Abstract
Objective
We discuss the evidence on the occurrence of de novo seizures in patients with COVID-19, the consequences of this catastrophic disease in people with epilepsy (PWE), and the electroencephalographic (EEG) findings in patients with COVID-19.
Methods
This systematic review was prepared according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. MEDLINE, Scopus, and Embase from inception to August 15, 2020 were systematically searched. These key words were used: “COVID” AND “seizure” OR “epilepsy” OR “EEG” OR “status epilepticus” OR “electroencephalography”.
Results
We could identify 62 related manuscripts. Many studies were case reports or case series of patients with COVID-19 and seizures. PWE showed more psychological distress than healthy controls. Many cases with new-onset focal seizures, serial seizures, and status epilepticus have been reported in the literature. EEG studies have been significantly ignored and underused globally.
Conclusion
Many PWE perceived significant disruption in the quality of care to them, and some people reported increase in their seizure frequency since the onset of the pandemic. Telemedicine is a helpful technology that may improve access to the needed care for PWE in these difficult times. De novo seizures may occur in people with COVID-19 and they may happen in a variety of forms. In addition to prolonged EEG monitoring, performing a through metabolic investigation, electrocardiogram, brain imaging, and a careful review of all medications are necessary steps. The susceptibility of PWE to contracting COVID-19 should be investigated further.
Keywords: Coronavirus, COVID-19, EEG, Epilepsy, Seizure
Introduction
Since late 2019, the world has been experiencing a catastrophic pandemic of a new coronavirus disease (COVID-19) caused by SARS-CoV2.[1]. Previous outbreaks of coronaviruses consist of the severe acute respiratory syndrome (SARS) in 2002 and the Middle East respiratory syndrome (MERS) in 2012.[2] Coronaviruses primarily target the human respiratory system. However, they have also been associated with neurological manifestations (e.g., seizures, change in mental status, and encephalitis).[2, 3]. Neurotropic and neuroinvasive capabilities of coronaviruses have been described before.[4].
The signs and symptoms of COVID-19 infection often appear after an incubation period of about five days [1]. The most frequent manifestations at the onset of COVID-19 illness are fever, cough, loss of smell and taste, and fatigue; other manifestations may include headache, hemoptysis, diarrhea, and dyspnea. In patients with severe disease, pneumonia, acute respiratory distress syndrome, organ failure, metabolic derangements, and acute cardiac injury may happen [1]. It is reasonable to expect that some patients with COVID-19 develop seizures as a consequence of hypoxemia, organ failure, metabolic derangements, drug-drug interactions, or even brain damage that may occur in patients with COVID-19 [2, 3, 5]. On the other hand, information on the susceptibility of people with epilepsy (PWE) to contracting COVID-19 and the consequences and challenges of this catastrophic disease in PWE are scarce.
In the current systematic review, we will discuss the evidence on the occurrence of de novo seizures in patients with COVID-19 (the types of seizures and the etiology), the consequences, and challenges of this catastrophic disease in PWE (e.g., access to care and seizure control), and finally, the electroencephalographic (EEG) findings in patients with COVID-19. We will also provide suggestions to tackle the abovementioned issues based on the best available evidence.
Methods
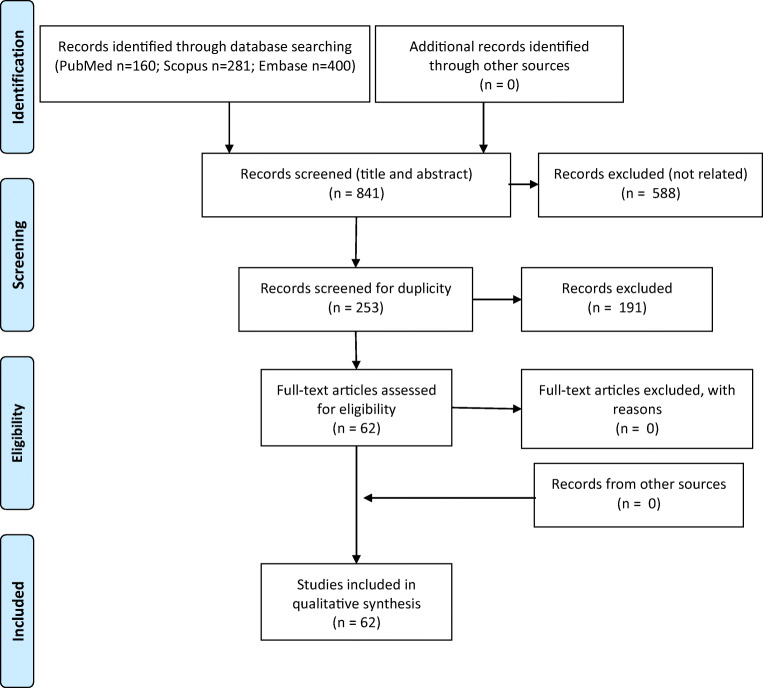
This manuscript was prepared according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [6, 7] (Fig. 1 and Table 1). MEDLINE (accessed from PubMed), Scopus, and Embase from inception to August 15, 2020 were systematically searched for related published articles. In all electronic databases, the following search strategy was implemented and these key words (in the title/abstract) were used: “COVID” AND “seizure” OR “epilepsy” OR “EEG” OR “status epilepticus” OR “electroencephalography”. Articles written in English were all included. To ensure literature saturation, the authors scanned the reference lists of the included studies or relevant articles identified through the search. All authors participated through each phase of the review independently (screening, eligibility, and inclusion). They obtained full reports for all titles that appeared to meet the inclusion criteria or where there was any uncertainty. They resolved any disagreement through discussions. Neither of the authors were blind to the journal titles nor the study authors or institutions.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the study
Table 1.
The search keywords included “COVID” and “Epilepsy/Seizure” in the title/abstract
| Medline (PubMed) | Scopus | Embase | ||||
|---|---|---|---|---|---|---|
| Keywords (& COVID) | Primary hints | Relevant articles | Primary hints | Relevant articles | Primary hints | Relevant articles |
| Epilepsy | 55 | 23 | 85 | 25 (24 duplicates)* | 154 | 28 (28 duplicates) |
| Seizure | 42 | 23 (11 duplicates) | 112 | 28 (21 duplicates) | 122 | 22 (22 duplicates) |
| EEG | 29 | 16 (6 duplicates) | 26 | 5 (5 duplicates) | 50 | 13 (13 duplicates) |
| Electroencephalography | 15 | 12 (7 duplicates) | 43 | 11 (11 duplicates) | 42 | 11 (11 duplicates) |
| Status epilepticus | 19 | 13 (9 duplicates) | 15 | 15 (15 duplicates) | 32 | 8 (8 duplicates) |
*Duplicates: already found in previous searches
The following data were extracted from the included studies: study authors, study location, study designs, main results, and limitations. The methodological quality of the included studies was assessed by the authors. The class of evidence was defined following the American Academy of Neurology criteria for classification of evidence in studies of causation (Appendix, Table 5) [8].
Table 5.
