Abstract
Vincristine-related secondary hypertension is rare. This study reports two children who were treated with vincristine for acute lymphoblastic leukemia (ALL) and posaconazole for fungal infections who experienced vincristine-related secondary hypertension. Blood pressure normalized in both children after halting the drugs and providing antihypertensive treatment. Thus, posaconazole can interact with vincristine and induce secondary hypertension in children with ALL. As an adverse event, this interaction is a rare occurrence.
Keywords: Vincristine, posaconazole, secondary hypertension, acute lymphoblastic leukemia, children, adverse event
Introduction
Vincristine is an important chemotherapeutic drug in the treatment of acute lymphoblastic leukemia (ALL) in children. Among its many side effects, secondary hypertension is possible but rare.1 A variety of drugs interact with vincristine to aggravate its side effects.2,3 This paper reports the cases of two children who received vincristine for ALL and posaconazole for fungal infections who developed vincristine-related secondary hypertension.
Case report
Case 1
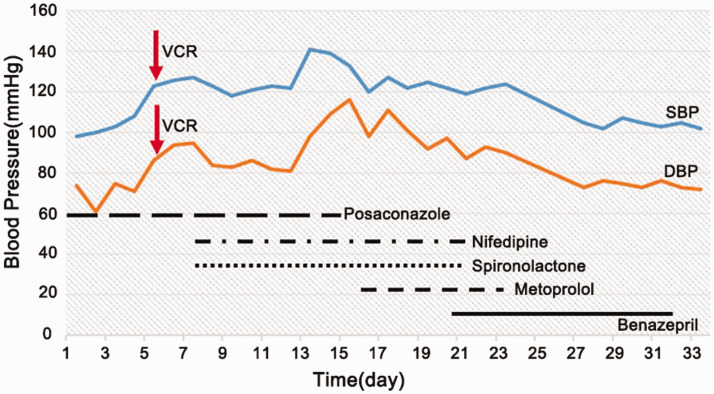
Case 1 was a 12-year-old girl who was diagnosed with ALL (B, IR) and treated with the CCCG-ALL-2015 chemotherapy regimen. She received VDLD re-induction chemotherapy [dexamethasone 8 mg/m2/day (days 1 to 7 and 15 to 21) + vincristine 1.5 mg/m2 (days 1, 8, and 15) + daunomycin 25 mg/m2 (day 1) + pegaspargase 2000 U/m2 (day 3) + intrathecal therapy (methotrexate 12.5 mg + cytarabine 35 mg + dexamethasone 5.0 mg; day 1)]. The patient had taken posaconazole orally for 4 months for pulmonary fungal infection. On the first day after the third vincristine dose, her blood pressure increased, peaking at 141/98 mmHg 2 weeks later. On the next day, she gradually developed abdominal distention, abdominal pain, dyschezia, dysuria, drowsiness, hand and foot numbness, hypodynamia of the extremities and myasthenia of both legs and experienced one generalized tetanic convulsion without fever. The patient’s biochemistry data were as follows: blood sodium, 117 mmol/L; blood potassium, 4.72 mmol/L; blood chlorine, 77 mmol/L; plasma osmolality, 260 mosm/L; urine sodium, 130 mmol/L; and serum albumin, 26.1 g/L. Blood urea nitrogen, creatinine, thyroid-stimulating hormone, free triiodothyronine, free thyroxine, and cortisol levels were all normal. Plain abdominal X-ray in the erect and supine positions revealed small intestine obstruction. Abdominal computed tomography illustrated that part of the small intestine was pneumatized and dilated, with multiple contents in the entire intestinal tract. No obvious abnormality was found via head magnetic resonance imaging. The patient was diagnosed with secondary hypertension, syndrome of inappropriate antidiuretic hormone secretion (SIADH), peripheral neuropathy, and paralytic ileus. Vincristine and posaconazole were stopped immediately; fluids were limited; hypertonic sodium was provided; diuresis was performed; and spironolactone, nifedipine, metoprolol, and benazepril were given successively as antihypertensive treatment. After 2 days of the aforementioned treatment, the child began to discharge soft yellow stool. After 4 days, her blood sodium level reached 130 mmol/L. After 17 days, extremity numbness, sensation of the limbs, and muscle tension of both legs had resolved. The hypertension in this patient was finally diagnosed with vincristine-related secondary hypertension. After 21 days, the patient’s blood pressure had returned to normal (Figure 1).
