Abstract
Purpose of Review
We performed a literature search to generate incidence and prevalence rates of narcolepsy in diverse populations based on current available data.
Recent Findings
With an onset in childhood, narcolepsy often has a delayed diagnosis due to symptoms of excessive daytime sleepiness not being recognized or being misdiagnosed. Clinical, electrophysiological, and biological tests are needed in order to diagnose narcolepsy. At the same time, the discovery of the link with the immunoregulatory human leukocyte antigen complex and the adverse events in relation to the H1N1 pandemic vaccines have shuffled the epidemiological numbers.
Summary
In this meta-review, we pooled incidence rates and prevalence rates reported in 30 countries or from 209 sets of data. Findings are reported per age, continent, and proxy race/ethnicity as well as period (i.e., before/after the pandemic). This meta-review showed that narcolepsy occurs in 0.87–1.21 of the world population, with specifically NT1 being investigated. Its pooled incidence rate in vaccinated samples is 1.58. There is furthermore an underreporting of narcolepsy in ethnic/race and gender minorities, of childhood narcolepsy type 2 and potential comorbid conditions masking the clinical complaints and hence timely diagnosis.
Keywords: Narcolepsy, Excessive daytime somnolence, Prevalence, Incidence
Introduction
Narcolepsy is a chronic sleep disorder characterized by excessive daytime sleepiness with or without cataplexy, or episodes of muscle weakness triggered by strong emotions: narcolepsy type 1 (NT1) and narcolepsy type 2 (NT2), respectively [1]. Both genetic and epidemiological evidence suggest an autoimmune mechanism in the destruction, or a highly specific loss, of orexin/hypocretin neurons, while influenza A infection and immunization have been proposed as the highest environmental risk factors [2•, 3].
Overnight polysomnography and the multiple sleep latency test (MSLT) reveal short sleep latencies and rapid eye movement (REM) periods characterizing the sleep architecture of individuals with narcolepsy [1]. Human leukocyte antigen (HLA) typing has been suggested as a useful test to screen familial risk [4••]; however, susceptibility for a number of neurodegenerative diseases, for example, Alzheimer disease, equally associates to this immunoregulatory complex.
To date, the diagnosis of narcolepsy is still secured by clinical, electrophysiological, and biological evaluations often leading to a delayed diagnosis, e.g., 8.7 to 22.1 years [5], at risk of misdiagnosis [6]. In 2010, Sweden and Finland flagged adverse events to the H1N1 pandemic vaccinations as narcolepsy [7]. At the same time, two upsurges are noticed in the scientific literature: studies applying the Brighton Collaboration case definitions towards narcolepsy diagnosis, and alternatively, studies debating the roles of HLA [8] and H1N1 [9] in narcolepsy. Consequently, both issues suggest that the year 2009 is a turning point for narcolepsy research.
In an era of increased sleepiness complaints at societal level; a new pandemic, COVID-19; and personalized medicine, we aim to systematically review the literature concerning the presentation of narcolepsy in diverse populations.
Methods
Procedure
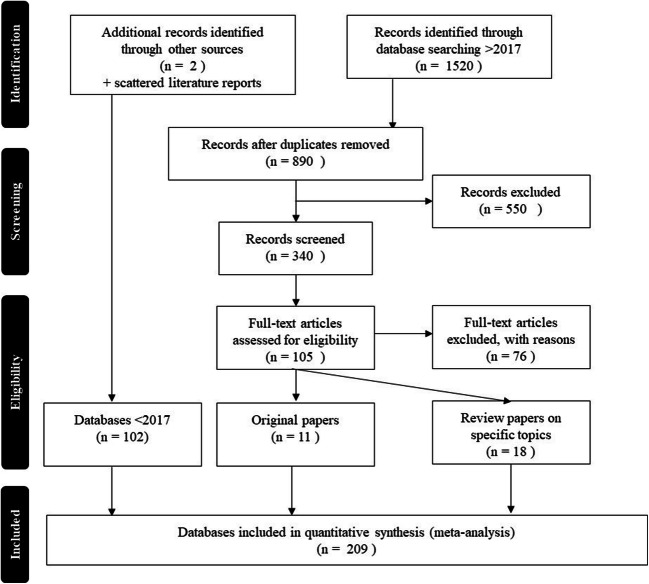
A systematic literature search in PubMed, Scopus, and Web of Science was executed. The terms narcolepsy combined with ethnicity, race, neurodevelopmental disorder, neurologic disorder, psychopathology, sleep disorder, and epidemiology were used, and studies were selected per PRISMA guidelines (Fig. 1). Because a substantial amount of studies “hit” on “gender” due to, for instance, matching on gender of study samples, we searched narcolepsy with “gender” in title separately. The search was limited to 2017 up until 31 August 2020. All types of study designs were allowed.
Fig. 1.
Flowchart of selected databases
Statistical Analyses
Statistica (TIBCO version 13) will be used for descriptive analyses. Means with standard deviations (SD) or percentages when applicable will be printed. Comprehensive meta-analysis software (version 3.3.070) was used for the meta-analyses.
We will report only the point estimate with 95% confidence intervals (95% CI) or standard error, and the number of datasets (k) included. Given that rates will be mainly population-based, i.e., huge ns, they will not be printed. The I-square (I2) with thresholds of 25%, 50%, and 75% will be considered low, moderate, and high heterogeneity, being the percentage of true observed total variance across studies. A two-tailed P value < 0.05 was applied as statistically significant.
Data Management: Continent, Race/Ethnicity, Age, and Pandemic Information
From the literature, we extracted when possible the event and sample size to (re)calculate the point estimate and its 95% CI such that rounding errors of reported rates would not jeopardize further statistical analysis. In addition, when possible, we (re)calculated incidence to prevalence rates, and vice versa.
Population-based data were divided into continents and ethnicity/race groups. Countries encountered were European continent: Czech-Republic, Denmark, Finland, France, Germany, Ireland, Italy, Norway, Slovakia, Spain, Switzerland, and the UK; North America: Brazil, Canada, Mexico, and the USA; and Asia: China, India, Iran, Israel, Japan, Kuwait, Korea, Saudi-Arabia, Singapore, Taiwan, and Turkey. Unless specifically reported, the ethnicity/race was inferred from the country/continent, that is, Amerindian: North, South, and Central America; Asian: Far East and South East Asia, Indian subcontinent, China, India, Japan, and Korea; Black: data were from the USA; and White/Caucasian: Europe, Middle East, and North Africa. This categorization is based on the National Institute of Health Diversity Programs definitions (see NOT-OD-15-053). Also, regarding age, we applied a categorization when possible: children < 19 years, adults > 19 years, and all-encompassing the child-adult age range as well as a combination of these three categories as a total age group (excluding overlaps of equivalent sets of data). Of note, the child-adult age range category reflects sets of data including ages below and above 19 years old that could not be split into children/adults separately. When reported, we also extracted data specific to NT1 and NT2. Lastly, we categorized data based on reports before the pandemic (i.e., roughly < 2009), during/after (i.e., period of the influenza and/or vaccinations, > 2009), and the mixture (i.e., including data from before and after 2009). Of note, studies will be used within their proper categorization for instance; we did not compile data from children and adult studies together to generate studies reporting child-adult age ranges.