American Academy of Neurology criteria for classification of evidence in studies of causation (Gronseth GS, Cox J, Gloss D, et al. on behalf of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. 2017. Clinical Practice Guideline Process Manual, 2017 ed. Minneapolis, MN: The American Academy of Neurology)
| Classification | Criteria |
|---|---|
| I |
Prospective cohort study with all relevant confounders controlled, masked or objective outcome assessments, and a) ≤ 2 primary outcomes, b) clearly defined inclusion/exclusion criteria c) ≥ 80% study completion rate. |
| II | Retrospective cohort study or case-control study meeting all other class I criteria. |
| III | Cohort study or case-control study meeting all class I or II criteria except a, b, or c above. |
| IV | Studies not meeting class I, II, or III criteria |
Standard protocol approvals, registrations, and patient consents
The Shiraz University of Medical Sciences Institutional Review Board approved this study and systematic review.
Results
Through the search strategy, we could identify 62 related manuscripts (Tables 2, 3, and 4) [9–70]. Only one paper provided class 2 evidence and 11 papers provided class 3 evidence; the rest of the publications provided class 4 evidence. Many studies were case reports or case series of patients with COVID-19 and seizures. The rest of the publications were surveys or observational studies (Tables 2, 3, and 4). Below, we summarize the results of this systematic review:
-
1.1.
Epilepsy related papers (Table 2): One cross-sectional study of 21 patients with active epilepsy and COVID-19 out of 1537 patients (1.4%) with level 2 of evidence showed that the cumulative incidence of COVID-19 in people with epilepsy was higher compared with the population without epilepsy (1.2% vs. 0.5%) [9]. Furthermore, the total case fatality rate was higher in PWE compared to patients without active epilepsy (23.8% vs. 3.6%; p < 0.001) [9]. However, these results were obtained based on a small number of PWE (21 persons) and should be interpreted with caution. In addition, in another study, among 5700 PWE, who were managed at the studied centers, only 14 people tested positive for SARS-CoV-2, without obvious impacts on their epilepsy [18]. Six surveys of PWE showed that many people perceived significant disruption in the quality and availability of care to them (31 to 95%), as well as increased stress and social isolation, and increase in seizure frequency (6 to 35%) since the onset of the pandemic [11, 12, 14–17]. One observational comparative study confirmed that PWE showed more psychological distress than healthy controls, and they spent significantly more time following the COVID-19 outbreak news [13]. Finally, four studies showed that telemedicine may improve access to specialized care for PWE in these difficult times [10, 20–22]. The development of an epilepsy electronic patient portal may promote improved patient-clinician partnerships and facilitate patient self-management [23]
-
1.2.
Seizure-related papers (Table 3): While one retrospective study reported no seizures in 304 patients with COVID-19 [25], multiple studies refuted that finding [24, 26–29]. Furthermore, many cases with new-onset focal seizures, serial seizures, and status epilepticus have been reported in the literature [30–49, 51, 63]. The etiology of seizure(s) in the reported patients was most likely multifactorial, as many of them had comorbidities (e.g., diabetes and kidney disease), multiorgan failure, metabolic derangements, hypoxemia, etc. In addition, all these patients received many medications [33–47]. However, some of the patients had specific neurological problems (e.g., encephalitis [33, 40, 70] and cerebrovascular events [38, 42, 43, 51, 59]). SARS-CoV-2 RNA was detected in the cerebrospinal fluid (CSF) in two patients [40, 53]; however, many other studies that tested for this had negative results [36, 42, 44, 46, 52, 59, 60, 64, 67, 70]. It should be emphasized that many centers did not investigate the patients with COVID-19, who had seizures, thoroughly. For example, many patients did not have EEG exams, and in a significant number of them CSF analysis and even brain imaging studies were not performed.
-
1.3.
EEG-related papers (Table 4): While change in mental status has frequently been reported in patients with COVID-19 [26, 27, 55, 60, 65, 66, 70], and while there are many reports on clinical and subclinical seizures and status epilepticus in these patients [33–47, 63, 66], EEG studies have been significantly ignored and underused in these patients globally. Three studies showed that EEG services have been significantly disrupted by the pandemic of COVID-19 [18, 19, 57]. Three other studies showed that many of the patients with COVID-19 and encephalopathy/seizures had epileptiform discharges/seizures in their EEG [29, 54, 55]. One study of two patients with COVID-19 and encephalopathy suggested a unique EEG pattern with continuous, slightly asymmetric, monomorphic, diphasic, delta slow waves with greater amplitude over both frontal areas and with a periodic organization [58]. Finally, two studies suggested that quantitative EEG (QEEG) features may be useful for diagnosis and prognostication of the neurological outcome in critically ill patients with COVID-19 [61, 62]. While studies investigating EEG in patients with COVID-19 did not find a consistent and specific neurophysiological pattern in critically ill patients, the authors should describe the EEG findings more appropriately, adhering to the standardized terminologies, in future studies [71].
Table 2.
COVID-19 in people with epilepsy
| Author/country | Methods | Results | Major limitations | Level of evidence |
|---|---|---|---|---|
| Cabezudo-García/Spain [9] | Cross-sectional study of 21 patients with active epilepsy and COVID-19 | The cumulative incidence of COVID-19 in patients with epilepsy was higher compared with the population without epilepsy (1.2% vs. 0.5%). Epilepsy was associated with fatality during hospitalization (odds ratio: 5.1 [95% CI: 1.3–24.0]). |
Small sample Size and hospital-based study |
II |
| Conde-Blanco/Spain [10] | Survey of 66 neurologists | During the pandemic, respondents handled their epilepsy clinics mainly with telephone calls (88%); only 4.5% used videoconference. | Self-report design | IV |
| Huang/ China [11] | Survey of 362 patients | 8.5% of patients had increased seizures. Stress, uncontrolled seizures, and inappropriate change in drug regimen were associated with increased seizures. | Self-report design | IV |
| Asadi-Pooya/Iran [12] | Survey of 100 patients | 31% expressed hardship obtaining their drugs; 6% expressed worsening of their seizure control status in the past 4 weeks. | Self-report design | IV |
| Hao/China [13] | Cross-sectional study of 252 patients with epilepsy and 252 controls | Patients with epilepsy showed more psychological distress than healthy controls and spent significantly more time following the COVID-19 outbreak. | Self-report design | IV |
| Miller/USA [14] | Survey of 94 patients | Significant disruption in epilepsy self-management was reported. Lack of ability to obtain medications or see epilepsy providers, as well as increased stress, and social isolation were reported; 35% reported an increase in seizure frequency since the onset of the pandemic. | Self-report design | IV |
| Aledo-Serrano/Spain [15] | Survey of 227 patients | Three patients with Dravet syndrome had confirmed COVID-19. All of them had mild symptoms, and none needed hospitalization or showed either seizure or behavioral worsening. However, 14% of the whole cohort reported seizure frequency increase and 30% reported behavioral deterioration during the lockdown. The main variables associated with seizure increase were age and difficulties finding antiseizure medications. | Self-report design | IV |
| Assenza/Italy [16] | Survey of 456 patients and 472 controls | Disruption in epilepsy management was reported in 95%; 18% reported seizure worsening. People with epilepsy had worse depressive and anxiety symptoms. | Self-report design | IV |
| Alkhotani/ Saudi Arabia [17] | Survey of 156 patients | 29.5% reported an increase in seizure frequency. 59% had an increase in self-reported stress. | Self-report design | IV |
| Granata/Europe [18] | Descriptive study of the changes required in hospitals to cope with the COVID-19 pandemic. | The epilepsy care activities were reduced to less than 10%. Elective epilepsy surgeries, including vagal nerve stimulator implantations, were canceled. Hospitalizations and EEG examinations were limited to emergencies. The outpatient visits were postponed, and follow-up visits mostly managed by telehealth. Among the 5700 people with epilepsy managed at the studied centers, only 14 tested positive for SARS-CoV-2, without obvious impact on their epilepsy. | Descriptive study | IV |
| Wirrell/Worldwide [19] | Cross-sectional, online survey of pediatric neurologists across the world | 92% reported changes to outpatient care, 91% with reduced access to EEG, 37% with altered management of infantile spasms, 92% with restrictions in ketogenic diet initiation, 93% with closed or severely limited epilepsy monitoring units, and 91% with canceled or limited epilepsy surgery. Telehealth use had increased. | Descriptive study | IV |
| Punia/USA [20] | Observational study of telemedicine’s practicality | Clinic visits accounted for 80.3% of the completed visits during the baseline phase compared with only 0.7% in the current phase. Virtual visits went from 19.7% during the baseline phase to 66.8% of the completed visits during the current phase. | Descriptive study | IV |
| von Wrede/Germany [21] | Survey of 239 patients with epilepsy on acceptance and appreciation of telemedicine | 82% of the participants were satisfied with the telemedicine appointments. | Descriptive study | IV |
| Panda/India [22] | Descriptive study of telemedicine’s practicality in 153 children | 96% of caregivers were satisfied with the quality of medical advice. | Descriptive study | IV |
| Power/Ireland [23] | The development of an epilepsy electronic patient portal | The system can promote improved patient-clinician partnerships and facilitate patient self-management. | – | IV |
Table 3.
