Abstract
Microbial communities infiltrate the respiratory tract of cystic fibrosis patients, where chronic colonization and infection lead to clinical decline. This report aims to provide an overview of the diversity of bacterial and fungal species from the airway secretion of three young CF patients with severe pulmonary disease. The bacterial and fungal microbiomes were investigated by culture isolation, metataxonomics, and metagenomics shotgun. Virulence factors and antibiotic resistance genes were also explored. A. fumigatus was isolated from cultures and identified in high incidence from patient sputum samples. Candida albicans, Penicillium sp., Hanseniaspora sp., Torulaspora delbrueckii, and Talaromyces amestolkiae were isolated sporadically. Metataxonomics and metagenomics detected fungal reads (Saccharomyces cerevisiae, A. fumigatus, and Schizophyllum sp.) in one sputum sample. The main pathogenic bacteria identified were Staphylococcus aureus, Pseudomonas aeruginosa, Burkholderia cepacia complex, and Achromobacter xylosoxidans. The canonical core CF microbiome is composed of species from the genera Streptococcus, Neisseria, Rothia, Prevotella, and Haemophilus. Thus, the airways of the three young CF patients presented dominant bacterial genera and interindividual variability in microbial community composition and diversity. Additionally, a wide diversity of virulence factors and antibiotic resistance genes were identified in the CF lung microbiomes, which may be linked to the clinical condition of the CF patients. Understanding the microbial community is crucial to improve therapy because it may have the opposite effect, restructuring the pathogenic microbiota. Future studies focusing on the influence of fungi on bacterial diversity and microbial interactions in CF microbiomes will be welcome to fulfill this huge gap of fungal influence on CF physiopathology.
Keywords: cystic fibrosis, lung microbiome, metagenomic, colonization, Aspergillus fumigatus, Burkholderia, lung microbiota
Introduction
Cystic fibrosis (CF) is the most common lethal autosomal recessive disorder in Caucasians and is characterized by a mutation in the cystic fibrosis transmembrane conductance regulator gene (CFTR) (Cutting, 2015; Lavelle et al., 2016). The codified CFTR protein is responsible for the transport of chloride ions over the apical membranes of epithelial cells from tissues such as airways, intestine, pancreas, kidneys, sweat glands, and male reproductive tract (Bell et al., 2020). In the sinopulmonary tract, the transport of chloride anions contributes to the hydration of the mucus, forming a physical barrier against microbial pathogens and particles, which can be expelled by expectoration or swallowed through the action of ciliary movements in the trachea (Patel et al., 2020). In addition, the CFTR protein also transports bicarbonate ions, controls the levels of acidity in fluid secretions, and interacts with other membrane proteins to maintain tight junctions. All these roles of CFTR protein allow mucociliary clearance (MCC), whose impairment leads to the production of more viscous mucus, predisposing patients to develop chronic infections of airways (Procianoy et al., 2020). As a result, acute episodes of lung infections are observed in CF, receiving the general designation of “pulmonary exacerbation” (De Koff et al., 2016).
To overcome the deficiency in MCC and to prevent microbial colonization in the respiratory tract, the prescription of inhaled and systemic antibiotics is the first line of treatment available (De Koff et al., 2016). Pulmonary microbial colonization is facilitated by the building up of mucus, which may be a nutrient source for anaerobic bacteria such as Streptococcus, Prevotella, and Veilonella, whose metabolism releases short-chain fatty acids that serve as substrates for Pseudomonas aeruginosa. These bacteria are recognized as keystone taxa because they facilitate the growth of canonical CF pathogens through cross-feeding pathways (Silveira et al., 2020; Flynn et al., 2020). However, despite the frequent detection of these taxa in CF patients, a very high inter-patient variability of the airway microbiome has been reported (Bacci et al., 2020).
The CF microbiome varies according to age and clinical conditions of each patient, and it is generally more diverse in the younger than in the elderly, with decreasing diversity directly correlated to the loss of pulmonary function (Cox et al., 2010; Coburn et al., 2015; Caverly and Lipuma, 2018). Studies have also pointed out fungi of the genera Candida and Aspergillus as members of CF microbiomes, mainly related to cases of pulmonary exacerbation (Bevivino et al., 2019; Soret et al., 2020). However, research and treatment have conventionally focused on bacterial pathogens with little attention to fungal species. The fungi have been sub-notified as agents of CF infections for several reasons: (i) they might be erroneously regarded as contaminants in laboratory cultures; (ii) there are usually limited resources to recover fungal species in routine analysis of clinical samples; (iii) there is a lack of robust clinical guidelines and laboratory tools to assure unambiguous taxonomic identification of fungi; and (iv) clinicians tend to get puzzled by laboratory reports on unfamiliar fungal genera, which leads to difficulties in evaluating their real significance concerning the clinical findings in CF cases (Martín-Gómez, 2020).
In the era of next-generation sequencing (NGS) technologies, knowledge about the microorganisms related to CF has greatly enhanced. Previously overlooked microorganisms have been revisited, and new paradigms emerged in the CF microbiome. Knowledge of microbial composition has been correlated with the degree of lung function and contributes to a more assertive clinical diagnosis (Coburn et al., 2015; Caverly and Lipuma, 2018; Quinn et al., 2019).
Taking into consideration the increased detection of fungal infections in patients with CF and the novel tools available to evaluate the microbiome, this study was carried out to describe the lung microbial diversity of three young CF patients who were culture-positive for filamentous fungi at different stages of the disease.
Casuistic and Methods
Participants and Sample Collection
Three young patients with CF were recruited at the ‘Ambulatório Multidisciplinar de Fibrose Cística (AMFC)’ at the ‘Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HC-FMRP-USP)’ during 2018. The patients were unequivocally diagnosed with CF, either by detection of two mutations in the CFTR gene or by an abnormally high result for the sweat chloride test (≥60 mmol/L). They were enrolled due to their high visit frequency to the hospital, severe lung disease and presented a history of fungal isolation in sputum and chronic infections by pathogenic bacteria. They were named patient A (male, 12 years old), patient B (female, 9 years old), and patient C (male, 17 years old). The patients also presented the loss of pulmonary function, according to the spirometric parameters (% of predicted forced expiratory volume in one second, FEV1%), with test results for patients A, B, and C of 39, 64, and 37%, respectively. During the study period, the patients received antibiotics for pulmonary exacerbation ( Supplementary Table S1 ). The patients were followed up to seven months, with a collection of sputum samples during routine clinical appointments and/or during hospitalization due to pulmonary exacerbation. A total of twelve sputum samples were collected with an average interval of 44 days and were distributed as follows: five from patient A (PA1 to 5), three from patient B (PB1 to 3), and four from patient C (PC1 to 4). The sputum samples were collected by expectoration into a sterile cup after mouth rinsing with water to prevent excessive salivary contamination. To each sputum sample, an equal volume of sterile dithiothreitol (DTT) solution (DTT 50 µg ml−1 dissolved in phosphate-buffered saline plus 0.1% gelatin) was added. After 30 min, the samples were vortexed to homogenize and cultured for bacterial isolation by seeding on blood agar, chocolate agar, salt mannitol agar, MacConkey agar, and Burkholderia cepacia selective agar at the routine clinical laboratory of the HC-FMRP-USP, with incubation at 37°C, under anaerobiosis for 24 to 48 h. Fungal isolation was performed by plating on Sabouraud Dextrose Agar (SDA—Acumedia, Michigan, USA) with 50 µg ml−1 chloramphenicol and in SDA with 50 µg ml−1 chloramphenicol plus 500 µg ml−1 cycloheximide. Seeded plates were incubated at both 25 and 37°C for up to three weeks. The remaining sputum samples were frozen at −80°C until DNA extraction for metagenomics analysis
Ethics Statement
The sputum samples from the three patients who volunteered for the study were collected at HC-FMRP-USP following the ethical guidelines of the hospital. This study was approved by the Ethics Committee of “Faculdade de Ciências Farmacêuticas de Ribeirão Preto da Universidade de São Paulo” (FCFRP-USP), under protocol number 2.492.043, in accordance with the HC-FMRP-USP as a co-participating institution. The parents of the patients signed a term of Written Informed Consent to permit the participation of the teenagers in this study.
Bacterial Identification and Molecular Identification of Fungal Isolates
The bacterial isolates were biochemically identified by VITEK®2 (bioMérieux, France) at HC-FMRP-USP. Fungal colonies were identified by conventional methods and DNA sequencing of the internal transcribed spacer (ITS) region. Aspergillus fumigatus was additionally identified by the sequencing of calmodulin (cmd)- and beta-tubulin (benA)-encoding genes. Briefly, fungal DNA was extracted as previously described (Tonani et al., 2018) and used for PCR amplification with Phusion High Fidelity DNA Polymerase (New England BioLabs Inc.) with the primers ITS1 and ITS4 for ITS (White et al., 1990); cmd5 and cmd6 for calmodulin (Hong et al., 2005); and bt2a and bt2b for β-tubulin (Glass and Donaldson, 1995). PCR-purified products were sequenced with the same primers in the ABI3730 DNA Analyzer (Applied Biosystems). Each DNA sequence was analyzed with ChromasPro Software (ChromasPro1.7.6, Technelysium Pty. Ltd., Tewantin QLD, Australia) and tested against publicly available DNA sequences in the NIH genetic sequence database (Altschul et al., 1990).
