Abstract
Talaromyces marneffei causes fatal invasive mycosis in Southeast Asia. Diagnosis by culture has limited sensitivity and can result in treatment delay. We describe the use of a novel Mp1p enzyme immunoassay (EIA) to identify blood culture–negative talaromycosis, subsequently confirmed by bone marrow cultures. This EIA has the potential to speed diagnosis, enabling early therapy initiation.
Keywords: Mp1p EIA, Penicillium marneffei, penicilliosis, Talaromyces marneffei, talaromycosis
Talaromycosis (penicilliosis) is an invasive mycosis caused by the dimorphic fungus Talaromyces marneffei (Tm), which is highly endemic in Southeast Asia [1], and has been diagnosed worldwide in returning travelers and immigrants from the region [2, 3]. Talaromycosis primarily affects individuals with advanced HIV disease, in whom it ranks as the third most common opportunistic infection, and is a leading cause of HIV-associated death [4–6]. Incidence is rapidly increasing in non-HIV-infected individuals who have malignancies, receive immunosuppressive therapy, or receive solid organ or bone marrow transplantations [7]. Human infection results from inhalation of aerosolized fungal spores from the environment. Infection can remain latent for years and can be reactivated in immunosuppressed individuals, causing disseminated disease involving the lungs, gastrointestinal tract, lymphatics, liver, spleen, blood, skin, and bone marrow [8]. The mortality rate despite antifungal therapy is up to 30% in HIV-infected [4, 5, 6] and 50% in non-HIV-infected individuals [9].
The gold standard for diagnosis is culture. However, this is poorly sensitive and takes up to 2 weeks to yield a positive result, leading to treatment delay [1, 8]. Identifying early disease, where interventions are most likely to be effective, is particularly challenging. While diagnosis can be made based on microscopy of lymph nodes or skin lesions, such diagnostic lesions are present in only 30% to 50% of patients [5, 9]. Blood culture remains the mainstay of diagnosis but misses 30% of infections in HIV-infected [5, 10] and 50% in non-HIV-infected patients [9]. A clinical trial cohort from Vietnam identified diagnostic delays of up to 6 months among patients [11]. Another cohort from China found diagnostic delays to be associated with increases in mortality from 24.3% to 50.6% [4]. Our research group has developed a novel monoclonal antibody–based enzyme immunoassay (EIA) that detects a Tm-specific protein Mp1p located throughout the cell wall of Tm [12, 13]. This antigen is abundantly secreted in the blood and urine of patients during infection, and we have recently demonstrated that this Mp1p EIA is more sensitive than conventional BACTEC blood cultures in detecting talaromycosis [14]. Here, we report 2 HIV-infected patients who presented with recurrent fevers and sepsis of unclear etiology, in whom multiple cultures of blood and other specimens were negative for pathogens. The diagnosis of talaromycosis was made based on a positive Mp1p EIA, which was confirmed by microscopy and cultures of bone marrow. The cases illustrate important features of this neglected infectious disease and the role of antigen detection in making early diagnosis.
PATIENT 1
Patient 1 was a 35-year-old man, an intravenous drug user, who presented to the Hospital for Tropical Diseases in Ho Chi Minh City (HCMC) with a 1-week history of high fevers, fatigue, vomitting, and diarrhea. He had been hospitalized 2 weeks before for fevers and was treated empirically for bacterial sepsis. He had been diagnosed with HIV infection 10 years previously, received antiretroviral therapy (ART) with tenofovir, lamivudine, and efavirenz, and had been switched to second-line therapy with tenofovir, lamivudine, and lopinavir/ritonavir 1 month before this admission due to treatment failure. His CD4+ T-cell count at that time was 10 cells/µL. He had recently completed therapy for tuberculous adenitis. On admission, he had pyrexia of 39°C, confusion, several shallow nontender ulcers on the hard palace, an enlarged cervical lymph node of 2 cm, and mild hepatosplenomegaly. His skin was normal. Laboratory tests revealed pancytopenia (white cells 2.6 × 109 L, hemoglobin 11 g/dL, platelets 43 × 109 L) and mild transaminitis (aspartate transaminase 64 U/L, alanine transaminase 54 U/L). Chest x-ray was normal. Abdominal ultrasound confirmed hepatosplenomegaly. He underwent a lumbar puncture. Cerebrospinal fluid (CSF) cell counts and chemistry were normal. CSF Indian ink and CSF culture were negative. Multiple sputum smears and cervical lymph node aspirate were negative for acid-fast bacilli (AFB). No pathogens were identified from 3 sets of blood cultures using the automated BACTEC system after 5 days of culture and stool cultures. He was again treated for suspected bacterial sepsis with 8 days of imipenem but remained febrile with no clinical improvement.
