine if targeting T cell oxidative metabolism would improve influenza survival in a mouse model of obesity. To target T cell oxidative metabolism, we chose to utilize the commonly prescribed diabetic drug metformin, which works by disrupting electron transport in the mitochondria, presumably by inhibiting complex I of the electron transport chain, and thereby decreasing mitochondrial oxidation (23). Consistent with previous reports (20), we found that T cells treated with metformin had decreased OCR in vitro. We also saw changes in several activation markers in activated T cells treated with metformin, including increased expression of both CD62L and CD44. CD62L is commonly expressed on naïve T cells (32) but its expression on memory T cells is associated with better recall response (33). CD44 is expressed on effector and effector memory T cells and is known to support T cell motility, increase tonic T cell activation, and improve T cell survival (34). CD69 was also found to be increased on activated T cells following metformin treatment, and is associated with resident memory T cells. On the other hand, activated T cells treated with metformin had decreased CD25 (IL-2 receptor) expression, suggesting decreased proliferation potential in response to IL-2 secretion. These changes in activation markers in response to metformin suggest a unique and direct effect of metformin on T cell function.
Based on these findings that metformin can directly affect T cell function and metabolism in vitro, we assessed the ability of metformin to influence the survival of influenza-infected lean versus obese mice. DIO mice display increased mortality when infected with influenza virus (11). Moreover, T cells play an important role in eliminating influenza by inducing the death of virus-infected lung endothelial cells and secreting cytokines to induce B cell differentiation to mount a humoral immune response (35). In obese mice, T cell effector and memory functions are impaired (10, 36); this is characterized by a decrease in lung resident memory T cells along with a decrease in lung effector T cell IFNγ and granzyme B production (10). We treated DIO mice with metformin prior to influenza challenge. The metformin dose we used was insufficient to reverse systemic metabolic dysfunction, as these mice did not lose weight nor normalize their blood glucose levels. Thus, the improved survival we saw in metformin-treated obese mice was not due to an improvement in the overall metabolic status of the mice. How metformin improved survival to influenza through modulation of T cell metabolism is not yet understood. Two possible mechanisms include i) improving T cell effector function as judged by the increase in the expression of the previously mentioned activation markers, and ii) enhancing the resolution of the infection induced inflammation through the reduction in CD25 expression, making it easier to control the expansion of the effector T cells by regulatory T cells and limiting the tissue damage induced by the inflammatory cytokines secreted by those cells.
One interesting observation from our study is that weight loss did not improve influenza survival in this study, consistent with our previous study of T cell memory where we saw that weight loss did not rescue T cell dysfunction or T cell metabolism in response to influenza reinfection challenge (10). In fact, obese mice that underwent weight loss (DIO diet switch group) had decreased survival following influenza infection compared to the DIO group. Why that occurred is unknown; however, one possibility is that it is a combination of persistent T cell dysfunction from obesity, coupled with a decrease in CD4+ T cell effector function, as we have previously reported following fasting or calorie restriction, secondary to decreased circulating leptin, in our undernutrition models (26, 27).
Certainly there are limitations to our study, which must be acknowledged. First, the comprehensive metabolic studies described here were performed only on splenic T cells. Other T cell populations may have a significant contribution in obesity-associated protective immune dysfunction, such as tissue resident T cells, which may provide a key first line of defense against pathogens in relevant tissues. However, the low numbers of tissue resident T cells hinder the ability to examine their metabolic phenotype using extracellular flux analysis. Second, although our study shows metformin to enhance the survival of obese mice following influenza infection and normalize the metabolism of T cells in obesity, we cannot exclude the possibility that the effect of metformin on other immune cells may also contribute to enhanced survival. Lastly, we were compelled to use a higher concentration of metformin ex vivo compared to the concentration used in our in vivo studies, given the fact that the culture of T cells with metformin ex vivo occurred for a very short period of time (24 hours) compared to in vivo treatment (8 weeks).
Altogether, we present evidence that obesity induced-changes in T cell metabolism can be targeted to improve immunity. In addition, we show that the antidiabetic drug metformin may have benefits beyond blood glucose management to improve immunity in obese individuals. Our future studies will focus on determining the mechanism by which obesity alters activated T cell glucose oxidation and identifying additional targets to normalize T cell immunity in obesity. Better understanding of how T cells are altered in obesity and can be targeted to restore immunity will have major public health implications for an ever-growing obese population, and are particularly relevant in the context of both seasonal and pandemic viral infections.
Supplementary Material
Acknowledgements
This work was funded by NIH R01- DK106090, the Translating Duke Health: Controlling the Immune System Initiative, and the Derfner Foundation.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Contributor Information
Yazan Alwarawrah, Department of Pediatrics, Duke University School of Medicine.
Amanda G. Nichols, Department of Pediatrics, Duke University School of Medicine
William D. Green, Department of Nutrition, Gillings School of Global Public Health, University of North Carolina
William Eisner, Department of Pediatrics, Duke University School of Medicine, Durham.