Metagenomic DNA Extraction
The total DNA from sputum samples was extracted using the commercial Quick-DNA Fungal/Bacterial Miniprep kit (Zymo Research, USA) according to the instructions of the manufacturer. DNA quality and quantity were measured by a Qubit® 3.0 fluorometer (Thermo Fisher Scientific, USA) following standard methods provided by the manufacturer.
Preparation of Samples for Metataxonomic Analysis
To assemble the community taxonomic composition of bacteria and fungi, the 16S rRNA V3–V4 and ITS2 (Internal Transcribed Spacer) regions were amplified from aliquots of 1.0 μl from the whole genomic DNA sample extract.
The 16S rRNA V3–V4 regions were amplified by a first PCR employing the universal primers: forward 5′–TCGTCGGCAGCGTCAGATGTGTATAAGA GACAGCCTACGGGNGGCWGCAG–3′, and reverse GTCTCGTGGGCTCG GAGATGTGTGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC–3′. The reaction media of each PCR contained 10 μl of GoTaq® Colorless Master Mix 2× (Promega, USA), 1.0 μl of metagenomic DNA and 0.3 μM of forward and reverse primers. The final volume of 20.0 μl was achieved by completing the reactional volume with ultrapure water.
The PCR conditions were characterized by a first denaturation step at 94°C/3 min followed by 25 cycles of denaturation at 94°C/30 s, annealing at 55°C/30 s, extension at 72°C/30 s, and a final extension step at 72°C/5 min.
To evaluate the quality and to confirm the amplification, agarose gel 2% (w/v) electrophoresis stained with UniSafe Dye 0.003% (v/v) was performed. The average amplicon size obtained for each sample was 500 bp.
Regarding the ITS2 amplification run for fungi, the forward 86-F 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGAATCATCGAATCTTTGAA-3′ and reverse ITS4R 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGTC CTCCGCTTATTGATATGC-3′ were used. The PCR components were the same as described above for 16S rRNA V3–V4 region amplification, although the PCR conditions to amplify ITS regions were slightly different: initially, a denaturation cycle at 95°C for 5 min was performed, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 56°C for 40 s, extension at 72°C for 1 min, and a final extension step at 72°C for 5 min. All PCRs were conducted on a Veriti™ Thermal Cycler (Applied Biosystems, USA).
The integrity checking of the generated amplicons was performed by agarose gel electrophoresis 2% (m/v) stained with UniSafe Dye 0.003% (v/v). The average amplicon size obtained for each sample was 400 bp.
The PCR amplicons were subsequently purified using Agencourt AMPure XP magnetic beads (Beckman Coulter, USA) following the manufacturers’ guidelines.
The indexation step, required to pool the samples and to insert them into the flow cell, was performed through a second PCR for the entire set of generated amplicons. In this reaction, the adapter’s sequences were inserted in the amplicon extremities. PCR was conducted with the Nextera XT index kit (Illumina®) following the manufacturers’ instructions.
The resulting libraries were purified using magnetic beads provided in the Agencourt AMPure XP kit (Beckman Coulter) to remove fragments <100 bp and residual primers. The libraries were quantified by RT-PCR using the KAPA-KK4824 Library Quantification Kit (Illumina®) according to the manufacturer’s recommendations. Next, an equimolar pool of samples was prepared by normalization of all samples to the final concentration of 3 nM. High-throughput DNA sequencing (HTS) was conducted on a MiSeq platform (Illumina®).
Metagenomic Sample Preparation and DNA Sequencing
The library preparation for metagenomics analysis was performed using the Nextera DNA Library Prep kit (Illumina®) to randomly fragment the metagenomic DNA using transposase activity. The adapters were inserted using the Nextera XT Index kit (Illumina®) following the manufacturers’ instructions. The libraries were quality-assured by removal of residual adapters, exceeding dNTPs and fragments <100 bp using AMPure XP magnetic beads (Beckman Coulter).
The libraries were quantified by RT-PCR using the KAPA-KK4824 Library Quantification Kit (Illumina®) following fabricant standards. A final concentration of 4 nM was used to normalize the libraries to pool them into the sequencer flow cell. The HTS was conducted on a NextSeq 500 platform (Illumina®) using the running kit NextSeq 500 MID Output (300 cycles) to generate 2 × 150 bp paired-end (PE) reads.
Raw Reads Processing: Contaminant Removal and Quality Control
The first preprocessing step was to overlap the PE reads using the BBmerge tool (Bushnell et al., 2017). The FASTQ files derived from the metataxonomic approach were addressed by the BaseSpace (Illumina®) pipeline with reads without adapters and Phred score values higher than 30, so these files were not subjected to any further quality processing.
On the other hand, the FASTQ files derived from metagenomic DNA sequencing were quality trimmed using the BBDUK tool to remove adapters and fragments <100 bp and Phred score <30 (parameters: hdist = 1, tpe, tbo, qtrim = rl, trimq = 30, maq = 30, minlen = 100).
Due to intrinsic characteristics, the sputum matrix was heavily concentrated with DNA from human cells. Thus, the human DNA reads were removed for the final processing of the Metagenomics Shotgun data. This was performed by downloading the human reference genome (version GRCh38.p12) from the NCBI database and indexing it on the Bowtie2 tool (Langmead and Salzberg, 2012). The FASTQ files were aligned against the indexed reference genome, resulting in two separate files: one with matches and the other with no matches. The unmatched FASTQ files were selected for downstream metagenomics analysis.
Community Taxonomic Composition
The community taxonomical composition was determined by two independent methods: (i) the QIIME1 open-reference method for amplicon analysis and (ii) MetaPhlan2-specific marker gene analysis for the metagenomics-derived data.
In the QIIME1 environment version 1.9.1 (Caporaso et al., 2010), the FASTQ files derived from metataxonomic HTS were converted to FASTA files, and the open-reference method was chosen to perform OTU (operational taxonomic units) picking using the UCLUST algorithm (Edgar, 2010) for sequence clustering. For fungal OTU picking, the UNITE database (version 8.0) was selected as a reference (Nilsson et al., 2019), and for bacterial OTU picking, the last version of the SILVA database (version 132) preformatted for QIIME was chosen (Quast et al., 2013).
The OTU tables were filtered to remove OTUs lower than 0.1% in relative abundance using built-in scripts provided by the pipeline. A bar plot of bacterial composition per patient was drawn in the R environment using the ggplot2 package. The statistical analyses related to alpha and beta-diversity measurements were performed on Microbiome Analyst (Dhariwal et al., 2017) environment selecting the default parameters of the “Marker Data Profiling (MDP)” pipeline.
The MetaPhlan2 pipeline (Segata et al., 2012) was chosen to process the shotgun-derived data using default parameters.
Annotation of Virulence and Antibiotic Resistance Genes
The search for virulence factors (VFs) in the genomes was conducted using the ShortBRED (Short, Better Representative Extract Dataset) tool (Huttenhower et al., 2012). Initially, a FASTA file containing the entire set of VFs curated on the Victors Database (Sayers et al., 2019) was downloaded. Then, the UniRef90 database (Suzek et al., 2007) was downloaded and used as a comprehensive reference database for additional comparisons of protein sequences. To create a VF custom reference database, first, the script ShortBRED-Identify was used. This script performs a global sequence homology identification of protein families related to the target sequences from the Victors Database. These sequences were collapsed to form a unique consensus sequence that possesses short peptide markers that best represented a given protein family. Moreover, applying the script ShortBRED-Quantify, the reads were aligned against the custom reference database represented by the newer protein markers, determined by the ShortBRED-Identify algorithm, quantified in terms of their absolute counts, normalized by marker gene length and finally represented by reads per kilobase per million (RPKM).
Regarding the search of antibiotic resistance genes (ARGs) in the metagenome, the same methodology for VF determination was employed, differing only due to the reference database chosen, which was the CARD (Comprehensive Antibiotic Resistance Database) (Jia et al., 2017).
Results
Three young patients with cystic fibrosis who were part of a long-term follow-up in the “Ambulatório Multidisciplinar de Fibrose Cística (AMFC)” at HC-FMRP-USP were monitored in this study in 2018. The youngest patient (patient B) had a fatal outcome due to severe and difficult-to-treat infection at the beginning of 2019.