Seizure in patients with COVID-19
| Author/country | Methods | Results | Major limitations | Level of evidence |
|---|---|---|---|---|
| Mao/ China [24] | Retrospective, observational case series of 214 COVID patients | 1 patient had a focal motor seizure. | Small sample size | III |
| Lu/China [25] | Multicenter retrospective study of 304 COVID patients | Neither acute symptomatic seizures nor status epilepticus was observed. | Small sample size | III |
| Pinna/USA [26] | Retrospective study of 50 patients with COVID-19 evaluated by the neurology service | 13 (26%) patients had seizure (new onset seizures or breakthrough). Altered mental status was seen in 60%. No further characterization was available. | Small sample size | III |
| Nalleballe/USA [27] | A database study | Of 40,469 COVID-19 patients, 22.5% had neuropsychiatric manifestations; 0.6% had seizures and 2.3% had encephalopathy. No further characterization was available. | Based on the database codes (the individual patient-level data could not be accessed to verify the data completeness) | IV |
| Radmard/USA[28] | A retrospective case series of 33 adults diagnosed with SARS-CoV-2, who required neurological evaluation | The encountered neurological problems were encephalopathy (12 patients, 36.4%), seizure (9 patients, 27.2%), and stroke (5 patients, 15.2%). | Small sample size | III |
| Anand/USA [29] | A retrospective case series of 7 patients | 3 patients had a prior history of well-controlled epilepsy, while 4 patients had new-onset seizures. 3 patients had no preceding symptoms of COVID-19 prior to seizures. Brain CT scan was uneventful in all. CSF analysis was done in one and was normal. EEG showed focal sharp waves in one and status epilepticus in one. | Small sample size | IV |
| Bhatta/USA [30] | A case report of tonic-clonic seizure (an 11-year-old boy) | CSF analysis was not done. EEG was not done. Brain CT scan was normal. The patient remained well. | – | IV |
| Farley/Grenada [31] | A case report of status epilepticus (an 8-year-old boy) | CSF analysis was not done. EEG showed diffuse cerebral dysfunction. Brain CT scan was normal. The patient remained well. | – | IV |
| Shawkat/USA [32] | A case report of a seizure (67-year-old man) | Tests were not done. The patient died. | – | IV |
| Lyons/Ireland [33] | A case report of tonic-clonic seizure (a 20-year-old man) | CSF analysis demonstrated a lymphocytic pleocystosis (21 cells/mm3, 99% mononuclear, 1% polymorphs). Brain imaging was normal. The patient remained well and was discharged home. Follow-up EEG was normal. | – | IV |
| Elgamasy/Germany [34] | A case report of focal motor seizures (a 73-year-old woman) | CSF analysis was normal. Brain imaging had nonspecific findings. EEG was normal. The patient was discharged home in a good condition. | – | IV |
| Kadono/Japan [35] | A case report of focal motor seizure (a 44-year-old man with known focal epilepsy) | Brain CT scan showed severe brain swelling of the right temporal lobe. CSF analysis was not done. EEG was not done. The patient was discharged home in a good condition. | – | IV |
| Farhadian/USA [36] | A case report of seizure (a 78-year-old woman with kidney transplant) | CSF analysis was normal. Levels of IL–6, IL–8, and IP–10 were elevated in both CSF and plasma. EEG showed mild generalized slowing. Brain imaging had nonspecific findings. CSF SARS-CoV-2 was negative. The patient was discharged home in a good condition. | – | IV |
| Kabashneh/USA [37] | A case report of seizure (a 54-year-old man with diabetes) | CSF analysis was normal. EEG showed mild generalized slowing. Brain imaging was normal. The patient had diabetic ketoacidosis, acute kidney injury, hypovolemic shock, and hyperammonemia. The patient was discharged home in a good condition. | – | IV |
| Efe/Turkey [38] | A case report of seizures (a 35-year-old woman) | Brain MRI showed hyperintense signal in the left temporal lobe (an encephalitis mimicking a glial tumor after surgery and pathology examination). CSF analysis was not done. EEG was not done. | – | IV |
| Haddad/USA [39] | A case report of seizures (a 41-year-old man with HIV) | CSF analysis was normal. EEG showed generalized slowing. Brain CT scan was normal. The patient was discharged home in a good condition. | – | IV |
| Moriguchi/Japan [40] | A case report of a seizure (a 24-year-old man) | CSF cell count was 12/mL (10 mononuclear and 2 polymorphonuclear cells). SARS-CoV-2 RNA was detected in the CSF. Brain MRI showed hyperintensity along the wall of the right lateral ventricle and hyperintense signal changes in the right mesial temporal lobe. EEG was not done. Outcome was not specified. | – | IV |
| Sohal/USA [41] | A case report of seizures (a 72-year-old man with hypertension, diabetes, and end-stage kidney disease on hemodialysis) | Brain CT scan showed chronic microvascular ischemic changes. EEG showed six left temporal seizures and left temporal sharp waves. CSF analysis was not done. The patient died. | – | IV |
| Dixon/UK [42] | A case report of seizures (a 59-year-old woman with aplastic anemia) | Brain MRI demonstrated brain stem swelling with symmetrical hemorrhagic lesions in the brain stem, amygdalae, putamen, and thalamic nuclei (acute necrotizing encephalopathy). CSF analysis did not show cells. CSF PCR for SARS-CoV-2 was negative. EEG was not done. The patient died. | – | IV |
| Klein/USA [43] | A case report of seizures (a 29-year-old woman) | Brain CT scan demonstrated left temporoparietal hemorrhagic venous infarct as well as venous thrombosis in distal left transverse and sigmoid sinuses. EEG was not done. CSF analysis was not done. Outcome was not specified. | – | IV |
| Zanin/Italy [44] | A case report of seizures (a 54-year-old woman with previous brain surgery for aneurysm) | EEG showed two seizures starting from right frontotemporal region. Brain MRI showed alterations of the periventricular white matter and bulbo-medullary junction. CSF examination was normal. The CSF RT-PCR for SARS-CoV-2 was negative. The patient was transferred to rehabilitation without sensorimotor deficits. | – | IV |
| Hepburn/USA [45] | 2 cases of seizures |
1 (a 76-year-old man): EEG showed focal electrographic seizures. CSF analysis was not done. Brain imaging had nonspecific findings. The patient was discharged to a long-term acute care hospital for further ventilator management. 2 (an 82-year-old man): Focal motor seizures (confirmed by EEG). CSF analysis was not done. Brain imaging had nonspecific findings. The patient had coagulopathy and acute kidney injury. The patient died. |
– | IV |
| Balloy/France [46] | A case report of status epilepticus (a 59-year-old man) | CSF analysis was unremarkable and CSF SARS-CoV2 RT-PCR was negative. Brain MRI was normal. EEG showed 2 focal seizures. The patient remained well and was discharged from ICU. | – | IV |
| Vollono/Italy [47] | A case report of status epilepticus (a 78-year-old woman) | Brain MRI showed old gliosis and atrophy involving the left temporo-parietal lobe. CSF analysis was not done. EEG showed focal non-convulsive status epilepticus. The patient was discharged home in a good condition. | – | IV |
| Abdi/Iran [48] | A case report (a 58-year-old man with decreased level of consciousness) | CSF analysis was normal. Brain MRI showed diffuse confluent white matter hyperintensities. EEG was not done. The patient died after suffering from status epilepticus. | – | IV |
