Abstract
Objective
To observe the evolution of chest high-resolution computed tomography (HRCT) manifestations in 105 patients with coronavirus disease 2019 (COVID-19).
Methods
One hundred five patients with confirmed COVID-19 were enrolled from 11 January to 9 February 2020. Sequential chest HRCT examinations were performed. Five stages were identified from the onset of initial symptoms: 0–3, 4–7, 8–14, 15–21, and >21 days (Stages A–E, respectively). A semi-quantitative CT scoring system was used to estimate the sum of lung abnormalities in each stage.
Results
In total, 393 CT scans were collected. The patients underwent 3.8 ± 1.5 CT examinations. Multiple lobes were involved in most cases. The proportion of consolidation and the total CT score gradually increased from Stage A to C and gradually decreased from Stage C to E. The total CT score of lung involvement was significantly higher in Stage C than in Stages B and D. The CT score of the lower lobe was significantly higher than the corresponding upper and middle lobes in Stages A to D.
Conclusions
Most patients with COVID-19 had a disease course of >14 days, and the lung lesions in most patients improved after 14 days since initial symptom onset.
Keywords: COVID-19, coronavirus infection, pneumonia, computed tomography, lung disease, disease course, imaging manifestations
Introduction
During the first week of December 2019, a few cases of pneumonia caused by a novel coronavirus appeared in Wuhan, Hubei Province, China. The patients had a history of visiting the nearby Huanan seafood market, where wild animals were sold.1 With the spread of the disease, such cases have also been found in other parts of China and abroad. As of 8 July 2020, 11,818,342 cases have been confirmed worldwide, including 544,157 deaths and 6,286,169 recoveries.
The novel coronavirus was originally named 2019 novel coronavirus (2019-nCoV); it was recently officially renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses. The disease caused by SARS-CoV-2 has been named coronavirus disease 2019 (COVID-19) by the World Health Organization. Like SARS-CoV, SARS-CoV-2 likely originated in bats; however, the intermediary animal host remains unclear. The nearest neighbor of the 2019-nCoV isolates from China is a bat SARS-like coronavirus (Bat-SL-CoVZC45).2
SARS-CoV-2 carries a high risk of human-to-human transmission, and such transmission has been reported in family clusters and among medical workers.3,4 The main route of transmission is respiratory droplets and contact transmission; the virus may also be transmitted through the conjunctiva and via aerosols.5 Current epidemiological surveys indicate that the latency period generally ranges from 3 to 7 days, with a maximum of 14 days. Affected patients present with pneumonia-like symptoms, including fever, fatigue, cough, expectoration, pharyngalgia, anorexia, and myalgia. Some patients also have gastrointestinal symptoms, including diarrhea, nausea, vomiting, and abdominal pain. In severe cases, patients may rapidly develop acute respiratory distress syndrome, septic shock, difficult-to-correct metabolic acidosis, and coagulation dysfunction.5,6
Increasing numbers of studies are suggesting the high clinical value of imaging examinations, especially high-resolution computed tomography (HRCT) of the chest, in patients with novel coronavirus pneumonia.7–9 Typical CT imaging manifestations include multiple patchy subsegmental or segmental ground-glass opacities (GGOs) and consolidation in the bilateral lungs.10 The imaging manifestations in patients with novel coronavirus pneumonia often change rapidly, and many patients thus undergo repeated CT examinations. Some patients with novel coronavirus pneumonia gradually improve, whereas others develop persistent hypoxemia or dyspnea and gradually worsen. The changes in the patient’s condition are often closely related to the severity of the lung lesions. Therefore, observing the evolution of the lung lesions by chest CT is very important to assess and predict the patient’s outcome.
This study was to performed observe the changes in the chest HRCT manifestations of 105 patients with pneumonia caused by SARS-CoV-2 from the time of the initial diagnosis. To our knowledge, this is the largest population evaluated to date among studies of the dynamic changes in imaging manifestations of novel coronavirus pneumonia.
Materials and methods
This study was approved by the ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The requirement for informed consent was waived because of the retrospective nature of the study. Anonymous data were collected and analyzed.
