Abstract
Sleep has a regulatory role in maintaining metabolic homeostasis and cellular functions. Inadequate sleep time and sleep disorders have become more prevalent in the modern lifestyle. Fragmentation of sleep pattern alters critical intracellular second messengers and neurotransmitters which have key functions in brain development and behavioral functions. Tryptophan metabolism has also been found to get altered in SD and it is linked to various neurodegenerative diseases. The kynurenine pathway is a major regulator of the immune response. Adequate sleep alleviates neuroinflammation and facilitates the cellular clearance of metabolic toxins produced within the brain, while sleep deprivation activates the enzymatic degradation of tryptophan via the kynurenine pathway, which results in an increased accumulation of neurotoxic metabolites. SD causes increased production and accumulation of kynurenic acid in various regions of the brain. Higher levels of kynurenic acid have been found to trigger apoptosis, leads to cognitive decline, and inhibit neurogenesis. This review aims to link the impact of sleep deprivation on tryptophan metabolism and associated complication in the brain.
Keywords: Kynurenine pathway, sleep deprivation, Kynurenic-acid, brain
Introduction
Sleep is an essential physiological process required for the survival and well-being. However, to date, the biological functions of sleep is understood fully are. Humans spend about one third of their life in sleeping.1 Sleep plays key roles in maintaining cellular homeostasis, energy conservation, metabolic waste clearance, and regulating immune functions.2 Quality sleep improves cognitive performance, vigilance, and psychological conditions.3 In humans, sleep has 2 phases: non-rapid eye movement (NREM); and rapid eye movement (REM). NREM sleep consists of: stage 1 (lightest phase of sleep and lasts for 1-5 minutes); stage 2 (characterized by sleep spindles and single long delta waves); stage 3 and 4 (Slow-Wave Sleep).4 NREM sleep is followed by REM sleep, which is distinguished by irregular eye movements, decline of muscle tone, tendency to dream and transmission of low-voltage brain waves, versus the non-REM phase.5 By limiting neuronal oxidative damage, apoptosis, alterations in neural and cytoskeleton proteins, both NREM and REM sleep types are neuroprotective.6 NREM disruption is found to contribute to hippocampal dependent memory decline.7 However, because of the lifestyle modifications and night shift work schedules, the prevalence of REM sleep disruption is higher than NREM,8,9 as a result it has been linked to early ageing and in the progression of most of the neurodegenerative diseases (Figure 1).
Figure 1.
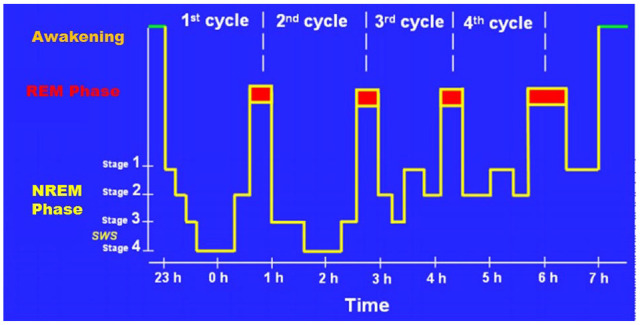
Stages of sleep. Sleep is composed of 2 phases: Rapid Eye Movement sleep (REM), and the Non-REM sleep (NREM). The NREM phase consists of 4 stages: Stage 1, which is transition from being awake to falling asleep; Stage 2, the period of light sleep during which the eyes movements stop, Stages 3 and 4, which are also called Slow Wave Sleep (SWS) (Reproduced with permission from Bernard10).
Sleep disorders have become a global and increasing health concern. Millions of people worldwide suffer from sleep deprivation, sleep apnea and insomnia on daily basis.11,12 The prevalence of insufficient sleep time and sleep deprivation (SD) has increased.13 SD is frequently experienced because of medical conditions, sleep disorders, work pressure, social and domestic responsibilities and lifestyle (eg, shift work, prolonged work hours, stress, and social media).14 Inadequate sleep or fragmented sleep alters the biochemical and physiological functions and has been strongly linked to neurodegenerative,15 metabolic,16 cardiovascular,17 and autoimmune diseases.18 REM SD has deleterious effects on general health. While it affects most of the organs, it has a major impact on various regions of brain like hippocampus, cortex, striatum, hypothalamus, etc and affect their functions.15 REM SD has a major impact on brain development, cognitive functions, and long term potentiation.19,20 This has been confirmed by several studies that sleep deprivation induces apoptosis and oxidative stress in different regions of rat brain.21 Oxidative stress is known to be associated with the development of neurodegenerative diseases as the brain is more vulnerable to reactive oxygen species (ROS) due to the high consumption of oxygen and lesser antioxidants defence.22 While chronic sleep deprivation in young, healthy volunteers increases appetite and energy consumption, activates immune system and sympathetic tone; it decreases parasympathetic tone.23,24 Increased blood pressure, cortisol levels, and