Abstract
Purpose:
To investigate platelet-to-lymphocyte ratio (PLR) in retinal vein occlusion (RVO) patients.
Methods:
In this study, we retrospectively reviewed data of 32 patients with RVO (RVO group) and 32 age- and sex-matched participants without RVO (control group) between January 2017 and March 2019. The PLR was determined by dividing the platelet count by the lymphocyte count.
Results:
Age and gender were comparable between the groups (p = 0.204 and p = 0.800, respectively). PLR was significantly elevated in the RVO group compared with the control group (137 (113–164) vs 101 (86–129), p = 0.001)). In the receiver operator characteristics curve analysis, the optimal cut-off value of PLR for predicting RVO was 123, with 69% sensitivity and 72% specificity.
Conclusion:
We report that PLR are elevated in RVO, suggesting that PLR may be a useful marker for RVO.
Keywords: inflammation, platelet-to-lymphocyte ratio, retinal vein occlusion, thrombus
Introduction
Retinal vein occlusion (RVO) is the second most common retinal vascular disorder following diabetic retinopathy. If left untreated, RVO may lead to loss of vision due to retinal ischemia and macular edema.1 Depending on the place of the vessel blockage, the RVO is divided into central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO).2 Although the mechanisms of RVO are not fully understood yet, high intraocular pressure or glaucoma, and short axial distance are believed to be localised risk factors.1,2 Furthermore, atherosclerotic diseases, diabetes mellitus, hypertension, systemic inflammatory diseases, dyslipidemia, oral contraceptive drug use, gravidity, smoking, hypercoagulable situations, and older age are thought to be systemic factors for RVO development.3 Several existing acceptable treatments such as laser photocoagulation, intravitreal steroids, and intravitreal vascular endothelial growth factor inhibitors have been reported for RVO.4–7 Given that RVO can cause vision loss, the identification of easily accessible markers for RVO is very important.
Platelet cells have a crucial part in the ethiopathogenesis of thrombo-occlusive diseases. The platelet hyperaggregability might be an important factor in the RVO development process.8 On the other hand, lymphocytes are important cells that play a role in inflammatory response, and activation of systemic inflammatory pathways, as seen e.g. in sarcoidosis and Behçet’s disease, are also involved in the pathogenesis of RVO.9,10 As RVO is a disease involving thrombotic and inflammatory processes, inflammatory markers may help to predict the risk of RVO. Platelet-to-lymphocyte ratio (PLR), which is an inexpensive marker calculated from routine complete blood count (CBC) test, is a new biomarker that shows the existence both thrombosis and inflammation. Higher PLR levels are associated with poor cardiovascular events.11–13 The PLR has also been investigated in many ophthalmic disorders such as glaucoma, dry eye disease, and uveitis.14–16 Given the significance of thrombogenesis and inflammation in the ethiopathogenesis of RVO, we purposed to explore a relationship between PLR and other routine CBC parameters and occurrence of RVO.
Materials and methods
This study was been conducted according to the tenets of Declaration of Helsinki. The study protocol was approved by the Ethical Committee of Hatay Mustafa Kemal University Tayfur Ata Sökmen Faculty of Medicine approved (2019/03) before the study was initiated. All patients provided informed consent.
We retrospectively reviewed the data of 32 subjects diagnosed with any type of RVO at our center (RVO group) and 32 age- and sex-matched individuals (control group) who came to our clinic for routine ophthalmologic examination between January 2017 and March 2019. Patients having CBC pending the onset diagnosis were assessed. CBC parameters of control subjects for routine control and CBC parameters of RVO patients for further investigation were requested by the internal medicine outpatient clinic. Exclusion criteria was composed of a history of any ocular surgery or trauma, diabetes, active smoking, kidney insufficiency, chronic obstructive lung disease, anemia, cancer, any cardiovascular disease, acute/chronic infections or chronic inflammatory diseases, stroke, and glaucoma. Patients using nonsteroidal anti-inflammatory, anticoagulant medications, and oral contraceptive pills were also excluded. All patients underwent a detailed ophthalmic exam. CRVO was identified as the existence of retinal hemorrhages together with retinal vein dilatation in four retinal quadrants. BRVO was identified as venous dilation and tortuosity with flame-shaped and dot-blot hemorrhages in a wedge-shaped region. Fundus fluorescein angiography was used for identifying retinal non-perfusion and classifying ischemia.