Study design and participants
Consecutive patients were retrospectively enrolled in this single-center study from 11 January 2020 to 9 February 2020. These patients met the Diagnosis and Treatment of New Coronavirus Pneumonia (trial version 7) issued by the National Health Commission of the People’s Republic of China,11 and their diagnoses were confirmed by reverse-transcription polymerase chain reaction. According to the Diagnosis and Treatment of New Coronavirus Pneumonia (trial version 7) issued by the Chinese National Health Committee, patients who developed severe pneumonia during their disease course were excluded. Severe pneumonia was defined as severe respiratory distress (respiratory rate of >30 breaths/minute), a requirement for oxygen treatment or mechanical ventilation, an oxygen saturation of <93% on room air, a partial arterial oxygen pressure/fraction of inspired oxygen ratio of ≤300 mmHg, shock, or pneumonia combined with other organ failure.
CT data acquisition and image reconstruction
All patients were examined using a Discovery CT750 HD scanner (GE Healthcare, Chicago, IL, USA) during a single inspiratory phase. Before the examination, the medical staff guided the patient to hold his or her breath and inhale. After complete exhalation, the patient held his or her breath, and a CT scan was performed from the bottom of the lung to the apex of the lung. The tube voltage was 120 kVp with automatic tube current modulation. The scan time was 2 s, the scan interval was 5 mm, and the scan layer thickness was 5 mm. After scanning, adaptive statistical iterative reconstruction of 30% and the standard algorithm were adopted, and the reconstruction layer thickness was 1.25 mm. No contrast agent was used during the CT scan. Most patients underwent multiple chest CT examinations, largely because the clinicians were still unclear about the evolution tendencies of the lung lesions associated with COVID-19 pneumonia. Additionally, some patients had symptoms such as hypoxemia or dyspnea due to rapid disease progression. Therefore, multiple chest CT examinations were required to observe the progress or absorption of pneumonia.
Chest CT evaluation and grouping
The major CT manifestations were described using internationally standard nomenclature defined by the Fleischner Society glossary, using terms such as GGO, consolidation, and fibrous strips.12 A semi-quantitative scoring system was used to estimate the pulmonary involvement of all of these abnormalities on the basis of the area involved.13 Each of the five lung lobes was visually scored from 0 to 5 as follows: 0, no involvement; 1, <5% involvement; 2, 5% to 25% involvement; 3, 26% to 49% involvement; 4, 50% to 75% involvement; and 5, >75% involvement. The total CT score was the sum of the individual lobar scores and ranged from 0 (no involvement) to 25 (maximum involvement). The number of affected lung lobes was also recorded. The images were transferred to an AW4.7 workstation (GE Healthcare) and anonymized. All images were evaluated by two radiologists with more than 10 years of work experience, and the final scores were determined by consensus.
Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, IL, USA). Measurement data are expressed as mean ± standard deviation (minimum–maximum), and categorical data are presented as the percentage of the group. A P-value of <0.05 was defined as statistically significant.
Results
Demographics and laboratory investigations
In total, 393 CT scans of 105 patients (49 men, 56 women) with confirmed COVID-19 were collected. The patients’ ages ranged from 23 to 72 years, and their mean age was 48.6 ± 13.1 years. Fever was the most common symptom (Table 1). The patients underwent 3.8 ± 1.5 (2–9) chest CT examinations, and the interval between the first and last scan was 16.6 ± 9.0 days (1–47 days).
Table 1.
Patients’ demographic characteristics and laboratory investigation findings.
| All patients (n = 105) | |
|---|---|
| Age, years | 48.6 ± 13.1 (23–72) |
| Sex | |
| Male | 49 (46.7) |
| Female | 56 (53.3) |
| Initial symptoms | |
| Fatigue | 8 (7.6) |
| Fever | 86 (81.9) |
| Cough | 10 (9.5) |
| Myalgia | 1 (0.95) |
| Dizziness | 1 (0.95) |
| Diarrhea | 1 (0.95) |
| Chest distress | 1 (0.95) |
| Laboratory investigations | |
| White blood cell count, G/L | 6.9 ± 5.7 (2.3–23.5) |
| Neutrophil count, G/L | 5.1 ± 5.7 (1.3–21.1) |
| Lymphocyte count, G/L | 1.3 ± 0.5 (0.4–2.4) |
| C-reactive protein, mg/L | 18.0 ± 15.4 (1.4–52.6)↑ |
| Erythrocyte sedimentation rate, seconds | 18.4 ± 22.0 (2.0–64.0) |
| Alanine aminotransferase, U/L | 26.8 ± 19.0 (11.0–67.0) |
| D-dimers, mg/L | 0.8 ± 0.7 (0.3–3.0)↑ |
| Creatinine, µmol/L | 67.8 ± 14.8 (43.0–94.0) |
| Ferritin, µg/L | 1006.2 ± 888.7 (41.6–2513.0)↑ |
| Number of scans | 3.8 ± 1.5 (2–9) |
| Interval between first and last scans, days | 16.6 ± 9.0 (1–47) |
Data are presented as mean ± standard deviation (minimum–maximum) or n (%).