The bacterial and fungal microbiotas/microbiomes of the patient’s lung were investigated by culture isolation, metataxonomics, and metagenomics shotgun sequencing. The culturable bacteria detected in the specimens of CF patients were for the patient A—methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and Burkholderia cepacia complex; for the patient B—B. cepacia complex; and for the patient C—Staphylococcus aureus, P. aeruginosa, Stenotrophomonas maltophilia, Achromobacter sp., Achromobacter xylosoxidans, and Chryseobacterium indologenes ( Supplementary Table S1 ). The results of fungal culture revealed the frequent isolation of Aspergillus fumigatus from the sputum of patients A and C. Additionally, Penicillium sp. and Torulaspora delbrueckii were isolated from patient A, and Candida albicans was isolated from patient C. From patient B, Penicillium sp., Hanseniaspora sp., Torulaspora delbrueckii, and Talaromyces amestolkiae were isolated from sputum samples ( Table 1 and timeline S1 ). Regarding A. fumigatus clinical isolates, nucleotide polymorphisms were observed in calmodulin (cmd) and beta-tubulin (tub) genomic DNA sequences, which indicated four different sequence patterns, which we named sequence types (ST) 1 to ST4. Patient A had ST1 isolated from sputum samples PA1 and PA5 and ST2 from sputum samples PA3 and PA5. Patient B had no A. fumigatus isolates. Patient C had ST1, ST2, and ST3 isolated from sputum sample PC1, and ST4 from sputum samples PC1, PC2, PC3, and PC4 ( Table 2 ). Focusing on sequence types, ST1 and ST2 were isolated from two different sputum samples from patient A, and ST4 was isolated from 4 sputum samples from patient B, thus indicating the colonization of patients A and C by A. fumigatus strains.
Table 1.
Molecular identification of fungal isolates.
| Patient | Sputum sample | ID | Species | ITS | Calmodulin | β-tubulin | |||||||||
| Ident. (%) | e-Value | Max score | Access GenBank | Ident. (%) | e-Value | Max score | Access GenBank | Ident. (%) | e-Value | Max score | Access GenBank | ||||
| PA | PA1 | LMC8001.01 | Aspergillus fumigatus | 100 | 0.0 | 933 | KC689325.1 | 100 | 0.0 | 952 | MK451408.1 | 100 | 0.0 | 917 | KJ175502.1 |
| PA2 | NI | NI | – | – | – | – | – | – | – | – | – | – | – | – | |
| PA3 | LMC8001.03 | A. fumigatus | 100 | 0.0 | 933 | KC689325.1 | 100 | 0.0 | 952 | MK451394.1 | 100 | 0.0 | 917 | KJ175502.1 | |
| PA4 | LMC8001.08 | Penicillium sp. | 99.81 | 0.0 | 968 | MK226539.1 | – | – | – | – | – | – | – | – | |
| PA5 | LMC8001.09 | A. fumigatus | 100 | 0.0 | 933 | KC689325.1 | 100 | 0.0 | 952 | MK451408.1 | 100 | 0.0 | 917 | KJ175502.1 | |
| PA5 | LMC8001.10 | A. fumigatus | 100 | 0.0 | 933 | KC689325.1 | 100 | 0.0 | 952 | MK451394.1 | 100 | 0.0 | 917 | KJ175502.1 | |
| PA5 | LMC8001.12 | Torulaspora delbrueckii | 100 | 0.0 | 1360 | KY105646.1 | – | – | – | – | – | – | – | – | |
| PB | PB1 | NI | NI | – | – | – | – | – | – | – | – | – | – | – | – |
| PB2 | LMC8002.01 | Penicillium sp. | 98.48 | 0.0 | 926 | MH864712.1 | – | – | – | – | – | – | – | – | |
| PB2 | LMC8002.02 | Hanseniaspora sp. | 99.37 | 0.0 | 1,146 | AJ512437.1 | – | – | – | – | – | – | – | – | |
| PB2 | LMC8002.03 | Torulaspora delbrueckii | 100 | 0.0 | 1,362 | KY105646.1 | – | – | – | – | – | – | – | – | |
| PB3 | LMC8002.04 | Talaromyces amestolkiae | 100 | 0.0 | 961 | MH856395.1 | – | – | – | – | – | – | – | – | |
| PC | PC1 | LMC8003.01 | A. fumigatus | 100 | 0.0 | 963 | KC689325.1 | 100 | 0.0 | 896 | MK451402.1 | 100 | 0.0 | 917 | KJ175502.1 |
| PC1 | LMC8003.02 | A. fumigatus | 100 | 0.0 | 963 | KC689325.1 | 99.79 | 0.0 | 952 | MK451394.1 | 99.8 | 0.0 | 924 | KJ175502.1 | |
| PC1 | LMC8003.06 | A. fumigatus | 100 | 0.0 | 963 | KC689325.1 | 100 | 0.0 | 952 | MK451394.1 | 100 | 0.0 | 917 | KJ175502.1 | |
| PC1 | LMC8003.09 | Candida albicans | 100 | 0.0 | 924 | KY101918.1 | – | – | – | – | – | – | – | – | |
| PC1 | LMC8003.10 | A. fumigatus | 100 | 0.0 | 963 | KC689325.1 | 100 | 0.0 | 952 | MK451408.1 | 100 | 0.0 | 917 | KJ175502.1 | |
| PC2 | LMC8003.11 | A. fumigatus | 100 | 0.0 | 963 | KC689325.1 | 99.79 | 0.0 | 952 | MK451394.1 | 99.8 | 0.0 | 924 | KJ175502.1 | |
| PC3 | LMC8003.13 | A. fumigatus | 100 | 0.0 | 963 | KC689325.1 | 99.79 | 0.0 | 952 | MK451394.1 | 99.8 | 0.0 | 924 | KJ175502.1 | |
| PC4 | LMC8003.14 | A. fumigatus | 100 | 0.0 | 963 | KC689325.1 | 99.79 | 0.0 | 952 | MK451394.1 | 99.8 | 0.0 | 924 | KJ175502.1 | |
ID, fungal idetification; NI, no isolation; -, not done; Ident., identity; ITS, internal transcribed spacer.
Table 2.
A. fumigatus clinical isolate nucleotide polymorphism.
| Patient | Sample | ID | Gene (bp) | ST | |||
|---|---|---|---|---|---|---|---|
| cmd | cmd | cmd | tub | ||||
| 195 | 360 | 568 | 328 | ||||
| PA | PA1 | LMC8001.01 | T | T | A | G | 1 |
| PA3 | LMC8001.03 | C | G | G | G | 2 | |
| PA5 | LMC8001.09 | T | T | A | G | 1 | |
| PA5 | LMC8001.10 | C | G | G | G | 2 | |
| PC | PC1 | LMC8003.01 | T | G | A | G | 3 |
| PC1 | LMC8003.02 | C | G | A | A | 4 | |
| PC1 | LMC8003.06 | C | G | G | G | 2 | |
| PC1 | LMC8003.10 | T | T | A | G | 1 | |
| PC2 | LMC8003.11 | C | G | A | A | 4 | |
| PC3 | LMC8003.13 | C | G | A | A | 4 | |
| PC4 | LMC8003.14 | C | G | A | A | 4 | |
ID, strain identification; cmd, calmodulin; tub, ß-tubulin; ST, sequence type.
Concerning the results of HTS, for metataxonomic characterization of sputum samples from CF patients A, B, and C, the BaseSpace pipeline (Illumina®) returned an average of 153,229 and 87,538 quality-processed PE reads of 16S rRNA and ITS, respectively. These reads were overlapped to be analyzed on the QIIME1 pipeline, which is useful for determining the microbial community composition and diversity. A low overlap was observed, and only R1 FASTQ files were maintained, following the guidelines of the BBmerge tool, which recommends working only with the R1 reads to assure high quality during downstream processing if there is <15% overlap.
Regarding the HTS of metagenomics shotgun data, the average number of raw PE reads generated was 10,601,533 per sample. All samples were overlapped and preprocessed to remove low-quality base calls and adapter sequences, resulting in an average of 4,540,817 quality-processed PE reads per sample. However, the majority of community DNA was derivate from the hosts (mean of 95.82%) remaining an average of 182,342 quality-filtered PE reads by sample for taxonomic and functional downstream analyses.
Community Composition Shifts
The structures of the microbial communities from CF patients were drawn from metataxonomic and metagenomic results to exploit the best of each technique, aiming to improve the resolution of the study. The metataxonomics focus on specific taxonomic markers, and the targeted amplification of these DNA regions allows for enrichment of OTUs that might otherwise be difficult to detect due to low relative abundances in shotgun datasets (Almeida and De Martinis, 2019). On the other hand, the analyses of single-copy species-specific reads from the shotgun results help in the assignment of OTUs at the species level.
