Abstract
Background
Low circulating vitamin D levels are associated with poor colorectal cancer (CRC) survival. We assess whether vitamin D supplementation improves CRC survival outcomes.
Methods
PubMed and Web of Science were searched. Randomised controlled trial (RCTs) of vitamin D supplementation reporting CRC mortality were included. RCTs with high risk of bias were excluded from analysis. Random-effects meta-analysis models calculated estimates of survival benefit with supplementation. The review is registered on PROSPERO, registration number: CRD42020173397.
Results
Seven RCTs (n = 957 CRC cases) were identified: three trials included patients with CRC at outset, and four population trials reported survival in incident cases. Two RCTs were excluded from meta-analysis (high risk of bias; no hazard ratio (HR)). While trials varied in inclusion criteria, intervention dose and outcomes, meta-analysis found a 30% reduction in adverse CRC outcomes with supplementation (n = 815, HR = 0.70; 95% confidence interval (CI): 0.48–0.93). A beneficial effect was seen in trials of CRC patients (progression-free survival, HR = 0.65; 95% CI: 0.36–0.94), with suggestive effect in incident CRC cases from population trials (CRC-specific survival, HR = 0.76; 95% CI: 0.39–1.13). No heterogeneity or publication bias was noted.
Conclusions
Meta-analysis demonstrates a clinically meaningful benefit of vitamin D supplementation on CRC survival outcomes. Further well-designed, adequately powered RCTs are needed to fully evaluate benefit of supplementation in augmenting ‘real-life’ follow-up and adjuvant chemotherapy regimens, as well as determining optimal dosing.
Subject terms: Outcomes research, Chemotherapy, Colon cancer, Rectal cancer, Cancer epidemiology
Background
Colorectal cancer (CRC) is the third most common cancer across the world, with 1.8 million cases and ~860,000 deaths each year.1 There is a 10-fold variation in incidence across the world with risk being highest in developed countries, suggesting that the disease may be largely preventable. Ecological variation in vitamin D levels between populations has been proposed as an environmental factor contributing to variation in CRC incidence.2
Controversy surrounds the role of vitamin D deficiency in the aetiology of several common cancers. The strongest available observational evidence supports a link between vitamin D and CRC.3–5 Numerous in vitro studies demonstrate vitamin D-induced growth arrest and apoptosis of CRC cells, modulation of the Wnt signalling pathway, DNA repair and immunomodulation,6 lending support to a causal relationship between vitamin D and cancer. However, observational data implicating vitamin D deficiency in CRC aetiology or survival are limited by potential bias: environmental risk factors associated with CRC are also associated with vitamin D status (co-causality; e.g. physical activity, obesity); heterogeneity in assay type and performance across studies; the development of CRC itself—or its treatment—may induce lower vitamin D levels (reverse causation).5 Mendelian randomisation is an approach that can provide evidence for causality, but studies have thus far failed to detect a causal association between blood 25-hydroxyvitamin D level and CRC risk.7 This may be due to weakness of the available genetic instruments, combined with powerful environmental influences, such as variation in exposure to vitamin D-making ultraviolet B (UVB) sunlight.
Large population trials to date, including the VITAL, VIDA and WHI trials, have shown that vitamin D supplementation did not provide any detectable difference in the incidence of CRC.8–10 Baron et al.11 also reported no reduction in risk of recurrent colorectal adenomas following 3–5 years of supplementation. However, several features of these studies may have limited the ability to detect any effect of supplementation on clinical endpoints.12,13 In brief, recruited subjects were predominantly already sufficient or replete for vitamin D, thereby blunting any health benefit that might be achieved; ‘off-protocol’ vitamin D supplementation was reported in control groups; population heterogeneity such as genetic (variable response or action of vitamin D due to participant genetics) and UVB exposure due to latitude of residence/outdoor activity was not considered as CRC incidence rate was low during follow-up.
In support of a causal effect, several studies have demonstrated an interaction between vitamin D-related genetic variation, 25-hydroxyvitamin D (25OHD) level and CRC or neoplasia risk or outcome, mitigating against potential confounding effects.14–17 In a sub-analysis of VITAL trial data, a lower rate of all cancer death was observed after 2 years of follow-up (hazard ratio (HR) = 0.75; 95% confidence interval (CI 0.59–0.96)). Furthermore, a recent meta-analysis found reduced total cancer mortality with vitamin D supplementation (HR = 0.87; 95% CI 0.79–0.96).18
Here, we present a systematic review and meta-analysis of randomised controlled trials examining the impact of vitamin D supplementation on progression and survival in patients with CRC.
Methods
Literature search
We performed two literature searches. First, to identify trials of vitamin D supplementation in CRC patients; second, to identify completed trials of vitamin D supplementation in non-cancer cohorts, which included cancer mortality as a trial outcome. The electronic databases PubMed19 and Web of Science20 were systematically searched for eligible trials from inception until 31 January 2020.