↑: Higher than upper limit of reference range.
The laboratory results are presented in Table 1. Most laboratory results were normal, with mildly elevated concentrations of C-reactive protein (18.0 ± 15.4 mg/L), D-dimers (0.8 ± 0.7 mg/L), and ferritin (1006.2 ± 888.7 µg/L).
CT image analysis
Based on clinical experience, five stages were identified from the onset of initial symptoms: Stage A (0–3 days, n = 104), Stage B (4–7 days, n = 73), Stage C (8–14 days, n = 85), Stage D (15–21 days, n = 59), and Stage E (>21 days, n = 26). One patient had a 4-day history of cough with fever and was in Stage B at the time of admission. The frequencies of different lesions in different stages are presented in Table 2. Two patients in Stage A and one patient in Stage E had no lesions. Multiple lobes were involved in most of the CT scans in all stages. Multiple lobe involvement was present in 86 (82.7%) patients in Stage A, 68 (93.2%) in Stage B, 77 (90.6%) in Stage C, 53 (89.8%) in Stage D, and 22 (84.6%) in Stage E. GGOs could be seen in all stages, and the proportion of GGOs in each stage was similar. The proportion of consolidation first increased and then decreased over time. GGOs and consolidation were present in 80 (76.9%) and 78 (75.0%) patients in Stage A, 50 (72.5%) and 57 (78.1%) in Stage B, 64 (75.3%) and 71 (83.5%) in Stage C, 43 (75.4%) and 31 (54.4%) in Stage D, and 18 (72.0%) and 4 (16.0%) in Stage E, respectively. The consolidations in some cases were absorbed.
Table 2.
Frequencies of different lesions in different stages.
| Stage A(n = 104) | Stage B(n = 73) | Stage C(n = 85) | Stage D(n = 59) | Stage E(n = 26) | |
|---|---|---|---|---|---|
| Involvement of lesions | |||||
| No involvement | 2 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.8) |
| Single lobe | 16 (15.4) | 5 (6.8) | 8 (9.4) | 6 (10.2) | 3 (11.5) |
| Multiple lobes | 86 (82.7) | 68 (93.2) | 77 (90.6) | 53 (89.8) | 22 (84.6) |
| GGOs | |||||
| None | 24 (23.1) | 19 (27.5) | 21 (24.7) | 14 (24.6) | 7 (28.0) |
| Yes | 80 (76.9) | 50 (72.5) | 64 (75.3) | 43 (75.4) | 18 (72.0) |
| Consolidation | |||||
| None | 26 (25.0) | 16 (21.9) | 14 (16.5) | 26 (45.6) | 21 (84.0) |
| Yes | 78 (75.0) | 57 (78.1) | 71 (83.5) | 31 (54.4) | 4 (16.0) |
Data are presented as n (%).
GGOs, ground-glass opacities.
As shown in Table 3, the total CT score also first increased and then decreased over time, and the total CT score of pulmonary involvement was significantly higher in Stage B than in Stage A (8.1 ± 4.3 vs. 6.7 ± 4.4, respectively; P = 0.001). Additionally, the total CT score of lung involvement was significantly higher in Stage C than in Stages B and D [9.2 ± 4.6 vs. 8.1 ± 4.3 (P = 0.001) and 9.2 ± 4.6 vs. 7.1 ± 4.5 (P = 0.04), respectively]. The number of involved lobes was significantly higher in Stage B than in Stage A (3.9 ± 1.3 vs. 3.4 ± 1.6, respectively; P = 0.02). Notably, the CT score of the lower lobe was significantly higher than the corresponding upper and middle lobes in Stages A, B, C, and D (P < 0.001 for all). In Stage E, the CT score of the right lower lobe was significantly higher than that of the right upper/middle lobes (P = 0.01 and P = 0.002, respectively), whereas no significant difference was observed in the CT score between the left lower lobe and left upper lobe.
Table 3.
