Abstract
Readmissions for pulmonary hypertension are poorly understood and understudied. We sought to determine national estimates and risk factors for 30-day readmission after pulmonary hypertension-related hospitalizations. We utilized the Healthcare Cost and Utilization Project Nationwide Readmission Database, which has weighted estimates of roughly 35 million discharges in the US. Adult patients with primary International Classification of Disease, Ninth Revision, Clinical Modification diagnosis codes of 416.0 and 416.8 for primary and secondary pulmonary hypertension with an index admission between 2012 and 2014 and any readmission within 30 days of the index event were identified. Predictors of 30-day readmission were identified using multivariable logistic regression with adjustment for covariates. Results showed that the national estimate for Primary Pulmonary Hypertension vs Secondary Pulmonary Hypertension-related index events between 2012 and 2014 with 30-day readmission was 247 vs 2550 corresponding to a national readmission risk estimate of 17% vs 18.3%, respectively. The presence of fluid and electrolyte disorders, renal failure, and alcohol abuse were associated with increased risk of readmission in Primary Pulmonary Hypertension, while factors associated with Secondary Pulmonary Hypertension readmissions included anemia, congestive heart failure, lung disease, fluid and electrolyte disorders, renal failure, diabetes, and liver disease. The median cost of Primary Pulmonary Hypertension admissions and readmissions were $46,132 (IQR: $25,384–$85,647) and $41,604.50 (IQR: $22,481.50–$84,420.50), respectively. The median costs of Secondary Pulmonary Hypertension admissions and readmissions were $34,893 (IQR: $19,670–$66,143) and $36,279 (IQR: $19,059–$74,679), respectively. In conclusion, approximately 19% of Primary Pulmonary Hypertension and Secondary Pulmonary Hypertension hospitalizations result in 30-day readmission, with significant costs accrued during the index hospitalization and readmission. With evolving clinical terminology and diagnostic codes, future study will need to better clarify underlying factors associated with readmissions amongst pulmonary hypertension sub-types, and identify methods and procedures to minimize readmission risk.
Keywords: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP), Nationwide Readmission Database, pulmonary hypertension, readmissions
Introduction
Pulmonary hypertension (PH) is a chronic, under-recognized, and life-threatening spectrum of disease that is often misdiagnosed and poorly understood. PH has been estimated to have a prevalence of 1% in the global population, which increases to 10% in individuals above the age of 65 years.1 While the prevalence of PH secondary to intrinsic heart and lung disease is significantly higher,2 the prevalence of a sub-group of PH, namely pulmonary arterial hypertension (PAH), is approximately 12.4 cases/million.3–5 The most recent PH surveillance summary from the Centers for Disease Control and Prevention in 2014 shows an increasing trend of PH-associated mortality and hospitalization rates.6 Recently, Sikachi et al. analyzed the Healthcare Cost and Utilization Project’s (HCUP) nationwide inpatient sample from 2000 to 2013 for PH-associated admissions and described an increased mean length of stay (LOS) from 5.89 days to 6.67 days (p = 0.004) and an increased mean cost of hospitalization by 209.5%.7,8
While these administrative datasets may provide valuable information in understanding the demographics and the economic burden of PH and its sub-types, they have been shown to be limited by changing clinical terminology and administrative coding systems.9 Despite this, with hospital readmissions currently the focus of much scrutiny across other cardiopulmonary diseases, few data exist regarding the causes and impact of readmissions in the context of PH. Identifying patient, clinical and hospital factors associated with increased risk of readmissions can discern areas for intervention in preventing readmissions. We, therefore, sought to examine HCUP’s Nationwide Readmissions Database (NRD) from 2012 through 2014 to determine the rate, predictors, and causes of 30-day readmission following PH-related hospitalization.
Methods
Data source
The NRD is a part of HCUP that is sponsored by the Agency for Healthcare Research and Quality (AHRQ) that collects discharge data from 22 geographically dispersed states.10 The NRD contains approximately 15 million discharges each year. Sampling weights permit national-level estimates for roughly 35 million discharges in the United States representing 49% of US hospitalizations for each year.10 The NRD data include International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, patient demographics and comorbidity data, hospital characteristics, measures of disease severity including All Patient Refined Diagnosis related group (APR-DRG) severity of illness, and risk of mortality sub classification and total costs.10,11 Since the NRD is a de-identified publicly available dataset, it is exempt from institutional review board approval.
Study sample
All adult hospitalizations (>18 years of age) with ICD-9-CM codes for PH and its subtypes (ICD-9-CM codes 416.0 for Primary PH (PPH) and 416.8 for Secondary PH (SPH)) between 1 January 2012 and 31 December 2014 were included in the analysis. Since the NRD does not track readmissions between years, data from December of each year was not captured by the NRD, allowing 30 days of follow-up for every patient. We excluded admissions of the patient if they died during the index hospitalization, patient age was < 18 years, the LOS was missing, the discharge month was December since 30-day readmission data would be lacking, or if the patients were residents of a different state. Within the NRD, patients are identified by state-specific linkage numbers and, therefore, cannot be tracked across states in the NRD.
Patient, clinical and hospital characteristics
Risk factors for readmissions were grouped as patient, clinical and hospital characteristics. Patient characteristics studied were age, sex, insurance status, household income, comorbidities, and severity of illness and risk of mortality. Age was stratified into groups of 18–39, 40–64, and 65 years or older. Insurance status was categorized as Medicare, Medicaid, private insurance, self-pay, or “other” (including no charge). Income levels were based on the estimated median household income of residents in the patient’s zip code and were divided into $1–$37,999, $38,000–$47,999, $48,000–$63,999, and $64,000 or greater. Pre-existing comorbidities were limited to chronic conditions determined on the basis of the 29 Elixhauser comorbidities provided by the AHRQ. APR-DRG severity of illness and risk of mortality sub-classifications were examined as measures of disease severity. Clinical variables included post-acute care disposition and LOS during index hospitalization. Post-acute care disposition was stratified as home or self-care, transfer to skilled nursing or intermediate care facility (ICF), and home health care and LOS were categorized as <3, 4–7, and > 7 days. Hospital-related characteristics such as bed size (small, medium, and large), location (urban vs rural), and teaching status were also identified and ownership categories included government (public), private (not-for-profit), and private (investor-owned).
Study outcomes
The primary outcome was all-cause readmission within 30 days of discharge, following an index hospitalization. Only the first readmission within 30 days after discharge from the index admission was included. Median cost for initial hospitalization and readmissions were tabulated.
Reasons for readmission (cardiovascular and non-cardiovascular) were determined on the basis of Clinical Classification Software (CCS) codes, a categorization scheme developed by the AHRQ based on ICD-9-CM coding. Predictors of 30-day readmissions of PH grouped by PPH and SPH were analyzed.
Statistical analysis
Categorical variables are reported as (n) total counts with percentages and continuous variables are reported with mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate, respectively. Univariate differences between PH types, namely PPH and SPH, were assessed using t-tests or Wilcoxon rank-sum (Mann–Whitney U) test/Kruskal–Wallis test for normally/non-normally distributed data, respectively. Percentage of 30-day readmissions was charted as number of all-cause readmissions on a day divided by total number of 30-day readmissions multiplied by 100.
To examine the association between risk factors and 30-day readmission, as represented by adjusted odds ratio (aOR), variables with a p-value of < 0.1 on univariate model were analyzed using multivariable logistic regression that accounted for the complex survey design of the NRD (i.e. weighting, stratification). Relevant readmission risk factors were selected apriori and included in the multivariable model (regardless of significance). These were age, sex, and severity of illness and risk of mortality. All analyses were performed in Stata 14.2 (StataCorp, College Station, TX). A two-sided p-value ≤ 0.05 was considered significant.
Results
Readmission risk and healthcare costs associated with index admissions and readmissions of PH and its sub-types
There were 14,659 index admissions associated with PH in the NRD between 2012 and 2014. These led to 2797 readmissions and a national readmission risk estimate of 19%. Of the total, 1433 vs 13,226 index admissions were associated with PPH vs SPH, respectively. There were 247 vs 2550 readmissions and risk estimates of 17% vs 18% for PPH and SPH, respectively (Table 1). Median index admission and readmission costs (IQR) for PH overall were $35,746 ($19,952.5–$68,417.5) and $36,748.5 ($19,535–$75,130), respectively; for PPH were $46,132 ($25,384–$85,647) and $41,604.5 ($22,481.5–$84,420.5), and for SPH were $34,893 ($19,670–$66,143) and $36,279 ($19,059–$74,679) (Table 1).
