Abstract
Polyhydroxyalkanoate (PHA) is the most promising solution to major ecological problem of plastic accumulation. The biodegradable and biocompatible properties of PHA make it highly demanding in the biomedical and agricultural field. The limited market share of PHA industries despite having tremendous demand as concerned with environment has led to knock the doors of scientific research for finding ways for the economic production of PHA. Therefore, new methods of its production have been applied such as using a wide variety of feedstock like organic wastes and modifying PHA synthesizing enzyme at molecular level. Modifying metabolic pathways for PHA production using new emerging techniques like CRISPR/Cas9 technology has simplified the process spending less amount of time. Using green solvents under pressurized conditions, ionic liquids, supercritical solvents, hypotonic cell disintegration for release of PHA granules, switchable anionic surfactants and even digestion of non-PHA biomass by animals are some novel strategies for PHA recovery which play an important role in sustainable production of PHA. Hence, this review provides a view of recent applications, significance of PHA and new methods used for its production which are missing in the available literature.
Keywords: Applications, Biodegradable plastic, Biosynthesis of PHA, Economic production, Molecular approach
Introduction
The consistent accumulation of conventional synthetic plastic in environment has compelled us to think that there is an urgent need to replace it with biodegradable plastics. Polyhydroxyalkanoate (PHA) is the most promising solution to this major ecological problem. The properties of PHA make it close to plastics hence PHA bioplastic makes a good alternate which is biodegradable and biocompatible. Chemically, PHAs are in vivo polymerized polyoxoesters of hydroxyalkanoates (Sowinski et al. 2010) which are produced by microorganisms when there is carbon rich and nutrient (nitrogen, phosphorous, magnesium or sulfur) limiting condition for their energy and carbon storage (Koller 2018a, b). PHAs are produced in the form of water insoluble granules within the cytoplasm of the cell. PHA help microorganisms to survive under environmental stress conditions, and it is the key mechanism for their survival (Saharan et al. 2014). During starvation, the presence of PHA protects the cellular components such as RNA and proteins (Vinet and Zhedanov 2010) and it also plays an important role in sporulation. It has potential protective mechanisms for bacterial cells when they are exposed to freezing and thawing (Obruca et al. 2016), osmotic shocks, oxidative pressure, desiccation, H2O2 supply, heavy metals and UV exposures (Obruca et al. 2020).
PHA was first discovered by Lemoigne in 1925 from Bacillus megaterium (Chee et al. 2010) in poly(3-hydroxybutyrate) (PHB) form. Other forms of PHA were discovered afterwards. In 1974, Wallen and Rohwedder (1974) extracted 3-hydroxyvalerate along with 3-Hydroxybutyrates and some higher molecular weight components from activated sewage sludge. Short chain length hydroxyacids of 11 different types were reported by Findlay and White (1983) from marine sediment extracts, also 3-Hydroxyheptanoate (3HHp) was detected in B. megaterium in same year. Since then the number of PHA being discovered is increasing, and nearly 150 different monomers of PHA have been discovered and the number is still increasing with the introduction of new techniques like modifying natural PHA by physical or chemical techniques and genetic modification of microorganisms to synthesize PHA with particular functional group (Tan et al. 2014). The developments have also been made in the sustainable production of PHA in many ways such as using inexpensive carbon sources, genetic engineering techniques and using different recovery methods which is discussed in this review in later sections. The use of recombinant strains should be focused as genetic and metabolic engineering has promoted novel methods for enhancing the PHA production. Many reviews on Polyhydroxyalkanoate and its production can be found but our review mainly focuses on the outlook of the biotechnological approaches, molecular engineering of the PHA producing strains and PHA recovery methods to increase overall yield of PHA which is not common in the currently available review articles.
Sources of PHA
Different species of bacteria and archaebacteria have been used to produce PHA in sufficient amount. New bacterial species which produce copolymers and recombinant strains have been used to produce large amount of PHA in industrial scale. Some bacterial species known for economical production of PHA are Ralstonia eutropha, Aspergillus eutrophus, Cupriavidus necator, Rhodobacter sphaeroides, Wautersia eutropha, Pseudomonas sp., Thermus thermophiles, Hydrogenophaga pseudoflava, Haloferax mediterranei, Saccharophagus degradans, Bacillus sp., Halomonas sp., Azohydromonas lata, Chromobacterium sp., Methylobacterium sp., Azotobacter sp., Burkholderia sp., Zobellella denitrificans, Dechloromonas sp., Comamonas sp., Aeromonas sp., Erwinia sp. and recombinant E. coli (Anjum et al. 2016).
Structure, classification and properties
PHA is a linear polyester which contains 3-hydroxy fatty acid monomers. Hydroxyl group from one monomer forms an ester bond with the carboxyl group of another monomer unit. The R group in the structure represents alkyl group. If the R group is methyl (CH3), the polymer is named as poly(3-hydroxybutyrate) as it will contain four carbons in the monomer unit, likewise if R is C3H7, it is called poly(3-hydroxyhexanoate) (Fig. 1). The chemical modification in PHA depends on the varying composition and size of the side chain substituents which are likely responsible for their different applications.
Fig. 1.
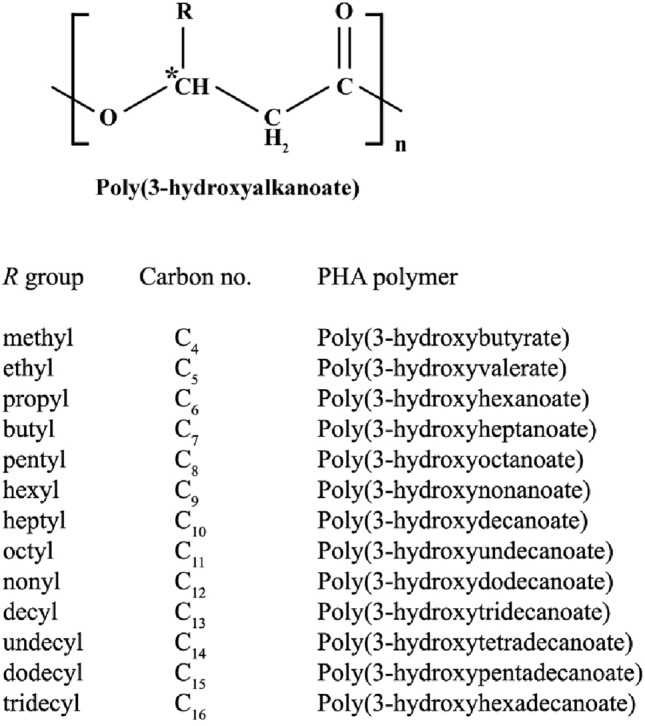
The general structure of PHA, where R can be different groups. If the R group is methyl (CH3) the polymer is named as poly(3-hydroxybutyrate) as it will contain four carbons in the monomer unit, likewise if R is C3H7, it is called poly(3-hydroxyhexanoate) (Tan et al. 2014)
Based on the number of carbons chain length, polyhydroxyalkanoates are classified into three classes on the basis of chain length, short chain length (scl) PHA, medium chain length (mcl) PHA and long chain length (lcl) PHA. Short chain length (scl) PHA consists of three to five carbon atoms. Some bacteria which produce scl-PHA are Azohydromonas lata and Cupriavidus necator. Medium chain length (mcl) PHA consists of 6–14 carbon atoms in the chain. Pseudomonas mendocina and Pseudomonas putida are two examples for mcl-PHA accumulating strains. Long chain length (lcl) PHA consists of more than 14 carbon atoms and some bacteria known for producing lcl-PHA are Aureispira marina and Shewanella oneidensis.
Depending on the type of PHA, the unique properties of PHAs vary physically and chemically. PHAs with different monomers have specific melting point, hydrophobicity, degree of crystallinity and glass transition temperature. Thermal and mechanical properties have been compared in Table 1. Properties of different types of PHAs are discussed below.
Table 1.
