Abstract
Background
Evidence supports streamlined approaches for inpatients with community-acquired pneumonia (CAP) including early transition to oral antibiotics and shorter therapy. Uptake of these approaches is variable, and the best approaches to local implementation of infection-specific guidelines are unknown. Our objective was to evaluate the impact of a clinical decision support (CDS) tool linked with a clinical pathway on CAP care.
Methods
This is a retrospective, observational pre–post intervention study of inpatients with pneumonia admitted to a single academic medical center. Interventions were introduced in 3 sequential 6-month phases; Phase 1: education alone; Phase 2: education and a CDS-driven CAP pathway coupled with active antimicrobial stewardship and provider feedback; and Phase 3: education and a CDS-driven CAP pathway without active stewardship. The 12 months preceding the intervention were used as a baseline. Primary outcomes were length of intravenous antibiotic therapy and total length of antibiotic therapy. Clinical, process, and cost outcomes were also measured.
Results
The study included 1021 visits. Phase 2 was associated with significantly lower length of intravenous and total antibiotic therapy, higher procalcitonin lab utilization, and a 20% cost reduction compared with baseline. Phase 3 was associated with significantly lower length of intravenous antibiotic therapy and higher procalcitonin lab utilization compared with baseline.
Conclusions
A CDS-driven CAP pathway supplemented by active antimicrobial stewardship review led to the most robust improvements in antibiotic use and decreased costs with similar clinical outcomes.
Keywords: antimicrobial stewardship, multidisciplinary, pathway, pneumonia
Community-acquired pneumonia (CAP) is a leading cause of hospitalization and death worldwide, and its economic burden in the United States remains >$17 billion annually [1, 2]. Recent data supporting streamlined treatment for CAP have shown both improved quality and reduced health care costs. For example, the early transition from intravenous (IV) to oral antibiotics has been shown not only to be safe and effective, but also to be associated with reductions in length of stay (LOS) [3]. Additionally, recent data challenge the need for empiric coverage for atypical bacterial organisms in all patients with CAP and support shorter durations of antibiotic therapy as safe and effective [4–8]. Finally, the biomarker procalcitonin was approved to help distinguish patients with bacterial vs viral lower respiratory tract infection and help guide clinicians to early cessation of antibiotics [9]. Only recently did the Infectious Diseases Society of America (IDSA) incorporate some of these changes into their national guidelines [10].
Although evidence suggests that incorporating local guidelines into inpatient CAP management leads to improvements in the process of care and patient outcomes, provider adherence remains variable, and the best approaches to implementation are unknown [11, 12]. Reasons for nonadherence include individual provider preference, lack of knowledge of existing best practices, and existence of conflicting guidelines [13]. To overcome these challenges, data suggest that the use of antimicrobial stewardship (AS) intervention can effectively reduce duration of therapy and curtail these barriers [14]. Additionally, locally developed clinical decision support (CDS) tools have been shown to be effective at improving health care process measures by giving providers an aid at decision-making points across diverse settings [15].
At our institution, CAP was recognized as a leading cause of admission that was both cost- and resource-intensive. Upon further investigation, we found that multiple conflicting order sets existed within our electronic health record (EHR), and there was wide variation in admission location, diagnostic workup, choice of empiric antibiotics, and duration of therapy. To address these issues, we assembled a multidisciplinary team and developed a CDS advisory with a linked order set as part of a CAP pathway with the goals of promoting early identification of patients with CAP, standardization of diagnostic and empiric treatment approaches, promotion of early intravenous (IV) to oral antibiotic transition, reducing coverage for atypical pathogens, and shorter overall durations of antibiotic therapy. We aimed to evaluate the impact of our CAP pathway on length of intravenous antibiotic therapy, total length of antibiotic therapy, laboratory utilization, hospital LOS, costs, and clinical outcomes for patients admitted with CAP.
METHODS
Setting
University of Utah Hospital is a 592-bed academic medical center in Salt Lake City, Utah. Adult patients who presented to the University of Utah emergency department with pneumonia and were admitted to the hospitalist or pulmonary services on either an acute care floor or intensive care unit (ICU) were included in the study. Diagnosis of pneumonia was based on International Classification of Disease, Ninth and Tenth Revision (ICD-9 and ICD-10), codes at discharge. Patients were excluded if they had a history of cystic fibrosis or solid organ transplant based on ICD-9 and ICD-10 codes, given the potential risk of drug-resistant pathogens and local practice favoring longer durations of therapy. Patients with an abscess diagnosis during the visit were excluded. Patients admitted to our separate cancer hospital were not included. Additionally, patients with an LOS >30 days were excluded, as these do not usually represent typical CAP cases [16]. See Appendices A and B for the full list of ICD-9/10 codes used for inclusion and exclusion criteria as well as the number of patients excluded per phase. When calculating total antibiotics, postdischarge antibiotic durations >21 days were not included as these usually represented chronic antibiotics for prophylaxis. Antibiotics with clear indications other than pneumonia were also not included.