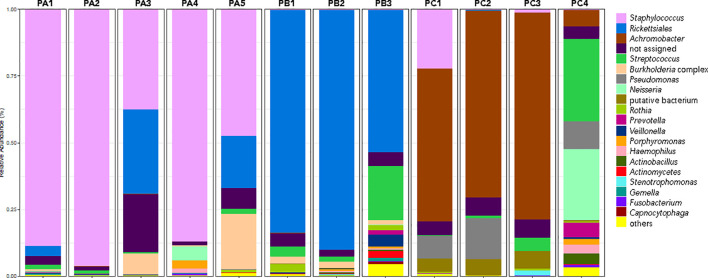
According to the metataxonomic findings, the patients presented variable bacterial compositions ( Figure 1 ). For patient A, the high prevalence of Staphylococcus was notorious among all sputum samples evaluated. Lower amounts are shown for the Rickettsiales and Burkholderia groups in samples PA1, PA3, and PA5.
Figure 1.
Shifts in CF patient-associated bacteria expressed by the top 21 most prevalent OTUs.
For patient B, Rickettsiales was the main bacterial order detected, with variable levels of Streptococcus and Burkholderia genera during the study ( Figure 1 ).
Patient C was chronically colonized by Achromobacter, but Staphylococcus sp. was also detected in PC1 and PC3 samples, with a higher prevalence in the former. Pseudomonas genus was present in all but PC3 sputum samples. The PC4 sample was the most diverse, with the additional detection of Streptococcus, Neisseria, Prevotella, Actinobacillus, and Haemophilus ( Figure 1 ).
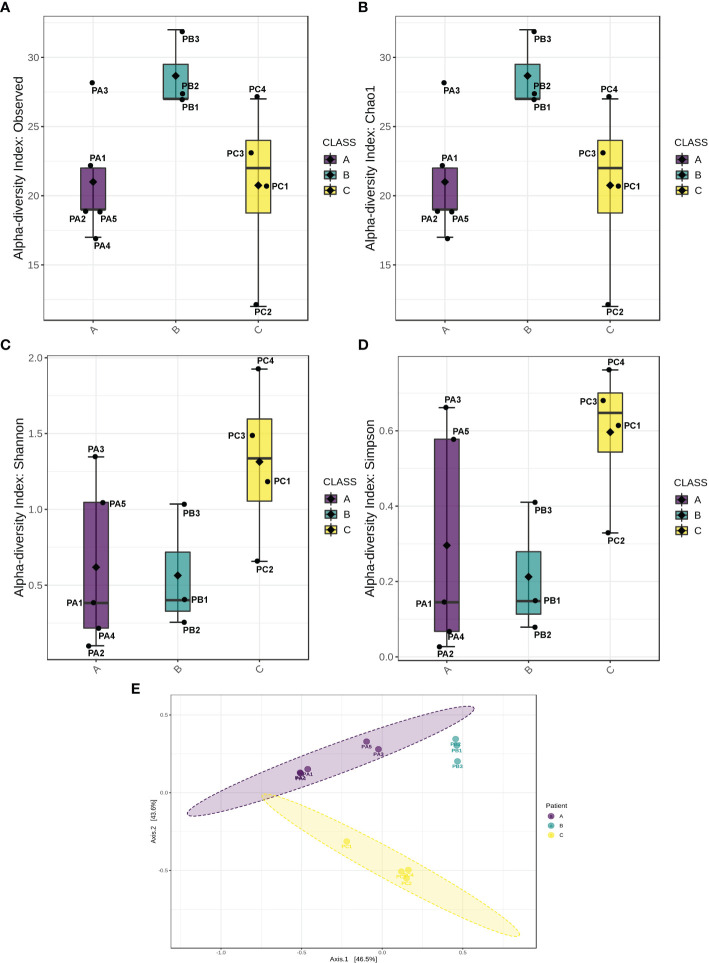
Regarding intraspecies diversity (α-index), it was higher for patient B samples, as revealed by the larger number of different OTUs detected ( Figure 2A ). The theoretically expected diversity expressed by the Chao1 index (Kim et al., 2017) was very close to the OTU index observed in this study ( Figure 2B ), indicating the good quality of sampling, which means that the number of groups undetected was similar to the number of groups estimated to be undetected at random. However, the lower values of Shannon and Simpson indexes ( Figures 2C, D ) measured for patient B and patient C samples indicate that the majority of OTUs harbored by these patients were represented by rare groups, which is corroborated by the observation of the low relative abundances of the minor OTUs ( Figure 1 ). Overall, the higher values observed for Shannon and Simpson metrics for patient C revealed a well-established microbiome, with a higher diversity in comparison with other patients, indicating a uniform relative abundance of OTUs. The comparison among the microbiota of patients (β-diversity) rendered a pattern of clustering per patient, indicating that each individual presented a distinguishable bacterial profile ( Figure 2E ).
Figure 2.
Biological diversity indexes. Indexes of α-diversity (intrasample comparison) represented by observed OTUs (A), Chao1 (B), Shannon (C), and Simpson indexes (D). (E) Index of β-diversity (intersample comparison).
Concerning the results of metataxonomics based on ITS gene markers, fungi were detected only in the PC4 sputum sample. This patient exhibited most reads assigned to Saccharomyces cerevisiae, Aspergillus fumigatus, and Schizophyllum sp. ( Figure 3 ). Notably, the fungi were present in the sample with the highest bacterial diversity ( Figures 1 , 2C, D ).
Figure 3.
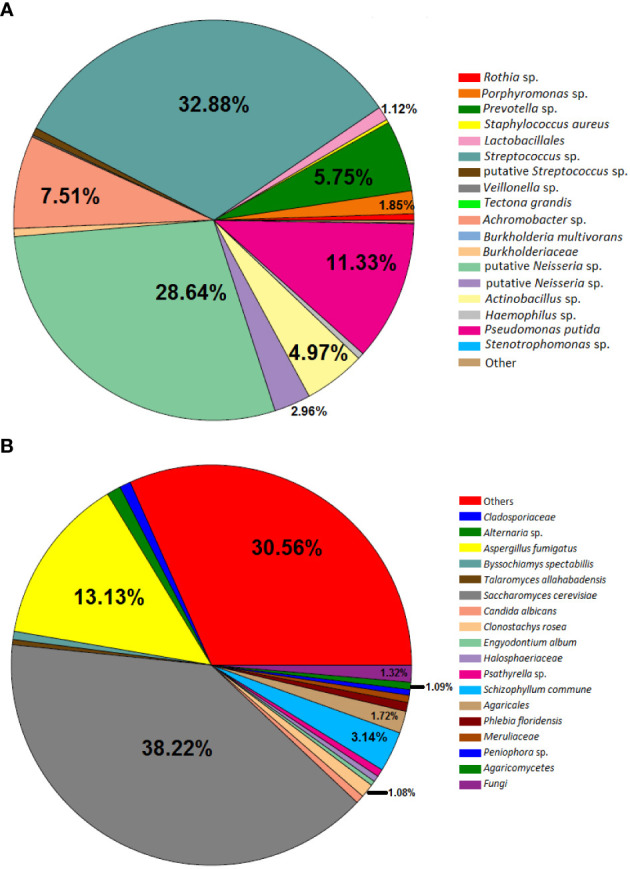
Pie chart depicting bacteria (A) and fungi (B) detected in Patient C in the sample PC4. The slices without the relative abundance information represent the taxa that have less than 1% relative abundance.
Clustering analysis based on metagenomic shotgun data revealed the occurrence of a particular microbiota in each patient, and clinical specimens presented complex microbial compositions ( Figure 4 ). Almost all samples of patient A were clustered together, except for the PA3 sample, possibly due to the “not assigned” OTUs. Additionally, the PA5 sample presented high levels of Burkholderia complex OTUs, analogous to some samples of patient B, justifying its proximity with the patient B cluster ( Figure 4 ). As patient C exhibited a very particular microbial profile, the samples were clustered independently from the other subjects.
Figure 4.
Species-level taxonomic assignment of fungi, bacteria, and viruses in the microbiome based on metagenomics data. Some taxa were not assigned to the species level, so only the genus level was expressed.
Exploring deeply the shotgun data, the patient A microbiota was characterized by the presence of S. aureus during the entire study and, in lower proportions, the cooccurrence of Neisseria sp., Neisseria flavescens, Haemophilus parainfluenzae, Ralstonia sp., and Burkholderia sp. ( Figure 4 ).
The patient B microbiota was marked by the dominance of Burkholderia cepacia complex and the co-occurrence of Rothia mucilaginosa, Stenotrophomonas maltophila, Veillonella sp., Streptococcus mitis/oralis/pneumoniae, Streptococcus salivarius, and Capnocytophaga sp. ( Figure 4 ).
Finally, patient C exhibited the most diverse microbiota, as illustrated by Figures 2C, D . Achromobacter sp. and A. xylosoxidans were present in all sputum samples. Pseudomonas aeruginosa and Bordetella sp. were observed in 75% (n = 3) of the sputum samples. PC4 was the most diverse sample, with the cooccurrence of low relative ratios of species from the genera Haemophilus, Streptococcus, Kingella, Prevotella, Neisseria, Porphyromonas, Veillonella, Actinobacillus, and Rothia ( Figure 4 ).
