Abstract
Background
Breakthrough infections of measles and mumps have raised concerns about the duration of vaccine-induced immunity, which might be improved by a third dose of measles-mumps-rubella vaccine (MMR3).
Methods
Here we compared (IgG) antibody levels against measles, mumps, and rubella in blood samples of 9-year-old children and young adults (18–25 years) following MMR2 and MMR3, respectively.
Results
We found that, in addition to antibody boosting for all 3 vaccine components, MMR3 resulted in lower antibody decay rates than MMR2; the declines were most prominent for mumps and rubella.
Conclusions
This study suggests that MMR3 provides long-lasting seroprotection against measles, mumps, and rubella.
Keywords: antibody response, humoral immunity, measles, mumps, rubella, seroprotection, vaccine, waning
Before routine vaccination was implemented, measles, mumps, and rubella were highly contagious diseases that were very common in children. Measles, characterized by its typical red skin rash, can lead to serious complications such as pneumonia and encephalitis [1]. Mumps, characterized by the swelling of the parotid glands, is generally perceived as a mild infectious disease, but may lead to complications such as viral meningitis, deafness, pancreatitis, or encephalitis. In adult men and women, mumps can cause orchitis and oophoritis, respectively [1]. Rubella, causing red skin rash, can result in significant congenital defects in the fetus after maternal infection during pregnancy [1]. Implementation of routine measles-mumps-rubella (MMR) vaccination has had a major impact on the incidence of these infectious diseases globally. In the Netherlands, routine MMR vaccination was implemented in 1987 by offering the first dose at 14 months of age (MMR1) and the second dose to children at 9 years of age (MMR2). However, vaccine-induced immunity has been shown to wane over time, and for mumps this has led to numerous outbreaks worldwide among vaccinated young adults [2–5]. Antibody levels against the mumps vaccine component have been shown to decline more rapidly compared with the measles and rubella vaccine components [6]. Although waning of measles and rubella antibody levels also occurs, measles and rubella outbreaks have only been reported in communities with pockets of unvaccinated persons [7, 8]. In addition, vaccinated health care workers who are treating measles cases are at risk and have occasionally been involved in measles outbreaks or clusters [9].
Recently, we performed a clinical study that was originally set up to investigate whether a third dose of MMR vaccine (MMR3) could improve immunity against mumps in young adults and be a good and safe intervention for controlling a mumps outbreak [10]. Here we present the first study to compare the effect of MMR2 receipt in 9-year-old children vs MMR3 receipt in young adults (18–25 years) with respect to the dynamics in antibody response and seroprotection rates against measles, mumps, and rubella.
METHODS
Study Cohorts
Children aged 9 years and young adults (18–25 years) were included from 2 different Dutch cohorts, which have been described elsewhere (hereafter referred to as the MMR2 [11] and MMR3 cohorts [10]). The MMR2 cohort includes 81 children enrolled in 2013–2014 who received MMR1 at 14 months of age and MMR2 at 9 years of age (M-M-RVAXPRO) subcutaneously in the upper arm. Apart from MMR2, these children also received an acellular pertussis vaccination as part of a study to analyze the immune response against pertussis; data on measles, mumps, and rubella antibody responses have not been published elsewhere [11]. From the participants of this cohort, plasma samples (15 mL) were taken just before and 1 month and 1 year after MMR2 receipt. The MMR3 cohort consists of 147 healthy young adults, 18–25 years of age, who were enrolled in 2016–2017 and had previously received MMR1 and MMR2 at the ages of, respectively, 14 months and 9 years. All 147 participants received a third dose of the MMR vaccine (M-M-RVAXPRO, containing measles virus, Enders’ Edmonston strain [live, attenuated]; not less than 1×103 50% cell culture infectious dose [CCID50]; mumps virus Jeryl Lynn strain [live, attenuated]; not less than 12.5×103 CCID50; rubella virus Wistar RA 27/3 strain [live, attenuated]; not less than 1×103 CCID50) in the deltoid muscle of the upper arm. Serum samples (8 mL) were taken from the participants just before and 4 weeks and 1 year after vaccination.