CT scores of pulmonary involvement in the five stages.
| Stage A(n = 104) | Stage B(n = 73) | Stage C(n = 85) | Stage D(n = 59) | Stage E(n = 26) | |
|---|---|---|---|---|---|
| Total CT score of pulmonary involvement | 6.7 ± 4.4 | 8.1 ± 4.31 | 9.2 ± 4.65,6 | 7.1 ± 4.5 | 6.6 ± 4.8 |
| Number of involved lobes | 3.4 ± 1.6 | 3.9 ± 1.31 | 4.1 ± 1.3 | 3.7 ± 1.5 | 3.8 ± 1.6 |
| CT score in each lobe | |||||
| Right upper lobe | 2.0 ± 1.12 | 2.3 ± 1.22 | 2.1 ± 1.02 | 2.1 ± 1.02 | 1.9 ± 0.82 |
| Right middle lobe | 1.8 ± 0.93 | 2.0 ± 0.93 | 1.9 ± 0.93 | 1.8 ± 0.83 | 1.8 ± 0.73 |
| Right lower lobe | 2.1 ± 0.9 | 2.6 ± 0.9 | 2.3 ± 0.9 | 2.1 ± 0.8 | 2.0 ± 1.0 |
| Left upper lobe | 1.6 ± 0.94 | 2.1 ± 1.04 | 1.8 ± 0.94 | 1.8 ± 1.04 | 1.7 ± 0.9 |
| Left lower lobe | 2.0 ± 0.8 | 2.4 ± 0.9 | 2.2 ± 0.9 | 1.9 ± 1.0 | 1.6 ± 0.8 |
Data are presented as mean ± standard deviation.
CT, computed tomography.
1Mann–Whitney U test showed a significant difference between Stage A and Stage B (P < 0.05).
2Wilcoxon test showed a significant difference between the right upper lobe and right lower lobe (P < 0.05).
3Wilcoxon test showed a significant difference between the right middle lobe and right lower lobe (P < 0.05).
4Wilcoxon test showed a significant difference between the left upper lobe and left lower lobe (P < 0.05).
5Mann–Whitney U test showed a significant difference between Stage C and Stage B (P < 0.05).
6Mann–Whitney U test showed a significant difference between Stage C and Stage D (P < 0.05).
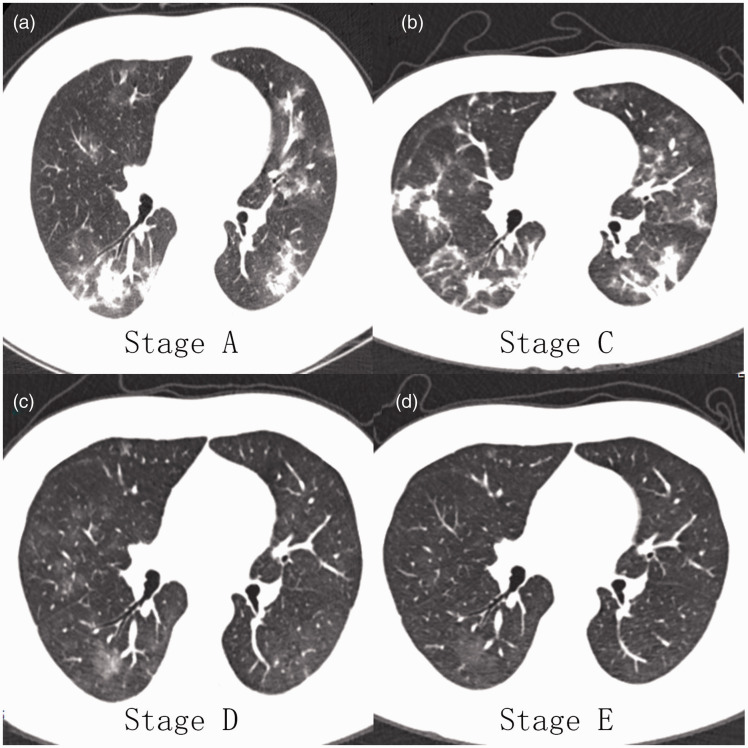
The CT images of a patient with novel coronavirus pneumonia are shown in Figure 1. GGOs and consolidation first increased from Stage A to Stage C, after which the consolidation gradually absorbed; the GGOs then gradually decreased in Stages D and E.
Figure 1.
Computed tomography images of a 45-year-old man with novel coronavirus pneumonia. (a) Ground-glass opacities (GGOs) and consolidation were distributed mainly under the pleura. (b) Increased GGOs and consolidation were randomly distributed. (c) The consolidation was almost absorbed, leaving mainly GGOs. (d) The density and extent of the GGOs had decreased.