Figure 1.
Changes in blood pressure in Case #1.
VCR, vincristine; SBP, systolic blood pressure; DBP: diastolic blood pressure.
Case 2
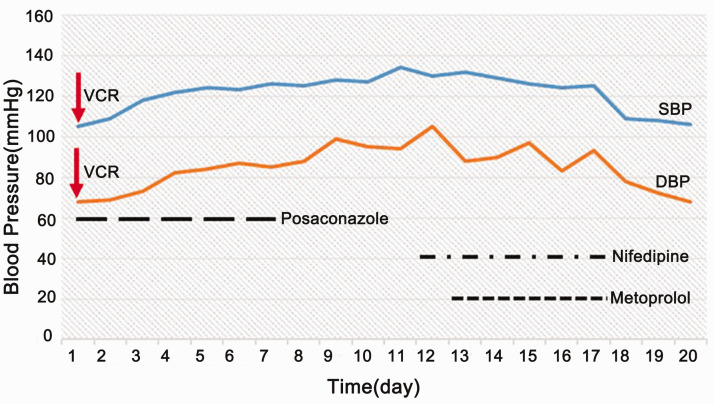
Case 2 was a 9-year-old boy who was diagnosed with recurrent ALL (B, IR) and treated with the CCCG Relapsed ALL chemotherapy regimen. The admission reported in this study was for induction remission chemotherapy [dexamethasone 6 mg/m2/day (days −2 to 0) + dexamethasone 20 mg/m2/day (days 1 to 5 and 15 to 19) + vincristine 1.5 mg/m2 (days 1, 8, and 15) + mitomycin 10 mg/m2 (days 1 to 2) + pegaspargase 2000 U/m2 (days 5 and 22) + intrathecal therapy (methotrexate 12.5 mg + cytarabine 35 mg + dexamethasone 5.0 mg; days 1, 8, and 22)]. Because of repeated diarrhea and a positive stool fungus culture during chemotherapy, the child was administered posaconazole for 9 days. The third vincristine dose was administered as scheduled. After 2 days, the patient’s blood pressure began to rise and peaked at 134/94 mmHg. After 6 days, the patient had abdominal distention and dyschezia. Vincristine-related secondary hypertension and paralytic ileus were considered. Vincristine and posaconazole were stopped, and nifedipine and metoprolol were given successively for antihypertensive treatment. After 2 weeks, the symptoms of abdominal distention and dyschezia had dissipated, and his blood pressure had returned to normal (Figure 2).
Figure 2.
Changes in blood pressure in Case #2.