| Gómez-Enjuto/Spain [49] | A case report (a 74-year-old man with refractory status epilepticus) | Brain CT scan showed reversible posterior leucoencephalopathy syndrome (PRES). CSF analysis was normal. EEG was not done. Outcome was not specified. | – | IV |
| Abdulsalam/Kuwait [50] | A case report (a 32-year-old man with convulsive status epilepticus) | Brain CT scan was normal. CSF analysis showed elevated protein (2212 mg/L). EEG was not done. The patient was discharged home in a good condition. | – | IV |
| Bolaji/UK [51] | A case report (a 63-year-old man with status epilepticus) | Brain CT scan showed extensive cerebral venous sinus thrombosis with bilateral venous cortical infarcts and acute cortical hemorrhage. CSF analysis was not done. EEG was not done. The patient was discharged to a rehabilitation center. | – | IV |
| Monti/Italy [52] | A case report (a 50-year-old man with acute onset of psychiatric symptoms and refractory status epilepticus) | EEG showed a delta brush pattern. CSF study showed pleocytosis and anti-NMDA receptors antibodies. CSF SARS-CoV2 PCR was negative. Brain MRI was unremarkable. Four months after the onset, the patient was discharged home in good condition. | – | IV |
CNS, central nervous system; CSF, cerebrospinal fluid; EEG, electroencephalography; CI, confidence interval; ICU, intensive care unit; CT, computerized tomography; MRI, magnetic resonance imaging; HIV, human immunodeficiency virus; PCR, polymerase chain reaction
Table 4.
Electroencephalography in patients with COVID-19
| Author/country | Methods | Results | Major limitations | Level of evidence |
|---|---|---|---|---|
| Helms/France [53] | 42 COVID-19 patients with EEG recording due to change in mental status | EEG showed nonspecific abnormalities or diffuse, especially bifrontal, slow activity. CSF analysis revealed inflammatory disturbances in 18/28 patients, including oligoclonal bands with mirror pattern and elevated IL-6. The CSF RT-PCR SARS-CoV-2 was positive in one patient. | Small sample size | III |
| Pilato/USA [54] | 8 COVID-19 patients with EEG monitoring | Generalized background slowing in all and generalized epileptiform discharges with triphasic morphology in 3 patients. | A case series | IV |
| Galanopoulou/USA [55] | Retrospective study of 22 patients | New onset encephalopathy (68%) and seizure-like events (64%) were reasons for EEG study. Four patients had prior epilepsy. Epileptiform discharges were present in 41%. Many patients had organ failure. | Small sample size | III |
| Petrescu/France [56] | Retrospective study of 40 EEGs in 36 COVID-19 patients | Generalized periodic discharges, multifocal periodic discharges or rhythmic delta activity were found in 13 recordings (32.5%). | Small sample size | III |
| Assenza/Italy [57] | Survey of 206 centers on EEG management data | The number of EEGs performed was reduced by 76 ± 20%. Half of the centers performed inpatient EEGs only for urgencies. | – | IV |
| Vellieux/France [58] | 2 patients with COVID-19 and encephalopathy and a unique EEG pattern |
1. A 37-year-old man, EEG showed continuous, slightly asymmetric, monomorphic, diphasic, delta slow waves with greater amplitude over both frontal areas and with a periodic organization. Brain MRI showed hypoxic encephalopathy. CSF analysis was normal. At hospital discharge, he had a mild left sensorimotor deficit secondary infarction. 2. A 42-year-old man, EEG was similar to above. CSF analysis was not done. Brain imaging was not done. The patient died. |
– | IV |
| De Stefano/Switzerland [59] | A case with altered mental status (a 56-year-old woman) | EEG showed a focal monomorphic theta slowing in bilateral frontal-central regions. MRI showed microbleeds located in bilateral white matter junction, various regions of corpus callosum, and internal capsule. CSF analysis excluded the encephalitis. SARS-Cov2 RNA-PCR in CSF was negative. Outcome was not specified. | – | IV |
| Cecchetti/Italy[60] | A series of 18 patients | EEG showed generalized slowing in 88.9%; an anterior (bifrontal) prevalence of slow waves was noted in 55.6%. Two patients had epileptiform discharges (no seizures). One subject underwent lumbar puncture with normal results and negative PCR test for SARS-CoV-2. | A case series | IV |
| Pati/USA [61] | A series of 10 patients with continuous electroencephalography (cEEG) | Patients with good outcome had higher temporal-variance with greater diversity in frequency bands and spatial extents. QEEG features may prognosticate neurological outcome in critically ill patients with COVID-19. | A case series | IV |
| Pastor/Spain [62] | A series of 20 patients with QEEG | Temporal lobes showed different distribution for QEEG bands. | A case series | IV |
| Somani/UK [63] | 2 patients with COVID-19 and de novo status epilepticus |
1. A 49-year-old woman, EEG monitoring showed multiple seizures starting from the midline and left fronto-central regions. Brain MRI was normal. CSF analysis was not done. The patient was discharged home in a good condition. 2. A 73-year-old woman, EEG showed bilateral independent periodic discharges over the left and right hemisphere that evolved to form recurrent seizures starting from either right or left fronto-central-parietal regions. Brain CT scan was normal. CSF analysis was not done. The patient died. |
– | IV |
| Abdel-Mannan/UK [64] | 27 children with COVID-19 pediatric multisystem inflammatory syndrome | In all 3 patients who underwent EEG, a mild excess of slow activity was seen. In the 2 patients whose CSF was tested, samples were acellular, with negative SARS-CoV-2 PCR. | Small sample size | III |
| Vespignani/France [65] | 26 COVID-19 patients who underwent EEG to assess unexplained altered mental status | 5 patients had EEGs with periodic discharges consisting of high-amplitude frontal monomorphic delta waves with no epileptic activity. | Small sample size | III |
| Scullen/USA [66] | 27 critically ill patients with COVID-19 | 74% had encephalopathy, 7% acute necrotizing encephalopathy, and 19% vasculopathy. 44% had EEG abnormalities; most of them had generalized encephalopathy; one patient had nonconvulsive status epilepticus. | Small sample size | III |
| Passini/Italy [67] | EEG findings in 15 patients with COVID-19 and encephalopathy | The EEGs were abnormal (slow) in all cases. No epileptiform abnormalities or triphasic waves were observed. SARS-CoV-2 was not detected in the CSF in any case. | Small sample size | III |
| Abenza-Abildúa/Spain [68] | A case report (a 56-year-old woman with change in mental status) | EEG showed generalized slowing. CSF analysis was normal. Brain MRI was uneventful. The patient remained well. | – | IV |
| Roy-Gash/France [69] | A case report (a 63-year-old woman with status epilepticus) | EEG showed focal status epilepticus. CSF analysis was not done. Brain CT scan showed left temporal hemorrhage with venous thrombosis. The patient died. | – | IV |
| Pilotto/Italy [70] | A case report (a 60-year-old man with change in mental status) | EEG showed generalized slowing. CSF analysis showed lymphocytic pleocytosis (18/μL). CSF PCR for SARS-CoV-2 was negative. Brain MRI was normal. The patient was discharged home in a good condition. | – | IV |