Discussion
Since the end of 2019, an outbreak of a novel pneumonia in Wuhan has been drawing tremendous attention around the world. Chinese government officials and researchers have taken swift measures to control the outbreak and conduct etiological studies. The causative agent of this pneumonia has been identified as SARS-CoV-2, a novel coronavirus. As its name suggests, its genetic structure is 82% similar to that of SARS-CoV.14
The most frequently affected organ of the novel coronavirus is the lung. In the previous version of the diagnosis and treatment guideline for pneumonia caused by novel coronavirus, the diagnosis was confirmed by the presence of viral nucleic acids in throat swabs, sputum, and alveolar lavage fluid. However, the more recent guideline indicates that if typical chest CT findings are observed in patients suspected to have COVID-19, the patient should be clinically diagnosed. This reflects the important role of chest CT in the diagnosis. Moreover, current clinical experience is showing that the chest CT manifestations change rapidly. Therefore, many patients in the present study underwent multiple repeated examinations during treatment. To our knowledge, this study is by far the largest case series of follow-up imaging among patients with COVID-19.
SARS-CoV-2 infects human respiratory epithelial cells through S-protein and has a strong infection capacity for humans. Coronavirus particles have a diameter of 60 to 140 nm and can directly reach the bottom of the lung. Because of lung volume and gravity, respiratory airflow, and other factors, the type II alveolar epithelial cells present at the bottom of the lung become the target cells of the coronavirus. Therefore, the lesions are mainly distributed under the pleura or along the vascular bronchial bundle.15 After the alveolar epithelial cells have become combined with the virus, the alveolar wall is damaged and large numbers of immune cells and lymphocytes accumulate, resulting in swelling around the lung leaflets and blood vessel congestion. The pulmonary lobules merge with each other, forming nodular or patchy GGOs. As the disease progresses, multiple lung segments become involved, the scope of the lesion expands, the alveolar wall shrinks and collapses, and the exfoliated and necrotic epithelium and the exuded inflammatory cells form a hyaline membrane. These changes manifest as coexisting imaging abnormalities such as GGOs, nodules, consolidation, and fibrous cord strips.12
Several studies have focused on the evolution of CT changes in patients with COVID-19 pneumonia. Pan et al.16 analyzed the disease course according to 4-day stages and found that the CT score was the highest on days 9 to 13 of the disease course; after 9 days, the CT score of the lower lung was significantly higher than that of the middle and upper lungs. Zhou et al.17 found that CT images of patients with COVID-19 pneumonia showed a predominantly peripheral, middle, lower, and posterior distribution. The early rapid progressive stage was 1 to 7 days from symptom onset, the advanced stage with peak levels of CT abnormalities occurred from 8 to 14 days, and the abnormalities started to improve after 14 days. Lei et al.18 reported that patients with COVID-19 pneumonia had a typical transition from the early stage to the advanced stage and then from the advanced stage to the dissipating stage. Single or multiple GGOs were distributed along the bronchovascular or subpleural regions in the pulmonary parenchyma in the early stage, higher-density consolidations were observed in the advanced stage, and the GGOs and consolidations were absorbed in the dissipating stage. Xiang et al.19 evaluated only six patients with COVID-19 pneumonia and reported that the CT score peaked at 5 to 9 days and that the CT scores of the lower lobes were significantly higher than those of the middle/upper lobes after 10 days from disease onset. These findings were consistent with those reported by Pan et al.16 Liang et al.20 reported that the majority of patients had involvement of three or more lobes. Bilateral involvement was more prevalent than unilateral involvement. The proportions of patients observed to have pure GGOs or GGOs and consolidation decreased over time, while the proportion of patients with GGOs and linear opacities increased. The total severity score showed an increasing trend in the first 2 weeks. Bernheim et al.21 divided the disease course into three stages (0–2, 3–5, and 6–12 days) and found that 56% of patients in the first stage had normal CT findings. As the time since onset of symptoms increased, the CT abnormalities became more frequent; these findings included consolidation, bilateral and peripheral disease, greater total lung involvement, linear opacities, the “crazy-paving” pattern, and the “reverse halo” sign.