Table 1.
Readmission risk estimates and healthcare costs associated with index admissions and readmissions of PH and its sub-types.
| PH and its sub-types | Number of index admissions | Number of 30-day readmissions | Readmission risk | Cost of index admission $ (IQR) | Cost of readmissions $ (IQR) |
|---|---|---|---|---|---|
| Total PH | 14,659 | 2797 | 19% | 35,746 (19,952.5–68,417.5) | 36,748.5 (19,535–75,130) |
| Primary PH | 1433 | 247 | 17% | 46,132 (25,384–85,647) | 41,604.5 (22,481.5–84,420.5) |
| Secondary PH | 13,226 | 2550 | 18% | 34,893 (19,670–66,143) | 36,279 (19,059–74,679) |
PH: pulmonary hypertension; IQR: interquartile range.
Temporal trend and reasons for 30-day PH readmissions
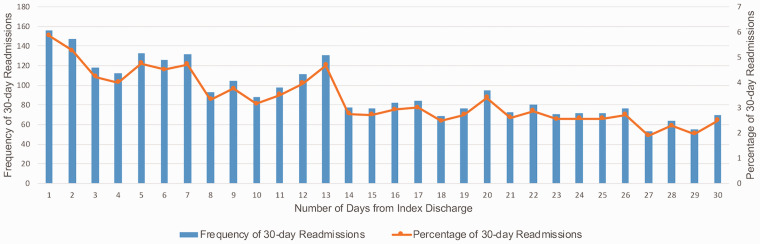
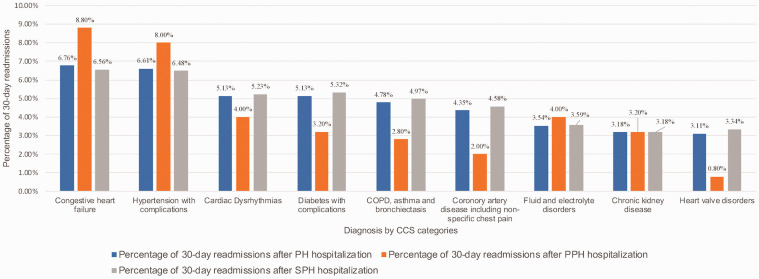
Fig. 1 depicts the temporal trend of frequency and percentage of 30-day readmissions in PH. Of all 30-day readmissions, 15% of total PH readmissions occurred within three days after discharge. Approximately 33% of readmissions were within the first seven days, and 58% were within the first 15 days. Fig. 2 depicts the reasons for 30-day readmissions by PH sub-type and CCS categories among those hospitalized with PH. Across PH and its sub-types, cardiac causes constituted 26% of the readmissions vs 74% defined as non-cardiac. As noted in Fig. 2, while cardiac causes such as congestive heart failure (CHF) (8.80% and 6.56% in PPH and SPH, respectively), and hypertension with complications (8% and 6.48% in PPH and SPH, respectively), were more common in the PPH group, heart and valve disorders as well as non-cardiac causes including chronic lung disease and diabetes were more common causes of readmission in the SPH group, as consistent with comorbidities leading to SPH.
Fig. 1.
Frequency and percentage of 30-day readmissions in pulmonary hypertension.
Fig. 2.
Reasons for 30-day readmissions by PH sub-type and clinical classification software categories among those hospitalized with PH.
COPD: chronic obstructive pulmonary disease; CCS: Clinical Classification Software; PH: pulmonary hypertension; PPH: Primary Pulmonary Hypertension; SPH: Secondary Pulmonary Hypertension.
Patient, clinical and hospital characteristics of index hospitalization
Table 2 depicts the patient, clinical and hospital characteristics for index hospital admissions associated with PH grouped by PH sub-types. In the total PH category, the mean age (±SD) was 63.90 (±16.20) years, whereas the mean age for PPH and SPH were 56.77 (±17.50) years and 64.67 (±15.90) years, respectively (p < 0.01). Across PH and its subtypes, more patients were female (73% and 67% in PPH and SPH, respectively) (p < 0.01). Most patients had Medicare insurance status (51% and 63% in PPH and SPH, respectively) (p < 0.01); 71% of PH patients had index admissions to hospitals with a large bed size, 65% were in large metropolitan areas with at least one million residents, 67% were metropolitan teaching hospitals, and 70% index admissions were in non-profit private hospitals.
Table 2.
Patient, clinical and hospital characteristics of index hospitalization.
| Characteristic | Total | Primary Pulmonary Hypertension (416.0) | Secondary Pulmonary Hypertension (416.8) | p-Values |
|---|---|---|---|---|
| Age, mean (±SD) | 63.90 ( ± 16.20) | 56.77 ( ± 17.50) | 64.67 ( ± 15.90) | <0.01 |
| Young adults (18–39 years), N (%) | 1243 (8.48%) | 265 (18.50%) | 978 (7.40%) | |
| Middle aged (40–64 years), N (%) | 5732 (39.10%) | 630 (44%) | 5102 (38.60%) | |
| Elderly (≥65 years), N (%) | 7684 (52.42%) | 538 (37.50%) | 7146 (54%) | |
| Sex | ||||
| Female, N (%) | 9898 (67.52%) | 1043 (72.80%) | 8855 (67%) | <0.01 |
| Male, N (%) | 4761 (32.48%) | 390 (27.20%) | 4371 (33%) | |
| Insurance type/expected primary payer | ||||
| Medicare, N (%) | 9182 (62.15%) | 738 (50.58%) | 8444 (63.41%) | <0.01 |
| Medicaid, N (%) | 1931 (13.07%) | 243 (16.66%) | 1688 (12.68%) | |
| Private, N (%) | 2743 (18.57%) | 377 (25.84%) | 2366 (17.77%) | |
| Self-pay, N (%) | 374 (2.53%) | 43 (2.95%) | 331 (2.49%) | |
| Other, N (%) | 450 (3.05%) | 47 (3.22%) | 403 (3.03%) | |
| Median household income | ||||
| ≥ $64,000, N (%) | 3108 (21.04%) | 364 (24.95%) | 2744 (20.61%) | <0.01 |
| $48,000–$63,999, N (%) | 3463 (23.44%) | 358 (24.54%) | 3105 (23.32%) | |
| $38,000–$47,999, N (%) | 3671 (24.85%) | 351 (24.06%) | 3320 (24.93%) | |
| ≤ 37,999, N (%) | 4281 (28.97%) | 362 (24.81%) | 3919 (29.43%) | |
| AHRQ comorbidities, N (%) | ||||
| Acquired immune deficiency syndrome | 80 (0.55%) | 6 (0.40%) | 74 (0.60%) | 0.492 |
| Alcohol abuse | 459 (3.13%) | 41 (2.90%) | 418 (3.20%) | 0.537 |
| Deficiency anemias | 3608 (24.61%) | 307 (21.40%) | 3301 (25%) | 0.003 |
| Rheumatoid arthritis/collagen vascular diseases | 1577 (10.76%) | 120 (8.40%) | 1457 (11%) | 0.002 |
| Chronic blood loss anemia | 124 (0.85%) | 14 (1%) | 110 (0.80%) | 0.568 |
| Congestive heart failure | 5903 (40.27%) | 510 (35.60%) | 5393 (40.80%) | <0.01 |
| Chronic pulmonary disease | 6023 (41.09%) | 489 (34.10%) | 5534 (41.80%) | <0.01 |
| Coagulopathy | 1371 (9.35%) | 187 (13%) | 1184 (9%) | <0.01 |
| Depression | 1759 (12%) | 173 (12.10%) | 1586 (12%) | 0.929 |
| Diabetes, uncomplicated | 4061 (27.70%) | 342 (23.90%) | 3719 (28.10%) | 0.010 |
| Diabetes with chronic complications | 1072 (7.31%) | 84 (5.90%) | 988 (7.50%) | 0.026 |
| Drug abuse | 706 (4.82%) | 80 (5.60%) | 626 (4.70%) | 0.154 |
