Abstract
Patients with portal hypertension may develop pulmonary hypertension. The economic implications of these comorbidities have not been systematically assessed. We compared healthcare resource utilization and costs in the United States between patients with co-existing portal hypertension and pulmonary hypertension (pulmonary hypertension cohort) and a matched cohort of portal hypertension patients without pulmonary hypertension (control cohort). In this retrospective analysis, adult pulmonary hypertension and control patients were identified from the Optum® Clinformatics® Data Mart database between 1 July 2014 and 30 June 2018. All patients had ≥2 claims with diagnosis codes for portal hypertension; pulmonary hypertension patients had ≥2 claims with diagnosis codes for pulmonary hypertension; controls could not have pulmonary hypertension diagnoses or any claims for pulmonary arterial hypertension-specific medications. Controls were matched to pulmonary hypertension patients by age, sex, Charlson comorbidity index score, and liver diseases. We assessed 12-month healthcare resource utilization and costs. Each cohort included 146 patients. During follow-up, pulmonary hypertension cohort patients were more likely than controls to experience a hospitalization (51% vs. 32%, P = 0.0014) and an emergency room visit (55% vs. 41%, P = 0.026). The average annual total cost was higher in pulmonary hypertension patients than for matched controls ($119,912 vs. $81,839, P < 0.0001). After covariate adjustment, costs for pulmonary hypertension cohort patients were 1.47 times higher than those for controls (P = 0.0197). These findings suggest that patients with portal hypertension and co-existing pulmonary hypertension are at a greater risk for hospitalization and incur higher mean annual total costs than portal hypertension patients without pulmonary hypertension.
Keywords: pulmonary arterial hypertension, portal hypertension, portopulmonary hypertension, healthcare resource utilization, hospitalization
Patients with advanced liver disease may also suffer from pulmonary complications, including pulmonary hypertension (PH).1 Some of these patients have pulmonary arterial hypertension (PAH), a rare disease characterized by increased vascular resistance and remodeling of the small pulmonary arteries, leading to right heart failure and death.2,3 Portopulmonary hypertension (PoPH) is a PAH subtype associated with portal hypertension.4,5 However, co-existence of portal hypertension and PH does not necessarily mean that the patient suffers from PoPH.6 Indeed, there are other reasons for the development of PH in patients who have advanced liver disease, including volume overload or hyperdynamic state.6,7
Regardless of the PH subtype or etiology, it is important to investigate the health and economic burden of PH in the setting of portal hypertension. However, most published literature is limited to patients with PoPH, rather than those with unspecified PH plus portal hypertension, which would also include PoPH. Of 3525 patients with PAH in the US Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management (REVEAL), 174 (5%) had PoPH.8 Data from REVEAL and the UK National PAH Registry have indicated worse outcomes with PoPH—including higher hospitalization rates and poorer survival—compared to idiopathic PAH.8,9
PoPH is most frequently encountered in patients with cirrhosis, the most common cause of portal hypertension.10 A prospective study of 1235 patients evaluated for liver transplant found that 66 (5%) met hemodynamic criteria for PoPH.6 No study has assessed the additional healthcare resource utilization (HCRU) and cost of care attributable to PH in patients who have portal hypertension, beyond the economic burden of portal hypertension and comorbid liver disease.
A recent survey of 74 physicians at 35 liver transplant centers reported variability in provider attitudes and practice patterns across the US regarding PoPH management, highlighting the need to standardize care in patients with this dual comorbidity.11 Understanding the current management and main cost drivers of portal hypertension in the setting of PH may identify strategies to improve quality of care. The objective of this study is to compare HCRU and costs between patients with portal hypertension and concomitant PH and patients with portal hypertension without PH.
Methods
Data source
Data were retrieved from the Optum® Clinformatics® Data Mart database, containing medical, pharmaceutical, and facility administrative claims for a privately insured US population. Most patients in the database have both medical and pharmacy benefits.
All data were anonymized and fully compliant with Health Insurance Portability and Accountability Act Privacy Rules. This retrospective claims database analysis contained no experiments on human or animal subjects requiring ethical approval.