Comparison of thermal and mechanical properties of different types of PHA (Singh et al. 2015)
| PHA composition | Mol% | Tm (°C) | Tg (°C) | Elastic modulus (GPa) | E:B* (%) | Tensile strength (MPa) |
|---|---|---|---|---|---|---|
| Homo polymer | ||||||
| 3HB | 100 | 171–179 | 2.5–10 | 1.1–3.5 | 04–5.0 | 19–40 |
| 4HB | 100 | 53 | − 48 | 149 | 1000 | 104 |
| Copolymers | ||||||
| 3HB:HV | 95:5 | 170 | 2.2 | – | – | – |
| 80–90:10–20 | 137–156 | − 1.0 to 1.7 | 0.8–1.2 | 50–100 | 20–30 | |
| 70–8-:20–30 | 138–139 | − 6 to − 0.1 | 1.37 | 30 | 70 | |
| 50–60:40–50 | 113–138 | − 16 to − 10 | – | – | – | |
| 30:70 | 87 | − 13 | – | – | – | |
| 3HB:4HB | 95:5 | 169 | − 2 | 1.23 | 10.7 | 1.36 |
| 84:16 | 150 | − 7 | – | – | – | |
| 76:24 | 161 | − 5 | 0.79 | 22.2 | 0.87 | |
| 62:38 | 152 | − 10 | 0.66 | 48 | 2.98 | |
| 3HB:3HHx | 88–90:10–12 | 96–127 | − 1.2 | 0.5 | 113–400 | 9.4–21 |
| 3HB:4HV | 90:10 | 159 | – | – | 242 | 24 |
| Tercopolymer | ||||||
| 3HB:3HV:4HB | 73:8:19 | 131 | − 10 | 0.1 | 316 | 12 |
| 63:4:33 | – | − 14 | 0.1 | 937 | 9 | |
| 49:18:33 | – | − 16 | 0.03 | 554 | 2 | |
| 87.3 | − 21.1 | 0.14 | 9 | 4 | ||
| 11:23–24:55–56 | 92–100 | − 15 to − 17 | 0.4–0.6 | 5-Mar | 10-Sep | |
| 10:40:50 | 88 | − 13.7 | 0.12 | 300 | 9 | |
| 55 | − 51.6 | 0.13 | 430 | 14 | ||
| 3HB:3HV:3HHx | 75:13:12 | 101 | − 1.9 | 0.07–0.1 | 740–833 | 12.8–14.3 |
| 70:25:5 | 129 | − 7.2 | – | – | – | |
| 67:20:13 | 58–68 | − 6 to − 3.6 | – | – | – | |
| 56:43:1 | 155 | − 5.5 | – | – | – | |
| 48:24:28 | 54 | − 5.1 | – | – | – | |
| 3HB:3HHx:3HHx | 94:3:3 | 153–168 | 2 | – | – | – |
| 89:6:5 | 145 | − 7 | – | – | – | |
| 65:18:17 | 145–165 | − 11 | 0.19 | 7.4 | 3.86 | |
| 3HB:3HHx:3HO | 94:3:3 | 126 | − 4 | 0.39 | 15 | 22 |
| Other copolymers | ||||||
| 3HB:3HA | 98:2 | 150–167 | 1 | 0.95 | 16 | 26 |
| 94–96:4–6 | 133 | − 8 | 0.22 | 680 | 17 | |
| 3HB:3HV:3HHx:3H2MB:3H2MV | 90:4:1:4:1 | 171 | 4.6 | – | – | – |
| 84:7:2:5:2 | 137 | 2.8 | – | – | – | |
| 74:24:2 | 101 | 2.68 | – | – | – | |
| 12:63–78:1–6:5–6:4–14 | 90–98 | − 12 to − 14 | – | – | – | |
| 11:63:6:6:14 | 89 | − 14 | – | – | – | |
| 3HB:3HV:3H2MV | 7:82:11 | 96 | – | – | – | – |
| 3HB:3HP:33HHx:3HHx | 52:0:27:21 | 139 | − 12 | – | – | – |
| 51:3:25:44 | 148 | − 11 | 0.04 | 10.3 | 1 | |
| 28:3:25:44 | 105 | − 16 | – | – | – | |
| 3HB:3HHx:3HO:3HD | 89:6:4:1 | 111 | − 6 | 0.07–0.21 | 188–493 | 10-Apr |
| 3HB:3HV:3HHD:3HOD | 83–87:5–8:4:4–5 | 115–120 | − 13 to − 14 | 0.2 | 701–723 | 18 |
| 3HHx:3HO:3HD:3HDD:3HDDE:3HTDE | 1–3:13–38:30–42:5–12:0–37:tr:0–23 | 37–40 | − 49 to − 53 | – | – | – |
| 3HHx:3HO:3HD:3HDD | 1–15:0–93:0–92:0–56 | 53–75 | − 37 to − 44 | 0.19–0.35 | 188–346 | 5.8–8.7 |
*Elongation to break
Short chain length PHA, polyhydroxybutyrate (PHB)
Among different PHAs, PHB is the most studied compound. The unique properties of PHB resemble those of polypropylene such as thermoplastic process ability and complete water resistance (Hrabak 1992). It is insoluble in water and soluble in chlorinated hydrocarbons like chloroform. It does not float on water and can undergo anaerobic biodegradation when descends under the water. The density of crystalline PHB is 1.26 g/cm3 whereas the density of amorphous PHB is 1.18 g/cm3. It is very stiff and brittle material. It shows good oxygen permeability and is relatively resistant to hydrolytic degradation and UV radiations but shows susceptibility towards acids and bases. Monomers and oligomers of 3HB and some of the other PHAs have been known to be present naturally in human and animal blood stream which suggests that they are highly biocompatible and non-toxic and can be used for medical purposes (Koller 2018a, b). It also aids in the process of inducing osteogenesis because of its piezo-electric effect. It has high tensile strength of 40 MPa and melting temperature of 179 °C above which it starts to degrade. In addition, it is not sticky when melted which makes it significant for use in many industrial applications like in textiles (Jangra et al. 2018).
PHB copolymers
One of the commonly used copolymer is poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(3HB-co-3HV)) which has more flexibility, toughness, increased elongation to break, lower melting point, lower crystallinity and lesser stiffness as compared to PHB (Ciesielski et al. 2015). Another copolymer, poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) [P(3HB-co-3HH)] shows good thermo-mechanical properties of flexibility, strength, toughness, ductility and elasticity in addition with the physicochemical properties of polyesters such as dyeability and printability. It can also be blended with Polylactic acid (PLA) and thermoplastic starch to add more beneficial properties.
PHA terpolymers
A better alternate to copolymers is the terpolymers. In terpolymers, properties are enhanced by addition of more than one secondary monomer in combination of different concentrations. For example, P(3HB-co-3HV-co-3HHx) has comparatively higher amorphousness, elongation at break 408% and a tensile strength of 12 MPa when at concentration of 39 mol% 3HV and 3 mol% 3HHx while P(3HB-co-3HV-co-4HB) at concentration of 93 mol% 4HB and 3 mol% 3HV has elongation at break 430% and 33 MPa toughness which is similar to low-density polyethylene (LDPE) (Anjum et al. 2016).
PHA biosynthesis
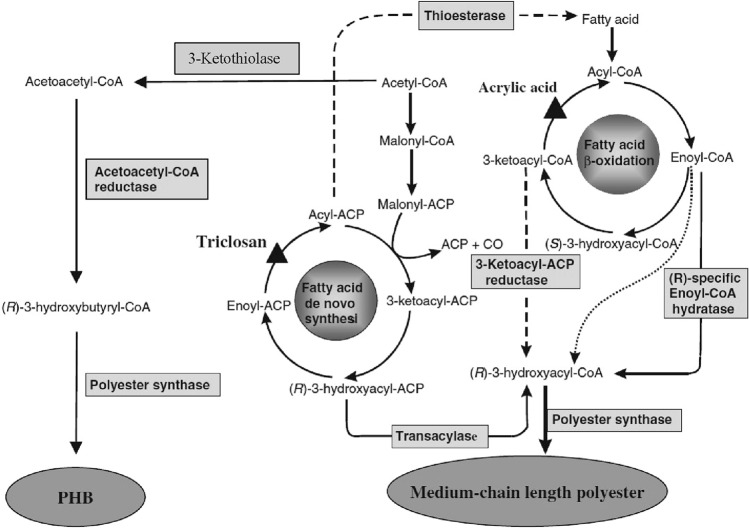
PHAs are intracellular energy and carbon materials produced by various prokaryotes under nutrient limiting conditions. PHA synthase is the main enzyme responsible for PHA production. A set of genes consisting of PHA synthase (PhaC), β-ketothiolase (PhaA) and NADPH-acetoacetyl-CoA reductase are co-localized and organized into PHA biosynthetic operon (Rehm 2003). The biosynthetic pathway of PHB, a scl-PHA starts when two molecules of acetyl-Co-A get condensed to form acetoacetyl-CoA with the help of the enzyme 3-ketothiolase (PhaA). The acetoacetyl-CoA is then reduced to (R)-3-hydroxybutyryl-CoA which is catalyzed by (R)-specific acetoacetyl-CoA reductase (PhaB) (Fig. 2). (R)-3-Hydroxybutyryl-CoA is finally polymerized to PHB by the enzyme PHA synthase (PhaC) (Urtuvia et al. 2018).