In summary, the shotgun clustering snapshot corroborated the metataxonomic assignment of OTUs and the β-diversity measurements, which unequivocally translated the real community structure assemblage and justified the high microbial diversity found in patient C.
Virulence and Antibiotic Resistance Genes
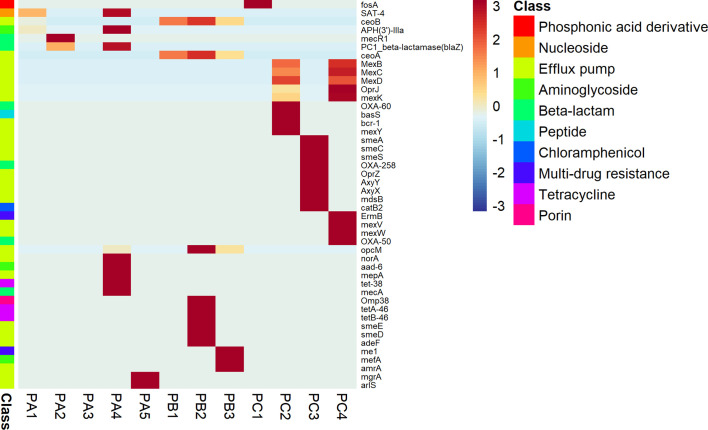
The alignment of quality-processed reads against the custom marker genes database created from the comparison of UniRef90 and Victor databases detected sets of reads matching against 33 coding genes related to putative virulence factors. As observed for microbial succession, the prevalence of specific virulence determinants was highly variable according to each patient sampling time. In general, patient A exhibited high amounts of reads related to Pseudomonas quinolone signal (PQS), adhesion/invasion, cytolytic toxins, and general pathogenicity-enhancing effectors. In contrast, the most diverse microbiota in terms of virulence repertoire were those from patients B and C, which varied in terms of the presence of type II secretory systems and exopolysaccharide synthesis, presence of type III secretory systems, endotoxin synthesis and cytolytic potential ( Figure 5 ).
Figure 5.
Disease-related potential. Virulence sequences of fragmented genes detected on the CF-associated microbiome.
Considering the ARG profiles, the alignments against the custom CARD and UniRef90 databases returned sets of reads matching 46 putative ARG coding genes. The antimicrobial profiles were variable according to the patient, but each one presented a huge diversity of efflux pump determinants. Patient A presented reads matching to nucleoside, beta-lactam, and aminoglycoside antibiotic classes, while the patient B microbiome was more related to general multidrug resistance effectors, porins with reduced permeability to antibiotics and tetracycline resistance. Finally, patient C presented genes conferring resistance to phosphonic acid derivatives, aminoglycosides, beta-lactams, chloramphenicol, and multidrug-related drugs ( Figure 6 ).
Figure 6.
Disease-related potential. ARG sequences of fragmented genes detected on the CF-associated microbiome.
Discussion
The lung microbiomes of three young patients with CF with compromised pulmonary function were studied. The investigation is particularly interesting once the microbial community is more dynamic in infants than in adults (Cox et al., 2010; Klepac-Ceraj et al., 2010; Frayman et al., 2017). Virulence factors and antibiotic resistance genes were also explored.
The bacteria isolated from sputum samples were S. aureus, P. aeruginosa, and B. cepacia complex from patient A; B. cepacia complex from patient B; and Achromobacter sp., A. xylosoxidans, Stenotrophomonas maltophilia, P. aeruginosa, and Chryseobacterium indologenes from patient C. Although metataxonomic and metagenomic analyses identified most of these species, reads of P. aeruginosa were not observed in patient A sputum samples. Additionally, metataxonomic and metagenomic analyses identified species of the genera Streptococcus, Neisseria, Rothia, Prevotella, and Haemophilus, among others. The differences between identification by both culture isolation and HTS strategy show that the culture complexity and fastidious nutritional requirements of some pathogens can lead to non-isolation in standard microbial culture (Zemanick et al., 2017), and in the intrinsic composition of metagenomic samples with a high proportion of host-DNA, which sometimes encompasses more than 99% of the dataset, as was observed in this study, limiting the resolution of community composition. A recent study advocates the use of culture-enrichment metagenomics associated with the traditional HTS methods to improve the identification of low-abundant organisms and microbiome characterization, which may overcome this limitation (Lim et al., 2014).
As mentioned above, patient B showed an early poor outcome that culminated in patient death. The same patient had the lowest lung microbiota diversity, and studies have linked the decreased lung microbiota diversity with a reduction in lung function (Cuthbertson et al., 2020), which can justify the poor outcome. Additionally, the increase in microbiota dominance occurs in the loss of microbiota diversity (Cuthbertson et al., 2020). Members of Pseudomonas, Staphylococcus, and Achromobacter genera found in this study are potential pathogenic microorganisms and are frequently isolated from the airways of CF patients (Coburn et al., 2015; Losada et al., 2016). B. cepacia complex members were also found in this study. These members are most frequently isolated from patients with CF and generally linked to pulmonary deterioration (Coburn et al., 2015), high risk to the host due to multidrug resistance, easy transmissibility, the potential to cause septicemia, and an unpredictable clinical outcome, varying from an asymptomatic infection up to fatal pneumonia known as “cepacia syndrome” (Zlosnik et al., 2015; Hector et al., 2016). A previous study in the same hospital showed patients with good clinical outcomes and favorable options for antibiotic therapy (da Costa Capizzani et al., 2017). Even so, possibly the highly impaired lung functions of these young patients are related to the early presence of very pathogenic bacteria, related to adverse outcomes.
Patient A’s lung microbiome had a prevalence of S. aureus and B. cepacia complex, which was shown by culture isolation, metataxonomics, and metagenomics from the sputum samples. S. aureus is a common pathogen recovered from CF samples, and the association of this species with exacerbations in CF is evidenced by high levels of inflammation due to stimulus in interleukin-6 (IL-6) recruitment and eventually provoking lung damage (Hurley, 2018).
The patient B microbiome was primarily dominated by B. cepacia complex. This species was identified by both culture isolation and metataxonomics/metagenomics. It is commonly identified in CF patients and represents a high risk to the host due to the carriage of several virulence factors (VFs), such as the expression of secretion systems, several colony variants, and the presence of lipopolysaccharides, which are VFs frequently related to pandrug-resistance phenotypes (Lopes et al., 2015).
Achromobacter xylosoxidans is also a prevalent CF-related microbe and has been considered an emergent bacterium. It was highly detected in patient C lung microbiota, although A. insuavis and A. ruhlandii species are currently known as key pathogens in CF disease as well. These bacteria present resistance against the innate immune response, high plasticity in front of antimicrobial agents, may acquire resistance in short periods of exposure to antimicrobials, and can alter the expression of a set of genes to promote chronic infection (Edwards et al., 2017).
According to the literature review, the lung microbiota in CF disease may be divided into emergent pathogen microbiota and core microbiota. Commonly, the core microbiota of CF pediatric patients is formed by the genera Streptococcus, Rothia, Prevotella, Actinomyces, Veillonella, Gemella, Neisseria, and Haemophilus, which are commensal bacteria and not necessarily related to CF disease (Prevaes et al., 2017). Metataxonomic and metagenomic analyses identified the canonical core CF microbiome species from the genera Streptococcus, Neisseria, Rothia, Prevotella, and Haemophilus, among others, in our study. Association studies showed that the taxa Pseudomonas aeruginosa, Rothia mucilaginosa, and Streptococcus pneumoniae were related to more deteriorated lung function (Paganin et al., 2015). Rothia sp. and Streptococcus sp. were detected at variable levels by metataxonomics in all sputum samples, but Pseudomonas aeruginosa was detected only in the patient C microbiome, concomitant with high levels of Achromobacter spp. The presence of all these taxa in the CF microbiome could predict a progressive loss in lung function.
Regarding the core microbiota, attention must be paid to H. influenza, which is a bacterium that naturally colonizes the respiratory tract during infancy but has recently been reported as an emergent pathogen frequently related to acute respiratory infections, contributing to the local inflammatory response and premature lung damage. The main concern with H. influenza pathogenicity is its strong ability for biofilm formation, which leads to high tolerance to antibiotics (Cardines et al., 2012). On the other hand, there are also reports describing the protective effect of Haemophilus spp. colonization to the lungs of adolescent patients with CF (Hector et al., 2016). Additionally, S. maltophilia and Ralstonia sp. were emergent pathogens detected among the sputum samples (considering both approaches of OTU assignment). Recent publications suggest that S. maltophilia plays several roles in lung damage, which can culminate with disease exacerbation, lung transplantation, and even death (Waters et al., 2013). The highly successful adaptation of S. maltophilia to CF is related to intrinsic antibiotic resistance, a decrease in biofilm formation, and pathogenicity in chronic disease. Additionally, S. maltophilia exhibits marked phenotypic and genotypic heterogeneity and complex regulatory networks among S. maltophilia clones in CF disease, which makes it difficult to identify therapeutic strategies to eradicate this bacterium (Esposito et al., 2017).