Venous blood samples were gathered by antecubital vein and evaluated within 2 h with hematology analyzer (Mindray, UK). The PLR values were analyzed by dividing the platelet count by the lymphocyte count.
Statistical analysis
Parametric data were assessed as mean ± standard deviation or median (25–75% interquartile range) as appropriate. Continuous variables were compared with the Student’s t-test or Mann–Whitney U test when appropriate. Categorical data were expressed as number and percentages. To compare categorical data, the chi-square test was used. Receiver operator characteristic (ROC) curve analysis was applied to define the best cut-off points of PLR, neutrophil-to-lymphocyte ratio (NLR), and platelet for predicting of RVO. The areas under the curve (AUC) were analyzed as measures of the correctness of the tests. Statistical analysis was performed using SPSS version 21.0 (SPSS, Inc., Chicago, Illinois, USA). A p < 0.05 was accepted as statistically important.
Results
The RVO group consisted of 13 female and 19 male patients and the control group included 14 female and 18 male participants. The mean age of the RVO group was 60 ± 10 years and the control group was 63 ± 12 years. There was no statistical difference between groups in terms of age and sex (p = 0.204 and p = 0.800, respectively). The existence of hypertension was similar in the RVO and control groups (p > 0.05). In RVO group, there were 29 (90.6%) unilateral RVO (17 right eye, 12 left eye) and 3 (9.4%) bilateral RVO patients. Again in this patient group, there were 23 (71.9%) patients with BRVO and 9 (28.1%) patients with CRVO. Seven (21.9%) patients had ischemic RVO and 25 (78.1%) patients had non-ischemic RVO. There was no significant difference according to intraocular pressures between RVO and control groups. Baseline characteristics of the groups are presented in Table 1.
Table 1.
Demographic and clinical characteristics of study population.
| Characteristics | Study groups |
p value | |
|---|---|---|---|
| Control (n = 32) | RVO (n = 32) | ||
| Age, years | 0.204 | ||
| Mean ± SD | 63 ± 12 | 60 ± 10 | |
| Range | (42–85) | (45–81) | |
| Gender, n (%) | 0.800 | ||
| Female | 14 (43.8) | 13 (40.6) | |
| Male | 18 (56.3) | 19 (59.4) | |
| Hypertension, n (%) | 10 (31.3) | 13 (40.6) | 0.434 |
| Side of RVO, n (%) | |||
| Unilateral | – | 29 (90.6) | |
| Right eye | – | 17 (53.1) | |
| Left eye | – | 12 (37.5) | |
| Bilateral | – | 3 (9.4) | |
| Location of RVO, n (%) | |||
| Branch RVO | – | 23 (71.9) | |
| Central RVO | – | 9 (28.1) | |
| Type of RVO, n (%) | |||
| Ischemic | 7 (21.9) | ||
| Non-ischemic | 25 (78.1) | ||
| Intraocular pressure (mmHg) | 15.95 ± 1.89 | 16.61 ± 2.97 | 0.306 |
RVO, retinal vein occlusion; SD, standard deviation.