| Hypertension | 9029 (61.59%) | 700 (48.80%) | 8329 (63%) | <0.01 |
| Hypothyroidism | 2530 (17.26%) | 227 (15.80%) | 2303 (17.40%) | 0.135 |
| Liver disease | 1162 (7.93%) | 115 (8%) | 1047 (7.90%) | 0.885 |
| Lymphoma | 149 (1.02%) | 16 (1.10%) | 133 (1%) | 0.691 |
| Fluid and electrolyte disorders | 4761 (32.48%) | 512 (35.70%) | 4249 (32.10%) | 0.006 |
| Metastatic cancer | 114 (0.78%) | 14 (1%) | 100 (0.80%) | 0.366 |
| Neurological disorders | 860 (5.87%) | 70 (4.90%) | 790 (6%) | 0.096 |
| Obesity | 4051 (27.63%) | 317 (22.10%) | 3734 (28.20%) | <0.01 |
| Paralysis | 139 (0.95%) | 16 (1.10%) | 123 (0.90%) | 0.489 |
| Peripheral vascular disorders | 1069 (7.29%) | 87 (6.10%) | 982 (7.40%) | 0.061 |
| Psychoses | 572 (3.90%) | 56 (3.90%) | 516 (3.90%) | 0.990 |
| Renal failure | 4124 (28.13%) | 308 (21.50%) | 3816 (28.90%) | <0.01 |
| Solid tumor without metastasis | 254 (1.73%) | 20 (1.40%) | 234 (1.80%) | 0.303 |
| Valvular disease | 2604 (17.76%) | 171 (11.90%) | 2433 (18.40%) | <0.01 |
| Weight loss | 644 (4.39%) | 82 (5.70%) | 562 (4.20%) | 0.010 |
| All patient refined diagnosis related group: risk of mortality subclass | ||||
| Minor likelihood of dying, N (%) | 171 (1.17%) | 119 (8.3%) | 597 (4.5%) | <0.01 |
| Moderate likelihood of dying, N (%) | 5549 (37.85%) | 204 (14.2%) | 6527 (49.3%) | |
| Major likelihood of dying, N (%) | 7338 (50.06%) | 947 (66.1%) | 4938 (37.3%) | |
| Extreme likelihood of dying, N (%) | 1600 (10.91%) | 163 (11.4%) | 1163 (8.8%) | |
| All patient refined diagnosis related group: severity of illness subclass | ||||
| Minor loss of function, N (%) | 716 (4.88%) | 3 (0.20%) | 168 (1.30%) | <0.01 |
| Moderate loss of function, N (%) | 6731 (45.92%) | 596 (41.60%) | 4953 (37.40%) | |
| Major loss of function, N (%) | 5885 (40.15%) | 639 (44.60%) | 6699 (50.70%) | |
| Extreme loss of function, N (%) | 1326 (9.05%) | 195 (13.60%) | 1405 (10.60%) | |
| Disposition of patient at discharge | ||||
| Home or self care | 9076 (61.90%) | 897 (62.60%) | 8179 (61.85%) | <0.01 |
| Transfer to skilled nursing or intermediate care facility | 1569 (10.71%) | 102 (7.12%) | 1467 (11.09%) | |
| Home health care | 3215 (21.94%) | 321 (22.40%) | 2894 (21.89%) | |
| Total length of stay in days, mean ( ± SD) | 6.52 ( ± 7.56) | 8.01 ( ± 10.10) | 6.36 ( ± 7.20) | <0.01 |
| Length of stay of index hospitalization, N (%) | ||||
| ≤ 3days | 5619 (38.33%) | 471 (32.87%) | 5148 (38.92%) | <0.01 |
| 4–7 days | 5095 (34.76%) | 473 (33.01%) | 4622 (34.95%) | |
| > 7 days | 3945 (26.91%) | 489 (34.12%) | 3456 (26.13%) | |
| Hospital bed size | ||||
| Small, N (%) | 1292 (8.81%) | 101 (7%) | 1191 (9%) | <0.01 |
| Medium, N (%) | 2925 (19.95%) | 206 (14.40%) | 2719 (20.60%) | |
| Large, N (%) | 10,442 (71.23%) | 1126 (78.60%) | 9316 (70.40%) | |
| Hospital urban–rural designation | ||||
| Large metropolitan areas with at least one million residents, N (%) | 9583 (65.37%) | 1010 (70.50%) | 8573 (64.80%) | <0.01 |
| Small metropolitan areas with less than one million residents, N (%) | 4245 (28.96%) | 380 (26.50%) | 3865 (29.20%) | |
| Micropolitan areas, N (%) | 669 (4.56%) | 35 (2.40%) | 634 (4.80%) | |
| Not metropolitan or micropolitan (non-urban residual), N (%) | 162 (1.11%) | 8 (0.60%) | 154 (1.20%) | |
| Teaching status of hospital | ||||
| Metropolitan non-teaching, N (%) | 4045 (27.59%) | 314 (21.90%) | 3731 (28.20%) | <0.01 |
| Metropolitan teaching, N (%) | 9783 (66.74%) | 1076 (75.10%) | 8707 (65.80%) | |
| Non-metropolitan, N (%) | 831 (5.67%) | 43 (3%) | 788 (6%) | |
| Control/ownership of hospital | ||||
| Government, nonfederal, N (%) | 2619 (17.87%) | 230 (16.10%) | 2389 (18.10%) | <0.01 |
| Private, not-profit, N (%) | 10,308 (70.32%) | 1104 (77%) | 9204 (69.60%) | |
| Private, invest-own, N (%) | 1732 (11.82%) | 99 (6.90%) | 1633 (12.30%) | |
AHRQ: Agency for Healthcare Research and Quality. Values that are statistically significant with a p-value less than or equal to 0.05 are in bold
Compared to SPH, patients with PPH were more likely to have coagulopathy (13% vs 9%; p < 0.01), fluid and electrolyte disorders (36% vs 32%; p = 0.006), and weight loss (5.70% vs 4.20%; p = 0.010). Patients with SPH when compared to PPH were more likely to have anemia (25% vs 21.40%; p = 0.003), collagen vascular disease (11% vs 8.40%; p = 0.002), CHF (41% vs 36%; p < 0.01), chronic pulmonary disease (42% vs 34%; p < 0.01), uncomplicated diabetes (28% vs 24%; p = 0.010), diabetes with chronic complications (7.50% vs 6%; p = 0.026), hypertension (63% vs 49%; p < 0.01), obesity (28% vs 22%; p < 0.01), renal failure (29% vs 21.50%; p < 0.01), and valvular disease (18% vs 12%; p < 0.01). PPH patients had worse APR-DRG risk of mortality severity index when compared to SPH with 78% PPH patients having major or extreme likelihood of dying when compared to 46% SPH patients.
Approximately 62% of patients in both PPH and SPH were discharged home following index hospitalization and approximately 22% of patients in both sub-types were discharged with home health care. Patients with SPH were more likely to be discharged to a skilled nursing or ICF when compared to PPH (11% vs 7%; p < 0.01). PPH patients had a longer mean (±SD) LOS of index hospitalization when compared to SPH patients (8.01 (±10) days vs 6.36 (±7.2) days; p < 0.01); 34% of PPH patients had a LOS of >7 days compared to 26% in SPH patients (p < 0.01).