Fig. 2.
Metabolic routes towards biopolyester synthesis. Triangles depict targets for inhibitors enabling biopolyester synthesis. The biosynthetic pathway of PHB, a scl-PHA starts when two molecules of acetyl-Co-A get condensed to form acetoacetyl-CoA with the help of the enzyme 3-ketothiolase. The acetoacetyl-CoA is then reduced to (R)-3- hydroxybutyryl-CoA which is catalyzed by (R)-specific acetoacetyl-CoA reductase (Rehm 2006)
On the other hand, for mcl-PHA production, the intermediates of fatty acid metabolism are converted to (R)-3-hydroxyacyl-CoA. If the fatty acid β oxidation pathway is not followed, acetyl CoA is generated by the oxidation of carbon source and the intermediates of fatty acid de novo pathway are directed towards PHA biosynthesis with the help of the enzyme transacylase (PhaG). The (R)-3-hydroxyacyl moiety of the respective ACP (acyl carrier protein) thioester is transferred to CoA by this enzyme (Hoffmann et al. 2002). Whereas if the carbon source is oxidized by β-oxidation pathway, then enoyl-CoA is oxidized to (R)-3-hydroxyacyl-CoA by the enzyme enoyl-CoA hydratase (PhaJ) (Fukui et al. 1998). The PhaG and PhaJ encoding genes are co-regulated but not present within the PHA synthase operon.
The formation of PHA granules is done with the help of different proteins including phasins. Phasins are amphiphilic molecules that are responsible for the size and number of the granules of PHA formed (Pfeiffer and Jendrossek 2014).
Production of PHA in sustainable way
The ability of PHA to get modified by chemical and molecular-biological ways and attaining desired properties makes it applicable in many ways. Most suitable and popular use of PHA is in making bioplastic. Bioplastic comprises only 1% of the total annual production of 359 million tons of synthetic plastic. However, the awareness about environmental hazard caused by conventional plastic has risen the demand of bioplastic and the target set for its production has increased from current capacity of 2.11 million tons to 2.43 million tons in next 4 years globally. Currently PHA based bioplastic is only 1.2% of the total production of bioplastic. The main cause that PHA bioplastic is not so common is its higher production cost than conventional plastic. The PHA production costs two to three times more than the conventional plastic (Bioplastic market data 2019). Sustainable production of PHA includes engineering aspects for upstream processing to make adequate substrates accessible for microbial conversion, the intrinsic biotechnological cultivation process and feeding strategy, and the downstream processing to recover PHA as an intracellular product from the non-PHA biomass. Various microbiological, genetic, raw material-related, or process engineering strategies have been applied for the sustainable production, some of which are discussed here in detail.
Use of cheaper carbon sources
Microorganisms can utilize a number of carbon sources and use them when they are available in excess, for synthesizing PHAs. Huge number of different carbon sources from cheaper sources such as industrial waste, byproducts and agricultural wastes has been used in past few years for the production of PHA from different bacteria (Table 2). The market price of PHA based bioplastic was 15–17 times higher as compared to synthetic plastics in beginning but the efforts to reduce the production cost has actually reduced it but it is three times higher than the synthetic polymers. The use of industrial and agricultural waste for the production of PHA not only makes its production process economic but also helps in management of wastes generated in thousands of tonnes every year and reducing the environmental pollution.
Table 2.
PHA production from different bacterial isolates using various substrates
| S. no | Carbon source used | Bacterial source | PHA yield | PHA production | References |
|---|---|---|---|---|---|
| 1 | Coconut oil | Pseudomonas mendocina | 58% | – | Basnett et al. (2018) |
| 2 | Palm oil | Cupriavidus necator | 9.7% | 97 g/l | Khunthongkaew et al. (2018) |
| 3 | Apple pulp | Pseudomonas citronellolis | 30% | 0.025 g/l | Rebocho et al. (2019) |
| 4 | Glycolate | Recombinant E. coli | 1% | – | Insomphun et al. (2016) |
| 5 | Wheat bran | Halomonas boliviensis | 50% | 4 g/l | Van-Thuoc et al. (2008) |
| 6 | Whey | P. hydrogenovora | 12.7% | 1.27 g/l | Koller et al. (2008) |
| 7 | Rice bran | Haloferax mediterranei | 55.6% | 77.8 g/l | Huang et al. (2006) |
| 8 | Potato starch | Ralstonia eutropha | 52.51% | 94 g/l | Haas et al. (2014) |
| 9 | Pineapple peel waste | Ralstonia eutropha | 60% | – | Castro et al. (2016) |
| 10 | Glucose | Bacillus sp. | – | 3.09 g/l | Mohapatra et al. (2015) |
| 11 | Olive oil deodorizer distillate | Pseudomonas resinovorans | 62% | 4.7 ± 0.3 g/l | Cruz et al. (2016) |
| 12 | Soyabean oil | Pseudomonas aeruginosa | 50.27% | 0.98 g/l | Abid et al. (2016) |
| 13 | Synthetic wastewater | Pseudomonas pseudoflava | 57% | 20 g/l | Reddy et al. (2017) |
| 14 | Crude glycerol and activated sludge |
Alphaproteobacteria Betaproteobacteria |
80% | 236 mg/l | Fauzi et al. (2019) |
| 15 | Rice mill effluent | Acinetobacter junii | 94.28% | 2.64 ± 0.18 g/l | Sabapathy et al. (2018) |
| 16 | Macroalgal-derived carbohydrates | Haloferax mediterranei | 54.89% | 2.2 ± 0.12 g/l | Ghosh et al. (2019) |
| 17 | Aegle marmelos | Bacillus aerophilus | 11.62% | 2.47 g/l | Sabapathy et al. 2019 |
| 18 | Camelina oil | Pseudomonas resinovorans | 40% (w/w) | – | Bustamante et al. 2019 |
| 19 | Glycerol | Cupriavidus eutrophus B-10646 | 78% | 85.8 g/l | Volova et al. (2019) |
| 20 | Peanut oil | Cupriavidus necator | 51% | 0.56 g/g | Arauz et al. (2019) |
| 21 | Agro-industrial effluents | Halomonas sp. SF2003 | 31% | 1.89 g/l | Lemechko et al. (2019) |
| 22 | Low-rank coal liquefaction products | Pseudomonas oleovorans Rhodococcus ruber |
8% 2.4% |
-– | Fuchtenbusch, and Steinbuchel (1999) |
| 23 | Waste frying oil | Cupriavidus necator | – | 1.2 g/l | Verlinden et al. (2011) |
| 24 | Spent coffee ground | Burkholderia cepacia | 54.78% | 2.69 g/l | Obruca et al. (2014) |
| 25 | Lipid waste from animal-processing industry | Cupriavidus necator | – | 0.65 g/g | Koller and Braunegg (2015) |
Molecular engineering
PHA synthase is the key enzyme involved in the biosynthesis of PHA as discussed in the above section. Along with PHA synthase, other enzymes such as 3-ketothiolase (PhaA), (R)-specific acetoacetyl-CoA reductase (PhaB), transacylase (PhaG) and enoyl-CoA hydratase (PhaJ) are also important part of conversion of substrates into final PHA polymer. The genes corresponding to the respective enzyme involved in the process are responsible for the type and the amount of PHA produced by the microbe using different substrates. Using this fact, PHA production can be increased by altering the genes involved in the PHA production process. Modifying the enzyme at molecular level has been used by many researchers. CRISPR/CAS9 technology has been recently used for manipulating genes. Chimeric enzymes have been prepared with improved properties and higher stability. Increasing the protein expression is an important strategy to get higher level of production of PHA. In addition, cloning of the genes and harboring the recombinant strain has been highly accepted.
Engineering metabolic genes can be used to increase the PHA production by many ways such as (a) overexpression of PHA synthase operon, (b) inhibiting PHA depolymerase (phaZ) enzyme of the cell, (c) providing sufficient amount of NADH/NADPH for synthesis, (d) inhibiting beta oxidation pathways to favor the PHA synthesis, (e) enhancing cell size by modifying cell morphology, and (h) deleting pathways competing with PHA synthesis. The combinations of two or three ways can be used in a single recombinant strain to maximize the production.