CSF, cerebrospinal fluid; EEG, electroencephalography; CT, computerized tomography; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; QEEG, quantitative EEG
Discussion
The evidence on the susceptibility of PWE to contracting COVID-19 and the consequences of this catastrophic disease in PWE is scarce. A recent systematic review suggested that patients with pre-existing neurological disorders (including epilepsy) and COVID-19 may develop exacerbation of their neurological problems and also severe COVID-19 [72]. This significant concern should be addressed in the future through well-designed prospective cohort studies with all relevant confounders controlled. However, evidence showed that many PWE perceived significant disruption in the quality and availability of care to them, and some people reported increase in their seizure frequency since the onset of the pandemic [11, 12, 14, 16, 17]. Previous global experiences confirm this piece of evidence. A study of 227 PWE during the SARS outbreak in 2003 in Taiwan demonstrated that 22% of them did not receive their medications due to loss of contact with their healthcare providers; 12% of them experienced seizure control status worsening [73]. In addition, many PWE may have increased stress and social isolation, even more than that in others, during such circumstances as COVID-19 pandemic [12–15]. During pandemics and other difficult and catastrophic circumstances (e.g., wars and mass displacements), healthcare workers should focus not only on seizure control status of their patients, but also on the mental health of PWE [13]. In such times, stress is associated with increased seizures in PWE [11], and stress management strategies may play a significant role in helping these patients cope with their ongoing problems more successfully. Research shows that people who follow COVID-19 news the most, experience more anxiety and stress [74, 75]. Healthcare providers should advise their patients to avoid following COVID-19 news frequently. In addition, it might be helpful to use digital communication methods such as social networks to prevent social isolation [75]. Similarly, many patients with epilepsy may have depressive symptoms, particularly during such difficult times, and several factors related to epilepsy (e.g., seizure frequency and quality of life) are significantly correlated with depressive symptoms [76]. Therefore, it is helpful to screen all PWE for anxiety, stress, and depression, and treat any psychological problems that patients may have, particularly during a pandemic or other difficult circumstances.
Telemedicine is a viable and helpful technology that may improve access to the needed care for PWE in these difficult times [10, 20, 22]. During a pandemic such as what we are experiencing now, telemedicine, particularly video consultations, ought to be promoted in order to reduce the risk of disease transmission [77]. Telemedicine has been shown to improve access to care for PWE in difficult circumstances (e.g., people living in rural areas) [78]. However, many countries do not have the infrastructure or the regulatory frameworks to authorize, integrate, and reimburse telemedicine services [77].
Another important issue is the occurrence of new-onset seizures in people with COVID-19. While seizure is not a common manifestation of COVID-19, it may happen in a variety of forms (e.g., focal motor, tonic-clonic, convulsive status epilepticus, and nonconvulsive status epilepticus) [28, 30–47]. On the other hand, many patients with severe COVID-19 may have changes in their mental status [26, 27]. When visiting a patient who is in a critical medical condition and has a change in mental status, the treating healthcare professional should make sure that nonconvulsive status epilepticus is not a part of the clinical scenario. Prolonged EEG monitoring is required in these circumstances [3]. Electroencephalography is probably one of the most useful tests to help identify the etiology of seizures and/or change in mental status in critically ill patients, including those with COVID-19; and at the same time, it is probably the most neglected test so far in the management process of these patients globally. It is helpful to describe the EEG findings in a systematic and consistent manner in order to characterize any possible distinguishing and/or prognosticating EEG patterns in patients with COVID-19. Finally, quantitative EEG may also be useful for diagnosis and prognostication of the neurological outcome in critically ill patients with COVID-19 [61, 62].
Seizures may happen as a consequence of hypoxia, metabolic derangements, organ failure, medications, or brain damage that could happen in people with COVID-19 [3, 5]. If a patient with COVID-19 develops a seizure, one should try to determine the etiology of the seizure and manage the cause (e.g., hypoxia and metabolic derangements) immediately. In addition to prolonged EEG monitoring, performing a thorough metabolic investigation, electrocardiogram, brain imaging, and a careful review of all medications (for adverse drug reactions and also drug-drug interactions) are necessary steps. CSF analysis is also necessary, at least to investigate other causes for acute symptomatic seizures that may happen concomitantly or that may mimic the clinical picture of COVID-19 (e.g., herpes simplex virus-1 encephalitis) [79]. In addition, it is often necessary to start an antiseizure medication (ASM) for a patient with COVID-19 and seizure(s); this is to abort prolonged seizures and also to prevent further seizures from happening [3]. It should be emphasized that patients with acute symptomatic seizures do not need long-term ASM therapy after the period of acute illness, unless a subsequent seizure happens [80]. For a comprehensive review on these issues, please refer to the references [3, 5, 81].
Conclusion
What we know and should pay more attention to
Many PWE perceived significant disruption in the quality and availability of care to them, and some people reported increase in their seizure frequency since the onset of the pandemic. In addition, many PWE may have increased stress and anxiety. During pandemics and other difficult and catastrophic circumstances, healthcare workers should focus not only on seizure control status of their patients, but also on the mental health of PWE.
Telemedicine is a viable and helpful technology that may improve access to the needed care for PWE in these difficult times. The current COVID-19 pandemic is a strong call to develop the required infrastructure and to adopt the appropriate regulatory frameworks for implementation of telemedicine services globally [77].
De novo seizures may occur in people with COVID-19, and they may happen in a variety of forms (e.g., focal motor, tonic-clonic, convulsive status epilepticus, nonconvulsive status epilepticus, and post SARS-CoV2 autoimmune encephalitis associated with new-onset refractory status epilepticus (NORSE) [82, 83]). Furthermore, many patients with severe COVID-19 may have change in mental status. If a patient with COVID-19 develops a seizure or change in mental status, one should try to determine the etiology and manage the cause immediately. In addition to prolonged EEG monitoring, performing a thorough metabolic investigation, electrocardiogram, brain imaging, CSF analysis, and a careful review of all medications are necessary steps.