We performed a retrospective observational pre–post intervention study evaluating the impact of (1) CAP educational training, (2) the use of a standardized EHR order set triggered by a CDS advisory, and (3) prospective audit and feedback of patients with CAP from the antimicrobial stewardship program (ASP).
CAP Pathway Design
A multidisciplinary team including physicians (emergency medicine, pulmonary and critical care, infectious diseases/antimicrobial stewardship, hospitalists), pharmacists, value engineering, information technology, and the quality department reviewed current guidelines and available literature and developed a standardized CAP order set for patients presenting to the emergency department (ED). The CAP pathway refers to all patients admitted to the hospital with CAP who have been started on a standard treatment protocol via the CDS advisory or order set. The pathway is initiated in the emergency room when a provider signs an order for both a chest radiograph and any antibiotic. This triggers a CDS advisory to the provider stating, “If this antibiotic is for pneumonia, click ‘Open order set.’” Upon selection, this order set provides guidance on appropriate triage (ICU vs floor vs home), tools for assessing risk for drug-resistant bacterial pathogens, appropriate diagnostic testing, and recommended empiric antibiotic therapy [17]. Procalcitonin is ordered once on admission to help the medical team determine whether antibiotics are needed if alternative diagnoses are considered. For patients admitted with CAP who were not initiated on the CAP pathway via the CDS advisory in the ED, the admitting team could access the CAP order set via the general admission order set. Patients not at increased risk for antimicrobial resistance admitted to the medicine floor are given a single IV dose of ceftriaxone and IV azithromycin, followed by an automatic transition to oral cefuroxime after 24 hours, for a total antibiotic duration of 5 days. Azithromycin is automatically discontinued after 24 hours unless Legionella urine antigen returns positive [5].
Phase 1: Educational Only
Before the initiation of the CDS advisory, CAP pathway educational training was provided to providers, nurses, respiratory therapists, and pharmacists. Training sessions lasted between 15 and 30 minutes and included background, purpose of the project, and diagnostic testing strategies including the use of procalcitonin. CDS advisory education and antibiotic overview were given to ED and internal medicine residents, advanced practice clinicians, and attendings over a series of conferences, division meetings, and email communications between April and September 2017. Less formal education and reinforcement was continued intermittently for new staff and rotating residents until October 2018.
Phase 2: CDS Advisory and ASP
The CDS advisory was initially validated by a single ED provider (C.H.) for a total of 3 months. The CDS advisory went live for all providers starting September 30, 2017. The ASP started audit and feedback of patients admitted to the hospitalist teams with CAP on October 1, 2017. The ASP team consisted of a 0.5 full-time equivalent (FTE) infectious diseases physician and 1 FTE infectious diseases pharmacist. The ASP MD and PharmD co-led the program. The ASP MD had 6 years of experience leading and conducting ASP interventions, and the PharmD had 2 years of active ASP experience. Prospective audit and feedback consisted of ASP review of CAP patients on hospitalists’ teams on weekdays, with direct feedback provided on appropriate diagnostic testing, antibiotic choice, and duration. Interventions on CAP patients admitted to the ICU generally occurred after their transfer to the hospitalist service. If a patient was on IV antibiotics but deemed clinically appropriate to transition to oral antibiotics, the ASP provided this feedback to the primary team. Additionally, the ASP provided recommendations for stopping azithromycin when atypical pathogens were felt to be unlikely etiologies.
Phase 3: CDS Advisory Only
After 6 months of prospective audit and feedback, stewardship resources were required on other projects. From April 2018, the ASP was unable to give consistent audit and feedback for CAP patients.