Overexpression of PHA synthase operon and inhibiting competing pathways to favor the PHA synthesis
The most widely accepted method for enhancing the production is overexpression of the concerned enzyme. This can be attained by selecting strong promoters for increasing the transcriptional levels of the PHA synthase enzyme. This was shown by a recent study in which three promoters were selected for integration into upstream of phaC operon in Pseudomonas mendocina NK-01 which resulted in enhanced transcriptional levels of phaC1 and phaC2. Further they deleted phaZ gene coding for PHA depolymerase from the recombinant strains which would inhibit the PHA utilization by the bacteria and led to the higher production of PHA (Zhao et al. 2019).
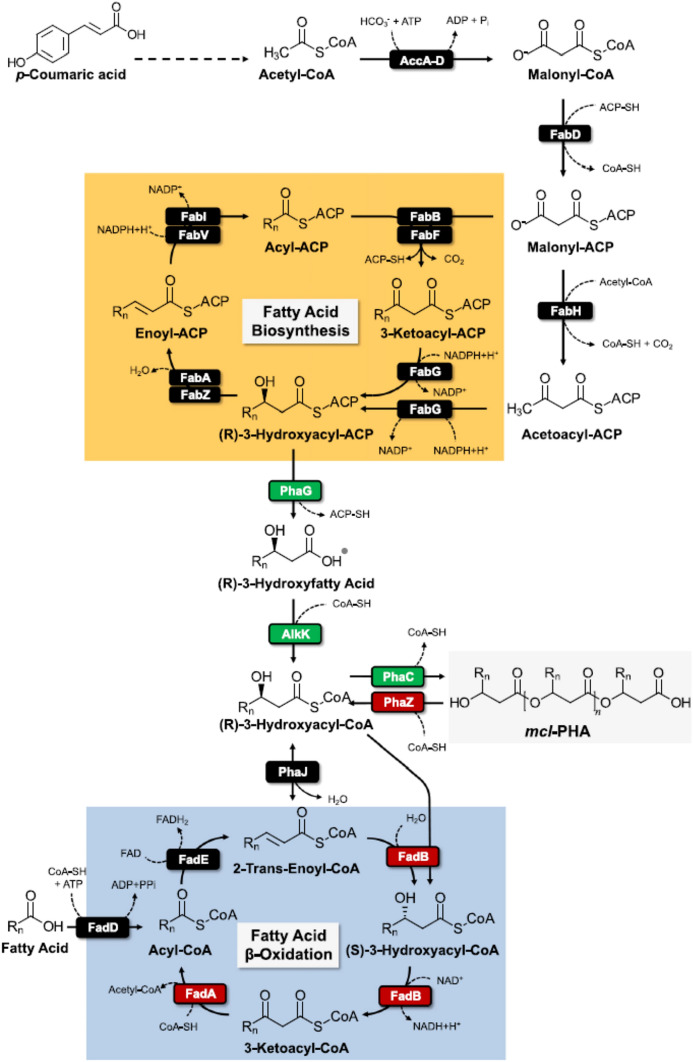
Another approach to increase PHA production by metabolic engineering is deletion of PHA depolymerase. PHA depolymerase is an enzyme secreted by the bacteria to degrade PHA to utilize it as a carbon and energy source. Deletion of phaZ gene coding for enzyme PHA depolymerase leads to accumulation of PHA in the cell. Researchers working in the field of PHA production may use other approaches like overexpression of certain genes favoring PHA production along with the phaZ knockout. For example, PHA production by deleting phaZ and fadBA1 and fadBA2 (responsible for fluxing 3‐hydroxyacyl‐CoA towards β‐oxidation pathway) genes has been studied recently (Salvachua et al. 2019). This increased the PHA accumulation in the cell to some extent. The modified strains showed a 53% and 200% increase in mcl‐PHA titre (g l−1) and a 20% and 100% increase in yield (g mcl‐PHA per g cell dry weight) as compared with the wild-type strain. To further increase the production, genes such as phaG, alkK, phaC1 and phaC2 were overexpressed to boost the substrate availability to PHA synthesis (Fig. 3).
Fig. 3.
The mcl-PHA production pathway in P. putida KT2440 via fatty acid biosynthesis and competing fatty acid β-oxidation pathway. Red boxes indicate genes targeted for deletion, and green boxes indicate genes targeted for overexpression. AccA-D, acetyl-CoA carboxylase; FabD, malonyl CoA-ACP transacylase; FabH, 3-ketoacyl-ACP synthase; FabG, 3-ketoacyl-ACP reductase; FabA and FabZ, 3-hydroxyacyl-ACP dehydratase; FabI and FabV, enoyl-ACP reductase; FabB and FabF, 3-oxoacyl-ACP synthase; PhaG, hydroxyacyl-ACP acyl-transferase; AlkK, acyl-CoA-synthase; PhaC1 and PhaC2, PHA polymerases; PhaZ, PHA depolymerase; PhaJ, R-specific enoyl-CoA hydratase; FadB, enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase; FadA, 3-ketoacyl-CoA thiolase; FadE, acyl-CoA dehydrogenase; FadD, long-chain acyl-CoA synthetase (Salvachua et al. 2019)
The approach of inserting the PHA synthesis genes and not the genes encoding PHA depolymerase into non-PHA producing microorganisms like E. coli to increase PHA productivity has been practiced since decades (Langenbach et al. 1997). Further improvements like rearranging the three genes of phaCAB operon in the host organism can lead to production of ultrahigh molecular weight PHA (Hiroe et al. 2012).
Enhancing cell size by modifying cell morphology
Modifications in cell morphology related genes has been a recent approach to increase the number and size of the bacterial cell and hence increasing PHA accumulation in the cells. Overexpression of genes such as ftsZ (responsible for Z ring formation and cytokinesis during bacterial cell division), sulA (inhibits cell division by interacting with FtsZ through GTP hydrolysis), mreB (maintains cell shape), minC and minD (inhibits FtsZ ring) can affect the PHA accumulation of the cell. For example, a study conducted by Zhao et al. (2019), used a minCD knocked out mutant Pseudomonas mendocina and overexpressed ftsZ, mreB and sulA genes in the mutant strain which resulted in improved PHA production. The reason being that minCD knock out resulted in increased cell length which resulted in increased PHA yield by 45.62% (i.e., from 0.28 to 0.41 g/l) whereas overexpressing mreB gene changed the shape and increased the width of the cell. Increasing ftsZ expression achieved multiple fission of the cell with 60.87% increase in PHA yield (i.e., from 0.23 to 0.37 g/l). Therefore, the combined effect increased the overall potential of the cell to produce high amount of PHA (Zhao et al. 2019). Similar results were observed in another study in which E. coli mutant strain showed long filamentous shaped cells after knocking out envC and nlpD genes which are responsible for degrading peptidoglycan. Deletion of minCD gene compelled the cell to divide by multiple fission rather than binary fission. Over expression of sulA gene in these E. coli mutant strains showed combined effect of multiple fission and elongated shape with accumulation of larger PHB granules in the cell accommodating 70% PHB in 9 g/l cell dry mass (CDM) which was significantly higher than the wild-type strain that produced 51% PHB from 8 g/l CDM (Wu et al. 2016). Besides increasing the cell size for higher PHA accumulation, the size of PHA granule can also be altered as smaller size of PHA granules make their recovery and purification process incommodious and costly. The number and size of PHA granules can be regulated by regulating the amount of phasin protein (PhaP) present on the surface of PHA granule. The deletion of some phasin genes (phaP) can result in production of enlarged PHA granules but size of the bacterial cell can restrict the process. Therefore Shen et al. (2019), in their study suggested that overexpression of minCD genes with phaP deletion could be done to achieve greater cell size with large PHA granules. They observed that 4HB mol% of PHA produced by H. bluephagenesis TDH4-minCD-ΔphaP1 was 14% more than the wild-type strain.
Providing sufficient amount of NADH/NADPH for synthesis
By increasing the NADH/NADPH availability to the bacteria, PHA biosynthesis can be increased (Zheng et al. 2017). As discussed in the biosynthesis of PHA that acetyl CoA acts as precursor for PHA synthesis and it requires NADPH as a cofactor. Low levels of NADPH lead to conversion of acetyl CoA to acetic acid by an alternate pathway. But high levels of NADPH favor the catalytic action of NADPH-dependent oxoacyl-CoA reductases which reduces the oxoacyl-CoA thioesters to corresponding (R)-hydroxyacyl-CoA thioesters, a process also known as “pseudo-fermentation” (Koller 2018a, b). NADPH levels can be increased by overexpression of gapN gene which codes for NADP-dependent glyceraldehyde 3-phosphate dehydrogenase. In addition to increasing the levels of NADPH, ackA-pta gene responsible for acetic acid production can be deleted and hence favoring more acetyl CoA flow towards PHA production (Heras et al. 2016).
