Abstract
Despite no significant differences of growth differentiation factor-15 expressions in peripheral, right atrial, and right ventricular blood, in the pulmonary arterial blood, there was a significantly high level of growth differentiation factor-15 in Group I pulmonary arterial hypertension patients subsequently developing heart failure. During right heart catheterization, collecting pulmonary blood samples is suggested to measure growth differentiation factor-15.
Keywords: growth differentiation factor-15 (GDF-15), intracardiac, pulmonary biomarkers, heart failure hospitalization
To the editor
Growth differentiation factor-15 (GDF-15), a stress-responsive member of the transforming growth factor-β cytokine superfamily, participates in differentiation, remodeling, and repair.1,2 GDF-15 is abundantly expressed in plexiform lesions in patients with pulmonary arterial hypertension (PAH) and affects the proliferation and apoptosis of pulmonary endothelial cells.3 The expression of peripheral GDF-15 increases in patients with tissue hypoxia and PAH.1,3,4 Given that circulating levels of GDF-15 are influenced by renal function and co-existence of systemic disease, this value may not be a timely or reliable indicator of disease prognosis with respect to PAH.5 In clinical practice, right heart catheterization (RHC) is mandatory at diagnosis and subsequent follow-up of PAH. RHC-derived blood samples provide direct information on the intracardiac and pulmonary expressions of biomarkers and insight into the pathophysiology of PAH. However, whether GDF-15 levels of the intracardiac and pulmonary arterial blood have a clinical impact remains indeterminate. This study aimed to evaluate the association of GDF-15 expression of the intracardiac and pulmonary arterial blood with the clinical outcomes of patients with WHO Group I PAH.
In this longitudinal prospective study, we enrolled patients with Group I PAH at a pulmonary pressure above 25 mmHg and pulmonary vascular resistance > 3 woods as detected by RHC. Among the 76 subjects, 25 were idiopathic, 28 had connective tissue disease, and 23 had congenital heart disease (CHD)-related pulmonary hypertension. Each patient provided informed consent. This study was conducted according to the recommendations of the 1975 Declaration of Helsinki on Biomedical Research involving human subjects and was approved by the local ethics committee (IRB: B-ER-106-056). We prospectively collected the subjects’ peripheral, right atrial, right ventricular, and main pulmonary arterial blood samples separately during RHC. Blood samples collected in EDTA tubes were centrifuged at 3000 r/min for 15 min at 4℃ and the derived sera was temporarily stored at –80℃. For GDF-15 measurement, a Quidel Triage ELISA kit (BIOSITE, San Diego, CA) was applied according to the manufacturer’s instructions. RHC and echocardiography were conducted at diagnosis, while no PAH-specific therapies including prostacyclin, endothelin ETA receptor antagonist, or phosphodiesterase type 5 inhibitor were prescribed at the time. The average follow-up duration was four years. The primary endpoint was hospitalization for heart failure (HF), which has been reviewed by a blinded reviewer to determine that the hospitalization was for HF. The continuous data were represented as the means ± standard deviations or as the medians and interquartile ranges. Dichotomous data were represented as numbers and percentages. Chi-square tests or Fisher’s exact tests were used for the categorical variables. Comparisons were conducted using Student’s t tests and nonparametric tests for continuous variables that were and were not normally distributed. The Kaplan–Meier method was used with a log-rank test to compare the survival rates. Using Cox regression analyses, we further studied the factors that were associated with the hospitalization for HF. The parameters sensitively associated with hospitalization for HF (p < 0.05) were included in multivariable analysis. In Model 1, GDF-15 was counted as a continuous variant, while in Model 2, GDF-15 > 1000 ng/l was counted as a binary variant. Age and gender were adjusted. The SPSS software (version 22.0, IBM SPSS Inc., Chicago, IL) was used for the statistical analyses.