Data Collection and Analysis
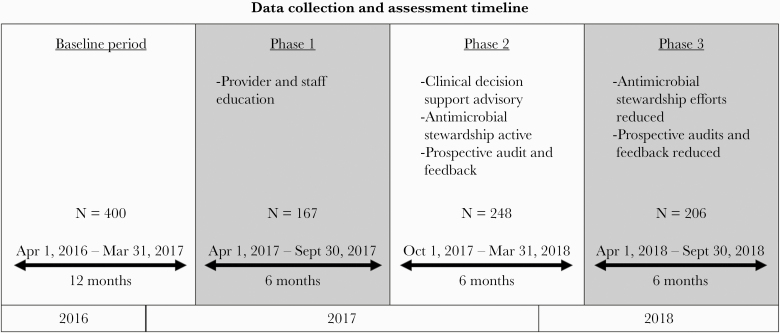
The study consisted of a 12-month pre-intervention baseline phase followed by 3 intervention phases of 6 months (Figure 1). The baseline period took place from April 1, 2016, to March 31, 2017. Phase 1: Provider education alone occurred from April 1, 2017, to September 30, 2017. Phase 2: CDS-triggered CAP pathway coupled with active AS prospective audit and feedback occurred from October 1, 2017, to March 31, 2018. Phase 3: The CDS-triggered CAP pathway continued, but AS was no longer providing prospective audit and feedback, as their efforts were required for other institutional activities. Phase 3 occurred from April 1, 2018, to September 30, 2018. We obtained all data from the enterprise data warehouse [18].
Figure 1.
Data collection and assessment timeline.
Visit characteristics included patient age, gender, Charlson comorbidity index (CCI), severity of illness on presentation using the CURB-65 score (confusion, urea >7 mmol/L, respiratory rate ≥30/min, systolic blood pressure <90 mmHg, and age ≥65), whether a flu test was ordered, viral pneumonia diagnosis, ICU stay, ICU escalation after floor admission, ventilation status, and number of laboratory tests per visit (Table 1).
Table 1.
Visit Characteristics by Phase
| Patient Characteristics | Baseline | Phase 1 | Phase 2 | Phase 3 | P Value |
|---|---|---|---|---|---|
| No. of visits | 400 | 167 | 248 | 206 | |
| No. of patients | 378 | 161 | 233 | 199 | |
| Age, y | 59.56 (18.76) | 59.63 (18.76) | 62.21 (18.12) | 64.25 (17.03) | .01 |
| CCI | 3.9 (3.36) | 4.17 (3.2) | 4.59 (3.32) | 4.74 (3.63) | .01 |
| CURB-65 | 1.25 (1.04) | 1.42 (0.97) | 1.41 (1.04) | 1.48 (1.02) | .03 |
| Flu test ordered, No. (%) | 212 (53) | 49 (29.34) | 196 (79.03) | 51 (24.76) | <.01 |
| Viral pneumonia diagnosis, No. (%) | 28 (7) | 4 (2.4) | 47 (18.95) | 3 (1.46) | <.01 |
| ICU stay, No. (%) | 150 (37.5) | 62 (37.13) | 84 (33.87) | 86 (41.75) | .39 |
| ICU escalation, No. (%) | 33 (8.25) | 11 (6.59) | 13 (5.24) | 11 (5.34) | .39 |
| Ventilation status, No. (%) | 43 (10.75) | 10 (5.99) | 13 (5.24) | 11 (5.34) | .02 |
| No. of laboratory tests per visit | 41.34 (42.97) | 42.83 (47.23) | 38.89 (40.46) | 42.48 (39.51) | .76 |
Values are expressed as mean (SD) unless specified otherwise. P values are based on the Kruskal-Wallis test, analysis of variance, or chi-square test, as appropriate.
Abbreviations: CCI, Charlson comorbidity index; CURB 65 = confusion, urea >7mmol/L, respiratory rate ≥30/min, systolic blood pressure <90 mmHg, and age ≥65 score; ICU, intensive care unit.
The primary outcomes were length of IV antibiotic therapy and total length of antibiotic therapy. Length of IV antibiotic therapy was calculated as calendar days of inpatient IV antibiotics. We defined total length of antibiotic therapy as calendar days of inpatient antibiotics (both IV and oral) plus calendar days of postdischarge antibiotic [19]. Postdischarge antibiotic days were calculated by prescription duration on the discharge order and were manually validated by a physician (C.C.) and a pharmacist (T.T.), with an interrater rater reliability of 85% calculated based on 20 orders. All outpatient prescriptions were reviewed for accuracy to exclude chronic prophylactic antibiotics and confirm the indication if present.