Another recent study revealed that Halomonas bluephagenesis required NADH and not NADPH as a cofactor and increase in NADH/NAD + ratio under limited oxygen conditions could enhance the PHA production. NADH inhibits citrate synthase which is required for conversion of acetyl CoA to citrate which follows TCA cycle. If NADH amount was considerably increased, then it would be fluxed for PHA synthesis (Ling et al. 2018). Therefore, to increase the NADH levels, Ling et al. (2018) blocked the electron transfer flavoprotein subunits α and β encoded by etf operon to stop electron transport pathway which ultimately led to enhanced level with 94% of PHA as compared to 84% of PHA originally accumulated in the cell.
Using CRISPR/Cas9 technology
New developments in genome-editing tools, particularly clustered regularly interspaced short palindromic repeats (CRISPR)-based tools, have made a revolutionary change in genome-editing techniques. CRISPR-based genome editing, derived from the RNA-guided immune systems found in many bacteria, has been successfully adapted to manipulate organisms at genetic level, very efficiently and rapidly, without any damage to organism. The approach has been applied to many organisms such as Streptococcus pneumoniae, E. coli, Streptomyces, Lactobacillus reuteri, Clostridium, Bacillus subtilis and Corynebacterium glutamicum which also includes bacteria for efficient production of PHA. For example, in a recent study, genome-editing method using an electroporation-based CRISPR/Cas9 technique has been developed in R. eutropha (Xiong et al. 2018). They have disrupted five putative restriction endonuclease genes concerned with restriction modification system of the bacteria which limits its electroporation efficiency. This made them to optimize cas9 expression enabling genome editing via homologous recombination based on CRISPR-Cas9 in R. eutropha. They were able to edit five genes very efficiently, this can be applied for higher production of PHA. As comparison to conventional methods, which would involve integration of a suicide plasmid via a single-crossover recombination event, transferring it to R. eutropha from a host and then selecting transconjugants with desired result, the method is very laborious, time consuming and do not produce efficient results. Similarly, in another study, CRISPR/Cas9 was used to construct eight mutants to study combined effect of four different genes on glucose metabolism in H. bluephagenesis TD01 (Qin et al. 2018). The modified strain produced P(3HB-co-3HV) more efficiently and PHA production was increased to 16-folds.
Using different extraction methods
PHA recovery is an important factor which influences the total cost of the production process. PHAs are produced intracellularly, so they must be extracted using different solvents but use of expensive solvents makes the process not feasible for industrial use. Solvent-based, chemical, enzymatic, and mechanical recovery methods have been developed to make it cheaper. Recently, novel strategies have been implemented to use “green solvents” under pressurized conditions, ionic liquids, supercritical solvents, hypotonic cell disintegration for release of PHA granules, switchable anionic surfactants, and even digestion of non-PHA biomass by animals (Koller 2020). The right choice of recovery method influences the economic value of the whole process. Some of the methods are discussed here.
Sodium hypochlorite extraction
Digestion with sodium hypochlorite solution has been among the most common and widely used methods. The harvested and dried cells are treated with 30% sodium hypochlorite and incubated for 1 h at 37 °C. After treatment with sodium hypochlorite, the cells are washed with water cold acetone and ethanol. The extracted PHA crystals are dissolved in chloroform and boiled at 100 °C for 20 min. The final powdered PHA can then be analyzed for purity (Marjadi and Dharaiya 2014).
Extraction using DMSO
Dimethyl sulfoxide (DMSO) being a non-toxic solvent can be used for extracting PHA from bacteria Caston et al. (2015) used DMSO to extract PHA from C. necator. The harvested cells were treated with 50 ml DMSO at 70 °C with agitation and aliquots of cell suspension added periodically until maximum production was obtained. The PHB was separated by precipitation by ethanol for 160 min at 4 °C. This method eliminates the requirement for use of halogenated solvents.
Solvent extraction
PHA extraction has been carried out using various organic solvents and this method had been used from past many years till now. In this method, the harvested bacterial cells were treated with methanol at high temperature for 1 h and granules were kept for drying after removing methanol. Finally, a mixture of methanol and water in the ratio 7:3 was used to precipitate out PHA (Sadasivam et al. 2018).
Extraction using ionic liquids
Ionic liquids (ILs) have been used for the extraction of PHA as they are also non-toxic. IL, 1-ethyl-3-methylimidazolium diethylphosphate ([C2mim]-[(C2)2OPO3]) has been used by Dubey et al. (2018) for extracting PHA from Halomonas hydrothermalis. [C2mim][(C2)2OPO3] served as a good solvent because of its high hydrogen bond basicity (β = 1.07). They recovered PHA with a yield of 60% ± 2%. It was quite an effective method for the extraction of PHA.
SDS sonication method
Sodium dodecyl sulfate (SDS) is an anionic detergent and used for the lysis of bacterial cells. SDS was used for sonication of cells which were then centrifuged to recover PHA (Arikawa et al. 2017). It was then washed with water and ethanol and measured by checking the dried weight. The purity was checked by a laser-scattering instrument and it was found that SDS at a concentration of 3.3% gave purity greater than 96%.
Ultrasound-assisted extraction
Ultrasound-assisted extraction process is mainly suitable for intracellular medium chain length PHA. The method uses a mixture of acetone as a solvent and heptane as a marginal non-solvent in the medium for extraction. Treatment was given with the sonication frequency of 37 kHz. Ishak et al. (2016) used the method for extracting PHA from Pseudomonas putida. They reported ultrasonic energy output of 1151 ± 3 J ml−1 gave maximum of 74 × 10−3 g PHA g−1 dried biomass min−1 at 50:50 acetone/heptane ratio for irradiation time.
Enzyme digestion of non-PHA cellular materials (NPCM)
Digestion of non-PHA cellular materials (NPCM) such as nucleic acids, lipids and phospholipids, peptidoglycan, proteins, glycoproteins and in case of Gram-negative bacteria, lipopolysaccharides and other carbohydrates has been done to extract the PHA granules from the cells. Proteolytic enzymes and also cocktail of enzymes are used for the digestion of NPCM. The process is carried out in mild conditions with low-energy requirements but usually not preferred for industrial use because of the expensive enzymes involved in it. A study used a fungal strain Aspergillus oryzae which produces a number enzymes mainly proteases along with pectinases, lipases and phosphatases, for digesting NPCM (Kachrimanidou et al. 2016). A combination of enzyme and chemical treatment has also resulted in good recovery of PHA. Chemical treatment with SDS and EDTA combined with enzymatic action of Alcalase has resulted in good recovery of PHA with a degree of purity of higher than 90% (Martino et al. 2014). This treatment also eliminated a step of pretreatment of heat.
Use of supercritical fluids (SCF)
Super critical fluids (SCF) are fluids which have low viscosity, like a gas, high density, like a liquid, and almost non-existent surface tension. These properties of SCF make them suitable for use in cell disruption of bacteria and yeast without any damage to the enzymes such as alcohol dehydrogenase, invertase, glucose-6-phosphate dehydrogenase, fumarase, and protein activities. Supercritical CO2 (scCO2) is the best studied SCF. Abrupt discharge of scCO2 penetrates and expands within the cell which results in cell wall disruption due to rapid pressure force. The process is very simple, inexpensive, and offers minimum damage without any heat generation. It makes PHA recovery easier as CO2 simply evaporates after extraction (Khosravi-Daravi et al. 2004).
Use of animals for PHA extraction
More recently, a new method of using animals for PHA recovery has been successfully applied. The method involves feeding rats with freeze-dried PHA producing C. necator H16 cells. It was found that the rat was able to digest cytoplasmic content excluding PHA due to the absence of PHA depolymerize (PHA digesting enzyme). This method was also applied to mealworm beetles which were fed with C. necator biomass and PHA granules could be recovered from their feaces (Kunasundari et al. 2017). The biological method of extracting PHA is eco-friendly without the use of toxic solvents and chemicals.
Extraction of PHA with solvents such as chlorinated hydrocarbons, i.e., chloroform, 1,2-dichloroethane or some cyclic carbonates such as ethylene carbonate and 1,2-propylene carbonate is generally not regarded as environmentally friendly. Other factors such as high capital and operational costs do not encourage the use of such solvents. However, sequential surfactant-hypochlorite treatment promotes better and rapid recovery of PHA and results in 50% reduction of overall cost when compared to solvent extraction (Kunasundari et al. 2011). Though the operating cost is low, the process is more complex and involves relatively high cost of surfactants used. On the other hand, use of enzymes for digestion is attractive because of its mild operating conditions and high specificity, recovery of PHA with good quality could be expected. But the high cost of enzymes again makes the process not favorable. Use of super critical fluids is a good option in this context as the process is simple, inexpensive, rapid and environmentally friendly. However, biological recovery process of PHA from bacterial cells coupled with insect farming has also joined the debate as it minimizes the use of solvents, chemicals, and water. The proteins, lipids, and minerals from the bacterial cells do not end up as wastes but in turn are used as nutrition by the animals thus reducing the costs of both PHA recovery and insect production.