What we do not know yet and should be investigated further
The evidence on the susceptibility of PWE to contracting COVID-19 and the consequences of this catastrophic disease in PWE is scarce. This significant concern should be addressed in the future through well-designed prospective cohort studies with all relevant confounders controlled.
SARS-CoV-2 RNA was detected in the CSF in two patients, but many other studies that tested for this had negative results. This is important to clarify the role of neurtropism and neuroinvasiveness of this virus in causing seizures in future studies.
It is not clear whether there is a unique EEG pattern for COVID-19 encephalopathy. It is helpful to describe the EEG findings in a systematic and consistent manner in order to characterize any possible distinguishing and/or prognosticating EEG patterns in patients with COVID-19.
Contributions
Ali A. Asadi-Pooya: Designed and conceptualized the study; collected the data; analyzed the data; drafted and revised the manuscript.
Leila Simani, Mina Shahisavandi, Zohreh Barzegar: Collected the data; revised the manuscript.
Appendix
Funding
This study is supported by Shiraz University of Medical Sciences.
Data availability
Data sharing is not applicable to this article.
Compliance with ethical standards
Conflict of interest
Ali A. Asadi-Pooya, M.D.: Honoraria from Cobel Daruo, RaymandRad and Tekaje; Royalty: Oxford University Press (book publication). Others: none.
Ethical approval
The Shiraz University of Medical Sciences Institutional Review Board approved this study and systematic review.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Role of the funding source
Shiraz University of Medical Sciences had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
None of the authors listed on the manuscript are employed by a government agency. All are academicians. None of the authors are submitting this manuscript as an official representative or on behalf of the government.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ali A. Asadi-Pooya, Email: aliasadipooya@yahoo.com
Leila Simani, Email: l.simani62@gmail.com.
Mina Shahisavandi, Email: shahisavandimina@gmail.com.
Zohreh Barzegar, Email: zohrehbarzegar1375@gmail.com.
References
- 1.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asadi-Pooya AA. Seizures associated with coronavirus infections. Seizure. 2020;79:49–52. doi: 10.1016/j.seizure.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asadi-Pooya AA, Attar A, Moghadami M, Karimzadeh I (2020) Management of COVID-19 in people with epilepsy: drug considerations. Neurol Sci:1–7. 10.1007/s10072-020-04549-5 [DOI] [PMC free article] [PubMed]
- 6.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JPA, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 8.Gronseth GS CJ, Gloss D, Merillat S, Dittman J, Armstrong MJ, on behalf of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology et al (2017) Clinical practice guideline process manual, 2017 edn. The American Academy of Neurology, Minneapolis, 2017
- 9.Cabezudo-García P, Ciano-Petersen NL, Mena-Vázquez N, Pons-Pons G, Castro-Sánchez MV, Serrano-Castro PJ (2020) Incidence and case fatality rate of COVID-19 in patients with active epilepsy. Neurology. 10.1212/WNL.0000000000010033 [DOI] [PubMed]
- 10.Conde-Blanco E, Centeno M, Tio E, Muriana D, García-Peñas JJ, Serrano P, et al. Emergency implementation of telemedicine for epilepsy in Spain: results of a survey during SARS-CoV-2 pandemic. Epilepsy Behav. 2020;111:107211. doi: 10.1016/j.yebeh.2020.107211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S, Wu C, Jia Y, Li G, Zhu Z, Lu K, Yang Y, Wang F, Zhu S. COVID-19 outbreak: the impact of stress on seizures in patients with epilepsy. Epilepsia. 2020;61:1884–1893. doi: 10.1111/epi.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asadi-Pooya AA, Farazdaghi M, Bazrafshan M. Impacts of the COVID-19 pandemic on Iranian patients with epilepsy. Acta Neurol Scand. 2020;142:392–395. doi: 10.1111/ane.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao X, Zhou D, Li Z, Zeng G, Hao N, Li E, Li W, Deng A, Lin M, Yan B. Severe psychological distress among patients with epilepsy during the COVID-19 outbreak in southwest China. Epilepsia. 2020;61:1166–1173. doi: 10.1111/epi.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller WR, Von Gaudecker J, Tanner A, Buelow JM. Epilepsy self-management during a pandemic: experiences of people with epilepsy. Epilepsy Behav. 2020;111:107238. doi: 10.1016/j.yebeh.2020.107238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aledo-Serrano Á, Mingorance A, Jiménez-Huete A, Toledano R, García-Morales I, Anciones C, Gil-Nagel A. Genetic epilepsies and COVID-19 pandemic: lessons from the caregiver perspective. Epilepsia. 2020;61:1312–1314. doi: 10.1111/epi.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assenza G, Lanzone J, Brigo F, Coppola A, Di Gennaro G, Di Lazzaro V, et al. Epilepsy care in the time of COVID-19 pandemic in Italy: risk factors for seizure worsening. Front Neurol. 2020;11:737. doi: 10.3389/fneur.2020.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alkhotani A, Siddiqui MI, Almuntashri F, Baothman R. The effect of COVID-19 pandemic on seizure control and self-reported stress on patient with epilepsy. Epilepsy Behav. 2020;112:107323. doi: 10.1016/j.yebeh.2020.107323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granata T, Bisulli F, Arzimanoglou A, Rocamora R (2020) Did the COVID-19 pandemic silence the needs of people with epilepsy? Epileptic Disord. 10.1684/epd.2020.1175 [DOI] [PMC free article] [PubMed]
- 19.Wirrell EC, Grinspan ZM, Knupp KG, Jiang Y, Hammeed B, Mytinger JR et al (2020) Care delivery for children with epilepsy during the COVID-19 pandemic: an international survey of clinicians. J Child Neurol 35:924–933 [DOI] [PMC free article] [PubMed]
- 20.Punia V, Nasr G, Zagorski V, Lawrence G, Fesler J, Nair D, Najm I. Evidence of a rapid shift in outpatient practice during the COVID-19 pandemic using telemedicine. Telemed J E Health. 2020;26:1301–1303. doi: 10.1089/tmj.2020.0150. [DOI] [PubMed] [Google Scholar]
- 21.von Wrede R, Moskau-Hartmann S, Baumgartner T, Helmstaedter C, Surges R. Counseling of people with epilepsy via telemedicine: experiences at a German tertiary epilepsy center during the COVID-19 pandemic. Epilepsy Behav. 2020;112:107298. doi: 10.1016/j.yebeh.2020.107298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panda PK, Dawman L, Panda P, Sharawat IK. Feasibility and effectiveness of teleconsultation in children with epilepsy amidst the ongoing COVID-19 pandemic in a resource-limited country. Seizure. 2020;81:29–35. doi: 10.1016/j.seizure.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power K, McCrea Z, White M, Breen A, Dunleavy B, O'Donoghue S et al (2020) The development of an epilepsy electronic patient portal: facilitating both patient empowerment and remote clinician-patient interaction in a post-COVID-19 world. Epilepsia. 10.1111/epi.16627 [DOI] [PMC free article] [PubMed]