Secondary outcomes included clinical, process, and cost outcomes. Clinical outcomes were hospital LOS, all-cause inpatient mortality, and 30-day readmissions. Process outcomes included length of inpatient IV azithromycin therapy, inpatient azithromycin therapy, inpatient atypical antibiotic therapy (ie, azithromycin, doxycycline, levofloxacin), procalcitonin utilization, and Legionella and Streptococcus pneumoniae urine antigen utilization. Cost data were collected from our institutionally derived Value Driven Outcomes tool [20]. This tool allocates care costs to individual clinical encounters based on actual cost, time- and quantity-based allocations, and other costing methods [18–20]. We received estimates of costs for visit, facility utilization, pharmacy, and laboratory tests with aggregated cost estimates rather than separate costs for each laboratory test. Facility utilization, pharmacy, and laboratory subcosts are also reported. All costs were converted to 2015 dollars to account for inflation, then normalized to the baseline total cost (Table 2).
Table 2.
Outcome Measures by Phase
| Outcome | Baseline | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|---|
| Primary outcomes | ||||
| Length of intravenous antibiotic therapy, d | 3.49, 3.19–3.82 | 3.16, 2.68–3.74, P = .32 | 2.73, 2.36–3.16, P < .01 | 2.81, 2.41–3.28, P = .02 |
| Total length of antibiotic therapy, d | 6.4, 5.97–6.86 | 6.04, 5.33–6.86, P = .45 | 5.54, 4.96–6.19, P = .03 | 5.98, 5.31–6.73, P = .34 |
| Clinic outcomes | ||||
| Length of stay, d | 4.94, 4.53–5.39 | 4.54, 3.87–5.33, P = .37 | 4.46, 3.87–5.13, P = .21 | 4.68, 4.03–5.43, P = .54 |
| Inpatient mortality, % | 6.28, 4.26–9.17 | 6.51, 3.31–12.41, P = .93 | 4.31, 2.2–8.27, P = .31 | 4.28, 2.07–8.65, P = .35 |
| 30-d readmission, % | 12.81, 9.85–16.5 | 10.03, 5.98–16.34, P = .39 | 13.73, 9.03–20.33, P = .78 | 12.39, 7.91–18.9, P = .90 |
| Process outcomes | ||||
| Length of inpatient intravenous azithromycin therapy, d | 1.2, 1.09–1.33 | 1.09, 0.91–1.31, P = .35 | 1, 0.86–1.18, P = .05 | 0.99, 0.84–1.17, P = .05 |
| Length of inpatient azithromycin therapy, d | 1.91, 1.77–2.06 | 1.71, 1.48–1.96, P = .18 | 1.56, 1.37–1.76, P < .01 | 1.48, 1.3–1.69, P < .01 |
| Length of inpatient atypical antibiotic therapy, d | 2.35, 2.18–2.53 | 1.97, 1.72–2.26, P = .03 | 1.89, 1.68–2.13, P < .01 | 1.73, 1.52–1.96, P < .01 |
| Procalcitonin lab utilization, % | 47.91, 42.99–52.88 | 52.76, 43.67–61.68, P = .37 | 75.72, 68.73–81.57, P < .01 | 64.96, 56.58–72.51, P < .01 |
| Legionella and Streptococcus pneumoniae urine antigen utilization, % | 72.06, 67.41–76.28 | 76.41, 68.36–82.93, P = .33 | 78.58, 71.28–84.44, P = .13 | 78.2, 70.97–84.03, P = .14 |
| Cost outcomes | ||||
| Total cost per visit | 1, 0.9–1.12 | 0.79, 0.65–0.97, P = .05 | 0.8, 0.67–0.95, P = .03 | 0.84, 0.69–1.01, P = .11 |
| Facility utilization cost | 0.52, 0.47–0.58 | 0.43, 0.36–0.53, P = .11 | 0.44, 0.37–0.52, P = .11 | 0.47, 0.4–0.57, P = .41 |
| Pharmacy cost | 0.15, 0.13–0.18 | 0.1, 0.07–0.14, P = .05 | 0.1, 0.08–0.14, P = .03 | 0.12, 0.08–0.16, P = .16 |
| Laboratory cost | 0.1, 0.09–0.12 | 0.06, 0.05–0.08, P < .01 | 0.05, 0.04–0.07, P < .01 | 0.05, 0.04–0.06, P < .01 |
Values are expressed as estimated marginal mean, 95% confidence interval, P value based on gamma and logistic regression models adjusted for age, Charlson comorbidity index, CURB-65, and flu season. P values are based on the following comparisons: Baseline/Phase 1, Baseline/Phase 2 and Baseline/Phase 3. Cost data are normalized based on baseline total cost per visit.