Study design and sample
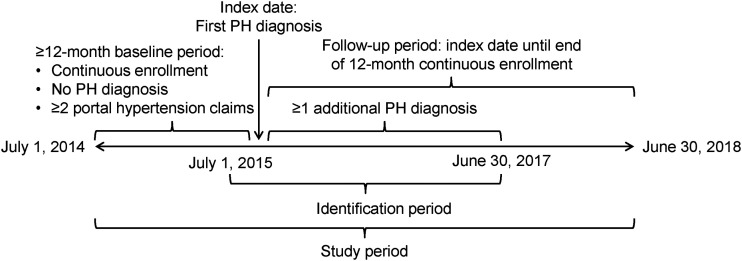
We retrieved data for the study period between 1 July 2014 and 20 June 2018 (Fig. 1). For each patient in the portal hypertension with PH group (PH cohort), we defined the index date as the date, within the identification period of 1 July 2015 through 30 June 2017, of the first claim associated with an International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM or ICD-10-CM, respectively) diagnosis code for PH: ICD-9-CM 416.0, 416.8, or 416.9, or ICD-10-CM I27.0, I27.2, I27.89, or I27.9 (Supplementary Table 1). For each patient in the control cohort (i.e. with portal hypertension but no PH), we selected a random index date from a similar distribution as the index date for PH cohort patients. The date range of the identification period allowed for 12 months prior to the index date for each patient to evaluate baseline characteristics. The follow-up period for each patient was ≥ 12 months following his/her index date. To avoid the influence of end-of-life costs unrepresentative of most patients, we restricted our analyses to patients who remained alive throughout the follow-up period.
Fig. 1.
Study design. Control patients had no PH diagnosis during the study period.
PH: pulmonary hypertension.
Adult patients ( ≥ 18 years of age at the index date) were eligible if they had continuous health plan enrollment for ≥ 12 months prior to the index date and ≥12 months following the index date (a gap in enrollment of ≤ 30 days was permitted), and ≥2 medical claims for portal hypertension (ICD-9-CM 572.3 or ICD-10-CM K76.6) during the baseline period. Inclusion in the PH group required ≥2 medical claims dated 1 day to 12 months apart within the identification period with a diagnosis code for PH. Inclusion in the control group required no PH diagnosis and no prescription claim for PAH-specific medications (including prostacyclin pathway agents, endothelin receptor antagonists, phosphodiesterase type-5 inhibitors, or riociguat) at any time during the study period. We identified drugs from pharmacy claims by National Drug Code and from medical claims by Healthcare Common Procedure Coding System codes as reported in Supplementary Table 1.
We excluded patients in the PH cohort with a diagnosis or Current Procedural Terminology code indicative of liver transplant (Supplementary Table 1) at any time during the study period, because most patients who have portal hypertension and PH are considered ineligible for liver transplant due to poor and unpredictable outcomes, including a higher risk of perioperative mortality in the presence of increased hemodynamic pressures.12
Demographic variables recorded for each patient were age, sex, geographic region, and type of insurance (Commercial or Medicare). We calculated the Charlson comorbidity index (CCI) following methods of Quan et al.,13 using updated weights from Quan et al.14 The following liver-related conditions were identified based on diagnosis codes: hepatitis B or C viral infection, autoimmune hepatitis, cirrhosis, alcoholic cirrhosis, biliary cirrhosis, cirrhosis alcoholic + viral hepatitis, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis (Supplementary Table 1).
Outcomes
We assessed patients’ HCRU and costs during the 12-month follow-up period, including for hospital admissions, emergency room (ER) visits, outpatient office/clinic visits, and pharmacy claims. HCRU and costs were categorized as all-cause and PH-specific. We identified PH-specific claims based on associated PH diagnosis codes.
Hospital admissions and readmissions were evaluated as rates per 100 person-years and mean number per patient. We defined readmissions at two time points: first as admissions occurring within 30 days of discharge from a preceding hospitalization and second as those occurring at any time post-discharge during the 12-month follow-up. Risk of hospitalization was assessed over a minimum of 12 months after index date, and after this 12-month period, each patient was censored at the end of continuous enrollment, all-cause death, or end of data (30 June 2018), whichever occurred earliest.
We converted cost measures to mean per-patient annual costs. All costs were inflation-adjusted to 2018 US dollars based on the Consumer Price Index. PAH-related medication costs, for patients in the PH group only, were summed from pharmacy claims for PAH-related therapies, as previously defined.
Statistical analysis
We constructed a balanced 1:1 matched study using propensity score matching. Propensity scores were derived from a logistic regression model, with the dependent variable being PH case or control with the following matching covariates: age, sex, CCI score, and liver diseases (as defined previously). We used 1:1 matching without replacement and a caliper width equal to 0.2 of the propensity scores. Descriptive statistics are reported as mean and standard deviation (SD) for continuous variables and counts and percentages for categorical variables. Counts of <10 when summed across both groups were suppressed and reported as not available in accordance with privacy guidelines.15
In univariate analyses, comparison of baseline characteristics, HCRU, and costs between PH cohort patients and control cohort patients used Student’s t-tests or Wilcoxon–Mann–Whitney tests for continuous variables and chi-squared or Fisher’s exact tests for categorical variables. Kaplan–Meier analysis was used to graph time to first all-cause hospitalization in both groups, without covariate adjustment.