PATIENT 2
Patient 2 was a 28-year-old HIV-infected man referred to our hospital with 3 months of worsening fevers, fatigue, exertional dyspnea, and weight loss. His symptoms began within 1 week of initiating ART with tenofovir, lamivudine, and efavirenz. His CD4+ T-cell count at that time was 19 cells/µL. Outpatient work-up included multiple negative sputum AFB smears, negative blood cultures, and normal chest x-rays. On admission he had pyrexia of 38.0°C and cachexia. There was no lymphadenopathy; his skin was normal. He had severe pancytopenia (white cells 0.9 × 109 L, hemoglobin 8.3 g/dL, platelets 21 × 109 L). Liver enzymes were normal. Abdominal ultrasound revealed hepatosplenomegaly and multiple intra-abdominal lymph nodes that were 1–2 cm in diameter. Multiple sputum AFB smears and blood cultures were again negative. Chest x-ray was normal. The patient was treated for suspected bacterial sepsis with ceftriaxone for 7 days without improvement.
STATEMENT OF PATIENT CONSENT AND ETHICS
Both patients gave written consent and participated in a prospective study using the novel Mp1p EIA to screen for talaromycosis in hospitalized patients with AIDS. They gave a separate written consent for this report. The study was approved by the Hospital for Tropical Diseases (approval number 52/HDDD; November 30, 2018).
Based on an established optical density (OD) cutoff of 0.515, the EIA tests were positive in the serum, plasma, and urine samples of both patients (Patient 1: ODs = 1.05, 1.91, 2.84; Patient 2: ODs = 2.96, 3.07, 2.92, respectively). As the Mp1p EIA was being evaluated in a research study, antifungal therapy was not yet started. However, the study protocol dictated that a bone marrow biopsy be offered to evaluate for deep-seated infection in all antigen-positive culture-negative patients who also had evidence of bone marrow suppression. The microscopy of the bone marrow aspirates in both patients showed numerous intra- and extracellular yeast cells, some revealing a mid-line septum characteristic of Tm (Figure 1). Treatment with amphotericin B deoxycholate was initiated. Both patients had rapid improvement after 1 week of therapy. Amphotericin B was switched to itraconazole early in patient 1 due to reduced creatinine clearance, but he continued to improve and was discharged home. Patient 2 developed a suswedden onset of severe abdominal pain and distention. Abdominal x-ray showed free air. He unfortunately succumbed to septic shock due to suspected peforated viscous. Tm was isolated from bone marrow cultures of both patients (Figure 2).
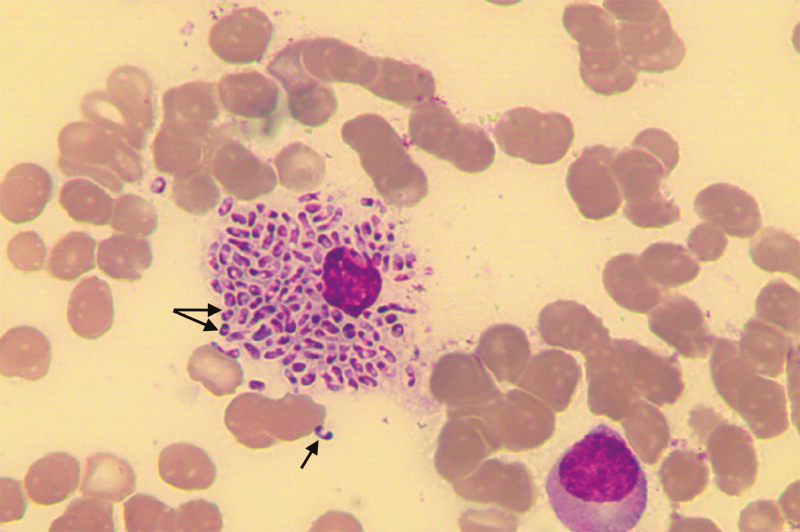
Figure 1.