Laboratory findings of subjects with RVO and controls are reported in Table 2. Platelet distribution width, mean platelet volume (MPV), lymphocyte count, NLR, and serum glucose levels were comparable between the groups. Platelet count was 289 ± 64 in RVO group and 240 ± 65 in control group (p = 0.003). PLR values were considerably higher in RVO group than in the control group (137 (113–164) vs 101 (86–129), p = 0.001); Figure 1). In terms of type of RVO, the PLR values were higher in ischemic-type RVO than in the non-ischemic-type RVO, and the difference was statistically significant (134 (98–162) vs 164 (131–212), p = 0.042)). We also explored if there was a relation between PLR and the vision at presentation. Of the 32 RVO cases, only 3 had good presenting vision (>20/40) and 29 had poor presenting vision (<20/40). We did not find a statistically significant relationship between PLR and the presenting vision (140 (112–163) for poor vision vs 128 (127–191) for good vision, p = 0.872)). C-reactive protein values were elevated in RVO group than control group, but this difference did not reach statistical significance (3.13 (0.57–3.27) vs 0.40 (0.40–3.13), p = 0.245). Considering the ROC curve analysis, the best cut-off value of PLR for estimating RVO was >123, with 69% sensitivity and 72% specificity (AUC = 0.726, 95% confidence interval = 0.602–0.850, p = 0.002, Figure 2). Additional ROC curve analysis demonstrated that the platelet count also significantly predicted RVO (p = 0.01), but the AUC for platelet was lower than PLR (AUC for platelet 0.688; Figure 2).
Table 2.
Laboratory characteristics of patients with RVO and controls.
| Characteristics | Study groups |
p value | |
|---|---|---|---|
| Control (n = 32) | RVO (n = 32) | ||
| Platelet-to-lymphocyte ratio | 101 (86–129) | 137 (113–164) | 0.001 |
| White blood cell count (×109/L) | 8.30 ± 1.65 | 8.44 ± 2.01 | 0.776 |
| Neutrophil count (×109/L) | 5.18 ± 1.57 | 5.39 ± 1.85 | 0.639 |
| Neutrophil-to-lymphocyte ratio | 2.36 ± 0.89 | 2.70 ± 1.24 | 0.218 |
| Red cell distribution width (%) | 14.2 ± 1.44 | 14.14 ± 1.59 | 0.891 |
| Platelet count (×109/L) | 240 ± 65 | 289 ± 64 | 0.003 |
| Lymphocyte count (×109/L) | 2.31 ± 0.60 | 2.23 ± 0.79 | 0.627 |
| Platelet distribution width (%) | 16.11 ± 0.61 | 15.67 ± 1.61 | 0.163 |
| Mean platelet volume (fL) | 10.21 ± 1.07 | 10.09 ± 1.09 | 0.654 |
| C-reactive protein (mg/dL) | 0.40 (0.40–3.13) | 3.13 (0.57–3.27) | 0.245 |
| Fasting blood glucose (mg/dL) | 97 (91–108) | 99 (93–117) | 0.567 |
RVO, retinal vein occlusion.
Figure 1.
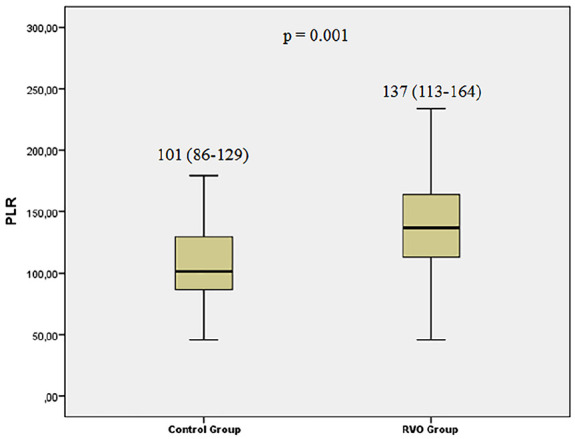
Comparison of platelet-to-lymphocyte ratio (PLR) levels between the retinal vein occlusion (RVO) and control groups.
Figure 2.