A comprehensive list of search terms directly relevant to the scope of this systematic review was created. For vitamin D, we included a wide range of terms, including vitamin D, 25-hydroxyvitamin D, calcidiol, cholecalciferol and 25OHD. For the intervention, the following terms were used: supplementation, intervention, treatment, placebo and RCT. For the patient population, we included terms: CRC, bowel, digestive, colon and rectum. Last, for the outcome we included terms: survival, prognosis, mortality and recurrence (Supplementary Table 1). For trials in non-cancer cohorts, the CRC terms were omitted (i.e. CRC, bowel, digestive, colon and rectum). We considered all human research original full-text articles with no restriction on follow-up duration or language, but excluded case reports, reviews and prior meta-analyses. The two searches returned 768 and 3333 articles, respectively. Bibliographies from obtained articles, relevant reviews and clinicaltrials.gov were searched with no further relevant and reported trials identified. To ensure all relevant trials had been included, we checked results against two recent meta-analyses of vitamin D supplementation and all cancer mortality,18,21 which did not yield any further trials. Titles/abstracts were screened by two researchers (P.G.V.-S. and L.F.B.), who then screened full texts for eligibility. The trial ‘PICO’ inclusion criteria were: (i) participants: individuals over the age of 18 years (with/without diagnosis of CRC); (ii) intervention: vitamin D supplementation; (iii) comparators: a placebo/lower dose of vitamin D; (iv) outcome: all measures of survival, for example, progression-free survival, overall survival (OS) and CRC-specific survival. Only randomised controlled trials were included. Disagreements at any stage were resolved by discussion with the senior author (M.G.D.). The review is registered on PROSPERO, registration number CRD42020173397.
Data extraction
Data extraction was conducted by two investigators (L.F.B. and P.G.V.-S.). The data from eligible trials were extracted into a prospectively designed database, including the following information: trial name, publication year, location, sample size, the trial duration, the active intervention (dose and frequency) and comparator (placebo or lower dose), treatment duration and total follow-up duration, the primary and secondary outcomes and the measured outcome (e.g. HR for OS, disease-free survival (DFS) or relapse-free survival and colorectal/disease-specific survival (DSS)). The most fully adjusted HR were extracted. Where the relevant HR were not reported, we contacted the trial authors by email to obtain these (N = 4 contacted, two authors provided relevant HR). For population trials, we included HR for CRC mortality from the time of randomisation in those trial subjects who developed CRC.
Quality assessment
An assessment of the methodological quality of the included trials was conducted using the 2010 CONSORT statement by two authors (L.F.B. and P.G.V.-S.) and disagreement resolved by discussion. Each trial was assessed for adherence against the CONSORT checklist as per previously reported methods.22–24 Adherence against 22 items was assessed and any trial with a high level of missing items (>50%) was considered at high risk of bias and excluded from quantitative assessment through meta-analysis.
Statistical analysis
The main analysis was a trial level meta-analysis of supplementation and CRC outcomes for all eligible trials. Secondary pre-specified sub-group meta-analyses were individually performed for colorectal-specific survival and DFS and for CRC and population trials. The extracted HRs and 95% CIs were used to calculate the pooled HR estimates. Standard errors were used to calculate weighting for each trial. The Hartung–Knapp–Sidik–Jonkman method was used to calculate pooled HR because of the a priori expected heterogeneity between trials, due to differences among populations and methodological dissimilarities between trials. This method was preferred over the DerSimonian and Laird random-effects model given the small number of trials included in the meta-analysis.25,26 The I2 statistic was calculated to quantify the degree of heterogeneity between trials and assess impact on the meta-analysis.27 Publication and selection bias was investigated by checking for asymmetry in the funnel plots and running the Egger’s regression test.28 All analyses were performed in R29 with the R-package ‘metafor’ used for meta-analyses.30
Results
Literature search
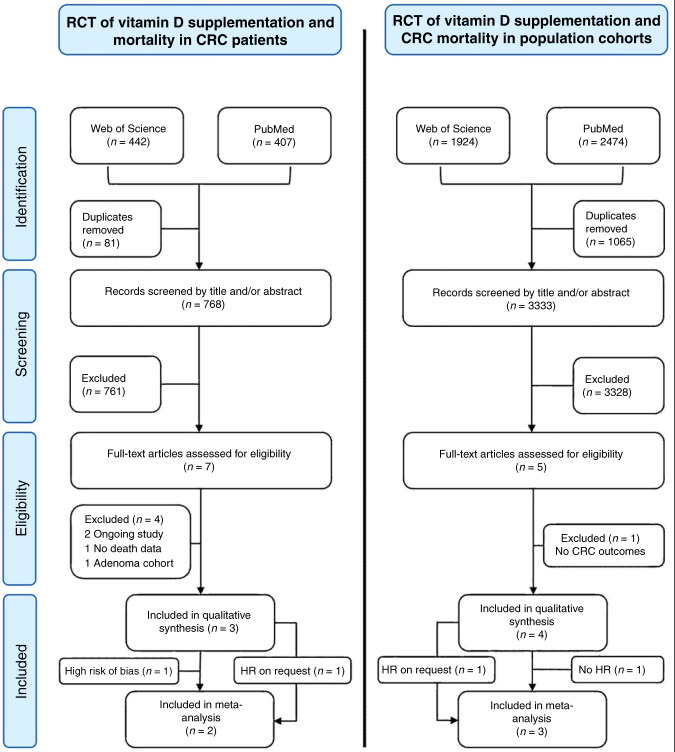
A flowchart illustrating trial selection process is shown in Fig. 1. After removal of duplicates, the two searches (in CRC patients and population trials) yielded 768 and 3333 trials, respectively. Full texts of seven trials in CRC patients and five population trials were considered for inclusion and assessed for eligibility. Full-text review and subsequent correspondence with trial authors yielded three relevant trials in CRC patients31–33 and four population trials for systematic review8,34,35 (Table 1).