Abbreviation: CURB 65 = confusion, urea >7mmol/L, respiratory rate ≥30/min, systolic blood pressure <90 mmHg, and age ≥65 score.
We used generalized linear models to compare outcomes for the 3 phases with their baseline levels while adjusting for the following covariates: age, CCI, CURB-65, and admission during flu season (October–March). We used gamma regression with log link for continuous outcomes and logistic regression for binary outcomes. We chose visit-level regression models over an aggregated interrupted time series analysis because of the short intervention phase duration and availability of visit-level risk adjustment variables. Statistical analyses were done in R, version 3.5.1. P values <.05 were considered significant.
RESULTS
A total of 400 patient visits were included in the baseline period and 623 in the intervention phases (Phase 1: 167; Phase 2: 248; Phase 3: 206). Patient characteristics within the study phases are summarized in Table 1. The CAP order set was used for 55.24% of visits in Phase 2 and 44.17% of visits in Phase 3. Compared with baseline, patients in Phase 3 were significantly older, with higher mean CURB-65 and CCI scores (Table 1). During the flu season, patients were tested for flu more often, with more cases of viral pneumonia present.
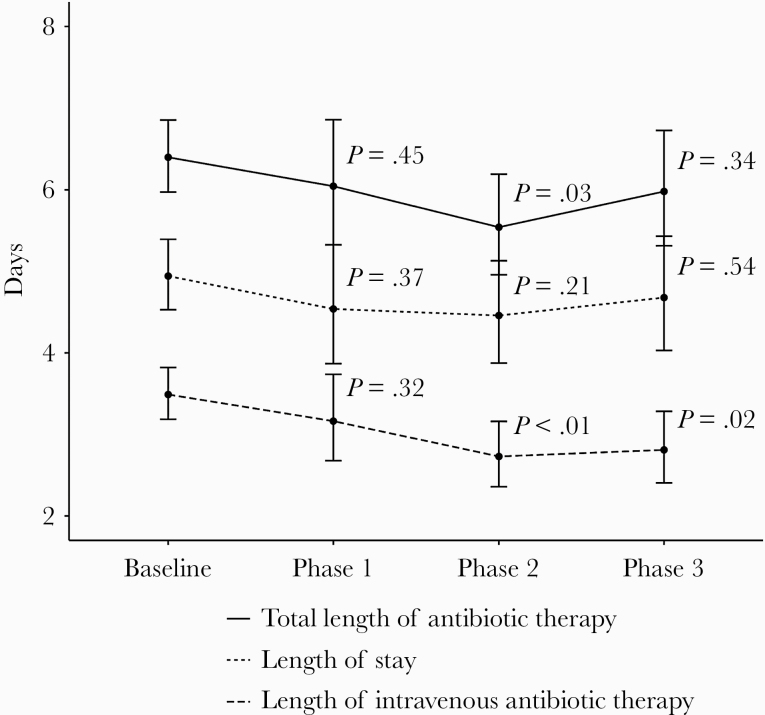
Process and outcome measures by phase are listed in Table 2. Length of IV antibiotic therapy days was unchanged in Phase 1 as compared with baseline, but was significantly lower in both Phase 2 and Phase 3. A similar trend was seen for the length of azithromycin therapy. Length of inpatient atypical antibiotic therapy was significantly reduced in all 3 phases compared with baseline. Total length of antibiotic therapy was decreased in Phase 2, but not in Phases 1 or 3, as compared with baseline.
LOS did not significantly change in any of the phases as compared with baseline. We saw a nonsignificant decrease in LOS from 4.94 (95% CI, 4.53–5.39) days at baseline to 4.46 (95% CI, 3.87–5.13) days in Phase 2 (P = .21) (Figure 2). There was no change in inpatient mortality or 30-day readmissions between any of the phases.
Figure 2.
Intravenous antibiotic therapy length, total antibiotic therapy length, and length of stay by phase. Estimated marginal means, 95% confidence intervals, and P values are based on gamma regression models adjusted for age, Charlson comorbidity index, CURB-65 (confusion, urea >7mmol/L, respiratory rate ≥30/min, systolic blood pressure <90 mmHg, and age ≥65 score), and flu season, with visits with length of stay >30 days excluded. P values are based on the following comparisons: Baseline/Phase 1, Baseline/Phase 2, and Baseline/Phase 3.