blood glucose levels are reported to be associated with chronic sleep restricted individuals.25,26 Sleep restores the brain energy during active waking27 and clears the metabolic waste from the central nervous system by regulating glymphatic system.28 Preclinical and clinical studies have shown that SD alters glymphatic clearance and results in the deposition of toxins like amyloid β (Aβ), α-synuclein and other aberrant proteins in brain.29-34 Interestingly, chronic SD also disrupts blood brain barrier cohesion and alters its permeability.35 The combination of increased blood-brain barrier permeability and reduced glymphatic clearance due to SD, predisposes neurodegenerative processes.35,36 Increased metabolic waste has been attributed to the impairment in brain function and development of diseases.37-40 Thus, sleep disorder or SD impairs biochemical and behavioral function in brain and evolve as risk factor for neurological disorders
Sleep Deprivation, Tryptophan, and Kynurenine Pathway
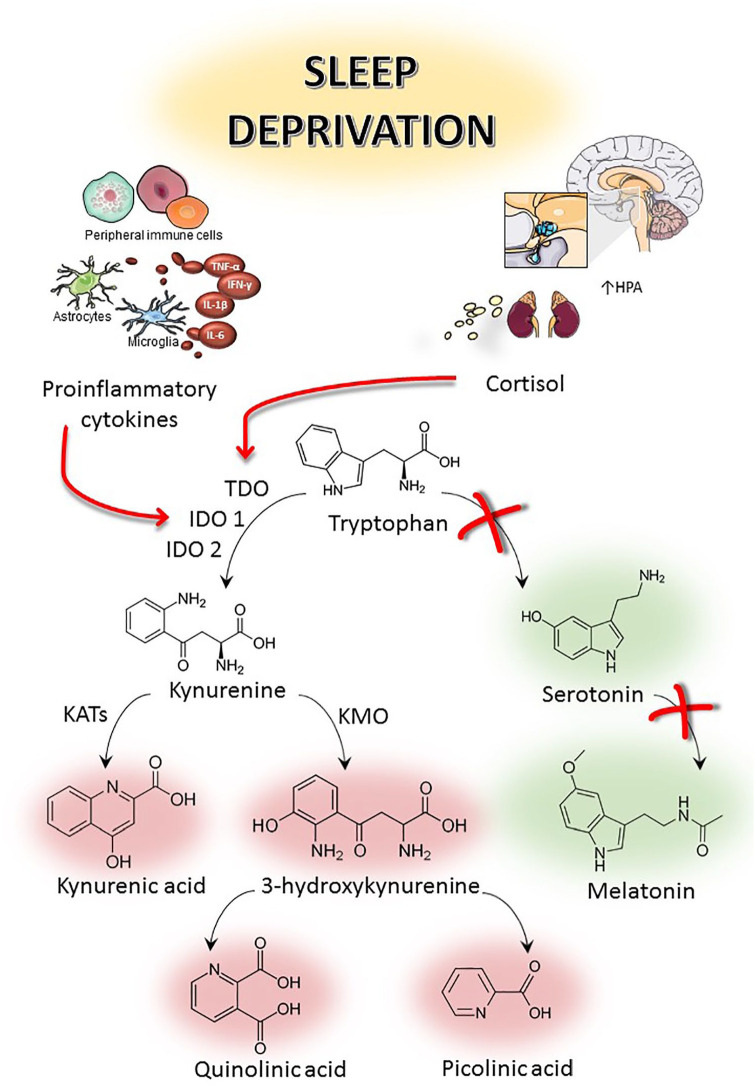
The essential amino acid tryptophan (TRP) is catabolised via 2 distinct biochemical pathways: (1) the serotonin pathway (5%) and (2) the kynurenine pathway (KP) (the majority, 95%).41 The kynurenine pathway is a major regulator of the immune response.42 Tryptophan is converted to kynurenine by 1 of 3 rate-limiting enzymes: indoleamine-2,3-dioxygenase-1 (IDO1), or indoleamine-2,3-di-oxygenase-2 like protein (IDO2), or tryptophan 2,3-dioxygenase (TDO). TDO is highly expressed in the liver and is activated in response to corticosteroid stress hormones, while IDO1 expression is activated by inflammatory stimuli such as Interferon gamma (IFN-γ), Tumor necrosis factor alpha (TNF-α), amyloid beta, etc.43 Sleep is important for maintaining inflammatory homeostatic functions and its loss is linked to the alterations in the immune response. Inflammation activates the kynurenine pathway, leading to increased production of 3-hydroxykynurenine (3HK) and quinolinic acid (QUIN), major neurotoxic metabolites. Under physiological conditions, 40% of the kynurenine is produced within the brain, and 60% is taken up from the periphery through the blood brain barrier.44 SD triggers the activation of TDO through the increase of the stress hormone, corticosterone, via hypothalamic-pituitary-adrenal (HPA) axis and IDO1 through inflammatory signalling (TNF-α, IFN-γ) leading to the catabolism of tryptophan.19,45,46 This increased production of kynurenine metabolites alters various neurotransmitter systems within the cortical and striatal regions.47-49 Kynurenine metabolites reduce dopamine levels in substantia nigra and in the striatum,48,50 acetylcholine in the cortex,51,52 and gamma aminobutyric acid (GABA) in rat striatum and cortex of rat brains.47,53 Dysregulation of kynurenine metabolism associated with SD is known to impact cognitive function and to promote the development and progression of neurodegeneration.41,54,55 In rat model of chronic sleep deprivation, tryptophan (TRP) and kynurenine (KYN) are increased in plasma.56,57 These peripheral tryptophan and kynurenine are taken up into the brain and used as extra substrate resulting in an overproduction of Kynurenic acid (KYNA) in the presynaptic neurons of hypothalamus, hippocampus, and cerebral cortex in SD rats.55 SD alters the immune response resulting in increased levels of inflammatory mediators such as Interleukin 6 (IL-6), TNF-α, IFN-γ, and C-reactive protein (CRP).58-62 Increase in inflammatory mediators activate IDO which metabolizes tryptophan to kynurenine (Figure 2). Increase in KYN/TRP ratio infers increased expression of IDO. KYN is further metabolized to 3-hydroxykynurenine (3HK) by kynurenine monooxygenase (KMO), an enzyme also found up regulated in inflammatory conditions.63 Further, this leads to an increased production of the excitotoxin, quinolinic acid, by macrophages and activated microglia.64-66 Imbalance between neurotoxic and neuroprotective and immune-modulator kynurenine pathway metabolites have been reported in many neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Multiple sclerosis (MS), Amyotrophic lateral sclerosis (ALS), Huntington disease (HD).43,67-71 Increased kynurenine levels or catabolic enzyme activity are not the only mechanisms that determine the formation of KYNA in the brain. Accumulation of pro-oxidant factors after sleep deprivation also promotes non-enzymatic degradation of kynurenine to KYNA, which is a stable metabolite, and the enzymes responsible for its breakdown are absent in human.72,73 This data indicates that sleep loss is associated with higher levels of inflammation and kynurenine metabolism is increasingly linked with inflammation and neurodegeneration.