Applications of PHA
With time, the applications of PHA have expanded rapidly in medical implants, drug delivery carriers, nutritional supplements, printing and photographic materials, drugs and fine chemicals. Nowadays, PHA has also been used as a type of biofuel. PHA genes and proteins have been modified to regulate the metabolisms and to increase the potential of industrial microorganisms for specific drug targeting and protein purifications (Chen 2009).
PHA in medical use
PHA has been widely used to develop many medical implant devices including sutures, meniscus repair devices, suture fasteners, tacks, staples, surgical mesh, repair patches, bone plates and bone plating systems, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair/regeneration devices, nerve guides, articular cartilage repair devices, tendon repair devices, ocular cell implants, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, spinal fusion cages, bone graft substitutes, skin substitutes, bone dowels and wound dressings. The flexibility to alter monomer component of PHA makes it more efficient for the selective mechanical properties, its degradation and biocompatibility. PHA provides ideal environment for cells to grow and differentiate when used for tissue engineering. The improved chemical and physical properties are desirable for blood-contact material with less platelet adhesion, reduced erythrocyte contact and hemolysis reactivity as compared with PHB and PHBV films. PHB conduits have been proved to be useful in repairing a 10 mm gap in rat sciatic nerves with good regeneration and biocompatibility and to produce bone tissue with favorable response.
PHA polymers are used to entrap or microencapsulate drugs. Multiple layers of PHA are made with at least one layer of magnetic substance added which can be altered according to feasible microcapsule structure for drug delivery (Yano et al. 2006). 3-Hydroxybutyrate is known to be present in human blood as ketone bodies which can be used as alternative energy source for brain when glucose is limited (Martin et al. 2002).
PHA as a packaging material
Gas barrier property of PHA makes it best suitable for use in food or beverage packaging. It can be used in coating and making films for milk bottles. Packaging consists of the major portion (1.6 million tonnes, 40%) of the total bioplastic market. There is a continuous increase in the use of bioplastics in others sectors including household items, health and safety (22%, 0.9 million tonnes), automobile industry (14%, 0.6 million tonnes) and building sector (13%, 0.5 million tonnes) (Bioplastic market data 2019). However, according to an article published by European Bioplastics in 2016, it is estimated that global bioplastics production capacity is set to 17 times increase from around 4.2 million tonnes in 2016 to approximately 6.1 million tonnes in 2021.
PHA in agriculture
In agriculture, the development towards making it sustainable has led to the use of degradable plastics as mulching material. PHA films have been used well during last few years for mulching in water efficient agricultural practices. The film can be degraded with time based on the climate and crop conditions. PHA is also known to help bacteria during stress conditions. It has been reported that using PHA producing bacteria as plant growth promoters has proved to be very beneficial because they are able to resist the stress conditions such as carbon limitation, desiccation and variations in osmolarity or temperature. 3-Hydroxybutyrate acts as a chemical chaperone which can help the bacteria to survive under unfavorable conditions of heat and oxidative stress (Vinet and Zhedanov 2010).
Conclusion
The biodegradability of PHA has made its use not just limited to bioplastics but it has now been used in many areas such as medical, cosmetics, and agriculture. Advancement in genome engineering strategies has significantly increased the scope of study in different organisms for PHA production. Identifying new carbon sources ensures the sustainability of the production. The use of green solvents, enzymes, ionic liquids, supercritical solvents, anionic surfactants and even animals have made the recovery process of PHA easier and eco-friendly. All these new methods provide more scope to a researcher to choose best methods considering its environmental feasibility and cost effectiveness without compromising its degree of purity and molecular weight. Therefore, this review gives a broad view of various methods recently used by researchers for cost-effective and efficient production of PHA, which could also help in further improvement of the present methods with respect to future perspective of this eco-friendly polymer. In addition, it is believed that in the coming future if cost effective raw materials and economic production methods are used, the market size of PHA industries would be increased further and would contribute towards getting a plastic free world.
Acknowledgements
The financial support from Department of Biotechnology, Ministry of Science and Technology, Govt. of India, to Department of Biotechnology, Himachal Pradesh University, Shimla (India), is thankfully acknowledged. The financial assistance from CSIR (Council of Scientific and Industrial Research), in the form of Senior Research Fellow (SRF) is thankfully acknowledged.
Author contributions
All the authors have seen and approved the manuscript and its contents, and are aware of the responsibilities connected to the authorship. RS and RG had the idea for the article, RS performed the literature search, data analysis, writing and editing, revisions and RG had critically investigated and revised the work.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Rutika Sehgal, Email: rutikasehgal@yahoo.in, https://www.researchgate.net/profile/Rutika_Sehgal.
Reena Gupta, Email: reenagupta_2001@yahoo.com, https://www.researchgate.net/profile/Reena_Gupta22.
References
- Abid S, Raza ZA, Hussain T. Production kinetics of polyhydroxyalkanoates by using Pseudomonas aeruginosagammaraymutantstrainEBN-8culturedonsoyabeanoil. Biotech. 2016;6:142–152. doi: 10.1007/s13205-016-0452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum A, Zuber M, Zia KM, Noreen A, Anjum MN, Tabasum S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent developments. Int J Biol Macromol. 2016;89:161–174. doi: 10.1016/j.ijbiomac.2016.04.069. [DOI] [PubMed] [Google Scholar]
- Arauz AOP, Rabiela AEA, Torres AV, Hernandez AIR, Hernandez NC, Porras BV, Cuellar MRL. Production and characterization of biodegradable films of a novel polyhydroxyalkanoate (PHA) synthesized from peanut oil. Food Packag Shelf Life. 2019 doi: 10.1016/j.fpsl.2019.01.001. [DOI] [Google Scholar]
- Arikawa H, Sato S, Fujiki T, Matsumoto K. Simple and rapid method for isolation and quantitation of Polyhydroxyalkanoate by SDS-sonication treatment. J Biosci Bioeng. 2017;20:1–5. doi: 10.1016/j.jbiosc.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Basnett P, Marcello E, Lukasiewicz B, Panchal B, Nigmatullin R, Knowles JC, Roy I. Biosynthesis and characterization of a novel, biocompatible medium chain length polyhydroxyalkanoate by Pseudomonas mendocina CH50 using coconut oil as the carbon source. J Mater Sci: Mater Med. 2018;29:179–190. doi: 10.1007/s10856-018-6183-9. [DOI] [PubMed] [Google Scholar]
- Bustamante D, Tortajada M, Ramon D, Rojas A. Camelina oil as a promising substrate for mcl-PHA production in Pseudomonas sp. cultures. Appl Food Biotechnol. 2019;6:61–70. [Google Scholar]