Factors associated with 30-day readmissions for PH
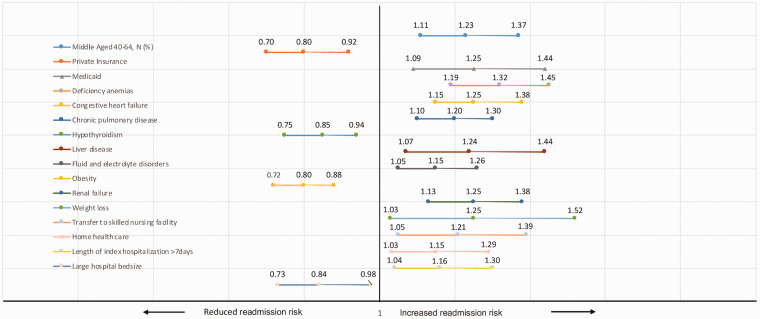
Factors associated with PH readmissions on univariate and multivariable analyses are listed in Table 3. In multivariable analysis, middle-aged patients (age 40–64 years) had increased odds of 30-day readmissions (aOR: 1.23; 95% CI: 1.11–1.37) when compared to the age category 18–39 years. Patients with private insurance status had decreased odds of 30-day readmissions (aOR: 0.74; 95% CI: 0.65–0.85) and patients with Medicaid had increased odds of 30-day readmissions (aOR: 1.25; 95% CI: 1.09–1.44) when compared to patients with Medicare. Comorbidities associated with an increased odds of 30-day readmissions include iron/B12/folate deficiency anemia’s (aOR: 1.32; 95% CI: 1.19–1.45), CHF (aOR: 1.25; 95% CI: 1.15–1.38), chronic pulmonary disease (aOR: 1.20; 95% CI: 1.10–1.30), liver disease (aOR: 1.24; 95% CI: 1.07–1.44), fluid and electrolyte disorders (aOR: 1.15; 95% CI: 1.05–1.26), renal failure (aOR: 1.25; 95% CI: 1.13–1.38), and weight loss (aOR: 1.25; 95% CI: 1.03–1.52). The comorbidities of hypothyroidism (aOR: 0.85; 95% CI: 0.75–0.94) and obesity (aOR: 0.80; 95% CI: 0.72–0.88) were associated with a decreased risk of readmission.
Table 3.
Risk factors for 30-day readmissions in Total PH.
| Characteristics | 30-day readmissions |
Multivariate OR |
|||||
|---|---|---|---|---|---|---|---|
| Not readmitted | Readmitted | p-Values | Unadjusted odds ratio (95% CI) | p-Values | Adjusted odds ratio (95% CI) | p-Values | |
| Age, mean ( ± SD) | 63.93 ( ± 16.3) | 63.76 ( ± 16.1) | 0.625 | ||||
| Young adults (18–39 years), N (%) | 1017 (8.60%) | 226 (8.10%) | 0.051 | 1 (Reference) | 1 (Reference) | ||
| Middle aged (40–64 years), N (%) | 4582 (38.60%) | 1150 (41.10%) | 1.05 (1.01–1.10) | 0.015 | 1.23(1.11–1.37) | <0.01 | |
| Elderly (≥ 65 years), N (%) | 6263 (52.80%) | 1421 (50.80%) | 0.97 (0.95–1.00) | 0.057 | |||
| Sex | |||||||
| Female | 8034 (67.70%) | 1864 (66.60%) | 0.270 | 0.95 (0.87–1.04) | 0.270 | ||
| Male | 3828 (32.30%) | 933 (33.40%) | 1 (Reference) | ||||
| Insurance type/expected primary payer | |||||||
| Medicare, N (%) | 7371 (61.80%) | 1811 (64.20%) | <0.01 | 1 (Reference) | 1 (Reference) | ||
| Medicaid, N (%) | 1490 (12.50%) | 441 (15.60%) | 1.20 (1.07–1.36) | 0.002 | 1.25 (1.09–1.44) | 0.001 | |
| Private, N (%) | 2328 (19.50%) | 415 (14.70%) | 0.73 (0.65–0.82) | <0.01 | 0.80 (0.70–0.92) | 0.002 | |
| AHRQ comorbidities, N (%) | |||||||
| Deficiency anemias | 2734 (23%) | 874 (31.20%) | <0.01 | 1.51 (1.38–1.67) | <0.01 | 1.32 (1.19–1.45) | <0.01 |
| Congestive heart failure | 4601 (38.80%) | 1302 (46.50%) | <0.01 | 1.37 (1.26–1.49) | <0.01 | 1.25 (1.15–1.38) | <0.01 |
| Chronic pulmonary disease | 4741 (40%) | 1282 (45.80%) | <0.01 | 1.27 (1.17–1.38) | <0.01 | 1.20 (1.10–1.30) | <0.01 |
| Hypothyroidism | 2088 (17.6%) | 442 (15.8%) | 0.023 | 0.88 (0.78–0.98) | 0.024 | 0.85 (0.75–0.94) | 0.004 |
| Liver disease | 883 (7.40%) | 279 (10%) | <0.01 | 1.38 (1.20–1.59) | <0.01 | 1.24 (1.07–1.44) | 0.006 |
| Fluid and electrolyte disorders | 3733 (31.50%) | 1028 (36.80%) | <0.01 | 1.26 (1.16–1.38) | <0.01 | 1.15 (1.05–1.26) | 0.003 |
| Obesity | 3332 (28.10%) | 719 (25.70%) | 0.011 | 0.88 (0.80–0.97) | 0.011 | 0.80 (0.72–0.88) | <0.01 |
| Renal failure | 3159 (26.60%) | 965 (34.50%) | <0.01 | 1.45 (1.33–1.58) | <0.01 | 1.25 (1.13–1.38) | <0.01 |
| Weight loss | 485 (4.10%) | 159 (5.70%) | <0.01 | 1.41 (1.17–1.70) | <0.01 | 1.25 (1.03–1.52) | 0.021 |
| All patient refined diagnosis related group: risk of mortality subclass | |||||||
| Minor likelihood of dying, N (%) | 627 (5.30%) | 89 (3.20%) | <0.01 | ||||
| Moderate likelihood of dying, N (%) | 5535 (46.70%) | 1196 (42.80%) | |||||
| Major likelihood of dying, N (%) | 4597 (38.80%) | 1288 (46%) | |||||
| Extreme likelihood of dying, N (%) | 1102 (9.30%) | 224 (8%) | |||||
| All patient refined diagnosis related group: severity of illness subclass | |||||||
| Minor loss of function, N (%) | 153 (1.30%) | 18 (0.60%) | <0.01 | ||||
| Moderate loss of function, N (%) | 4650 (39.20%) | 899 (32.10%) | |||||
| Major loss of function, N (%) | 5761 (48.60%) | 1577 (56.40%) | |||||
| Extreme loss of function, N (%) | 1297 (10.90%) | 303 (10.80%) | |||||
| Disposition of patient at discharge | |||||||
| Home or self care | 7455 (62.86%) | 1621 (57.95%) | <0.01 | 1 (Reference) | 1 (Reference) | ||
| Transfer to skilled nursing or intermediate care facility | 1197 (10.09%) | 372 (13.3%) | 1.42 (1.25–1.62) | <0.01 | 1.21 (1.05–1.39) | 0.008 | |
| Home health care | 2510 (21.17%) | 705 (25.21%) | 1.28 (1.16–1.41) | <0.01 | 1.15 (1.03–1.29) | 0.008 | |
| Total length of stay in days, mean (± SD) | 6.41 (± 7.40) | 6.96 ( ± 8.40) | 0.001 | ||||
| Length of stay of index hospitalization, N (%) | |||||||
| ≤ 3days | 4648 (39.20%) | 971 (34.70%) | <0.01 | 1 (Reference) | 1 (Reference) | ||
| 4–7 days | 4084 (34.40%) | 1011 (36.10%) | 1.18 (1.07–1.30) | 0.001 | 1.09 (0.98–1.21) | 0.078 | |
| >7 days | 3130 (26.40%) | 815 (29.10%) | 1.25 (1.13–1.38) | <0.01 | 1.16 (1.04–1.30) | 0.009 | |
| Hospital bed size | |||||||
| Small, N (%) | 1017 (8.60%) | 275 (9.80%) | <0.01 | 1 (Reference) | 1 (Reference) | ||
| Medium, N (%) | 2297 (19.40%) | 628 (22.50%) | 1.01 (0.86–1.18) | 0.890 | |||
| Large, N (%) | 8548 (72.10%) | 1894 (67.70%) | 0.82 (0.71–0.94) | 0.006 | 0.84 (0.73–0.98) | 0.035 | |
| Hospital urban–rural designation | |||||||
| Large metropolitan areas with at least one million residents, N (%) | 7723 (65.10%) | 1860 (66.50%) | 0.140 | 1 (Reference) | |||
| Small metropolitan areas with less than one million residents, N (%) | 3466 (29.20%) | 779 (27.90%) | 0.93 (0.85–1.02) | 0.144 | |||
| Micropolitan areas, N (%) | 550 (4.60%) | 119 (4.30%) | 0.90 (0.73–1.10) | 0.304 | |||
| Not metropolitan or micropolitan (non-urban residual), N (%) | 123 (1%) | 39 (1.40%) | 1.31 (0.91–1.90) | 0.138 | |||
| Teaching status of hospital | |||||||
| Metropolitan non-teaching, N (%) | 3219 (27.10%) | 826 (29.50%) | 0.037 | 0.89 (0.80–0.97) | 0.001 | 0.92 (0.83–1.02) | 0.123 |
| Metropolitan teaching, N (%) | 7970 (67.20%) | 1813 (64.80%) | 0.91 (0.76–1.10) | 0.357 | |||
| Non-metropolitan, N (%) | 673 (5.70%) | 158 (5.60%) | 1 (Reference) | 1 (Reference) | |||
| Control/ownership of hospital | |||||||
| Government, nonfederal, N (%) | 2144 (18.10%) | 475 (17%) | 0.014 | 1 (Reference) | 1 (Reference) | ||
| Private, not-profit, N (%) | 8359 (70.50%) | 1949 (69.70%) | 1.05 (0.94–1.17) | 0.367 | |||
| Private, investor-owned, N (%) | 1359 (11.5%) | 373 (13.3%) | 1.24 (1.06–1.44) | 0.006 | 1.14 (0.97–1.35) | 0.110 | |
AHRQ: Agency for Healthcare Research and Quality. Values that are statistically significant with a p-value less than or equal to 0.05 are in bold.