Results
Review Papers Published on Diverse Narcolepsy Populations Since 2017
In total, 17 review papers have been published (Table 1): a majority are narrative reviews, but there are also three systematic reviews and two meta-reviews. The meta-analyses focused on the role of HLA-DQB1*06:02 [4••] and the H1N1 pandemic [17••]. The remainder showed a nearly equal distributed objective, i.e., reviews with a general aim [3, 10•, 11, 15, 16]; a health focus [12, 14, 19–22]; and a focus on associations with cognitive, behavioral, and emotional functioning [13, 18, 23•, 24]. Particularly, those with an interest in health-related issues often started from, or are published with, a case report. From Dodd et al. [11], data was extracted to calculate pooled effect sizes.
Table 1.
Review papers on narcolepsy retrieved based on our search terms
| Author | Year | Aim | Conclusion |
|---|---|---|---|
| Pillen et al. [10•] | 2017 | Narrative review with focus on the diagnosis and management of cataplexy | The diagnosis of cataplexy is made almost solely on clinical grounds, based on history taking and (home) videos. Cataplexy shows remarkable differences in childhood compared to adults, with profound facial hypotonia and complex active motor phenomena. |
| Capittini et al. [4••] | 2018 | Meta-review of genetic test in four major ethnic groups: Asians, Afro-Americans, Amerindians, and Caucasians | Data support the preponderant role of HLA-DQB1*06:02 in susceptibility to NT1/NT2 across all ethnicities. HLA-DQB1*06:02 negativity should make clinicians cautious in excluding other diagnoses. |
| Dye et al. [3] | 2018 | Narrative review of epidemiology and pathophysiology of childhood narcolepsy | Both genetic and environment factors play a crucial role in the pathophysiology of narcolepsy. Increased cases of narcolepsy in children and adolescents were observed after the H1N1 pandemic. Potential role of autoimmune-mediated processes in the loss of hypocretin neurons |
| Dodd et al. [11] | 2018 | *Systematic review of incidence rates of diagnosed narcolepsy for periods defined by influenza virus circulation and vaccination campaign dates | Simulations showed that the individual-level relative risk of narcolepsy was underestimated using ecological methods comparing post- versus pre-vaccination periods; this effect was attenuated with higher vaccine coverage and a shorter interval from disease onset to diagnosis. |
| Kallweit et al. [12] | 2018 | Narrative review, including six cases on multiple sclerosis (MS) | Narcolepsy and MS are rarely associated. In addition to NT2 secondary to hypothalamic demyelination, some patients present a coexistence of MS with NC without detectable hypothalamic lesions. |
| Ludwig et al. [13] | 2018 | Systematic review on the effect of narcolepsy (and idiopathic hypersomnia) on intellectual functioning, academic achievement, behavior, and emotion | The variability in results suggests that further research using standardized and validated assessment instruments is required to determine if there is an association. Behavior and emotion appear to be significantly affected by narcolepsy. |
| Maia Palhano et al. [14] | 2018 | Narrative review with focus on precocious puberty and obesity | The incidence of overweight or obesity ranges from 25% to 74% in patients with narcolepsy type I, while precocious puberty is present in 17% of children with narcolepsy with cataplexy. However, the mechanisms involved in the association of narcolepsy with obesity and precocious puberty have not been fully elucidated yet. |
| Plazzi et al. [15] | 2018 | Narrative review with focus on burden of illness | Pediatric narcolepsy is also associated with comorbidities including rapid weight gain, precocious puberty, and attention deficit hyperactivity disorder, and increased risk for deficits in social functioning, depression, and anxiety. School performance is also typically impaired, requiring special education services. |
| Ray et al. [16] | 2018 | Narrative review with cases from a sleep clinic at a tertiary care center | Narcolepsy, although rarely reported from India, should be suspected in young non-obese patients complaining of EDS and confirmed by performing MSLT following overnight PSG. |
| Sarkanen et al. [17••] | 2018 | Meta-review on the incidence of narcolepsy after H1N1 influenza | During the first year after vaccination, the relative risk of narcolepsy was increased 5- to 14-fold in children and adolescents and 2 to 7-fold in adults. |
| Schiappa et al. [18] | 2018 | Narrative review with focus on the emotional experience/emotional brain circuits | Neurophysiological and neurochemical findings support the hypothesis of the involvement of the limbic system in the physiopathology of cataplexy |
| Weil et al. [19] | 2018 | Narrative review and a case study with focus on hypothalamic region tumors | Overall 26 cases: most symptomatic narcolepsy cases were reported in children (70%). Half of the patients (13 of 25, 52%) developed narcolepsy after surgery, whereas 11 patients (44%) were symptomatic at the time of the tumor diagnosis. |
| Gohil et al. [20] | 2019 | Narrative review and a case study with focus on growth hormone (GH) deficiency | The siblings developed increased sleepiness following initiation of GH therapy; the authors propose that hypothalamic dysfunction may be the link between GH deficiency and sleep disorders in these children. |
| Hershner et al. [21] | 2019 | Narrative review with focus on perioperative risk | The evidence is sparse and based on case reviews, case series, and retrospective reviews. |
| Antelmi et al. [22] | 2020 | Narrative review with focus on REM sleep behavior disorder (RBD) | RBD reportedly affects 30%–60% of patients with Narcolepsy type 1 (NT1), but it may be seen also in Narcolepsy type 2 (NT2). |
| BaHammam et al. [23•] | 2020 | Narrative review with focus on neuropsychiatric correlates | Comorbid neuropsychiatric manifestations in patients with narcolepsy include depression, anxiety, psychosis, rapid eye movement (REM) sleep behavior disorder, and cognitive impairment. |
| Kim et al. [24] | 2020 | A systematic review on ADHD | The prevalence of ADHD symptoms was > 30%, making it an important comorbidity of narcolepsy |
Incidence—Prevalence Rates in Diverse Populations
In our endeavor, to be complete, we extracted data from two papers published before 2017 reporting epidemiological data (Fig. 1) and scattered literature reports if the data was not yet included: Longstreth et al. [25] and Wijnans et al. [26]. Our PRISMA search generated 11 new papers from which data was used. Table 2 and Table 3 show the different rates extracted from that literature collection approach. Ultimately, a total of 209 sets of data were analyzed with > 5% of the data extracted representing samples of Sweden, Netherlands, Finland, the UK, Spain, Korea, Denmark, the USA, Canada, and Taiwan.
Table 2.