Wright’s stained smear of the bone marrow aspirate of Patient 1 showing numerous yeast cells measuring 5–6 µm inside an engorged histiocyte. The arrows show the actively dividing yeast cells, revealing a midline septum characteristic of Talaromyces marneffei.
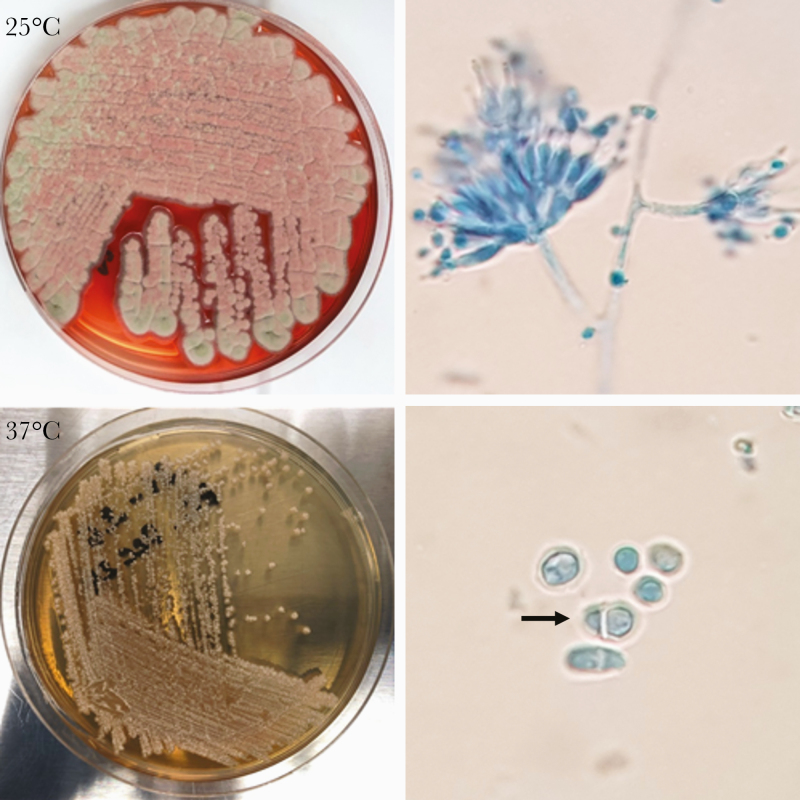
Figure 2.
Talaromyces marneffei subcultures from the bone marrow of Patient 2. At 25°C, T. marneffei produces powdery greenish mold colonies and a bright red pigment that diffuses into Sabouraud Dextrose Agar (SDA) medium. Tape preparation of the mold colonies shows septate hyphae with conidiophores bearing phialides and round conidia under the microscope. At 37°C, T. marneffei produces white yeast colonies on SDA media without the red pigmentation. Microscopic examination shows oval yeast cells, 1 with a central septum (arrow).
DISCUSSION
These cases demonstrate several important features of talaromycosis. First, infection has an indolent course with nonspecific signs and symptoms that are indistinguishable from infections due to salmonellosis, invasive mycoses, tuberculosis, and other mycobacteria. Further, coinfections with these pathogens occur frequently in this profoundly immunosuppressed population [5, 10], posing a challenge for diagnosis and management. Second, even in the settings of advanced HIV disease and disseminated infection, blood cultures can be negative in talaromycosis. While a bone marrow biopsy is clinically indicated, these are rarely performed in low-income, high–tuberculosis burden settings such as ours. Was it not for the Mp1p EIA performed in these cases, the diagnosis would have been missed; the most likely outcome would have been commencement of 9 months of antituberculosis chemotherapy. This would almost certainly have resulted in the death of Patient 1. These cases highlight the need for rapid, more sensitive, non-culture-based diagnostics for talaromycosis. Several real-time polymerase chain reaction (PCR) assays have been developed to detect Tm directly from blood [15–18]; however, their sensitivities have been suboptimal (60%–85%) due to DNA loss during extraction. EIA-based antigen detection has become a standard diagnostic for other mycoses including aspergillosis, cryptococcosis, and histoplasmosis [19–21]. Among 2 recently developed monoclonal antibody–based assays that detect Tm antigen [14, 22], our Mp1p EIA is advantageous, as it targets a Tm-specific antigen abundantly secreted in the blood and urine of patients. In our study of 372 culture-positive cases and 517 controls, we have demonstrated that the Mp1p EIA is more sensitive than blood cultures (86.3% vs 72.8%; P < .001) and has a specificity of 98.1% [14]. The test is more sensitive in urine than in plasma specimens, and testing plasma and urine in combination further increases sensitivity [14]. Our patients had high Mp1p ODs in the urine, higher than in the serum and plasma of Patient 1. This supports our previous finding and suggests that, similar to histoplasmosis, urine is an excellent specimen for Tm antigen testing. Our cases illustrate how the Mp1p EIA can guide management of immunocompromised patients living in or having traveled to Tm-endemic countries who present with undifferentiated fevers. Our cases provide further support for the Mp1p EIA as a rapid diagnostic tool for talaromycosis. A prospective Tm antigen screening study is underway to define the diagnostic utility of antigen testing in populations at risk for talaromycosis (ClinicalTrials.gov: NCT04033120).