Upon evaluation of clinical and hospital factors, patients transferred to skilled nursing or ICF (aOR: 1.21; 95% CI: 1.05–1.39) and patients discharged with home health care services (aOR: 1.15; 95% CI: 1.03–1.29) had a higher risk of readmission when compared to patients discharged home. Patients with index hospitalization LOS > 7 days had a higher risk of readmission when compared to index hospitalization LOS ≤ 3 days (aOR: 1.16; 95% CI: 1.04–1.30). PH patients admitted to hospitals with large bed size had a reduced risk of readmission when compared to hospitals with small bed size (aOR: 0.84; 95% CI: 0.73–0.98). Readmitted patients had worse APR-DRG severity of illness and higher risk of mortality subclass; 54% of readmitted patients had major or extreme likelihood of dying compared to 48% of non-readmitted patients; 67% of readmitted patients had major or extreme loss of function compared to 59% of non-readmitted patients. Significant factors in multivariable analysis associated with 30-day readmissions for PH are demonstrated in Fig. 3. We then assessed risk factors associated with 30-day readmission by PH subtype, namely PPH (Table 4) and SPH (Table 5). Significant factors in multivariable analysis associated with 30-day readmissions for PPH and SPH are demonstrated in supplemental figures 1 and 2, respectively.
Fig. 3.
Risk factors for 30-day readmissions in pulmonary hypertension.
Table 4.
Risk factors for 30-day readmissions in PPH.
| Characteristics | 30-day readmissions |
Multivariate OR |
|||||
|---|---|---|---|---|---|---|---|
| Not readmitted | Readmitted | p-Values | Unadjusted odds ratio (95% CI) | p-Values | Adjusted odds ratio (95% CI) | p-Values | |
| Age, mean (± SD) | |||||||
| Young adults (18–39 years), N (%) | 228 (19.2%) | 37 (15.0%) | 0.073 | 1 (Reference) | 1 (Reference) | ||
| Middle aged (40–64 years), N (%) | 506 (42.7%) | 124 (50.2%) | 1.16 (1.01–1.33) | 0.030 | 1.37 (1.03–1.81) | 0.028 | |
| Elderly (≥ 65 years), N (%) | 452 (38.1%) | 86 (34.8%) | 0.95 (0.86–1.05) | 0.331 | |||
| Sex | |||||||
| Female | 872 (73.5%) | 171 (69.2%) | 0.168 | 0.81 (0.60–1.09) | 0.168 | ||
| Male | 314 (26.5%) | 76 (30.8%) | 1 (Reference) | ||||
| AHRQ comorbidities, N (%) | |||||||
| Alcohol abuse | 27 (2.3%) | 14 (5.7%) | 0.004 | 2.58 (1.33–5) | 0.005 | 2.29 (1.14–4.59) | 0.019 |
| Fluid and electrolyte disorders | 398 (33.6%) | 114 (46.2%) | <0.01 | 1.7 (1.28–2.24) | <0.01 | 1.41 (1.05–1.89) | 0.021 |
| Renal failure | 235 (19.8%) | 73 (29.6%) | 0.001 | 1.7 (1.24–2.31) | 0.001 | 1.51 (1.09–2.09) | 0.013 |
| All patient refined diagnosis related group: risk of mortality subclass | |||||||
| Minor likelihood of dying | 108 (9.1%) | 11 (4.5%) | 0.020 | ||||
| Moderate likelihood of dying | 170 (14.3%) | 34 (13.8%) | |||||
| Major likelihood of dying | 766 (64.6%) | 181 (73.3%) | |||||
| Extreme likelihood of dying | 142 (12.0%) | 21 (8.5%) | |||||
| All patient refined diagnosis related group: severity of illness subclass | |||||||
| Minor loss of function | 3 (0.3%) | 0 (0.0%) | 0.009 | ||||
| Moderate loss of function | 516 (43.5%) | 80 (32.4%) | |||||
| Major loss of function | 508 (42.8%) | 131 (53.0%) | |||||
| Extreme loss of function | 159 (13.4%) | 36 (14.6%) | |||||
| Disposition of patient at discharge | |||||||
| Home or self care | 750 (63.24%) | 147 (59.51%) | 1 (Reference) | ||||
| Transfer to skilled nursing or intermediate care facility | 78 (6.58%) | 24 (9.72%) | 1.56 (0.95–2.54) | 0.080 | |||
| Home health care | 257 (21.67%) | 64 (24.91%) | 1.26 (0.91–1.74) | 0.160 | |||
| Total length of stay in days, mean (±SD) | 7.67 (± 9.5) | 9.65 (± 12.4) | 0.005 | ||||
| Length of stay of index hospitalization, N (%) | |||||||
| ≤3days | 417 (34.5%) | 64 (25.6%) | 0.002 | 1 (Reference) | 1 (Reference) | ||
| 4–7 days | 400 (33.1%) | 78 (31.2%) | 1.27 (0.89–1.82) | 0.190 | |||
| >7 days | 392 (32.4%) | 108 (43.2%) | 1.79 (1.28–2.52) | 0.001 | 1.52 (1.06–2.18) | 0.020 | |
| Hospital bed size | |||||||
| Small, N (%) | 77 (6.5%) | 24 (9.7%) | 0.131 | 1 (Reference) | 1 (Reference) | ||
| Medium, N (%) | 167 (14.1%) | 39 (15.8%) | 0.75 (0.42–1.33) | 0.326 | |||
| Large, N (%) | 942 (79.4%) | 184 (74.5%) | 0.62 (0.38–1.01) | 0.050 | 0.6 (0.36–0.97) | 0.040 | |
AHRQ: Agency for Healthcare Research and Quality. Values that are statistically significant with a p-value less than or equal to 0.05 are in bold.
Table 5.