Meta-review of Narcolepsy: pooled incidence rates (95% CI)
| Diverse population | Children | Adults | Child-adult age range | All ages combined | Diverse population | Children | Adults | Child-adult age range | All ages combined |
|---|---|---|---|---|---|---|---|---|---|
| A. Before the H1N1 pandemic | B. During/after the H1N1 pandemic in unvaccinated populations | ||||||||
| Europe |
1.14 (0.42–1.87) |
1.12 (0.77–1.48) |
- |
1.12 (0.74–1.51) |
Europe |
0.96 (0.81–1.12) |
1.11 (0.76–1.47) |
4.39 (3.37–5.41) |
1.00 (0.88–1.12) |
| k = 6; I2: 98.47 | k = 5; I2: 97.23 | - | k = 11; I2: 98.87 | k = 15; I2: 99.58 | k = 14; I2: 98.05 | k = 1; I2: 0 | k = 30; I2: 99.35 | ||
| North America |
0.66 (0.50–0.82) |
0.77 (0.68–0.87) |
1.37 (0.91–1.83) |
0.82 (0.60–1.04) |
North America |
0.69 (0.36–1.03) |
0.76 (0.65–0.86) |
- |
0.75 (0.66–0.84) |
| k = 1; I2: 0 | k = 1; I2: 0 | k = 1; I2: 0 | k = 3; I2: 75.90 | k = 2; I2: 56.92 | k = 2; I2: 0 | - | k = 4; I2: 0 | ||
| Asia |
0.36 (0–0.78) |
0.48 (0–1.09) |
0.60 (0–1.52) |
0.45 (0.25–0.66) |
Asia |
0.81 (0.25–1.37) |
1.58 (0.65–2.51) |
- |
1.18 (0.72–1.64) |
| k = 2; I2: 97.63 | k = 2; I2: 99.46 | k = 2; I2: 96.56 | k = 6; I2: 98.19 | k = 3; I2: 96.23 | k = 3; I2: 98.32 | - | k = 6; I2: 98.49 | ||
| Caucasian |
1.06 (0.44–1.69) |
1.05 (0.80–1.29) |
0.73 (0–1.94) |
1.00 (0.72–1.28) |
Caucasian |
0.92 (0.78–1.07) |
1.04 (0.74–1.34) |
- |
0.96 (0.85–1.07) |
| k = 7; I2: 98.53 | k = 6; I2: 0 | k = 2; I2: 96.11 | k = 14; I2: 98.61 | k = 17; I2: 99.51 | k = 16; I2: 97.75 | - | k = 33; I2: 99.28 | ||
| Asian |
0.36 (0–0.78) |
0.48 (0–1.09) |
1.08 (0.76–1.40) |
0.52 (0.28–0.76) |
Asian |
0.81 (0.25–1.37) |
1.58 (0.65–2.51) |
- |
1.18 (0.72–1.64) |
| k = 2; I2: 97.63 | k = 2; I2: 99.46 | k = 1; I2:0 | k = 5; I2: 98.54 | k = 3; I2: 96.23 | k = 3; I2: 98.32 | - | k = 6; I2: 98.49 | ||
| World |
0.90 (0.33–1.48) |
0.91 (0.65–1.16) |
0.84 (0.02–1.67) |
0.87 (0.66–1.09) |
World |
0.88 (0.75–1.01) |
1.12 (0.85–1.39) |
4.39 (3.37–5.41) |
0.98 (0.87–1.08) |
| k = 9; I2: 99.45 | k = 8; I2: 98.93 | k = 3; I2: 96.03 | k = 20; I2: 99.14 | k = 20; I2: 99.43 | k = 19; I2: 97.79 | k = 1; I2: 0 | k = 40; I2: 99.24 | ||
| C. During/after the H1N1 pandemic in vaccinated populations | D. Before and during/after the H1N1 pandemic in unvaccinated populations | ||||||||
| Europe |
8.82 (5.4–12.25) |
1.46 (0.92–2.01) |
1.49 (1.10–1.89) |
4.59 (3.7–5.48) |
Europe |
0.63 (0.33–1.02) |
0.99 (0.70–1.28) |
0.89 (0.62–1.17) |
0.89 (0.62–1.17) |
| k = 9; I2: 96.81 | k = 8; I2: 92.33 | k = 1; I2: 0 | k = 17; I2: 97.30 | k = 11; I2: 96.76 | k = 13; I2: 98.54 | k = 14; I2: 99.3 | k = 14; I2: 99.26 | ||
| North America | - | - | - | - | North America | - | - |
0.69 (0.51–0.87) |
0.69 (0.51–0.87) |
| - | - | - | - | - | - | k = 3; I2: 91.1 | k = 3; I2: 91.11 | ||
| Asia |
0.13 (0.10–0.16) |
0.59 (0.53–0.66) |
- |
0.36 (0–0.81) |
Asia |
1.37 (1.27–1.48) |
8.15 (7.90–9.41) |
0.29 (0.27–0.32) |
3.27 (0.70–5.83) |
| k = 1; I2: 0 | k = 1; I2: 0 | - | k = 2; I2: 99.37 | k = 1; I2: 0 | k = 1; I2: 0 | k = 1; I2: 0 | k = 3; I2: 99.95 | ||
| Caucasian |
8.82 (5.4–12.25) |
1.46 (0.92–2.01) |
1.49 (1.10–1.89) |
4.59 (3.7–5.48) |
Caucasian |
0.63 (0.33–1.02) |
0.99 (0.70–1.28) |
0.86 (0.63–1.08) |
0.86 (0.63–1.08) |
| k = 9; I2: 96.81 | k = 8; I2: 92.33 | k = 1; I2: 0 | k = 17; I2: 97.30 | k = 11; I2: 96.76 | k = 13; I2: 98.54 | k=; I2: 99.1 | k = 17; I2: 99.11 | ||
| Asian |
0.13 (0.10–0.16) |
0.59 (0.53–0.66) |
- |
0.36 (0–0.81) |
Asian |
1.37 (1.27–1.48) |
8.15 (7.90–9.41) |
0.29 (0.27–0.32) |
3.27 (0.70–5.83) |
| k = 1; I2: 0 | k = 1; I2: 0 | - | k = 2; I2: 99.37 | k = 1; I2: 0 | k = 1; I2: 0 | k = 17; I2: 0 | k = 3; I2: 99.95 | ||
| World |
7.85 (4.87–10.84) |
0.93 (1.15–8.24) |
1.49 (1.10–1.89) |
1.58 (1.33–1.82) |
World |
0.70 (0.37–1.02) |
1.51 (0.9–2.12) |
0.82 (0.62–1.03) |
1.21 (0.91–1.51) |
| k = 10; I2: 98.32 | k = 9; I2: 92.88 | k = 1; I2: 0 | k = 20; I2: 98.11 | k = 12; I2: 97.23 | k = 15; I2: 99.68 | k = 18; I2: 99.3 | k = 20; I2: 99.69 | ||
Table 3.