Patient Consent Statement
The patient’s written consent was obtained before any study handling. The design of both the MMR2 and MMR3 studies was approved by local ethical committees (ie, respectively, VCMO, Rotterdam, the Netherlands, and METC, Noord-Holland, the Netherlands). Both studies conform to the principles outlined in the Declaration of Helsinki and the Good Clinical Practice Guidelines.
Determination of Antibody Responses
Serum IgG antibodies for each of the 3 vaccine components were determined in parallel by a fluorescent bead-based multiplex immunoassay, as previously described [10]. Briefly, purified measles virus (strain Edmonston [in-house]), purified mumps vaccine strain (Jeryl Lynn [in-house]), and rubella virus (strain HPV-77; GenWay) were used as antigens. For each assay, a reference (RUBI-1–94, calibrated against the international standards for measles and an in-house standard for mumps), controls, and blanks were included. Antibody concentrations were obtained by interpolation of the mean fluorescent intensity in the reference serum curve using a logistic-5PL regression type and expressed in international units per mL (IU/mL) for measles and rubella and RIVM units (RU/mL) for mumps. An antibody concentration of ≥0.12 IU/mL for measles [12] and ≥10 IU/mL for rubella [13] was used as the cutoff for seroprotection for clinical protection. For mumps, there is no international agreement, nor an accurate serological correlate of protection. Here we used a surrogate level of protection of ≥102 RU/mL, which was previously assessed as the most appropriate cutoff for seroprotection against mumps virus infection [10].
In order to accurately bridge quantitative antibody data obtained from either plasma samples, collected by cell preparation tubes (CPTs; MMR2 study), to serum samples, collected by serum (clot activator) tubes (MMR3 study), measles-, mumps-, and rubella-specific IgG concentrations were compared with paired plasma and serum samples from 20 different individuals. Subsequently, measured IgG concentrations from plasma samples were corrected for dilution differences due to the use of CPT, for accurate comparison with antibody concentrations measured in serum samples.
Statistical Analysis
For each time point following MMR2 and MMR3 receipt, geometric mean IgG concentrations (GMCs) with 95% confidence intervals were calculated. The antibody concentrations were log-transformed to achieve normal distribution. Differences in antibody responses between the various time points were analyzed with a paired-samples t test. Subsequently, the proportion of participants with antibody concentrations above the cutoff level for seroprotection was calculated and compared at each time point with the NcNemar’s test for paired data. All reported P values are 2-sided; P values <.05 were considered statistically significant. Data were analyzed using IBM SPSS Statistics, version 24.
Linear effects models were employed to assess the effects of MMR2 vs MMR3 receipt and sampling time on longitudinal log-transformed IgG concentrations separately for measles, mumps, and rubella. Model selection was performed by means of likelihood ratio test. The final 3 models for measles, mumps, and rubella were specified as the interaction between sampling time and study type (MMR2/MMR3), taking into account IgG measurements per individual. The random effects part was specified as random intercept and random slope on time. Using this linear mixed model, we were able to provide a maximal likelihood estimation of fold change of IgG measurements across studies (MMR2/MMR3) for any combination of time points. The corresponding 95% confidence intervals were calculated by delta method. The setup and application of the linear mixed model were performed using R, version 3.4.3.
RESULTS
Antibody responses to MMR2 were measured in 81 children aged 9 years (male sex, 48.1%; female sex, 51.9%), and the responses to MMR3 were measured in 147 young adults (mean age at baseline [range], 22.4 [18–25] years; male sex, 46.3%; female sex, 53.7%) (Table 1, Figure 1).
Table 1.