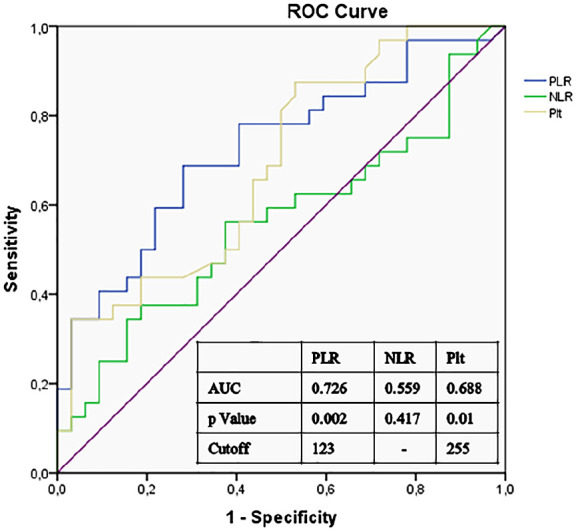
Receiver operator characteristic (ROC) curve of platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and platelet count (Plt) for the development of retinal vein occlusion.
Discussion
Our study demonstrated that PLR was significantly elevated in RVO patients and that higher PLR may be a powerful marker for RVO development.
RVO is the most widespread retinal vascular disorder after diabetic retinopathy, and it may lead to loss of vision because of the retinal ischemia, macular edema, and neovascular glaucoma.1,2 Even though it is easy to diagnose, the pathogenetic mechanisms of RVO are not clearly established. In the current literature, some reports indicate a relationship between platelet activity and inflammation markers with the occurrence of RVO, while other studies have not verified this relationship.8–10,17–23 Nevertheless, it is widely accepted that thrombo-inflammatory cascades take part in the pathogenesis of RVO.24
The platelet hyperaggregability could be one of the key factors in the RVO development process.8 Kuhli-Hattenbach and colleagues25 investigated whether adenosine diphosphate (ADP)-induced platelet hyperaggregability is associated with RVO, given that ADP can contribute to the growth and stability of a thrombus by activating platelets. They found that in patients with RVO, platelets were significantly hyperreactive after induction of very low concentrations of ADP, when compared with healthy individuals. MPV is another marker of platelet activation. In studies investigating the relationship between MPV and RVO, contradictory outcomes have been reported. In some studies, MPV was considerably elevated in patients with RVO compared with control group,17–20 whereas in other studies, there was no significant relationship between MPV and RVO.21,22 Ornek and colleagues21 did not discover elevated MPV levels in patients with RVO compared with controls. On the other hand, Dursun and colleagues23 demonstrated that higher NLR was associated with the development of RVO. More recently, in a study conducted by Kumral and colleagues22 there was no significant difference in MPV and NLR between RVO and control groups. The authors concluded that MPV and NLR were not associated with a higher risk of RVO. In concordance with the results of the study by Kumral et al., MPV and NLR values were not different between the groups in our study, suggesting that MPV is not a helpful RVO biomarker. However, Kumral and colleagues did not examine PLR values. Previous studies showed the relationship between PLR and various ocular diseases such as glaucoma,14 dry eye disease,15 and uveitis.16 Although the limitation in our study is that the number of subjects was too low to make conclusive statements or to calculate a cut-off value in this population, we suggest that PLR may be used for risk prediction in patients RVO.
In our study population, the lymphocyte counts were lower in the RVO group than the control group, but this difference was not statistically significant. On the other hand, the platelet counts were considerably higher in patients with RVO. However, the statistical significance of PLR was more meaningful than platelet count only. Although p values for both platelet count and PLR were significant in predicting RVO in the ROC curve, the AUC of the PLR was greater than the platelet count (AUC = 0.726 and 0.688, respectively). Therefore, PLR was emphasized in our study.
Given the role of both increased thrombogenity and inflammation in the pathogenesis of RVO, as a marker containing both platelet activity (platelet) and inflammation (lymphocyte), PLR can be proposed as a novel potentially useful tool in RVO patients.