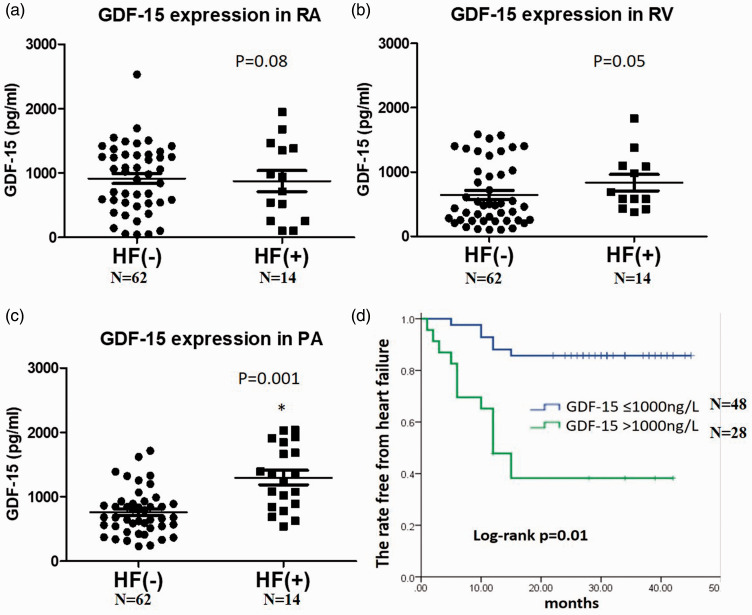
Among the 76 patients, 14 reached the endpoint. The average ages and etiologies of those with PAH were similar between the two groups, whereas the hospitalized patients were prone to be male. In the clinical and echocardiographic parameters, there were lower systolic blood pressures (102.8 ± 8.4 vs 119.8 ± 13, p = 0.03) but larger echo-derived right atria areas (15.7 ± 6.9 vs 14.5 ± 7.9, p = 0.04) in the patients with decompensated HF compared with those free from decompensation (Table 1). Regarding the RHC of both groups, all of the patients had increased right heart, PA pressures and pulmonary vascular resistance with low wedge pressures while their cardiac indexes were generally below 2.5 l/m2. Both groups of patients had lower six-minute walk distances. Most of the patients were at an intermediate risk of REVEAL scores while there were more patients at a high risk in the group of HF hospitalization. Patients with decompensated HF had a higher ratio of digoxin use (28.5% vs 12.9%, p = 0.03). Despite a relatively higher level of GDF-15 in the peripheral, right atrial, and right ventricular blood in patients developing HF compared with those free from HF, it was not insignificant (Fig. 1a and b). In contrast, there was a significantly high expression of GDF-15 in the pulmonary arterial blood in patients subsequently developing HF compared with those free from HF (1299.8 (576–2083) vs 761.4 (308–1197), p = 0.0001) (Fig. 1c). Of note, using the cut-off value of 1000 ng/l, GDF-15 level in the pulmonary arterial blood sensitively predicted the subsequent hospitalization for HF in those patients (Fig. 1d). In the Cox regression, only GDF-15 level in the pulmonary arterial blood above 1000 ng/l and REVEAL score were sensitively associated with the end-point of hospitalization for HF (HR: 5.46, CI: 1.69–17.6, p = 0.004 vs HR: 1.41, CI: 1.13–1.77, p = 0.003, respectively) (Table 2), compared with systolic blood pressure, right atria area, and digoxin use.
Table 1.
The baseline clinical, echocardiographic, functional, hemodynamic, and serologic parameters of patients with Group I pulmonary arterial hypertension (PAH).
| HF hospitalization (–) N = 62 | HF hospitalization (+) N = 14 | p Values | |
|---|---|---|---|
| Clinical parameters | |||
| Age (y/o) | 53.1 ± 14.9 | 53.4 ± 13.2 | 0.9 |
| Gender, N (%) | 19 (29) | 6 (43) | 0.08 |
| Body height (cm) | 149.8 ± 33.6 | 145.8 ± 44.7 | 0.7 |
| Body weight (kg) | 60.4 ± 13.7 | 51.4 ± 20.3 | 0.1 |
| Diabetes, N (%) | 5 (8) | 2 (14) | 0.4 |
| Systemic HTN, N (%) | 15 (24) | 1 (7) | 0.01 |
| Atrial fibrillation, N (%) | 3 (5) | 1 (7) | 0.4 |
| Smoking, N (%) | 0 (0) | 1 (7) | 0.1 |