In multivariable analyses, we compared the risk of adjusted all-cause hospitalization between the PH and control groups with Cox proportional hazards models, calculating the hazard ratio (HR) and 95% confidence interval (CI). A negative binominal regression model with follow-up time as the offset variable was used to compare the all-cause hospitalization rate ratio (RR) with 95% CI between the two groups, calculated as number of hospital admissions per person-time. To account for the skewed distribution of cost data, we used a generalized linear regression model with gamma distribution and log link function to compare the annual (i.e. 12-month post-index) total healthcare costs between PH cases and controls. To describe the percentage change in mean total healthcare costs between cases and controls, exponentiated β coefficients with 95% CI are reported.
We used the Instant Health Data platform (Boston Health Economics, LLC, Boston, MA) to create the data cut and develop analytic variables. Statistical analyses were undertaken with R, version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
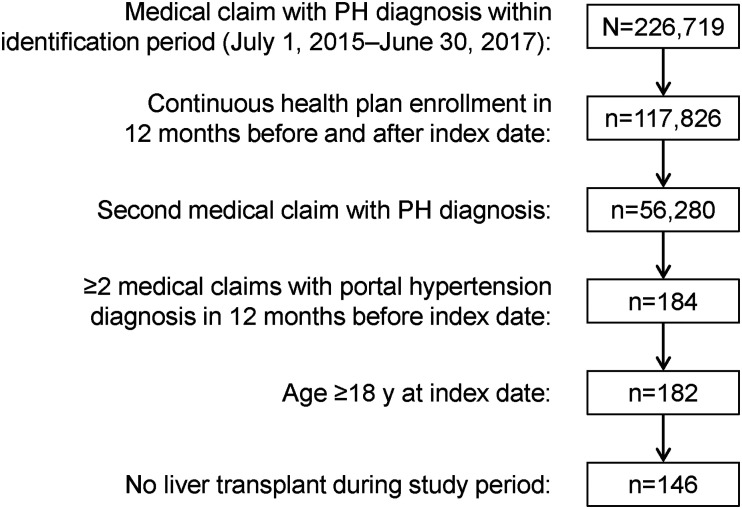
After the application of all eligibility criteria, 146 patients were identified for inclusion in the portal hypertension with PH group (PH cohort; Fig. 2), and 146 portal hypertension patients without PH were matched by propensity score for the control cohort. At baseline, 53% of PH patients were female, 81% had Medicare insurance, 45% resided in the US southern region, and 95% had cirrhosis (Table 1). The mean (SD) age of the PH cohort was 66.4 (9.3) years, with 63% of patients aged 65 years or older. Controls were similar in age, sex distribution, insurance type, US geographic region, CCI score, and liver diseases at baseline (Table 1).
Fig. 2.
Ascertainment of the PH cohort (portal hypertension with PH). PH: pulmonary hypertension.
Table 1.
Baseline characteristics for portal hypertension patients with PH and controls with portal hypertension but without PH, matched by propensity score.
| PH patients (n = 146) | Matched controls (n = 146) | P | |
|---|---|---|---|
| Age, years, mean (SD) | 66.41 (9.30) | 64.22 (10.90) | 0.0655 |
| Female, n (%) | 77 (52.74) | 75 (51.37) | 0.9067 |
| Insurance type, n (%) | |||
| Commercial | 28 (19.18) | 42 (28.77) | |
| Medicare | 118 (80.82) | 104 (71.23) | 0.0747 |
| US geographic region, n (%) | |||
| Midwest | 36 (24.66) | 23 (15.75) | |
| Northeast | 13 (8.90) | 9 (6.16) | 0.1575 |
| South | 65 (44.52) | 80 (54.79) | |
| West | 32 (21.92) | 34 (23.29) | |
| CCI score, mean (SD) | 7.73 (2.14) | 7.62 (2.26) | 0.6707 |
| Liver disease, n (%)* | |||
| Autoimmune hepatitis | 6 (4.11) | 12 (8.22) | 0.2238 |
| Biliary cirrhosis | 12 (8.22) | 10 (6.85) | 0.8245 |
| Cirrhosis | 138 (94.52) | 141 (96.58) | 0.5704 |
| Cirrhosis, alcoholic | 57 (39.04) | 58 (39.73) | 1.00 |
| Cirrhosis, alcoholic + viral hepatitis | 14 (9.59) | 12 (8.22) | 0.8372 |
| Hepatitis C | 35 (23.97) | 33 (22.60) | 0.8899 |
| Non-alcoholic fatty liver disease | 68 (46.58) | 64 (43.84) | 0.7243 |
| Non-alcoholic steatohepatitis† | 20 (13.70) | 20 (13.70) | 1.00 |
CCI: Charlson comorbidity index; PH: pulmonary hypertension; SD: standard deviation.