Ralstonia spp. infections in CF are still poorly reported, probably because it is underestimated, since this pathogen can grow on Burkholderia cepacia selective agar, generally with high levels of contamination by other pathogens, such as P. aeruginosa and S. aureus (Green et al., 2017). The genus Ralstonia is a common cause of nosocomial infections in CF patients due to contamination of hospital devices (i.e. respiratory therapy) and distilled water used to prepare solutions to be injected in patients. As infection is commonly related to polymicrobial communities in CF, it is difficult to establish the role of Ralstonia species in pathogenicity, but a high prevalence of this potential pathogen has been reported in the CF microbiome when the lung microbiome diversity is too low (Prior et al., 2017).
Thus, despite the low number of samples here, the airway of the CF patients presented dominant bacterial genera and inter-individual variability in microbial community composition and diversity, confirming the results of individual signatures of multiple species recorded in other studies (Delhaes et al., 2012; Losada et al., 2016; Güemes et al., 2019). The individual variations of microbial composition are most likely attributed to the patient’s domestic environment and how patient’s microbiome will respond to therapeutic perturbation (Whelan et al., 2020).
The depleted mucociliary clearance and the thick mucus filling the airways of CF patients easily trap the inhaled fungal conidia. A. fumigatus is the most prevalent species involved in CF colonization and disease (Warris et al., 2019) and was culture isolated at a high frequency from the sputum samples of patients A and C. Additionally, A. fumigatus with the same sequence type was observed in different sputum samples of each patient. Persistent colonization occurs when the same species is identified twice or more times in the same year (Saunders et al., 2016). Thus, the lung airways of patients A and C have persistent colonization of the lung airways by A. fumigatus. On the other hand, transient colonization of Penicillium sp., Hanseniaspora sp., Torulaspora delbrueckii, and Talaromyces amestolkiae was observed in the lung airways of patient B once a single culture isolation was registered (Saunders et al., 2016). The isolation of A. fumigatus in respiratory secretions of CF patients is a common occurrence. However, the mean age of the patient at the date of the first isolation of A. fumigatus ranges from 9 to 16 years old (Whelan et al., 2020). Our results reinforce the literature data once 9-year-old patient B has no A. fumigatus isolation from sputum samples, whereas older patients (A and C) present persistent colonization by this fungal species.
The culture method was able to recover fungal species in most sputum samples once it is an enrichment-based strategy and appropriate selective media with longer incubation times, which are not routinely performed in clinical microbiology labs (Hong et al., 2017; Martín-Gómez, 2020). On the other hand, metataxonomic and metagenomic analyses detected fungal reads in only one sputum sample (PC4). It is important to note that fungi are generally present in low amounts in the lung microbiome of CF patients, representing approximately 1% of the total OTUs (Losada et al., 2016), hampering the identification of these microorganisms by the culture-independent method. Thus, considering only metagenomics data, without the enrichment of gene markers by metataxonomics, few fungal reads were assessed, suggesting that metagenomics may underestimate fungi detection since the higher bacterial biomass and accelerated proliferation may overwhelm the fungi reads. The concordance between the molecular methods was in the detection of S. cerevisiae reads. S. cerevisiae was previously identified in sputum from CF patients (Güngör et al., 2013; Ziesing et al., 2016). A recent study evaluated the fungal composition in low- and high-severity CF patients against healthy subjects through isolation methods and found that S. cerevisiae was present in high-severity or healthy subjects, but its meaning in the context of CF physiopathology is unknown.
It is important to highlight the high diversity of bacterial species in the PC4 sample in comparison with the other samples. However, the copresence of fungal members and its influence on bacterial diversity is a matter that needs future investigation. The high microbiome diversity is related to low ratios of pathogenic bacteria and to an improvement in lung function (Carmody et al., 2015; Losada et al., 2016). However, the presence of pathogenic fungi, such as A. fumigatus, one of the major fungi identified in PC4, may not be positive since there is evidence of its role in CF pathophysiology causing a significant decrease in FEV-1 and being frequently correlated with inflammatory episodes (Eickmeier et al., 2020).
Schizophyllum commune was the third most prevalent fungal OTU detected in the patient C metagenome. This basidiomycete is an environmental fungus that commonly grows on organic matter. However, a novel report linked this microorganism to allergic bronchopulmonary mycosis (ABPM) and pulmonary fungal ball (Chowdhary et al., 2013), characterizing it as an emergent pathogen.
Regarding the other fungal taxa detected, to the best of our knowledge, there are no conclusive reports on their influence in CF disease. The well-known yeast C. albicans is frequently isolated from the sputum culture of CF patients. It is believed that the use of inhaled steroids and antibiotics predisposes Candida sp. colonization (Williams et al., 2016).
In this work, we observed a wide diversity of VFs and ARGs found in all CF microbiomes. As pointed out by a previous assay, the abundance of VFs and ARGs in CF microbiomes are not directly related to a specific bacterial group but are spread among several members of the CF community, indicating that the emergence of these traits should be linked to a particular clinical condition of the CF patient (normal, mild or severe) and enhanced by the antibiotic therapy treatment provided in care units for long-term periods (Bacci et al., 2017).
Antibiotic stewardship indeed may potentiate resistance by triggering downstream responses on the bacterial community, such as the upregulation of efflux pumps in biofilm formation, making bacteria immune to antimicrobials or enhancing the rates of horizontal gene transfer among bacteria (Sherrard et al., 2014). On the other hand, susceptible bacteria can acquire temporary resistance to antibiotics due to microbial interactions in the lung environment. Resistant bacteria can modify antibiotics, diminishing their efficacy, or biofilm formation may limit and dilute the minimum inhibitory concentration values resulting in sublethal doses or fungi that may protect bacteria from forming hyphal barriers (Vandeplassche et al., 2019).
A recent study suggests that a therapy focusing on eliminating anaerobic bacteria re-establishes the canonical CF microbiota, improving lung function and respiratory health (Silveira et al., 2020). At the same time, evidence of a therapy targeted to the weakest cross-feeder, a susceptible bacterium that feeds the entire microbial community, reduces the viability of the resistant ones, improving treatment efficacy (Adamowicz et al., 2018). Currently, the simple detection of virulence factors and antibiotic resistance genes in CF microbiomes is unmeaning if microbial interactions and community response to therapy are not considered.
In summary, the lung airway microbiome of three young CF patients with fungal colonization presents a personal signature with low variation in the microbiome across pulmonary exacerbations and a core set of virulence factors and antibiotic resistance genes. Understanding the microbial community is crucial to improve therapy because it may have the opposite effect, restructuring the pathogenic microbiota. Future studies focusing on the influence of fungi on bacterial diversity and microbial interactions in CF microbiomes will be welcome to fulfill this huge gap in fungi influence in CF physiopathology.