Geometric Mean IgG Concentrations [95% CI] and Seroprotection Rates of Measles, Mumps, and Rubella Before and 1 Month and 1 Year After Receipt of a Second or Third Dose of Measles-Mumps-Rubella Vaccine (MMR2 or MMR3)
| Measles (Cutoff 0.12 IU/mL) | Mumps (Cutoff 102 RU/mL) | Rubella (Cutoff 10 IU/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MMR2 | Before MMR2 (n = 80) | 1 mo (n = 81) | 1 y (n = 78) | Before MMR2 (n = 80) | 1 mo (n = 81) | 1 y (n = 78) | Before MMR2 (n = 80) | 1 mo (n = 81) | 1 y (n = 78) |
| IgG [95% CI], RU/mL | 1.27 [1.06–1.52] | 1.67 [1.48–1.89] | 1.33 1.15–1.55 | 120 [94.6–153] | 423 [365–491] | 236 [198–281] | 56.3 [47.0–67.4] | 190 [172–210] | 80.9 [70.2–93.2] |
| Fold increase from baseline | - | 1.3 | 1.0 | - | 3.5 | 2.0 | - | 3.4 | 1.4 |
| Fold decrease 1 y vs 1 mo | - | - | 1.3 | - | - | 1.8 | - | - | 2.4 |
| Seroprotection, % | 100 | 100 | 100 | 50 | 98 | 90 | 98 | 100 | 100 |
| MMR3 | Before MMR3 (n = 147) | 4 wk (n = 147) | 1 y (n = 134) | Before MMR3 (n = 147) | 4 wk (n = 147) | 1 y (n = 134) | Before MMR3 (n = 147) | 4 wk (n = 147) | 1 y (n = 134) |
| IgG [95% CI], RU/mL | 0.69 [0.59–0.80] | 1.23 [1.10–1.38] | 1.04 [0.92–1.17] | 185 [163–211] | 306 [273–343] | 255 [224–290] | 36.7 [32.4–41.6] | 111 [100–122] | 64.8 [58.1–72.5] |
| Fold increase from baseline | - | 1.8 | 1.5 | - | 1.6 | 1.4 | - | 3.0 | 1.8 |
| Fold decrease 4 wk vs 1 y | - | - | 1.2 | - | - | 1.2 | - | - | 1.7 |
| Seroprotection, % | 97 | 100 | 100 | 81 | 94 | 90 | 95 | 100 | 100 |
Abbreviations: 1 mo and 4 wk, 1 month and 4 weeks after a second or third dose of measles-mumps-rubella vaccine dose (MMR2 or MMR3); 1 y, time point 1 year after MMR2 or MMR3; IgG, IgG concentration; IU/mL, international units per milliliter; RU/mL, RIVM units per milliliter.
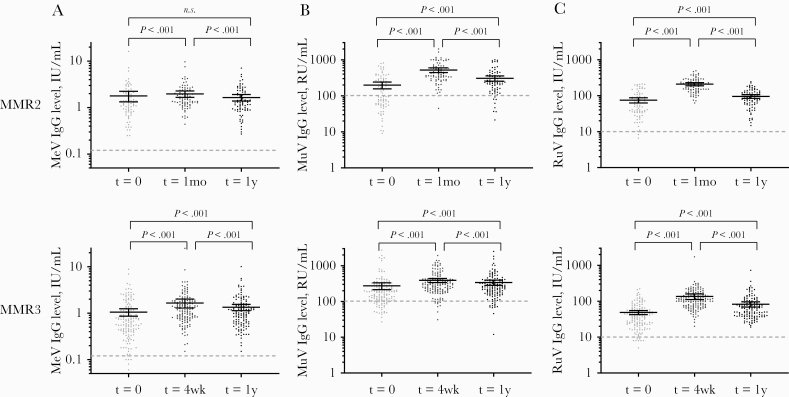
Figure 1.
IgG concentrations of measles (A), mumps (B), and rubella (C) before and 1 month and 1 year after MMR2 or MMR3 receipt. Geometric mean IgG concentrations with 95% confidence interval indicated as horizontal bars, were compared by paired-samples t test. Dashed lines indicate the antibody cutoff levels for seroprotection. Abbreviations: IU/mL, international units per milliliter; MeV-IgG, measles virus–specific IgG concentration; MuV-IgG, mumps virus–specific IgG concentration; RuV-IgG, rubella virus–specific IgG concentration; RU/mL, RIVM units per milliliter; t = 1mo and t = 4wk, 1 month and 4 weeks after a second or third dose of measles-mumps-rubella vaccine dose (MMR2 or MMR3); t = 1y, time point 1 year after MMR2 or MMR3.