Figure 2.
Relationship between sleep deprivation, hypothalamic-pituitary-adrenal (HPA) axis and tryptophan metabolism.
Abbreviations: IDO1, indoleamine-2,3-dioxygenase; IDO2, indoleamine-2,3-di-oxygenase-like protein; IFN-γ, interferon gamma; IL-1β, interleukin-1β; IL-6, interleukin-6; HPA, hypothalamic-pituitary-adrenal; KATs, kynurenine aminotransferases; KMO, kynurenine monooxygenase; TDO, tryptophan 2,3-dioxygenase; TNF-α, tumor necrosis factor-α.
Sleep Deprivation and Kynurenic Acid
Kynurenic acid is mainly produced by astrocytes within the central nervous system.74 Nanomolar levels of KYNA measured in the brain and cerebrospinal fluid of patients with schizophrenia.75,76 Animal studies have also shown an increased level of KYNA producing cognitive deficits,77 impairing auditory sensory gating78 and reduction in the dopaminergic and glutamatergic neurotransmission.48,79 KYNA has been shown to prevent the excitotoxicity induced neurodegeneration when administered at lower concentrations exogenously.71 However, the central nervous system (CNS) concentration required to produce neuroprotection is still not clear.80 Dysfunctional KMO activity may directly increase KYNA level and this is recorded in the cerebrospinal fluid (CSF)81 and post-mortem brains of patients with schizophrenia and bipolar disorder.76,82 The impact of a change in KYNA levels on cognitive function has been extensively studied as it blocks N-methyl-d-aspartate (NMDA) and affects human cortical development,83 desensitizes α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors84 and inhibits α7 nicotinic acetylcholine (α7nACh) receptors.85 Increased KYNA levels in the brain are associated with increased cognitive deficits, whereas decreased KYNA levels, have been reported to improve learning and memory because they impair both glutamatergic and cholinergic transmission.86,87 An increase in the levels of KYNA in the presynaptic area inhibits extra synaptic NMDA receptors, nicotinic acetylcholine receptors and AMPA receptor-mediated currents.44,84,88 This results in cognitive decline and long term potentiation in rodents.89 At nanomolar concentrations, KYNA disrupts hippocampus-based memory in rodents.86,87 A decrease in the brain KYNA by pharmacological interventions, ameliorated the cognitive functions in rats.87 KYNA alters acetylcholine and glutamate which are critically involved in learning and memory, sleep dependent synaptic plasticity, and regulation of circadian rhythm.90-93 Also, increased KYNA level restrains dopamine release into the synaptic cleft.48 Elevated levels of KYNA in the brainstem, cortex, and hippocampus have been found to impair contextual memory and disrupt the sleep wake cycle in rodents.94 Exogenous administration of KYNA was shown to impair rat performance in the behavioral paradigms like open-field, and Morris water-maze tests.86,95 Decline in glutamate levels have been associated with age related cognitive decline.96 Recently it was found that sleep deprivation impacts the hippocampal and cortical levels of KYNA in sex dependent manner.19 Increased levels of KYNA in hippocampus was found in male rats after sleep deprivation results in cognitive decline whereas female SD rats didn’t show any impairment in memory.19,94 This indicates that sex hormones also play an important role in the metabolism of tryptophan. Further, 96 hours SD in rats showed to suppresses neurogenesis in dentate gyrus region of hippocampus, which has been attributed to the accumulation of KYNA in the hippocampus.97,98 These evidences strengthen the correlation between increased levels of KYNA and sleep deprivation which might promote the progression of neurodegenerative diseases.