Fig. 1. PRISMA flowchart of the trial selection process.
Excluded RCTs of supplementation in CRC patients were the D-health trial (ongoing trial,40) D2dca trial (not yet published); trials by Lappe et al.52 solely reported cancer incidence, but not survival outcomes; Baron et al.11 reported CRC incidence in an adenoma cohort, but with only 14 CRC cases.53 The excluded RCT of supplementation in population cohorts was the VIDA trial as CRC deaths were not reported or available on request;10 the RECORD trial reported CRC deaths,39 but a hazard ratio (HR) was not reported or available on request, so was excluded from meta-analysis. The Golubic et al.31 trial was not included in the meta-analysis of trials in CRC patients due to a high risk of bias, see below.
Table 1.
Characteristics of included trials.
| Name (country, year) | Rx duration | Total/CRC casesa | Participant population | Intervention | Comparator | Primary outcome | Relevant secondary outcome | Follow-up from Rx start | CRC deaths | Overall survival | CRC-specific survival | Progression-free survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRC/adenoma trials | ||||||||||||
| Golubic et al.31 (Croatia, 2018) | 2 years | 71/71 | Metastatic CRC, age 24–79 years | 2000 IU/day vitamin D3 + standard chemotherapy | No placebo | OS | PFS | 46 months | Not known | HR = 1.01 (95% CI 0.39–2.61) | NA | HR = 1.11 (95% CI 0.69–1.77) |
| SUNSHINE32 (USA, 2019) | 23 months | 139/139 | Metastatic CRC | 4000 IU/day vitamin D3 + standard chemotherapy | 400 IU/day vitamin D3 | PFS | OS | 23 months | 111 | 24.3 vs. 24.3 months; P = 0.43 | NA | HR = 0.64 (95% CI 0–0.90) |
| AMATERASU33 (Japan, 2019) | 3.5 years | 201/201 | Epithelial carcinoma of digestive tract (stages 1–3) | 2000 IU/day vitamin D3 + standard chemotherapy | Placebo | PFS | OS | 3.5 years | Not known | HR = 0.95 (95% CI 0.57–1.57) | NA | HR = 0.69 (95% CI 0.39–1.24) |
| Population trials | ||||||||||||
| Trivedi et al.34 (UK, 2003) | 5 years | 2686/55 | Aged 65–85 years | 100,000 IU/4m vitamin D3 | Placebo | Fracture incidence, mortality | Colon-DSS | NA | 18 | NA | HR = 0.62 (95% CI 0.24–1.60) | NA |
| WHI trial7 (USA, 2006) | 7 years | 36,282/322 | Post-menopausal women, 50–79 years | 400 IU/day vitamin D3 + CaCO3 | Placebo | Hip fracture | CRC risk | 7 years | 75 | HR = 0.91 (95% CI 0.83–1.01); (total trial mortality) | HR0.82 (95% CI 0.52–1.29) | NA |
| RECORD trial39 (UK, 2012) | 24–62 months | 5292/71 | Aged >70 years, fracture | 800 IU/day vitamin D3 ± calcium | Placebo ± calcium | Fracture | OS, PFS | 24–62 months | 33 | HR = 0.80 (95% CI 0.61–1.11) (total trial mortality) | 20/41 deaths (vitamin D3) vs.13/30; P = 0.83† | NA |
| VITAL trial35 (USA, 2019) | 5 years | 25,871/98 | Men ≥50 years, women ≥55 years | 2000 IU/day vitamin D3 + omega-3 fatty acids | Placebo | Invasive cancer risk | Cancer mortality | 5.3 years | 25 | NA | HR = 0.65 (95% CI 0.28–1.50) | HR = 0.79 (95% CI 0.36–1.75) |
Rx intervention/comparator duration, IU International units, 4m every 4 months, CaCO3 calcium carbonate, OS overall survival, PFS progression-free survival, DSS disease (CRC)-specific survival, colon-DSS colon cancer disease-specific survival, HR hazard ratio with 95% CI given within parentheses, NA not available.
aCRC number given as recruited cases or incident cases.