The number of patients with hepatitis B was not reported because the sample size summed across both groups was less than 10.
ICD-10-CM code for non-alcoholic steatohepatitis was not available in the US until October 2015.
Healthcare resource utilization
All-cause
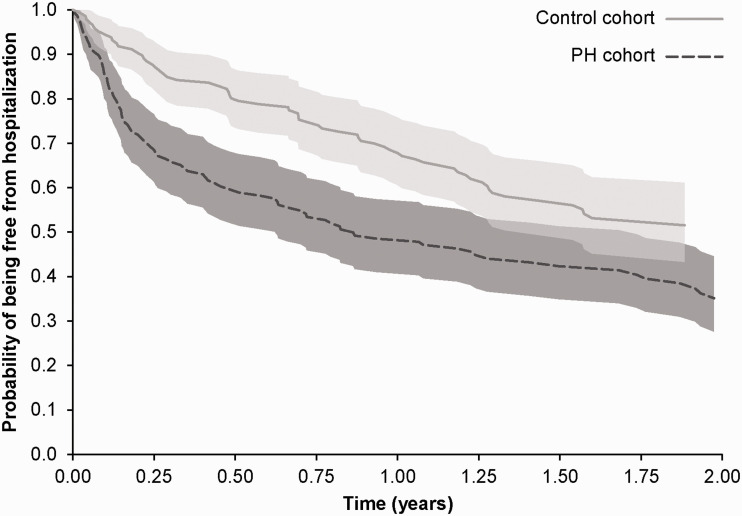
All-cause hospitalizations were significantly more frequent in the PH cohort than in the control cohort (Table 2). The proportion of patients hospitalized in the 12 months following index date was 51% in the PH group compared with 32% in the control group (P = 0.0014). Over the variable follow-up period (i.e. ≥12 months), the risk of all-cause hospitalization was 73% higher among PH cohort patients compared with controls (HR 1.73, 95% CI 1.23, 2.35; P = 0.0013) (Fig. 3). The rate of all-cause hospitalization in PH cohort patients was 118.0 per 100 person-years compared to 84.9 per 100 person-years in controls. The negative binominal regression model showed that the all-cause hospitalization rate was 51% higher in PH patients compared with controls (RR 1.51, 95% CI 1.1, 2.30; P = 0.0002).
Table 2.
All-cause healthcare resource utilization during the 12 months following index date in portal hypertension patients with PH and controls with portal hypertension but without PH, matched by propensity score.
| PH patients (n = 146) | Matched controls (n = 146) | P | |
|---|---|---|---|
| All-cause hospitalizations | |||
| Patients with hospitalization, n (%) | 75 (51.37) | 47 (32.19) | 0.0014 |
| Number of hospitalizations | |||
| Mean (SD) | 1.49 (2.22) | 0.80 (1.68) | 0.0006 |
| Median (IQR) | 1 (0–2) | 0 (0–1) | |
| By category, n (%) | 0.0082 | ||
| 0 | 71 (48.63) | 99 (67.81) | |
| 1 | 26 (17.81) | 20 (13.70) | |
| 2 | 19 (13.01) | 10 (6.85) | |
| ≥3 | 30 (20.55) | 17 (11.64) | |
| Readmissions, n (%) | |||
| Within 30 days after initial discharge | 19 (13.01) | 10 (6.85) | NA* |
| Within 12 months after initial discharge | 49 (33.56) | 27 (18.49) | 0.0051 |
| All-cause ER visits | |||
| Patients with ER visits, n (%) | 80 (54.79) | 60 (41.10) | 0.026 |
| Number of ER visits | |||
| Mean (SD) | 1.6 (2.91) | (1.41) (2.77) | 0.0886 |
| Median (IQR) | 1 (0–2) | 0 (0–2) | |
| All-cause outpatient office/clinic services | |||
| Patients with outpatient office/clinic services, n (%) | 118 (80.82) | 110 (75.34) | 0.3221 |
| Number of outpatient office/clinic services | |||
| Mean (SD) | 13.47 (12.79) | 11.1 (12.11) | 0.0777 |
| Median (IQR) | 10 (3–20) | 9 (1–16) | |
| All-cause pharmacy | |||
| Patients with ≥1 medication dispensed, n (%) | 128 (86.67) | 137 (93.84) | 0.1061 |
| Number of medications dispensed | |||
| Mean (SD) | 65.53 (55.28) | 49.28 (41.78) | 0.0446 |
| Median (IQR) | 55.5 (22.3–89.8) | 42.5 (17.3–64.8) |
ER: emergency room; IQR: interquartile range; NA: not available; PH: pulmonary hypertension; SD: standard deviation.