Risk factors for 30-day readmissions in SPH.
| Characteristics | 30-day readmissions |
Multivariate OR |
|||||
|---|---|---|---|---|---|---|---|
| Not readmitted | Readmitted | p-Values | Unadjusted odds ratio (95% CI) | p-Values | Adjusted odds ratio (95% CI) | p-Values | |
| Age, mean (±SD) | |||||||
| Young adults (18–39 years), N (%) | 789 (7.4%) | 189 (7.4%) | 0.142 | 1 (Reference) | |||
| Middle aged (40–64 years), N (%) | 4076 (38.2%) | 1026 (40.2%) | 1.04 (0.99–1.09) | 0.055 | |||
| Elderly (≥65 years), N (%) | 5811 (54.4%) | 1335 (52.4%) | 0.97 (0.94–1.00) | 0.059 | |||
| Sex | |||||||
| Female | 7162 (67.1%) | 1693 (66.4%) | 0.504 | 0.97 (0.88–1.06) | 0.504 | ||
| Male | 3514 (32.9%) | 857 (33.6%) | 1 (Reference) | ||||
| Insurance type/expected primary payer | |||||||
| Medicare, N (%) | 6757 (62.9%) | 1687 (65.5%) | <0.01 | 1 (Reference) | 1 (Reference) | ||
| Medicaid, N (%) | 1292 (12.0%) | 396 (15.4%) | 1.23 (1.08–1.39) | 0.001 | 1.40 (1.22–1.60) | <0.01 | |
| Private, N (%) | 2017 (18.8%) | 349 (13.5%) | 0.69 (0.61–0.79) | <0.01 | 0.70 (0.60–0.81) | 0.017 | |
| Median household income | |||||||
| ≥$64,000, N (%) | 2252 (21.0%) | 492 (19.1%) | 0.142 | 0.86 (0.76–0.98) | 0.020 | 0.94 (0.83–1.07) | 0.384 |
| $48,000–$63,999, N (%) | 2526 (23.5%) | 579 (22.5%) | 0.90 (0.80–1.01) | 0.100 | |||
| $38,000–$47,999, N (%) | 2656 (24.7%) | 664 (25.8%) | 0.99 (0.88–1.11) | 0.830 | |||
| ≤37,999, N (%) | 3127 (29.1%) | 792 (30.7%) | 1 (Reference) | 1 (Reference) | |||
| AHRQ comorbidities, N (%) | |||||||
| Deficiency anemias | 2492 (23.3%) | 809 (31.7%) | <0.01 | 1.52 (1.39–1.68) | <0.01 | 1.33 (1.20–1.47) | <0.01 |
| Congestive heart failure | 4183 (39.2%) | 1210 (47.5%) | <0.01 | 1.40 (1.28–1.52) | <0.01 | 1.27 (1.15–1.40) | <0.01 |
| Chronic pulmonary disease | 4341 (40.7%) | 1193 (46.8%) | <0.01 | 1.28 (1.17–1.4) | <0.01 | 1.21 (1.10–1.32) | <0.01 |
| Diabetes, uncomplicated | 2952 (27.7%) | 767 (30.1%) | 0.014 | 1.12 (1.02–1.23) | 0.014 | 1.10 (1.02–1.22) | 0.048 |
| Hypothyroidism | 1906 (17.9%) | 397 (15.6%) | 0.006 | 0.85 (0.75–0.95) | 0.006 | 0.81 (0.72–0.91) | 0.001 |
| Liver disease | 797 (7.5%) | 250 (9.8%) | <0.01 | 1.35 (1.16–1.56) | <0.01 | 1.27 (1.09–1.49) | 0.002 |
| Fluid and electrolyte disorders | 3335 (31.2%) | 914 (35.8%) | <0.01 | 1.23 (1.12–1.34) | <0.01 | 1.12 (1.02–1.23) | 0.020 |
| Obesity | 3062 (28.7%) | 672 (26.4%) | 0.019 | 0.89 (0.8–0.98) | 0.019 | 0.81 (0.73–0.90) | <0.01 |
| Renal failure | 2924 (27.4%) | 892 (35.0%) | <0.01 | 1.42 (1.3–1.56) | <0.01 | 1.22 (1.11–1.36) | <0.01 |
| Weight loss | 421 (3.9%) | 141 (5.5%) | <0.01 | 1.42 (1.17–1.73) | <0.01 | 1.27 (1.03–1.56) | 0.021 |
| All patient refined diagnosis related group: risk of mortality subclass | |||||||
| Minor likelihood of dying | 519 (4.9%) | 78 (3.1%) | <0.01 | ||||
| Moderate likelihood of dying | 5365 (50.3%) | 1162 (45.6%) | |||||
| Major likelihood of dying | 3831 (35.9%) | 1107 (43.4%) | |||||
| Extreme likelihood of dying | 960 (9.0%) | 203 (8.0%) | |||||
| All patient refined diagnosis related group: severity of illness subclass | |||||||
| Minor loss of function | 150 (1.4%) | 18 (0.7%) | <0.01 | ||||
| Moderate loss of function | 4134 (38.7%) | 819 (32.1%) | |||||
| Major loss of function | 5253 (49.2%) | 1446 (56.7%) | |||||
| Extreme loss of function | 1138 (10.7%) | 267 (10.5%) | |||||
| Disposition of patient at discharge | |||||||
| Home or self care | 6705 (62.82%) | 1474 (57.8%) | 1 (Reference) | 1 (Reference) | |||
| Transfer to skilled nursing or intermediate care facility | 1119 (10.48%) | 348 (13.65%) | 1.41 (1.23–1.61) | <0.01 | 1.17 (1.02–1.35) | 0.030 | |
| Home health care | 2253 (21.11%) | 641 (25.14%) | 1.28 (1.15–1.42) | <0.01 | 1.13 (1.02–1.27) | 0.023 | |
| Total length of stay in days, mean (±SD) | 6.27 (±7.1) | 6.70 (±7.8) | 0.007 | ||||
| Length of stay of index hospitalization, N (%) | |||||||
| ≤3days | 4275 (39.8%) | 920 (35.7%) | 0.001 | 1 (Reference) | 1 (Reference) | ||
| 4–7 days | 3698 (34.4%) | 938 (36.4%) | 1.18 (1.07–1.30) | 0.001 | 1.08 (0.97–1.18) | 0.242 | |
| >7 days | 2766 (25.8%) | 719 (27.9%) | 1.21 (1.08–1.35) | 0.001 | 1.06 (0.94–1.21) | 0.302 | |
| Hospital bed size | |||||||
| Small, N (%) | 940 (8.8%) | 251 (9.8%) | <0.01 | 1 (Reference) | 1 (Reference) | ||
| Medium, N (%) | 2130 (20.0%) | 589 (23.1%) | 1.03 (0.88–1.22) | 0.681 | |||
| Large, N (%) | 7606 (71.2%) | 1710 (67.1%) | 0.84 (0.72–0.98) | 0.023 | 0.88 (0.75–1.02) | 0.090 | |
AHRQ: Agency for Healthcare Research and Quality. Values that are statistically significant with a p-value less than or equal to 0.05 are in bold.
Furthermore, patients with index hospitalization LOS > 7 days, patients transferred to skilled nursing facility (SNF) or ICF, and patients discharged with home health services had higher APR-DRG risk of mortality subclass (OR: 4.00; 95% CI: 3.67–4.36) vs (OR: 3.47; 95% CI: 3.09–3.89) vs (OR: 2.41; 95% CI: 2.22–2.62) and higher APR-DRG severity of illness subclass (OR: 6.01; 95% CI: 5.45–6.61) vs (OR: 3.00; 95% CI: 2.65–3.40) vs (OR: 2.23; 95% CI: 2.05–2.43).
Discussion
While PH is an increasingly recognized condition, limited data exist with regard to risk for readmission and factors associated with readmissions in this population. In part, this may be attributed to several factors including an increasing recognition of PH, as well as evolving clinical definitions and administrative coding of PH and its subgroups. As an initial look into understanding the scope of PH readmissions, we analyzed the NRD to provide national estimates of readmissions following an index hospitalization for PH. We found nearly one-fifth of patients with PH were readmitted within 30 days, with approximately one-third occurring during week 1. Risk factors for readmission after hospitalization for PH were primarily cardiovascular in nature and were primarily associated with patient characteristics and complications related to comorbid conditions.
As has been described across many cardiopulmonary conditions, the presence of PH is a strong predictor of mortality and hospitalizations.12–15 PH is associated with worse morbidity and mortality in the context of chronic obstructive pulmonary disease (COPD) and left heart disease (PH-LHD) either from heart failure with reduced ejection fraction or heart failure with preserved ejection fraction.16–19 Moreover, PH-LHD patients have more severe symptoms and experience higher hospitalization rates with significant implications to quality of life and healthcare costs.16–19 This is consistent with our study, which showed that PH patients with CHF and COPD had an increased risk of readmission.