Sleep Deprivation and Serotonin
Tryptophan is the unique precursor for the biosynthesis of serotonin and melatonin which both play key regulating roles on the sleep-wake cycle. Melatonin and its analogs have been used for the treatment of various sleep disorders for many years.99-101 Serotonergic system is susceptible to SD. For example, SD for 1 night exerts an immediate but short-lived antidepressant effect which may be due to a transient increase in dopamine and serotonin metabolites.102,103 Levels of extracellular serotonin are highest during waking, lower in slow-wave sleep and lowest in REM sleep in all regions of brain including the hippocampus and cortex.103,104 This effect is, however, short lasting. SD stimulates the suprachiasmatic nucleus (SCN) and stimulates the release of serotonin in hamster.105 Toru et al reported an increase in the levels of 5-hydroxyindoleacetic acid (5-HIAA), the precursor of serotonin in the dorsal raphe nucleus and thalamus of 24 hour SD rats.106 Also, total SD increases the mean firing rate of serotonergic neurons in the dorsal raphe nucleus in cats.107 Chronic sleep restriction desensitizes Serotonin1A receptors,108 inactivation of Serotonin1A receptors alters synaptic plasticity and impairs learning and memory.109 In contrast, Bjorvatn et al, reported that 8 hours SD does not increase the levels of extracellular serotonin in hippocampus or cortex.110
Sleep Deprivation and Melatonin
Melatonin is the main hormone secreted by pineal gland having a critical role in circadian rhythm.111 Melatonin is synthesized from serotonin via acetylation in the presence of enzyme N-acetyl transferase (NAT). The activity of NAT at pineal gland is dependent on the noradrenergic input received from nervi conarii. Increase in the activity of nervi conarii initiated by the suprachiasmatic nucleus of the hypothalamus stimulates the synthesis of melatonin during night time.112 Day time melatonin levels are low. Optimal levels of melatonin is a powerful free-radical scavenger and combats oxidative stress.113 Melatonin improves sleep efficacy114 and enhances long term memory.115 Decrease in melatonin have been related to insomnia and sleep disorders in elderly people.116 Total sleep deprivation for 72 hours in mice decreases plasma melatonin level which results in increased expression of inflammatory cytokines and reduced antioxidants.117 Similarly, a decrease in melatonin production was observed in patients suffering from insomnia.116 Chronic decrease in melatonin was found in night time shift workers.118 In a clinical study, it was found that melatonin excretion increases in healthy males subjected to sleep deprivation by exposing them to white light.119 Exogenous administration of melatonin inhibits inflammatory mediators and increase the reduced glutathione levels in locus coeruleus nucleus in sleep deprived mice,120 reduces oxidative stress and improves memory consolidation in SD rats.121 Melatonin has also been found to be efficacious for sleep disorders in children with autism.122 Oral administration of melatonin has also shown to improve the sleep quality and increase the antioxidant capacity in patients with fibromyalgia.123 These data indicate the involvement of sleep deprivation in the metabolism of the melatonin which results in activation of inflammatory mediators and memory consolidation.
Sleep Deprivation and Hypothalamic-Pituitary-Adrenal Axis
Hypothalamic-pituitary-adrenal axis is responsible for regulation of the physiological response to stress.124,125 Stress stimuli induce the release of corticotropin-releasing hormone (CRH) from the paraventricular nucleus region of the hypothalamus. CRH triggers the release of adrenocorticotropic hormone (ACTH) from the pituitary gland. ACTH stimulates the adrenal cortex to release glucocorticoids (cortisol in humans, corticosterone in animals).126,127 Sleep plays a key role in modulating stress reactivity of the HPA axis.128 Preclinical and clinical studies have confirmed that the potential mechanism by which SD modulates the HPA axis is by increasing the levels of glucocorticoids.129-132 An increase in the levels of cortisol have been found in children with lower sleep efficiency133,134 and in healthy SD adults.130 Similarly, higher levels of glucocorticoids have been found in sleep deprived rodents and healthy young females.135-137 Higher levels of glucocorticoids in humans138 and animals139 during SD have an impact on KP metabolism.46 Increased corticosterone increases KP metabolism and leads to mass production of neurotoxic metabolites KYNA, 3HK and QUIN in brain and periphery.140,141 Stress influences KP metabolism by activation of the key enzyme TDO.142 Stress in pregnancy not only affects the development of the fetal brain but also increase the risk of offspring developing psychiatric disorders such as schizophrenia and depression143 which may be attributed to increase in the levels of KYNA.75 This sleep deprivation activates HPA axis and increased levels of the stress hormones play a crucial role in neurodegeneration by increasing the neurotoxic metabolites.
Conclusion
Sleep regulates the immune and endocrine systems, helps to encounter deleterious stimuli, improves central nervous system functioning and reduces the metabolic wastes. It has an essential role in learning and memory and synaptic plasticity formation. Sleep deprivation precipitates neurodegenerative diseases such as AD, PD, HD, and motor neuron diseases. Tryptophan is metabolized by majorly via kynurenine pathway. However inadequate sleep or sleep deprivation increases the expression of key enzymes such as TDO and IDO resulting in increased levels of KP metabolites such as KYNA, 3HK, and quinolinic acid which participate in the progression of neurodegenerative diseases. Exogenous administration of melatonin reduces the neuroinflammation and improves memory consolidation. Also, IDO1, and TDO enzymes can be potential targets in correcting sleep disorders and sleep deprivation induced neurodegeneration.
Acknowledgments
AB is supported by the IBRO-APRC Exchange Fellowships program, Macquarie University and Senior Research Fellowship from Indian Council of Medical Research (ICMR), Govt of India, New Delhi. GJG is supported by the National Health Medical Research Council (NHMRC), the Australian Research Council (ARC), the Hanbury Foundation, the Mason Foundation, and Macquarie University. This paper has been professionally edited by Red Fern Communications, Sydney Australia.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Contributions: AB wrote the first draft. ASP prepared the figures. VT, SBC and GJG revised the manuscript. All authors read the final version and approve it.