Data Availability Statement
The dataset generated for this study can be found in GenBank accession MT886813 to MT886844, MT891039 to MT891061, and MT911441 to MT911463. The metataxonomic sequence data are available in Bioproject PRJNA644204. The shotgun data are available in Bioproject PRJNA644285.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of “Faculdade de Ciências Farmacêuticas de Ribeirão Preto da Universidade de São Paulo” (FCFRP-USP), under protocol number 2.492.043, in accordance with the HC-FMRP-USP as a co-participating institution. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
OA performed the data curation, formal analysis, investigation, methodology, visualization, and wrote, reviewed, and edited the original draft. CC wrote, reviewed, and edited the manuscript. LuT was in charge of the methodology. PB was in charge of the methodology and wrote, reviewed, and edited the manuscript. AC conceptualized the study and wrote, reviewed, and edited the manuscript. EM supervised the study and wrote, reviewed, and edited the manuscript. LiT was responsible for the formal analysis, visualization, and writing and reviewing the manuscript. MK conceptualized the study, and was in charge of the methodology, project administration, supervision, formal analysis, funding acquisition, project administration, resources, visualization, writing, reviewing, and editing original draft. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by The São Paulo Research Foundation—FAPESP (Grant # 17/25300-8). OA is grateful to FAPESP for the Ph.D. fellowship (Grant #17/13759-6). This work was also partially funded by Coordenação de Aperfeiçoamento do Ensino Superior—CAPES (Finance code-001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors will always be indebted to each patient and their families due to their huge contribution to this study. The bioinformatics processing was performed using sources of The Multi-user Facility from the Center for Medical Genomics at the Clinics Hospital of the Medical School of Ribeirão Preto, coordinated by Professor Wilson Araújo da Silva Jr. The authors also acknowledge the staff Marcelo Gomes for his valuable technical assistance with the bioinformatic server maintenance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2020.598938/full#supplementary-material
References
- Adamowicz E. M., Flynn J., Hunter R. C., Harcombe W. R. (2018). Cross-feeding modulates antibiotic tolerance in bacterial communities. ISME J. 12, 2723–2735. 10.1038/s41396-018-0212-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida O. G. G., De Martinis E. C. P. (2019). Bioinformatics tools to assess metagenomic data for applied microbiology. Appl. Microbiol. Biotechnol. 103, 69–82. 10.1007/s00253-018-9464-9 [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- Bacci G., Mengoni A., Fiscarelli E., Segata N., Taccetti G., Dolci D., et al. (2017). A different microbiome gene repertoire in the airways of cystic fibrosis patients with severe lung disease. Int. J. Mol. Sci. 18, 1–16. 10.3390/ijms18081654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci G., Taccetti G., Dolce D., Armanini F., Segata N., Di Cesare F., et al. (2020). Untargeted Metagenomic Investigation of the Airway Microbiome of Cystic Fibrosis Patients with Moderate-Severe Lung Disease. Microorganisms 8, 1–18. 10.3390/microorganisms8071003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. C., Mall M. A., Gutierrez H., Macek M., Madge S., Davies J. C., et al. (2020). The future of cystic fibrosis care: a global perspective. Lancet Respir. Med. 8, 65–124. 10.1016/S2213-2600(19)30337-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevivino A., Bacci G., Drevinek P., Nelson M. T., Hoffman L., Mengoni A. (2019). Deciphering the Ecology of Cystic Fibrosis Bacterial Communities: Towards Systems-Level Integration. Trends Mol. Med. 25, 1110–1122. 10.1016/j.molmed.2019.07.008 [DOI] [PubMed] [Google Scholar]
- Bushnell B., Rood J., Singer E. (2017). BBMerge – Accurate paired shotgun read merging via overlap. PloS One 12, 1–15. 10.1371/journal.pone.0185056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Intensity normalization improves color calling in SOLiD sequencing. Nat. Publ. Gr 7, 335–336. 10.1038/nmeth0510-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardines R., Giufrè M., Pompilio A., Fiscarelli E., Ricciotti G., Di Bonaventura G., et al. (2012). Haemophilus influenzae in children with cystic fibrosis: Antimicrobial susceptibility, molecular epidemiology, distribution of adhesins and biofilm formation. Int. J. Med. Microbiol. 302, 45–52. 10.1016/j.ijmm.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Carmody L. A., Zhao J., Kalikin L. M., LeBar W., Simon R. H., Venkataraman A., et al. (2015). The daily dynamics of cystic fibrosis airway microbiota during clinical stability and at exacerbation. Microbiome 3, 1–11. 10.1186/s40168-015-0074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverly L. J., Lipuma J. J. (2018). Cystic fibrosis respiratory microbiota: Unraveling complexity to inform clinical practice. Expert Rev. Respir. Med. 12, 857–865. 10.1080/17476348.2018.1513331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A., Randhawa H. S., Gaur S. N., Agarwal K., Kathuria S., Roy P., et al. (2013). Schizophyllum commune as an emerging fungal pathogen: A review and report of two cases. Mycoses 56, 1–10. 10.1111/j.1439-0507.2012.02190.x [DOI] [PubMed] [Google Scholar]
- Coburn B., Wang P. W., Diaz Caballero J., Clark S. T., Brahma V., Donaldson S., et al. (2015). Lung microbiota across age and disease stage in cystic fibrosis. Sci. Rep. 5, 1–12. 10.1038/srep10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. J., Allgaier M., Taylor B., Baek M. S., Huang Y. J., Daly R. A., et al. (2010). Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PloS One 5 (6), e11044. 10.1371/journal.pone.0011044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson L., Walker A. W., Oliver A. E., Rogers G. B., Rivett D. W., Hampton T. H., et al. (2020). Lung function and microbiota diversity in cystic fibrosis. Microbiome 8, 45. 10.1186/s40168-020-00810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting G. R. (2015). Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 16, 45–56. 10.1038/nrg3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa Capizzani C. P., Caçador N. C., Torres LAGMM, Tonani L., Vandamme P., Darini A. L. C. (2017). Clinical and microbiological profile of chronic Burkholderia cepacia complex infections in a cystic fibrosis reference hospital in Brazil. Eur. J. Clin. Microbiol. Infect. Dis. 36 (11), 2263–2271. 10.1007/s10096-017-3058-9 [DOI] [PubMed] [Google Scholar]
- De Koff E. M., De Winter-De Groot K. M., Bogaert D. (2016). Development of the respiratory tract microbiota in cystic fibrosis. Curr. Opin. Pulm. Med. 22, 623–628. 10.1097/MCP.0000000000000316 [DOI] [PubMed] [Google Scholar]
- Delhaes L., Monchy S., Fréalle E., Hubans C., Salleron J., Sylvie Leroy S., et al. (2012). The airway microbiota in cystic fibrosis: A complex fungal and bacterial community-implications for therapeutic management. PloS One 7 (4), e36313. 10.1371/journal.pone.0036313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhariwal A., Chong J., Habib S., King I. L., Agellon L. B., Xia J. (2017). MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 45, W180–W188. 10.1093/nar/gkx295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Edwards B. D., Greysson-Wong J., Somayaji R., Waddell B., Whelan F. J., Storey D. G., et al. (2017). Prevalence and outcomes of achromobacter species infections in adults with cystic fibrosis: A North American cohort study. J. Clin. Microbiol. 55, 2074–2085. 10.1128/JCM.02556-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickmeier O., Zissler U. M., Wittschorek J., Unger F., Schmitt‐Grohé S., Schubert R., et al. (2020). Clinical relevance of Aspergillus fumigatus sensitization in cystic fibrosis. Clin. Exp. Allergy 50, 325–333. 10.1111/cea.13557 [DOI] [PubMed] [Google Scholar]
- Esposito A., Pompilio A., Bettua C., Crocetta V., Giacobazzi E., Fiscarelli E., et al. (2017). Evolution of stenotrophomonas maltophilia in cystic fibrosis lung over chronic infection: A genomic and phenotypic population study. Front. Microbiol. 8, 1590. 10.3389/fmicb.2017.01590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. M., Cameron L. C., Wiggen T. D., Dunitz J. M., Harcombe W. R., Hunter R. C. (2020). Disruption of Cross-Feeding Inhibits Pathogen Growth in the Sputa of Patients with Cystic Fibrosis. mSphere 5, 1–8. 10.1128/msphere.00343-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayman K. B., Armstrong D. S., Grimwood K., Ranganathan S. C. (2017). The airway microbiota in early cystic fibrosis lung disease. Pediatr. Pulmonol. 52, 1384–1404. 10.1002/ppul.23782 [DOI] [PubMed] [Google Scholar]
- Glass N. L., Donaldson G. C. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61, 1323–1330. 10.1128/aem.61.4.1323-1330.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. D., Bright-Thomas R., Kenna D. T., Turton J. F., Woodford N., Jones A. M. (2017). Ralstonia infection in cystic fibrosis. Epidemiol. Infect. 145, 2864–2872. 10.1017/S0950268817001728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güemes A. G. C., Lim Y. W., Quinn R. A., Conrad D. J., Benler S., Maughan H. (2019). Cystic fibrosis rapid response: Translating multi-omics data into clinically relevant information. MBio 10, 1–15. 10.1128/MBIO.00431-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güngör Ö, Tamay Z., Güler N., Erturan Z. (2013). Frequency of fungi in respiratory samples from Turkish cystic fibrosis patients. Mycoses 56, 123–129. 10.1111/j.1439-0507.2012.02221.x [DOI] [PubMed] [Google Scholar]