- 24.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L, Xiong W, Liu D, Liu J, Yang D, Li N, Mu J, Guo J, Li W, Wang G, Gao H, Zhang Y, Lin M, Chen L, Shen S, Zhang H, Sander JW, Luo J, Chen S, Zhou D. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61:e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinna P, Grewal P, Hall JP, Tavarez T, Dafer RM, Garg R, Osteraas ND, Pellack DR, Asthana A, Fegan K, Patel V, Conners JJ, John S, Silva ID. Neurological manifestations and COVID-19: experiences from a tertiary care center at the frontline. J Neurol Sci. 2020;415:116969. doi: 10.1016/j.jns.2020.116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nalleballe K, Reddy Onteddu S, Sharma R, Dandu V, Brown A, Jasti M, et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav Immun. 2020;S0889-1591(20):31008–31004. doi: 10.1016/j.bbi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radmard S, Epstein SE, Roeder HJ, Michalak AJ, Shapiro SD, Boehme A, Wilson TJ, Duran JC, Bain JM, Willey JZ, Thakur KT. Inpatient neurology consultations during the onset of the SARS-CoV-2 New York City pandemic: a single center case series. Front Neurol. 2020;11:805. doi: 10.3389/fneur.2020.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anand P, Al-Faraj A, Sader E, Dashkoff J, Abdennadher M, Murugesan R, et al. Seizure as the presenting symptom of COVID-19: a retrospective case series. Epilepsy Behav. 2020;112:107335. doi: 10.1016/j.yebeh.2020.107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatta S, Sayed A, Ranabhat B, Bhatta RK, Acharya Y. New-onset seizure as the only presentation in a child with COVID-19. Cureus. 2020;12:e8820. doi: 10.7759/cureus.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farley M, Zuberi J. COVID-19 precipitating status epilepticus in a pediatric patient. Am J Case Rep. 2020;21:e925776. doi: 10.12659/AJCR.925776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shawkat A, Merrell ET, Fadel GA, Amzuta I, Amin H, Shah AJ, et al. Multiple thrombotic events in a 67-year-old man 2 weeks after testing positive for SARS-CoV-2: a case report. Am J Case Rep. 2020;21:e925786. doi: 10.12659/AJCR.925786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyons S, O’Kelly B, Woods S, Rowan C, Brady D, Sheehan G, et al. Seizure with CSF lymphocytosis as a presenting feature of COVID-19 in an otherwise healthy young man. Seizure. 2020;80:113–114. doi: 10.1016/j.seizure.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elgamasy S, Kamel MG, Ghozy S, Khalil A, Morra ME, Islam SMS (2020) First case of focal epilepsy associated with SARS-coronavirus-2. J Med Virol. 10.1002/jmv.26113 [DOI] [PMC free article] [PubMed]
- 35.Kadono Y, Nakamura Y, Ogawa Y, Yamamoto S, Kajikawa R, Nakajima Y, Matsumoto M, Kishima H. A case of COVID-19 infection presenting with a seizure following severe brain edema. Seizure. 2020;80:53–55. doi: 10.1016/j.seizure.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farhadian S, Glick LR, Vogels CBF, Thomas J, Chiarella J, Casanovas-Massana A, Zhou J, Odio C, Vijayakumar P, Geng B, Fournier J, Bermejo S, Fauver JR, Alpert T, Wyllie AL, Turcotte C, Steinle M, Paczkowski P, dela Cruz C, Wilen C, Ko AI, MacKay S, Grubaugh ND, Spudich S, Barakat LA. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20:248. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabashneh S, Ali H, Alkassis S. Multi-organ failure in a patient with diabetes due to COVID-19 with clear lungs. Cureus. 2020;12:e8147. doi: 10.7759/cureus.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Efe IE, Aydin OU, Alabulut A, Celik O, Aydin K. COVID-19-associated encephalitis mimicking glial tumor. World Neurosurg. 2020;140:46–48. doi: 10.1016/j.wneu.2020.05.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haddad S, Tayyar R, Risch L, Churchill G, Fares E, Choe M, Montemuro P. Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient. IDCases. 2020;21:e00814. doi: 10.1016/j.idcr.2020.e00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sohal S, Mansur M. COVID-19 presenting with seizures. IDCases. 2020;20:e00782. doi: 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dixon L, Varley J, Gontsarova A, Mallon D, Tona F, Muir D, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7:e789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein DE, Libman R, Kirsch C, Arora R. Cerebral venous thrombosis: a typical presentation of COVID-19 in the young. J Stroke Cerebrovasc Dis. 2020;29:104989. doi: 10.1016/j.jstrokecerebrovasdis.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, Fontanella MM. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162:1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hepburn M, Mullaguri N, George P, Hantus S, Punia V, Bhimraj A et al (2020) Acute symptomatic seizures in critically ill patients with COVID-19: is there an association? Neurocrit Care:1–5. 10.1007/s12028-020-01006-1 [DOI] [PMC free article] [PubMed]
- 46.Balloy G, Mahé PJ, Leclair-Visonneau L, Péréon Y, Derkinderen P, Magot A, et al. Non-lesional status epilepticus in a patient with coronavirus disease 2019. Clin Neurophysiol. 2020;131:2059–2061. doi: 10.1016/j.clinph.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vollono C, Rollo E, Romozzi M, Frisullo G, Servidei S, Borghetti A, Calabresi P. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020;78:109–112. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdi S, Ghorbani A, Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J Neurol Sci. 2020;416:117001. doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gómez-Enjuto S, Hernando-Requejo V, Lapeña-Motilva J, Ogando-Durán G, Fouz-Ruiz D, Domingo-García J, Rodríguez-García E, Cemillán-Fernández CA. Verapamil as treatment for refractory status epilepticus secondary to PRES syndrome on a SARS-Cov-2 infected patient. Seizure. 2020;80:157–158. doi: 10.1016/j.seizure.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdulsalam MA, Abdulsalam AJ, Shehab D (2020) Generalized status epilepticus as a possible manifestation of COVID-19. Acta Neurol Scand. 10.1111/ane.13321 [DOI] [PMC free article] [PubMed]
- 51.Bolaji P, Kukoyi B, Ahmad N, Wharton C. Extensive cerebral venous sinus thrombosis: a potential complication in a patient with COVID-19 disease. BMJ Case Rep. 2020;13:e236820. doi: 10.1136/bcr-2020-236820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monti G, Giovannini G, Marudi A, Bedin R, Melegari A, Simone AM, Santangelo M, Pignatti A, Bertellini E, Trenti T, Meletti S. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. 2020;81:18–20. doi: 10.1016/j.seizure.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helms J, Kremer S, Merdji H, Schenck M, Severac F, Clere-Jehl R, Studer A, Radosavljevic M, Kummerlen C, Monnier A, Boulay C, Fafi-Kremer S, Castelain V, Ohana M, Anheim M, Schneider F, Meziani F. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24:491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pilato MS, Urban A, Alkawadri R, Barot NV, Castellano JF, Rajasekaran V et al (2020) EEG findings in coronavirus disease. J Clin Neurophysiol. 10.1097/WNP.0000000000000752 [DOI] [PubMed]