Meta-review of narcolepsy: prevalence rates (95% CI)
| Diverse population | Children | Adults | Child-adult age range | All ages combined | Diverse population | Children | Adults | Child-adult age range | All ages combined |
|---|---|---|---|---|---|---|---|---|---|
| A. Before the H1N1 pandemic | B. During/after the H1N1 pandemic in unvaccinated populations | ||||||||
| Europe |
11.07 (2.96–19.18) |
2.46 (2.16–2.76) |
0.09 (0–0.23) |
2.92 (2.64–3.19) |
Europe |
3.36 (2.55–4.18) |
7.42 (5.51–9.33) |
17.58 (13.5–21.67) |
5.69 (4.87–6.51) |
| k = 5; I2: 98.78 | k = 11; I2: 99.71 | k = 3; I2: 88.42 | k = 19; I2: 99.68 | k = 11; I2: 98.86 | k = 12; I2: 99.32 | k = 1; I2: 0 | k = 24; I2: 94.05 | ||
| North America |
6.63 (5.03–8.23) |
2.59 (0.10–5.79) |
26.03 (17.29–34.77) |
5.14 (2.40–7.87) |
North America |
6.91 (3.51–10.31) |
7.56 (6.55–8.57) |
- |
7.53 (6.64–8.43) |
| k = 1; I2: 0 | k = 4; I2: 98.79 | k = 1; I2: 0 | k = 6; I2: 98.51 | k = 2; I2: 57.79 | k = 2; I2: 0 | - | k = 4; I2: 0 | ||
| Asia |
4.53 (3.02–6.04) |
6.93 (5.06–8.80) |
0.35 (0.02–0.68) |
3.57 (3.09–4.06) |
Asia |
7.74 (0.97–14.52) |
9.09 (4.99–13.20) |
16.76 (11.21–2.32) |
8.22 (5.97–10.48) |
| k = 4; I2: 99.38 | k = 5; I2: 99.85 | k = 3; I2: 95.86 | k = 12; I2: 99.68 | k = 2; I2: 97.67 | k = 4; I2: 99.07 | k = 1; I2: 0 | k = 8; I2: 98.53 | ||
| Caucasian |
10.19 (3.35–17.04) |
3.84 (3.51–4.16) |
0.08 (0–0.17) |
2.42 (2.26–2.58) |
Caucasian |
3.73 (2.94–4.52) |
7.51 (5.70–9.31) |
17.29 (14.00–20.58) |
6.12 (5.35–6.89) |
| k = 6; I2: 98.78 | k = 16; I2: 99.75 | k = 6; I2: 92.40 | k = 29; I2: 99.75 | k = 13; I2: 98.68 | k = 14; I2: 99.28 | k = 2; I2: 0 | k = 29; I2: 99.11 | ||
| Asian |
1.60 (0.73–2.47) |
1.45 (0.61–2.28) |
1.08 (0.76–1.4) |
1.39 (0.95–1.83) |
Asian |
7.74 (0.97–14.52) |
9.09 (4.99–13.20) |
- |
7.38 (5.08–9.68) |
| k = 3; I: 98.18 | k = 4; I2: 99.30 | k = 1; I2: 0 | k = 8; I2: 98.78 | k = 2; I2: 97.67 | k = 4; I2: 99.07 | - | k = 7; I2: 0 | ||
| World |
7.30 (5.12–9.47) |
3.19 (2.91–3.47) |
0.16 (0.05–0.27) |
2.06 (1.92–2.19) |
World |
3.93 (3.22–4.65) |
7.69 (6.10–9.27) |
17.29 (14.00–20.58) |
6.13 (5.43–6.83) |
| k = 10; I2: 99.64 | k = 20; I2: 99.71 | k = 7; I2: 94.51 | k = 37; I2: 99.70 | k = 16; I2: 98.50 | k = 18; I2: 99.26 | k = 2; I2: 0 | k = 36; I2: 97.70 | ||
| C. During/after the H1N1 pandemic in vaccinated populations | D. Before and during/after the H1N1 pandemic in unvaccinated populations | ||||||||
| Europe |
21.72 (15.16–8.29) |
2.66 (1.71–3.61) |
8.36 (0–23.65) |
6.56 (5.38–7.74) |
Europe |
8.66 (4.20–13.11) |
11.15 (8.40–13.91) |
9.26 (6.46–12.06) |
9.26 (6.46–12.06) |
| k = 9; I2: 98.11 | k = 8; I2: 94.05 | k = 2; I2: 87.40 | k = 19; I2: 97.4 | k = 14; I2: 99.00 | k = 16; I2: 98.31 | k = 14; I2: 99.26 | k = 14; I2: 99.26 | ||
| North America | - | - |
4.32 (0–11.12) |
4.32 (0–11.12) |
North America | - | - |
6.91 (5.08–8.74) |
6.91 (5.08–8.74) |
| - | - | k = 1; I2: 0 | k = 1; I2: 0 | - | - | k = 3; I2: 90.44 | k = 3; I2: 90.44 | ||
| Asia |
0.65 (0.50–0.80) |
2.95 (2.63–3.28) |
- |
1.80 (0–4.05) |
Asia |
6.85 (6.33–7.38) |
21.40 (0–9.32) |
2.92 (2.66–3.19) |
13.12 (3.33–22.91) |
| k = 1; I2: 0 | k = 1; I2: 0 | - | k = 2; I2: 99.4 | k = 1; I2: 0 | k = 2; I2: 99.96 | k = 1; I2: 0 | k = 4; I2: 99.91 | ||
| Caucasian |
21.72 (15.16–8.29) |
2.66 (1.71–3.61) |
5.86 (0–12.97) |
6.51 (5.34–7.68) |
Caucasian |
8.66 (4.20–13.11) |
11.15 (8.40–13.91) |
8.84 (6.56–11.12) |
8.84 (6.56–11.12) |
| k = 9; I2: 98.11 | k = 8; I2: 94.05 | k = 3; I2: 76.72 | k = 20; I2: 97.20 | k = 14; I2: 99.00 | k = 16; I2: 98.31 | k = 17; I2: 99.10 | k = 17; I2: 99.10 | ||
| Asian |
0.65 (0.50–0.80) |
2.95 (2.63–3.28) |
- |
1.80 (0–4.05) |
Asian |
6.85 (6.33–7.38) |
21.40 (0–9.32) |
2.92 (2.66–3.19) |
13.12 (3.33–22.91) |
| k = 1; I2: 0 | k = 1; I2: 0 | - | k = 2; I2: 99.4 | k = 1; I2: 0 | k = 2; I2: 99.96 | k = 1; I2: 0 | k = 4; I2: 99.91 | ||
| World |
16.76 (12.84–0.67) |
2.97 (1.88–4.06) |
5.86 (0–12.97) |
4.22 (3.49–4.94) |
World |
8.46 (4.89–12.03) |
12.53 (7.95–17.12) |
8.50 (6.41–10.59) |
9.66 (7.01–12.31) |
| k = 10; I2: 98.23 | k = 9; I2: 97.70 | k = 3; I2: 76.72 | k = 22; I2: 97.7 | k = 15; I2: 98.98 | k = 18; I2: 99.53 | k = 18; I2: 99.27 | k = 21; I2: 99.62 | ||
Pooled Incidence Rates of Narcolepsy
Given that data were population-based, pooled sample sizes are substantial (hence not reported), and also, heterogeneity rapidly inflated as the number of countries (databases) increased (Table 2, but also the other tables).
Before the H1N1 pandemic, the pooled incidence rates (IR) ranged from 0.36 to 1.37 across diverse populations, with a global pooled IR of 0.87 (95% CI: 0.66–1.09) based on 20 datasets. The highest pooled IR upper boundary can be seen in European children (upper 95% CI: 1.87). During/after the H1N1 pandemic in unvaccinated populations, i.e., data collected in and after 2009 with varying time limits applied, the pooled IR ranged from 0.69 to 1.58 across diverse populations, with a global pooled IR of 0.98 (95% CI: 0.87–1.08) based on 40 datasets. An outlier is the hazard ratio of 4.39 in Norway covering child-adult age ranges [27]. During/after the influenza period, however, the highest upper boundary is seen for adults in Asia(n) (upper 95% CI: 2.51). Overall, the pooled IR between these two periods (Table 2 A versus B) in the world are comparable (p value = 0.823).
During/after the H1N1 pandemic in vaccinated populations, the world pooled IR almost doubled, but is not significantly different from the other periods (Table 2 A: p value = 0.4935 and B: p value = 0.4971). The lowest and highest pooled IR were reported in children, and in Asia(n) (pooled IR: 0.13) and in Europe (Caucasian) (pooled IR: 8.82) for this period, respectively.
About 20 sets of data generated IR overarching the before-after H1N1 pandemic period in unvaccinated samples (Table 2 D). Asia demonstrated the lowest and highest pooled IR depending on the age of the population, respectively, a pooled IR of 0.29 (95%CI: 0.27–0.32) when reflecting child-adult age ranges and a pooled IR of 1.37 (95% CI: 1.27–1.48) for children only. Korea [28] is an outlier here by reporting a crude incidence rate (adult pooled IR: 8.15). No significant differences were found upon comparing to the other world pooled IR in the other periods (p values for comparisons versus A = 0.7247, B = 0.7847, and C = 0.7356).
Pooled Prevalence Rates of Narcolepsy
Only a handful more studies reported prevalence rates. These studies, in contrast to those reporting incidence rates, often involve smaller sample sizes. In addition, we pooled data from case reports to enlarge the dataset for that population when possible (e.g., Ray et al. [16]). Based on 37 databases, a world pooled prevalence rates (PR) of 2.06 (95% CI: 1.92–2.19) before the H1N1 pandemic was found. The highest prevalence rate should be interpreted with caution since selection criteria applied in the county also included “doubtful” narcolepsy [29]. Nevertheless, higher prevalence rates can be noted in childhood.