Before the MMR2 receipt at 9 years of age, mumps-specific IgG concentrations were lower compared with before MMR3 receipt at the age of 18–25 years (P = .01; IgG geometric mean concentrations [GMC] of 120 RU/mL vs 185 RU/mL, respectively) (Table 1). The mumps seroprotection rate (ie, percentage of individuals above the used cutoff for presumed seroprotection) was also considerably lower before MMR2 receipt (50%) compared with before MMR3 receipt (81%). In contrast, measles- and rubella-specific IgG concentrations were higher before MMR2 receipt (for measles, 1.27 U/mL; for rubella, 56.3 U/mL) than before MMR3 receipt (for measles, 0.69 U/mL; for rubella, 36.7 U/mL; P ≤ .0001). Seroprotection rates for both measles and rubella were much higher when compared with mumps before MMR2 and MMR3 receipt, that is, 95%–100%. One month after MMR2 receipt, IgG concentrations had increased significantly by, respectively, 1.3-, 3.5-, and 3.4-fold for measles, mumps, and rubella (P < .0001). One year after MMR2 receipt, IgG concentrations against all 3 viruses had declined significantly compared with 1 month after MMR2 by, respectively, 1.3-, 1.8-, and 2.4-fold. However, the antibody concentrations of both mumps and rubella remained significantly higher than before MMR2, whereas that for measles returned to baseline. While antibody levels for both measles and rubella remained above the seroprotection levels, the seroprotection rate of mumps declined from 98% to 90% between 1 month and 1 year after MMR2 receipt.
One month after MMR3 receipt, IgG concentrations also increased significantly by, respectively, 1.8-, 1.6-, and 3.0-fold for measles, mumps, and rubella. One year after MMR3 receipt, IgG concentrations had declined again, but, interestingly, IgG concentrations against all 3 viruses were still higher than before MMR3 receipt. While the seroprotection rate of mumps had declined from 94% to 90% at 1 year after MMR3 receipt, the levels were still higher than before MMR3 receipt (81%).
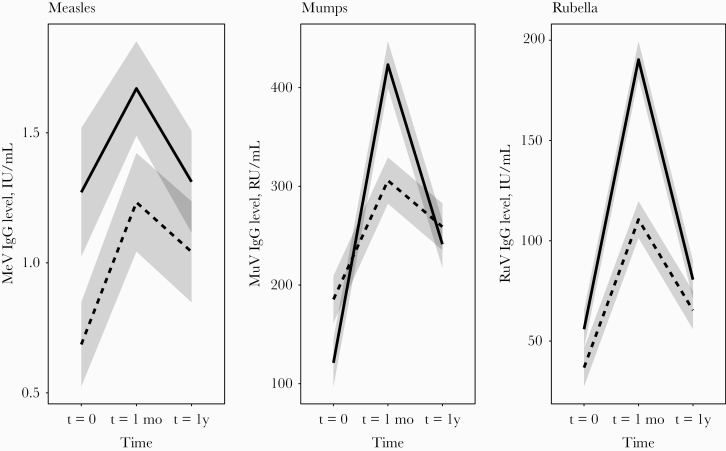
We further compared antibody dynamics after a second vs a third dose of MMR vaccine using a linear mixed model (Figure 2). This model showed that the individuals who generally had the lowest IgG concentrations before MMR2 or MMR3 receipt also showed the strongest rise in IgG concentrations after vaccination, both at 1 month and at 1 year. Therefore, when the antibodies increased strongly 1 month after vaccination, the antibody concentrations did not necessarily decrease more rapidly after 1 year. Furthermore, the model confirmed that the decline of both mumps- and rubella-specific IgG from 1 month to 1 year after vaccination was significantly more prominent after MMR2 receipt than after MMR3 receipt, despite that MMR2 resulted in a stronger initial rise of IgG concentrations (mumps: decline ratio MMR2/MMR3, 1.49; 95% CI, 1.31–1.67; rubella: decline ratio, 1.39; 95% CI, 1.20–1.59). Interestingly, decline of measles-specific antibody concentrations after either MMR2 or MMR3 receipt was low and comparable (decline ratio MMR2/MMR3, 1.08; 95% CI, 0.95–1.20).