- 55.Galanopoulou AS, Ferastraoaru V, Correa DJ, Cherian K, Duberstein S, Gursky J, Hanumanthu R, Hung C, Molinero I, Khodakivska O, Legatt AD, Patel P, Rosengard J, Rubens E, Sugrue W, Yozawitz E, Mehler MF, Ballaban-Gil K, Haut SR, Moshé SL, Boro A. EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: a small case series preliminary report. Epilepsia Open. 2020;5:314–324. doi: 10.1002/epi4.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrescu AM, Taussig D, Bouilleret V. Electroencephalogram (EEG) in COVID-19: a systematic retrospective study. Neurophysiol Clin. 2020;50:155–165. doi: 10.1016/j.neucli.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Assenza G, Lanzone J, Ricci L, Boscarino M, Tombini M, Galimberti CA et al (2020) Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci:1–6. 10.1007/s10072-020-04546-8 [DOI] [PMC free article] [PubMed]
- 58.Vellieux G, Rouvel-Tallec A, Jaquet P, Grinea A, Sonneville R, d’Ortho MP. COVID-19 associated encephalopathy: is there a specific EEG pattern? Clin Neurophysiol. 2020;131:1928–1930. doi: 10.1016/j.clinph.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Stefano P, Nencha U, De Stefano L, Mégevand P, Seeck M. Focal EEG changes indicating critical illness associated cerebral microbleeds in a Covid-19 patient. Clin Neurophysiol Pract. 2020;5:125–129. doi: 10.1016/j.cnp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cecchetti G, Vabanesi M, Chieffo R, Fanelli G, Minicucci F, Agosta F et al (2020) Cerebral involvement in COVID-19 is associated with metabolic and coagulation derangements: an EEG study. J Neurol:1–5. 10.1007/s00415-020-09958-2 [DOI] [PMC free article] [PubMed]
- 61.Pati S, Toth E, Chaitanya G. Quantitative EEG markers to prognosticate critically ill patients with COVID-19: a retrospective cohort study. Clin Neurophysiol. 2020;131:1824–1826. doi: 10.1016/j.clinph.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pastor J, Vega-Zelaya L, Martín Abad E. Specific EEG encephalopathy pattern in SARS-CoV-2 patients. J Clin Med. 2020;9:1545. doi: 10.3390/jcm9051545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Somani S, Pati S, Gaston T, Chitlangia A, Agnihotri S (2020) De Novo Status Epilepticus in patients with COVID-19. Ann Clin Transl Neurol. 10.1002/acn3.51071 [DOI] [PMC free article] [PubMed]
- 64.Abdel-Mannan O, Eyre M, Löbel U, Bamford A, Eltze C, Hameed B et al (2020) Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA 77:1–6 [DOI] [PMC free article] [PubMed]
- 65.Vespignani H, Colas D, Lavin BS, Soufflet C, Maillard L, Pourcher V et al (2020) Report of EEG Finding on Critically Ill Patients with COVID-19. Ann Neurol. 10.1002/ana.25814 [DOI] [PMC free article] [PubMed]
- 66.Scullen T, Keen J, Mathkour M, Dumont AS, Kahn L. Coronavirus 2019 (COVID-19)-associated Encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg. 2020;S1878-8750(20):31163–31163. doi: 10.1016/j.wneu.2020.05.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasini E, Bisulli F, Volpi L, Minardi I, Tappatà M, Muccioli L, Pensato U, Riguzzi P, Tinuper P, Michelucci R. EEG findings in COVID-19 related encephalopathy. Clin Neurophysiol. 2020;131:2265–2267. doi: 10.1016/j.clinph.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abenza-Abildúa MJ, Novo-Aparicio S, Moreno-Zabaleta R, Algarra-Lucas MC, Rojo Moreno-Arcones B, Salvador-Maya MÁ, Navacerrada-Barrero FJ, Ojeda-Ruíz de Luna J, Pérez-López C, Fraile-Vicente JM, Suárez-García I, Suarez-Gisbert E, Palacios-Castaño JA, Ramirez-Prieto MT. Encephalopathy in severe SARS-CoV2 infection: inflammatory or infectious? Int J Infect Dis. 2020;98:398–400. doi: 10.1016/j.ijid.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roy-Gash F, Marine M, Jean-Michel D, Herve V, Raphael B, Nicolas E. COVID-19-associated acute cerebral venous thrombosis: clinical, CT, MRI and EEG features. Crit Care. 2020;24:419. doi: 10.1186/s13054-020-03131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S et al (2020) Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol. 10.1002/ana.25783 [DOI] [PMC free article] [PubMed]
- 71.Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American clinical neurophysiology Society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 72.Kubota T, Kuroda N (2020) Exacerbation of neurological symptoms and COVID-19 severity in patients with preexisting neurological disorders and COVID-19: a systematic review. Clin Neurol Neurosurg:106349. 10.1016/j.clineuro.2020.106349 [DOI] [PMC free article] [PubMed]
- 73.Lai SL, Hsu MT, Chen SS. The impact of SARS on epilepsy: the experience of drug withdrawal in epileptic patients. Seizure. 2005;14:557–561. doi: 10.1016/j.seizure.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.World Health Organization . Mental health and psychosocial considerations during the COVID-19 outbreak, 18 March 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- 75.Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, Rasoulpoor S, Khaledi-Paveh B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob Health. 2020;16:57. doi: 10.1186/s12992-020-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tombini M, Assenza G, Quintiliani L, Ricci L, Lanzone J, Ulivi M, di Lazzaro V. Depressive symptoms and difficulties in emotion regulation in adult patients with epilepsy: association with quality of life and stigma. Epilepsy Behav. 2020;107:107073. doi: 10.1016/j.yebeh.2020.107073. [DOI] [PubMed] [Google Scholar]
- 77.Ohannessian R, Duong TA, Odone A. Global telemedicine implementation and integration within health systems to fight the COVID-19 pandemic: a call to action. JMIR Public Health Surveill. 2020;6:e18810. doi: 10.2196/18810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haddad N, Grant I, Eswaran H. Telemedicine for patients with epilepsy: a pilot experience. Epilepsy Behav. 2015;44:1–4. doi: 10.1016/j.yebeh.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 79.Lovati C, Osio M, Pantoni L. Diagnosing herpes simplex-1 encephalitis at the time of COVID-19 pandemic. Neurol Sci. 2020;41:1361–1364. doi: 10.1007/s10072-020-04461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bergey GK. Management of a first seizure. Continuum (Minneap Minn) 2016;22(1 Epilepsy):38–50. doi: 10.1212/CON.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 81.French JA, Brodie MJ, Caraballo R, Devinsky O, Ding D, Jehi L, Jette N, Kanner A, Modi AC, Newton CR, Patel AA, Pennell PB, Perucca E, Sander JW, Scheffer IE, Singh G, Williams E, Wilmshurst J, Cross JH. Keeping people with epilepsy safe during the COVID-19 pandemic. Neurology. 2020;94:1032–1037. doi: 10.1212/WNL.0000000000009632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dono F, Carrarini C, Russo M, De Angelis MV, Anzellotti F, Onofrj M et al (2020) New-onset refractory status epilepticus (NORSE) in post SARS-CoV-2 autoimmune encephalitis: a case report. Neurol Sci:1–4. 10.1007/s10072-020-04846-z [DOI] [PMC free article] [PubMed]
- 83.Emami A, Fadakar N, Akbari A, Lotfi M, Farazdaghi M, Javanmardi F, Rezaei T, Asadi-Pooya AA. Seizure in patients with COVID-19. Neurol Sci. 2020;41:3057–3061. doi: 10.1007/s10072-020-04731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article.