The pooled PR of the world almost tripled during/after the H1N1 pandemic in unvaccinated populations at 6.13 (95% CI: 5.43–6.83) (Table 3 A versus B: p value = 0.0004). The two largest pooled PRs reflect populations [27, 30] where the selection criteria and sampling may have skewed percentages.
Likely, due to the retrospective nature of data collection, fewer individual studies reported on vaccinated samples, contrary to, for instance, adverse event registries following pharmacological treatment. The pooled PR during/after the H1N1 pandemic in vaccinated populations for the world was 4.22 (95% CI: 3.49–4.94). Although the world pooled PR doubles in vaccinated populations, the 95% CI remains within the before H1N1 pandemic boundaries (Table 3 A versus C: p = 0.076). Also, high pooled PRs are noted in European/Caucasian children. The world PR in vaccinated and unvaccinated was found to be comparable (Table 3 B versus C: p = 0.1567) during the same period, i.e., > 2009.
The highest pooled PR for the world was found for the period overarching before and after the H1N1 pandemic, namely 9.66 (95% CI: 7.01–12.31) based on 21 datasets of unvaccinated samples. This pooled PR was also significantly different from the others (Table 3 D versus A = p < 0.00001, B = 0.0033, C = 0.0001). While the pooled PRs before 2009 tend to be high in childhood, a shift can be noted towards adulthood as regards data collected over the mixture of time periods.
Caution is needed in interpreting these pooled rates, because the year 2009 (or H1N1 pandemic circulation) is a crude time point applied in different ways throughout the literature, particularly in combination with the recollection of the onset of symptoms of narcolepsy. Data generated from vaccinated samples however were often provided through census data and/or registries for adverse events.
Prevalence Rates of Narcolepsy Type 1 and Narcolepsy Type 2 in Narcolepsy Samples
Fewer studies explicitly detailed on NT1 (maximum k = 13, Table 4) and NT2 (maximum k = 6, Table 5) in samples characterized by narcolepsy (Fig. 2). In samples exhibiting narcolepsy symptomatology before the H1N1 pandemic, the pooled PR of the world was 46.02 ± 3.5 (k = 13) for NT1 and 14.22 ± 2.91 (k = 6) for NT2, especially the first pooled PR might be biased by doubtful selection criteria applied before 2009. Yet this pooled PR remains high also for data reflecting the > 2009 period in unvaccinated samples: 53.86 ± 13.63 (k = 8) for NT1 and 38.27 ± 18.76 (k = 5) for NT2. Data reporting before as well as after 2009 prevalence rates in narcolepsy samples, pooled as 49.52 ± 31.26 (k = 3) for NT1 and 16.58 ± 9.25 (k = 3) for NT2 in the world. In a vaccinated sample of people presenting narcolepsy symptomatology across the world, NT1 (only Europe) was reported in 4.27 ± 0.51 (k = 3).
Table 4.
Pooled prevalence rates (± standard error) of NT1 per racial and ethnic categories
| Diverse population | Children | Adults | Child-adult age range | All ages combined | Diverse population | Children | Adults | Child-adult age range | All ages combined |
|---|---|---|---|---|---|---|---|---|---|
| A. Before the H1N1 pandemic | B. During/after the H1N1 pandemic in unvaccinated populations | ||||||||
| Caucasian | - | 50.03 ± 33.02 | 14.06 ± 3.30 | 40.62 ± 20.50 | Caucasian | 0.64 ± 0.25 | - | 65.20 ± 3.11 | 43.49 ± 26.45 |
| - | k = 3; I2: 99.66 | k = 1; I2: 0 | k = 4; I2: 99.50 | k = 1; I2: 0 | - | k = 2; I2: 0 | k = 3; I2: 99.53 | ||
| Asian | 15.00 ± 0.25 | 38.99 ± 38.59 | 82.82 ± 3.17 | 51.19 ± 19.49 | Asian | - | 59.92 ± 12.93 | 60.55 ± 11.10 | 60.16 ± 7.01 |
| k = 1; I2: 0 | k = 2; I2: 99.48 | k = 2; I2: 63.93 | k = 5; I2: 99.99 | - | k = 3; I2: 82.29 | k = 2; I2: 94.20 | k = 5; I2: 86.95 | ||
| Black | - | 32.90 ± 32.91 | - | 32.90 ± 32.91 | Black | - | - | - | - |
| - | k = 2; I2: 99.90 | - | k = 2; I2: 99.90 | - | - | - | - | ||
| Amerindian | - | - | 73.87 ± 6.89 | 73.87 ± 6.89 | Amerindian | - | - | - | - |
| - | - | k = 2; I2: 52.69 | k = 2; I2: 52.69 | - | - | - | - | ||
| World | 15.00 ± 0.25 | 12.23 ± 0.81 | 64.48 ± 15.37 | 46.02 ± 3.50 | World | 0.64 ± 0.25 | 59.92 ± 12.93 | 62.54 ± 6.18 | 53.86 ± 13.63 |
| k = 1; I2: 0 | k = 7; I2: 99.67 | k = 5; I2: 99.15 | k = 13; I2: 99.99 | k = 1; I2: 0 | k = 3; I2: 82.29 | k = 4; I2: 91.10 | k = 8; I2: 99.65 | ||
| C. During/after the H1N1 pandemic in vaccinated populations | D. Before and during/after the H1N1 pandemic in unvaccinated populations | ||||||||
| Caucasian | 4.26 ± 0.51 | 4.70 ± 4.69 | - | 4.27 ± 0.51 | Caucasian | 4.57 ± 1.08 | 90.55 ± 1.78 | 43.52 ± 39.95 | 43.52 ± 39.95 |
| k = 2; I2: 0 | k = 1; I2: 0 | - | k = 3; I2: 0 | k = 2; I2: 27.99 | k = 1; I2: 0 | k = 2; I2: 99.65 | k = 2; I2: 99.65 | ||
| Asian | - | - | - | - | Asian | - | 61.90 ± 9.79 | - | 61.90 ± 9.79 |
| - | - | - | - | - | k = 1; I2: 0 | - | k = 1; I2: 0 | ||
| World | 4.26 ± 0.51 | 4.70 ± 4.69 | - | 4.27 ± 0.51 | World | 4.57 ± 1.08 | 77.84 ± 14.23 | 43.52 ± 39.95 | 49.52 ± 31.26 |
| k = 2; I2: 0 | k = 1; I2: 0 | - | k = 3; I2: 0 | k = 2; I2: 27.99 | k = 2; I2: 87.94 | k = 2; I2: 99.65 | k = 3; I2: 99.38 | ||
Table 5.