Figure 2.
A linear mixed model was used to directly compare the dynamics in IgG antibody response to measles, mumps, rubella after a second measles-mumps-rubella vaccine dose (MMR2; solid lines) or a third MMR vaccine dose (MMR3; dashed lines). Abbreviations: t = 0, time point before MMR vaccination; t = 1, 1 month or 4 weeks after, respectively, a second or third dose of measles-mumps-rubella vaccine (MMR2 or MMR3); t = 1y, time point 1 year after MMR vaccination.
DISCUSSION
This is the first study to compare the dynamics of antibody responses to measles, mumps, and rubella of individuals who received 2 routine MMR vaccine doses with those of individuals who who received an additional third dose of the MMR vaccine at a young adult age (18–25 years). Before routine MMR2, at the age of 9 years, mumps-specific IgG concentrations were lower than in young adults, who received their last MMR2 dose at 9 years of age. Apparently, administration of MMR2 at the age of 9 years resulted in an increase of IgG concentrations against mumps virus, which remained elevated at the age of 18–25 years. The steep increase of mumps-specific antibodies shortly after MMR2 receipt indicates a secondary amnestic response, suggesting an active role of memory CD4+ T and B cells. Similar results were previously found for mumps in a large cross-sectional population-based serosurveillance study performed in the Netherlands in 2006/2007 (n = 7900), in which mumps-specific IgG concentrations were higher in 18–25-year-olds than in 9-year-old children before MMR2 receipt [14]. In the present study, before MMR2 receipt the seroprotection rate for mumps was estimated to be 50% This emphasizes the importance of routine MMR2 for children to provide protection against mumps. However, it should be noted that despite lower antimumps IgG concentrations, breakthrough infections among vaccinated children <9 years of age have hardly occurred in the Netherlands during mumps outbreaks [4]. Perhaps the risk of mumps virus introduction and exposure is simply smaller in this age group or the children still have a higher degree of immune protection despite low antibody concentrations. Possibly other immune compartments play an important role here.
It has been estimated that in order to achieve herd immunity and to prevent a mumps outbreak, ~86% of the population would need to have developed protective antibody levels against mumps [10]. In young adults who received their last MMR2 dose at 9 years of age, the seroprotection rate for mumps (81%) was still lower in this age group than the presumed threshold needed to achieve herd immunity (≥86%) [10]. This may explain why mumps outbreaks can occur in the age group of 18–25 years. Seroprotection rates of measles (97%) and rubella (95%) were clearly higher than that of mumps before MMR3, thereby reaching herd immunity to these 2 viruses. This suggests that the age group of 18–25 years, despite their 2 routine MMR vaccinations, is at risk for mumps, but not for measles and rubella. Previously, it was shown that the mumps component is the least effective in eliciting a response that would give rise to antibodies of high avidity [6]. In line with this, it has been found that mumps-specific memory B cells are detected at a lower frequency than measles- or rubella-specific B cells in vaccinees [15]. Nevertheless, seroprotection rates against all 3 vaccine components increased after receipt of both MMR2 and MMR3, but more importantly remained above the presumed herd immunity threshold for up to 1 year after MMR vaccination.
Interestingly, despite the higher IgG concentrations measured 1 month after MMR2 compared with MMR3 receipt, the decline of both mumps- and rubella-specific IgG concentrations appeared to be more prominent after MMR2 receipt. This suggests that antibodies are sustained longer after MMR3 receipt, indicating the persistence of long-lived plasma B cells.
In conclusion, an additional third dose of the MMR vaccine may be an adequate intervention for persons who are identified to be at risk of mumps during an outbreak in order to boost immunity to mumps, and it might also improve immunity to measles and rubella.