| Cancer, N (%) | 5 (8) | 2 (14) | 0.2 |
| Etiologies | |||
| IPAH, N (%) | 21 (33.8) | 4 (28.5) | 0.5 |
| CHD, N (%) | 20 (32.2) | 3 (21.4) | 0.6 |
| CTD, N (%) | 21 (33.8) | 7 (50) | 0.4 |
| Functional capacity | |||
| NYFc I, N (%) | 18 (29) | 2 (14) | 0.3 |
| NYFc II, N (%) | 31 (50) | 8 (57) | 0.8 |
| NYFc III, N (%) | 7 (11) | 3 (21) | 0.4 |
| NYFc IV, N (%) | 6 (9.6) | 1 (7) | 0.3 |
| 6MWD (m) | 407.1 ± 60 | 402.6 ± 74.9 | 0.8 |
| %Predicted DLCO | 58.6 ± 18.9 | 52.8 ± 28.9 | 0.5 |
| Serologic markers (peripheral blood) | |||
| Hemoglobin (mg/dl) | 14.4 ± 16.5 | 11.5 ± 5.5 | 0.2 |
| eGFR (mL/min/1.73 m2) | 96.3 ± 45 | 81.6 ± 48.2 | 0.3 |
| ALT (IU/l) | 25.1 ± 16.6 | 24.9 ± 16.3 | 0.9 |
| Bilirubin (mg/dl) | 1.2 ± 1.1 | 1.5 ± 1.85 | 0.7 |
| Na (mmol/l) | 133.1 ± 27.7 | 125.8 ± 39.4 | 0.5 |
| Echocardiographic parameters | |||
| LVEF (%) | 68.8 ± 9.7 | 72 ± 4.3 | 0.1 |
| RA area (cm2) | 14.5 ± 7.9 | 15.7 ± 6.9 | 0.04 |
| TAPSE (cm) | 1.4 ± 0.4 | 1.3 ± 0.3 | 0.2 |
| S’ (cm/s) | 10.4 ± 2.2 | 9.8 ± 2.1 | 0.3 |
| PAP (mmHg) | 60.6 ± 29.1 | 78.4 ± 38.4 | 0.5 |
| Pericardial effusion, N (%) | 22 (35.4) | 5 (35.7) | 0.1 |
| Right heart catheterization | |||
| Heart rate (bpm) | 84.9 ± 10.9 | 86.5 ± 12.5 | 0.6 |
| SBP (mmHg) | 119.8 ± 13. | 102.8 ± 8.4 | 0.03 |
| DBP (mmHg) | 71.3 ± 8.8 | 70.4 ± 7.8 | 0.6 |
| SaO2 (%) | 95.3 ± 2. | 94.7 ± 5.2 | 0.1 |
| RA pressure (mmHg) | 9.6 ± 5.5 | 10.9 ± 4.5 | 0.5 |
| mRV pressure(mmHg) | 29.6 ± 12.3 | 36.1 ± 8.3 | 0.1 |
| mPA pressure (mmHg) | 39.1 ± 17.9 | 44.1 ± 13.5 | 0.3 |
| Wedge (mmHg) | 11.5 ± 3.7 | 13.5 ± 2.5 | 0.07 |
| Cardiac index (l/m2) | 3.7 ± 1.5 | 3.1 ± 0.8 | 0.1 |
| PVR (woods) | 6 ± 5.3 | 8.9 ± 5.6 | 0.1 |
| Peripheral GDF-15 (pg/ml) | 809 (224–1521) | 856 (158–1376) | 0.28 |
| RA GDF-15 (pg/ml) | 817 (150–1483) | 975 (458–1233) | 0.08 |
| RV GDF-15 (pg/ml) | 643.5 (108–1573) | 835.3 (489–1208) | 0.05 |
| PA GDF-15(pg/ml) | 761.4 (308–1197) | 1299.8 (576–2083) | 0.0001 |
| Medications | |||
| Diuretics, N (%) | 37 (59.6) | 10 (71.4) | 0.3 |
| Digoxin, N (%) | 8 (12.9) | 4 (28.5) | 0.03 |
| CCB, N (%) | 3 (4.8) | 1 (7) | 0.4 |
| Anti-coagulants, N (%) | 7 (11.3) | 2 (14) | 0.5 |
| REVEAL risk score | |||
| Mean | 1.8 ± 0.6 | 6.6 ± 0.5 | 0.02 |
| Median | 2 | 8 | 0.01 |
| Range | 1–9 | 4–12 | 0.01 |
| High risk (> 8) | 10 (16.1) | 5 (35.7) | 0.01 |
| Intermediate risk (6–8) | 25 (40.3) | 5 (35.7) | 0.2 |
| Low risk (< 6) | 27 (43.6) | 4 (28.5) | 0.12 |
Notes: Normally distributed parameters are expressed as mean ± standard deviation. Non-normally distributed parameters are expressed as the medians and interquartile ranges. eGFR < 60 mL/min/1.73 m2 or renal insufficiency if missing eGFR.