In this study, we evaluated the CT findings of 105 patients with COVID-19 pneumonia confirmed by reverse-transcription polymerase chain reaction. The patients underwent a mean of 3.8 ± 1.5 (range: 2–9) chest CT examinations. At present, the course of COVID-19 pneumonia and when patients should be re-examined remain inconclusive. As mentioned above, different studies have staged the disease according to different time periods. We grouped the patients’ CT scans at different time intervals. The time interval grouping was based on current clinical experience and is consistent with the imaging interval described in the pneumonia diagnosis and treatment guidelines.10 This grouping is also partially similar to that described by Pan et al.16 and Zhou et al.17 Multiple lobe involvement was observed in most patients in all stages, and lower lobe lesions were significantly more common than upper and middle lobe lesions; this is also consistent with the findings reported by Pan et al.16 and Liang et al.20 As mentioned above, most studies have suggested that patients with COVID-19 pneumonia undergo a typical transition from the early stage to the advanced stage and then from the advanced stage to the dissipating stage; however, the time to reach the peak of the disease course varies among different studies, and the patients in our study showed a similar tendency. GGOs and consolidation were the common chest CT manifestations; the proportion of GGOs in each stage was similar, while consolidation and the total CT score first increased and then decreased over time. The total CT score was significantly higher in Stage B than in Stage A, while the total CT score was significantly higher in Stage C than in Stage B. These findings suggest that the lung lesions of most patients significantly improved after 14 days and that most patients with new coronavirus pneumonia had a disease course of more than 14 days.
This study had three main limitations. First, we did not consider the effects of factors such as age, sex, medical history (including hypertension, hyperlipidemia, hyperglycemia, and malignant tumors), and lifestyle habits (including smoking and drinking history) on patient outcomes. Second, although 105 patients with novel coronavirus pneumonia were included in this study, the number of patients in Stages B to E was limited. Future studies should include more patients and extend the observation time to ensure that the study results are more representative. Third, some studies have suggested that factors such as age, the C-reactive protein concentration, and the interleukin 6β concentration may be related to patient outcomes. However, we did not perform a correlation analysis between the laboratory data and patient outcomes.
In conclusion, we followed up and evaluated 393 chest CT scans of 105 patients with confirmed COVID-19 pneumonia and found that most patients had a disease course of more than 14 days and that the lung lesions of most patients improved significantly after 14 days. The purpose of this study is to help clinicians better understand the disease course and properly perform chest CT re-examinations for patients with COVID-19 pneumonia to minimize the radiation dose.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Hanxiong Guan https://orcid.org/0000-0001-9675-4496
References
- 1.Hui DS, Azhar EI, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020; 91: 264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik YS, Sircar S, Bhat S, et al. Emerging novel coronavirus (2019-nCoV)—current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q 2020; 40: 68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395: 514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382: 1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang DW, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang YC, Zhang HQ, Xu YY, et al. CT manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020; 295: 208–209. doi: 10.1148/radiol.2020200280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Y, Guan H. Imaging changes in patients with 2019-nCov. Eur Radiol 2020; 30: 3612–3613. doi: 10.1007/s00330-020-06713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanne JP. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology 2020; 295: 16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020; 7: 4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health Commission of the People’s Republic of China. Diagnosis and treatment protocols of pneumonia caused by a novel coronavirus (trial version 7).
- 12.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 13.Chang YC, Yu CJ, Chang SC, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology 2005; 236: 1067–1075. doi: 10.1148/radiol.2363040958. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 2020; 92: 418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollura DJ, Asnis DS, Crupi RS, et al. Imaging findings in a fatal case of pandemic swine-origin influenza A (H1N1). Am J Roentgenol 2009; 193: 1500–1503. doi: 10.2214/AJR.09.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan F, Ye TH, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020; 295: 715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou SC, Zhu TT, Wang YJ, et al. Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China. Eur Radiol 2020; 5: 1–9. doi: 10.1007/s00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei PG, Fan B, Mao JJ, et al. The progression of computed tomographic (CT) images in patients with coronavirus disease (COVID-19) pneumonia. J Infect 2020; 80: e30–e31. doi: 10.1016/j.jinf.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang Y, Yang QX, Sun HH, et al. Chest CT findings and their dynamic changes in patients with COVID-19. J South Med Univ 2020; 40: 327–332. doi: 10.12122/j.issn.1673-4254.2020.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang T, Liu Z, Wu CC, et al. Evolution of CT findings in patients with mild COVID-19 pneumonia. Eur Radiol 2020; 30: 4865–4873. doi: 10.1007/s00330-020-06823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernheim A, Mei XY, Huang MQ, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology 2020; 295: 200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]