ORCID iD: Gilles J Guillemin  https://orcid.org/0000-0001-8105-4470
https://orcid.org/0000-0001-8105-4470
References
- 1. Hirshkowitz M, Whiton K, Steven M, et al. National sleep foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1:233-243. [DOI] [PubMed] [Google Scholar]
- 2. Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deak MC, Stickgold R. Sleep and cognition. Wiley Interdiscip Rev Cogn Sci. 2010;1:491-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Memar P, Faradji F. A novel multi-class EEG-based sleep stage classification system. IEEE Trans Neural Syst Rehabil Eng. 2018;26:84-95. [DOI] [PubMed] [Google Scholar]
- 5. McNamara P, Johnson P, McLaren D, Harris E, Beauharnais C, Auerbach S. REM and NREM sleep mentation. Int Rev Neurobiol. 2010;92:69-86. [DOI] [PubMed] [Google Scholar]
- 6. Ranjan A, Biswas S, Mallick BN. Cytomorphometric changes in the dorsal raphe neurons after rapid eye movement sleep deprivation are mediated by noradrenalin in rats. Behav Brain Funct. 2010;6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lucey BP, Bateman RJ. Amyloid-β diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging. 2014;35:S29-S34. [DOI] [PubMed] [Google Scholar]
- 8. Kang SH, Yoon IY, Lee SD, Han JW, Kim TH, Kim KW. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36:1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez CL, Jaimchariyatam N, Budur K. Rapid eye movement sleep behavior disorder: a review of the literature and update on current concepts. Chest. 2017;152:650-662. [DOI] [PubMed] [Google Scholar]
- 10. Bernard A. Effect of chronic sleep deprivation on skin status in healthy young women. In: ASMDA 2011, 14th Applied Stochastic Models and Data Analysis International Conference, June 2011, Rome, Italy. [Google Scholar]
- 11. Chattu VK, Manzar MD, Kumary S, Burman D, Spence DW, Pandi-Perumal SR. The Global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2018;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riemann D. Epidemiology of sleep disorders, sleep deprivation, dreaming and spindles in sleep. J Sleep Res. 2019;28:e12822. [DOI] [PubMed] [Google Scholar]
- 13. Ma B, Chen J, Mu Y, et al. Proteomic analysis of rat serum revealed the effects of chronic sleep deprivation on metabolic, cardiovascular and nervous system. PLoS One. 2018;13:e0199237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519-528. [PMC free article] [PubMed] [Google Scholar]
- 15. Pillai JA, Leverenz JB. Sleep and neurodegeneration: a critical appraisal. Chest. 2017;151:1375-1386. [DOI] [PubMed] [Google Scholar]
- 16. Micić DD, Šumarac-Dumanović M, Šušić V, Pejković D, Polovina S. Sleep and metabolic disorders. Glas Srp Akad Nauka Umet Odeljenje Med Nauka. 2011;52:5-25. [PubMed] [Google Scholar]
- 17. Yazdanpanah MH, Homayounfar R, Khademi A, Zarei F, Shahidi A, Farjam M. Short sleep is associated with higher prevalence and increased predicted risk of cardiovascular diseases in an Iranian population: fasa PERSIAN cohort study. Sci Rep. 2020;10:4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baratta AM, Buck SA, Buchla AD, et al. Sex differences in hippocampal memory and kynurenic acid formation following acute sleep deprivation in rats. Sci Rep. 2018;8:6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073-1076. [DOI] [PubMed] [Google Scholar]
- 21. Biswas S, Mishra P, Mallick BN. Increased apoptosis in rat brain after rapid eye movement sleep loss. Neuroscience. 2006;142:315-331. [DOI] [PubMed] [Google Scholar]
- 22. Kim GH, Kim JE, Rhie SJ, Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. 2015;24:325-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choudhary AK, Alam T, Dhanvijay AKD, Kishanrao SS. Sleep restriction progress to cardiac autonomic imbalance. Alex J Med. 2018;54:149-153. [Google Scholar]
- 24. Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. [DOI] [PubMed] [Google Scholar]
- 25. Buxton OM, Cain SW, O’Connor SP, et al. Metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choudhary AK, Dhanvijay AKD, Alam T, Kishanrao SS. Sleep restriction and its influence on blood pressure. Artery Res. 2017;19:42-48. [Google Scholar]
- 27. Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347-360. [DOI] [PubMed] [Google Scholar]
- 28. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fagotti J, Targa ADS, Rodrigues LS, et al. Chronic sleep restriction in the rotenone Parkinson’s disease model in rats reveals peripheral early-phase biomarkers. Sci Rep. 2019;9:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kang J-E, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shokri-Kojori E, Wang GJ, Wiers CE, et al. β-amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115:4483-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sprecher KE, Bendlin BB, Racine AM, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36:2568-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tabuchi M, Lone SR, Liu S, et al. Sleep interacts with aβ to modulate intrinsic neuronal excitability. Curr Biol. 2015;25:702-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gómez-González B, Hurtado-Alvarado G, Esqueda-León E, Santana-Miranda R, Rojas-Zamorano JÁ, Velázquez-Moctezuma J. REM sleep loss and recovery regulates blood-brain barrier function. Curr Neurovasc Res. 2013;10:197-207. [DOI] [PubMed] [Google Scholar]
- 36. Hurtado-Alvarado G, Domínguez-Salazar E, Pavon L, Velázquez-Moctezuma J, Gómez-González B. Blood-brain barrier disruption induced by chronic sleep loss: low-grade inflammation may be the link. J Immunol Res. 2016;2016:4576012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Askenasy JJ. Sleep in Parkinson’s disease. Acta Neurol Scand. 1993;87:167-170. [DOI] [PubMed] [Google Scholar]