- Caston IS, Kelly CA, Fitzgerald AVL, Leeke GA, Jenkins M, Overton TW. Development of a rapid method to isolate polyhydroxyalkanoates from bacteria for screening studies. J Biosci Bioeng. 2015;20:1–4. doi: 10.1016/j.jbiosc.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Castro OV, Calderon JC, Leon E, Segura A, Arias M, Perez L, Sobral PJA. Characterization of a polyhydroxyalkanoate obtained from pineapple peel waste using Ralstonia eutropha. J Biotechnol. 2016;231:232–238. doi: 10.1016/j.jbiotec.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Chee JY, Tan Y, Samian MR, Sudesh K. Isolation and characterization of a Burkholderia sp. USM (JCM15050) capable of producing polyhydroxyalkanoate (PHA) from trigycerides, fatty acids and glycerols. J Polym Environ. 2010;18:584–592. [Google Scholar]
- Chen GQ. A microbial polhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev. 2009;38:2434–2446. doi: 10.1039/b812677c. [DOI] [PubMed] [Google Scholar]
- Ciesielski S, Mozejko J, Pisutpaisal N. Plant oils as promising substrate for polyhydroxyalkanoates production. Clean J Prod. 2015;106:408–421. [Google Scholar]
- Cruz MV, Freitas F, Paiva A, Mano F, Dionisio M, Ramos AM, Reis AM. Valorization of fatty acids-containing wastes and byproducts into short and medium chain length polyhydroxyalkanoates. New Biotechnol. 2016;33:206–215. doi: 10.1016/j.nbt.2015.05.005. [DOI] [PubMed] [Google Scholar]
- de las Heras AM, Portugal-Nunes DJ, Rizza N, Sandström AG, Gorwa-Grauslund MF. Anaerobic poly-3-hydroxybutyrate production from xylose in recombinant Saccharomyces cerevisiae using a NADH-dependent acetoacetyl-CoA reductase. Microb Cell Fact. 2016;15:197. doi: 10.1186/s12934-016-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey S, Bharmoria P, Gehlot PS, Agrawal V, Kumar A, Mishra S. 1-Ethyl-3- methylimidazolium diethylphosphate based extraction of bioplastic “Polyhydroxyalkanoates” from bacteria: green and sustainable approach. ACS Sustain Chem Eng. 2018;6:766–773. [Google Scholar]
- Fauzi AHM, Chua ASM, Yoon LW, Nittami T, Yeoh HK. Enrichment of PHA- accumulators for sustainable PHA production from crude glycerol. Process Saf Environ. 2019;122:200–208. [Google Scholar]
- Findlay RH, White DC. Polymeric beta hydroxyalkanoates from environmental samples and Bacillus megaterium. Appl Environ Microbiol. 1983;45:71–78. doi: 10.1128/aem.45.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchtenbusch B, Steinbuchel A. Biosynthesis of polyhydroxyalkanoates from low-rank coal liquefaction products by Pseudomonas oleovorans and Rhodococcus ruber. Appl Microbiol Biotechnol. 1999;52:91–95. doi: 10.1007/s002530051492. [DOI] [PubMed] [Google Scholar]
- Fukui T, Shiomi N, Doi Y. Expression and characterization of (R)-specific enoyl coenzyme A hydratase involved in Polyhydroxyalkanoate biosynthesis by Aeromonas caviae. J Bacteriol. 1998;180:667–673. doi: 10.1128/jb.180.3.667-673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Gnaim R, Greiserman S, Fadeev L, Gozin M, Golberg A. Macroalgal biomass subcritical hydrolysates for the production of Polyhydroxyalkanoate (PHA) by Haloferax mediterranei. Bioresour Technol. 2019;271:166–173. doi: 10.1016/j.biortech.2018.09.108. [DOI] [PubMed] [Google Scholar]
- Haas R, Jin B, Zepf FT. Production of Poly(3-hydroxybutyrate) from waste potato starch. Biosci Biotechnol Bioschem. 2014;72:1–4. doi: 10.1271/bbb.70503. [DOI] [PubMed] [Google Scholar]
- Hiroe A, Tsuge K, Nomura CT, Itaya M, Tsuge T. Rearrangement of gene order in the phaCAB operon leads to effective production of ultrahigh-molecular-weight poly [(R)-3-hydroxybutyrate] in genetically engineered Escherichia coli. Appl environ microbiol. 2012;78:3177–3184. doi: 10.1128/AEM.07715-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann N, Amara AA, Beermann BB, Qi Q, Hinz HJ, Rehm BH. Biochemical characterization of the Pseudomonas putida 3-hydroxyacyl ACP:CoA transacylase, which diverts intermediates of fatty acid de novo biosynthesis. J Biol Chem. 2002;77:42926–42936. doi: 10.1074/jbc.M207821200. [DOI] [PubMed] [Google Scholar]
- Hrabak O. Industrial production of poly-β-hydroxybutyrate. FEMS Microbiol Rev. 1992;103:251–256. [Google Scholar]
- https://www.european-bioplastics.org/market/
- Huang TY, Duan KJ, Huang SY, Chen CW. Production of polyhydroxyalkanoates from inexpensive extruded rice bran and starch by Haloferax mediterranei. J Ind Microbiol Biotechnol. 2006;33:701–706. doi: 10.1007/s10295-006-0098-z. [DOI] [PubMed] [Google Scholar]
- Insomphun C, Kobayashi S, Fujiki T, Numata K. Biosynthesis of polyhydroxyalkanoates containing hydroxyl group from glycolate in Escherichia coli. AMB Express. 2016;6:29–37. doi: 10.1186/s13568-016-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak AK, Annuar MSM, Heidelberg T, Gumel AM. Ultrasound-assisted rapid extraction of bacterial intracellular medium-chain-length poly(3-hydroxyalkanoates) (mcl- PHAs) in medium mixture of solvent/marginal non-solvent. Arab J Sci Eng. 2016;41:33–44. [Google Scholar]
- Jangra MR, Ikbal NKS, Jangra S, Pippal A, Sikka VK. Recent updates on the economic use of poly hydroxy butyrate (PHB): a green alternative to plastics. Biosci Biotech Res Comm. 2018;11:97–109. [Google Scholar]
- Kachrimanidou V, Kopsahelis N, Vlysidis A, Papanikolaou S, Kookos IK, et al. Downstream separation of poly (hydroxyalkanoates) using crude enzyme consortia produced via solid state fermentation integrated in a biorefinery concept. Food Bioprod Proc. 2016;100:323–334. [Google Scholar]
- Khosravi-Darani K, Vasheghani-Farahani E, Shojaosadati SD, Yamini Y. Effect of process variables on supercritical fluid disruption of Ralstonia eutropha cells for poly-(R)-hydroxybutyrate recovery. Biotechnol Progr. 2004;20:1757–1765. doi: 10.1021/bp0498037. [DOI] [PubMed] [Google Scholar]
- Khunthongkaew P, Murugan P, Sudesh K, Iewkittayakorn J. Biosynthesis of polyhydroxyalkanoates using Cupriavidus necator H16 and its application for particleboard production. J polym Res. 2018;25:131–140. [Google Scholar]
- Koller M. Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules. 2018;23:362–382. doi: 10.3390/molecules23020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller M. Chemical and biochemical engineering approaches in manufacturing polyhydroxyalkanoate (PHA) biopolyesters of tailored structure with focus on the diversity of building blocks. Chem Biochem Eng Q. 2018;32:413–438. [Google Scholar]
- Koller M. Established and advanced approaches for recovery of microbial polyhydroxyalkanoate (PHA) biopolyesters from surrounding microbial biomass. Eurobiotech j. 2020;4:113–126. [Google Scholar]
- Koller M, Braunegg G. Biomediated production of structurally diverse poly (hydroxyalkanoates) from surplus streams of the animal processing industry. Polimery. 2015 doi: 10.14314/polimery.2015.298. [DOI] [Google Scholar]
- Koller M, Bona R, Chiellini E, Fernandes EG, Horvat P, Kutschera C, Hesse P, Braunegg G. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Bioresour Technol. 2008;99:4854–4863. doi: 10.1016/j.biortech.2007.09.049. [DOI] [PubMed] [Google Scholar]
- Kunasundari B, Sudesh K. isolation and recovery of microbial polyhydroxyalkanoates. EXPRESS Polym Lett. 2011;7:620–634. [Google Scholar]
- Kunasundari B, Arza CR, Maurer FHJ, Murugaiyah V, Kaur G, Sudesh K. Biological recovery and properties of poly(3-hydroxybutyrate) from Cupriavidus necatorH16. Sep Purif Technol. 2017;172:1–6. doi: 10.1016/j.seppur.2016.07.043. [DOI] [Google Scholar]
- Langenbach S, Rehm BR, Steinbuchel A. Functional expression of the PHA synthase gene pha C1 from Pseudomonas aeruginosa in Escherichia coli results in poly (3-hydroxyalkanoate) synthesis. FEMS microbiol lett. 1997;150:303–309. doi: 10.1016/s0378-1097(97)00142-0. [DOI] [PubMed] [Google Scholar]
- Lemechko P, Magali LF, Bruzaud S. Production of poly(3-hydroxybutyrate-co-3- hydroxyvalerate) using agro-industrial effluents with tunable proportion of 3-hydroxyvalerate monomer units. Int J Biolog Macromol. 2019;128:429–434. doi: 10.1016/j.ijbiomac.2019.01.170. [DOI] [PubMed] [Google Scholar]