Our study also showed an increased risk of readmission in patients with renal failure and fluid and electrolyte disturbances, which is consistent with prior data showing a correlation between the presence of renal failure, hyponatremia, hypervolemia, and PH.20–23
In addition, alcohol abuse was associated with a significantly higher odds of readmission in the PPH subgroup. This was independent of hypervolemia, fluid and electrolyte disturbances, and renal disease,24,25 although it could possibly suggest the presence of portopulmonary hypertension.26,27 The presence of diabetes and anemia also significantly increased the odds of readmission as previously described.28–30 Finally, we have found an “obesity readmission paradox” wherein there is a 20% reduction in odds for readmission in patients with obesity. Conversely, weight loss is associated with a nearly 30% increase in odds of readmission, which is consistent with prior data in PH.31,32
LOS is an indicator of process of care and patient-related factors and can hence influence readmission risk.33 Sud et al analyzed the Canadian Institute for Health Information Discharge Abstract Database for associations between LOS and 30-day readmissions in hospitalized patients with CHF and described that a longer LOS for CHF was associated with higher 30-day all-cause readmission risk.33 In our study, we similarly found that PH patients with index hospitalization LOS of more than seven days had an increased risk of readmission. In addition, we also found that patients transferred to SNF or ICF and patients discharged with home health services had an increased risk of readmissions as compared to those discharged home. In subgroup analyses, patients with index hospitalization LOS > 7 days, patients transferred to SNF/ICF, or patients discharged with home health services had higher APR-DRG risk of mortality and severity of illness.
Our study also elucidated a substantial burden of PH readmissions in middle-aged adults and patients with Medicaid insurance, consistent with data in the general population, while patients with private insurance had a reduced risk of readmission.34 This suggests that procedures targeted at the Medicaid population should be evaluated to help mitigate risk of readmission.
Beyond the direct costs accrued during the index hospitalization, our analysis demonstrates the substantial economic burden incurred by the US healthcare system secondary to PH readmissions. This finding from our study is consistent with Burke et al.,35 which analyzed the Optum Research Database for PH readmissions in commercially insured patients and found that the mean cost of initial hospitalization was $46,070 when compared to a mean readmission cost of $73,066.35 Furthermore, Lacey et al. examined the same database for PAH and reported a mean readmission cost of $71,622.36 However, these results were based on patients with specific healthcare coverage plans in the Optum Research Database and were not representative of all patients with PH in the US.35 Furthermore, NRD provides a more comprehensive representation of utilization of healthcare coverage plans by the PH population.
Limitations
Our study must be interpreted in the context of its limitations, most of which are inherent to any national survey data analysis. Foremost, our study is limited by the use of administrative data dependent on the use of ICD-9 codes to identify index PH admissions. Beyond the potential for coding errors, which may lead to misclassification of PH, the coding terminology is archaic and does not reflect current clinical definitions or classification schema, as previously described.9 Not only does PH encompass a broad group of conditions with many subgroups, but its recognition has increased exponentially over the decades, along with the complexity associated with correctly ascribing diagnostic categories. For example, in practice, the presence of some degree of underlying lung disease (e.g. Interstitial Lung Disease (ILD)) does not necessarily negate the presence of “true” Group I PAH (as can be seen, for example, in scleroderma). Thus, appropriate categorization is challenging on the individual patient level, and is likely compounded when examining administrative data sets. Moreover, the terminology PPH and SPH do not equate to updated terms Group I PAH vs. Groups 2–5, respectively, as there are entities such as connective tissue disease-PAH which in the past would have been considered a “secondary” form of PH (i.e. SPH) which is indeed Group 1 PAH. Despite this, our work provides initial insights into the economic burden and clinical characteristics associated with PH readmissions, which may be improved with updates including the use of the ICD-10 aligned more closely to PH diagnostic definitions. Furthermore, misclassification may be particularly problematic with regard to rare diagnoses such as PAH which then may lead to inaccurate summary statistics, readmissions rates, etc. That said, it was reassuring that the PPH group was smaller in number, more likely female, younger, and with less percentage of certain comorbidities (including chronic lung disease, valve disease, obesity) as compared to SPH, as would be expected in non-Group 1 disease (i.e. Groups 2, 3 PH).
Other limitations include changes in the treatment of PH with the advent of new medications and treatment approaches, which have been shown to impact PH disease progression, including hospitalization, since the years in study.37–39 Furthermore, there are no patients in the entire NRD dataset who are dead between index admission and readmission, thus competing risks due to death cannot be evaluated. We also restricted our comorbidity analysis to Elixhauser comorbidities that, although not fully representative or inclusive, can in turn lead to issues with collinearity and lack of fit when adding the data into a logistic model. To avoid this, pre-existing comorbidities were apriori limited to chronic conditions determined on the basis of the 29 Elixhauser comorbidities provided by the AHRQ. Only those risk factors with a p-value of <0.1 on univariate analysis were entered into a multivariate logistic regression model to examine the association between risk factors and 30-day readmission, as represented by aOR. Furthermore, factors such as treatment compliance and objective lab values are not captured by the NRD and cannot be assessed in association with risk of readmission. Also, as there is no possible linkage between years as the data are derived from three unique datasets, we excluded patients who were discharged in the month of December in order to ensure adequate 30-day follow-up. And while NRD tracks admissions related to a specific patient in one state, there is no way to capture hospitalizations if a patient is readmitted in another state. Finally, we focused on 30-day readmissions though overall readmissions including those after 30 days may be systematically different.
Conclusions
In conclusion, 30-day readmission for PH is common and associated with high healthcare costs, with the majority readmitted for non-cardiovascular causes. We demonstrate important risk factors for 30-day readmission among individuals with PH, while acknowledging limitations to administrative data specifically in the context of PH. Despite the inherent limitations, the results of this investigation serve as a foundation to study this topic in the current era of PH therapy, clinical classification, and ICD-10 coding era. Further research should focus on optimization of coding schema utilization, and identification of effective interventions (perhaps initially in the high-risk populations, i.e. Medicaid and SNF-discharged patients), to reduce PH, and specifically PAH readmissions, including the role of remote monitoring, early post-discharge follow-up strategies, among others.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020966889 for Risk factors for 30-day readmission in adults hospitalized for pulmonary hypertension by Priyanka T. Bhattacharya, Asif M. Abdul Hameed, Shubhadeep T. Bhattacharya, Julio A. Chirinos, Wei-Ting Hwang, Edo Y. Birati, Jonathan N. Menachem, Saurav Chatterjee, Jay S. Giri, Steven M. Kawut, Stephen E. Kimmel and Jeremy A. Mazurek in Pulmonary Circulation
Supplemental material, sj-pdf-2-pul-10.1177_2045894020966889 for Risk factors for 30-day readmission in adults hospitalized for pulmonary hypertension by Priyanka T. Bhattacharya, Asif M. Abdul Hameed, Shubhadeep T. Bhattacharya, Julio A. Chirinos, Wei-Ting Hwang, Edo Y. Birati, Jonathan N. Menachem, Saurav Chatterjee, Jay S. Giri, Steven M. Kawut, Stephen E. Kimmel and Jeremy A. Mazurek in Pulmonary Circulation
Conflict of interest: J.A.C. has received consulting honoraria from Sanifit, Microsoft, Fukuda Denshi, Bristol Myers Squibb, OPKO Healthcare, Ironwood Pharmaceuticals, Pfizer, Akros Pharma, Merck, Edwards Lifesciences, and Bayer. J.A.C. is named as inventor in the University of Pennsylvania patent for the use of inorganic nitrates/nitrites for the treatment of HF and Preserved Ejection Fraction, and a patent application for the use of novel neoepitope biomarkers of tissue fibrosis in heart failure. S.E.K. has received consulting fees from several pharmaceutical companies, all unrelated to the content of this study. J.A.M has received advisory board honoraria from Actelion Pharmaceuticals and United Therapeutics, speaker honoraria from Abbott and research support from Actelion Pharmaceuticals, Complexa, Corvia Medical and Tenax Therapeutics.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplemental material: Supplemental material for this article is available online.
Author contributions
All authors have contributed to this work in the following manner:
P.T.B. and J.A.M. conceived of the project and study design.
P.T.B., A.M.A.H. and S.T.B. contributed to data acquisition.