- 38. Herzog–Krzywoszanska R, Krzywoszanski L. Sleep disorders in Huntington’s disease. Front Psychiatry. 2019;10:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ju YES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. 2014;10:115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ju YES, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leklem JE. Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am J Clin Nutr. 1971;24:659-672. [DOI] [PubMed] [Google Scholar]
- 42. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191-1193. [DOI] [PubMed] [Google Scholar]
- 43. Guillemin GJ, Williams KR, Smith DG, Smythe GA, Croitoru-Lamoury J, Brew BJ. Quinolinic acid in the pathogenesis of Alzheimer’s disease. Adv Exp Med Biol. 2003;527:167-176. [DOI] [PubMed] [Google Scholar]
- 44. Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;12:64-82. [DOI] [PubMed] [Google Scholar]
- 45. Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11:198-209. [DOI] [PubMed] [Google Scholar]
- 47. Beggiato S, Tanganelli S, Fuxe K, Antonelli T, Schwarcz R, Ferraro L. Endogenous kynurenic acid regulates extracellular GABA levels in the rat prefrontal cortex. Neuropharmacology. 2014;82:11-18. [DOI] [PubMed] [Google Scholar]
- 48. Rassoulpour A, Wu HQ, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762-765. [DOI] [PubMed] [Google Scholar]
- 49. Wu HQ, Pereira EFR, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci. 2010;40:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beskid M, Finkiewicz-Murawiejska L. Quinolinic acid: effects on brain catecholamine and c-AMP content during L-dopa and reserpine administration. Exp Toxicol Pathol. 1992;44:66-69. [DOI] [PubMed] [Google Scholar]
- 51. Metcalf RH, Riddell DL, Boegman RJ. Acetylcholine content and distribution in rat cortical synaptosomes after in vivo exposure to quinolinic acid. Neurosci Lett. 1990;108:219-224. [DOI] [PubMed] [Google Scholar]
- 52. Zmarowski A, Wu HQ, Brooks JM, et al. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci. 2009;29:529-538. [DOI] [PubMed] [Google Scholar]
- 53. Santamaria A, Rios C, Pérez P, et al. Quinolinic acid neurotoxicity: in vivo increased copper and manganese content in rat corpus striatum after quinolinate intrastriatal injection. Toxicol Lett. 1996;87:113-119. [DOI] [PubMed] [Google Scholar]
- 54. Yamashita M, Yamamoto T. Tryptophan circuit in fatigue: from blood to brain and cognition. Brain Res. 2017;1675:116-126. [DOI] [PubMed] [Google Scholar]
- 55. Yamashita M, Yamamoto T. Tryptophan and kynurenic acid may produce an amplified effect in central fatigue induced by chronic sleep disorder. Int J Tryptophan Res. 2014;7:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baratta AM, Kanyuch NR, Cole CA, Valafar H, Deslauriers J, Pocivavsek A. Acute sleep deprivation during pregnancy in rats: rapid elevation of placental and fetal inflammation and kynurenic acid. Neurobiol Stress. 2020;12:100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davies SK, Ang JE, Revell VL, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A. 2014;111:10761-10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cho HJ, Eisenberger NI, Olmstead R, Breen EC, Irwin MR. Preexisting mild sleep disturbance as a vulnerability factor for inflammation-induced depressed mood: a human experimental study. Transl Psychiatry. 2016;6:e750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678-683. [DOI] [PubMed] [Google Scholar]
- 61. Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165-170. [DOI] [PubMed] [Google Scholar]
- 62. Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119-2126. [DOI] [PubMed] [Google Scholar]
- 63. Walker AK, Budac DP, Bisulco S, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38:1609-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279:1356-1365. [DOI] [PubMed] [Google Scholar]
- 65. Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15-23. [DOI] [PubMed] [Google Scholar]
- 66. Saito K, Markey SP, Heyes MP. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992;51:25-39. [DOI] [PubMed] [Google Scholar]
- 67. da Silveira TL, Zamberlan DC, Arantes LP, et al. Quinolinic acid and glutamatergic neurodegeneration in Caenorhabditis elegans. Neurotoxicology. 2018;67:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Myint AM, Kim YK, Verkerk R, Scharpé S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98:143-151. [DOI] [PubMed] [Google Scholar]
- 69. Raison CL, Dantzer R, Kelley KW, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Szalardy L, Zadori D, Toldi J, Fulop F, Klivenyi P, Vecsei L. Manipulating kynurenic acid levels in the brain - on the edge between neuroprotection and cognitive dysfunction. Curr Top Med Chem. 2012;12:1797-1806. [PubMed] [Google Scholar]
- 71. Urenjak J, Obrenovitch TP. Neuroprotective potency of kynurenic acid against excitotoxicity. Neuroreport. 2000;11:1341-1344. [DOI] [PubMed] [Google Scholar]
- 72. Moroni F, Russi P, Lombardi G, Beni M, Carlà V. Presence of kynurenic acid in the mammalian brain. J Neurochem. 1988;51:177-180. [DOI] [PubMed] [Google Scholar]
- 73. Turski WA, Schwarcz R. On the disposition of intrahippocampally injected kynurenic acid in the rat. Exp Brain Res. 1988;71:563-567. [DOI] [PubMed] [Google Scholar]
- 74. Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:842-853. [DOI] [PubMed] [Google Scholar]
- 75. Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96-98. [DOI] [PubMed] [Google Scholar]
- 76. Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521-530. [DOI] [PubMed] [Google Scholar]
- 77. Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28:1454-1462. [DOI] [PubMed] [Google Scholar]
- 79. Konradsson-Geuken A, Wu HQ, Gash CR, et al. Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience. 2010;169:1848-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Salvati P, Ukmar G, Dho L, et al. Brain concentrations of kynurenic acid after a systemic neuroprotective dose in the gerbil model of global ischemia. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:741-752. [DOI] [PubMed] [Google Scholar]