- Hector A., Kirn T., Ralhan A., Graepler-Mainka U., Berenbrinker S., Riethmueller J., et al. (2016). Microbial colonization and lung function in adolescents with cystic fibrosis. J. Cyst. Fibros. 15, 340–349. 10.1016/j.jcf.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Hong S.-B., Go S.-J., Shin H.-D., Frisvad J. C., Samson R. A. (2005). Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 97, 1316–1329. 10.1080/15572536.2006.11832738 [DOI] [PubMed] [Google Scholar]
- Hong G., Miller H. B., Allgood S., Lee R., Lechtzin N., Zhang S. X. (2017). Use of selective fungal culture media increases rates of detection of fungi in the respiratory tract of cystic fibrosis patients. J. Clin. Microbiol. 55, 1122–1130. 10.1128/JCM.02182-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley M. N. (2018). Staphylococcus aureus in cystic fibrosis: Problem bug or an innocent bystander? Breathe 14, 87–90. 10.1183/20734735.014718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhower C., Gevers D., Knight R., Abubucker S., Badger J. H., Chinwalla A. T., et al. (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B., Raphenya A. R., Alcock B., Waglechner N., Guo P., Tsang K. K., et al. (2017). CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 45, D566–D573. 10.1093/nar/gkw1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. R., Shin J., Guevarra R. B., Lee J. H., Kim D. W., Seol K. H., et al. (2017). Deciphering diversity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 27, 2089–2093. 10.4014/jmb.1709.09027 [DOI] [PubMed] [Google Scholar]
- Klepac-Ceraj V., Lemon K. P., Martin T. R., Allgaier M., Kembel S. W., Knapp A. A., et al. (2010). Relationship between cystic fibrosis respiratory tract bacterial communities and age, genotype, antibiotics and Pseudomonas aeruginosa. Environ. Microbiol. 12, 1293–1303. 10.1111/j.1462-2920.2010.02173.x [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle G. M., White M. M., Browne N., McElvaney N. G., Reeves E. P. (2016). Animal Models of Cystic Fibrosis Pathology: Phenotypic Parallels and Divergences. BioMed. Res. Int. 2016, 1–14. 10.1155/2016/5258727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. W., Evangelista J. S., 3rd, Schmieder R., Bailey B., Haynes M., Furlan M., et al. (2014). Clinical insights from metagenomic analysis of sputum samples from patients with cystic fibrosis. J. Clin. Microbiol. 52 (2), 425–437. 10.1128/jcm.02204-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes S. P., Azevedo N. F., Pereira M. O. (2015). Microbiome in cystic fibrosis: Shaping polymicrobial interactions for advances in antibiotic therapy. Crit. Rev. Microbiol. 41, 353–365. 10.3109/1040841X.2013.847898 [DOI] [PubMed] [Google Scholar]
- Losada P. M., Chouvarine P., Dorda M., Hedtfeld S., Mielke S., Schulz A., et al. (2016). The cystic fibrosis lower airways microbial metagenome. ERJ Open Res. 2, 00096–02015. 10.1183/23120541.00096-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Gómez M. T. (2020). Taking a look on fungi in cystic fibrosis: More questions than answers. Rev. Iberoam. Micol. 37, 17–23. 10.1016/j.riam.2019.10.004 [DOI] [PubMed] [Google Scholar]
- Nilsson R. H., Larsson K. H., Taylor A. F. S., Bengtsson-Palme J., Jeppesen T. S., Schigel D., et al. (2019). The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, D259–D264. 10.1093/nar/gky1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganin P., Fiscarelli E. V., Tuccio V., Chiancianesi M., Bacci G., Morelli P., et al. (2015). Changes in cystic fibrosis airway microbial community associated with a severe decline in lung function. PloS One 10, 1–19. 10.1371/journal.pone.0124348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. D., Bono T. R., Rowe S. M., Solomon G. M. (2020). CFTR targeted therapies: Recent advances in cystic fibrosis and possibilities in other diseases of the airways. Eur. Respir. Rev. 29, 1–13. 10.1183/16000617.0068-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevaes S. M. P. J., De Steenhuijsen Piters W. A. A., De Winter-De Groot K. M., Janssens H. M., Tramper-Stranders G. A., Chu M. L. J. N., et al. (2017). Concordance between upper and lower airway microbiota in infants with cystic fibrosis. Eur. Respir. J. 49, 1602235. 10.1183/13993003.02235-2016 [DOI] [PubMed] [Google Scholar]
- Prior A. R., Gunaratnam C., Humphreys H. (2017). Ralstonia species – do these bacteria matter in cystic fibrosis? Paediatr. Respir. Rev. 23, 78–83. 10.1016/j.prrv.2016.09.005 [DOI] [PubMed] [Google Scholar]
- Procianoy E., de Abreu e Silva F. A., Maróstica P. J. C., Quinton P. M. (2020). Chloride Conductance, Nasal Potential Difference and Cystic Fibrosis Pathophysiology. Lung 198, 151–156. 10.1007/s00408-019-00293-6 [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2013). The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, 590–596. 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn R. A., Adem S., Mills R. H., Comstock W., Goldasich L. D., Humphrey G., et al. (2019). Neutrophilic proteolysis in the cystic fibrosis lung correlates with a pathogenic microbiome. Microbiome 7, 1–13. 10.1186/s40168-019-0636-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders R. V., Modha D. E., Claydon A., Gaillard E. A. (2016). Chronic Aspergillus fumigatus colonization of the pediatric cystic fibrosis airway is common and may be associated with a more rapid decline in lung function. Med. Mycol. 54 (5), 537–543. 10.1093/mmy/myv119 [DOI] [PubMed] [Google Scholar]
- Sayers S., Li L., Ong E., Deng S., Fu G., Lin Y., et al. (2019). Victors: A web-based knowledge base of virulence factors in human and animal pathogens. Nucleic Acids Res. 47, D693–D700. 10.1093/nar/gky999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Waldron L., Ballarini A., Narasimhan V., Jousson O., Huttenhower C. (2012). Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods 9, 811–814. 10.1038/nmeth.2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard L. J., Tunney M. M., Elborn J. S. (2014). Antimicrobial resistance in the respiratory microbiota of people with cystic fibrosis. Lancet 384, 703–713. 10.1016/S0140-6736(14)61137-5 [DOI] [PubMed] [Google Scholar]
- Silveira C. B., Cobian-guemes A. G., Uranga C., Baker J. (2020). Multi-omics study of keystone species in the cystic fibrosis lung microbiome. 10.21203/rs.3.rs-18792/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soret P., Vandenborght L. E., Francis F., Coron N., Enaud R., Avalos M., et al. (2020). Respiratory mycobiome and suggestion of inter-kingdom network during acute pulmonary exacerbation in cystic fibrosis. Sci. Rep. 10, 1–14. 10.1038/s41598-020-60015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzek B. E., Huang H., McGarvey P., Mazumder R., Wu C. H. (2007). UniRef: Comprehensive and non-redundant UniProt reference clusters. Bioinformatics 23, 1282–1288. 10.1093/bioinformatics/btm098 [DOI] [PubMed] [Google Scholar]
- Tonani L., Morosini N. S., Dantas de Menezes H., da Silva M. E. N. B., Wainwright M., Braga G. Ú. L., et al. (2018). In vitro susceptibilities of Neoscytalidium spp. sequence types to antifungal agents and antimicrobial photodynamic treatment with phenothiazinium photosensitizers. Fungal Biol. 122, 436–448. 10.1016/j.funbio.2017.08.009 [DOI] [PubMed] [Google Scholar]
- Vandeplassche E., Tavernier S., Coenye T., Crabbé A. (2019). Influence of the lung microbiome on antibiotic susceptibility of cystic fibrosis pathogens. Eur. Respir. Rev. 28, 190041. 10.1183/16000617.0041-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warris A., Bercusson A., Armstrong-James D. (2019). Aspergillus colonization and antifungal immunity in cystic fibrosis patients. Med. Mycol. 57 (2), S118–S126. 10.1093/mmy/myy074 [DOI] [PubMed] [Google Scholar]
- Waters V., Atenafu E. G., Lu A., Yau Y., Tullis E., Ratjen F. (2013). Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J. Cyst. Fibros. 12, 482–486. 10.1016/j.jcf.2012.12.006 [DOI] [PubMed] [Google Scholar]
- Whelan F. J., Waddell B., Syed S. A., Shekarriz S., Rabin H. R., Parkins M. D., et al. (2020). Culture-enriched metagenomic sequencing enables in-depth profiling of the cystic fibrosis lung microbiota. Nat. Microbiol. 5 (2), 379–390. 10.1038/s41564-019-0643-y [DOI] [PubMed] [Google Scholar]
- White T. J., Bruns T., Lee S., Taylor J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR Protocols: a Guide to Methods and Applications. Eds. Michael A., Innis D. H. G., Sninsky J. J., White T. J. (INC Harcourt Brace Jovanovich, New York, N.Y: Academic Press; ), 315–322. [Google Scholar]
- Williams C., Ranjendran R., Ramage G. (2016). Pathogenesis of Fungal Infections in Cystic Fibrosis. Curr. Fungal Infect. Rep. 10, 163–169. 10.1007/s12281-016-0268-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemanick E. T., Wagner B. D., Robertson C. E., Ahrens R. C., Chmiel J. F., Clancy J. P., et al. (2017). Airway microbiota across age and disease spectrum in cystic fibrosis. Eur. Respir. J. 50, 1–13. 10.1183/13993003.00832-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziesing S., Suerbaum S., Sedlacek L. (2016). Fungal epidemiology and diversity in cystic fibrosis patients over a 5-year period in a national reference center. Med. Mycol. 54, 781–786. 10.1093/mmy/myw035 [DOI] [PubMed] [Google Scholar]
- Zlosnik J. E. A., Zhou G., Brant R., Henry D. A., Hird T. J., Mahenthiralingam E., et al. (2015). Burkholderia species infections in patients with cystic fibrosis in British Columbia, Canada: 30 years’ experience. Ann. Am. Thorac. Soc. 12, 70–78. 10.1513/AnnalsATS.201408-395OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated for this study can be found in GenBank accession MT886813 to MT886844, MT891039 to MT891061, and MT911441 to MT911463. The metataxonomic sequence data are available in Bioproject PRJNA644204. The shotgun data are available in Bioproject PRJNA644285.