The statistical software could not calculate a P value (the chi-squared and Fisher’s exact test failed to converge).
Fig. 3.
Kaplan–Meier curves with 95% confidence intervals for all-cause hospitalization in portal hypertension patients with PH (PH cohort) and controls with portal hypertension but without PH, matched by propensity score. PH: pulmonary hypertension.
A significantly higher proportion of PH patients than controls had ER visits: 55% vs. 41%, respectively; P = 0.026 (Table 2). There were no statistically significant between-groups differences in the proportion of patients with outpatient office/clinic services (80% vs. 75%, respectively; P = 0.3221) or the proportion of patients with any type of medication dispensed for any reason (88% vs. 94%, respectively; P = 0.1061). As expected, due to the additional management burden of PH, patients with portal hypertension and PH had a significantly higher average number of medications dispensed compared with controls during the 12 months following index date: mean (SD) 65.53 (55.28) vs. 49.28 (41.78), respectively (P = 0.0446).
PH-related
Among PH patients, approximately 34% had a PH-related hospitalization, 16% had a PH-related ER visit, and 33% had PH-related outpatient office/clinic service use during the 12-month follow-up (Table 3). Among PH patients with at least one PH-related hospitalization, 14% and 38% of patients were readmitted for another PH-related hospitalization within 30 days and 12 months post-discharge, respectively.
Table 3.
PH-related healthcare resource utilization during the 12 months following index date in portal hypertension patients with PH.
| PH patients (n = 146) | |
|---|---|
| PH-related hospitalizations | |
| Patients with hospitalizations | 50 (34.25) |
| Number of hospitalizations | |
| Mean (SD) | 0.63 (1.21) |
| Median (IQR) | 0 (0–1) |
| By category, n (%) | |
| 0 | 96 (62.75) |
| 1 | 31 (21.23) |
| 2 | 8 (5.48) |
| ≥3 | 11 (7.53) |
| Readmissions, n (%) | |
| Within 30 days after initial discharge | 7 (4.79) |
| Within 12 months after initial discharge | 19 (13.01) |
| PH-related ER visits | |
| Patients with ER visits, n (%) | 23 (15.75) |
| Number of ER visits | |
| Mean (SD) | 0.21 (0.61) |
| Median (IQR) | 0 (0–0) |
| PH-related outpatient office/clinic services | |
| Patients with outpatient office/clinic services, n (%) | 48 (32.88) |
| Number of outpatient office/clinic services | |
| Mean (SD) | 0.69 (1.29) |
| Median (IQR) | 0 (0–1) |
| PAH-specific medications, n (%) | |
| Any PAH medication | 20 (13.70) |
| Endothelin receptor antagonist | 9 (6.16) |
| Phosphodiesterase type-5 inhibitors | 15 (10.27) |
| Prostacyclin-pathway drug | 8 (5.48) |
| Soluble guanylyl cyclase stimulator | 2 (1.37) |
ER: emergency room; IQR: interquartile range; PH: pulmonary hypertension; SD: standard deviation.
In the PH cohort, 86% of patients had no prescription fill for a PAH-specific medication in the one-year follow-up. Among PH patients treated with PAH-specific drugs (n = 20), phosphodiesterase type-5 inhibitors were the most commonly prescribed (75%), followed by endothelin receptor antagonists (45%), prostacyclin pathway agents (40%), and riociguat (10%).