All authors contributed to data analysis and interpretation with the majority of analysis performed by P.T.B., W.-T.H., J.A.M. and S.T.B.
P.T.B., A.M.A.H. and J.A.M. prepared the draft manuscript and all authors contributed to the writing and editing of the manuscript.
All authors have reviewed and agree with the content of the article. None of the article contents are under consideration for publication in any other journal or have been published in any journal.
ORCID iDs
Priyanka T. Bhattacharya https://orcid.org/0000-0001-6922-7084
Asif M. Abdul Hameed https://orcid.org/0000-0001-5928-5029
References
- 1.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016; 4: 306–322. [DOI] [PubMed] [Google Scholar]
- 2.Wijeratne DT, Lajkosz K, Brogly SB, et al. Increasing incidence and prevalence of World Health Organization Groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes 2018; 11: e003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest 2011; 139: 128–137. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 5.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. [DOI] [PubMed] [Google Scholar]
- 6.George MG, Schieb LJ, Ayala C, et al. Pulmonary hypertension surveillance: United States, 2001 to 2010. Chest 2014; 146: 476–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikachi RR, Sahni S, Mehta D, et al. Nationwide trends in inpatient admissions of pulmonary hypertension in the United States from 2000 to 2013. Adv Respir Med 2017; 85: 77–86. [DOI] [PubMed] [Google Scholar]
- 8.Anand V, Roy SS, Archer SL, et al. Trends and outcomes of pulmonary arterial hypertension-related hospitalizations in the United States: analysis of the nationwide inpatient sample database from 2001 through 2012. JAMA Cardiol 2016; 1: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 9.Link J, Glazer C, Torres F, et al. International Classification of Diseases coding changes lead to profound declines in reported idiopathic pulmonary arterial hypertension mortality and hospitalizations: implications for database studies. Chest 2011; 139: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HCUP National Readmissions Database (NRD). Healthcare Cost and Utilization Project (HCUP) 2012–2014 Agency for Healthcare Research and Quality, Rockville, MD, www.hcup-usahrqgov/nisoverviewjsp (2012–2014, accessed 19 October 2020).
- 11.HCUP National Readmissions Database (NRD). Healthcare Cost and Utilization Project (HCUP) 2012–2014, www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp (2012–2014, accessed 19 October 2020).
- 12.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: D109–D116. [DOI] [PubMed] [Google Scholar]
- 13.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant 2012; 31: 373–380. [DOI] [PubMed] [Google Scholar]
- 14.Burrows B, Kettel LJ, Niden AH, et al. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med 1972; 286: 912–918. [DOI] [PubMed] [Google Scholar]
- 15.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 1995; 107: 1193–1198. [DOI] [PubMed] [Google Scholar]
- 16.Dzudie A, Kengne AP, Thienemann F, et al. Predictors of hospitalisations for heart failure and mortality in patients with pulmonary hypertension associated with left heart disease: a systematic review. BMJ Open 2014; 4: e004843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation 2012; 126: 975–990. [DOI] [PubMed] [Google Scholar]
- 18.Haddad F, Kudelko K, Mercier O, et al. Pulmonary hypertension associated with left heart disease: characteristics, emerging concepts, and treatment strategies. Prog Cardiovasc Dis 2011; 54: 154–167. [DOI] [PubMed] [Google Scholar]
- 19.Vachiery JL, Adir Y, Barbera JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 2013; 62: D100–D108. [DOI] [PubMed] [Google Scholar]
- 20.Haddad F, Peterson T, Fuh E, et al. Characteristics and outcome after hospitalization for acute right heart failure in patients with pulmonary arterial hypertension. Circ Heart Fail 2011; 4: 692–699. [DOI] [PubMed] [Google Scholar]
- 21.Kawar B, Ellam T, Jackson C, et al. Pulmonary hypertension in renal disease: epidemiology, potential mechanisms and implications. Am J Nephrol 2013; 37: 281–290. [DOI] [PubMed] [Google Scholar]
- 22.Rabinovitz A, Raiszadeh F, Zolty R. Association of hyponatremia and outcomes in pulmonary hypertension. J Card Fail 2013; 19: 550–556. [DOI] [PubMed] [Google Scholar]
- 23.Hansen L, Burks M, Kingman M, et al. Volume management in pulmonary arterial hypertension patients: an expert pulmonary hypertension clinician perspective. Pulm Ther 2018; 4: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med 2017; 377: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 25.Moses E, Rigas K. Metabolic abnormalities in alcoholic patients: focus on acid base and electrolyte disorders. J Alcohol Drug Depend 2015; 3: 1. [Google Scholar]
- 26.Fritz JS, Fallon MB, Kawut SM. Pulmonary vascular complications of liver disease. Am J Respir Crit Care Med 2013; 187: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surani SR, Mendez Y, Anjum H, et al. Pulmonary complications of hepatic diseases. World J Gastroenterol 2016; 22: 6008–6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abernethy AD, Stackhouse K, Hart S, et al. Impact of diabetes in patients with pulmonary hypertension. Pulm Circ 2015; 5: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krasuski RA, Hart SA, Smith B, et al. Association of anemia and long-term survival in patients with pulmonary hypertension. Int J Cardiol 2011; 150: 291–295. [DOI] [PubMed] [Google Scholar]
- 30.Grinnan D, Farr G, Fox A, et al. The role of hyperglycemia and insulin resistance in the development and progression of pulmonary arterial hypertension. J Diabetes Res 2016; 2016: 2481659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samareh Fekri M, Torabi M, Azizi Shoul S, et al. Prevalence and predictors associated with severe pulmonary hypertension in COPD. Am J Emerg Med 2018; 36: 277–280. [DOI] [PubMed] [Google Scholar]
- 32.Mazimba S, Holland E, Nagarajan V, et al. Obesity paradox in group 1 pulmonary hypertension: analysis of the NIH-Pulmonary Hypertension registry. Int J Obes (Lond) 2017; 41: 1164–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sud M, Yu B, Wijeysundera HC, et al. Associations between short or long length of stay and 30-day readmission and mortality in hospitalized patients with heart failure. JACC Heart Fail 2017; 5: 578–588. [DOI] [PubMed] [Google Scholar]
- 34.Strom JB, Kramer DB, Wang Y, et al. Short-term rehospitalization across the spectrum of age and insurance types in the United States. PLoS One 2017; 12: e0180767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke JP, Hunsche E, Regulier E, et al. Characterizing pulmonary hypertension-related hospitalization costs among Medicare Advantage or commercially insured patients with pulmonary arterial hypertension: a retrospective database study. Am J Manag Care 2015; 21: s47–s58. [PubMed] [Google Scholar]
- 36.Lacey M, Hunsche E, Buzinec P, et al. Hospitalization costs related to pulmonary hypertension (PH) among Medicare advantage or commercially insured patients with pulmonary arterial hypertension (PAH) in the United States. Value Health 2013; 16: A233. [Google Scholar]
- 37.Galie N, Barbera JA, Frost AE, et al. Initial use of Ambrisentan plus Tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 38.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 39.Channick RN, Delcroix M, Ghofrani HA, et al. Effect of macitentan on hospitalizations: results from the SERAPHIN trial. JACC Heart Fail 2015; 3: 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020966889 for Risk factors for 30-day readmission in adults hospitalized for pulmonary hypertension by Priyanka T. Bhattacharya, Asif M. Abdul Hameed, Shubhadeep T. Bhattacharya, Julio A. Chirinos, Wei-Ting Hwang, Edo Y. Birati, Jonathan N. Menachem, Saurav Chatterjee, Jay S. Giri, Steven M. Kawut, Stephen E. Kimmel and Jeremy A. Mazurek in Pulmonary Circulation
Supplemental material, sj-pdf-2-pul-10.1177_2045894020966889 for Risk factors for 30-day readmission in adults hospitalized for pulmonary hypertension by Priyanka T. Bhattacharya, Asif M. Abdul Hameed, Shubhadeep T. Bhattacharya, Julio A. Chirinos, Wei-Ting Hwang, Edo Y. Birati, Jonathan N. Menachem, Saurav Chatterjee, Jay S. Giri, Steven M. Kawut, Stephen E. Kimmel and Jeremy A. Mazurek in Pulmonary Circulation