Pooled prevalence rates (± standard error) of NT2 per racial and ethnic categories
| Diverse population | Children | Adults | Child-adult age range | All ages combined | Diverse population | Children | Adults | Child-adult age range | All ages combined |
|---|---|---|---|---|---|---|---|---|---|
| A. Before the H1N1 pandemic | B. During/after the H1N1 pandemic in unvaccinated populations | ||||||||
| Caucasian | - | 7.87 ± 2.44 | - | 7.87 ± 2.44 | Caucasian | - | - | 10.61 ± 2.20 | 10.61 ± 2.20 |
| - | k = 1; I2: 0 | - | k = 1; I2: 0 | - | - | k = 1; I2: 0 | k = 1; I2: 0 | ||
| Asian | - | 22.2 ± 5.5 | 17.18 ± 3.17 | 17.98 ± 2.76 | Asian | - | 51.59 ± 15.87 | 27.78 ± 5.18 | 45.48 ± 15.36 |
| - | k = 1; I2: 0 | k = 2; I2: 63.93 | k = 3; I2: 55.07 | - | k = 3; I2: 96.15 | k = 1; I2: 0 | k = 4; I2: 97.72 | ||
| Black | - | 5.34 ± 0.99 | - | 5.34 ± 0.99 | Black | - | - | - | - |
| - | k = 1; I2: 0 | - | k = 1; I2: 0 | - | - | - | - | ||
| Amerindian | - | - | 20.37 ± 5.39 | 20.37 ± 5.39 | Amerindian | - | - | - | - |
| - | - | k = 1; I2: 0 | k = 1; I2: 0 | - | - | - | - | ||
| World | - | 9.52 ± 3.19 | 17.34 ± 2.41 | 14.22 ± 2.91 | World | - | 51.59 ± 15.87 | 18.56 ± 8.56 | 38.27 ± 18.76 |
| - | k = 3; I2: 79.08 | k = 3; I2: 46.71 | k = 6; I2: 95.12 | - | k = 3; I2: 96.15 | k = 2; I2: 89.27 | k = 5; I2: 99.55 | ||
| C. During/after the H1N1 pandemic in vaccinated populations | D. Before and during/after the H1N1 pandemic in unvaccinated populations | ||||||||
| Caucasian | - | - | - | - | Caucasian | 4.00 ± 2.00 | 92 ± 0.12 | 7.83 ± 7.83 | 7.83 ± 7.83 |
| - | - | - | - | k = 2; I2: 99.90 | k = 1; I2: | k = 2; I2: 91.03 | k = 2; I2: 91.03 | ||
| Asian | - | - | - | - | Asian | - | 40 ± 10.07 | - | 40 ± 10.07 |
| - | - | - | - | - | k = 1; I2: 0 | - | k = 1; I2: 0 | ||
| World | - | - | - | - | World | 4.00 ± 2.00 | 66.97 ± 25.98 | 7.83 ± 7.83 | 16.58 ± 9.25 |
| - | - | - | - | k = 2; I2: 99.90 | k = 2; I2: 96.25 | k = 2; I2: 91.03 | k = 3; I2: 92.43 | ||
Fig. 2.
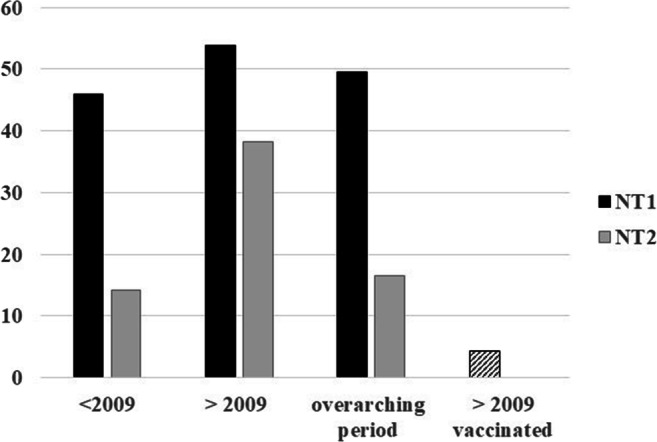
World Pooled Prevalence rate for narcolepsy type 1 and narcolepsy type 2. NT1, narcolepsy type 1 (black bar); NT2, narcolepsy type 2 (gray bar). Striped bar: only NT1
Given that study samples rather report the PR as found in a convenience sample, contrary to PRs generated from epidemiological studies, extreme caution is warranted in interpreting these pooled PR of NT1 and NT2. However, several tendencies become clear when looking at Tables 4 and 5, that is, an underreporting of specific PR in children and minorities, or alternatively an overreliance on adult and “white” samples. More studies are needed in NT2 samples in general, and similarly in children and minorities. Contrary to NT1 pooled PRs, the H1N1 pandemic may have boosted the NT2 prevalence, or narcolepsy without cataplexy symptomatology.
Discussion
This meta-review showed that narcolepsy occurs in 0.87–1.21 of the world population, with specifically NT1 being investigated. There is furthermore an underreporting of narcolepsy in minorities and of NT2 in children. This meta-review reported pooled IR and PR rates within the range of previously published epidemiological studies. The upper-boundaries in particular may have clinical relevance towards concerted efforts improving timely diagnosis and management.
Narcolepsy is a life-long, severe, multifaceted disease often arising in childhood or adolescence as characterized by excessive daytime sleepiness. Although hypersomnia is not uncommon in the general population, the complex series of tests and the detailed history taking towards diagnosis of the specific types of narcolepsy [1, 31, 32] may jeopardize epidemiological “numbering.” That is, between the age of diagnosis and the age of onset, there is the patient’s recollection, complaints, and subjectivity of excessive somnolence, which, in the absence of cataplexy, may make the diagnosis challenging. As a consequence, diagnosis may occur 10–16 years later [33].
More recently, the H1N1 pandemic [28, 34] and/or AS03-adjuvanted vaccines [35, 36] may have shifted some numbers [17, 37]. Our pooled data confirms these aspects. Firstly, a high preponderance in childhood was demonstrated. Secondly, mirroring the inconsistency in the literature, a doubling or tripling was shown alongside the 2009 influenza circulation and/or vaccinations. These vaccines, and their timed campaigns, however differed across countries, and consequently, their adverse events may or may not have reached registries or other databases.
Our findings concur that the incidence rate of narcolepsy varies by age, country, and period [11]. Although vaccination doubled the pooled IR/PR in the world, overall rates were comparable. We may, however, infer that while originally European children showed a high occurrence, a shift has occurred over the H1N1 pandemic period towards increased rates in Asia. Combined with outliers, caution is still warranted towards overgeneralization or even causality assumptions. Namely, methods of calculation (e.g., the denominator) and similarly selection criteria applied vary widely across the studies—countries—included, as expressed by very high I2s. This applies even more so to the pooled PR findings, since individual study data often reports frequencies in convenience samples contrary to epidemiological databases. Alternatively, such large-scale prevalence studies have to rely on carefully designed surveys such as Ohayon et al. [38].
The gap in epidemiological data concerning minorities and gender disparities was striking, as shown in this meta-review. Hence, our current knowledge and therefore management strategies represent mainly European and Caucasian samples. For instance, the first (racist) report in an African-American sample dates from 1945, and only in the 1990s, some other data surfaced [25, 39]. A dominance of a handful of countries correspondingly is apparent. Regarding, the variable “gender” that is commonly applied for matching purposes in studies investigating narcolepsy samples, few specific epidemiological (or treatment [40]) studies were found. In addition, NT2 populations particularly in childhood seem to be overlooked. The reviews published since 2017 moreover highlight the gap in knowledge regarding co-occurrence of disorders that may mask symptomatology of narcolepsy. Especially given the associated features of narcolepsy, such as hypnagogic/hypnopompic hallucinations, sleep paralysis, and obesity, misdiagnosis might be common [41, 42].