†P value was not reported in this paper; χ2 test was performed here.
The main characteristics of included trials are summarised in Table 1. In brief, Golubic et al.31 found no effect of supplementation on OS in stage IV patients at 46 months (2000 IU/day; baseline median 25OHD 13.2 ng/ml, 70 (98.6%) of 71 patients insufficient (<20 ng/ml) at baseline, survival HR = 1.01; 95% CI: 0.39–2.61). In the SUNSHINE trial,32 70% (n = 87) patients had insufficient 25OHD at baseline, and 4000 IU/day supplementation increased median 25OHD from 16.1 to 34.8 ng/ml (87 (70%) with improved median progression-free survival from 11.0 to 13.0 months in stage IV CRC patients (HR = 0.64; 95% CI: 0–0.90; median follow-up 23 months). In the AMATERASU trial,33 41% (n = 173) patients had insufficient 25OHD at baseline, with 2000 IU/day supplementation associated with a non-significant improvement in survival with supplementation after median follow-up 3.5 years in stage I–III patients (25OHD ~20 ng/ml at baseline, ~60 ng/ml at follow-up; HR = 0.69; 95% CI: 0.39–1.24). In the population trials, 400 IU/day in the Women’s Health Initiative trial8 did not impact CRC mortality (baseline median 25OHD 18.4 ng/ml; proportion of insufficient participants at baseline not given; HR = 0.82; 95% CI: 0.52–1.29), with similar results reported by Trivedi et al.34 (100,000 IU/4-monthly; proportion of insufficient participants at baseline not given; follow-up 25OHD 29.7 vs. 21.4 ng/ml with placebo; HR = 0.62; 95% CI: 0.24–1.60). The VITAL trial35 authors provided relevant data on request, with a trend towards increased DSS and PFS in 98 incident CRC cases (only 2001 (13%) insufficient for 25OHD at baseline; 25OHD 29.8 ng/ml to 41.8 ng/ml in treatment arm; DSS HR = 0.65; 95% CI: 0.28–1.50); PFS HR = 0.79; 95% CI: 0.36–1.75). In the RECORD trial36 of secondary fracture prevention, there was no impact on CRC death in 71 incident CRC cases, with 20 CRC deaths in the vitamin D group and 13 in the placebo/calcium groups (baseline 25OHD 15.2 ng/ml; HR not available on request).
Quality assessment
Adherence to the CONSORT 2010 checklist37 was assessed for the seven trials identified in the literature search, with high rates of adherence for all but the Golubic trial (Supplementary Table 2). In particular, it was noted that this trial was not placebo controlled, with no reported mechanism to implement the random allocation sequence, no eligibility criteria for participants given and no description of level and method of blinding given. As a result, this trial was considered at high risk of bias and excluded from overall meta-analysis.
Meta-analysis of vitamin D supplementation and survival outcomes