Study limitations
This study has a number of limitations, the main limitations including its retrospective nature and the relatively small sample size, because only the participants who had CBC variables pending at the early period of the disease were admitted, and rigorous exclusion criteria were applied. Another limitation is that we could not evaluate the body mass indices. Varieties in body mass indices may have influenced the blood platelet data. Finally, given that studies have shown that platelet volume indices are increased in patients with arterial hypertension compared to those without the disease,26,27 the presence of hypertension on both groups may affect the CBC parameters. However, there was no significant difference in the proportion of individuals with hypertension between RVO and control groups, so we assume that this did not affect the outcome of the study. Finally, our results need to be verified with further prospective clinical trial in a larger cohort.
Conclusion
This study demonstrated that elevated PLR values were linked to RVO. Therefore, PLR may be a valuable marker for RVO. To understand the value of this marker in the predicting and prognosis of RVO, further prospective studies should be conducted.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Bengi Ece Kurtul  https://orcid.org/0000-0001-5194-8772
https://orcid.org/0000-0001-5194-8772
Contributor Information
Bengi Ece Kurtul, Department of Ophthalmology, Tayfur Ata Sökmen Faculty of Medicine, Hatay Mustafa Kemal University, 31060, Alahan, Hatay, Turkey.
Ayșe İdil Çakmak, Department of Ophthalmology, Tayfur Ata Sökmen Faculty of Medicine, Hatay Mustafa Kemal University, Hatay, Turkey.
Ahmet Elbeyli, Department of Ophthalmology, Tayfur Ata Sökmen Faculty of Medicine, Hatay Mustafa Kemal University, Hatay, Turkey.
Deniz Özarslan Özcan, Department of Ophthalmology, Tayfur Ata Sökmen Faculty of Medicine, Hatay Mustafa Kemal University, Hatay, Turkey.
Sait Coșkun Özcan, Department of Ophthalmology, Tayfur Ata Sökmen Faculty of Medicine, Hatay Mustafa Kemal University, Hatay, Turkey.
Veysel Cankurtaran, Department of Ophthalmology, Tayfur Ata Sökmen Faculty of Medicine, Hatay Mustafa Kemal University, Hatay, Turkey.
References
- 1. Koizumi H, Ferrara DC, Bruè C, et al. Central retinal vein occlusion case-control study. Am J Ophthalmol 2007; 144: 858–863. [DOI] [PubMed] [Google Scholar]
- 2. Wong TY, Scott IU. Retinal vein occlusion. N Engl J Med 2010; 363: 2135–2144. [DOI] [PubMed] [Google Scholar]
- 3. Turello M, Pasca S, Daminato R, et al. Retinal vein occlusion: evaluation of “classic” and “emerging” risk factors and treatment. J Thromb Thrombolysis 2010; 29: 459–464. [DOI] [PubMed] [Google Scholar]
- 4. Battaglia Parodi M, Saviano S, Ravalico G. Grid laser treatment in macular branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 1999; 237: 1024–1027. [DOI] [PubMed] [Google Scholar]
- 5. Bandello F, Augustin A, Tufail A, et al. A 12-month, multicenter, parallel group comparison of dexamethasone intravitreal implant versus ranibizumab in branch retinal vein occlusion. Eur J Ophthalmol 2018; 28: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitry D, Bunce C, Charteris D. Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion. Cochrane Database Syst Rev 2013; 1: CD009510. [DOI] [PubMed] [Google Scholar]
- 7. Thach AB, Yau L, Hoang C, et al. Time to clinically significant visual acuity gains after ranibizumab treatment for retinal vein occlusion: BRAVO and CRUISE trials. Ophthalmology 2014; 121: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 8. Leoncini G, Bruzzese D, Signorello MG, et al. Platelet activation by collagen is increased in retinal vein occlusion. Thromb Haemost 2007; 97: 218–227. [PubMed] [Google Scholar]