Procalcitonin testing was increased in Phases 2 and 3 as compared with baseline; however, there was no significant change in use of Legionella and Streptococcus pneumoniae urine antigens.
When compared with baseline, the CAP pathway with active AS in Phase 2 showed a significant reduction of 20% in normalized cost per visit. A cost reduction of 21% was also seen in Phase 1, and a nonsignificant reduction of 16% was seen in Phase 3. Savings were most notable in the area of pharmacy and laboratory cost.
Discussion
We were able to demonstrate that a multifaceted decision support–triggered CAP pathway combined with AS prospective audit and feedback led to shorter durations of IV antibiotic therapy, shorter total length of antibiotic therapy, and a 20% overall hospital cost decrease, without an increase in patient harm.
Previous studies have shown that short courses of antibiotics for patients hospitalized with CAP are safe, effective, and have the potential to improve adherence, reduce antimicrobial resistance, and decrease cost [6, 8, 9, 17]. Although current IDSA CAP guidelines suggest conversion to oral therapy based on stability criteria, studies in inpatients with CAP report similar outcomes with automatic oral switches regardless of stability criteria [21, 22]. Oosterheert et al. evaluated early transition from IV to oral antibiotics for severe CAP and showed that an automatic switch to oral after 3 days of IV antibiotics was associated with earlier discharge, a 2-day reduction in LOS, and decreased drug and treatment costs with similar clinical outcomes [3]. Additionally, Castro-Guardiola and colleagues found that inpatients with nonsevere CAP treated with oral antibiotics from the time of admission had similar clinical outcomes to those changed from IV to oral based on stability criteria, in addition to fewer adverse events and shorter hospital stays [22]. As most patients admitted to the medicine floor are able to tolerate an oral diet and their oral home medications, we advocated for an even earlier IV to oral antibiotic transition of 24 hours. This intervention was associated with a decrease in mean IV antibiotic duration from 3.49 days to 2.73 days, translating to ~1 less dose of IV antibiotics for most patients. Our preset duration of 5 days and the automatic transition from IV to oral antibiotics likely helped reduce some of the mental load our physicians face in the multiple decisions needed in the care of hospitalized patients.
Phase 2 with the CAP pathway and active AS showed the most significant improvements as compared with baseline, with reductions in length of IV antibiotic therapy, total length of therapy, and atypical antibiotic duration, as well as significant cost savings. We attribute these findings to this phase being the most intensive, with ASP conducting prospective audit and feedback of all patients with CAP in addition to the CAP pathway. Phase 1 was associated with cost reduction without associated reduction in decreased antibiotic duration. The source of cost reduction in Phase 1 is unclear; however, we believe that increased attention to CAP and standardization of practices caused by education may have led to a decrease in cost. Days on IV antibiotics and atypical antibiotic duration continued to remain significantly shorter in Phase 3 compared with baseline, despite less robust AS involvement. Although direct comparisons between phases were not made, days on IV antibiotics and atypical antibiotic duration appeared to increase in Phase 3 as compared with Phase 2. Additionally, significant cost-savings and reductions in total length of therapy were not present in Phase 3 as compared with baseline after ASP resources were shifted to other areas. Multiple studies have evaluated the benefits of syndrome-specific ASP, finding that ASP leads to increases in guideline-concordant therapy and shorter durations of therapy [17, 23, 24]. While it is not clear whether the apparent increases in antibiotic duration seen in Phase 3 are related to lack of intensive AS involvement or alert fatigue related to the CDS advisory, our findings highlight the synergistic effect of syndrome-specific pathways with additional ASP prospective audit and feedback, but suggest that ongoing dedicated ASP resources are imperative for sustainability.
Another unique aspect of our project was the early discontinuation of atypical coverage after a single dose of IV azithromycin. The IDSA/American Thoracic Society (ATS) guidelines from 2018 recommend treatment with a beta-lactam and macrolide for patients admitted with CAP; however, recent guidelines from the Dutch Working Party on Antibiotic Policy (SWAB) and Dutch Association of Chest Physicians (NVALT) suggest that monotherapy with a beta-lactam is appropriate for most patients with CAP admitted to the medicine floor [5, 21]. This topic continues to be heavily debated, as the risks and benefits of antibiotic use need to be balanced with rising rates of antimicrobial resistance and adverse events [4]. Musher et al. evaluated the reported epidemiologic causes of CAP and determined that Mycoplasma and Chlamydia were very uncommon causes of CAP leading to hospitalization of adults [25]. Furthermore, randomized controlled trials have shown no benefit in survival or clinical efficacy with empiric atypical coverage for hospitalized patients [26]. With the rising concern about both resistance patterns and adverse events, we avoided including fluoroquinolones as part of our standard antibiotic regimen. We saw significant reductions in length of atypical antibiotic therapy starting in Phase 1 and continuing throughout all intervention periods without associated increases in LOS, mortality, or 30-day readmissions, suggesting that macrolide therapy in addition to a beta-lactam may not be necessary for all cases of CAP.