VCR, vincristine; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Discussion
The anti-tumor activity of vincristine is related to its antimicrotubule activity during mitosis in tumor cells, but vincristine also interferes with the microtubule activity of the axons and causes neurotoxicity, including autonomic, cranial, peripheral, and other neurotoxicities.4 Posaconazole is a potent antifungal agent that can overcome resistance to fluconazole and voriconazole.5
Azole antifungal drugs can slow the metabolism of vincristine through CYP3A4 inhibition in the liver and its elimination through P-glycoprotein (P-gp). Therefore, when azole antifungal drugs are used in combination with vincristine, they often lead to an elevated blood concentration of vincristine, thus enhancing its toxicity.3,6–9 Differing from voriconazole and itraconazole, posaconazole is metabolized through the uridine diphosphate glucosyltransferase 1A4 pathway in liver microsomes instead of CYP3A4. Therefore, many physicians believe that posaconazole has lower risks of drug interactions than other azole drugs. Nevertheless, pharmacokinetic experiments of midazolam confirmed that posaconazole is also a potent inhibitor of CYP3A410 and that posaconazole can aggravate the side effects of vincristine by inhibiting the CYP3A4 pathway and P-gp–mediated vincristine elimination.8 Moriyama et al.3 reported that the median onset time of side effects caused by an interaction between posaconazole and vincristine was 13.5 days after starting posaconazole and that the median number of vincristine doses before the appearance of adverse events was three. In the present case report, both children with ALL had received several doses of vincristine without obvious side effects. However, after starting posaconazole to treat fungal infection, SIADH, convulsion, peripheral neuropathy, paralytic ileus, and secondary hypertension occurred after the third dose of vincristine, which was consistent with the literature.3,6–9
Hypertension is often observed in children with ALL who receive induction remission chemotherapy or re-induction chemotherapy (VDLD/VDLP regimens). Clinicians often consider that such hypertension is caused by adrenal glucocorticoids or other factors, ignoring that hypertension is a possible yet rare side effect of vincristine possibly caused by its effects on autonomic nerves, thereby leading to an imbalanced blood pressure regulation mechanism.2,3 When vincristine and azoles are used simultaneously, the incidence of secondary hypertension is as high as 36.4%.3 In this paper, both children developed secondary hypertension, and one case was refractory. Neither patient had a history of hypertension. After excluding hypertension caused by infective endocarditis, organic diseases of the central nervous system, water-electrolyte disturbance, and dexamethasone, vincristine-related secondary hypertension exacerbated by the combined use of posaconazole was considered. After discontinuing both drugs and providing active symptomatic treatment, blood pressure gradually returned to normal within approximately 2 to 3 weeks in both patients. Subsequently, the two children resumed chemotherapy, and posaconazole was no longer given. No vincristine-related side effects, including hypertension, occurred. Although secondary hypertension caused by vincristine and posaconazole gradually resolved after timely treatment discontinuation and active antihypertensive treatment, there was still a risk of cerebrovascular accident, which could have delayed the restart of chemotherapy. Therefore, great attention should be paid by clinicians to the side effects of vincristine and the possible drug interactions.
Nifedipine is a calcium channel blocker and a commonly used antihypertensive drug in pediatrics. It is metabolized through the CYP3A4 pathway, and it is also a P-gp inhibitor. Therefore, the combined use of nifedipine and vincristine can increase the area under the concentration-time curve of vincristine, increasing its plasma concentration and intensifying its side effects.11 Therefore, when vincristine is used in combination with posaconazole and nifedipine, its side effects should be closely monitored. In Case #1, nifedipine was used for antihypertensive treatment after hypertension occurred, and thus, the combination of vincristine, posaconazole, and nifedipine was used. Vincristine-related side effects such as refractory hypertension occurred early with severe symptoms and a prolonged disease course. Hypertension and other symptoms disappeared when those drugs were stopped. Sathiapalan et al.12 reported that although most side effects are reversible, some children experience permanent nerve damage and cerebrovascular accident, which seriously reduce their quality of life. It is worth noting that posaconazole can also increase the plasma concentration of calcium channel blockers that are metabolized by CYP3A4 (e.g., verapamil, diltiazem, nifedipine, nicardipine, felodipine). Therefore, such drugs should be avoided in combination with vincristine and posaconazole. If such a combination is still needed, calcium channel blocker-related side effects should be closely monitored, and the dosage of calcium channel blockers should be reduced if necessary.13,14
Our study had limitations. Hypertension is a common side effect of dexamethasone, and both patients received dexamethasone concomitantly with vincristine and posaconazole. However, when secondary hypertension occurred in the children, their cortisol levels were normal detected. Therefore, it was more likely that posaconazole aggravate vincristine-related hypertension.