IPAH: idiopathic pulmonary artery hypertension; CHD: congenital heart disease; CTD: connective tissue disease; NYFc: New York functional class; 6MWD: six-minute walk distance; ALT: alanine aminotransferase; LVEF: left ventricular ejection fraction; RA: right atrium; TAPSE: tricuspid annular plane systolic excursion; S’: Tissue Doppler tricuspid annulus velocity; PAP: pulmonary arterial pressure; RV: right ventricular; PA: pulmonary arterial; PVR: pulmonary vascular resistance; CCB: calcium channel blocker; SBP: systolic blood pressure; GDF-15: growth differentiation factor-15; HTN: hypertension; DLCO: diffusing capacity for carbon monoxide; DBP = diastolic blood pressure; mRV= mean right ventricular; mPA = mean pulmonary arterial; eGFR = estimated Glomerular filtration rate.
Fig. 1.
The expression of GDF-15 in the blood samples of (a) right atrium, (b) right ventricle, and (c) pulmonary artery between Group I patients with and without hospitalization for heart failure. (d) Using the cut-off value of 1000 ng/l, GDF-15 levels in the blood around the pulmonary artery sensitively predicted the subsequent hospitalization for heart failure in those patients (Student t test for Panel a–c; Kaplan–Meier plot for panel d) *p < 0.05 (total N = 76).
GDF-15: growth differentiation factor-15; HF: heart failure.
Table 2.
Univariate and multivariable predictors of hospitalization for heart failure.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
|||||
| Parameters | HR | p | HR | p | HR | p |
| Age | 1.001 (0.97–1.03) | 0.92 | ||||
| Gender | 0.49 (0.22–1.15) | 0.1 | ||||
| SBP | 0.96 (0.92–0.99) | 0.04 | 0.96 (0.92–1.01) | 0.128 | 0.97 (0.93–1.02) | 0.26 |
| Right atria area | 1.02 (0.96–1.08) | 0.05 | ||||
| Digoxin use. | 2.71 (1.11–6.58) | 0.02 | 1.46 (0.47–4.53) | 0.512 | 1.25 (0.37–4.16) | 0.71 |
| REVEAL risk score | 4.62 (2.21–9.68) | 0.0001 | 4.39 (1.57–12.2) | 0.005 | 1.41 (1.13–1.77) | 0.003 |
| GDF-15 | 4.02 (2.01–10.03) | 0.0001 | 3.48 (1.01–11.98) | 0.04 | ||
| GDF-15 > 1000 ng/l | 5.84 (2.23–15.28) | 0.0001 | 5.46 (1.69–17.6) | 0.004 | ||
Notes: Model 1: GDF-15 as a continuous variant; Model 2: GDF-15 > 1000 ng/l as a binary variant. Adjusted by age and gender.
SBP: systolic blood pressure; GDF-15: growth differentiation factor-15.
GDFs family abundantly expressed in various organs including neurons, muscles, pulmonary, and cardiac circulations.1,6 Among them, GDF-15 has been reported with a high expression in plexiform lesions in PAH and affects the proliferation and apoptosis of pulmonary endothelial cells.1–3,7 As a stress-responsive protein, GDF-15 is upregulated under a pathologic stress, including acute injury, tissue hypoxia, inflammation or oxidative stress.6,8 Although Yu and colleagues also indicated that GDF-11 may promote abnormal proliferation in pulmonary endothelial cell, to date, GDF-15 remains more remarkable in PAH.1,9 Serum levels are also increased in scleroderma and systemic sclerosis (SSc)-PAH patients compared with SSc patients without PAH and positively correlate with PA pressure.4 Circulating GDF-15 levels are elevated and independently related to the risk of death or transplantation in patients with idiopathic PAH.1 The information provided by GDF-15 is additive to that of established hemodynamic and biochemical markers. In addition to idiopathic and SSc-PAH, circulating GDF-15 levels also increased in patients with CHD-related PAH compared with those with CHD free from PAH.8 Similarly, a higher level of circulating GDF-15 has been reported as a predictive factor for the development of chronic thromboembolic pulmonary hypertension secondary to acute pulmonary embolism.10 To note, the increased right ventricular afterload could induce stresses on cardiomyocytes and subsequently trigger the activation of GDF-15.1 Nevertheless, whether GDF-15 can be widely applied to patients with Group I PAH remains unknown. Also, although Nickel et al. reported an abundant expression of GDF-15 in plexiform lesions in patients with PAH, the clinical applicability of intracardiac and pulmonary GDF-15 requires exploration.3 In this study, we found that in patients with Group I PAH, the level of GDF-15 significantly increased in the blood around pulmonary artery compared with those in the peripheral, right atrial, and right ventricular blood. The level of GDF-15 in the pulmonary arterial blood was also associated with hospitalization for HF in these patients. Upon clinical observations, although patients with PAH have similar pulmonary arterial pressures, the outcomes and probabilities of subsequent HF vary.11 Our findings support that GDF-15 level in the pulmonary arterial blood could sensitively reflect the cardiac stress and be a potential surrogate marker for decompensated HF in patients with PAH.