Nonetheless, data reported here, and hence in the literature, are challenged by several biases towards accurate “numbering.” Firstly, the ascertainment and recall bias of excessive somnolence may affect the reported rates. In other words, in the absence of a simple (non-invasive) objective test, the data principally depends on the patient’s complaint. Yet, in the case of H1N1 vaccination, the adverse event may have been “captured.” Secondly, the studies included noticeably have a selection bias given the age at onset/diagnosis gap, the health care triage system, and the time periods investigated, that is, the criteria applied in recruitment varies greatly. Subsequently, a confirmation bias might be present. We, therefore, agree with Verstraeten et al. [36] that the H1N1 influenza has certainly complicated the clinical picture, which is directly observable in how studies report on their samples or the data collected. The process of sampling over time, or the unsystematic collection of data in somnolent samples potentially introduces a sampling bias as well. While the search for markers such as the HLA gene complex in an era of an influenza may exemplify a potential measurement bias. Lastly, the influenza may have introduced a chronology bias in data. For these reasons, studies and epidemiological reports would become more homogeneous if consensus criteria for reporting of narcolepsy would be outlined, eventually advancing its scientific investigation given that the burden of illness is omnipresent [15, 43].
This meta-review has some limitations to address. Most are related to the lack of consensus in reporting data, e.g., regarding age at onset, whether a sleep study was performed at some point, regards comorbidities or treatments, and even the period under investigation. Consequently, our categorizations remain crude. Similarly, our race and ethnicity categorization remains a proxy unless clearly stipulated in the (few) study. Lastly, several incidence rates and prevalence rates were difficult to trace and therefore directly used (i.e., not recalculated) which may have created some degree of error in our rates.
In conclusion, the H1N1 influenza may have created an increased risk of narcolepsy as reflected by the rates before/after 2009 but similarly plausible is that a certain degree of research bias is present. Nevertheless, though the number of studies varied, vaccination doubled the rate of narcolepsy occurrence and the pooled prevalence rates of narcolepsy and NT1 are akin suggesting some concurrence. By the same token, the childhood preponderance shifted along with the H1N1 influenza period to Asia.
Compliance with Ethical Standards
Conflict of Interest
The author has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Sleep and Health Disparities
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.American Academy of Sleep Medicine International Classification of Sleep Disorders. 3rd ed. Darien: IL American Academy of Sleep Medicine; 2014. https://www.textbooks.com/International-Classification-of-Sleep-Disorders-3rd-Edition/9780991543410/American-Academy-of-Sleep-Medicine.php?CSID=AZBSBMWWUKJWMCTOACC2KUSMB.
- 2.• Bonvalet M, Ollila HM, Ambati A, Mignot E. Autoimmunity in narcolepsy. Curr Opin Pulm Med. 2017;23(6):522–9. 10.1097/MCP.0000000000000426This paper summarizes the recent findings in narcolepsy focusing on the environmental and genetic risk factors in disease development. [DOI] [PMC free article] [PubMed]
- 3.Dye TJ, Gurbani N, Simakajornboon N. Epidemiology and pathophysiology of childhood narcolepsy. Paediatr Respir Rev. 2018;25:14–8. 10.1016/j.prrv.2016.12.005. [DOI] [PubMed]
- 4.•• Capittini C, De Silvestri A, Terzaghi M, Scotti V, Rebuffi C, Pasi A, et al. Correlation between HLA-DQB1*06:02 and narcolepsy with and without cataplexy: approving a safe and sensitive genetic test in four major ethnic groups. A systematic meta-analysis. Sleep Med. 2018;52:150–7. 10.1016/j.sleep.2018.08.024This paper provides meta-analytic data on HLA testing for narcolepsy diagnosis in four major ethnical groups: Asians, Afro-Americans, Amerindians, and Caucasians. [DOI] [PubMed]
- 5.Çökmüş FP, Aydın O, Dikici DS, Sapmaz ŞY. Quickly diagnosed and treated prepubertal type 1 narcolepsy case. Psychiatry Clin Psychopharmacol. 2018;28(2):227–9. 10.1080/24750573.2017.1408230.
- 6.Gupta AK, Sahoo S, Grover S. Narcolepsy in adolescence—a missed diagnosis: a case report. Innov Clin Neurosci. 2017;14(7–8):20–3. [PMC free article] [PubMed]
- 7.Harris T, Wong K, Stanford L, Fediurek J, Crowcroft N, Deeks S. Did narcolepsy occur following administration of AS03-adjuvanted A(H1N1) pandemic vaccine in Ontario, Canada? A review of post-marketing safety surveillance data. Euro Surveill. 2014 Sep 11;19(36):20900. 10.2807/1560-7917.es2014.19.36.20900. [DOI] [PubMed]
- 8.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355(9197):39–40. 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed]
- 9.Mahlios J, De la Herran-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol. 2013;23(5):767–73. 10.1016/j.conb.2013.04.013. [DOI] [PMC free article] [PubMed]
- 10.• Pillen S, Pizza F, Dhondt K, Scammell TE, Overeem S. Cataplexy and its mimics: Clinical recognition and management. Curr Treat Options Neurol. 2017 Jun;19(6):23. 10.1007/s11940-017-0459-0. This paper reviews the diagnosis and management of cataplexy: attacks of bilateral loss of muscle tone, triggered by emotions and with preserved consciousness. [DOI] [PubMed]
- 11.Dodd CN, de Ridder M, Huang WT, Weibel D, Giner-Soriano M, Perez-Vilar S, et al. Incidence rates of narcolepsy diagnoses in Taiwan, Canada, and Europe: the use of statistical simulation to evaluate methods for the rapid assessment of potential safety issues on a population level in the SOMNIA study. PLoS One. 2018;13(10):e0204799. 10.1371/journal.pone.0204799. [DOI] [PMC free article] [PubMed]
- 12.Kallweit U, Bassetti CLA, Oberholzer M, Fronczek R, Béguin M, Strub M, et al. Coexisting narcolepsy (with and without cataplexy) and multiple sclerosis: six new cases and a literature review. J Neurol. 2018;265(9):2071–8. 10.1007/s00415-018-8949-x. [DOI] [PubMed]
- 13.Ludwig B, Smith S, Heussler H. Associations between neuropsychological, neurobehavioral and emotional functioning and either narcolepsy or idiopathic hypersomnia in children and adolescents. JCSM: 2018;14(4):661–74. 10.5664/jcsm.7066. [DOI] [PMC free article] [PubMed]
- 14.Maia Palhano AC, Kim LJ, Moreira GA, Santos Coelho FM, Tufik S, Levy AM. Narcolepsy, precocious puberty and obesity in the pediatric population: a literature review. Pediatr Endocrinol Rev. 2018;16(2):266–74. 10.17458/per.vol16.2018.Narcolepsypubertyobesity. [DOI] [PubMed]
- 15.Plazzi G, Clawges HM, Owens JA. Clinical characteristics and burden of illness in pediatric patients with narcolepsy. Pediatr Neurol. 2018;85:21–32. 10.1016/j.pediatrneurol.2018.06.008. [DOI] [PubMed]