- 81. Olsson SK, Samuelsson M, Saetre P, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J Psychiatry Neurosci. 2010;35:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sathyasaikumar KV, Stachowski EK, Wonodi I, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bagasrawala I, Zecevic N, Radonjić NV. N-methyl d-aspartate receptor antagonist kynurenic acid affects human cortical development. Front Neurosci. 2016;10:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Prescott C, Weeks AM, Staley KJ, Partin KM. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci Lett. 2006;402:108-112. [DOI] [PubMed] [Google Scholar]
- 85. Albuquerque EX, Schwarcz R. Kynurenic acid as an antagonist of α7 nicotinic acetylcholine receptors in the brain: facts and challenges. Biochem Pharmacol. 2013;85:1027-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Potter MC, Elmer GI, Bergeron R, et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35:1734-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Silva-Adaya D, Pérez-De La, Cruz V, Villeda-Hernández J, et al. Protective effect of L-kynurenine and probenecid on 6-hydroxydopamine-induced striatal toxicity in rats: implications of modulating kynurenate as a protective strategy. Neurotoxicol Teratol. 2011;:303-312. [DOI] [PubMed] [Google Scholar]
- 89. DeAngeli NE, Todd TP, Chang SE, Yeh HH, Yeh PW, Bucci DJ. Exposure to kynurenic acid during adolescence increases sign-tracking and impairs long-term potentiation in adulthood. Front Behav Neurosci. 2015;8:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Aton SJ, Seibt J, Dumoulin M, et al. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Queiroz CM, Tiba PA, Moreira KM, et al. Sleep pattern and learning in knockdown mice with reduced cholinergic neurotransmission. Braz J Med Biol Res. 2013;46:844-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci. 2006;27:141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xu M, Chung S, Zhang S, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18:1641-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pocivavsek A, Baratta AM, Mong JA, Viechweg SS. Acute kynurenine challenge disrupts sleep-wake architecture and impairs contextual memory in adult rats. Sleep. 2017;40:zsx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chess AC, Bucci DJ. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav Brain Res. 2006;170:326-332. [DOI] [PubMed] [Google Scholar]
- 96. Zahr NM, Mayer D, Pfefferbaum A, Sullivan EV. Low striatal glutamate levels underlie cognitive decline in the elderly: evidence from in vivo molecular spectroscopy. Cereb Cortex N Y. 2008;18:2241-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Guzmán-marín R, Suntsova N, Stewart DR, Gong H, Szymusiak R, McGinty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol. 2003;549:563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kanai M, Funakoshi H, Takahashi H, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Quera-Salva MA, Claustrat B. Melatonin: physiological and pharmacological aspects related to sleep: the interest of a prolonged-release formulation (Circadin®) in insomnia. Encephale. 2018;44:548-557. [DOI] [PubMed] [Google Scholar]
- 100. Xie Z, Chen F, Li WA, et al. A review of sleep disorders and melatonin. Neurol Res. 2017;39:559-565. [DOI] [PubMed] [Google Scholar]
- 101. Lerchl A, Reiter RJ. Treatment of sleep disorders with melatonin. BMJ. 2012;345:e6968. [DOI] [PubMed] [Google Scholar]
- 102. Boland EM, Rao H, Dinges DF, et al. Meta-analysis of the antidepressant effects of acute sleep deprivation. J Clin Psychiatry. 2017;78:e1020-e1034. [DOI] [PubMed] [Google Scholar]
- 103. Zant JC, Leenaars CHC, Kostin A, Van Someren EJW, Porkka-Heiskanen T. Increases in extracellular serotonin and dopamine metabolite levels in the basal forebrain during sleep deprivation. Brain Res. 2011;1399:40-48. [DOI] [PubMed] [Google Scholar]
- 104. Portas CM, Bjorvatn B, Ursin R. Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies. Prog Neurobiol. 2000;60:13-35. [DOI] [PubMed] [Google Scholar]
- 105. Grossman GH, Mistlberger RE, Antle MC, Ehlen JC, Glass JD. Sleep deprivation stimulates serotonin release in the suprachiasmatic nucleus. Neuroreport. 2000;11:1929-1932. [DOI] [PubMed] [Google Scholar]
- 106. Toru M, Mitsushio H, Mataga N, Takashima M, Arito H. Increased brain serotonin metabolism during rebound sleep in sleep-deprived rats. Pharmacol Biochem Behav. 1984;20:757-761. [DOI] [PubMed] [Google Scholar]
- 107. Gardner JP, Fornal CA, Jacobs BL. Effects of sleep deprivation on serotonergic neuronal activity in the dorsal raphe nucleus of the freely moving cat. Neuropsychopharmacology. 1997;17:72-81. [DOI] [PubMed] [Google Scholar]
- 108. Roman V, Walstra I, Luiten PGM, Meerlo P. Too little sleep gradually desensitizes the serotonin 1A receptor system. Sleep. 2005;28:1505-1510. [PubMed] [Google Scholar]
- 109. Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci U S A. 2000;97:14731-14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bjorvatn B, Grønli J, Hamre F, et al. Effects of sleep deprivation on extracellular serotonin in hippocampus and frontal cortex of the rat. Neuroscience. 2002;113:323-330. [DOI] [PubMed] [Google Scholar]
- 111. Bumb JM, Schwarz E, Enning F, et al. Sleep deprivation in humans: effects on melatonin in cerebrospinal fluid and serum. Sleep Biol Rhythms 2014;12:69-72. [Google Scholar]
- 112. Lumsden SC, Clarkson AN, Cakmak YO. Neuromodulation of the pineal gland via electrical stimulation of its sympathetic innervation pathway. Front Neurosci. 2020;14:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Murali R, Thanikaivelan P, Cheirmadurai K. Melatonin in functionalized biomimetic constructs promotes rapid tissue regeneration in Wistar albino rats. J Mater Chem B. 2016;4:5850-5862. [DOI] [PubMed] [Google Scholar]