Acknowledgments
We thank all participants who made this work possible as well as the study team of the Spaarne Hospital, Jacqueline Zonneveld, José de Droog-Laane, and Greetje van Asselt, for recruiting and enrolling participants and managing visits for the MMR3 study; the laboratory staff members at the Regional Laboratory Kennemerland for processing laboratory samples for the MMR3 study; Alienke Wijmenga-Monsuur for preparing clinical documents and for providing ethical approval for the MMR3 study; and Gaby Smits (both RIVM) for excellent technical assistance with the IgG analysis for both the MMR2 and MMR3 samples. Furthermore, we would like to thank Saskia van der Lee (RIVM), an investigator on the MMR-2 study, for providing us the plasma samples of the MMR2 study.
Financial support. This work was supported by the Dutch Ministry of Health, Welfare and Sport.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. None of the authors has conflicts of interest to disclose. No conflicts of interests to declare.
References
- 1. Plotkin SA, Orenstein W, Offit PA, Edwards KM.. Plotkin’s Vaccines. 7th ed Philadelphia: Elsevier; 2017. [Google Scholar]
- 2. Cohen C, White JM, Savage EJ, et al. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerg Infect Dis 2007; 13:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dayan GH, Quinlisk MP, Parker AA, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008; 358:1580–9. [DOI] [PubMed] [Google Scholar]
- 4. Sane J, Gouma S, Koopmans M, et al. Epidemic of mumps among vaccinated persons, the Netherlands, 2009–2012. Emerg Infect Dis 2014; 20:643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marin M, Marlow M, Moore KL, Patel M. Recommendation of the Advisory Committee on Immunization Practices for use of a third dose of mumps virus-containing vaccine in persons at increased risk for mumps during an outbreak. MMWR Morb Mortal Wkly Rep 2018; 67:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kontio M, Jokinen S, Paunio M, et al. Waning antibody levels and avidity: implications for MMR vaccine-induced protection. J Infect Dis 2012; 206:1542–8. [DOI] [PubMed] [Google Scholar]
- 7. Patel M, Lee AD, Clemmons NS, et al. National update on measles cases and outbreaks—United States, January 1-October 1, 2019. MMWR Morb Mortal Wkly Rep 2019; 68:893–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hahne S, Macey J, van Binnendijk R, et al. Rubella outbreak in the Netherlands, 2004–2005: high burden of congenital infection and spread to Canada. Pediatr Infect Dis J 2009; 28:795–800. [DOI] [PubMed] [Google Scholar]
- 9. Hahne SJ, Nic Lochlainn LM, van Burgel ND, et al. Measles outbreak among previously immunized healthcare workers, the Netherlands, 2014. J Infect Dis 2016; 214:1980–6. [DOI] [PubMed] [Google Scholar]
- 10. Kaaijk P, Wijmenga-Monsuur AJ, van Houten MA, et al. A third dose of measles-mumps-rubella vaccine to improve immunity against mumps in young adults. J Infect Dis 2020; 221:902–9. [DOI] [PubMed] [Google Scholar]
- 11. van der Lee S, Sanders EAM, Berbers GAM, Buisman AM. Whole-cell or acellular pertussis vaccination in infancy determines IgG subclass profiles to DTaP booster vaccination. Vaccine 2018; 36:220–6. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization. Immunization, Vaccines and Biologicals. The Immunological Basis for Immunization Series. Module 7: Measles, Update Geneva: World Health Organization; 2009. Available at: https://www.who.int/immunization/documents/immunological_basis_series/en/
- 13. Skendzel LP. Rubella immunity. Defining the level of protective antibody. Am J Clin Pathol 1996; 106:170–4. [DOI] [PubMed] [Google Scholar]
- 14. Smits G, Mollema L, Hahné S, et al. Seroprevalence of mumps in the Netherlands: dynamics over a decade with high vaccination coverage and recent outbreaks. PLoS One 2013; 8:e58234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Latner DR, McGrew M, Williams N, et al. Enzyme-linked immunospot assay detection of mumps-specific antibody-secreting B cells as an alternative method of laboratory diagnosis. Clin Vaccine Immunol 2011; 18:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]