This report has some limitations. First, among the studied population, only 14 patients developed decompensated HF, which attenuated the statistical power. Second, given no absolute GDF-15 reference values, our cut-off value may not be widely applicable to all other PAH populations. Second, different from a previous study showing an association between high peripheral GDF-15 levels and an increased risk of mortality,1 in this cohort, peripheral GDF-15 failed to differentiate patients from decompensated HF. Instead, GDF-15 level in the pulmonary arterial blood strengthened the differences. Third, in this study, ELISA was used to measure the level of GDF-15 in the blood collected from peripheral, right atrium, right ventricle and pulmonary artery but alternative measurements including reverse transcription-PCR, immuno-blot analysis, or immuno-staining could better determine the mRNA expressions and protein levels of GDF-15 and further corroborate the results of ELISA. Also, other GDF family proteins should be measured in the future experiments.
Collectively, the GDF-15 level in the pulmonary arterial blood can be a prognostic marker in patients with Group I PAH. This study demonstrates using the pulmonary arterial blood, GDF-15 could facilitate in identifying patients with an increased risk of decompensated HF. Currently, we are continuously following those patients to evaluate if any changes of hemodynamic or serologic parameters after the treatment of PAH.
Footnotes
Data sharing: The datasets of this study are available from the corresponding author on reasonable request.
Author contributions: W.-T.C., J.-Y.S., C.-H.H., Y.-W.L., and Z.-C.C. contributed to the conception and design of the study. W.-T.C., J.Y.S., C.-H.H., Y.-W.L., J.-N.R., and Z.C.C. contributed to the acquisition, analyses, and interpretation of data. W.-T.C. drafted the manuscript and all authors critically revised it. All authors gave final approval and agreed to be accountable for all aspects of the work ensuring integrity and accuracy.
Ethical approval: Our study was complied with the 1964 Declaration of Helsinki and its later amendments, and approved by the Ethics Committee of National Cheng Kung University Hospital (IRB: B-ER-106-056).
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by National Health Research Institute, Taiwan (NHRI-EX106- 10618SC), Taiwan Society of Cardiology, and Chi-Mei Medical Center.
References
- 1.Geenen LW, Baggen VJM, Kauling RM, et al. Growth differentiation factor-15 as candidate predictor for mortality in adults with pulmonary hypertension. Heart 2020; 106: 467–473. [DOI] [PubMed] [Google Scholar]
- 2.Dib H, Tamby MC, Bussone G, et al. Targets of anti-endothelial cell antibodies in pulmonary hypertension and scleroderma. Eur Respir J 2012; 39: 1405–1414. [DOI] [PubMed] [Google Scholar]
- 3.Nickel N, Jonigk D, Kempf T, et al. GDF-15 is abundantly expressed in plexiform lesions in patients with pulmonary arterial hypertension and affects proliferation and apoptosis of pulmonary endothelial cells. Respir Res 2011; 12: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meadows CA, Risbano MG, Zhang L, et al. Increased expression of growth differentiation factor-15 in systemic sclerosis-associated pulmonary arterial hypertension. Chest 2011; 139: 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair V, Robinson-Cohen C, Smith MR, et al. Growth differentiation factor-15 and risk of CKD progression. J Am Soc Nephrol 2017; 28: 2233–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickel N, Kempf T, Tapken H, et al. Growth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2008; 178: 534–541. [DOI] [PubMed] [Google Scholar]
- 7.Tiwari KK, Moorthy B, Lingappan K. Role of GDF15 (growth and differentiation factor 15) in pulmonary oxygen toxicity. Toxicol In Vitro 2015; 29: 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li G, Li Y, Tan XQ, et al. Plasma growth differentiation factor-15 is a potential biomarker for pediatric pulmonary arterial hypertension associated with congenital heart disease. Pediatr Cardiol 2017; 38: 1620–1626. [DOI] [PubMed] [Google Scholar]
- 9.Yu X, Chen X, Zheng XD, et al. Growth differentiation factor 11 promotes abnormal proliferation and angiogenesis of pulmonary artery endothelial cells. Hypertension 2018; 71: 729–741. [DOI] [PubMed] [Google Scholar]
- 10.Xu JX, Yu HZ, Wu Q, et al. The study of growth differentiation factor-15 in chronic thromboembolic pulmonary hypertension following acute pulmonary thromboembolism. Chin J Tuberc Respir Dis 2016; 39: 876–880. [DOI] [PubMed] [Google Scholar]
- 11.Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]