- 114. Paredes SD, Marchena AM, Bejarano I, et al. Melatonin and tryptophan affect the activity–rest rhythm, core and peripheral temperatures, and interleukin levels in the ringdove: changes with age. J Gerontol A Biol Sci Med Sci. 2009;64:340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ran D, Xie B, Gan Z, Sun X, Gu H, Yang J. Melatonin attenuates hLRRK2-induced long-term memory deficit in a Drosophila model of Parkinson’s disease. Biomed Rep. 2018;9:221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Riemann D, Klein T, Rodenbeck A, et al. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res. 2002;113:17-27. [DOI] [PubMed] [Google Scholar]
- 117. Gao T, Wang Z, Dong Y, et al. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J Pineal Res. 2019;67:e12574. [DOI] [PubMed] [Google Scholar]
- 118. Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol. 2001;171:557-564. [DOI] [PubMed] [Google Scholar]
- 119. Åkerstedt T, Fröberg JE, Friberg Y, Wetterberg L. Melatonin excretion, body temperature and subjective arousal during 64 hours of sleep deprivation. Psychoneuroendocrinology. 1979;4:219-225. [DOI] [PubMed] [Google Scholar]
- 120. Jameie SB, Mesgar S, Aliaghaei A, et al. Neuroprotective effect of exogenous melatonin on the noradrenergic neurons of adult male rats’ locus coeruleus nucleus following REM sleep deprivation. J Chem Neuroanat. 2019;100:101656. [DOI] [PubMed] [Google Scholar]
- 121. Zhang L, Zhang HQ, Liang XY, Zhang HF, Zhang T, Liu FE. Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: role of oxidative stress, BDNF and CaMKII. Behav Brain Res. 2013;256:72-81. [DOI] [PubMed] [Google Scholar]
- 122. Cuomo BM, Vaz S, Lee EAL, Thompson C, Rogerson JM, Falkmer T. Effectiveness of sleep-based interventions for children with autism spectrum disorder: a meta-synthesis. Pharmacotherapy. 2017;37:555-578. [DOI] [PubMed] [Google Scholar]
- 123. Castaño MY, Garrido M, Delgado-Adámez J, Martillanes S, Gómez M, Rodríguez AB. Oral melatonin administration improves the objective and subjective sleep quality, increases 6-sulfatoxymelatonin levels and total antioxidant capacity in patients with fibromyalgia. J Appl Biomed. 2018;16:186-191. [Google Scholar]
- 124. Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev. 1992;16:115-130. [DOI] [PubMed] [Google Scholar]
- 125. Vargas I, Lopez-Duran N. Investigating the effect of acute sleep deprivation on hypothalamic-pituitary-adrenal-axis response to a psychosocial stressor. Psychoneuroendocrinology. 2017;79:1-8. [DOI] [PubMed] [Google Scholar]
- 126. Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865-871. [DOI] [PubMed] [Google Scholar]
- 127. van Dalfsen JH, Markus CR. The influence of sleep on human hypothalamic–pituitary–adrenal (HPA) axis reactivity: a systematic review. Sleep Med Rev. 2018;39:187-194. [DOI] [PubMed] [Google Scholar]
- 128. Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197-210. [DOI] [PubMed] [Google Scholar]
- 129. Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int J Endocrinol. 2010;2010:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Minkel J, Moreta M, Muto J, et al. Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychol. 2014;33:1430-1434. [DOI] [PubMed] [Google Scholar]
- 131. Suchecki D, Tiba PA, Tufik S. Hormonal and behavioural responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol. 2002;14:549-554. [DOI] [PubMed] [Google Scholar]
- 132. von Treuer K, Norman TR, Armstrong SM. Overnight human plasma melatonin, cortisol, prolactin, TSH, under conditions of normal sleep, sleep deprivation, and sleep recovery. J Pineal Res. 1996;20:7-14. [DOI] [PubMed] [Google Scholar]
- 133. Mrug S, Tyson A, Turan B, Granger DA. Sleep problems predict cortisol reactivity to stress in urban adolescents. Physiol Behav. 2016;155:95-101. [DOI] [PubMed] [Google Scholar]
- 134. Räikkönen K, Matthews KA, Pesonen AK, et al. Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. J Clin Endocrinol Metab. 2010;95:2254-2261. [DOI] [PubMed] [Google Scholar]
- 135. Honma A, Revell VL, Gunn PJ, et al. Effect of acute total sleep deprivation on plasma melatonin, cortisol and metabolite rhythms in females. Eur J Neurosci. 2019; 51:366-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Olayaki LA, Sulaiman SO, Anoba NB. Vitamin C prevents sleep deprivation-induced elevation in cortisol and lipid peroxidation in the rat plasma. Niger J Physiol Sci. 2015;30:5-9. [PubMed] [Google Scholar]
- 137. Victor OO, Odigie MO, Aigbiremolen AA, et al. Hypothalamo-pituitary-gonadal changes in sleep deprivation induced with kolaviron in male wistar rats. Asian J Res Med Pharm Sci. 2017;1-11. [Google Scholar]
- 138. Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865-870. [PubMed] [Google Scholar]
- 139. Sgoifo A, Buwalda B, Roos M, Costoli T, Merati G, Meerlo P. Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology. 2006;31:197-208. [DOI] [PubMed] [Google Scholar]
- 140. Gibney SM, Fagan EM, Waldron AM, O’Byrne J, Connor TJ, Harkin A. Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int J Neuropsychopharmacol. 2014;17:917-928. [DOI] [PubMed] [Google Scholar]
- 141. Miura H, Ozaki N, Shirokawa T, Isobe K. Changes in brain tryptophan metabolism elicited by ageing, social environment, and psychological stress in mice. Stress Amst Neth. 2008;11:160-169. [DOI] [PubMed] [Google Scholar]
- 142. Notarangelo FM, Schwarcz R. Restraint stress during pregnancy rapidly raises kynurenic acid levels in mouse placenta and fetal brain. Dev Neurosci. 2016;38:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Babenko O, Kovalchuk I, Metz GAS. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48:70-91. [DOI] [PubMed] [Google Scholar]