In conclusion, posaconazole can interact with vincristine and induce secondary hypertension in children with ALL. As an adverse event, this interaction is rare. Simultaneous treatment with vincristine and azole antifungal drugs such as posaconazole should be avoided. Calcium channel blockers such as nifedipine should be prohibited when treating hypertension during vincristine treatment. Nevertheless, the cases reported in this study highlight the need for clinicians to pay attention to drug interactions, especially for drugs with rare and severe toxicities.
Footnotes
Ethical statement and informed consent: The patients and their parents provided informed consent for their inclusion in this study, and the Ethics Committee of the Seventh Affiliated Hospital of Sun Yat-Sen University approved this case report.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Hongman Xue https://orcid.org/0000-0002-4652-5369
References
- 1.Gidding CE, Kellie SJ, Kamps WA, et al. Vincristine revisited. Crit Rev Oncol Hematol 1999; 29: 267–287. [DOI] [PubMed] [Google Scholar]
- 2.Pekpak E, Ileri T, Ince E, et al. Toxicity of Vincristine Combined With Posaconazole in Children With Acute Lymphoblastic Leukemia. J Pediatr Hematol Oncol 2018; 40: e309–e310. [DOI] [PubMed] [Google Scholar]
- 3.Moriyama B, Henning SA, Leung J, et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses 2012; 55: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomber S, Dewan P, Chhonker D. Vincristine induced neurotoxicity in cancer patients. Indian J Pediatr 2010; 77: 97–100. [DOI] [PubMed] [Google Scholar]
- 5.Hof H. A new, broad-spectrum azole antifungal: posaconazole–mechanisms of action and resistance, spectrum of activity. Mycoses 2006; 49: 2–6. [DOI] [PubMed] [Google Scholar]
- 6.Van Schie RM, Bruggemann RJ, Hoogerbrugge PM, et al. Effect of azole antifungal therapy on vincristine toxicity in childhood acute lymphoblastic leukaemia. J Antimicrob Chemother 2011; 66: 1853–1856. [DOI] [PubMed] [Google Scholar]
- 7.Eiden C, Palenzuela G, Hillaire-Buys D, et al. Posaconazole-increased vincristine neurotoxicity in a child: a case report. J Pediatr Hematol Oncol 2009; 31: 292–295. [DOI] [PubMed] [Google Scholar]
- 8.Jain S, Kapoor G. Severe life threatening neurotoxicity in a child with acute lymphoblastic leukemia receiving posaconazole and vincristine. Pediatr Blood Cancer 2010; 54: 783. [DOI] [PubMed] [Google Scholar]
- 9.Zhong LP, Xue HM, Zhu DB, et al. [Antifungal azoles exacerbate vinblastine-related hyponatremia in ALL children]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2014; 22: 1386–1390. [DOI] [PubMed] [Google Scholar]
- 10.Groll AH, Townsend R, Desai A, et al. Drug-drug interactions between triazole antifungal agents used to treat invasive aspergillosis and immunosuppressants metabolized by cytochrome P450 3A4. Transpl Infect Dis 2017; 19. [DOI] [PubMed] [Google Scholar]
- 11.Fedeli L, Colozza M, Boschetti E, et al. Pharmacokinetics of vincristine in cancer patients treated with nifedipine. Cancer 1989; 64: 1805–1811. [DOI] [PubMed] [Google Scholar]
- 12.Sathiapalan RK, El-Solh H. Enhanced vincristine neurotoxicity from drug interactions: case report and review of literature. Pediatr Hematol Oncol 2001; 18: 543–546. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Theuretzbacher U, Clancy CJ, et al. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clin Pharmacokinet 2010; 49: 379–396. [DOI] [PubMed] [Google Scholar]
- 14.Gharibi S, Kimble B, Vogelnest L, et al. Pharmacokinetics of posaconazole in koalas (Phascolarctos cinereus) after intravenous and oral administration. J Vet Pharmacol Ther 2017; 40: 675–681. [DOI] [PubMed] [Google Scholar]