Finally, these cases demonstrate how ART unmasks talaromycosis in patients with advanced HIV disease. Both patients developed symptoms of infection within 1 week of initiating ART. Such unmasking immune reconstitution inflammatory syndrome (IRIS) is frequently seen in talaromycosis [23–25]. Among 440 talaromycosis patients who participated in our itraconazole vs amphotericin B clinical trial (IVAP) [11], 40% developed talaromycosis within 4 weeks of ART initiation. We believe there is a role for antigen screening in detecting subclinical infections at the time of ART initiation. This may permit prevention of disease through preemptive antifungal therapy. Such a screen-and-preemptive strategy has been shown to reduce mortality and be cost-effective in HIV-associated cryptococcal meningitis [21, 26–28] and can serve as a model for talaromycosis prevention.
Acknowledgments
We thank Prof. Kwok-Yung Yuen and Mr. Jian-Piao Cai from the University of Hong Kong for providing in-kind test reagents (antibodies and the recombinant Mp1p) and for technical support for the Mp1p EIA. We thank the patients who participated in this study and gave permission for us to report the details of their infections.
Financial support. This study was supported by the Wellcome Trust in the UK (Grant No. 110307 / Z / 15 / Z to Day and Le), the Wellcome Trust Intermediate Fellowship (WT097147MA to Day), the National Institutes of Health (R01AI143409 to Le), the Duke University Center for AIDS Research (CFAR), an NIH-funded program (P30 AI064518 to Le), and donations from Marina Man-Wai Lee and the Hong Kong Hainan Commercial Association South China Microbiology Research Fund (to Chan).
Potential conflicts of interest. John Perfect reports research grants from Merck, Astellas, F2G, Scynexis, Pfizer, Amplyx, Ampili, Minnetronix, and Matinas outside the submitted work. Thuy Le has received investigator-initiated research funding from Gilead Sciences outside of the submitted work. The remaining authors declare that they have no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Study concept and design: Day and Le. Obtaining funding: Day and Le. Clinical and microbiology data acquisition: Ly, Thanh, Thu, Nga, Chau. Mp1p EIA performance and quality assurance: Thu, Chan. Patient care and follow-up: Ly, Thanh, Nga, Chau. Drafting the manuscript: Thanh, Ly, Le. Critical revision of the manuscript for intellectual content: Chan, Day, Perfect, Nga, Chau. All authors contributed to and approved the final manuscript.