- Ling C, Qiao GQ, Shuai BW, Olavarria K, Yin J, Xiang RJ, Song KN, Shen YH, Guo Y, Chen GQ. Engineering NADH/NAD+ ratio in Halomonas bluephagenesis for enhanced production of Polyhydroxyalkanoates (PHA) MetaboL Eng. 2018 doi: 10.1016/j.ymben.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Marjadi D, Dharaiya N. Recovery and characterization of poly(3-hydroxybutyric acid) synthesized in Staphylococcus epidermidis. Afric J Environ Sci Technol. 2014;8:319–329. [Google Scholar]
- Martin DP, Peoples OP, Williams SF, Zhong LH (2002) US Patent No. 6380244
- Martino L, Cruz MV, Scoma A, Freitas F, Bertin L, et al. Recovery of amorphous polyhydroxybutyrate granules from Cupriavidus necator cells grown on used cooking oil. Int J Biol Macromol. 2014;71:117–123. doi: 10.1016/j.ijbiomac.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Mohapatra S, Mohanta P, Sarkar B, Daware A, Kumar C, Samantaray D. Production of polyhydroxyalkanoates (PHAs) by Bacillus strain isolated from waste water and its biochemical characterization. Proc Natl Acad Sci India Sect Biolog Sci. 2015 doi: 10.1007/s40011-015-0626-6. [DOI] [Google Scholar]
- Obruca S, Benesova P, Petrik S, Oborna J, Prikryl R, Marova I. Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Proc Biochem. 2015;49:1409–1414. [Google Scholar]
- Obruca S, Sedlacek P, Krzyzanek V, Mravec F, Hrubanova K, Samek O, Kucera D, Benesova P, Marova I. Accumulation of poly(3-hydroxybutyrate) helps bacterial cells to survive freezing. PLoS ONE. 2016 doi: 10.1371/journal.pone.0157778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obruca S, Sedlacek P, Slaninova E, Fritz I, Daffert C, Meixner K, Sedrlova Z, Koller M. Novel unexpected functions of PHA granules. Appl Microbiol Biotechnol. 2020;104:4795–4810. doi: 10.1007/s00253-020-10568-1. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D, Jendrossek D. PhaM is the physiological activator of poly(3- hydroxybutyrate) (PHB) synthase (PhaC1) in Ralstonia eutropha. Appl Environ Microbiol. 2014;80:555–563. doi: 10.1128/AEM.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Ling C, Yiqing Z, Yang T, Yin J, Guo Y, Chen GQ. CRISPR/Cas9 editing genome of extremophile Halomonas spp. Metab Eng. 2018 doi: 10.1016/j.ymben.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Rebocho AT, Pereira JR, Freitas F, Neves LA, Alves VD, Sevrin C. Production of medium-chain length polyhydroxyalkanoates by Pseudomonas citronellolis grown in Apple pulp waste. Appl Food Biotechnol. 2019;6:71–82. [Google Scholar]
- Reddy MV, Mawatari Y, Onodera R, Nakamura Y, Yajima Y, Chang YC. Polyhydroxyalkanotes (PHA) production from synthetic waste using Pseudomonas pseudoflava: PHA synthase enzyme activity analysis from P. pseudoflava and P. palleronii. Bioresour Technol. 2017;234:99–105. doi: 10.1016/j.biortech.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Rehm BH. Polyester synthases: natural catalysts for plastics. Biochem J. 2003;376:15–33. doi: 10.1042/BJ20031254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm BH. Genetics and biochemistry of Polyhydroxyalkanoate granule self-assembly: the key role of polyester synthases. Biotechnol Lett. 2006;28:207–213. doi: 10.1007/s10529-005-5521-4. [DOI] [PubMed] [Google Scholar]
- Sabapathy PC, Devaraj S, Parthiban A, Kathirvel P. Bioprocess optimization of PHB homopolymer and copolymer P3(HB-co-HV) by Acinetobacter junii Bp25 utilizing rice mill effluent as sustainable substrate. Environ Technol. 2018;39:1441–1430. doi: 10.1080/09593330.2017.1330902. [DOI] [PubMed] [Google Scholar]
- Sabapathy PC, Devaraj S, Parthiban A, Pugazhendhi A, Kathirvel P. Aegle marmelos: A novel low-cost substrate for the synthesis of Polyhydroxyalkanoate by Bacillus aerophilus RSL-7. Biocatal Agric Biotechnol. 2019 doi: 10.1016/j.bcab.2019.101021. [DOI] [Google Scholar]
- Sadasivam S, Sigamani S, Venkatachalam H, Ramamurthy D. A new method for the production of polyhydroxyalkanoates by Bacillus sp. and detect the presence of PHA synthase. Smart Sci. 2018 doi: 10.1080/23080477.2018.1437332. [DOI] [Google Scholar]
- Saharan BS, Grewal B, Kumar A. Biotechnological production of polyhydroxyalkanoates: a review on trends and latest developments. Chin J Biol. 2014;2014:1–18. [Google Scholar]
- Salvachua D, Rydzak T, Auwae R, Capite AD, Black BA, Bouvier JT, et al. Metabolic engineering of Pseudomonas putida for increased Polyhydroxyalkanoate production from lignin. Microbial biotechnol. 2019;13:290–298. doi: 10.1111/1751-7915.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Ning ZY, Lan YX, Chen JC, Chen GQ. Manipulation of Polyhydroxyalkanoate granular sizes in Halomonas bluephagenesis. Metab Eng. 2019;54:117–126. doi: 10.1016/j.ymben.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Singh M, Kumar P, Ray S, Kalia VC. Challenges and opportunities for customizing polyhydroxyalkanoates. Indian J Microbiol. 2015;55:235–249. doi: 10.1007/s12088-015-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GYA, Chen CL, Li L, Ge L, Wang L, Razaad IMN, Li Y, Zhao L, Mo Y, Wang JY. Start a research on biopolymer polyhydroxyalkanoate (PHA): a review. Polymers. 2014;6:706–754. [Google Scholar]
- Urtuvia V, Villegas P, Fuentes S, Gonzalez M, Seegaer M. Burkholderia xenovorans LB400 possesses a functional polyhydroxyalkanoate anabolic pathway encoded by the pha genes and synthesizes poly(3-hydroxybutyrate) under nitrogen-limiting conditions. Int Microbiol. 2018;21:47–57. doi: 10.1007/s10123-018-0004-3. [DOI] [PubMed] [Google Scholar]
- Van-Thuoc D, Quikkaguaman J, Mamo G, Mattiasson B. Utilization of agricultural residues for poly(3-hydroxybutyrate) production by Halomonas boliviensis LC1. J Appl Microbiol. 2008;104:420–428. doi: 10.1111/j.1365-2672.2007.03553.x. [DOI] [PubMed] [Google Scholar]
- Verlinden RAJ, Hill DJ, Kenward MA, Williams CD, Piotrowska-Seget Z, Radecka IK. Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. AMB Express. 2011;1:11–19. doi: 10.1186/2191-0855-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinet L, Zhedanov A. A “missing” family of classical orthogonal polynomials. J Phys A Math Theor. 2010;54:450–472. [Google Scholar]
- Volova T, Demidenko A, Kiselev E, Baranovskiy S, Shishatskaya E, Zhila N. Polyhydroxyalkanoate synthesis based on glycerol and implementation of the process under conditions of pilot production. Appl Microbiol Biotechnol. 2019;103:225–237. doi: 10.1007/s00253-018-9460-0. [DOI] [PubMed] [Google Scholar]
- Wallen LL, Rohwedder WK. Poly-β-hydroxyalkanoate from activated sludge. Environ Sci Technol. 1974;8:576–579. [Google Scholar]
- Wu H, Chen J, Chen GQ. Engineering the growth pattern and cell morphology for enhanced PHB production by Escherichia coli. Appl Microbiol Biotechnol. 2016;100:9907–9916. doi: 10.1007/s00253-016-7715-1. [DOI] [PubMed] [Google Scholar]
- Xiong B, Li Z, Liu L, Zhao D, Zhang X, Bi C. Genome editing of Ralstonia eutropha using an electroporation-based CRISPR-Cas9 technique. Biotechnol Biofuels. 2018;11:172–181. doi: 10.1186/s13068-018-1170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Nomoto T, Kozaki S, Imamura T, Honma T, Canon KK (2006) US Patent No. 2006263432
- Zhao F, Gong T, Liu X, Fan X, Huang R, Ma T, Wang S, Gao W, Yang C. Morphology engineering for enhanced production of medium-chain-length polyhydroxyalkanoates in Pseudomonas mendocina NK-01. Appl Microbiol Biotechnol. 2019;103:1713–1724. doi: 10.1007/s00253-018-9546-8. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Yuan Q, Yang X, Ma H. Engineering Escherichia coli for poly-(3-hydroxybutyrate) production guided by genome-scale metabolic network analysis. Enzyme Microb Technol. 2017;106:60–66. doi: 10.1016/j.enzmictec.2017.07.003. [DOI] [PubMed] [Google Scholar]