The success of our intervention is likely multifactorial. First, our organization has a strong “value culture” with numerous quality improvement projects focused on reducing overutilization and promoting best practices [20]. The value equation used by the University of Utah was adapted from the concept proposed by the Harvard Business School in which value equals quality of care plus service over cost [27]. In this case, value is enhanced by improving quality through reducing days on IV antibiotics, reducing total length of therapy, and reducing normalized cost. Second, we had strong support from the hospital staff, ASP, hospitalist group, medical ICU, and ED, as well as organizational leadership. This allowed us to effectively develop the CDS advisory and CAP pathway, disseminate education on the project, obtain feedback from front-line providers, and perform audit and feedback. Interestingly, we were able to see improvements despite an older and sicker population. It remains unclear if our CDS advisory helped ED providers to more effectively triage patients with CAP and prevent hospital admission for the less sick patients, or if we are seeing an increasing trend in more acutely ill patients admitted to our hospital.
Our study has several limitations. Foremost, this was a single-center, quasi-experimental study and was subject to the inherent limitations therein. We had limited time frames in each phase, which may introduce bias related to CAP seasonal variability. The duration of Phase 2 with additional ASP intervention was limited due to resource reallocations, suggesting difficulty with long-term sustainability that may limit external validity and widespread adoption. It is also difficult to isolate the individual effects of the CDS-driven CAP pathway and ASP prospective audit and feedback, though a synergistic impact of clinical pathways and compliance feedback on CAP has been reported elsewhere, suggesting the necessity of this combination [12]. Although we could track order set usage, we were unable to determine compliance. If providers felt that alternative diagnoses were appropriate, they could easily discontinue or modify the order set. Additionally, we did not account for the fixed cost of the ASP in the financial analysis. Although providers’ time and effort are reimbursed to focus on many stewardship interventions in addition to CAP, some of the calculated cost-savings generated from this pathway are offset by stewardship resources. Finally, we did not include solid organ transplant patients or those with active malignancy, limiting the application of our findings to immunocompromised populations.
Future directions include work to better understand provider variation and reasons for nonadherence to the pathway. This information will inform future efforts to increase provider usage of the CAP pathway and adherence to recommendations so that less intensive AS review is needed. Additionally, we aim to incorporate narrow-spectrum penicillins in future iterations of our pathway in an effort to minimize antibiotic-associated adverse events. Finally, future work will focus on evaluating discharge antibiotic prescriptions for CAP patients. Recent data suggest that discharge antibiotics account for the majority of excess duration of therapy for CAP, with each excess day associated with a 5% increased risk of antibiotic-associated adverse events [19]. Minimizing excess antibiotic durations has the potential to improve health care value by reducing readmissions and repeat health care visits related to adverse events.
Conclusions
The early initiation of a CDS-driven CAP pathway supplemented by ASP review appears to improve health care value through decreased IV antibiotic length of therapy, decreased total length of therapy, and decreased costs with similar clinical outcomes.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was unfunded.
Potential conflicts of interest. K.K. reports honoraria, consulting, or sponsored research related to clinical decision support or standards-based interoperability with McKesson InterQual, Hitachi, Premier, Klesis Healthcare, Vanderbilt University, the University of Washington, the University of California at San Francisco, and the US Office of the National Coordinator for Health IT (via ESAC, JBS International, A+ Government Solutions, Hausam Consulting, and Security Risk Solutions). These relationships have no direct relevance to the manuscript but are reported in the interest of full disclosure. K.K. was also a co-developer of the University of Utah value-driven outcomes tool used for cost analyses in the submitted work. The other authors have no financial relationships or conflicts of interest relative to this article to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This study was classified by the University of Utah Institutional Review Board as a quality improvement project and did not require review and oversight or written informed consent.