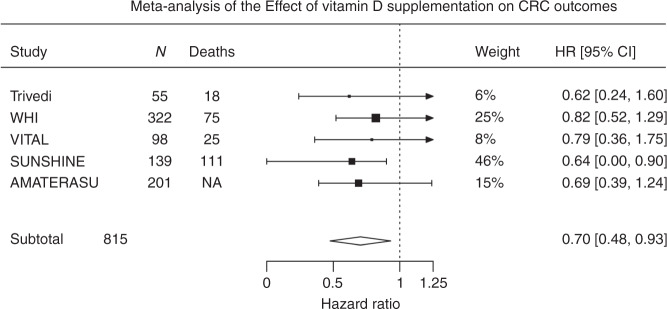
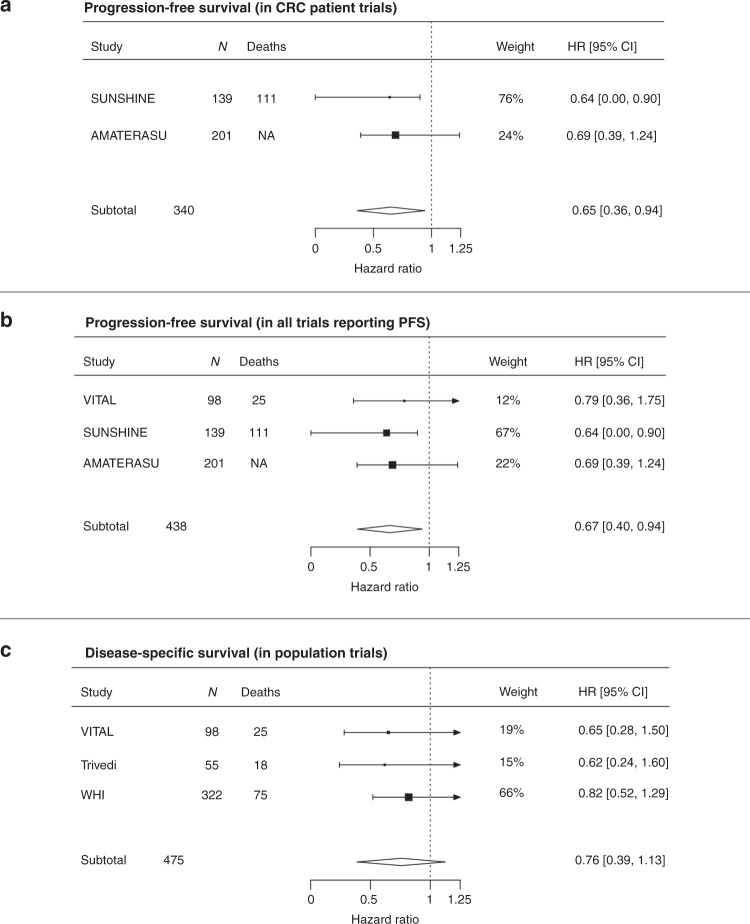
All included trials demonstrated a beneficial effect. Overall meta-analysis in five trials, comprising 815 participants revealed a beneficial effect of vitamin D supplementation on cancer outcomes in patients with CRC (HR = 0.70; 95% CI: 0.48–0.93; Fig. 2). Sub-group meta-analyses demonstrated a consistent favourable effect with vitamin D supplementation. In trials recruiting patients with CRC at outset, CRC progression or death was reduced by 35% (HR = 0.65; 95% CI:0.36–0.94; Fig. 3a), and by 33% across the three trials reporting PFS (HR = 0.67; 95% CI: 0.40–0.94; Fig. 3b). In the population trials, disease-specific survival improved by 24% (HR = 0.76; 95% CI: 0.39–1.13; Fig. 3c). Results were not quantitatively changed when the excluded Golubic et al.31 trial was included in the meta-analysis (Supplementary Fig. 1).
Fig. 2. Meta-analysis of the effect of vitamin D supplementation on CRC outcomes.
HRs used are for disease (CRC)-specific survival for the Trivedi and Women’s Health initiative (WHI) trials and progression-free survival for the VITAL, SUNSHINE and AMATERASU trials. HR confidence interval in the SUNSINE trial was one-sided. There was no evidence of heterogeneity with τ: 0.026; I2 (total heterogeneity/total variability): 0.85% and P = 0.98.
Fig. 3. Sub-group meta-analyses results.
HRs used are for disease (CRC) specific survival for the Trivedi and Women’s Health initiative (WHI) trials and progression-free survival for the VITAL, SUNSHINE and AMATERASU trials. Statistical testing in the SUNSINE trial was one-sided. There was no evidence of heterogeneity in sub-group meta-analyses presented in a–c with τ < 0.03; I2 (total heterogeneity/total variability): <0.6% and P > 0.88.
Testing for trial heterogeneity and publication bias
Despite the different interventions and outcomes in the included trials, there was no evidence of heterogeneity with τ: 0.026 and I2: 0.85% in the overall meta-analysis. No evidence of publication bias was seen, with Egger’s regression test for funnel plot asymmetry P = 0.87 (Supplementary Fig. 2).38
Discussion
This is the first systematic review with meta-analysis of randomised controlled trials to examine the effect of vitamin D supplementation on survival outcomes in patients with CRC. We found that supplementation imparts a 30% reduction in adverse survival outcomes overall, with a 24% reduction in CRC-specific death and a 33% in disease progression or death. The effect on survival was consistently observed in sub-group analyses both in trials specifically including CRC patients and in population trials reporting outcomes in incident CRC cases.