- 9. Ohara K, Okubo A, Sasaki H, et al. Branch retinal vein occlusion in a child with ocular sarcoidosis. Am J Ophthalmol 1995; 119: 806–807. [DOI] [PubMed] [Google Scholar]
- 10. El Fekih L, Khaldi N, Hmaied W, et al. Retinal venous occlusion in Behcet’s disease. Rev Med Interne 2007; 28: 742–745. [DOI] [PubMed] [Google Scholar]
- 11. Kurtul A, Yarlioglues M, Murat SN, et al. Usefulness of the platelet-to-lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Am J Cardiol 2014; 114: 342–347. [DOI] [PubMed] [Google Scholar]
- 12. Kurtul A, Murat SN, Yarlioglues M, et al. Association of platelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes. Am J Cardiol 2014; 114: 972–978. [DOI] [PubMed] [Google Scholar]
- 13. Demircelik MB, Kurtul A, Ocek H, et al. Association between platelet-to-lymphocyte ratio and contrast-induced nephropathy in patients undergoing percutaneous coronary intervention for acute coronary syndrome. Cardiorenal Med 2015; 5: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ozgonul C, Sertoglu E, Mumcuoglu T, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as novel biomarkers of primary open-angle glaucoma. J Glaucoma 2016; 25: e815–e820. [DOI] [PubMed] [Google Scholar]
- 15. Celik T. Assessment of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with dry eye disease. Ocul Immunol Inflamm 2018; 26: 1219–1222. [DOI] [PubMed] [Google Scholar]
- 16. Ozgonul C, Sertoglu E, Ayyildiz O, et al. Novel biomarkers for patients with idiopathic acute anterior uveitis: neutrophil to lymphocyte ratio and platelet to lymphocyte ratio. Int J Ophthalmol 2017; 10: 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sahin A, Sahin M, Yüksel H, et al. The mean platelet volume in patients with retinal vein occlusion. J Ophthalmol 2013; 2013: 236371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Onder HI, Kilic AC, Kaya M, et al. Relation between platelet indices and branch retinal vein occlusion in hypertensive patients. Indian J Ophthalmol 2013; 61: 160–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yilmaz T, Yilmaz A. Altered platelet morphological parameters in patients with retinal vein occlusion. Eur Rev Med Pharmacol Sci 2016; 20: 1934–1939. [PubMed] [Google Scholar]
- 20. Beyazyıldız E, Çıtırık M, Şimşek M, et al. Branch retinal vein occlusion associated with platelet activation. Turk J Med Sci 2019; 49: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ornek N, Ogurel T, Ornek K, et al. Mean platelet volume in retinal vein occlusion. Eur Rev Med Pharmacol Sci 2014; 18: 2778–2782. [PubMed] [Google Scholar]
- 22. Kumral ET, Yenerel NM, Ercalik NY, et al. Neutrophil/lymphocyte ratio and mean platelet volume in branch retinal vein occlusion. Saudi J Ophthalmol 2016; 30: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dursun A, Ozturk S, Yucel H, et al. Association of neutrophil/lymphocyte ratio and retinal vein occlusion. Eur J Ophthalmol 2015; 25: 343–346. [DOI] [PubMed] [Google Scholar]
- 24. Browning DJ. Retinal vein occlusions: evidence-based management (Chapter 2). New York: Springer, 2012, pp. 33–37. [Google Scholar]
- 25. Kuhli-Hattenbach C, Hellstern P, Kohnen T, et al. Platelet activation by ADP is increased in selected patients with anterior ischemic optic neuropathy or retinal vein occlusion. Platelets 2017; 28: 720–723. [DOI] [PubMed] [Google Scholar]
- 26. Coban E, Yazicioglu G, Berkant Avci A, et al. The mean platelet volume in patients with essential and white coat hypertension. Platelets 2005; 16: 435–438. [DOI] [PubMed] [Google Scholar]
- 27. Boos CJ, Beevers GD, Lip GY. Assessment of platelet activation indices using the ADVIATM 120 amongst “high-risk” patients with hypertension. Ann Med 2007; 39: 72–78. [DOI] [PubMed] [Google Scholar]