References
- 1. Postma DF, van Werkhoven CH, van Elden LJ, et al. ; CAP-START Study Group Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med 2015; 372:1312–23. [DOI] [PubMed] [Google Scholar]
- 2. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010; 122:130–41. [DOI] [PubMed] [Google Scholar]
- 3. Oosterheert JJ, Bonten MJ, Schneider MM, et al. . Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ 2006; 333:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garin N, Genné D, Carballo S, et al. . β-lactam monotherapy vs β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med 2014; 174:1894–901. [DOI] [PubMed] [Google Scholar]
- 5. Wiersinga WJ, Bonten MJ, Boersma WG, et al. . Management of community-acquired pneumonia in adults: 2016 guideline update from the Dutch Working Party on Antibiotic Policy (SWAB) and Dutch Association of Chest Physicians (NVALT). Neth J Med 2018; 76:4–13. [PubMed] [Google Scholar]
- 6. Uranga A, España PP, Bilbao A, et al. . Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med 2016; 176:1257–65. [DOI] [PubMed] [Google Scholar]
- 7. Dunbar LM, Wunderink RG, Habib MP, et al. . High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis 2003; 37:752–60. [DOI] [PubMed] [Google Scholar]
- 8. el Moussaoui R, de Borgie CA, van den Broek P, et al. . Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ 2006; 332:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schuetz P, Wirz Y, Sager R, et al. . Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18:95–107. [DOI] [PubMed] [Google Scholar]
- 10. Metlay JP, Waterer GW, Long AC, et al. . Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Capelastegui A, España PP, Quintana JM, et al. . Improvement of process-of-care and outcomes after implementing a guideline for the management of community-acquired pneumonia: a controlled before-and-after design study. Clin Infect Dis 2004; 39:955–63. [DOI] [PubMed] [Google Scholar]
- 12. Almatar M, Peterson GM, Thompson A, et al. . Clinical pathway and monthly feedback improve adherence to antibiotic guideline recommendations for community-acquired pneumonia. PLoS One 2016; 11:e0159467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Almatar MA, Peterson GM, Thompson A, et al. . Community-acquired pneumonia: why aren’t national antibiotic guidelines followed? Int J Clin Pract 2015; 69:259–66. [DOI] [PubMed] [Google Scholar]
- 14. Foolad F, Huang AM, Nguyen CT, et al. . A multicentre stewardship initiative to decrease excessive duration of antibiotic therapy for the treatment of community-acquired pneumonia. J Antimicrob Chemother 2018; 73:1402–7. [DOI] [PubMed] [Google Scholar]
- 15. Bright TJ, Wong A, Dhurjati R, et al. . Effect of clinical decision-support systems: a systematic review. Ann Intern Med 2012; 157:29–43. [DOI] [PubMed] [Google Scholar]
- 16. Brock GN, Barnes C, Ramirez JA, Myers J. How to handle mortality when investigating length of hospital stay and time to clinical stability. BMC Med Res Methodol 2011; 11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Webb BJ, Dascomb K, Stenehjem E, et al. . Derivation and multicenter validation of the drug resistance in pneumonia clinical prediction score. Antimicrob Agents Chemother 2016; 60:2652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim WS, van der Eerden MM, Laing R, et al. . Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaughn VM, Flanders SA, Snyder A, et al. . Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: a multihospital cohort study. Ann Intern Med 2019; 171:153–63. [DOI] [PubMed] [Google Scholar]
- 20. Lee VS, Kawamoto K, Hess R, et al. . Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA 2016; 316:1061–72. [DOI] [PubMed] [Google Scholar]
- 21. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44:S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castro-Guardiola A, Viejo-Rodríguez AL, Soler-Simon S, et al. . Efficacy and safety of oral and early-switch therapy for community-acquired pneumonia: a randomized controlled trial. Am J Med 2001; 111:367–74. [DOI] [PubMed] [Google Scholar]
- 23. Avdic E, Cushinotto LA, Hughes AH, et al. . Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis 2012; 54:1581–7. [DOI] [PubMed] [Google Scholar]
- 24. Stenehjem E, Hersh AL, Buckel WR, et al. . Impact of implementing antibiotic stewardship programs in 15 small hospitals: a cluster-randomized intervention. Clin Infect Dis 2018; 67:525–32. [DOI] [PubMed] [Google Scholar]
- 25. Musher DM, Abers MS, Bartlett JG. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of Pneumococcus. Clin Infect Dis 2017; 65:1736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eliakim-Raz N, Robenshtok E, Shefet D, et al. . Empiric antibiotic coverage of atypical pathogens for community-acquired pneumonia in hospitalized adults. Cochrane Database Syst Rev 2012;. 2012:CD004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Porter ME, Teisberg EO.. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.