Healthcare costs
All-cause
Mean all-cause healthcare costs during the 12 months post-index date were higher in PH patients than in controls for hospitalization ($33,622 vs. $22,930; P = 0.0015), ER visits ($8324 vs. $5308; P = 0.0313), pharmacy ($39,727 vs. $23,729; P = 0.0004), total medical service costs ($80,185 vs. $58,110; P < 0.0001), and total healthcare costs ($119,912 vs. $81,839; P < 0.0001) (Table 4). The difference in all-cause outpatient office/clinic service costs between PH patients and controls did not achieve statistical significance ($3044 vs. $2431; P = 0.1549). In the generalized linear regression model, the annual average total healthcare costs of PH patients were 1.47 (95% CI 1.06, 2.02; P = 0.0197) times higher than those of controls.
Table 4.
All-cause healthcare costs during the 12 months following index date in portal hypertension patients with PH and controls with portal hypertension but without PH, matched by propensity score.
| Annual per-patient costs, $
(inflation-adjusted to 2018 US dollars) |
P | ||
|---|---|---|---|
| PH patients (n = 146) | Matched controls (n = 146) | ||
| All-cause hospitalizations | |||
| Mean (SD) | 33,622 (61,177) | 22,930 (57,793) | 0.0015 |
| Median (IQR) | 5759 (0–39,287) | 0 (0–13,429) | |
| All-cause ER visits | |||
| Mean (SD) | 8324 (23,933) | 5308 (13,386) | 0.0313 |
| Median (IQR) | 901 (0–6614) | 0 (0–4431) | |
| All-cause outpatient office/clinic services | |||
| Mean (SD) | 3044 (7482) | 2431 (3471) | 0.1549 |
| Median (IQR) | 1601 (495–3619) | 1426 (4–2911) | |
| All-cause pharmacy | |||
| Mean (SD) | 39,727 (71,796) | 23,729 (61,878) | 0.0004 |
| Median (IQR) | 11,189 (3295–34,427) | 4313 (767–18,796) | |
| All-cause medical costs* | |||
| Mean (SD) | 80,185 (105,319) | 58,110 (102,249) | <0.0001 |
| Median (IQR) | 20,140 (8104–52,054) | 20,140 (8104–52,054) | |
| All-cause healthcare costs† | |||
| Mean (SD) | 119,912 (132,546) | 81,839 (33,309) | <0.0001 |
| Median (IQR) | 69,766 (39,546–147,910) | 35,681 (13,470–72,444) | |
ER: emergency room; IQR: interquartile range; PH: pulmonary hypertension; SD: standard deviation.
Sum of costs for hospital admission, outpatient office/clinic, ER, long-term care facility, skilled nursing facility, rehabilitation, and other medical services.
Sum of costs for all medical services and pharmacy claims.
PH-related
PH patients had mean annual total PH-related healthcare costs of $41,803 (Table 5), representing approximately 35% of all-cause healthcare costs. PH-related hospitalization accounted for nearly half (45%) of average annual PH-related healthcare costs. Annual costs for PAH-related medications were heavily right-skewed, indicating that most PH-related pharmacy costs were incurred by a small proportion of patients.
Table 5.
PH-related healthcare costs during the 12 months following index date in portal hypertension patients with PH (n = 146).
| Annual per-patient costs, $ (inflation -adjusted to 2018 US dollars) | |
|---|---|
| PH-related hospitalizations | |
| Mean (SD) | 18,800 (49,019) |
| Median (IQR) | 0 (0–17,686) |
| PH-related ER visits | |
| Mean (SD) | 2854 (18,955) |
| Median (IQR) | 0 (0–0) |
| PH-related outpatient office/clinic services | |
| Mean (SD) | 133.42 (288) |
| Median (IQR) | 0 (0–118) |
| PH-related pharmacy* | |
| Mean (SD) | 14,190 (46,901) |
| Median (IQR) | 0 (0–0) |
| PH-related medical costs† | |
| Mean (SD) | 27,613 (62,216) |
| Median (IQR) | 5183 (727–30,819) |
| PH-related healthcare costs‡ | |
| Mean (SD) | 41,803 (81,818) |
| Median (IQR) | 6593 (727–38,331) |
ER: emergency room; IQR: interquartile range; PH: pulmonary hypertension; SD: standard deviation.
Sum of pharmacy claims for any of the following medications: endothelin receptor antagonists, phosphodiesterase type-5 inhibitors, prostacyclin-pathway drugs, and soluble guanylate cyclase stimulator.
Sum of costs for hospital admission, outpatient office/clinic, ER, long-term care facility, skilled nursing facility, rehabilitation, and other medical services.
Sum of costs for all medical services and pharmacy claims.