We included two RCTs of supplementation in patients with a diagnosis of CRC and demonstrated a 35% reduction in CRC progression or death with supplementation. We also recognised that incident cases of CRC occur in large population trials, providing an additional source of trial evidence. We included three population trials totalling almost 65,000 participants in our meta-analysis, with a suggestive benefit from supplementation on CRC-specific survival (HR = 0.76; 95% CI: 0.39–1.12). Two relevant trials were not included as HRs for CRC outcomes were not available after requests to the author,10,39 while we identified several ongoing trials yet to publish results, or example, the D-Health trial.40
The VITAL trial authors recently performed a review and meta-analysis of supplementation and all cancer mortality based on incident cancers in population supplementation trials,18,35 reporting a reduction in total cancer mortality with supplementation (HR = 0.83; 95% CI: 0.67–1.02). A similar meta-analysis by Zhang et al.21 found a similar effect (HR = 0.84; 95% CI: 0.74–0.95), yet combining all cancers may be flawed given that ‘cancer’ is a not a single disease, but a hugely heterogeneous group of individual and specific diseases. The current literature review is the first to assimilate evidence from trials specifically including patients with a diagnosis of CRC, but also large population trials that reported survival outcomes in incident CRC cases. A consistent reduction in adverse survival outcomes irrespective of the trial inclusion criteria, supplementation dose or survival outcome measure is supportive of a true causal effect, which supports observational data linking 25OHD level and cancer outcomes.16,17
There are a number of limitations in the currently available trial data impacting on this analysis. First, our literature search demonstrates a lack of well-designed and adequately powered randomised controlled trials investigating vitamin D supplementation and CRC outcomes. All included trials in the current meta-analysis were small, each including <500 CRC cases amounting to only 815 cases in meta-analysis. Next, the population trials included here did not report any data on stage, site or subtype of incident CRC cases or adjuvant therapy used, which are known to impact survival outcomes and the variables used for the HR adjustment are not consistently reported. Third, observational data strongly supports an association between genetic factors related to vitamin D metabolism or function and survival outcomes,14,16,17 yet no trial to date has considered the relevance of genetic heterogeneity to the impact of vitamin D on cancer death. Finally, we acknowledge that pooling estimates from trials with differing methodology may limit the conclusions that can be drawn. For example, in the population trials, the two groups are comparable at point of randomisation, but may not be comparable at point of diagnosis of CRC, which could bias outcomes. However, variability in inclusion criteria, interventions or outcomes generally results in a more heterogeneous estimate and is likely to increase statistical uncertainty and hence results tend towards the null. Nonetheless, our summary findings (i.e. direction and magnitude of effect size) remain largely unchanged when the analysis was limited according to trial methodology or outcome.
We acknowledge that translation of results from supplement RCTs to a real-life healthcare setting is not always straight forward. While vitamin D is cheap and generally safe, vitamin D intoxication or other adverse effects of supplementation must be considered. Poor compliance may also impact on real-life benefit. Lower 25OHD level is strongly associated with CRC survival in observational data,14,16,17 providing a strong rationale for supplementation trials in cancer patients with survival outcomes as the defined endpoint yet observational studies of vitamin D supplementation or intake and survival do not provide consistent evidence of benefit from vitamin D. A Norwegian study recently reported better CRC survival in incident CRC cases with pre-diagnostic vitamin D intake of >400 IU/day (HR = 0.75; 95% CI: 0.61–0.92).41 Similarly, the Cancer Prevention Study-II reported a trend towards greater OS in those with higher total or dietary vitamin D intake (HR = 0.88; 95% CI: 0.57–1.35 and HR = 0.90; 95% CI: 0.67–1.21), yet even in quartile four, the intake was low (~>245 IU/day).42 Jeffreys et al.43 reported a non-significant reduction in mortality after CRC diagnosis in women who had been prescribed vitamin D supplementation in the 5 years preceding CRC diagnosis (13% of 4122 cases prescribed supplements; HR = 0.90; 95% CI: 0.78–1.04), yet some other studies have found no benefit from low-dose supplementation.44–46 Crucially, all of these studies assess low doses of supplementation or intake and do not consider vitamin D-related genetic variants that have been shown to influence the association between vitamin D and survival.14,16,17 The lack of consistent findings in the observational data support further well-powered trials investigating the role of appropriate supplementary doses of vital D in CRC patients with insufficient 25OHD levels at baseline. The above findings, together with the clear benefit of 4000 IU over 400 IU in the SUNSHINE study, suggest that an intake of 400 IU/day is inadequate. Indeed, it is noted that the reference nutrient intake for vitamin D of 400 IU/day is recommended for the UK population, with this intake given as the average amount needed by 97.5% of the population to maintain a serum 25OHD concentration ≥10 ng/l when UVB sunshine exposure is minimal.5 The optimal dose for survival benefit remains unclear and requires further investigation, but given that data from several publications and national bodies indicates 2000–4000 IU/day to be safe,5,47–51 we believe that doses of ~2000–4000 IU should be considered for future trials.
In conclusion, this meta-analysis demonstrates a clinically meaningful beneficial effect from vitamin D supplementation on survival outcomes in patients with CRC. Further well-designed, adequately powered RCTs are needed to fully evaluate the benefit of supplementation in augmenting ‘real-world’ follow-up and adjuvant chemotherapy regimens, as well as determining optimal dosing.
Supplementary information
Author contributions
P.G.V.-S.: conceptualisation, methodology, formal analysis, writing—original draft, visualisation, writing—review and editing. L.F.B.: methodology, formal analysis, writing—original draft. J.P.B.: conceptualisation, writing—review and editing. E.T.—methodology, writing—review and editing. L.Z.: conceptualisation, methodology, writing—review and editing. F.V.N.D.: conceptualisation, methodology, writing—review and editing. S.M.F.: supervision, writing—review and editing. M.G.D.: conceptualisation, project administration, funding acquisition, supervision, writing—review and editing.
Ethics approval and consent to participate
Not required; data were collected for previously published studies.
Data availability
Available at reasonable requests from pvaughan@ed.ac.uk.