Kaitlin Kiernan, Department of Immunology, Duke University School of Medicine, Durham.
Jonathan Warren, Department of Pediatrics, Duke University School of Medicine.
Laura P. Hale, Department of Pathology, Duke University School of Medicine
Melinda A. Beck, Department of Nutrition, Gillings School of Global Public Health, University of North Carolina
Nancie J. MacIver, Departments of Pediatrics, Immunology, and Pharmacology & Cancer Biology, Duke University School of Medicine
References
- 1.Engin A The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv Exp Med Biol. 2017;960:1–17. [DOI] [PubMed] [Google Scholar]
- 2.Pi-Sunyer X The medical risks of obesity. Postgrad Med. 2009;121(6):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13(9):981–1000. [DOI] [PubMed] [Google Scholar]
- 4.Andersen CJ, Murphy KE, Fernandez ML. Impact of Obesity and Metabolic Syndrome on Immunity. Adv Nutr. 2016;7(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8(12):709–16. [DOI] [PubMed] [Google Scholar]
- 6.Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One. 2010;5(3):e9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis. 2011;52(3):301–12. [DOI] [PubMed] [Google Scholar]
- 8.Neidich SD, Green WD, Rebeles J, Karlsson EA, Schultz-Cherry S, Noah TL, et al. Increased risk of influenza among vaccinated adults who are obese. Int J Obes (Lond). 2017;41(9):1324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond). 2012;36(8):1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebeles J, Green WD, Alwarawrah Y, Nichols AG, Eisner W, Danzaki K, et al. Obesity-Induced Changes in T-Cell Metabolism Are Associated With Impaired Memory T-Cell Response to Influenza and Are Not Reversed With Weight Loss. J Infect Dis. 2019;219(10):1652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137(5):1236–43. [DOI] [PubMed] [Google Scholar]
- 12.Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology. 2009;126(2):268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green WD, Beck MA. Obesity Impairs the Adaptive Immune Response to Influenza Virus. Ann Am Thorac Soc. 2017;14(Supplement_5):S406–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alwarawrah Y, Kiernan K, MacIver NJ. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front Immunol. 2018;9:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36(2):81–91. [DOI] [PubMed] [Google Scholar]
- 18.Ma EH, Bantug G, Griss T, Condotta S, Johnson RM, Samborska B, et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017;25(2):345–57. [DOI] [PubMed] [Google Scholar]
- 19.Ren W, Liao Y, Ding X, Jiang Y, Yan J, Xia Y, et al. Slc6a13 deficiency promotes Th17 responses during intestinal bacterial infection. Mucosal Immunol. 2019;12(2):531–44. [DOI] [PubMed] [Google Scholar]
- 20.Lee CF, Lo YC, Cheng CH, Furtmuller GJ, Oh B, Andrade-Oliveira V, et al. Preventing Allograft Rejection by Targeting Immune Metabolism. Cell Rep. 2015;13(4):760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milner JJ, Rebeles J, Dhungana S, Stewart DA, Sumner SC, Meyers MH, et al. Obesity Increases Mortality and Modulates the Lung Metabolome during Pandemic H1N1 Influenza Virus Infection in Mice. J Immunol. 2015;194(10):4846–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.REED LJ, MUENCH H. A SIMPLE METHOD OF ESTIMATING FIFTY PER CENT ENDPOINTS12. American Journal of Epidemiology. 1938;27(3):493–7. [Google Scholar]
- 23.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paich HA, Sheridan PA, Handy J, Karlsson EA, Schultz-Cherry S, Hudgens MG, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity (Silver Spring). 2013;21(11):2377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman NM, Boothby MR, Chi H. Metabolic coordination of T cell quiescence and activation. Nat Rev Immunol. 2019. [DOI] [PubMed] [Google Scholar]
- 26.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol. 2014;192(1):136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerriets VA, Danzaki K, Kishton RJ, Eisner W, Nichols AG, Saucillo DC, et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol. 2016;46(8):1970–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PloS one. 2012;7(9):e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18(4):1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieman HL, Horn SR, Jacobs SR, Altman BJ, Kornbluth S, Rathmell JC. An essential role for the Glut1 PDZ-binding motif in growth factor regulation of Glut1 degradation and trafficking. Biochem J. 2009;418(2):345–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272(5258):60–6. [DOI] [PubMed] [Google Scholar]
- 33.Hengel RL, Thaker V, Pavlick MV, Metcalf JA, Dennis G, Jr., Yang J, et al. Cutting edge: L-selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J Immunol. 2003;170(1):28–32. [DOI] [PubMed] [Google Scholar]
- 34.Baaten BJ, Li CR, Bradley LM. Multifaceted regulation of T cells by CD44. Commun Integr Biol. 2010;3(6):508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hufford MM, Kim TS, Sun J, Braciale TJ. The effector T cell response to influenza infection. Curr Top Microbiol Immunol. 2015;386:423–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010;184(6):3127–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.