Discussion
This study represents the first systematic attempt to define the incremental HCRU and cost in patients with portal hypertension and PH that is attributable to PH as distinct from cirrhosis and portal hypertension. Reflecting the complexity of management of these patients,16 average annual healthcare costs were 47% higher in patients with PH than in controls without PH. This increase was attributable mainly to PAH-specific medications and higher costs for hospitalization, a recognized aspect of worsening PAH.17
The magnitude of this incremental cost attributable to PH is smaller than the 160% to 300% excess costs reported in previous retrospective database studies comparing health-plan enrollees with PAH with those without PAH.18,19 This likely reflects that the patients in our study’s control group had portal hypertension, which is itself associated with higher HCRU and costs.20–22
A high rate of hospitalizations and readmissions in patients with PAH in the US has been previously documented.23,24 The present study revealed an increased risk and rate of hospitalization for patients with PH compared with controls. In the PH cohort, mean PH-related hospitalization costs were 1.3-fold higher than PH-related pharmacy costs.
Most patients (86%) in our PH cohort had no prescription fills for PAH-specific medications in the year following index date. This may reflect the inclusion in the cohort of an unknown proportion of patients with forms of PH other than PAH, for whom PAH-specific therapies would not be prescribed. There may also have been prescriber uncertainty about the benefit–risk balance of these agents in this patient population.7
In REVEAL, the proportion of patients with PoPH who were treated with PAH therapy was approximately 10% higher than in our analysis.8 This difference could be due to REVEAL enrolling patients at specialist centers and only including PoPH patients, whereas the Data Mart healthcare claims database covers a variety of clinical settings, and we included all patients with PH and portal hypertension. The 2017 survey of physicians at US liver transplant centers described in the Introduction found considerable heterogeneity in clinical practice and medications used to treat PoPH.11 The authors concluded that this reflects how little is known about the optimal management of PoPH and called for more data from clinical trials and real-world experience to improve and standardize care. We believe that this need exists for all patients with PH and portal hypertension, not just those with PoPH.
Our study has some limitations. It is a retrospective analysis of data for patients identified from insurance claims based on ICD codes, rather than from definitive hemodynamic assessments for PAH using right heart catheterization. Therefore, we cannot distinguish between patients who had PAH, and therefore PoPH, and those with PH in the setting of portal hypertension. The non-specific nature of ICD codes for PH25–28 means that this study likely included patients with PAH as well as PH due to other causes associated with advanced liver disease, such as volume overload and/or hyperdynamic state.7 As PoPH is underdiagnosed,16 the control group may have contained patients with undiagnosed PH. Despite propensity score matching, unmeasured confounders may impact the results. Claims data are collected for insurance payments not research and may be subject to coding error. Results may not be generalizable outside the US or to US patients without commercial insurance. Finally, we are unable to draw conclusions regarding causality of the associations we found.
Our findings indicate that there is a significant health and economic burden of co-existing portal hypertension and PH; these patients are at a greater risk for hospitalization and incur higher mean annual total healthcare costs than portal hypertension patients without the additional burden of concomitant PH.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020962917 for Burden of pulmonary hypertension in patients with portal hypertension in the United States: a retrospective database study by Sandeep Sahay, Yuen Tsang, Megan Flynn, Peter Agron and Robert Dufour in Pulmonary Circulation
Acknowledgments
Medical writing and editorial support were provided by W. Mark Roberts, PhD, Montréal, Québec, Canada, and Ify Sargeant of Twist Medical LLC and funded by Actelion Pharmaceuticals US, Inc.
Footnotes
Contributorship: YT acquired and analyzed the data. All authors contributed to study conception and design, data interpretation, manuscript drafting and/or critical revision, approved the final manuscript, and agree to be held accountable for all aspects of the work.
Conflict of interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SS has served on advisory committees for Actelion, Bayer and United Therapeutics, and has received consultancy, steering committee, and speaker bureau fees from Actelion, Bayer and United Therapeutics; travel/accommodation reimbursement from Actelion for presenting at the International Society for Heart and Lung Transplantation meeting 2019; and grant support from the ACCP Chest PAH Research Award 2017. YT, MF, PA, and RD are employees of Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson, and hold stock in Johnson & Johnson.
Ethical approval: This study is a retrospective claims database analysis and thus does not contain any experiments on human or animal subjects for which ethical approval is required.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson, provided funding and support for the analyses presented in this article and for medical writing assistance. The sponsor was involved in data analysis and interpretation, and the decision to publish the finished manuscript.
Guarantor: YT.