Competing interests
The authors declare no competing interests
Funding information
This work was supported by funding for the infrastructure and staffing of the Edinburgh CRUK Cancer Research Centre; CRUK programme grant C348/A18927 (M.G.D.). P.G.V.-S. was supported by a NES SCREDS clinical lectureship. J.P.B. is supported by an ECAT-linked CRUK ECRC Clinical training award (C157/A23218). E.T. is supported by a CRUK Career Development Fellowship (C31250/A22804). F.V.N.D. is supported by a CSO Senior Clinical Fellowship. This work was also funded by a grant to M.G.D. as Project Leader with the MRC Human Genetics Unit Centre Grant (U127527202 and U127527198 from 1/4/18).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Peter G. Vaughan-Shaw, Louis F. Buijs, Susan M. Farrington, Malcolm G. Dunlop
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-020-01060-8.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 3.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 5.SACN. Vitamin D and Health (Scientific Advisory Committee on Nutrition, 2016).
- 6.Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem. J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, Timofeeva M, Farrington SM, Vaughan-Shaw P, Svinti V, Walker M, et al. Exploring causality in the association between circulating 25-hydroxyvitamin D and colorectal cancer risk: a large Mendelian randomisation study. BMC Med. 2018;16:142. doi: 10.1186/s12916-018-1119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N. Engl. J. Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scragg RKR. Overview of results from the Vitamin D Assessment (ViDA) study. J. Endocrinol. Invest. 2019;42:1391–1399. doi: 10.1007/s40618-019-01056-z. [DOI] [PubMed] [Google Scholar]
- 11.Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, Snover DC, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N. Engl. J. Med. 2015;373:1519–1530. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughan-Shaw PG, Zgaga L, Theodoratou E, Blackmur JP, Dunlop MG. Whether vitamin D supplementation protects against colorectal cancer risk remains an open question. Eur. J. Cancer. 2019;115:1–3. doi: 10.1016/j.ejca.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Lappe JM, Heaney RP. Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinology. 2012;4:95–100. doi: 10.4161/derm.19833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zgaga L, Theodoratou E, Farrington SM, Din FV, Ooi LY, Glodzik D, et al. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J. Clin. Oncol. 2014;32:2430–2439. doi: 10.1200/JCO.2013.54.5947. [DOI] [PubMed] [Google Scholar]
- 15.Barry, E. L., Peacock, J. L., Rees, J. R., Bostick, R. M., Robertson, D. J., Bresalier, R. S. et al. Vitamin D receptor genotype, vitamin D3 supplementation, and risk of colorectal adenomas: a randomized clinical trial. JAMA Oncol.10.1001/jamaoncol.2016.5917 (2016). [DOI] [PMC free article] [PubMed]
- 16.Vaughan-Shaw, P. G., Zgaga, L., Ooi, L. Y., Theodoratou, E., Timofeeva, M., Svinti, V. et al. Low plasma vitamin D is associated with adverse colorectal cancer survival after surgical resection, independent of systemic inflammatory response. Gut69, 103–111 (2020) [DOI] [PMC free article] [PubMed]
- 17.Vaughan-Shaw PG, O’Sullivan F, Farrington SM, Theodoratou E, Campbell H, Dunlop MG, et al. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br. J. Cancer. 2017;116:1092–1110. doi: 10.1038/bjc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann. Oncol. 2019;30:733–743. doi: 10.1093/annonc/mdz059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NCBI. PubMedhttp://www.ncbi.nlm.nih.gov/pubmed/advanced (2015).
- 20.JISC. Web of Sciencehttp://wok.mimas.ac.uk/ (2015).
- 21.Zhang, Y., Fang, F., Tang, J. J., Jia, L., Feng, Y. N., Xu, P. et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ366, 10.1136/bmj.l4673 (2019). [DOI] [PMC free article] [PubMed]
- 22.McCormick F, Cvetanovich GL, Kim JM, Harris JD, Gupta AK, Abrams GD, et al. An assessment of the quality of rotator cuff randomized controlled trials: utilizing the Jadad score and CONSORT criteria. J. Shoulder Elb. Surg. 2013;22:1180–1185. doi: 10.1016/j.jse.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Jones A, Lepage L, Group C. Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA. 2001;285:1992–1995. doi: 10.1001/jama.285.15.1992. [DOI] [PubMed] [Google Scholar]
- 24.Sut N, Senocak M, Uysal O, Koksalan H. Assessing the quality of randomized controlled trials from two leading cancer journals using the CONSORT statement. Hematol. Oncol. Stem Cell Ther. 2008;1:38–43. doi: 10.1016/s1658-3876(08)50059-8. [DOI] [PubMed] [Google Scholar]
- 25.Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann. Intern. Med. 2014;160:267–270. doi: 10.7326/M13-2886. [DOI] [PubMed] [Google Scholar]
- 26.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 29.R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2013).