- 16.Ray A, Kanabar K, Upadhyay V, Sharma SK. A four year experience in narcolepsy from a sleep clinic at a tertiary care centre with a short review of contemporary indian literature. Indian J Med Res. 2018;148(6):748–51. 10.4103/ijmr.IJMR_888_16. [DOI] [PMC free article] [PubMed]
- 17.•• Sarkanen TO, Alakuijala APE, Dauvilliers YA, Partinen MM. Incidence of narcolepsy after H1N1 influenza and vaccinations: systematic review and meta-analysis. Sleep Med Rev. 2018;38:177–86. 10.1016/j.smrv.2017.06.006This paper provides an analyzis of the magnitude of H1N1 vaccination-related risk and examined if there was any association with H1N1 infection itself. [DOI] [PubMed]
- 18.Schiappa C, Scarpelli S, D'Atri A, Gorgoni M, De Gennaro L. Narcolepsy and emotional experience: a review of the literature. Behavioral And Brain Functions: BBF. 2018;14(1):19. 10.1186/s12993-018-0151-x. [DOI] [PMC free article] [PubMed]
- 19.Weil AG, Muir K, Hukin J, Desautels A, Martel V, Perreault S. Narcolepsy and hypothalamic region tumors: presentation and evolution. Pediatr Neurol. 2018;84:27–31. 10.1016/j.pediatrneurol.2017.12.016. [DOI] [PubMed]
- 20.Gohil A, Eugster E. Growth hormone deficiency and excessive sleepiness: a case report and review of the literature. Pediatr Endocrinol Rev: PER. 2019;17(1):41–6. 10.17458/per.vol17.2019.ge.ghdeficiencyandsleepiness. [DOI] [PMC free article] [PubMed]
- 21.Hershner S, Dauvilliers Y, Chung F, Singh M, Wong J, Gali B, et al. Knowledge gaps in the perioperative management of adults with narcolepsy: a call for further research. Anesth Analg. 2019;129(1):204–11. 10.1213/ane.0000000000004088. [DOI] [PubMed]
- 22.Antelmi E, Pizza F, Franceschini C, Ferri R, Plazzi G. REM sleep behavior disorder in narcolepsy: A secondary form or an intrinsic feature? Sleep Med Rev. 2020 Apr;50:101254. 10.1016/j.smrv.2019.101254. [DOI] [PubMed]
- 23.• BaHammam AS, Alnakshabandi K, Pandi-Perumal SR. Neuropsychiatric correlates of narcolepsy. Curr Psychiatry Rep. 2020 Jun 5;22(8):36. 10.1007/s11920-020-01159-y. This paper summarizes recent evidence for the association between narcolepsy and neuropsychiatric disorders.The review discusses the use of stimulants and anticataplectic medications that may pose diagnostic difficulties in terms of underlying neuropsychiatric comorbidities. [DOI] [PubMed]
- 24.Kim J, Lee GH, Sung SM, Jung DS, Pak K. Prevalence of attention deficit hyperactivity disorder symptoms in narcolepsy: a systematic review. Sleep Med. 2020;65:84–8. 10.1016/j.sleep.2019.07.022. [DOI] [PubMed]
- 25.Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30(1):13–26. 10.1093/sleep/30.1.13. [DOI] [PubMed]
- 26.Wijnans L, Lecomte C, de Vries C, Weibel D, Sammon C, Hviid A, et al. The incidence of narcolepsy in Europe: before, during, and after the influenza A(H1N1)pdm09 pandemic and vaccination campaigns. Vaccine. 2013;31(8):1246–54. 10.1016/j.vaccine.2012.12.015. [DOI] [PubMed]
- 27.Trogstad L, Bakken IJ, Gunnes N, Ghaderi S, Stoltenberg C, Magnus P, et al. Narcolepsy and hypersomnia in Norwegian children and young adults following the influenza A(H1N1) 2009 pandemic. Vaccine. 2017;35(15):1879–85. 10.1016/j.vaccine.2017.02.053. [DOI] [PubMed]
- 28.Choe YJ, Bae GR, Lee DH. No association between influenza A(H1N1)pdm09 vaccination and narcolepsy in South Korea: an ecological study. Vaccine. 2012;30(52):7439–42. 10.1016/j.vaccine.2012.10.030. [DOI] [PubMed]
- 29.Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep. 2002;25(2):197–202. 10.1093/sleep/25.2.197. [DOI] [PubMed]
- 30.Qasrawi SQ, Albarrak AM, Alharbi AS, Nashwan S, Almeneessier AS, Pandi-Perumal SR, et al. Narcolepsy in Saudi patients before and after the 2009 H1N1 vaccination. The experience of 2 referral centers. Saudi Med J. 2017;38(12):1196–200. 10.15537/smj.2017.12.21046. [DOI] [PMC free article] [PubMed]
- 31.Lopez R, Arnulf I, Drouot X, Lecendreux M, Dauvilliers Y. French consensus. Management of patients with hypersomnia: Which strategy? Rev Neurol. 2017;173(1–2):8–18. 10.1016/j.neurol.2016.09.018. [DOI] [PubMed]
- 32.Monaca C, Franco P, Philip P, Dauvilliers Y. French consensus. Type 1 and type 2 narcolepsy: investigations and follow-up. Rev Neurol. 2017;173(1):25–31. 10.1016/j.neurol.2016.09.016. [DOI] [PubMed]
- 33.Hale L, Guan S, Emanuele E. Epidemiology of narcolepsy. In: Goswami M, Thorpy MJ, Pandi-Perumal SR, editors. Narcolepsy: a clinical guide. Cham: Springer International Publishing; 2016. p. 37–43.
- 34.Duffy J, Weintraub E, Vellozzi C, DeStefano F. Narcolepsy and influenza A(H1N1) pandemic 2009 vaccination in the United States. Neurology. 2014;83(20):1823–30. 10.1212/wnl.0000000000000987. [DOI] [PMC free article] [PubMed]
- 35.Stassijns J, Bollaerts K, Baay M, Verstraeten T. A systematic review and meta-analysis on the safety of newly adjuvanted vaccines among children. Vaccine. 2016;34(6):714–22. 10.1016/j.vaccine.2015.12.024. [DOI] [PubMed]
- 36.Verstraeten T, Cohet C, Dos Santos G, Ferreira GL, Bollaerts K, Bauchau V, et al. Pandemrix™ and narcolepsy: A critical appraisal of the observational studies. Hum Vaccin Immunother. 2016;12(1):187–93. 10.1080/21645515.2015.1068486. [DOI] [PMC free article] [PubMed]
- 37.Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7(3):e33536. 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed]
- 38.Ohayon MM, Priest RG, Zulley J, Smirne S, Paiva T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology. 2002;58(12):1826–33. 10.1212/wnl.58.12.1826. [DOI] [PubMed]
- 39.Mignot E, Lin X, Arrigoni J, Macaubas C, Olive F, Hallmayer J, et al. DQB1*0602 and DQA1*0102 (DQ1) are better markers than DR2 for narcolepsy in Caucasian and black Americans. Sleep. 1994;17(8 Suppl):S60–7. 10.1093/sleep/17.suppl_8.s60. [DOI] [PubMed]
- 40.Won C, Mahmoudi M, Qin L, Purvis T, Mathur A, Mohsenin V. The impact of gender on timeliness of narcolepsy diagnosis. JCSM 2014;10(1):89–95. 10.5664/jcsm.3370. [DOI] [PMC free article] [PubMed]
- 41.Yeh JY, Shyu YC, Lee SY, Yuan SS, Yang CJ, Yang KC, et al. Comorbidity of narcolepsy and psychotic disorders: a nationwide population-based study in Taiwan. Front Psychiatry. 2020;11:205. 10.3389/fpsyt.2020.00205. [DOI] [PMC free article] [PubMed]
- 42.Morse AM. Narcolepsy in children and adults: a guide to improved recognition, diagnosis and management. Medical Sciences (Basel, Switzerland). 2019;7(12):106. 10.3390/medsci7120106. [DOI] [PMC free article] [PubMed]
- 43.Nordstrand SH, Hansen BH, Kamaleri Y, Nilsen KB, Rootwelt T, Karlsen TI, et al. Changes in quality of life in individuals with narcolepsy type 1 after the H1N1-influenza epidemic and vaccination campaign in Norway: a two-year prospective cohort study. Sleep Med. 2018;50:175–80. 10.1016/j.sleep.2018.05.037. [DOI] [PubMed]