ORCID iD: Sandeep Sahay https://orcid.org/0000-0002-0672-1680
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Fritz JS, Fallon MB, Kawut SM. Pulmonary vascular complications of liver disease. Am J Respir Crit Care Med 2013; 187: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019; 53: 1801887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006; 44: 1502–1510. [DOI] [PubMed] [Google Scholar]
- 7.AbuHalimeh B, Krowka MJ, Tonelli AR. Treatment barriers in portopulmonary hypertension. Hepatology 2019; 69: 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest 2012; 141: 906–915. [DOI] [PubMed] [Google Scholar]
- 9.Sithamparanathan S, Nair A, Thirugnanasothy L, et al. Survival in portopulmonary hypertension: outcomes of the United Kingdom National Pulmonary Arterial Hypertension Registry. J Heart Lung Transplant 2017; 36: 770–779. [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 11.DuBrock HM, Salgia RJ, Sussman NL, et al. Portopulmonary hypertension: a survey of practice patterns and provider attitudes. Transplant Direct 2019; 5: e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krowka MJ, Fallon MB, Kawut SM, et al. International Liver Transplant Society Practice Guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation 2016; 100: 1440–1452. [DOI] [PubMed] [Google Scholar]
- 13.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 14.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. [DOI] [PubMed] [Google Scholar]
- 15.Rudolph BA, Shah GH, Love D. Small numbers, disclosure risk, security, and reliability issues in Web-based data query systems. J Public Health Manag Pract 2006; 12: 176–183. [DOI] [PubMed] [Google Scholar]
- 16.Savale L, Manes A. Pulmonary arterial hypertension populations of special interest: portopulmonary hypertension and pulmonary arterial hypertension associated with congenital heart disease. Eur Heart J Suppl 2019; 21: K37–K45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galiè N, Simonneau G, Barst RJ, et al. Clinical worsening in trials of pulmonary arterial hypertension: results and implications. Curr Opin Pulm Med 2010; 16: S11–S19. [DOI] [PubMed] [Google Scholar]
- 18.Kirson NY, Birnbaum HG, Ivanova JI, et al. Excess costs associated with patients with pulmonary arterial hypertension in a US privately insured population. Appl Health Econ Health Policy 2011; 9: 293–303. [DOI] [PubMed] [Google Scholar]
- 19.Said Q, Martin BC, Joish VN, et al. The cost to managed care of managing pulmonary hypertension. J Med Econ 2012; 15: 500–508. [DOI] [PubMed] [Google Scholar]
- 20.Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology 2019; 156: 254–272 e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neff GW, Duncan CW, Schiff ER. The current economic burden of cirrhosis. Gastroenterol Hepatol (N Y) 2011; 7: 661–671. [PMC free article] [PubMed] [Google Scholar]
- 22.Desai AP, Mohan P, Nokes B, et al. Increasing economic burden in hospitalized patients with cirrhosis: analysis of a national database. Clin Transl Gastroenterol 2019; 10: e00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke JP, Hunsche E, Regulier E, et al. Characterizing pulmonary hypertension-related hospitalization costs among Medicare Advantage or commercially insured patients with pulmonary arterial hypertension: a retrospective database study. Am J Manag Care 2015; 21: s47–s58. [PubMed] [Google Scholar]
- 24.Anand V, Roy SS, Archer SL, et al. Trends and outcomes of pulmonary arterial hypertension-related hospitalizations in the United States: analysis of the Nationwide Inpatient Sample database from 2001 through 2012. JAMA Cardiol 2016; 1: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 25.Link J, Glazer C, Torres F, et al. International classification of diseases coding changes lead to profound declines in reported idiopathic pulmonary arterial hypertension mortality and hospitalizations: implications for database studies. Chest 2011; 139: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathai SC, Mathew S. Breathing (and coding?) a bit easier: changes to international classification of disease coding for pulmonary hypertension. Chest 2018; 154: 207–218. [DOI] [PubMed] [Google Scholar]
- 27.Mathai SC, Ryan Hemnes A, Manaker S, et al. Identifying patients with pulmonary arterial hypertension (PAH) using administrative claims algorithms. Ann Am Thorac Soc 2019; 16: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillmeyer KR, Lee MM, Link AP, et al. Accuracy of algorithms to identify pulmonary arterial hypertension in administrative data: a systematic review. Chest 2019; 155: 680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020962917 for Burden of pulmonary hypertension in patients with portal hypertension in the United States: a retrospective database study by Sandeep Sahay, Yuen Tsang, Megan Flynn, Peter Agron and Robert Dufour in Pulmonary Circulation