- 30.Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 31.Antunac Golubic Z, Barsic I, Librenjak N, Plestina S. Vitamin D supplementation and survival in metastatic colorectal cancer. Nutr. Cancer. 2018;70:413–417. doi: 10.1080/01635581.2018.1445766. [DOI] [PubMed] [Google Scholar]
- 32.Ng K, Nimeiri HS, McCleary NJ, Abrams TA, Yurgelun MB, Cleary JM, et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: the SUNSHINE randomized clinical trial. JAMA. 2019;321:1370–1379. doi: 10.1001/jama.2019.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urashima M, Ohdaira H, Akutsu T, Okada S, Yoshida M, Kitajima M, et al. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: the AMATERASU randomized clinical trial. JAMA. 2019;321:1361–1369. doi: 10.1001/jama.2019.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manson JE, Bassuk SS, Buring JE, Group VR. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J. Steroid Biochem. Mol. Biol. 2019;198:105522. doi: 10.1016/j.jsbmb.2019.105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial) J. Clin. Endocrinol. Metab. 2012;97:614–622. doi: 10.1210/jc.2011-1309. [DOI] [PubMed] [Google Scholar]
- 37.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D-3 and/or calcium (RECORD Trial) J. Clin. Endocrinol. Metab. 2012;97:614–622. doi: 10.1210/jc.2011-1309. [DOI] [PubMed] [Google Scholar]
- 40.Neale RE, Armstrong BK, Baxter C, Duarte Romero B, Ebeling P, English DR, et al. The D-Health trial: a randomized trial of vitamin D for prevention of mortality and cancer. Contemp. Clin. Trials. 2016;48:83–90. doi: 10.1016/j.cct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Oyeyemi SO, Braaten T, Skeie G, Borch KB. Competing mortality risks analysis of prediagnostic lifestyle and dietary factors in colorectal cancer survival: the Norwegian Women and Cancer Study. BMJ Open Gastroenterol. 2019;6:e000338. doi: 10.1136/bmjgast-2019-000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang B, McCullough ML, Gapstur SM, Jacobs EJ, Bostick RM, Fedirko V, et al. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: the Cancer Prevention Study-II Nutrition Cohort. J. Clin. Oncol. 2014;32:2335–2343. doi: 10.1200/JCO.2014.55.3024. [DOI] [PubMed] [Google Scholar]
- 43.Jeffreys M, Redaniel MT, Martin RM. The effect of pre-diagnostic vitamin D supplementation on cancer survival in women: a cohort study within the UK Clinical Practice Research Datalink. BMC Cancer. 2015;15:670. doi: 10.1186/s12885-015-1684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis C, Xun P, He K. Vitamin D supplementation and quality of life following diagnosis in stage II colorectal cancer patients: a 24-month prospective study. Support Care Cancer. 2016;24:1655–1661. doi: 10.1007/s00520-015-2945-9. [DOI] [PubMed] [Google Scholar]
- 45.Inoue-Choi M, Greenlee H, Oppeneer SJ, Robien K. The association between postdiagnosis dietary supplement use and total mortality differs by diet quality among older female cancer survivors. Cancer Epidemiol. Biomark. Prev. 2014;23:865–875. doi: 10.1158/1055-9965.EPI-13-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng K, Meyerhardt JA, Chan JA, Niedzwiecki D, Hollis DR, Saltz LB, et al. Multivitamin use is not associated with cancer recurrence or survival in patients with stage III colon cancer: findings from CALGB 89803. J. Clin. Oncol. 2010;28:4354–4363. doi: 10.1200/JCO.2010.28.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am. J. Clin. Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 48.Stamp TC, Haddad JG, Twigg CA. Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet. 1977;1:1341–1343. doi: 10.1016/s0140-6736(77)92553-3. [DOI] [PubMed] [Google Scholar]
- 49.Jones G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008;88:582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 50.Vieth R. Vitamin D toxicity, policy, and science. J. Bone Miner. Res. 2007;22(Suppl. 2):V64–V68. doi: 10.1359/jbmr.07s221. [DOI] [PubMed] [Google Scholar]
- 51.Azzi A, Brigelius-Flohe R, Kelly F, Lodge JK, Ozer N, Packer L, et al. On the opinion of the European Commission “Scientific Committee on Food” regarding the tolerable upper intake level of vitamin E (2003) Eur. J. Nutr. 2005;44:60–62. doi: 10.1007/s00394-005-0549-8. [DOI] [PubMed] [Google Scholar]
- 52.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am. J. Clin. Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 53.Calderwood AH, Baron JA, Mott LA, Ahnen DJ, Bostick RM, Figueiredo JC, et al. No evidence for posttreatment effects of vitamin D and calcium supplementation on risk of colorectal adenomas in a randomized trial. Cancer Prev. Res. (Philos.) 2019;12:295–304. doi: 10.1158/1940-6207.CAPR-19-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available at reasonable requests from pvaughan@ed.ac.uk.