References
- 1. Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis 2017; 17:e334–43. [DOI] [PubMed] [Google Scholar]
- 2. Antinori S, Gianelli E, Bonaccorso C, et al. . Disseminated Penicillium marneffei infection in an HIV-positive Italian patient and a review of cases reported outside endemic regions. J Travel Med 2006; 13:181–8. [DOI] [PubMed] [Google Scholar]
- 3. Cristofaro P, Mileno MD. Penicillium marneffei infection in HIV-infected travelers. AIDS Alert 2006; 21:140–2. [PubMed] [Google Scholar]
- 4. Hu Y, Zhang J, Li X, et al. . Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia 2013; 175:57–67. [DOI] [PubMed] [Google Scholar]
- 5. Le T, Wolbers M, Chi NH, et al. . Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis 2011; 52:945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang J, Meng S, Huang S, et al. . Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in Southern China: a retrospective cohort study. Clin Microbiol Infect 2019; 25:233–41. [DOI] [PubMed] [Google Scholar]
- 7. Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 2016; 5:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vanittanakom N, Cooper CR Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev 2006; 19:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of Penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis 2013; 13:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larsson M, Nguyen LH, Wertheim HF, et al. . Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in Northern Vietnam. AIDS Res Ther 2012; 9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le T, Kinh NV, Cuc NTK, et al. ; IVAP Investigators A trial of itraconazole or amphotericin B for HIV-associated talaromycosis. N Engl J Med 2017; 376:2329–40. [DOI] [PubMed] [Google Scholar]
- 12. Cao L, Chan CM, Lee C, et al. . MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect Immun 1998; 66:966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang YF, Cai JP, Wang YD, et al. . Immunoassays based on Penicillium marneffei Mp1p derived from Pichia pastoris expression system for diagnosis of penicilliosis. PLoS One 2011; 6:e28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thu NTM, Chan JFW, Ly VT, et al. . Superiority of a novel Mp1p antigen detection enzyme immunoassay compared to standard BACTEC blood culture in the diagnosis of talaromycosis. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu S, Li X, Calderone R, et al. . Whole blood nested PCR and real-time PCR amplification of Talaromyces marneffei specific DNA for diagnosis. Med Mycol 2016; 54:162–8. [DOI] [PubMed] [Google Scholar]
- 16. Pornprasert S, Praparattanapan J, Khamwan C, et al. . Development of TaqMan real-time polymerase chain reaction for the detection and identification of Penicillium marneffei. Mycoses 2009; 52:487–92. [DOI] [PubMed] [Google Scholar]
- 17. Hien HTA, Thanh TT, Thu NTM, et al. . Development and evaluation of a real-time polymerase chain reaction assay for the rapid detection of Talaromyces marneffei MP1 gene in human plasma. Mycoses 2016; 59:773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Zheng Y, Wu F, et al. . Evaluation of quantitative real-time PCR and Platelia galactomannan assays for the diagnosis of disseminated Talaromyces marneffei infection. Med Mycol 2020; 58:181–6. [DOI] [PubMed] [Google Scholar]
- 19. Marr KA, Datta K, Mehta S, et al. . Urine antigen detection as an aid to diagnose invasive aspergillosis. Clin Infect Dis 2018; 67:1705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nacher M, Blanchet D, Bongomin F, et al. . Histoplasma capsulatum antigen detection tests as an essential diagnostic tool for patients with advanced HIV disease in low and middle income countries: a systematic review of diagnostic accuracy studies. PLoS Negl Trop Dis 2018; 12:e0006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rajasingham R, Wake RM, Beyene T, Katende A, Letang E, Boulware DR, . Cryptococcal meningitis diagnostics and screening in the era of point-of-care laboratory testing. J Clin Microbiol 2019; 57:e01238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prakit K, Nosanchuk JD, Pruksaphon K, et al. . A novel inhibition ELISA for the detection and monitoring of Penicillium marneffei antigen in human serum. Eur J Clin Microbiol Infect Dis 2016; 35:647–56. [DOI] [PubMed] [Google Scholar]
- 23. Thanh NT, Vinh LD, Liem NT, et al. . Clinical features of three patients with paradoxical immune reconstitution inflammatory syndrome associated with Talaromyces marneffei infection. Med Mycol Case Rep 2018; 19:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ho A, Shankland GS, Seaton RA. Penicillium marneffei infection presenting as an immune reconstitution inflammatory syndrome in an HIV patient. Int J STD AIDS 2010; 21:780–2. [DOI] [PubMed] [Google Scholar]
- 25. Hall C, Hajjawi R, Barlow G, Thaker H, Adams K, Moss P. . Penicillium marneffei presenting as an immune reconstitution inflammatory syndrome (IRIS) in a patient with advanced HIV. BMJ Case Rep 2013; 2013: bcr2012007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jarvis JN, Lawn SD, Wood R, Harrison TS. Cryptococcal antigen screening for patients initiating antiretroviral therapy: time for action. Clin Infect Dis 2010; 51:1463–5. [DOI] [PubMed] [Google Scholar]
- 27. Meya D, Rajasingham R, Nalintya E, et al. . Preventing cryptococcosis-shifting the paradigm in the era of highly active antiretroviral therapy. Curr Trop Med Rep 2015; 2:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mfinanga S, Chanda D, Kivuyo SL, et al. ; REMSTART trial team Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 2015; 385: 2173–82. [DOI] [PubMed] [Google Scholar]